Abstract
Objectives:
We sought to evaluate the relevance of pediatric dairy fat recommendations for children at risk for nonalcoholic fatty liver disease (NAFLD) by studying the association between dairy fat intake and the amount of liver fat. The effects of dairy fat may be mediated by odd chain fatty acids (OCFA), such as pentadecanoic acid (C15:0), and monomethyl branched chain fatty acids (BCFA), such as iso-heptadecanoic acid (iso-C17:0). Therefore, we also evaluated the association between plasma levels of OCFA and BCFA with the amount of liver fat.
Methods:
Observational, cross-sectional, community-based sample of 237 children ages 8–17. Dairy fat intake was assessed by three 24-hour dietary recalls. Plasma fatty acids were measured by gas chromatography–mass spectrometry. Main outcome was hepatic steatosis measured by whole liver magnetic resonance imaging proton density fat fraction (MRI-PDFF).
Results:
Median dairy fat intake was 10.6 grams/day (range 0.0 – 44.5 g/d). Median liver MRI-PDFF was 4.5% (range 0.9% – 45.1%). Dairy fat intake was inversely correlated with liver MRI-PDFF (r = − 0.162; p = .012). In multivariable log linear regression, plasma C15:0 and iso-C17:0 were inverse predictors of liver MRI-PDFF (B = −0.247, p=0.048; and B=−0.234, p=0.009).
Conclusions:
Dairy fat intake, plasma C15:0, and plasma iso-C17:0 were inversely correlated with hepatic steatosis in children. These hypothesis-generating findings should be tested through clinical trials to better inform dietary guidelines.
Keywords: nutrition, milk, nonalcoholic steatohepatitis, obesity
INTRODUCTION
Pediatric dietary recommendations regarding dairy fat do not directly address NAFLD (nonalcoholic fatty liver disease). This is a critical gap considering NAFLD is the most common chronic liver disease in children and is thought to be strongly influenced by diet. The American Academy of Pediatrics (AAP) recommends that children ≥ 2 years consume low-fat or nonfat milk to reduce cardiovascular disease and limit excessive weight gain.1 However, longitudinal studies contradict this guideline, demonstrating that in children, consumption of nonfat milk is associated with greater weight gain than is observed with consumption of whole fat milk.2 The role of dietary fat in the development and treatment of NAFLD is controversial. Some pediatric gastroenterologists recommend decreasing dietary fat intake which typically includes eliminating whole milk and other sources of dairy fat1; however, dairy fat is not simply a source of cholesterol or calories, but rather a complex mixture of bioactive fatty acids. This mixture includes odd-chain fatty acids (OCFA) and monomethyl branched chain fatty acids (BCFA) which may have a beneficial effect on hepatic steatosis and type 2 diabetes.3
Observational studies in adults demonstrate an association between whole fat dairy consumption and multiple health benefits.4 Several authors suggest that OCFA, such as pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) mediate the beneficial effects of dairy fat.3 These OCFA are biomarkers of dairy fat intake and in adults are also associated with a lower incidence of type 2 diabetes. Furthermore, authors have speculated that the link between OCFA and decreased diabetes may be through OCFA-mediated reduction in liver fat.5 In addition to OCFA, BCFA such as iso-heptadecanoic acid (iso-C17:0), are present in dairy fat comprising 2% of total fatty acids in cow’s milk. BCFA are saturated fatty acids containing methyl-branches in the iso- or anteiso- positions. The plasma levels of BCFA are lower in adults with obesity6 but their levels in relation to hepatic steatosis are unknown.
We sought to evaluate the relevance of the pediatric dairy fat recommendation by studying whether dairy fat intake is associated with hepatic steatosis in children at risk for NAFLD. We designed a prospective observational study with the following aims: 1) to evaluate the association between dairy fat intake and the amount of liver fat, and 2) to evaluate the association between plasma levels of dairy associated OCFA and BCFA with the amount of liver fat. We hypothesized that higher intake of dairy fat is associated with lower liver fat and that plasma OCFAs and BCFAs correlate negatively with liver fat.
METHODS
Study Cohort
This was an observational, cross-sectional study of children at risk for NAFLD, ages 8 through 17. Participants were recruited from primary care offices and community health centers from July 2015 through December 2017. In order to focus on children at risk for NAFLD we oversampled males and children with obesity.7,8,9 The oversampling approach was to enroll a study population that was comprised of 60 to 65% males and that at least 60% of participants had a body mass index (BMI) percentile ≥ 95th percentile. Exclusion criteria were: (1) inability to complete MRI evaluation (claustrophobia, MRI-contraindicated metal implants, or body circumference greater than the imaging chamber), (2) established diagnosis of chronic liver disease, (3) chronic use of medications known to cause hepatic steatosis or raise liver chemistry, (4) chronic diseases that may have secondary effects on the liver, (5) substance abuse, and (6) pregnancy. The study was approved by the Institutional Review Board of the UC San Diego. Written informed consent from the parent or legal guardian and assent from the participants were obtained.
Clinical and Laboratory Evaluation
Participants made a fasting visit to the Altman Clinical and Translational Research Institute at UC San Diego. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the subjects standing, wearing light clothing without shoes. Body mass index was calculated as weight (kilograms) divided by height squared (meters). Blood was collected after a 12-hour fast for fatty acid analysis (see below) and the following routine laboratory measures: complete blood count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), glucose, insulin, glycosylated hemoglobin, total cholesterol, HDL-cholesterol and triglycerides.
Dietary Evaluation
All participants completed three 24-hour dietary recalls. Recalls were performed on non-consecutive days, capturing two weekdays and one weekend day within a two-week period. Recalls were done by a multiple-pass interview approach performed by a registered dietician using Nutrition Data System for Research (NDSR; University of Minnesota Nutrition Coordinating Center, Minneapolis, MN). Assessment of dairy fat intake in grams per day was done using published NDSR methodology.10 Data from the three recalls were averaged to yield per day estimates. Total calories per day were calculated and were considered unreliable and excluded if reported intake was less than 50% of the recommended daily caloric intake for age.11,12 We also calculated daily intake (grams per day) of protein, fat, carbohydrates, and sugars.
Fatty Acid Analysis
In addition to dietary recall data, plasma fatty acid profiles provide an objective measure of recent dietary intake. We measured OCFA and BCFA associated with dairy fat including C15:0, C17:0, iso-C17:0, and anteiso-C17:0.13,14 We also measured 20 other fatty acids selected as the most abundant fatty acids in human plasma.15 A complete list is shown in Table, Supplemental Digital Content 1. Plasma total fatty acids were extracted using a Folch-based methanol/chloroform/saline extraction at a ratio of 1:2:1 with inclusion of d31 C16:0, d3 C14:0, d3 C15:0, d3 C17:0, d3 C18:0, d2 C18:1n9, d3 C22:0, and d4 C24:0 as internal standards.16 Briefly, 250 μl MeOH, 500 μl CHCl3, 250 μl saline, and the fatty acid isotope internal standards were added to 100 μl plasma. This was vortexed for 10 minutes followed by centrifugation at 10,000 g for 5 minutes at 4°C. The lower chloroform phase was dried under nitrogen and then derivatized to form fatty acid methyl esters (FAMEs) via addition of 500 μl 2% H2SO4 in MeOH and incubation at 50°C for 2 hours. FAMEs were extracted via addition of 100 μl saturated salt solution and 500 μl hexane and were then analyzed using a Select FAME column (100m × 0.25mm i.d.) installed in an Agilent 7890A GC interfaced with an Agilent 5975C MS using the following temperature program: 80 °C initial, increase by 20 °C/min to 170 °C, increase by 1 °C/min to 204 °C, then 20 °C/min to 250 °C and hold for 10 min. Fatty acids were quantified from deuterated internal standards or external standard curves.
MRI Evaluation
MRI examinations were performed on a 3 Tesla scanner (GE Discovery 750, General Electric Healthcare, Wakeshaw WI) using a previously described advanced magnitude-based confounder-corrected chemical-shift-encoded acquisition and reconstruction technique to estimate proton density fat fraction (PDFF).17,18,19 T1 weighting was minimized by using a gradient-recalled-echo sequence with a low (10°) flip angle relative to a repetition time (TR) of ≥ 150 ms. Six gradient-recalled echoes were collected at successive nominally out-of-phase and in-phase echo times to allow correction for T2* signal decay.20,21 Computer-generated parametric PDFF maps were calculated using least-squares fitting analysis based on a six-fat-peak spectral model to correct for inter-peak spectral interference.22 For each PDFF parametric map, a 1-cm radius circular region of interest was manually placed in each of the 9 Couinaud liver segments, and a composite mean PDFF value was calculated.8
Data Analysis
Demographics and other study population characteristics were described. Categorical data were summarized as percentages and counts. One-way analysis of variance was used to calculate mean, median, range, and standard deviation of all continuous data. Correlation analysis was performed using Pearson’s correlation for parametric data and Spearman’s rank order correlation for non-parametric data. After bivariate analysis, stepwise multiple linear regression was used to determine the relationship between dairy fat intake (predictor variable) and hepatic MRI-PDFF (outcome variable) while controlling for age, sex, and BMI z-score as potential confounders. This relationship was also evaluated by sensitivity analyses to further control for other dietary factors (total calorie intake, total fat intake, total carbohydrate intake, total sugar intake). To support these analyses, a minimum sample size of 200 participants was projected. In addition, multivariate stepwise linear regression analyses were used to examine fatty acid predictors of MRI-PDFF while controlling for confounders. Variables that were not normally distributed were transformed logarithmically for regression analyses (liver MRI-PDFF and plasma fatty acids). Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 24.0 (Armonk, NY: IBM Corp) and R 3.5.0 (Vienna, Austria, R foundation for statistical computing). A p value < 0.05 was considered significant for all inference testing.
RESULTS
Study Cohort
Flow for screening, exclusion and inclusion is shown in Figure, Supplemental Digital Content 2. Characteristics of the study population are shown in Table 1. We studied 237 children with a mean age of 12.9 ± 2.6 years. There were more males than females (62% vs 38%). Obesity was present in 71% of participants. Mean ALT (SD) was 35 (49) U/L.
Table 1.
Characteristics of Study Population (n = 237)
| Characteristic | Value | |
|---|---|---|
| Demographics | ||
| Age, y, mean (SD) | 12.9 (2.6) | |
| Sex, n (%) | ||
| Male | 146 (62) | |
| Female | 91 (38) | |
| Race, n (%) | ||
| American Indian | 15 (6) | |
| Black | 18 (8) | |
| White | 104 (44) | |
| Other | 100 (42) | |
| Ethnicity, n (%) | ||
| Hispanic | 178 (75) | |
| Non-Hispanic | 59 (25) | |
| Anthropometrics | ||
| Weight, kg, mean (SD) | 71.0 (24.7) | |
| Height, cm, mean (SD) | 157.3 (13.8) | |
| BMI, kg/m2, mean (SD) | 28.0 (6.9) | |
| BMI percentile, mean (SD) | 89.6 (20.8) | |
| BMI z score, mean (SD) | 1.7 (1.0) | |
| Nutrition (per day) | ||
| Total Calories, mean (SD) | 1704 (377) | |
| Fat, gm, mean (SD) | 62 (23) | |
| Protein, gm, mean (SD) | 72 (21) | |
| Carbohydrates, gm, mean (SD) | 214 (53) | |
| Sugars, gm, mean (SD) | 85 (42) | |
| Dairy Fat, gm, mean (SD) | 12.1 (7.9) | |
| Laboratories | ||
| ALT, U/L, mean (SD) | 35 (49) | |
| AST U/L, mean (SD) | 31 (27) | |
| GGT U/L, mean (SD) | 25 (28) | |
| Glucose mg/dL, mean (SD) | 87.0 (12) | |
| HbA1c, percent, mean (SD) | 5.4 (0.7) | |
| Insulin μIU/mL, mean (SD) | 27 (23) | |
| Triglycerides mg/dL, mean (SD) | 114 (69) | |
| HDL-cholesterol, mg/dL, mean (SD) | 45 (11) | |
| LDL-cholesterol, mg/dL, mean (SD) | 88 (25) | |
| Liver MRI-PDFF, percent, mean (SD) | 7.9 (8.1) | |
SD = standard deviation; BMI = body mass index; ALT = alanine aminotransferase; AST = aspartate aminotransferase; GGT = gamma-glutamyl transferase; U/L = units/liter; MRI-PDFF = magnetic resonance imaging proton density fat fraction; HDL = high density lipoprotein; LDL = low density lipoprotein.
Dairy Fat Intake
The median dairy fat intake in study participants was 10.6 grams per day (range 0.0 – 44.5 grams). The distribution of dairy fat consumed per day is shown in Figure, Supplemental Digital Content 3. Dairy fat intake was not significantly associated with age (p = 0.148) or sex (p = 0.911) (Figure, Supplemental Digital Content 4). Dairy fat intake (grams/day) was significantly negatively correlated with body mass index z-score (r = −0.20, p = .002). Dairy fat intake was also significantly negatively correlated with ALT (r = −0.18, p = 0.007), but not HDL (r = 0.07, p = .286), LDL (r = −0.07, p = .275), or triglycerides (r = −0.09, p = .151).
Dairy Fat Intake and Liver Fat
The median MRI liver PDFF was 4.5% (range 0.9% – 45.1%). There was a significant negative correlation between grams of dairy fat consumed per day and liver MRI-PDFF (r = − 0.162; p = .012) (Figure 1). A multiple linear regression model was calculated to predict liver MRI-PDFF based upon dairy fat intake after controlling for age, sex, ethnicity and BMI z-score. The model was significant with R2 = 0.297; p < 0.001 (Table 2). The inverse relationship between the amount of dairy fat consumed and liver MRI-PDFF was significant after controlling for the other elements of the model (beta −0.152, t −2.675, p = 0.008) (Table 2). In this model, an additional one cup of whole milk (7.93g of dairy fat) would be associated with liver MRI PDFF that is 6.5 percentage points lower. In sensitivity analyses, further controlling for total calories, total fat, total carbohydrate, or total sugar intake attenuated but did not eliminate the relationship between dairy fat intake and liver MRI-PDFF (with total calorie intake, beta −0.133, t −2.145, p = 0.033; with total fat intake, beta −0.127, t −2.138, p = 0.034; with total carbohydrate intake, beta −0.141, t −2.372, p = 0.019; with total sugar intake, beta −0.148, t-2.602, p=0.012).
Figure 1. Distribution of daily dairy fat intake by liver parameters.
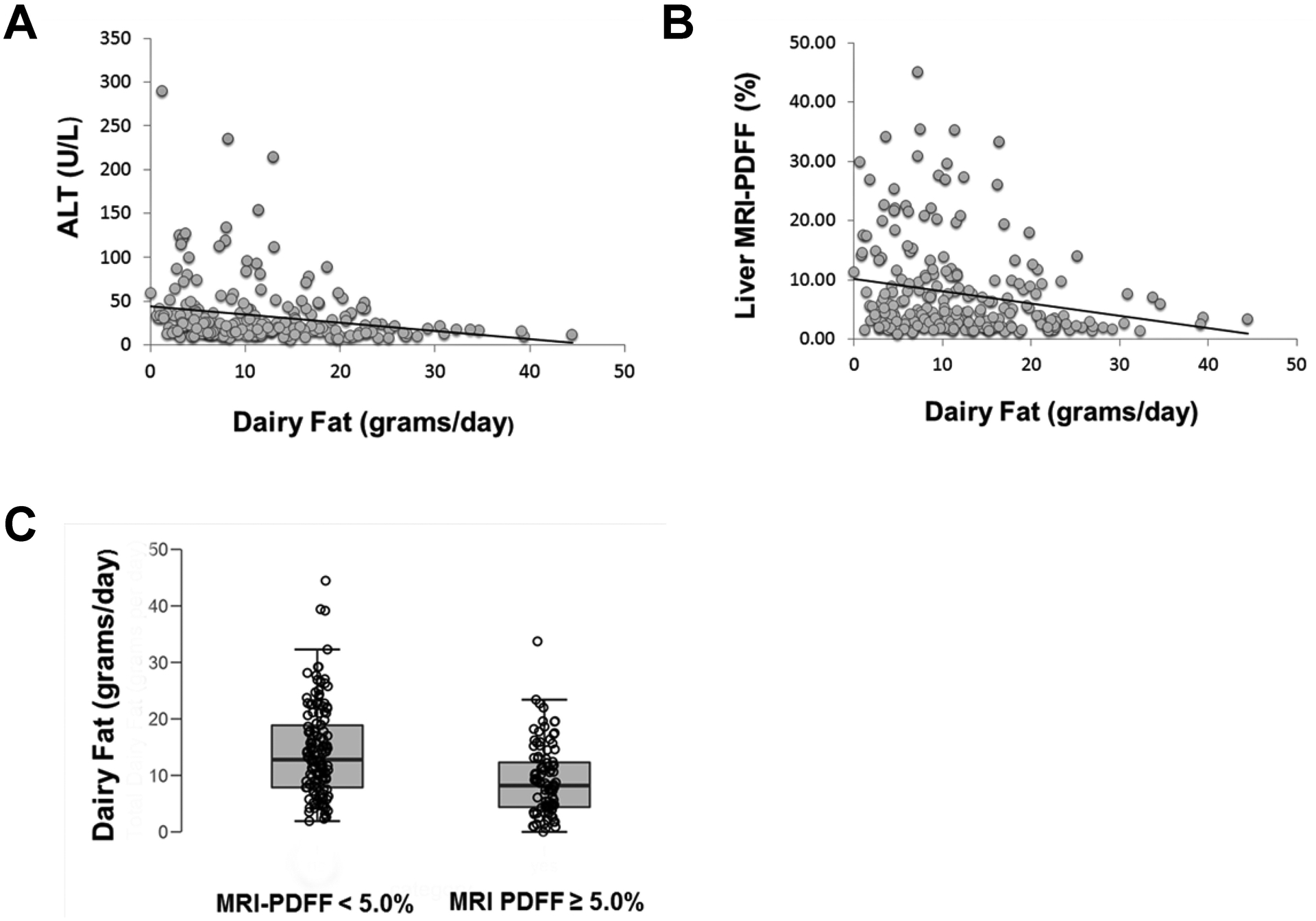
Panel A shows ALT; dairy fat was significantly negatively correlated with ALT (r = −0.18, p = 0.007). Panel B shows liver MRI-PDFF; dairy fat was significantly negatively correlated with liver MRI-PDFF (r = − 0.162; p = .012). Panel C shows liver MRI-PDFF separated by children above and below 5% liver MRI-PDFF. Children with MRI liver-PDFF ≥ 5% had significantly lower dairy fat intake than children with liver MRI-PDFF < 5% (mean 9.1 grams/day vs 14.2 grams/day; p < 0.001)
Table 2.
Multivariate Regression Model of Dairy Fat Intake as a Predictor of Liver MRI-PDFF
| β | t | p | |
|---|---|---|---|
| Dairy fat, gm/d | −0.152 | −2.675 | 0.008 |
| Age, y | −0.372 | −4.006 | <0.001 |
| Sex (male) | 0.125 | 2.111 | 0.036 |
| Ethnicity (Hispanic) | 0.401 | 4.623 | <0.001 |
| BMI z-score | 0.451 | 5.200 | <0.001 |
| Intercept | 2.040 | 5.695 | <0.001 |
BMI = body mass index; MRI-PDFF = magnetic resonance imaging proton density fat fraction. Variables that were not normally distributed were transformed logarithmically for regression analyses (dairy fat and liver MRI-PDFF). Model R2 = 0.297, p<0.001.
Plasma Fatty Acids and Liver Fat
The correlation of plasma fatty acids with liver MRI-PDFF was evaluated. Of the 29 plasma fatty acids measured, 20 were significantly correlated with liver MRI-PDFF in univariate analysis (Table 3). Of these, only three, C15:0, C17:0, and iso-C17:0, had inverse correlations with liver MRI-PDFF. A stepwise multivariable log linear model of plasma fatty acids adjusted for age, sex, ethnicity, and BMI z-score was significantly predictive of liver MRI-PDFF (Table 4, R2 = 0.481; p < 0.001). The covariates sex (male vs female) and BMI z-score were independent predictors of liver MRI-PDFF. Only five plasma fatty acids remained independently associated with liver MRI-PDFF. Negative predictors of liver MRI-PDFF were plasma levels of C15:0, iso-C17:0 and C20:4n6. Positive predictors of liver MRI-PDFF were plasma levels of C16:1 and C18:0.
Table 3.
Univariate Linear Regression of Plasma Fatty Acid Concentrations as Predictors of Liver MRI-PDFF
| Fatty Acid | Name | β | t | p |
|---|---|---|---|---|
| Significant Negative Correlations | ||||
| Iso-C17:0 | iso-heptadecanoic acid | −0.275 | −5.838 | <0.001 |
| C15:0 | Pentadecanoic acid | −0.115 | −2.354 | 0.006 |
| C17:0 | Heptadecanoic acid | −0.107 | −2.113 | 0.017 |
| Significant Positive Correlations | ||||
| C16:1 | Palmitoleic acid | 0.345 | 7.502 | <0.001 |
| C20:3, n-6 | Dihomo-γ-linoleic acid | 0.305 | 6.528 | <0.001 |
| C16:0 | Palmitic acid | 0.281 | 5.972 | <0.001 |
| C18:0 | Stearic acid | 0.266 | 5.617 | <0.001 |
| C18:1 | Oleic acid | 0.261 | 5.505 | <0.001 |
| C20:1n9 | 11-eicosenoic | 0.253 | 5.307 | <0.001 |
| C14:0 | Myristic acid | 0.218 | 4.530 | <0.001 |
| C20:2 | 11,14-eicosadienoic acid | 0.181 | 3.738 | <0.001 |
| C17:1 | Heptadecenoate | 0.179 | 3.685 | <0.001 |
| C18:3, n-3 | α-linolenic acid | 0.160 | 3.293 | <0.001 |
| C20:0 | Arachidic acid | 0.156 | 3.202 | 0.001 |
| C14:1 | Myristoleic | 0.147 | 3.012 | 0.003 |
| C18:2 | Linoleic acid | 0.144 | 2.954 | 0.003 |
| C18:3, n-6 | γ-linolenic acid | 0.136 | 2.779 | <0.001 |
| C12:0 | Lauric acid | 0.116 | 2.360 | 0.019 |
| C20:5, n-3 | Eicosapentaenoic acid | 0.102 | 2.313 | 0.019 |
| No Significant Correlations | ||||
| iso-C16:0 | iso-palmitic acid | −0.000 | −0.003 | 0.998 |
| Anteiso- C17:0 | anteiso-heptadecanoic acid | 0.018 | 0.372 | 0.512 |
| Iso-C18:0 | iso-octadecanoate | −0.026 | −0.527 | 0.599 |
| C20:3, n-3 | 11,14,17-eicosatrienoic acid | 0.068 | 1.389 | 0.166 |
| C20:4, n-6 | Arachidonic acid | −0.042 | −0.849 | 0.397 |
| C22:6, n-3 | Docosahexaenoic acid | 0.059 | 1.202 | 0.230 |
| C22:1n9 | Erucic | 0.0027 | 0.544 | 0.587 |
| C22:2 | 13,16-docosadienoic acid | −0.035 | −0.714 | 0.476 |
| C22:0 | Behenic acid | 0.017 | 0.345 | 0.730 |
| C24:1n9 | Nervonic acid | 0.027 | 0.544 | 0.587 |
Table 4.
Multivariate Model of Plasma Fatty Acids as Predictors of Liver MRI-PDFF
| Fatty Acids | β | t | p |
|---|---|---|---|
| C15:0 | −0.247 | −2.00 | 0.041 |
| C16:1 | 0.322 | 5.90 | < 0.001 |
| Iso-C17:0 | −0.234 | −2.63 | 0.009 |
| C18:0 | 0.426 | 4.68 | < 0.001 |
| C20:4n6 | −0.281 | −4.60 | < 0.001 |
| Covariates | |||
| Age, y | −0.242 | −2.834 | 0.007 |
| Sex (male) | 0.139 | 2.852 | 0.005 |
| Ethnicity (Hispanic) | 0.277 | 3.743 | <0.001 |
| BMI z-score | 0.312 | 5.891 | <0.001 |
Model R2 = 0.481; p < 0.001. BMI = body mass index; MRI-PDFF = magnetic resonance imaging proton density fat fraction. C15:0 = pentadecanoic acid; C16:1 = palmitoleic acid; iso-C17:0 = iso-heptadecanoic acid, C18:0 = stearic acid, C20:4n6 = arachidonic acid methyl ester.
DISCUSSION
We evaluated a large community-based sample of children at risk for NAFLD and assessed the relationship between dietary dairy fat intake and hepatic steatosis. We also evaluated plasma fatty acids in relationship to hepatic steatosis. We found that the average daily intake of dairy fat was significantly inversely correlated with liver MRI-PDFF. In plasma, the concentrations of the OCFA C15:0 and the BCFA iso-C17:0 were significant negative predictors of liver MRI-PDFF. In addition to C15:0 and iso-C17:0, liver MRI-PDFF was negatively associated with arachidonic acid (C20:4n6) and was positively associated with stearic acid (C18:0) and palmitoleic acid (C16:1).
We found that dairy fat intake was inversely correlated with liver MRI-PDFF in children. We also observed that dairy fat intake was negatively associated with BMI z-score, which is consistent with studies that have shown lower weight gain in children drinking whole milk.2 Importantly, the negative association between dairy fat intake and liver fat was independent of BMI z-score. There have been no prior pediatric studies of the relationship between dairy fat intake and liver fat; however, our data build upon two prior adult studies of this relationship. Kratz et al performed an observational study of 17 adults with NAFLD and 15 controls and found a significant negative correlation between dairy fat intake and computed tomography measured liver-spleen ratio, a surrogate measure for liver fat.23 In the second, an interventional crossover study, Dugan et al evaluated 37 adults who consumed non-dairy control foods in one phase and three servings of dairy per day in the other phase.24 This study reported significantly lower AST and ALT at the end of the dairy consumption period compared to the control foods phase; however, there were no measurements of hepatic fat performed. In our study, we also found that higher dairy fat intake was associated with lower ALT values.
The OCFA, pentadecanoic acid (C15:0) was negatively associated with hepatic steatosis. C15:0 is produced by microbial fatty acid oxidation and de novo lipogenesis in the bovine rumen and the primary source of this for humans is the consumption of dairy fat.25,26 In adults, C15:0 is an established circulating marker of dairy fat intake,27,28,29 and interventional studies show that circulating levels of C15:0 can be modified by changing the dietary intake of dairy fat.30,31,32 There are no prior pediatric data on the relationship between the plasma measures of C15:0 and liver fat. Two studies in adults have evaluated C15:0 concentration levels in subjects with NAFLD. Yoo et al evaluated plasma fatty acid profiles in 106 adults with NAFLD and found that plasma C15:0 was significantly lower in patients with higher NAFLD activity scores. Similarly, Kratz et al reported that plasma C15:0 was significantly lower in 17 adults with NAFLD than in 15 controls without NAFLD. In much larger, longitudinal studies, higher plasma levels of C15:0 were associated with lower incidence of both cardiovascular disease and type 2 diabetes.33,34 In a mouse model, supplementation with C15:0 decreased the severity of NAFLD.35 In humans, whether increasing the intake of C15:0 could potentially influence NAFLD is not yet known.
We also demonstrated that lower levels of iso-C17:0 were associated with higher amounts of hepatic steatosis. Prior studies of fatty acid composition in subjects with NAFLD have not evaluated this isomer. Iso-C17:0 is a branched chain fatty acid made by rumen microbiota and found primarily in dairy products.36 In addition to dietary sources, iso-C17:0 can be synthesized de novo from branched chain amino acids (BCAA).6 In adults, hepatic steatosis was associated with elevated plasma BCAA.6 One reason for simultaneous high BCAA and low BCFA can be a disturbance in the conversion from BCAA to BCFA. Thus, our finding that plasma iso-C17:0 concentrations were significantly lower in children with NAFLD may be due to both lower dietary intake of iso-C17:0 and reduced metabolism of BCAA to BCFA.
In addition to perceptions regarding dairy fat, there are widely held notions regarding the healthfulness of other dietary fats. Dietary advice has progressed from limiting all dietary fat to preferencing “good” fats such as monounsaturated fats and avoiding “bad” fats such as saturated fats. However, following such dietary recommendations is difficult because we do not eat or drink foods in isolation; rather diets are a complex mixture of many foods. In turn the fatty acid composition of foods can have a wide range of effects. In addition to C15:0 and iso-C17:0, there were three other fatty acids associated with liver MRI-PDFF. Stearic acid, C18:0, was the most strongly positively associated with hepatic steatosis. Stearic acid is a saturated fat that is highly prevalent in many foods, especially beef and pork.37 However, stearic acid is also present in dairy fat as well as plant-based oils. Palmitoleic acid, C16:1, was also strongly positively associated with hepatic steatosis. Palmitoleic acid is a monounsaturated fat also found in many foods. The top sources are beef, pork, avocado, butter, salmon, and olive oil.38 Conversely, arachidonic acid was inversely associated with liver MRI-PDDF. Arachadonic acid is an omega-6 polyunsaturated fatty acid found in many foods including eggs, fish, and butter.39 It is difficult to disentangle the source of each of these fatty acids in the individual diet for each participant and thus it remains challenging to develop fully optimized dietary recommendations to prevent or treat NAFLD.
Strengths of this study include the sample size, detailed phenotyping, and the measurement of liver fat by liver MRI-PDFF. We used a validated approach to assess quantitative dairy fat intake. There are known limitations to the self-report of dietary intake such as recall bias. Dietary choice may be confounded by many factors and the analyses did not control for these. In addition, dairy fat can be consumed in numerous forms such as milk, cheese, yogurt, butter, or as part of mixed foods. A larger study would be required to test for the effect of specific foods. To mitigate the limitations of self-reporting, we measured plasma fatty acids known to be associated with dairy fat intake by mass spectroscopy.40 Importantly, the inverse relationship between the dairy associated fatty acids C15:0 and iso-C17:0 was independent of other fatty acids as well as sex and BMI z-score. This study was cross-sectional and observational and thus did not evaluate causality. The findings should be considered as hypothesis generating.
In conclusion, we found that dairy fat intake as well as the plasma levels of the OCFA, pentadecanoic acid (C15:0), and the BCFA, iso-heptadecanoic acid (iso-C17:0), were inversely correlated with hepatic steatosis in children. These findings have implications for dietary guidelines and future biomarker studies. Clinical trials of whole fat dairy would be needed to know whether consuming higher amounts of dairy fat decreases liver fat in children. Such studies should consider to what extent changes are mediated by increases in circulating OCFA and BCFA.
Supplementary Material
Figure of the Distribution of Dairy Fat Intake by Age, Sex, and BMI z-score
Figure of the Distribution of Dairy Fat Intake versus ALT and Liver MRI-PDFF
Table of Plasma Fatty Acids Measured
Figure of Study Flow Diagram
Figure of the Distribution of Daily Dairy Fat Intake
What is Known
Pediatric nutrition guidelines recommend that children 2 years and older consume low-fat or nonfat milk to reduce cardiovascular disease and limit excessive weight gain
These guidelines do not directly address nonalcoholic fatty liver disease (NAFLD)
What is New
In children at risk for NAFLD, the average daily intake of dairy fat was significantly inversely correlated with the amount of liver fat
Higher amounts of plasma fatty acids associated with dairy fat, specifically the odd chain fatty acid, C15:0, and the branched chain fatty acid, iso-C17:0, were associated with lower amounts of liver fat
Funding:
The project was partially supported by grants from the National Institutes of Health, Grants UL1TR000100 and UL1TR001442, National Dairy Council, and the Camille and Henry Dreyfus Foundation. The funders did not participate in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the National Dairy Council, or the Camille and Henry Dreyfus Foundation.
Conflict of Interest Statement:
Sawh, Wallace, Shapiro, Goyal, Newton, Yu, Bross, Durelle, Knott, Gangoiti, Barshop, Gengatharan, Meurs, Schlein, Metallo: no conflicts of interest
Middleton: consults for Bracco, Kowa, Median, Merge Healthcare, Novo Nordisk, Quantitative Insights; and has grant funding from Gilead and Guerbet.
Sirlin: grant funding from Bayer, GE, Philips and Siemens; consults for AMRA, Boehringer and Guerbet; is on the speaker’s bureau for Resoundant and has lab service agreements with Gilead, ICON, Intercept, Shire and Synageva.
Schwimmer: grant funding from Galmed, Intercept, and Genfit.
Abbreviations:
- ALT
Alanine aminotransferase
- AAP
American Academy of Pediatrics
- AST
Aspartate Aminotransferase
- BCAA
Branched Chain Amino Acids
- BCFA
Branched Chain Fatty Acids
- FAMEs
Fatty Acid Methyl Esters
- GGT
Gamma-Glutamyl Transpeptidase
- NAFLD
Non-Alcoholic Fatty Liver Disease
- NDSR
Nutrition Data System for Research
- OCFA
Odd Chain Fatty Acids
- PDFF
Proton Density Fat Fraction
REFERENCES
- 1.Gidding SS, Dennison BA, Birch LL et al. Dietary Recommendations for Children and Adolescents A Guide for Practitioners Consensus Statement From the American Heart Association Endorsed by the American Academy of Pediatrics. Circulation 2005; 112:2061–2075. [DOI] [PubMed] [Google Scholar]
- 2.Beck AL, Heyman M, Chao C, et al. Full fat milk consumption protects against severe childhood obesity in Latinos. Prev Med Rep 2017; 23(8):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura F, Fretts A, Marklund M, et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLOS Medicine 2018; 15(10): e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kummer K, Jensen PN, Kratz M, et al. Full-Fat Dairy Food Intake is Associated with a Lower Risk of Incident Diabetes Among American Indians with Low Total Dairy Food Intake. The Journal of Nutrition 2019; 149(7): 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yakoob MY, Shi P, Willett WC, et al. Circulating Biomarkers of Dairy Fat and Risk of Incident Diabetes Mellitus Among Men and Women in the United States in Two Large Prospective Cohorts. Circulation 2016; 133(17): 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace M, Green CR, Roberts LS, et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat Chem Biol 2018; 14(11): 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006; 118: 1388–1393. [DOI] [PubMed] [Google Scholar]
- 8.Yu E, Golshan S, Harlow K, et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. Journal of Pediatrics 2019; 207:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spear BA, Barlow SE, Ervin C, et al. Recommendations for Treatment of Child and Adolescent Overweight and Obesity. Taveras Pediatrics 2007; 120(4): S254–S288. [DOI] [PubMed] [Google Scholar]
- 10.Dugan CE, Barona J, Fernandez ML. Increased Dairy Consumption Differentially Improves Metabolic Syndrome Markers in Male and Female Adults. Metabolic Syndrome and Related Disorders 2014; 12(1): 62–69. [DOI] [PubMed] [Google Scholar]
- 11.Evans EW, Jacques PF, Dallal GE, et al. The role of eating frequency on total energy intake and diet quality in a low-income, racially diverse sample of schoolchildren. Public Health Nutr 2015; 18(3): 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips KM, Patterson KY, Rasor AS, et al. Quality-control materials in the USDA National Food and Nutrient Analysis Program (NFNAP). Anal Bioanal Chem 2006; 384: 1341–1355. [DOI] [PubMed] [Google Scholar]
- 13.Yuzyuk T, Lozier B, Schwarz EL, Viau K, Kish-Trier E, De Biase I. Intra-individual variability of long-chain fatty acids (C12-C24) in plasma and red blood cells. Prostaglandins Leukot Essent Fatty Acids. 2018;135:30–38. [DOI] [PubMed] [Google Scholar]
- 14.Albani V, Celis-Morales C, O’Donovan CB, et al. Within-person reproducibility and sensitivity to dietary change of C15:0 and C17:0 levels in dried blood spots: Data from the European Food4Me Study. Mol Nutr Food Res. 2017;61(10): 10.1002/mnfr.201700142. [DOI] [PubMed] [Google Scholar]
- 15.Abdelmagid SA, Clarke SE, Nielsen DE, et al. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults [published correction appears in PLoS One. 2015;10(5):e0128167]. PLoS One. 2015;10(2):e0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metallo CM, Gameiro PA, Bell EL, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2011: 481(7381): 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 2015; 61:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging 2008; 26:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CY, McKenzie CA, Yu H, et al. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med 2007; 58:354–364. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Shimakawa A, McKenzie CA, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008; 60:1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging 2007; 26:1153–1161. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed 2011; (24): 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr 2013; 52: 1–24. [DOI] [PubMed] [Google Scholar]
- 24.Dugan CE, Aguilar D, Park YK, et al. Dairy Consumption Lowers Systemic Inflammation and Liver Enzymes in Typically Low-Dairy Consumers with Clinical Characteristics of Metabolic Syndrome. Journal of the American College of Nutrition 2016; 35(3):255–261. [DOI] [PubMed] [Google Scholar]
- 25.Crowe FL, Key TJ, Appleby PN, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. The American Journal of Clinical Nutrition 2008; 87(5): 1405–1413. [DOI] [PubMed] [Google Scholar]
- 26.Smedman A, Gustafsson IB, Berglund L, et al. Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. The American Journal of Clinical Nutrition 1999; 69(1): 22–29. [DOI] [PubMed] [Google Scholar]
- 27.Risérus U, Marklund M. Milk fat biomarkers and cardiometabolic disease. Current Opinion in Lipidology 2017; 28(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolk A, Vessby B, Ljung H, et al. Evaluation of a biological marker of dairy fat intake, The American Journal of Clinical Nutrition 1998; 68(2): 291–295. [DOI] [PubMed] [Google Scholar]
- 29.Wolk A, Furuheim M, Vessby B. Fatty Acid Composition of Adipose Tissue and Serum Lipids Are Valid Biological Markers of Dairy Fat Intake in Men. The Journal of Nutrition 2001; 131(3): 828–833. [DOI] [PubMed] [Google Scholar]
- 30.Vessby B, Uusitupa M, Hermansen K, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU study. Diabetologica 2001; 44(3): 312–319. [DOI] [PubMed] [Google Scholar]
- 31.Brevik A, Veierød MB, Drevon CA, et al. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur. J. Clin. Nutr 2005; 59: 1417–1422. [DOI] [PubMed] [Google Scholar]
- 32.Wennersberg MH, Smedman A, Turpeinen AM, et al. Dairy products and metabolic effects in overweight men and women: results from a 6-mo intervention study, The American Journal of Clinical Nutrition 2009; 90(4): 960–996. [DOI] [PubMed] [Google Scholar]
- 33.Otto MC, Lemaitre RN, Song X, et al. Serial measures of circulating biomarkers of dairy fat and total and cause-specific mortality in older adults: the Cardiovascular Health Study. The American Journal of Clinical Nutrition 2018; 108(3) 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Otto MC, Lemaitre RN, et al. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA), The American Journal of Clinical Nutrition 2013; 97(4): 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo W, Gjuka D, Stevenson HL, et al. Fatty acids in non-alcoholic steatohepatitis: Focus on pentadecanoic acid. PLoS ONE 2017; 12(12): e0189965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlaeminck B, Fievez V, Tamminga S, et al. Milk Odd- and Branched-Chain Fatty Acids in Relation to the Rumen Fermentation Pattern. Journal of Dairy Science 2006; 89(10): 3954–3964. [DOI] [PubMed] [Google Scholar]
- 37.Wholefoodcatalog.info. 2020. Foods High In Arachidonic Acid | Whole Food Catalog. [online] Available at: <https://wholefoodcatalog.info/nutrient/arachidonic_acid/foods/high/> [Accessed 18 July 2020].
- 38.Wholefoodcatalog.info. 2020. Palmitoleic Acid Content Of Foods | Whole Food Catalog. [online] Available at: <https://wholefoodcatalog.info/nutrient/palmitoleic_acid/foods/> [Accessed 18 July 2020].
- 39.Wholefoodcatalog.info. 2020. Foods High In Arachidonic Acid | Whole Food Catalog. [online] Available at: <https://wholefoodcatalog.info/nutrient/arachidonic_acid/foods/high/> [Accessed 18 July 2020].
- 40.Foster E, Bradley J. Methodological considerations and future insights for 24-hour dietary recall assessment in children. Nutrition Research 2018; 51:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure of the Distribution of Dairy Fat Intake by Age, Sex, and BMI z-score
Figure of the Distribution of Dairy Fat Intake versus ALT and Liver MRI-PDFF
Table of Plasma Fatty Acids Measured
Figure of Study Flow Diagram
Figure of the Distribution of Daily Dairy Fat Intake


