Abstract
Background:
Prenatal exposure to metals has been individually associated with birth outcomes. However, little is known about the effect of metal mixture, particularly at low exposure levels.
Objectives:
To estimate individual and joint effects of metal mixture components on birth outcomes.
Methods:
We used data from 1,391 mother- pairs in Project Viva (1999–2002). We measured 11 metals in maternal 1st trimester erythrocyte; abstracted birth weight from medical records; calculated gestational age from last menstrual period or ultrasound; and obtained birth length (n=729) and head circumference (n=791) from research measurements. We estimated individual and joint effects of metals using multivariable linear and Bayesian kernel machine regressions.
Results:
In both single metal and metal mixture analyses, exposure to higher concentrations of arsenic was associated with lower birth weight in males, zinc with higher head circumference in females, and manganese with higher birth length in sex-combined analysis. We also observed sex-specific metal interactions with birth outcomes. Arsenic and manganese showed a synergistic association with birth weight in males, in whom an interquartile range (IQR) increase in arsenic was associated with 25.3g (95%CI:−79.9, 29.3), 47.9g (95%CI:−98.0, 2.1), and 72.2g (95%CI:−129.8, −14.7) lower birth weight when manganese concentrations were at 25th, 50th, and 75th percentiles, respectively. Lead and zinc showed an antagonistic association with head circumference in males, where an IQR increase in lead was associated with 0.18cm (95%CI:−0.35, −0.02), 0.10cm (95%CI:−0.25, 0.04), 0.03cm (95%CI:−0.2, 0.14) smaller head circumference when zinc concentrations were at 25th, 50th, and 75th percentiles, respectively. Exposure to higher concentrations of arsenic was also associated with lower gestational age in males when concentrations of manganese and lead were higher.
Discussion:
Maternal erythrocyte concentrations of arsenic, manganese, lead, and zinc were individually and interactively associated with birth outcomes. The associations varied by infant sex and exposure level of other mixture components.
Keywords: Metals, environmental exposure, birth outcome, arsenic, manganese, lead, pregnancy, mixture analysis
1. INTRODUCTION
Size at birth is an important predictor of early childhood mortality and morbidity and a valuable indicator of adulthood risk for certain chronic diseases, including diabetes and cardiovascular diseases (1). Prenatal environmental exposure to certain metals and metalloids has been individually associated with body size at birth. However, fetal exposure to environmental chemicals rarely happens in isolation. Metals are ubiquitous in the environment, and many metals such as cadmium, mercury, and lead and some metalloids such as arsenic and selenium (for simplicity, we will refer all elements as metals) can easily cross the placenta (2–4). Metals can interact with each other and their overall effect can be different from their individual effect. Moreover, the growing fetus may be more susceptible to chemical mixture during the intrauterine period. Therefore, it is of paramount importance to assess the joint effect of metals on birth outcomes.
Epidemiological studies across the world have linked prenatal exposure to certain metals with birth parameters. For instance, exposure to arsenic (As), lead (Pb), cadmium (Cd), and manganese (Mn) have been associated with lower birth weight (5–9), shorter gestational age (5, 6, 10), higher risk of preterm birth (11–13), smaller head circumference (5, 7, 8, 14), and shorter birth length (7) in populations exposed to metals at various levels. Some other studies have reported inverted “U-shaped” associations for maternal blood manganese with birth measurements (15, 16) or, negative associations for prenatal zinc (Zn) supplementation with preterm birth (17–19). Most of these studies have assessed metals individually, although a few recent studies have examined the joint effect of certain metal mixture components on neonatal characteristics. These studies, however, did not yield consistent findings on the mixture effect (20–26), and were mostly focused on populations exposed to relatively high levels of metals (5, 6, 27, 28). Studies focusing on metal mixture in populations exposed to relatively lower levels, similar to the background population averages, with no apparent source of exposure are sparse (29, 30), yet they represent the most generalizable health effects of exposure. Furthermore, fewer studies have examined sex-specific effect of metal mixture on birth outcomes despite ample evidence suggesting sex-specific vulnerability to metal toxicity (7, 12, 23, 31, 32).
In the current study, we aimed to estimate the individual and joint effects of metal mixture components on weight, length, head circumference, and gestational age at delivery in infant sex-combined and sex-stratified analyses in Project Viva, a longitudinal pre-birth cohort of pregnant mothers and their children in Eastern Massachusetts. These individuals were not selected based on exposure and thus, are expected to have no history of metal exposure above the background population levels. We estimated these associations at levels relevant to the current U.S. population averages.
2. METHODS
2.1. Study population
Project Viva is a prospective pre-birth cohort (enrolled 1999–2002) that recruited women during their initial prenatal visit in the first trimester at the obstetrical offices of Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice located in eastern Massachusetts. The details of recruitment procedures, inclusion and exclusion criteria have been previously published (33). Briefly, trained research staff obtained written informed consent immediately following the women’s initial clinical prenatal visit, administered a brief interview, provided self-administered questionnaires to obtain demographic and lifestyle information, and collected blood samples for biomarker analyses. Study participation and attrition flow chart is presented in Figure A1. Of the 2128 singleton liveborns, 1423 mothers (of which 15 mothers participated twice in two separate pregnancy periods) provided blood samples at the 1st trimester and were selected for erythrocyte metal measurements.
Mothers provided written informed consent and protocols were approved by the Institutional Review Boards at Harvard Pilgrim Health Care Institute.
2.2. Erythrocyte metal concentrations
Blood samples collected in early pregnancy (mean 11.3 ± 2.8 weeks gestation), were centrifuged at 2000 rpm for 10 minutes at 4°C to separate erythrocytes from plasma; a separate aliquot of erythrocytes was provided for mercury analysis. All aliquots were stored at −70°C prior to metal analyses. For analyses, sample handling was performed in an ISO class 6 clean room with an ISO class 5 laminar flow clean hood. We used triple quadrupole Inductively Coupled Plasma-Mass Spectrometry (ICP-MS; Agilent 8800 ICP-QQQ) to quantify 19 metal(oid)s on a single run in MS/MS mode, which provides higher sensitivity and lower background interference than traditional ICP-MS. We also quantified mercury (Hg) separately using a Direct Mercury Analyzer 80 (Milestone Inc.). For simplicity, we referred all elements as metals in this manuscript.
For quality control (QC), we included procedural blanks, conducted initial and continuous calibration verification, and repeated analysis of 2% of samples. We analyzed matrix-appropriate certified reference materials once per study and Seronorm-Blood L3 (Trace Elements in whole blood Level 3, SERO, Billingstad, Norway) once daily to monitor measurement accuracy. Recoveries of QC standards were between 90% and 110% for most of the elements except Al, Sn and Sb for which measured values were below the detection limit out of the acceptable range. Intra-day coefficient of variation (CV) was calculated using analysis of in-house QC pools at three different concentration levels before and after every 10 samples (n=7). For most of the metals, intra-day CV was <5% except for Se and Sb where it was <10%. Inter-day CV for all elements was <15% except for concentrations near the limit of detection (LOD). Intra-class correlation coefficient (ICC), which is a composite measure of reliability, was estimated from a one-way random effects model measuring absolute agreement with multiple raters/measurements (34, 35), and used to assess both the degree of correlation and agreement between measurements. For this analysis, we included 11 metals with ICC >0.50 and the percent above LOD >70% (Table A1). These metals included arsenic (As), barium (Ba), cadmium (Cd), cesium (Cs), copper (Cu), magnesium (Mg), manganese (Mn), lead (Pb), selenium (Se), zinc (Zn), and mercury (Hg).
The measurement of metals was funded by the Children’s Health Exposure Analysis Resource (CHEAR) award (2017–1740, NIH grant U2CES026561) and was carried out at the Mount Sinai CHEAR Network Laboratory.
2.3. Ascertainment of birth outcomes
Women’s first day of last menstrual period (LMP) was recorded at study enrollment. Research staff abstracted date of birth from medical records. We calculated gestational age by subtracting the date of the last menstrual period from the date of birth, or by ultrasound (9.6% of participants) where an ultrasound was available and differed from LMP by more than 10 days (33). We obtained birth weight (in grams) from medical records. Research staff measured birth length (n = 739) and head circumference (n = 802) in the hospital after delivery.
2.4. Covariates
We selected covariates that could potentially predict metal concentrations and birth outcomes based on a priori knowledge and causal diagram using a directed acyclic graph (Figure A1) (36). These included maternal age (continuous), education (college graduate; yes, no), pre-pregnancy BMI (continuous), household income ($70,000 per year; yes, no), smoking status (never-smoker, smoked during pregnancy, former smoker), race/ethnicity (white race; yes, no), and parity (nulliparous; yes, no) (37–42). While infant sex can influence fetal concentrations of certain metals (43, 44), it is not clear if it also influences maternal blood metal concentrations in early pregnancy and acts as a confounder. However, there are ample evidence suggesting that infant sex can modify the effect of metals on birth outcomes (20, 28). Hence, we conducted both sex-combined and sex-stratified analysis. In sex-combined analysis, we additionally adjusted the models for infant sex. All continuous variables were transformed to z-scores prior to statistical analyses.
2.5. Statistical analysis
Complete covariate and metals data were available for 1406 participants (17 participants had missing data on erythrocyte Hg), of which we excluded 14 participants with extreme observation for one or more metals based on Rosner’s test for multiple outliers (45), and one participant with birth weight less than 500g, which leaves our final analytical sample to 1391 (Figure A2). Instrument estimated concentrations for all metals, including concentrations below the LOD, were used in analysis (46). Before analysis, metal concentrations were right shifted by 1 ng/g and natural log-transformed to account for right-skewness and avoid negative values (20 for As, 4 for Ba) and subsequently centered to achieve a common scale. We examined descriptive statistics and distributional plots for all variables. We examined correlations between metals by calculating Spearman’s rank correlation coefficients. We performed both sex-combined (adjusted for infant sex) and sex-stratified analyses of all models. All statistical analyses were performed using R (version 3.6.1; R Foundation for Statistical Computing).
2.5.1. Single metal linear regression analysis
We first examined the associations of individual metals with birth measurements using multivariable linear regression models adjusting for maternal age, education, pre-pregnancy BMI, parity, smoking status, race/ethnicity, household income, infant sex, and gestational age at delivery. We included individual metals, one at a time, as a continuous variable in all models. Point estimates were scaled to represent changes in outcome for each interquartile range (IQR) increase in natural log concentration of metals. We interpreted effect estimates based on the magnitude and precision instead of relying solely on their statistical significance. We reported estimates and 95% confidence intervals (95% CI) for all analyses.
2.5.2. Metal mixture analysis using Bayesian Kernel Machine Regression (BKMR)
To estimate the effect of mixture components, we implemented Bayesian Kernel Machine Regression (BKMR), a novel statistical approach (47) that has been previously implemented in environmental health studies related to metal mixtures (20, 25, 48). Our initial analysis using BKMR included six metals (As, Cd, Mn, Pb, Zn, and Hg) that were selected a priori based on prior studies on individual metals and birth measurements (5–7, 15, 20, 23, 25, 27, 28). One important advantage of BKMR is that it allows for estimation of potential non-linear and interactive effects of correlated mixture components accounting for uncertainty. In BKMR, the exposure-response function can be flexibly modeled, and does not required to be specified a priori. The BKMR model is given by -
where Yi is a continuous birth outcome (weight, length, gestational age, or head circumference), xi = (Asi, Cdi, Mni, Pbi, Zni, Hgi) denotes the mixture exposure composed by respectively arsenic, cadmium, manganese, lead, zinc, and mercury erythrocyte log-transformed and centered concentrations. zi = (zi1,…,ziP)T contains a set of potential confounders, and ei ~ N(0, σ2). The h() denotes an exposure-response function that accommodates nonlinearity or interaction among mixture components. We applied hierarchical variable selection approach in BKMR by grouping metals into toxic (As, Cd, Pb, Hg, Ba) and non-toxic or trace metals (Mn, Zn, Se, Cu, Cs, Mg) to rank the importance of exposure groups in association with respective birth outcomes. We used the R package “bkmr” (version 0.2.0) to perform the analysis (47).We specified our models to include 100,000 Markov chain Monte Carlo (MCMC) iterations using the Gaussian kernel.
2.5.3. Metal mixture analysis using multivariable linear regression models
To assess the reproducibility of the BKMR results and to test for potential interactions between mixture components, we modeled six metals (As, Cd, Mn, Pb, Zn, and Hg) simultaneously in the same linear regression models for respective birth outcomes, adjusting for covariates. To test for potential nonlinear associations suggested by BKMR, we included a quadratic term in the model. Quadratic terms with a p-value <0.05 were retained in the final model. We tested for metal interactions suggested by BKMR by including two- and three-way interaction terms in the model. A three-way interaction term was considered when BKMR suggested three two-way interactions involving three metals. Models with a three-way interaction also included all lower-level two-way interactions. Significant interaction between metals were defined as p-for-interaction <0.10. To interpret interaction effects, we estimated marginal effects for an IQR increase in one metal at 25th, 50th, and 75th percentiles of the other metal(s) using the R package “margins” (version 0.3.26). We used the delta method to estimate 95% confidence intervals of marginal effects (49).
2.5.4. Sensitivity analyses
We performed additional sensitivity analyses 1) fitting the same models for birth weight, length, and head circumference, adjusting for same covariates except gestational age, because prior studies have indicated that gestational age could be within the causal pathways between metals and birth measurements (6). Therefore, adjusting for gestational age could under- or overestimate the true effect (50); and 2) testing all eleven metals in BKMR analysis.
3. RESULTS
3.1. Study population characteristics
Demographic characteristics of participating mother-infant pairs from Project Viva are presented in Table 1. Mothers were enrolled at a mean age of 32 years. Over 12.2% of the mothers reported smoking during pregnancy. Our analytical sample was similar in demographic and outcome distributions to the study cohort except that the proportion of women self-identifying as “Whites” was little lower in the analytical sample (data not shown) than the overall study cohort.
Table 1:
Selected characteristics of mother-infant pairs from Project Viva included in this analysis
| Characteristics | Full cohort (n =1391) mean ± SD or n (%) |
Male (n = 718) mean ± SD or n (%) |
Female (n = 673) mean ± SD or n (%) |
|---|---|---|---|
| Age, years, | 32.3 ± 4.7 | 32.2 ± 4.7 | 32.4 ± 4.6 |
| Maternal Race/ethnicity | |||
| White | 1014 (72.9) | 522 (72.7) | 492 (73.1) |
| Black | 175 (12.6) | 91 (12.7) | 84 (12.5) |
| Hispanic | 95 (6.8) | 52 (7.2) | 43 (6.4) |
| Other | 107 (7.7) | 53 (7.4) | 54 (8.0) |
| Parity | |||
| Nulliparous | 667 (48.0) | 333 (46.4) | 334 (49.6) |
| Multiparous | 724 (52.0) | 385 (53.6) | 339 (50.4) |
| Education | |||
| <College graduate | 428 (30.8) | 244 (44.0) | 184 (27.3) |
| ≥College graduate | 963 (69.2) | 474 (66.0) | 489 (72.7) |
| Household oncome | |||
| <$70,000/year | 547 (39.3) | 286 (39.8) | 261 (38.8) |
| ≥$70,000/year | 844 (60.7) | 432 (60.2) | 412 (61.2) |
| Pre-pregnancy BMI, kg/m2 | |||
| <25 | 862 (62.0) | 443 (61.7) | 419 (62.3) |
| 25– <30 | 309 (22.2) | 167 (23.3) | 142 (21.1) |
| >=30 | 220 (15.8) | 108 (15.0) | 112 (16.6) |
| Smoking | |||
| Never smoker | 949 (68.2) | 481 (67.0) | 468 (69.5) |
| Former smoker | 272 (19.6) | 142 (19.8) | 130 (19.3) |
| Smoking in pregnancy | 170 (12.2) | 95 (13.2) | 75 (11.1) |
| Birth Outcomes | |||
| Gestational age at delivery, weeks | 39.5 ± 1.8 | 39.5 ± 1.8 | 39.5 ± 1.9 |
| Birth weight, g | 3492.8 ± 567.0 | 3574.7 ± 593.1 | 3405.8 ± 524.4 |
| Head circumference, cm a | 34.2 ± 1.3 | 34.5 ± 1.3 | 33.8 ± 1.2 |
| Birth length, cm a | 49.9 ± 2.1 | 50.4 ± 2.1 | 49.5 ± 2.0 |
Sample size for head circumference was 791 (male = 394, female = 397) and for birth length was 729 (male = 363, female = 366)
The distribution of erythrocyte metal concentrations and data quality-control parameters are presented in Table A1. Metals showed a weak to moderate (r= 0.03 to 0.67) correlations. The highest correlations were observed between Cu and Zn (r = 0.53 to 0.61) and As and Hg (r= 0.56 to 0.67) (Figure A3).
3.2. Single metal linear regression models
3.2.1. Associations with birth weight
Multivariable linear regression models adjusting for gestational age and other covariates revealed that exposure to higher concentrations of As, Cd, and Pb was associated with lower birth weight (Figure 1, Table A2). Specifically, each IQR increase in As (1.1 ng/g) was associated with 29.3 g (95% CI: −59.5, 1.0), Cd (IQR= 0.28 ng/g) with 26.6 g (95% CI: −51.8, −1.4), and Pb (IQR= 10.1 ng/g) with 33.9 g (95% CI: −65.3, −2.5) lower birth weight. The effect of As was stronger in males (β= −54.2 g; 95% CI: −98.9, −9.4 per IQR increase) than females (β= −2.6 g; 95% CI: −42.6, 37.4 per IQR increase) (p-for-interaction = 0.107). The associations were weaker in models not adjusted for gestational age (β= −36.11g; 95% CI: −74.31, 2.09 per IQR increase in males and β = 0.26; 95% CI: −49.32, 49.84 per IQR increase in females) but the As*infant sex interaction term was statistically significant (p-for-interaction = 0.04) (Table A3).
Figure 1.
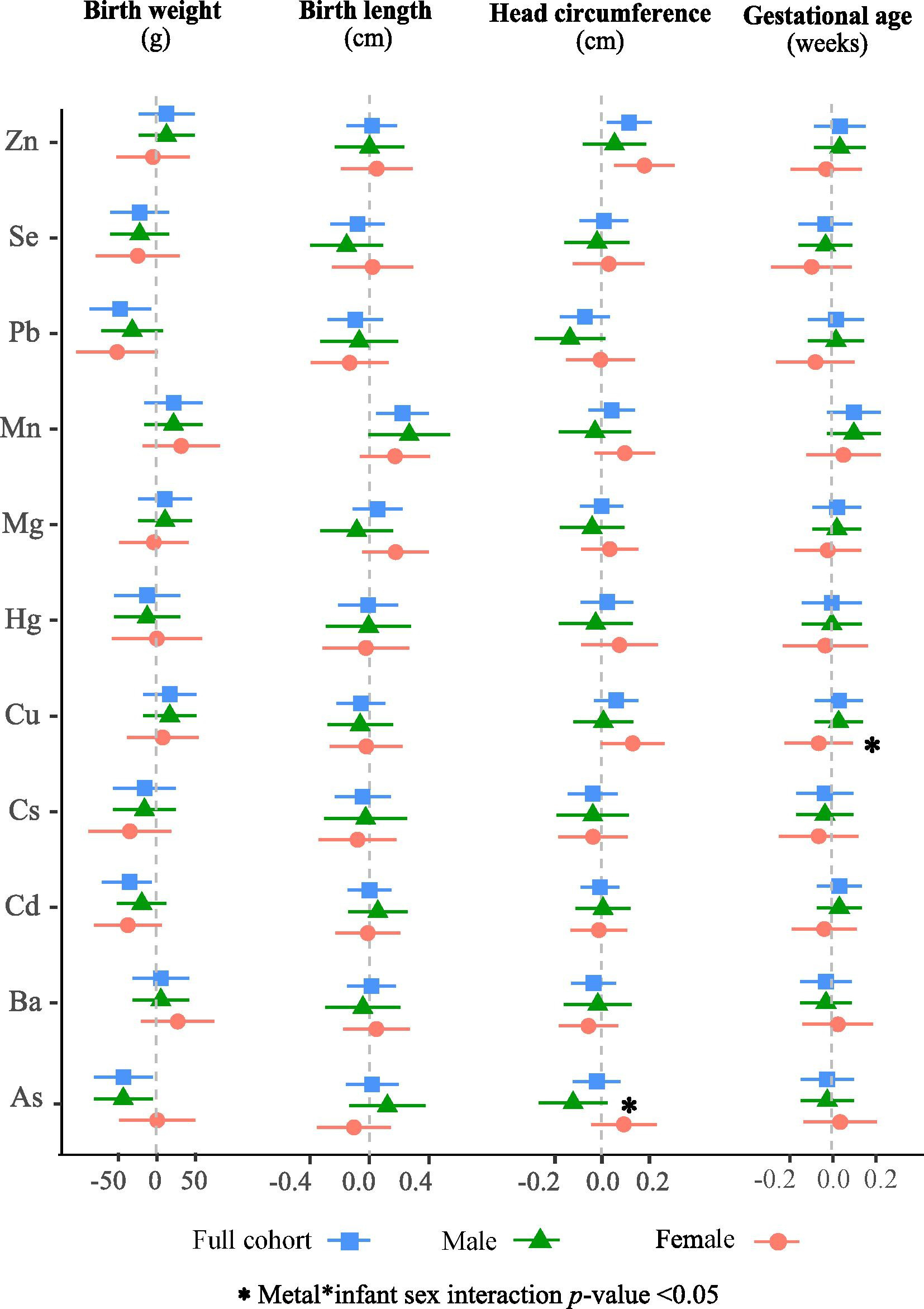
Adjusted associations of an IQR increase in erythrocyte concentrations of individual metal on birth outcomes in Project Viva in infant sex-combined and sex stratified analyses. Models were adjusted for gestational age at delivery (except when gestational age was an outcome) and potential confounders. The sex-combined analysis was also adjusted for infant sex.
3.2.2. Associations with birth length
Exposure to higher concentrations of Mn was associated with longer birth length (Figure 1, Table A2). Specifically, in gestational age adjusted models, an IQR increase in Mn (7.3 ng/g) was associated with 0.22 cm (95% CI: 0.05, 0.40) longer birth length in sex-combined analysis; the associations did not differ in stratified analysis by infant sex. Similar but stronger associations (β= 0.31 cm; 95% CI: 0.11, 0.51 per IQR increase) were observed in model without adjusting for gestational age (Table A3).
3.2.3. Associations with head circumference
Exposure to higher erythrocyte concentrations of Zn was associated with larger head circumference (Figure 1, Table A2). Specifically, an IQR increase in Zn (2330 ng/g) was associated with 0.12 cm (95% CI: 0.02, 0.21) larger head circumference. The association appeared stronger in females (β= 0.18 cm; 95% CI: 0.05, 0.31 per IQR increase) compared to males (β= 0.05 cm; 95% CI: −0.08, 0.19 per IQR increase). Similar association patterns were observed in models not adjusted for gestational age (Table A3). In addition, we observed marginally significant positive associations for Mn and Cu with head circumference in females.
In contrast, we observed marginally significant negative associations for As and Pb with head circumference, especially in males (Figure 1, Table A2). For instance, an IQR increase in As was associated with 0.13 cm (95% CI: −0.28, 0.02) smaller head circumference in males compared to a small and opposite association in females (β= 0.09 cm; 95% CI: −0.05, 0.24) in gestational age adjusted models (p-for-interaction = 0.037). Similarly, an IQR increase in Pb was associated with 0.14 cm (95% CI: −0.29, 0.01) smaller head circumference in males compared to null association in females (β= 0.00 cm; 95% CI: −0.15, 0.15).
3.2.4. Associations with gestational age
Single metal linear regression models showed no associations between erythrocyte metals and gestational age (Figure 1, Table A2, A3).
3.3. Metal mixture analyses using BKMR
3.3.1. Associations with birth weight
Mixture analysis using BKMR revealed a negative and linear association for As with birth weight, especially in males (Figure A4), with the association being stronger at higher concentration of other mixture components (Figure 2, Table A4). For instance, an IQR increase in As was associated with a lower birth weight of −0.08 (95% CI: −0.19, 0.03), −0.13 (95% CI: −0.20, −0.0004), and −0.13 (95% CI: −0.24, −0.01) SDs (1 SD = 567.0 g) when the concentrations of Cd, Mn, Pb, Zn, and Hg were fixed at 25th, 50th, and 75th percentiles, respectively in males. A similar pattern was observed when the models were not adjusted for gestational age (Table A5) or when all 11 metals were included in the BKMR analysis (Table A6). BKMR also suggested potential interaction between As and Mn with birth weight, especially in males (Figure A5). Additionally, we observed non-significant negative associations for Pb and positive associations for Mn with birth weight, particularly in females (Figure 2, Tables A4 – A6).
Figure 2.
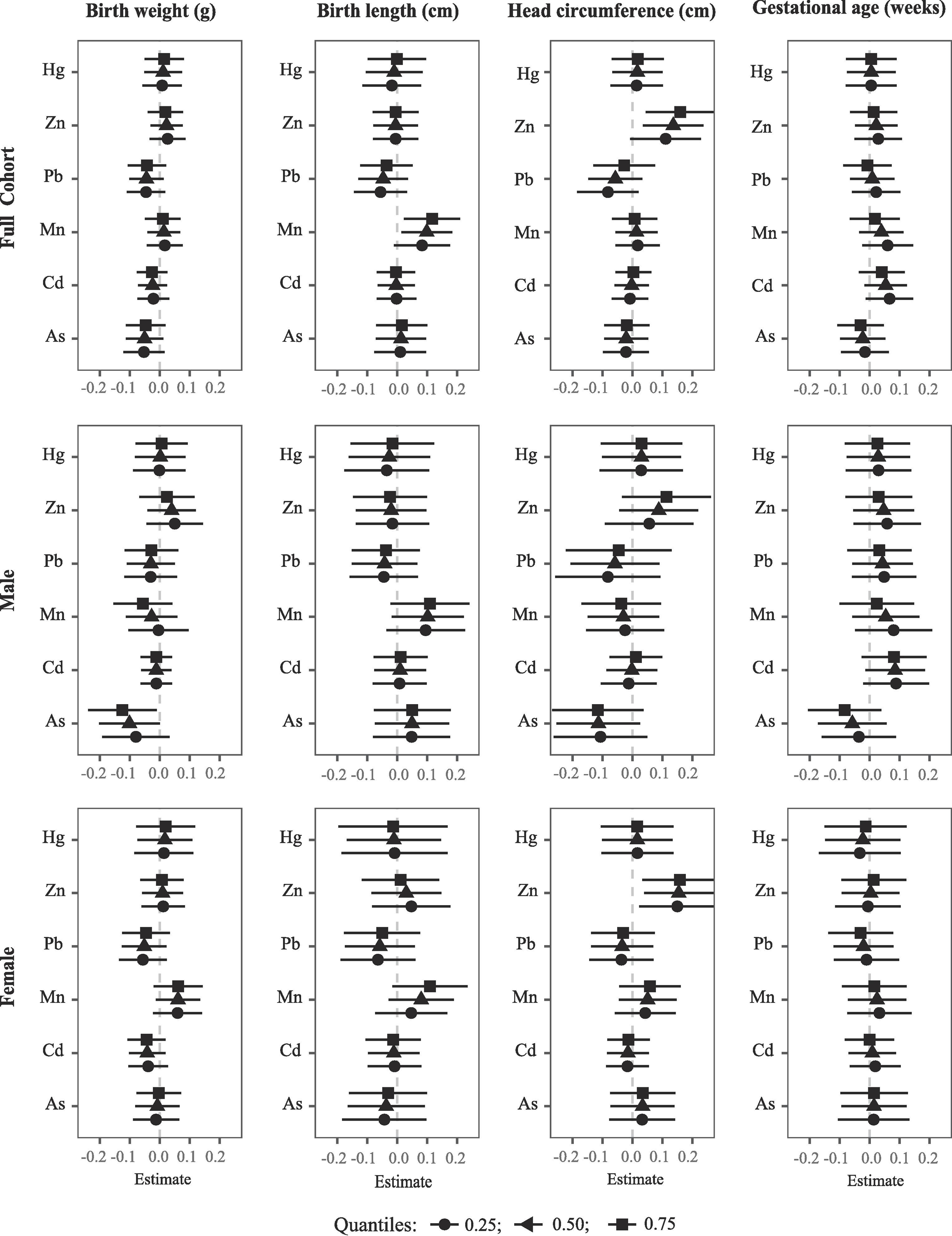
Mixture analyses with BKMR illustrating the effect of metal mixture components on birth outcomes (estimates and 95% confidence intervals, gray dashed line at the null) in Project viva in sex-combined and sex-stratified analyses. This plot compares birth measurements when a mixture component is at the 75th percentile vs. 25th percentile, when all the other mixture components are fixed at the 25th, 50th, and 75th percentiles. Models were adjusted for gestational age (except when gestational age is an outcome) and potential confounders. The sex-combined analysis was also adjusted for infant sex.
3.3.2. Associations with birth length
Exposure to Mn was positively and linearly associated with birth length (Figure A4); the associations appeared stronger at higher concentrations of other mixture components (Figure 2, Table A4). For instance, an IQR increase in Mn was associated with 0.08 (95% CI: −0.01, 0.18), 0.10 (95% CI: 0.01, 0.18), and 0.12 (95% CI: 0.02, 0.21) SDs longer birth length (1 SD = 2.1 cm) when the concentrations of As, Cd, Pb, Zn, and Hg were fixed at 25th, 50th, and 75th percentiles, respectively. Similar pattern was observed in sex-stratified analyses, although the associations lacked statistical significance. In models not adjusted for gestational age (Table A5) or, when all 11 metals were included in BKMR analyses (Table A6), the association patterns remained consistent. BKMR also suggested potential interactions for Hg with Mn and Zn in relation to birth length in females. For instance, the positive association of Mn was stronger at higher concentrations of Hg, whereas the positive association of Zn was stronger at lower concentration of Hg (Figure A6).
3.3.3. Associations with head circumference
Erythrocyte concentrations of Zn showed a nonlinear “J-shaped” association with head circumference (Figure A4). At a higher exposure range (>25th percentile), exposure to higher concentrations of Zn was associated with larger head circumference; the associations appeared stronger at higher concentrations of other mixture components (Figure 2). Specifically, an IQR increase in Zn was associated with 0.11 (95% CI: −0.01, 0.23), 0.14 (95% CI: −0.04, 0.24), and 0.16 (95% CI: −0.04, 0.28) SDs larger head circumference (1 SD = 1.3 cm) when the concentrations of As, Cd, Mn, Pb, and Hg were fixed at 25th, 50th, and 75th percentiles, respectively (Table A4). In sex stratified analysis, the associations appeared stronger in females than males. Similar patterns were observed in models not adjusted for gestational age (Table A5) or when all 11 metals were included in BKMR analysis (Table A6). In addition, BKMR suggested a negative association for As in males and a similar direction of association for Pb in sex-combined analysis, especially at lower concentrations of other metals (Figure 2, Tables A4 – A6). Our results also suggested potential interaction between Pb and Zn with head circumference such that the negative association of Pb was stronger at lower concentrations of Zn in males (Figure A7).
3.3.4. Associations with gestational age
BKMR suggested an “inverted U-shaped” association for Cd with gestational age, particularly in males and a similar association pattern for Zn in females (Figure A4); none of the associations were statistically significant (Figure 2, Tables A4 – A6). BKMR also suggested potential sex-specific interactions between mixture components with gestational age. For instance, As showed a negative exposure-response relationship with gestational age in males, which appeared to be stronger at higher concentrations of Mn. On the contrary, Cd showed a positive exposure-response relationship in females, which appeared to be stronger at lower concentrations of Zn (Figure A8).
3.4. Metal Mixture analyses using Multivariable Linear Regression Models
3.4.1. Associations with birth weight
Multivariable linear regression models simultaneously including all six metals (As, Cd, Mn, Pb, Hg, Zn) in the same model confirmed synergistic effect between As and Mn with birth weight in males. Specifically, the negative association between As and birth weight was stronger at higher concentrations of Mn (Figure 3A, Tables 2, A7). Specifically, each IQR increase in As was associated with 25.3g (95% CI: −79.9, 29.3), 47.9g (95% CI: −98.0, 2.1), and 72.2g (95% CI: −129.8, −14.7) lower birth weight when the concentrations of Mn were at 25th, 50th, and 75th percentiles, respectively in males (p-for-interaction = 0.067).
Figure 3.
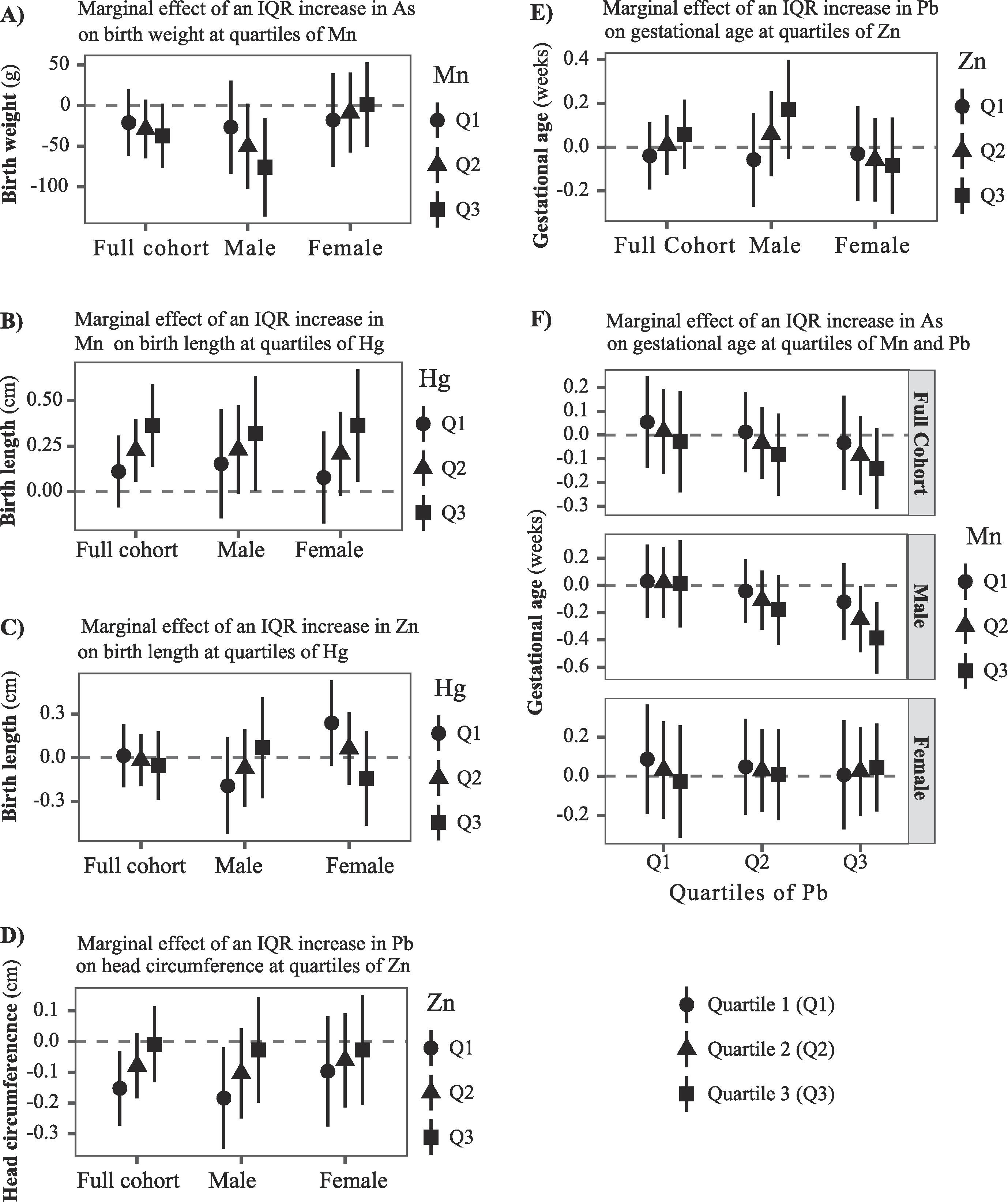
Multiple metal linear regression analyses illustrating interactions between metal mixture components with respective birth measurements (estimates and 95% confidence intervals, gray dashed line at the null) in Project viva in sex-combined and sex-stratified analyses. The plot illustrates the marginal effect for an IQR increase in A) As on birth weight at quartiles of Mn; B-C) Mn and Zn on birth length at quartiles of Hg, respectively; D) Pb on head circumference at quartiles of Zn; E) Pb on gestational age at quartiles of Zn; and F) As on gestational age at quartiles of Pb and Mn. All six metals (As, Cd, Mn, Pb, Zn, and Hg) were included in the same model along with their two- or three-way interactions. Models were adjusted for gestational age (except when gestational age is an outcome) and potential confounders. The sex-combined analysis was also adjusted for infant sex.
Table 2.
Multiple metals linear regression analyses presenting interactions between mixture components with respective birth measurements in Project viva in sex-combined and sex-stratified analysis. Estimates (95% CI) were calculated as the marginal effect for an IQR increase in erythrocyte concentration of selected metal at 25th, 50th, and 75th percentiles of other metal(s).
| Outcome | Effect of | at quartiles of metal 1 |
at quartiles of metal 2 | Full cohort (n= 1391) |
Male (n= 718) |
Female (n= 673) |
|---|---|---|---|---|---|---|
| Birth weight (g) | As | Mn-Q1 (13.1 ng/g) | ---- | −20.9 (−61.2, 19.5) | −25.3 (−79.9, 29.3) | −18.9 (−79.3, 41.6) |
| As | Mn-Q2 (16.2 ng/g) | ---- | −28.7 (−64.5, 7.1) | −47.9 (−98.0, 2.1) | −9.1 (−60.8, 42.6) | |
| As | Mn-Q3 (20.3 ng/g) | ---- | −37.1 (−76.6, 2.3) | −72.2 (−129.8, −14.7)* | 1.4 (−53.2, 56.0) | |
| As*Mn interaction p-value | 0.369 | 0.067 | 0.437 | |||
|
| ||||||
| Birth length (cm) a | Mn | Hg-Q1 (1.6 ng/g) | ---- | 0.11 (−0.09, 0.31) | 0.17 (−0.17, 0.52) | 0.08 (−0.18, 0.33) |
| Mn | Hg-Q2 (3.3 ng/g) | ---- | 0.23 (0.05, 0.40)* | 0.26 (−0.02, 0.54) | 0.21 (−0.02, 0.44) | |
| Mn | Hg-Q3 (6.4 ng/g) | ---- | 0.36 (0.14, 0.59)* | 0.36 (0.00, 0.72)* | 0.36 (0.05, 0.67)* | |
| Mn*Hg interaction p-value | 0.045 | 0.378 | 0.078 | |||
| Zn | Hg-Q1 (1.6 ng/g) | ---- | 0.01 (−0.2, 0.23) | −0.19 (−0.52, 0.14) | 0.24 (−0.05, 0.53) | |
| Zn | Hg-Q2 (3.3 ng/g) | ---- | −0.02 (−0.19, 0.16) | −0.07 (−0.34, 0.19) | 0.06 (−0.19, 0.31) | |
| Zn | Hg-Q3 (6.4 ng/g) | ---- | −0.05 (−0.29, 0.18) | 0.07 (−0.28, 0.42) | −0.14 (−0.47, 0.19) | |
| Zn*Hg interaction p-value | 0.623 | 0.229 | 0.043 | |||
|
| ||||||
| Head Circumference (cm) a | Pb | Zn-Q1 (9273.3 ng/g) | ---- | −0.15 (−0.27, −0.03)* | −0.18 (−0.35, −0.02)* | −0.10 (−0.28, 0.08) |
| Pb | Zn-Q2 (10403.6 ng/g) | ---- | −0.08 (−0.18, 0.03) | −0.10 (−0.25, 0.04) | −0.06 (−0.21, 0.09) | |
| Pb | Zn-Q3 (11601.8 ng/g) | ---- | −0.01 (−0.13, 0.11) | −0.03 (−0.20, 0.15) | −0.03 (−0.21, 0.15) | |
| Mn*Hg interaction p-value | 0.024 | 0.066 | 0.463 | |||
|
| ||||||
| Gestational age (weeks) | As | Mn-Q1 (13.1 ng/g) | Pb-Q1 (13.5 ng/g) | 0.05 (−0.14, 0.25) | 0.03 (−0.24, 0.30) | 0.09 (−0.19, 0.37) |
| As | Mn-Q1 (13.1 ng/g) | Pb-Q2 (17.7 ng/g) | 0.01 (−0.16, 0.18) | −0.04 (−0.28, 0.19) | 0.05 (−0.20, 0.29) | |
| As | Mn-Q1 (13.1 ng/g) | Pb-Q3 (23.6 ng/g) | −0.03 (−0.23, 0.17) | −0.12 (−0.40, 0.16) | 0.01 (−0.27, 0.29) | |
| As | Mn-Q2 (16.2 ng/g) | Pb-Q1 (13.5 ng/g) | 0.01 (−0.16, 0.19 ) | 0.02 (−0.24, 0.28) | 0.03 (−0.22, 0.28) | |
| As | Mn-Q2 (16.2 ng/g) | Pb-Q2 (17.7 ng/g) | −0.03 (−0.18, 0.12) | −0.11 (−0.33, 0.11) | 0.03 (−0.18, 0.24) | |
| As | Mn-Q2 (16.2 ng/g) | Pb-Q3 (23.6 ng/g) | −0.09 (−0.25, 0.08) | −0.25 (−0.49, −0.01)* | 0.03 (−0.20, 0.25) | |
| As | Mn-Q3 (20.3 ng/g) | Pb-Q1 (13.5 ng/g) | −0.03 (−0.24, 0.19) | 0.01 (−0.31, 0.33) | 0.03 (−0.31, 0.26) | |
| As | Mn-Q3 (20.3 ng/g) | Pb-Q2 (17.7 ng/g) | −0.08 (−0.26, 0.09) | −0.18 (−0.44, 0.08) | 0.01 (−0.23, 0.24) | |
| As | Mn-Q3 (20.3 ng/g) | Pb-Q3 (23.6 ng/g) | −0.14 (−0.31, 0.02) | −0.39 (−0.65, −0.13)* | 0.04 (−0.18, 0.27) | |
| As*Mn*Pb interaction p-value | 0.786 | 0.08 | 0.237 | |||
| Pb | Zn-Q1 (9273.3 ng/g) | ---- | −0.04 (−0.19, 0.11) | −0.06 (−0.27, 0.16) | −0.03 (−0.25, 0.19) | |
| Pb | Zn-Q2 (10403.6 ng/g) | ---- | 0.01 (−0.13, 0.15) | 0.06 (−0.13, 0.25) | −0.06 (−0.25, 0.13) | |
| Pb | Zn-Q3 (11601.8 ng/g) | ---- | 0.06 (−0.10, 0.22) | 0.17 (−0.05, 0.40) | −0.08 (−0.30, 0.14) | |
| Pb*Zn interaction p-value | 0.202 | 0.032 | 0.611 | |||
Sample size for head circumference was 791 (male = 394, female = 397) and for birth length was 729 (male = 363, female = 366)
Models were adjusted for gestational age at delivery (except when gestational age was an outcome), maternal age, education, pre-pregnancy BMI, parity, smoking status, race/ethnicity, and household income. The sex-combined analysis was additionally adjusted for infant sex. Models included all six metals (As, Cd, Mn, Pb, Zn, Hg) and selected two-way or three-way interactions and quadratic terms, where indicated. Models with a three-way interaction also included all lower-level two-way interactions. Standard errors were estimated based on the delta method. P-for-interaction <0.10 was considered statistically significant.
p-value <0.05
3.4.2. Associations with birth length
Mn and Hg showed a synergistic effect on birth length such that the positive association of Mn was stronger at higher concentrations of Hg (Figure 3B, Tables 2, A7). Specifically, an IQR increase in Mn was associated with 0.12 cm (95% CI: −0.10, 0.34), 0.25 cm (95% CI: 0.06, 0.44), and 0.40 cm (95% CI: 0.15, 0.65) larger birth length in sex-combined analysis when the concentrations of Hg were at 25th, 50th, and 75th percentiles, respectively (p-for-interaction = 0.045). Similar pattern was observed in sex-stratified analysis, although the Mn*Hg interaction term was not statistically significant in males.
In contrast, Zn and Hg showed an antagonistic association with birth length. Specifically, Zn showed a non-significant positive association with birth length in females, which reversed at higher concentrations of Hg (Figure 3C, Tables 2, A7). For example, an IQR increase in Zn was associated with 0.24 cm (95% CI: −0.05, 0.53), 0.06 cm (95% CI: −0.19, 0.31), and −0.14 cm (95% CI: −0.47, 0.19) differences in birth length when the concentrations of Hg were at 25th, 50th, and 75th percentiles, respectively (p-for-interaction = 0.043).
3.4.3. Associations with head circumference
Higher erythrocyte concentrations of Pb was associated with lower head circumference with potential interactions with Zn. Specifically, the head circumference lowering effect of Pb was attenuated at higher concentrations of Zn (Figure 3D, Tables 2, A7). For example, an IQR increase in Pb was associated with 0.16 cm (95% CI: −0.29, −0.03), 0.08 cm (95% CI: −0.20, 0.03), and 0.01 cm (95% CI: −0.14, 0.12) smaller head circumference when the concentrations of Zn were at 25th, 50th, and 75th percentiles, respectively (p-for-interaction = 0.024). Similar pattern was observed in males (p-for-interaction = 0.066). Our results also suggested potential non-linear association for Zn with head circumference in sex-combined analysis, as the quadratic term for Zn was marginally significant (p-value = 0.096).
3.4.4. Associations with gestational age
Antagonistic interaction between Zn and Pb was also suggested for gestational age in males (Figure 3E, Tables 2, A7) such that the negative association of Pb on gestational age was attenuated at higher concentrations of Zn (p-for-interaction = 0.032). In addition, our results suggested a three-way interaction between As, Mn, and Pb with gestational age in males. Specifically, exposure to higher concentrations of As was associated with shorter gestational age when the concentrations of both Mn and Pb were also higher (Figure 3F, Tables 2, A7). For example, an IQR increase in As was associated with −0.05 weeks (95% CI: −0.14, 0.25), 0.11 weeks (95% CI: −0.33, 0.11), 0.25 weeks (95% CI: −0.49, −0.01), and 0.39 weeks (95% CI: −0.65, −0.13) changes in gestational age when the concentrations of both Mn and Pb were at 25th percentiles, both Mn and Pb were at 50th percentiles, Mn was at 50th percentile and Pb at 75th percentile, and both Mn and Pb were at 75th percentiles, respectively in males (p-for three-way interaction = 0.08). Multi-pollutant models also suggested a nonlinear association for Cd with gestational age in sex-combined analysis (p-for quadratic term = 0.046) and in males (p-for quadratic term = 0.034) (Table A7).
4. DISCUSSION
In this prospective study of 1,391 mother-infant pairs from Eastern Massachusetts, maternal erythrocyte concentrations of As, Mn, Pb, and Zn in early pregnancy were associated with birth measurements; the associations varied by infant sex and exposure level of other metal mixture components. Specifically, As showed a significant negative and linear association with birth weight, especially in males; the association appeared stronger at higher concentrations of Mn. Mn showed a significant positive and linear association with birth length; the association was stronger at higher concentrations of Hg. Zn showed a “J-shaped” association with head circumference such that at a higher exposure levels (>25th percentile), higher concentration of Zn was associated with larger head circumference. We also observed an antagonistic effect between Pb and Zn with head circumference such that the negative association of Pb on head circumference was attenuated at higher concentrations of Zn. Similarly, As was associated with lower gestational age in males when the concentrations of Mn and Pb were higher. Note that these associations were observed at background exposure level in a population with no apparent source of exposure.
Few studies have examined metal mixture with birth outcomes. A direct comparison of studies is hindered by the differences in exposure distribution, population characteristics, biological matrices wherein metals were quantified, and the mixture modeling approaches used by the studies. Despite these limitations, our observed findings on As and birth measurements were corroborated by a prospective study in Oklahoma where maternal blood As was associated with lower birth weight, shorter gestational age, and smaller head circumference in a population where co-exposure to Mn and Pb were also recorded (5). In the New Hampshire Birth Cohort study, maternal toenail As showed an inverted “U-shaped” relation with head circumference (20), but a positive association with birth weight (20). Other prospective studies using a mixture approach reported null associations for As with birth measurements (24, 26, 51). On the contrary, prospective studies on individual metals, overwhelmingly reported negative associations for As with birth weight (6, 10, 14, 28, 52, 53), gestational age (6, 37, 53), preterm birth (13, 54), and head circumference (20, 27, 28, 55). It is important to note that a majority of these later studies were conducted in populations exposed to higher levels of As, although a handful of recent studies were conducted in populations with relatively low levels of exposure (5, 28, 52, 56).
Our study suggested sex-specific positive and linear associations for Mn with birth length and weight, while no associations with head circumference and gestational age. Prior studies have reported an inverted U-shaped association for maternal blood Mn with birth weight (15, 16, 57) and postpartum toenail Mn with head circumference (20), while null associations for birth length {Guan, 2014 #2728;Yamamoto, 2019 #2725}. Although, Mn is considered an essential trace element, it is toxic over an ultra-trace amount, which could potentially explain the observed inverted “U-shaped” association for Mn with some birth measurements. Interpolation of maternal blood Mn level (median (25th – 75th percentile) = 24.76 (24.32 – 25.20) μg/L) from erythrocyte Mn (erythrocyte reflects about 66% of blood Mn (58)) indicated that the average concentration of Mn in this population was lower than that in most other populations (58). Therefore, it is possible that we have captured the ascending segment of the otherwise inverted U-shaped exposure-response relationship for Mn with birth measurements. Other potential explanations behind inconsistent findings include different exposure biomarkers and statistical models (single metal vs metal mixture analysis) used across studies.
Erythrocyte Zn showed a “J-shaped” association with head circumference. Zn is an essential metal that plays an important role in cellular growth, specifically in the production of enzymes necessary for nucleic acid synthesis and metabolism, gene expression, cell division, and tubulin growth in the brain (59). Lack of zinc has been implicated in impaired DNA, RNA, and protein synthesis during brain development (60). Zinc insufficiency during pregnancy and lactation has been associated with nervous system abnormalities of the offspring (60). Reports on prenatal exposure to Zn and head circumference are sparse. However, Zn supplementation at 5 to 44 mg per day during pregnancy was associated with reduced risk of preterm birth, with no effect on birth measurements (19).
Certain heavy metals such as As, Cd, Hg, and Pb are known to generate free radicals, leading to oxidative stress and cellular damage (61). Oxidative stress during the prenatal period can disrupt placental development, function, and remodeling (62), leading to impaired fetal growth (62, 63). On the contrary, trace metals such as Zn, Mn, Se, and Cu are important constituents of antioxidant enzymes that play a role in cellular protection against oxidative stress (64–66). Interestingly, our results indicated potential antagonism between Zn and Pb with head circumference with the negative effect of Pb on head circumference was attenuated at higher concentration of Zn. Similar antagonism between Pb and Zn was also observed for gestational age in males. Pb is a known developmental neurotoxicant (67), with no safe level of exposure (68). Experimental studies showed that Pb-induced neurotoxicity can be attenuated by Zn. For example, in young rats administered Pb from birth, Zn prevented selective depletion of oligodendrocyte without diminishing blood Pb level (69). In cultured neuronal cells, intracellular Zn was reported to offset Pb-induced reduction in astroglia cell number in a dose dependent manner, although not enough to completely mask Pb-induced neurotoxicity (70). Whether prenatal Zn supplementation can offset the neurotoxic effect of Pb in newborn and children remains to be studied.
Zn also showed antagonism with Hg in relation to birth length among females. Specifically, the positive effect of Zn on birth length was attenuated at higher concentrations of Hg. Similar antagonism between Zn and Cd was suggested for gestational age in males by BKMR, which was not confirmed by multi-pollutant linear regression models. Our observed non-linear positive association for Cd with gestational age also merit further investigation, as prior studies have reported that maternal blood Cd was associated with higher risk of preterm birth (71). Surprisingly, we did not find an antagonism for metals known to induce oxidative stress (As, Cd, Hg, or Pb) with Se despite the fact that Se is an important constituent of several antioxidant enzymes, including glutathione peroxidase, thioredoxin reductase, and iodothyronine deiodinases (72). Prior studies have reported a dose-dependent interaction for Se with As such that at low concentrations, Se decreased the toxicity of As via formation and excretion of As-Se complex (73), while at a higher concentration, Se enhanced the toxicity of As by reacting with S-adenosylmethionine and glutathione, and modifying the structure and activity of arsenite methyltransferase (73). Further research using a mixture approach is needed to assess the role of Se as an antioxidant, especially at a low exposure level.
Our results showed super-additivity between As and Mn with birth weight in males, suggesting that prenatal exposure to As and Mn may have a synergistic effect, i.e. the toxicity of one metal may exacerbate in presence of the other metal. Similar sex-specific super-additivity among As, Mn, and Pb was observed with gestational age in males, where the negative effect of As on gestational age was exacerbated at higher concentrations of Mn and Pb. Prior studies have reported two-way synergisms between As*Mn, As*Pb, and Pb*Mn with neurodevelopmental outcomes in populations exposed to higher levels of metals (48, 74–76), although a three-way interaction was not assessed. Overall, our results suggested that co-exposure to As, Mn, Pb may have sex-specific synergistic effect on certain birth parameters (e.g., weight, gestational age), which may be absent or minimal with exposure to a single metal at a similar dose.
Sex difference in associations between metals and birth outcomes was also reported in prior studies (20, 28), although findings for specific metal or birth parameter were not always consistent. It has been suggested that male fetuses may be more susceptible to As toxicity and females to Pb and Mn (20, 28). Possible explanations underlying such differences include heterogeneity in the structure and function of the placenta (77, 78) and differences in the uptake and excretion rates of certain metals between male and female fetuses (43, 44).
Our results may be relevant to other US birth cohorts where exposure to metals are minimum. Our study participants were not selected based on exposure and thus, are expected to have no history of metal exposure above the background population averages. However, it is possible that exposure to certain metals rich in seafood and shellfish (e.g. Hg, As) were higher in this coastal community than average U.S. population. Indeed, interpolation of maternal blood Hg from erythrocyte Hg (70–95% of blood Hg are found in the erythrocyte (79, 80)) suggested that the average concentration of Hg in this population was little higher than that of pregnant mothers from NHANES, 1999–2016 (81).
In this study, metals were measured in the erythrocyte, which could be an ideal biological matrix for some but not all metals. For instance, erythrocyte Cu is more reflective of long-term exposure (82), whereas erythrocyte Cd can reflect both recent and cumulative exposure (83). Erythrocyte Mn accounts for about 66% of Mn level in the whole blood (58), and is 10–20 times the level in the serum (84). The level of Zn in the erythrocyte is higher and more stable than that in the plasma (85, 86). Unlike toenail or hair As, erythrocyte As may not be a suitable biomarker for cumulative exposure (87). As in blood represents total As, which rapidly gets excreted in the urine, with 50% to 90% cleared in 2–4 days (88). Another limitation of blood As is that it does not differentiate between more toxic inorganic As from less toxic organic form (89). However, blood As has been strongly correlated with urinary (r= 0.85) and drinking water As (r= 0.75), and suggested to be a useful biomarker for chronic exposure when the source of exposure remains consistent (87).
Erythrocyte Pb serves as a reliable biomarker for recent exposure, as the majority of Pb in the blood remains attached to the hemoglobin (90). Blood Pb level is reported to be higher in pregnant than non-pregnant women (90). Pregnancy is associated with increased bone remodeling, a process that endogenously release stored Pb from bones (constitutes 95% of the total body burden of Pb) to the circulation (90, 91). Pregnancy is also associated with a physiological increase in body fluid, leading to low hematocrit levels (92), which in turn, could potentially influence blood level of certain metals. In NHANES (1999–2016), lower blood levels of Pb, Cd, and Hg were reported in pregnant than non-pregnant women (81). However, pregnancy-related hemodynamic changes are less pronounced in early pregnancy (92), a time window when metals were measured in this cohort (11.3 ± 2.8 weeks of gestation). Therefore, we argue that potential misclassification of exposure due to physiological changes in pregnancy were likely minimum in this study.
Our study has several strengths. We implemented a novel flexible statistical method, BKMR, to quantify and visualize the joint effect of the mixture components with potential nonlinear and nonadditive effects (48). While we obtained similar main effect associations for metals by using traditional linear regression models, by adopting BKMR we overcame important limitations of traditional analyses such as single metal effect estimation and increased false discovery when fitting many regression models. Our results were also fairly robust to sensitivity analysis without adjusting for gestational age; yet, we presented results from gestational age adjusted models as primary findings to be more conservative in our estimates.
Potential limitations include relatively small sample size for head circumference and birth length, for which complete data were not collected from all participants. However, this disadvantage might be at least partially offset by the research quality of these measurements, for which clinical measures are notoriously inaccurate. Additionally, our study has one of the largest sample sizes in birth cohorts with multiple metal biomarkers, although stratified analyses by infant sex might be underpowered to detect small effect sizes or interactions between mixture components. We did not adjust for multiple testing, as our objective in this study was to interpret effect estimates based on the magnitude and precision instead of relying solely on their statistical significance. Hence, we cannot rule out the possibility of false positive findings. In addition, we did not include all measured elements in the final analysis because of low detection rates for some elements. Hence, the power to detect an association for some metals may have been undermined by the poor reliability of data.
While our results could have been potentially confounded by nutritional factors, the objective of this study was to estimate the effect of metal mixture using biomarkers, incorporating all possible sources of exposure, including diet. The moderate positive correlation between As and Hg (Spearman’s ρ = 0.56) in this coastal U.S. population suggest that erythrocyte concentrations of non-speciated As may be more reflective of exposure to organic As from seafood consumption than more toxic inorganic As. As such, we cannot rule out the possibility that our observed associations for As were driven by seafood-associated co-pollutants, other than Hg, which may also impact fetal growth (e.g., PCBs, etc.) (93). Our study included 30 paired pregnancies (15 mothers participated twice in separate pregnancies), which were treated as independent events during the analysis. While duplicate pregnancies could potentially introduce correlations and bias our estimates, we kept them in the analysis to preserve sample size. We also argue that the number of paired pregnancies was too small to bias our estimates in a meaningful way. Our observed non-linear associations for certain metals with birth outcomes could potentially be driven by a limited number of participants with extreme concentrations; hence, should be interpreted with caution. Finally, our observed associations between metals and birth outcomes are merely associational and does not indicate a causal relation.
5. CONCLUSIONS
In this prospective U.S. birth cohort of mother-infant pairs with low prenatal exposure profiles, maternal 1st trimester erythrocyte concentrations of arsenic, manganese, lead, and zinc were individually and interactively associated with birth measurements, where the associations varied by infant sex and exposure level of other metals.
Supplementary Material
HIGHLIGHTS.
As, Mn, Pb and Zn in maternal RBC were associated with birth outcomes
Associations varied by infant sex and exposure level of other mixture components
Birth weight lowering effect of As was stronger at higher levels of Mn in males
Head circumference lowering effect of Pb was attenuated at higher levels of Zn
As was associated with lower gestational age at higher levels of Mn and Pb in males
Acknowledgements
The authors would like to thank the participants Project Viva for their time and willingness to participate in the study; all members of the Project Viva team at the Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute for collecting and managing data, in particular, Inbar Brenner, Karen Ruderman, Marleny Ortega, and Chelsea Jenter for providing administrative support for this project; and the Children’s Health Exposure Analysis Resource (CHEAR) Data Center of the Department of Environmental Medicine and Public Health at Icahn School of Medicine at Mount Sinai for performing the quantification of metal concentrations in red blood cells. Persons named in the Acknowledgments section have provided the corresponding author with written permission to be named in the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health, National Institute of Environmental Health Sciences, or the Environmental influences on Child Health Outcomes (ECHO) program.
Funding source
This work was supported by the US National Institutes of Health grants R01 HD034568, UH3 OD023286, ES000002, and R01 ES031259. The Children’s Health Exposure Analysis Resource (CHEAR) funded the measurement of elements (CHEAR award #2017–1740) supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number (U2CES026561) and was carried out at the Mount Sinai CHEAR Network Laboratory with data processed by the CHEAR Data Center (U2CES026555).
Abbreviations
- As
Arsenic
- Ba
Barium
- Cd
Cadmium
- Cs
Cesium
- Cu
Copper
- Mg
Magnesium
- Mn
Manganese
- Pb
Lead
- Se
Selenium
- Zn
Zinc
- Hg
Mercury
- BKMR
Bayesian Kernel Machine Regression
- SD
standard deviation
- CI
confidence intervals
- CV
coefficient of variation
- QC
quality control
- LOD
limit of detection
- ICP-MS
inductively coupled mass spectrometry.
Footnotes
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
Bibliography
- 1.Wilcox AJ. On the importance--and the unimportance--of birthweight. International journal of epidemiology. 2001;30(6):1233–41. [DOI] [PubMed] [Google Scholar]
- 2.Concha G, Nermell B, Vahter MV. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environmental health perspectives. 1998;106(6):355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gundacker C, Hengstschlager M. The role of the placenta in fetal exposure to heavy metals. Wien Med Wochenschr. 2012;162(9–10):201–6. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. Journal of exposure science & environmental epidemiology. 2014;24(5):537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claus Henn B, Ettinger AS, Hopkins MR, Jim R, Amarasiriwardena C, Christiani DC, et al. Prenatal Arsenic Exposure and Birth Outcomes among a Population Residing near a Mining-Related Superfund Site. Environ Health Perspect. 2016;124(8):1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman ML, Valeri L, Kile ML, Mazumdar M, Mostofa G, Qamruzzaman Q, et al. Investigating causal relation between prenatal arsenic exposure and birthweight: Are smaller infants more susceptible? Environment international. 2017;108:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor CM, Golding J, Emond AM. Moderate Prenatal Cadmium Exposure and Adverse Birth Outcomes: a Role for Sex-Specific Differences? Paediatric and perinatal epidemiology. 2016;30(6):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai MS, Liao KW, Chang CH, Chien LC, Mao IF, Tsai YA, et al. The critical fetal stage for maternal manganese exposure. Environ Res. 2015;137:215–21. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Sakurai K, Eguchi A, Yamazaki S, Nakayama SF, Isobe T, et al. Association between blood manganese level during pregnancy and birth size: The Japan environment and children’s study (JECS). Environ Res. 2019;172:117–26. [DOI] [PubMed] [Google Scholar]
- 10.Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, et al. Estimating Effects of Arsenic Exposure During Pregnancy on Perinatal Outcomes in a Bangladeshi Cohort. Epidemiology (Cambridge, Mass). 2016;27(2):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eum JH, Cheong HK, Ha EH, Ha M, Kim Y, Hong YC, et al. Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environmental health : a global access science source. 2014;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E. Very low maternal lead level in pregnancy and birth outcomes in an eastern Massachusetts population. Ann Epidemiol. 2014;24(12):915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman ML, Kile ML, Rodrigues EG, Valeri L, Raj A, Mazumdar M, et al. Prenatal arsenic exposure, child marriage, and pregnancy weight gain: Associations with preterm birth in Bangladesh. Environment international. 2018;112:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169(3):304–12. [DOI] [PubMed] [Google Scholar]
- 15.Guan H, Wang M, Li X, Piao F, Li Q, Xu L, et al. Manganese concentrations in maternal and umbilical cord blood: related to birth size and environmental factors. European journal of public health. 2014;24(1):150–7. [DOI] [PubMed] [Google Scholar]
- 16.Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, et al. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20(3):367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori R, Ota E, Middleton P, Tobe-Gai R, Mahomed K, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2012(7):Cd000230. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Hu YF, Hao JH, Chen YH, Wang Y, Zhu P, et al. Maternal Serum Zinc Concentration during Pregnancy Is Inversely Associated with Risk of Preterm Birth in a Chinese Population. The Journal of nutrition. 2016;146(3):509–15. [DOI] [PubMed] [Google Scholar]
- 19.Mahomed K, Bhutta Z, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2007(2):CD000230. [DOI] [PubMed] [Google Scholar]
- 20.Signes-Pastor AJ, Doherty BT, Romano ME, Gleason KM, Gui J, Baker E, et al. Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environmental epidemiology (Philadelphia, Pa). 2019;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SS, Meeker JD, Aung MT, Yu Y, Mukherjee B, Cantonwine DE, et al. Urinary trace metals in association with fetal ultrasound measures during pregnancy. Environmental epidemiology (Philadelphia, Pa). 2020;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govarts E, Remy S, Bruckers L, Den Hond E, Sioen I, Nelen V, et al. Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. International journal of environmental research and public health. 2016;13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassidy-Bushrow AE, Wu KH, Sitarik AR, Park SK, Bielak LF, Austin C, et al. In utero metal exposures measured in deciduous teeth and birth outcomes in a racially-diverse urban cohort. Environ Res. 2019;171:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabrera-Rodriguez R, Luzardo OP, Gonzalez-Antuna A, Boada LD, Almeida-Gonzalez M, Camacho M, et al. Occurrence of 44 elements in human cord blood and their association with growth indicators in newborns. Environment international. 2018;116:43–51. [DOI] [PubMed] [Google Scholar]
- 25.Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, et al. Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environment international. 2020;138:105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe CG, Claus Henn B, Eckel SP, Farzan SF, Grubbs BH, Chavez TA, et al. Prenatal Metal Mixtures and Birth Weight for Gestational Age in a Predominately Lower-Income Hispanic Pregnancy Cohort in Los Angeles. Environ Health Perspect. 2020;128(11):117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kippler M, Wagatsuma Y, Rahman A, Nermell B, Persson LA, Raqib R, et al. Environmental exposure to arsenic and cadmium during pregnancy and fetal size: a longitudinal study in rural Bangladesh. Reprod Toxicol. 2012;34(4):504–11. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, Karagas MR. Relation between in Utero Arsenic Exposure and Birth Outcomes in a Cohort of Mothers and Their Newborns from New Hampshire. Environ Health Perspect. 2016;124(8):1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, Meeker JD. Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reprod Toxicol. 2016;66:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews KW, Savitz DA, Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. Am J Ind Med. 1994;26(1):13–32. [DOI] [PubMed] [Google Scholar]
- 31.Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311(1–2):3–12. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. American journal of human biology : the official journal of the Human Biology Council. 2010;22(3):330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: project viva. Int J Epidemiol. 2015;44(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological methods. 1996;1(1):30–46. [Google Scholar]
- 35.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. [DOI] [PubMed] [Google Scholar]
- 36.Sauer BC, Brookhart MA, Roy J, VanderWeele T. A review of covariate selection for non-experimental comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22(11):1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, et al. Estimating Effects of Arsenic Exposure During Pregnancy on Perinatal Outcomes in a Bangladeshi Cohort. Epidemiology. 2016;27(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehra R, Keene DE, Kershaw TS, Ickovics JR, Warren JL. Racial and ethnic disparities in adverse birth outcomes: Differences by racial residential segregation. SSM Popul Health. 2019;8:100417- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cnattingius S, Forman MR, Berendes HW, Graubard BI, Isotalo L. Effect of age, parity, and smoking on pregnancy outcome: A population-based study. Am J Obstet Gynecol. 1993;168(1, Part 1):16–21. [DOI] [PubMed] [Google Scholar]
- 40.Kramer MS, Olivier M, McLean FH, Dougherty GE, Willis DM, Usher RH. Determinants of fetal growth and body proportionality. Pediatrics. 1990;86(1):18–26. [PubMed] [Google Scholar]
- 41.Nieto A, Matorras R, Serra M, Valenzuela P, Molero J. Multivariate analysis of determinants of fetal growth retardation. Eur J Obstet Gynecol Reprod Biol. 1994;53(2):107–13. [DOI] [PubMed] [Google Scholar]
- 42.Arbuckle TE, Liang CL, Morisset A-S, Fisher M, Weiler H, Cirtiu CM, et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere. 2016;163:270–82. [DOI] [PubMed] [Google Scholar]
- 43.Llop S, Lopez-Espinosa M-J, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311(1):3–12. [DOI] [PubMed] [Google Scholar]
- 44.Vahter M, Berglund M, Åkesson A, Lidén C. Metals and Women’s Health. Environ Res. 2002;88(3):145–55. [DOI] [PubMed] [Google Scholar]
- 45.Rosner B Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;25(2):165–72. [Google Scholar]
- 46.Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. Am J Epidemiol. 2006;163(4):374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, et al. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect. 2017;125(6):067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greene WH. Econometric analysis. 4th ed. ed2000. [Google Scholar]
- 50.VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng G, Zhong H, Guo Z, Wu Z, Zhang H, Wang C, et al. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population-based study. Biological trace element research. 2014;160(3):437–44. [DOI] [PubMed] [Google Scholar]
- 52.Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, et al. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environmental health : a global access science source. 2013;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123(2):186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almberg KS, Turyk ME, Jones RM, Rankin K, Freels S, Graber JM, et al. Arsenic in drinking water and adverse birth outcomes in Ohio. Environ Res. 2017;157:52–9. [DOI] [PubMed] [Google Scholar]
- 55.Lin Z, Chen X, Xi Z, Lin S, Sun X, Jiang X, et al. Individual heavy metal exposure and birth outcomes in Shenqiu county along the Huai River Basin in China. Toxicology research. 2018;7(3):444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howe CG, Farzan SF, Garcia E, Jursa T, Iyer R, Berhane K, et al. Arsenic and birth outcomes in a predominately lower income Hispanic pregnancy cohort in Los Angeles. Environ Res. 2020;184:109294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L, Ding G, Gao Y, Wang P, Shi R, Huang H, et al. Manganese concentrations in maternal-infant blood and birth weight. Environmental science and pollution research international. 2014;21(9):6170–5. [DOI] [PubMed] [Google Scholar]
- 58.Milne DB, Sims RL, Ralston NV. Manganese content of the cellular components of blood. Clin Chem. 1990;36(3):450–2. [PubMed] [Google Scholar]
- 59.Chaffee BW, King JC. Effect of zinc supplementation on pregnancy and infant outcomes: a systematic review. Paediatric and perinatal epidemiology. 2012;26 Suppl 1:118–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfeiffer CC, Braverman ER. Zinc, the brain and behavior. Biol Psychiatry. 1982;17(4):513–32. [PubMed] [Google Scholar]
- 61.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–208. [DOI] [PubMed] [Google Scholar]
- 62.Jauniaux E, Burton GJ. [The role of oxidative stress in placental-related diseases of pregnancy]. J Gynecol Obstet Biol Reprod (Paris). 2016;45(8):775–85. [DOI] [PubMed] [Google Scholar]
- 63.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27(2):141–69. [DOI] [PubMed] [Google Scholar]
- 64.Bocca B, Ciccarelli S, Agostino R, Alimonti A. Trace elements, oxidative status and antioxidant capacity as biomarkers in very low birth weight infants. Environ Res. 2017;156:705–13. [DOI] [PubMed] [Google Scholar]
- 65.Li L, Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid Med Cell Longev. 2018;2018:7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59(4):627–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grandjean P Even low-dose lead exposure is hazardous. Lancet. 2010;376(9744):855–6. [DOI] [PubMed] [Google Scholar]
- 69.Reyners H, Gianfelici de Reyners E, Maisin JR, Winneke G, Csicsaky M. Effects of heavy metals (Cd, Tl, Zn and Pb) on glial cells. Neurobehav Toxicol Teratol. 1982;4(6):651–4. [PubMed] [Google Scholar]
- 70.Rowles TK, Womac C, Bratton GR, Tiffany-Castiglioni E. Interaction of lead and zinc in cultured astroglia. Metab Brain Dis. 1989;4(3):187–201. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez-Nahm S, Nihlani K, J SH, R LM, H GS, Hoyo C. Associations between Maternal Cadmium Exposure with Risk of Preterm Birth and Low after Birth Weight Effect of Mediterranean Diet Adherence on Affected Prenatal Outcomes. Toxics. 2020;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tinggi U Selenium: its role as antioxidant in human health. Environ Health Prev Med. 2008;13(2):102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun HJ, Rathinasabapathi B, Wu B, Luo J, Pu LP, Ma LQ. Arsenic and selenium toxicity and their interactive effects in humans. Environment international. 2014;69:148–58. [DOI] [PubMed] [Google Scholar]
- 74.Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27(2):210–6. [DOI] [PubMed] [Google Scholar]
- 75.Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernández-Avila M, et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect. 2012;120(1):126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodrigues EG, Bellinger DC, Valeri L, Hasan MOSI, Quamruzzaman Q, Golam M, et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environmental Health. 2016;15(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenfeld CS. Sex-Specific Placental Responses in Fetal Development. Endocrinology. 2015;156(10):3422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biology of sex differences. 2013;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J-W, Chang C-C, Wang S-L. Mercury Concentration of Whole Blood and Red Blood Cell in Taiwan Residents. Epidemiology. 2011;22(1). [Google Scholar]
- 80.Mortensen ME, Caudill SP, Caldwell KL, Ward CD, Jones RL. Total and methyl mercury in whole blood measured for the first time in the U.S. population: NHANES 2011–2012. Environ Res. 2014;134:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watson CV, Lewin M, Ragin-Wilson A, Jones R, Jarrett JM, Wallon K, et al. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999–2016. Environ Res. 2020;183:109208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li YV, Zhang John H. Metal Ion in Stroke. Li YV, Zhang John H., editor2012. [Google Scholar]
- 83.Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev. 2010;15(2):101–9. [PubMed] [Google Scholar]
- 84.Leach RM, Lilburn MS. Manganese metabolism and its function. World Rev Nutr Diet. 1978;32:123–34. [DOI] [PubMed] [Google Scholar]
- 85.Falchuk KH. Chapt 28. Harrison’s Principles of Internal Medicine. 13th ed ed. New York, NY: McGraw-Hill; 1994. p. 481–82. [Google Scholar]
- 86.Walther LE, Winnefeld K, Sölch O. Determination of iron, copper, zinc, magnesium and selenium in plasma and erythrocytes in neurosurgical patients. J Trace Elem Med Biol. 2000;14(2):92–5. [DOI] [PubMed] [Google Scholar]
- 87.Hall M, Chen Y, Ahsan H, Slavkovich V, van Geen A, Parvez F, et al. Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology. 2006;225(2–3):225–33. [DOI] [PubMed] [Google Scholar]
- 88.Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A Review of Arsenic Poisoning and its Effects on Human Health. Critical Reviews in Environmental Science and Technology. 1999;29(3):281–313. [Google Scholar]
- 89.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114(11):1790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barbosa F Jr., Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113(12):1669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS. Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environ Res. 1990;52(2):126–45. [DOI] [PubMed] [Google Scholar]
- 92.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24(1):11–4. [DOI] [PubMed] [Google Scholar]
- 93.Ouidir M, Buck Louis GM, Kanner J, Grantz KL, Zhang C, Sundaram R, et al. Association of Maternal Exposure to Persistent Organic Pollutants in Early Pregnancy With Fetal Growth. JAMA Pediatr. 2020;174(2):149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


