Abstract
Tumor necrosis factor (TNF) drives chronic inflammation and cell death in the intestine, and blocking TNF is a therapeutic approach in inflammatory bowel disease (IBD). Despite this knowledge, the pathways that protect the intestine from TNF are incompletely understood. Here we demonstrate that group 3 innate lymphoid cells (ILC3s) protect the intestinal epithelium from TNF-induced cell death. This occurs independent from interleukin (IL)-22, and rather we identify that ILC3s are a dominant source of heparin-binding epidermal growth factor-like growth factor (HB-EGF). ILC3s produce HB-EGF in response to prostaglandin E2 (PGE2) and engagement of the EP2 receptor. Mice lacking ILC3-derived HB-EGF exhibit increased susceptibility to TNF-mediated epithelial cell death and experimental intestinal inflammation. Finally, human ILC3s produce HB-EGF and are reduced from the inflamed intestine. These results define an essential role for ILC3-derived HB-EGF in protecting the intestine from TNF and indicate that disruption of this pathway contributes to IBD.
Introduction
Tumor necrosis factor (TNF) is a pleiotropic cytokine that promotes essential host defense against pathogens, cell survival and tissue regeneration under homeostatic condition1,2. However, if dysregulated and overexpressed, TNF is a major driver of chronic inflammation and target of biologic therapies in the clinic1,2. Excessive TNF production in the intestine targets the epithelium, drives increased cell death, and is sufficient to elicit substantial tissue inflammation1,2. Further, blockade of TNF provides therapeutic benefit in a subset of IBD patients3–7. Despite the importance of this pathway to intestinal health and inflammation, the mechanisms that control the beneficial versus detrimental outcomes of TNF in the intestine are incompletely understood.
Innate lymphoid cells (ILCs) are tissue-resident innate lymphocytes, which do not express rearranged antigen-specific receptors, and are well-defined critical regulators of immunity, inflammation and tissue homeostasis8–10. ILCs are subdivided into three subgroups based on their transcription factor expression profile, including T-bet+ group 1 ILCs (ILC1s), GATA3+ group 2 ILCs (ILC2s) and RORγt+ group 3 ILCs (ILC3s). These subsets of ILCs critically protect epithelial barriers in the mucosa during homeostasis or following infectious or inflammatory challenge8–10. For example, ILC2s protect the airway and intestinal epithelial barriers through production of the epidermal growth factor receptor (EGFR) ligand amphiregulin11,12. ILC3s similarly protect these barriers in part through multiple anti-microbial and tissue-protective responses elicited by IL-2213–15. However, limited knowledge exists on how ILCs respond to, or control the outcome of, excessive TNF production. This may be particularly important as ILCs are known to be altered in the context of multiple chronic inflammatory diseases in humans8,9,16.
In this study, we hypothesized that ILC3s critically control the outcome of TNF production in the intestine as this cell type is enriched in the healthy gastrointestinal tract, coordinates multiple aspects of mucosal immunity, and translational studies have found that ILC3s are fundamentally altered in the intestine of individuals with IBD, chronic infections, or cancer9,15–19. Utilizing an assay of in vivo high-dose delivery of exogenous TNF, we determined that mice lacking ILC3s exhibit excessive cell death in the intestinal epithelium. This ILC3-mediated protection occurred independent of IL-22. Rather, single cell RNA-sequencing revealed that a tissue-protective growth factor–HB-EGF–is dominantly produced by ILC3s in the intestinal mucosa. Gain-of-function and loss-of-function approaches demonstrated that ILC3-intrinsic HB-EGF production is dependent on a prostaglandin E2 (PGE2)-EP2 axis, which can occur in an autocrine manner following exposure to IL-1β. ILC3-derived HB-EGF is required to protect the intestinal epithelium from TNF-induced cell death, and limits disease in experimental models of acute and chronic intestinal inflammation. Finally, analyses of human samples revealed a similar pathway of induction and potential dysregulation of HB-EGF+ ILC3s in IBD. These data define a new tissue-protective mechanism by which PGE2 acts on ILC3s to induce HB-EGF and protect the intestinal epithelium from TNF, which advances our understanding of intestinal inflammation and could provoke opportunities for therapeutic intervention by supporting HB-EGF+ ILC3s in IBD.
Results
ILC3s protect the intestinal epithelium from TNF through an IL-22-independent mechanism
To explore whether ILC3s control the outcome of excessive TNF in the intestine, we employed mice lacking ILC3s and administered a single dose of recombinant TNF, an in vivo assay that induces rapid intestinal epithelial cell death predominantly at the villus tips20–22. RORγt-deficient mice, which lack ILC3s, T helper (Th)17 cells and most secondary lymphoid tissues23, exhibited a significant increase in TNF-induced intestinal epithelial cell death as characterized by immunofluorescence staining of cleaved-caspase 3 (CC3) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) of the small intestine relative to wild-type control mice (Fig. 1a,b). To dissect the contribution of T cells versus ILC3s in protecting against TNF-induced cell death, we simultaneously employed Rag1−/− mice that lack adaptive immunity and observed comparable levels of epithelial cell death relative to wild-type controls (Fig. 1a,b). Further, depletion of ILC3s from Rag1−/− mice with an anti-CD90.2 monoclonal antibody resulted in a significant increase of TNF-induced cell death within the epithelium as quantified by increased CC3 and TUNEL staining of the small intestine relative to controls (Fig. 1c,d and Extended Data Fig. 1a). ILC3s express high levels of CD90.2 and were robustly depleted by this approach, while ILC2s expressed lower amounts of CD90.2 and were partially reduced (Extended Data Fig. 1b–d). To directly investigate whether ILC3s are sufficient to protect the intestinal epithelium from TNF, we employed Rag2−/−Il2rg−/− mice that lack adaptive immunity and all ILC subsets, and observed significantly increased intestinal epithelial cell death in the small intestine relative to Rag1−/− mice after TNF exposure, while Rag2−/−Il2rg−/− mice reconstituted with sort-purified ILC3s displayed significantly reduced cell death reflected by TUNEL staining (Extended Data Fig. 1e). We next explored whether this protection was mediated by IL-22 and found that depletion of this cytokine did not alter the level of TNF-induced cell death in the epithelium despite a significant reduction in IL-22-dependent antimicrobial peptides (Fig. 1c,d and Extended Data Fig. 1f). Collectively, these data demonstrate that ILC3s protect the intestinal epithelium from TNF-induced cell death independent from IL-22.
Figure 1. ILC3s protect the intestinal epithelium from TNF-induced cell death.

a and b. Representative immunofluorescence sections and quantifications of cleaved-caspase 3 (CC3) (a) or TUNEL (b) staining of the small intestine from noted mice 4 hours post recombinant TNF injection (N=3 individual mice per group, n=18 fields per group). c and d. Representative immunofluorescence sections and quantifications of CC3 (c) or TUNEL (d) staining of small intestine from noted mice 4 hours post recombinant TNF injection (N=3 individual mice per group, n=18 fields per group). Data in a-d are representative of two independent experiments, shown as the means ± S.E.M. Statistics are calculated by one-way ANOVA. WT, wild type. Scale bars=50 μm.
ILC3s are a dominant source of HB-EGF in the intestine that protects the epithelium from TNF
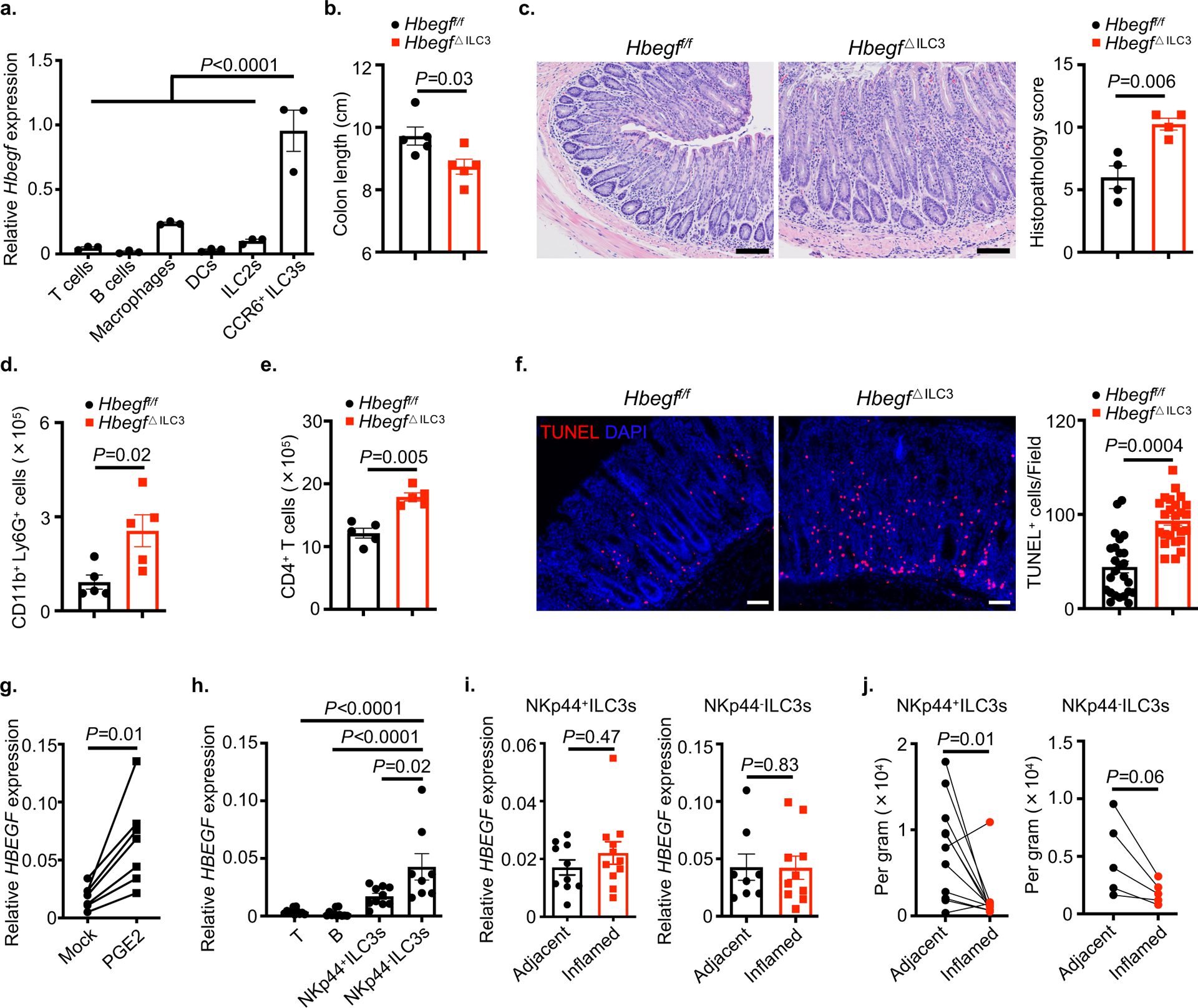
To interrogate the mechanism by which ILC3s protect the intestinal epithelium from TNF, we performed single-cell RNA-sequencing (scRNA-seq) on 11,556 sort-purified ILCs from the small intestine of mice treated with PBS control or recombinant TNF. Nine distinct clusters of cells were identified and classified into groups based on expression of signature genes, including ILC1s (Tbx21 and Il12rb2), ILC2s (Gata3 and Il5), and three distinct ILC3 subsets (Rorc and Il22) (Fig. 2a and Extended Data Fig. 2). The family of EGFR ligands are widely known to regulate multiple biological functions including cell proliferation, differentiation and cell death24. Prior studies determined that ILC2s protect the epithelial barrier during tissue damage through production of the EGFR ligand amphiregulin11,12, prompting us to ask whether ILC3s protect the epithelium from TNF through a similar mechanism. ILC3s in our data set had limited expression of Areg relative to ILC2s, as well as most other EGFR ligands, but unexpectedly expressed abundant levels of Hbegf (Fig. 2b and Supplementary Table 1), a ligand for the EGFR that is implicated in wound healing, angiogenesis and tissue repair25–27. Among these populations, Hbegf was dominantly expressed by ILC3s and significantly increased upon in vivo exposure to TNF, particularly among the CCR6+ ILC3 subset (Fig. 2c, d), which represent lymphoid tissue inducer (LTi)-like cells and are present within lymphoid clusters along the mammalian intestine23. CCR6+ ILC3s exhibited the highest expression of Hbegf relative to other lymphocytes or myeloid cells in the healthy small intestine (Fig. 2e). Utilizing a LacZ reporter for HB-EGF28, we also determined with unbiased flow cytometry analyses that the majority of hematopoietic HB-EGF+ cells in both the small and large intestine lack lineage markers for T cells, B cells, macrophages and dendritic cells, and rather are CD127+, CD90.2+ and CCR6+ ILC3s (Fig. 2f and Extended Data Fig. 3a). HB-EGF+ ILC3s are present at significantly higher total numbers relative to HB-EGF+ macrophages, dendritic cells, CD4+ T cells or ILC2s in the healthy mouse intestine (Fig. 2g,h and Extended Data Fig. 3b–e). Therefore, ILC3s are a dominant source of HB-EGF in the mammalian intestine that is significantly upregulated following in vivo exposure to TNF.
Figure 2. ILC3s are a major cellular source of HB-EGF in the intestine.
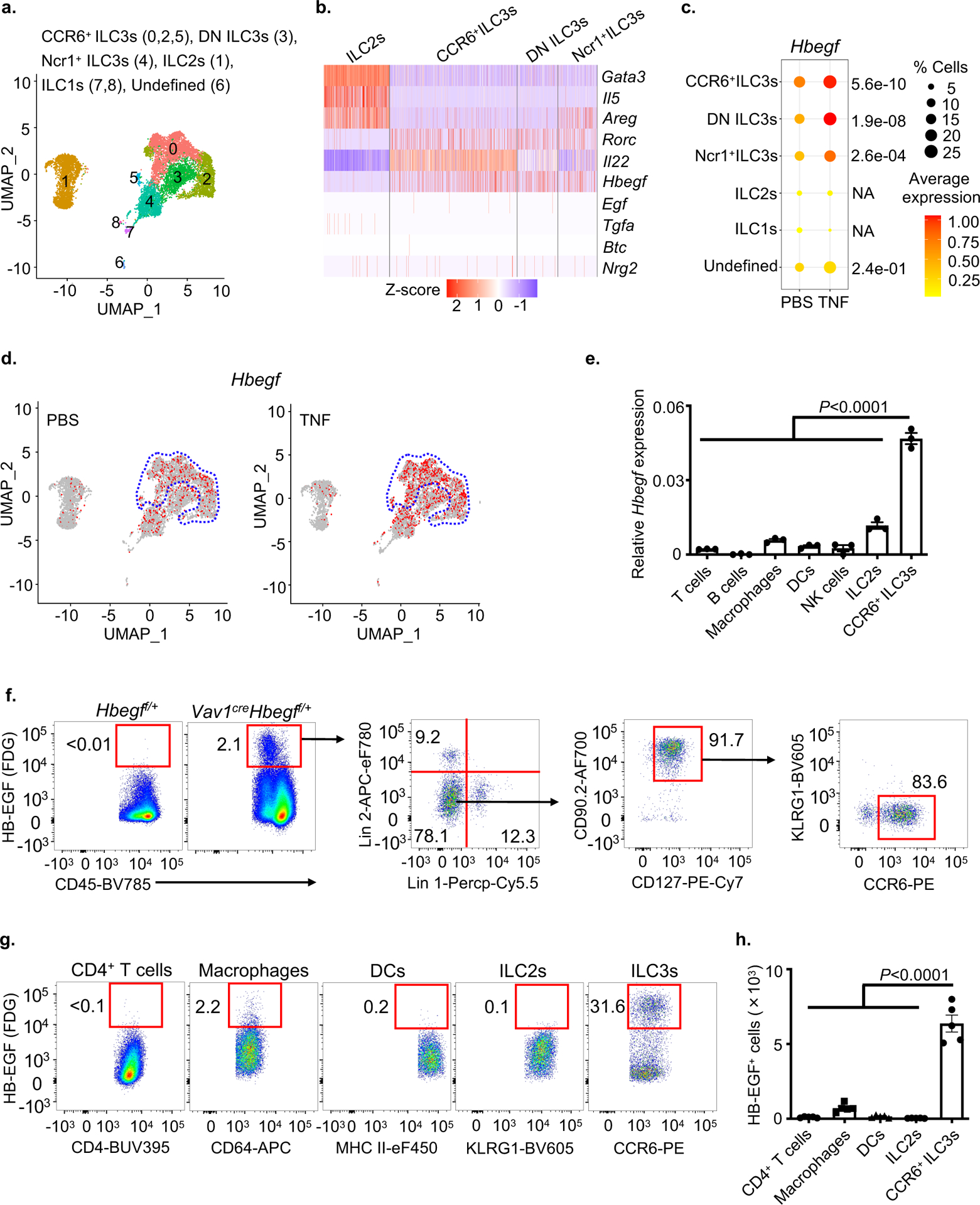
a-d. Total ILCs were sort-purified from the small intestine of PBS- or TNF-treated mice and analyzed by scRNA-Seq. Uniform manifold approximation and projection (UMAP) embedding of 11,556 cells (dots), colored by cluster (a). Heatmap showing expression Z-scores of the indicated genes in ILC2s and ILC3s from TNF-treated mice (b). Dot plot shows the mean expression (color) of Hbegf in clusters grouped by cell type as noted, dot size represents the proportion of cells in a cluster with the gene detected (c). UMAP shows the expression of Hbegf gene in all clusters of each treatment condition and the dotted line indicates CCR6+ ILC3 clusters (d). e. Expression of Hbegf in the indicated sort-purified immune cells as determined by qPCR (n=3 individual mice). Relative to Actb. f. Flow cytometry plots show HB-EGF expression in small intestine lamina propria of noted mice as measured by conversion of the fluorescent LacZ substrate fluorescein di-β-d-galactopyranoside (FDG). g and h. Flow cytometry plots (g) and quantification of cell number (h) of HB-EGF+ cells in the small intestine of noted mice as measured by LacZ activity (n=5 individual mice). Lineage 1: CD3ε, CD5, CD8α, NK1.1, TCRγδ. Lineage 2: CD11b, CD11c, B220. Data in e-h are representative of two independent experiments, shown as the means ± S.E.M. Statistics are calculated by one-way ANOVA. DN, double-negative of CCR6 and Ncr1.
We next examined for a protective role of HB-EGF and observed that recombinant HB-EGF significantly reduced in vitro TNF-induced cell death of an intestinal epithelial cell line (Fig. 3a and Extended Data Fig. 4a), which is consistent with prior studies29,30. The EGFR family of receptor tyrosine kinases contains EGFR/ErbB1/HER1, ErbB2/HER2/neu, ErbB3/HER3 and ErbB4/HER4, and HB-EGF directly binds ErbB1 or ErbB4 to activate a downstream PI3K-Akt signaling pathway31–33. We next interrogated whether HB-EGF protects from intestinal epithelial cell death through similar mechanisms, and observed that ErbB1 and Akt signaling is required for recombinant HB-EGF protection, while ErbB4 is not essential but could synergize with ErbB1 signaling to protection from cell death (Fig. 3b and Extended Data Fig. 4b). To interrogate the function of ILC3-derived HB-EGF, we selectively deleted Hbegf in ILC3s by employing RorccreHbegff/f (Hbegf△ILC3) mice. Following administration of recombinant TNF, Hbegf△ILC3 mice exhibited a significant increase in cell death at the intestinal epithelium as quantified by CC3 and TUNEL staining of the small intestine, relative to littermate controls (Fig. 3c,d). HB-EGF also protected from TNF-mediated activation of cleaved caspase 8 (Fig. 3e and Extended Data Fig. 4c). These data demonstrate that ILC3-derived HB-EGF is essential to protect the intestinal epithelium from TNF-induced cell death, and that this protection occurs through ErbB1 and Akt signaling.
Figure 3. ILC3-derived HB-EGF protects the intestinal epithelium from TNF.
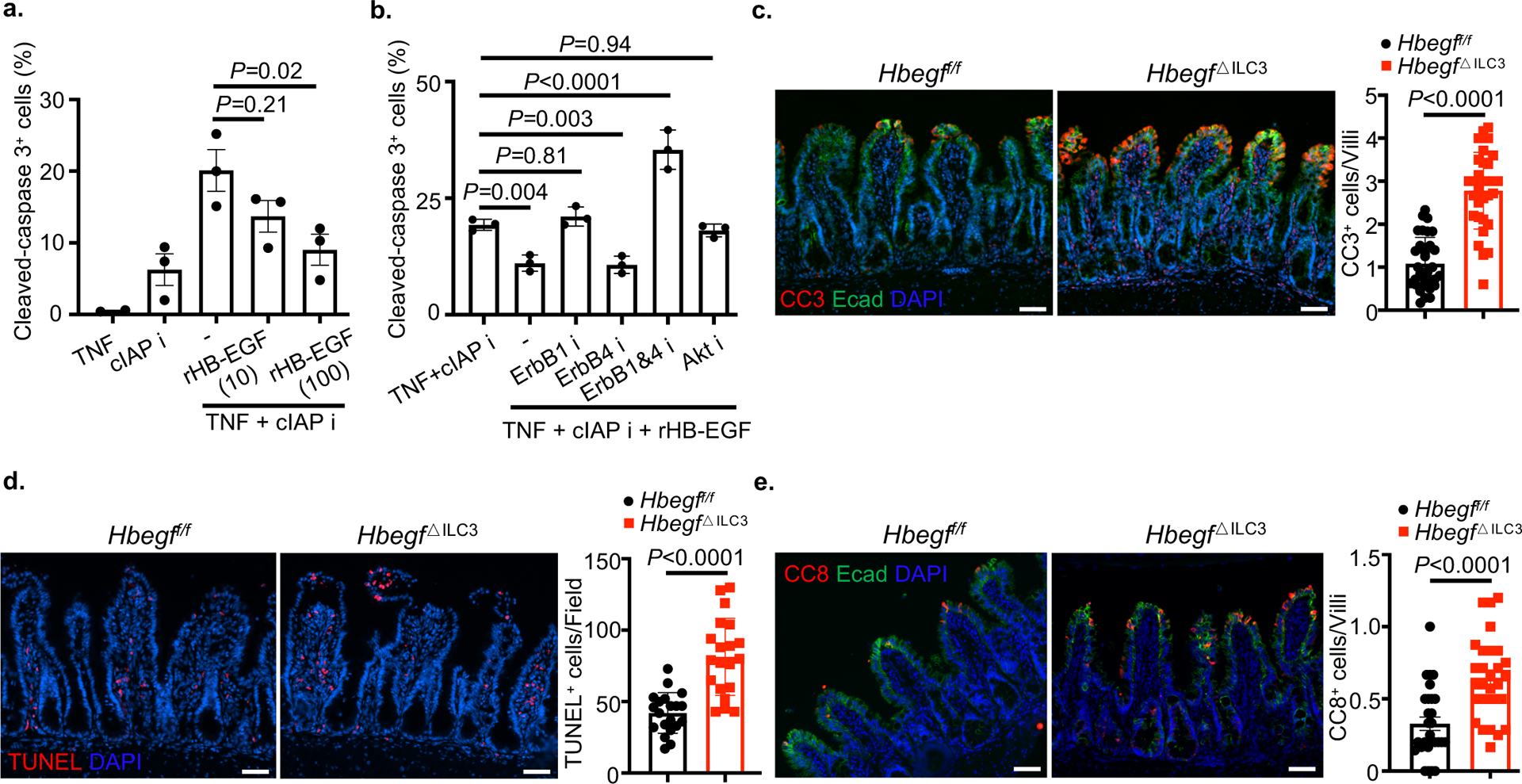
a and b. Cell death was induced in the murine rectum epithelial cell line CMT-93 by TNF and a cIAP inhibitor. Bar graph of frequencies of cleaved-caspase 3 (CC3) in the presence or absence of recombinant HB-EGF (a). Bar graph of frequencies of CC3 in the presence of various inhibitors (b). c-e. Representative immunofluorescence sections and quantifications of cleaved-caspase 3 (c) or TUNEL (d) or cleaved-caspase 8 (e) staining of small intestine 4 hours post recombinant TNF injection (N=3 individual mice per group. n=31 fields per group of c, n=20 fields per group of d, n=28 fields per group of e). Data in a and b are pooled from three independent experiments, shown as the means ± S.E.M, calculated by one-way ANOVA. Data in c-e are representative of two independent experiments, shown as the means ± S.E.M, calculated by unpaired two-tailed Student’s t-test. i, inhibition. Scale bars=50 μm.
An IL-1-PGE2 axis stimulates ILC3s through the EP2 receptor to promote HB-EGF
We next sought to understand what signals regulate ILC3-intrinsic HB-EGF production. Sort-purified ILC3s stimulated with TNF did not upregulate Hbegf (Extended Data Fig. 5a), suggesting that TNF acts in an indirect manner. Therefore, we analyzed other pathways upregulated by TNF treatment in our scRNA-Seq data set, focusing on CCR6+ ILC3s. Remarkably, we observed that Ptgs2, which encodes cyclooxygenases-2 (COX-2), was robustly upregulated after in vivo TNF treatment (Fig. 4a,b, Extended Data Fig. 5b and Supplementary Table 2). Further, CCR6+ ILC3s constitutively express Ptges, which encodes microsomal prostaglandin E synthase-1 (mPGES-1) (Fig. 4c and Extended Data Fig. 5c). The activities of COX-2 and mPGES-1 critically control the synthesis of PGE2, a prostanoid fatty acid derivative of arachidonic acid34 that often functions in an autocrine or paracrine manner to coordinate cellular responses within a local microenvironment35,36. This provokes a hypothesis that the autocrine PGE2 synthesis coordinates ILC3-derived HB-EGF production. To test this hypothesis, we first confirmed the PGE2 synthesis pathway in ILC3s and observed that IL-1β selectively induces Ptgs2 transcript and COX-2 protein in ILC3s (Fig. 4d,e). This is consistent with prior reports that IL-1β is substantially increased in the intestine following in vivo TNF exposure and induction of inflammatory cell death22. Further, we found ILC3s exhibit constitutively high mPGES-1 protein relative to other lymphocytes or macrophages (Fig. 4f), and sort-purified ILC3s produced significant levels of PGE2 in culture supernatants when stimulated with recombinant IL-1β (Fig. 4g). Therefore, ILC3s express machinery to produce PGE2, and this is engaged in response to sensing of IL-1β.
Figure 4. ILC3s produce PGE2 in response to IL-1β.
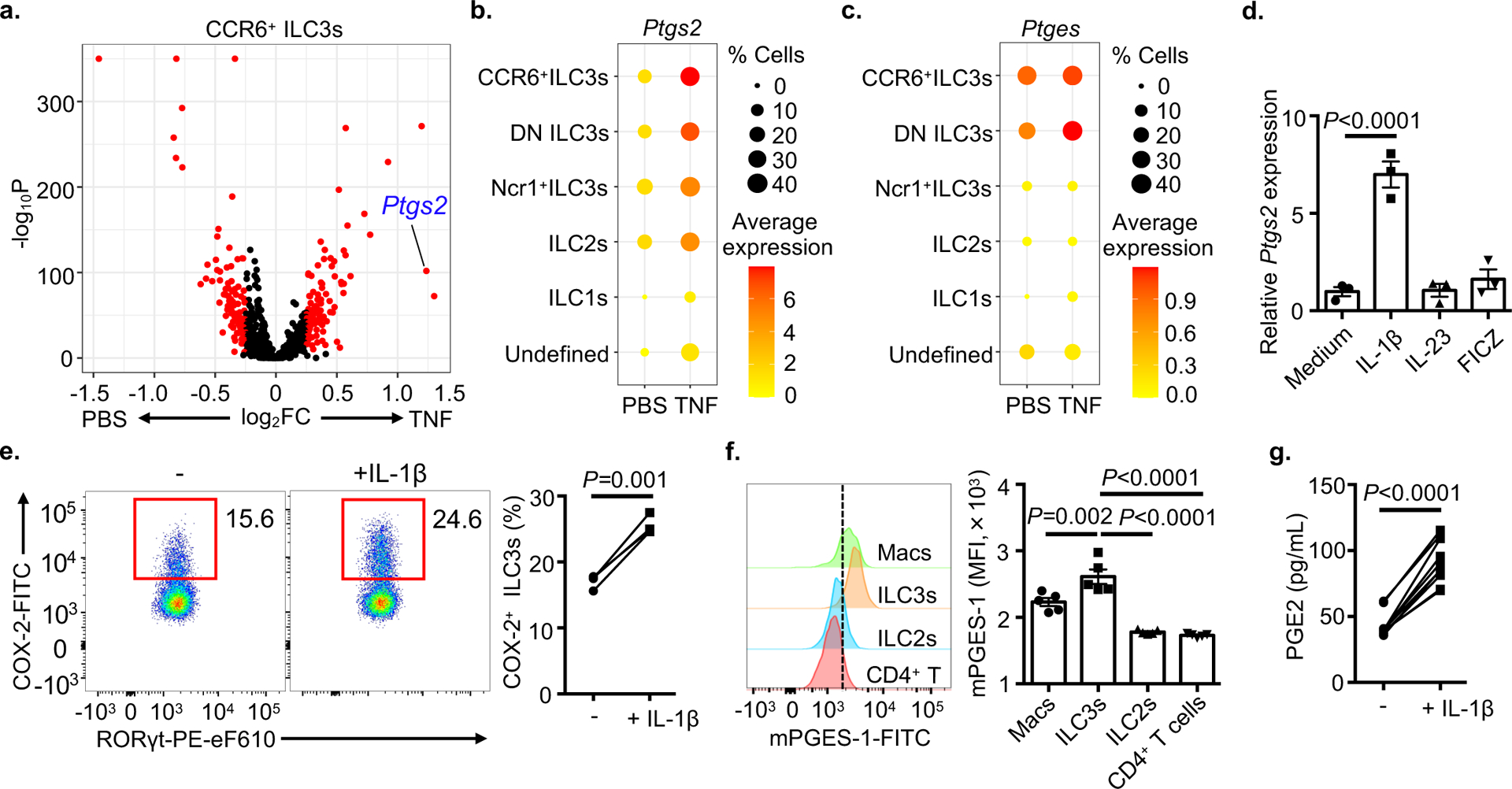
a. Volcano plot of differential expression between PBS and TNF in CCR6+ ILC3s of scRNA-Seq data set. b and c. Dot plot show the mean expression (color) of Ptgs2 (b) or Ptges (c) in clusters grouped by cell type as noted. d. Ptgs2 transcript in sort-purified ILC3s in the presence of various stimulus, relative to Hprt (n=3 individual mice). e. Flow cytometry plots with graph of frequency of COX-2+ ILC3s from the SI-LPs of wild type mice following ex vivo culture (n=4 individual mice). f. Histograms and bar graph examination of mPGES-1 expression in indicated cells from the small intestine of wild type mice (n=5 individual mice). g. Sort-purified ILC3s were stimulated with or without IL-1β and PGE2 in supernatants were determined by ELISA (n=10 individual mice). Data in d-f are representative of two or three independent experiments, data in g are pooled from three independent experiments, shown as the means ± S.E.M. Statistics are calculated by paired two-tailed Student’s t-test (e and g) or one-way ANOVA (d and f).
We next examined for a direct link between these pathways and observed that PGE2 is sufficient to significantly induce Hbegf expression in sort-purified ILC3s (Fig. 5a), indicating that PGE2 is produced by ILC3s in response to IL-1β, and that PGE2 exposure promotes Hbegf expression in ILC3s. This was selective as other pathways, such as retinoic acid, did not induce Hbegf expression in ILC3s, and PGE2 stimulation of ILC3s did not promote expression other effector cytokines (Extended Data Fig. 5d,e). PGE2 exerts its effects through four types of G protein-coupled receptors, EP1-434,37. EP2 (Ptger2) was the dominant receptor expressed in intestinal ILC3s, while EP4 was enriched in ILC2s, and EP1 and EP3 were minimally expressed (Fig. 5b). Consistent with this, an EP2 agonist significantly increased expression of Hbegf in sort-purified ILC3s (Extended Data Fig. 5f), while an EP2 antagonist was sufficient to block PGE2-mediated upregulation of Hbegf (Fig. 5c), and comparable changes were not observed with an EP4 agonist or antagonist respectively. In vivo delivery of an EP2 agonist was also sufficient to significantly increase the frequency of HB-EGF+ ILC3s (Fig. 5d and Extended Data Fig. 5g). To verify HB-EGF protein expression in ILC3s, we sort-purified ILC3s from Rag1−/− mice with or without in vivo EP2 agonist treatment and performed immunoblot analysis. Consistently, ILC3s express pro-HB-EGF in comparison to ILC2s, and more abundant protein was observed with EP2 agonist treatment (Extended Data Fig. 5h). To determine whether ILC3-intrinsic EP2 is necessary for production of HB-EGF in vivo, we deleted EP2 in ILC3s by crossing Rorccre with Ptger2f/f mice38 and observed a significant reduction of Hbegf expression in sort purified ILC3s relative to littermate controls (Fig. 5e and Extended Data Fig. 5i). To determine the functional significance of PGE2-EP2 axis in ILC3s, we performed the in vivo TNF-induced cell death assay and observed that RorccrePtger2f/f mice exhibited a significant increase of intestinal epithelial cell death in the small intestine characterized by CC3 and TUNEL staining relative to littermate controls, and this was significantly limited by pre-treatment of mice with recombinant HB-EGF (Fig. 5f,g). Finally, to test whether ILC3-derived PGE2 can act in an autocrine manner, we stimulated sort-purified ILC3s with IL-1β and observed a significant upregulation of Hbegf, which was blocked by addition of an EP2 antagonist (Extended Data Fig. 5j). These data demonstrate that PGE2 engagement of the EP2 receptor on ILC3s critically promotes HB-EGF expression, and further that this can occur in an autocrine manner following sensing of IL-1β.
Figure 5. PGE2 elicits ILC3-intrinsic HB-EGF production through the EP2 receptor.
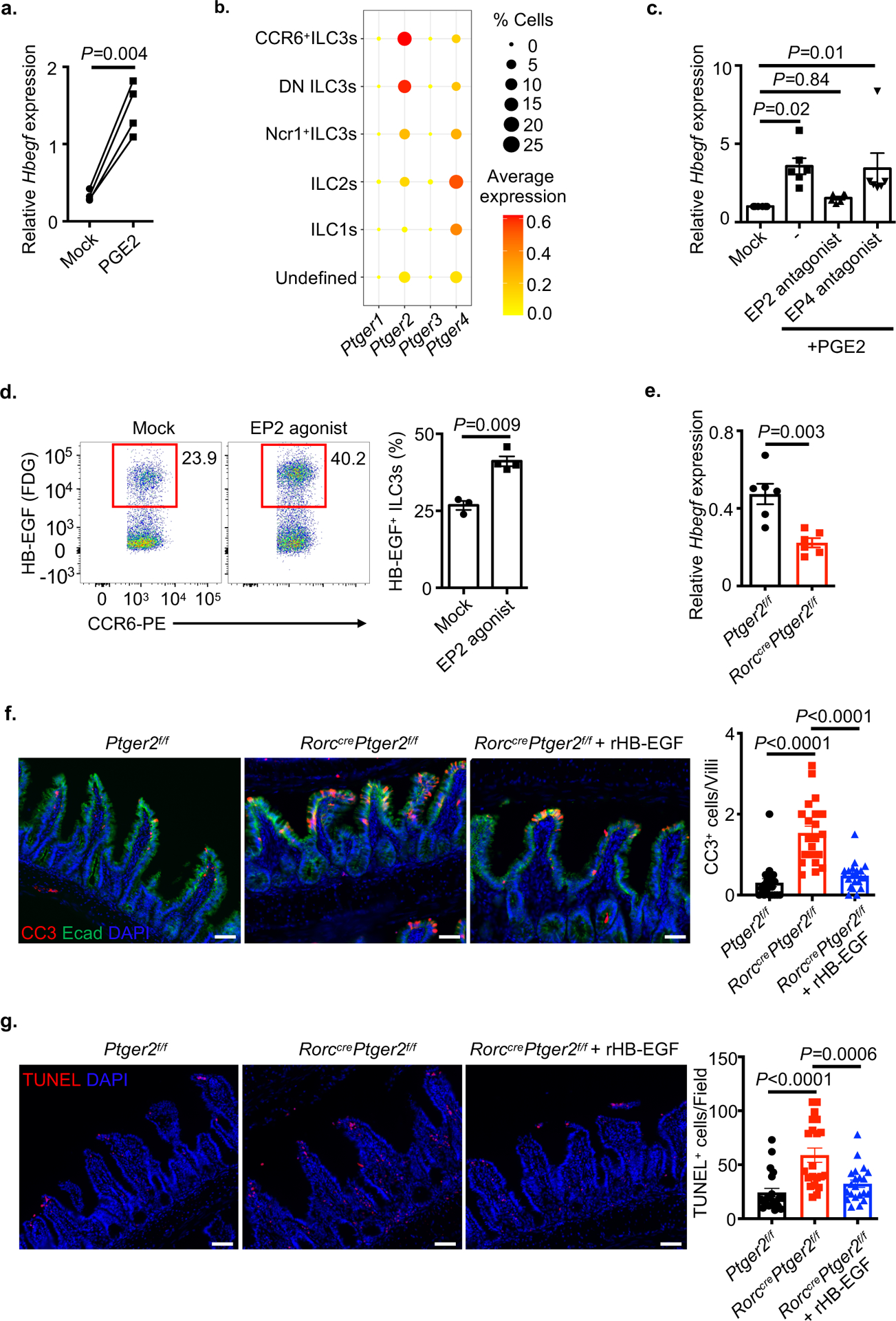
a. qPCR analysis of Hbegf expression in sort-purified ILC3s with or without PGE2 treatment, relative to Hprt (n=4 individual mice). b. Dot plot shows the mean expression (color) of Ptger1-4 in clusters grouped by cell type as noted from the small intestine of TNF-treated mice. c. Sort-purified ILC3s were stimulated with PGE2 in the presence of EP2 or EP4 antagonist, Hbegf expression was then examined by qPCR, relative to Hprt (n=6 individual mice). d. Flow cytometry plots with graph of the frequencies of HB-EGF+ ILC3s in small intestine of RorccreHbegff/+ mice with or without EP2 agonist in vivo treatment (n=3 individual mice in Mock, n=4 individual mice in EP2 agonist). e. ILC3-intrinsic HB-EGF of indicated mice at steady state was determined by qPCR, relative to Hprt (n=6 individual mice). f and g. Representative immunofluorescence sections and quantifications of cleaved-caspase 3 (CC3) (f) (N=3 individual mice per group, n=21 fields per group) or TUNEL (g) (N=3 mice per group, n=21 fields per group) staining of the small intestine from noted mice 4 hours post recombinant TNF injection. Data in a, d, f and g are representative of two or three independent experiments, data in c and e are pooled from two independent experiments, shown as the means ± S.E.M. Statistics are calculated by paired (a) or unpaired (d and e) two-tailed Student’s t-test or one-way ANOVA (c, f and g). Scale bars=50 μm.
ILC3-derived HB-EGF protects from acute intestinal damage and inflammation
We next sought to understand the functional significance of ILC3-derived HB-EGF in the context of intestinal health, damage and inflammation. At steady state, we did not observe any alterations in ILC3 or T cell responses in the intestine (Extended Data Fig. 6a–c). Cell death in the epithelium is a hallmark of intestinal inflammation and may contribute to the pathogenesis of IBD4,6,39. To investigate whether deficiency of HB-EGF in ILC3s sensitizes mice to acute experimental intestinal inflammation, we administered dextran sulfate sodium (DSS) to Hbegf△ILC3 mice and observed significantly exacerbated intestinal damage and inflammation, characterized by continuous weight loss, shorter colon length and severe disruption of epithelial crypt architecture relative to littermate controls (Fig. 6a–c). Rorccre will also target T cells, however few HB-EGF+ T cells were observed in the intestine and Cd4creHbegff/f (Hbegf△T cell) mice exhibited comparable responses to littermate controls following exposure to DSS (Extended Data Fig. 7a–c). To explore why ILC3-derived HB-EGF is important to protect from acute intestinal inflammation, we profiled other immune cell types and observed an increase in neutrophils at tissue recovery stage without impacting CD4+ T cells of Hbegf△ILC3 mice relative to littermate controls (Fig. 6d, e). Functions of the intestinal epithelium, such as anti-microbial peptides production, tight junction proteins, intestinal stem cell signature genes and proliferative ability were also unchanged in the absence of ILC3-specific HB-EGF (Extended Data Fig. 8a–d). In contrast, Hbegf△ILC3 mice exhibited a significant increase in cell death as determined by TUNEL staining, relative to littermate controls (Fig. 6f). To test if exogenous HB-EGF could provide protection during intestinal damage and inflammation, wild-type mice were intraperitoneally treated with recombinant HB-EGF (rHB-EGF) over the course of DSS exposure. This treatment resulted in significantly reduced weight loss, increased colon length and improved restoration of epithelial architecture relative to PBS-treated controls (Fig. 6g–i). These results demonstrate that ILC3-derived HB-EGF is essential to protect the intestinal epithelium during acute experimental tissue damage and inflammation, and that administration of exogenous HB-EGF can provide tissue protection and ameliorate disease severity.
Fig 6. ILC3-derived HB-EGF protects from acute intestinal damage and inflammation.
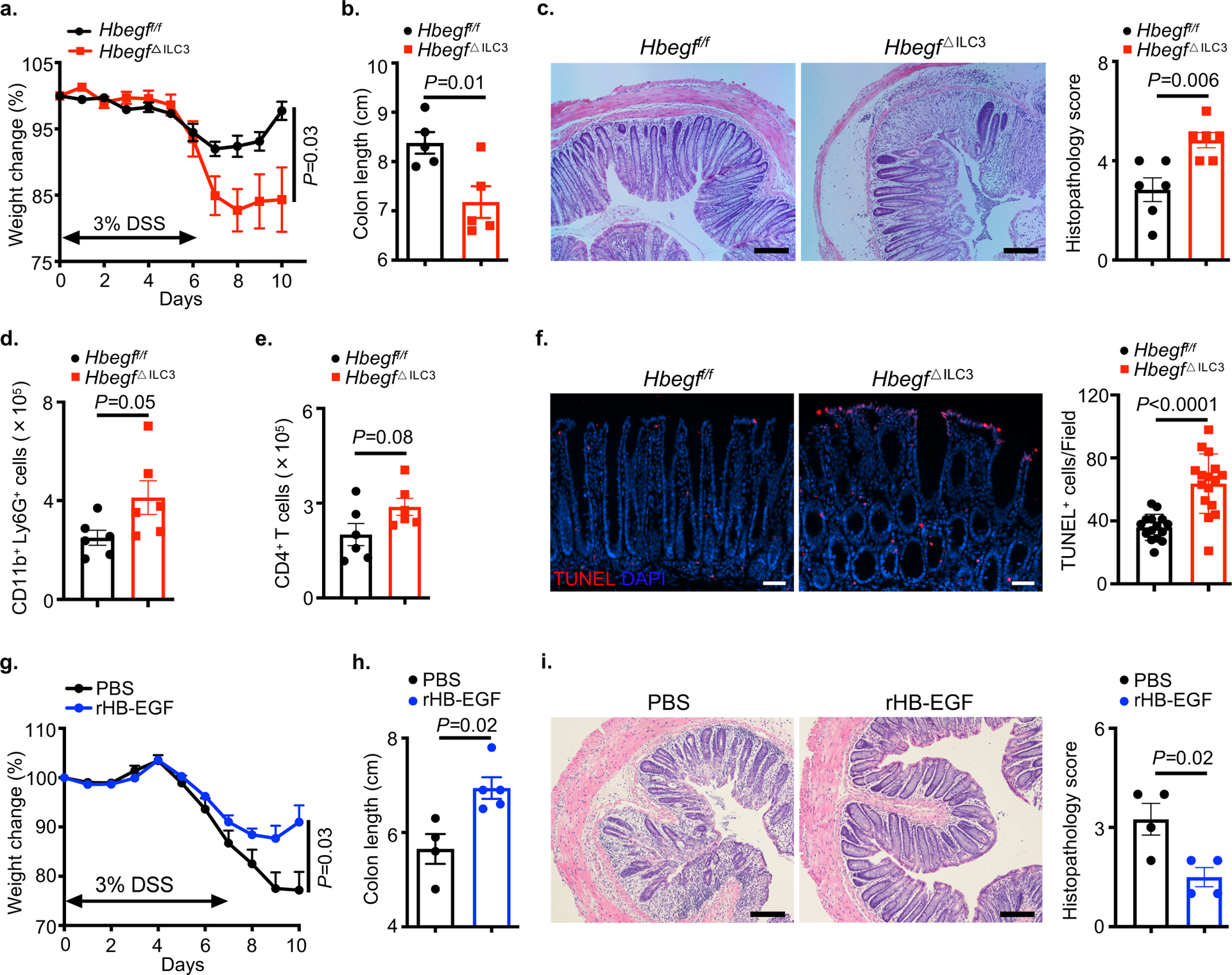
a-f. Mice were given 3% DSS for 6 days, followed by normal water for recovery. Disease and recovery were monitored by weight loss (a), colon shortening (b), hematoxylin and eosin (H&E) staining with histopathology scores of the distal colon (c), lamina propria neutrophil infiltration (d) and CD4+ T cell response (e) at day 10 (n=6 individual mice). Intestinal epithelial cell death was examined by TUNEL staining at day 6 (f) (N=4 individual mice per group, n=16 fields per group). g-i. Wild-type mice were exposed to 3% DSS in the drinking water for 7 days and treated with PBS or 1 mg/kg recombinant HB-EGF intraperitoneally every other day starting on day 2 of DSS exposure. Mice were examined for weight loss (g) over the course of treatment, and colon length (h) and H&E staining with histopathology scores of the distal colon (i) were assessed on day 10 (n=4 individual mice in PBS, n=5 individual mice in rHB-EGF). Data are representative of two or three independent experiments, shown as the means ± S.E.M. Statistics are calculated by unpaired two-tailed Student’s t-test. Scale bars=50 μm in f. Scale bars=100 μm in c and i.
ILC3-derived HB-EGF is essential to protect the intestinal epithelium from chronic inflammation.
TNF is the key driver of the intestinal epithelial cell death in the setting of chronic inflammation3,6,40. Therefore, we next asked whether ILC3-derived HB-EGF protects the epithelium in the context of chronic intestinal inflammation. We evaluated the expression profile of HB-EGF in the large intestine of IL-10-deficient mice that exhibited spontaneous chronic inflammation characterized by rectal prolapse. Consistent with healthy mice, CCR6+ ILC3s displayed the highest expression of Hbegf relative to other lymphocytes or myeloid cells in the large intestine of IL-10-deficient mice (Fig. 7a). We next utilized a mouse model of chronic intestinal inflammation induced by colonization with Helicobacter hepaticus and blockade of the regulatory response with an anti-IL10R antibody (Extended Data Fig. 8e). Notably, Hbegf△ILC3 mice exhibited exacerbated disease phenotype characterized by significantly shorter colon length, elevated histopathology, increased neutrophil infiltration and elevated CD4+ T cell responses relative to littermate controls (Fig. 7b–e). Further, significantly increased cell death was also observed in Hbegf△ILC3 mice (Fig. 7f). These findings demonstrate that ILC3-derived HB-EGF is essential to protect the intestinal epithelium and limit chronic intestinal inflammation.
Fig 7. HB-EGF-producing ILC3s protect from chronic intestinal inflammation and are altered in IBD.
a. Expression of Hbegf in the indicated sort-purified immune cells from large intestine of IL-10-deficient mice as determined by qPCR (n=3 individual mice). Relative to Hprt. b-f. Mice were orally infected with H. hepaticus and blockade of the regulatory response with anti-IL10R antibody (n=5 individual mice). Colon shortening (b), hematoxylin and eosin (H&E) staining with histopathology scores of the distal colon (c) (n=4 individual mice), lamina propria neutrophil infiltration (d) and CD4+ T cell response (e), and intestinal epithelial cell death was examined by TUNEL staining (f) (N=4 mice per group, n=25 fields per group) at day 21. g. Sort-purified ILC3s from human tonsils were stimulated with or without PGE2 and HBEGF transcript was determined by qPCR (n=7 individual donors). h-j, Human lamina propria lymphocytes were sort-purified from adjacent non-inflamed versus matched inflamed surgical resection tissues from the small intestine of Crohn’s disease patients (n=11 individual donors). HBEGF in the indicated cells of non-inflamed tissues were determined by qPCR, relative to ACTB (h). HBEGF in the NKp44−ILC3s or NKp44+ILC3s of adjacent versus inflamed tissues were determined by qPCR (i). Quantification of NKp44−ILC3s or NKp44+ILC3s numbers per gram of tissue between adjacent and inflamed tissues (j). Data in a-f are representative of two independent experiments, data in g-j are pooled from two or three independent experiments, shown as the means ± S.E.M. Statistics are calculated by one-way ANOVA (a) or unpaired two-tailed Student’s t-test (b-f), statistic in g and j are performed using two-tailed Wilcoxon matched-pairs test (paired), statistics in h and i are performed using two-tailed Mann–Whitney U-test (unpaired), Scale bars=50 μm in f. Scale bars=100 μm in c.
Human ILC3s produce HB-EGF in response to PGE2 and are reduced in the inflamed intestine
IBD is a chronic inflammatory disorder of the gastrointestinal tract, and prior studies have demonstrated a substantial alteration of ILC3s in the intestine of IBD patients9,15–18. This provoked us to examine whether the PGE2-ILC3-HB-EGF pathway is functional in humans. Sort-purified ILC3s from human tonsils were found to respond to PGE2 when cultured in vitro as noted by a significant increase in HBEGF expression (Fig. 7g). In the non-inflamed human intestine, ILC3s also express higher levels of HBEGF relative to other lymphocytes, with a particular enrichment in the NKp44− subset of ILC3s (Fig. 7h). Expression of HBEGF was unaltered when comparing these ILC3 subsets sort-purified from the non-inflamed versus inflamed region of surgically resected IBD tissues, but both populations exhibited an overall significant reduction from the inflamed region of the intestine (Fig. 7i,j and Supplementary Table 3). This is important as our collective data sets provoke a model whereby ILC3-derived HB-EGF is elicited in response to sensing of intestinal damage and IL-1β or autocrine PGE2, subsequently providing essential protection from TNF-mediated inflammation (Extended data Figure 9). These data sets demonstrate that the PGE2-HB-EGF pathway is conserved between humans and mouse ILC3s, and that an overall reduction of HB-EGF-producing ILC3s occurs in inflamed intestinal tissues during IBD.
Discussion
Our findings provoke a novel paradigm of tissue-protection in the gastrointestinal tract. ILC3s sense tissue damage or inflammation through IL-1β, which can then be amplified by a PGE2-EP2 axis, subsequently inducing production of HB-EGF and protecting the intestinal epithelium from TNF-induced cell death. We demonstrate in mouse models that this pathway is essential to protect the epithelium and limit both acute and chronic intestinal inflammation. Furthermore, this pathway is functional in human ILC3s and is reduced in the inflamed intestine of IBD patients. These collective findings advance our understanding of ILC biology, provoke a broad appreciation for the importance of HB-EGF in gastrointestinal health and inflammation, and suggest that selective harnessing HB-EGF+ ILC3s could be an approach to limit TNF-induced damage and chronic inflammation.
These data sets fundamentally advance our understanding of how ILC subsets regulate barrier immunity, inflammation and repair. For example, our results complement prior studies defining a critical role for ILC2-derived amphiregulin in promoting repair of the epithelial barrier in the lung and intestine11,12, while making the new finding that HB-EGF is the major EGFR ligand produced by ILC3s. This also complements prior research on IL-22, which is dominantly produced by ILC3s and critically regulates intestinal homeostasis, host defense and tissue repair after barrier damage9,13–15. However, it is also known that IL-22 can drive inflammation in several contexts, such as during chronic inflammation in the absence of IL-1041. In this study, we surprisingly found that transient blockade of endogenous IL-22 does not impact our in vivo assay of TNF-induced intestinal epithelial cell death. This is supported by a recent publication showing that recombinant IL-22 pre-treatment increases in vivo TNF-induced epithelial cell death through the induction of ER stress42, suggesting that IL-22 is more likely to play a pathologic role in the context of excessive TNF production. Thus, the role of IL-22 in regulation of intestinal epithelium is complex and context dependent.
Our results identify a previously unrecognized tissue-protective mechanism by which a PGE2-ILC3-HB-EGF axis critically protects intestinal epithelium from TNF-induced cell death. Intriguingly, this autocrine signaling of PGE2 would ensure robust activation of CCR6+ ILC3s that exist within uniform lymphoid clusters throughout the intestine43. ILC3s are replaced from this lymphoid cluster by other lymphocytes upon maturation or in chronic inflammatory settings, which may have important consequences for tissue health and the outcome of TNF production. Our data demonstrate that HB-EGF+ ILC3s are reduced from the inflamed intestine of individuals with IBD, a disease characterized or provoked by excessive cell death in the epithelium3,4,6, suggesting that these findings are linked and a loss of ILC3s increases susceptibility of the intestinal epithelium to TNF-induced damage.
Like other members of the EGFR ligand family, HB-EGF is synthesized as a transmembrane-anchored protein (known as pro-HB-EGF) composed of signal peptide, heparin-binding, EGF-like, juxtamembrane, transmembrane, and cytoplasmic domains24,28,33. Ectodomain shedding is recognized as a major pathway controlling the availability of EGFR ligands, including HB-EGF24,44. The membrane-bound pro-HB-EGF is cleaved at the juxtamembrane domain, resulting in the shedding of bioactive soluble HB-EGF. The mechanisms of ectodomain shedding of pro-HB-EGF are complex, involving sheddases from a disintegrin and metalloprotease (ADAM) and matrix metalloproteinase, and the pro-inflammatory cytokine IL-1β44,45. Both pro-HB-EGF and soluble HB-EGF are biologically active, the former acts on neighboring cells through juxtacrine signaling, whereas the latter can move to distant locations26,28. Our study revealed that ILC3s are a major cellular source of HB-EGF in the gastrointestinal tract and observed pro-HB-EGF protein expression in ILC3s. Despite of these findings, it remains unclear whether ILC3s protect the intestinal epithelium through pro-HB-EGF or soluble HB-EGF. If the latter, it is possible this could occur through constitutive shedding of pro-HB-EGF on ILC3s, or via increase ectodomain shedding of pro-HB-EGF on ILC3s in response to IL-1β signal. Several of these contexts could also occur or be differentially regulated during states of health and inflammation. Additional studies are required to comprehensively address these questions, which are currently hindered by technical challenges and limited availability of essential tools.
Finally, our findings indicate that novel strategies to manipulate ILC3s-HB-EGF axis could be important for preventing or limiting the pathogenesis of multiple TNF-mediated chronic inflammatory diseases, including IBD. This will be difficult to accomplish as many of the pathways associated with induction of ILC3-derived HB-EGF are also broadly active on other cell types and have the ability to promote inflammation, including IL-1β and PGE23,34. Delivery of these factors would also be unlikely to yield therapeutic benefit in IBD because ILC3s are largely depleted in the context of the inflamed human intestine. Delivery of exogenous HB-EGF could also be problematic as it is linked to cancer development or progression46. Therefore, in the development of potential therapeutics, it will be essential to consider the context dependent role for these pathways in intestinal health, inflammation and mucosal healing. For example, PGE2 is a prostanoid fatty acid derivative of arachidonic acid and is generally recognized as a mediator of active inflammation. However numerous studies have demonstrated its anti-inflammatory and tissue repair function through multiple mechanisms47–49. These include directly suppressing the production of pro-inflammatory cytokines, resolving inflammation in chronic settings, and promoting mucosal healing or tissue repair after injury. In addition, PGE2 administration can alleviate DSS-induced intestinal inflammation in mice50. Our results indicate that selective engagement of the PGE2-ILC3-HB-EGF pathway could critically protect the intestine from TNF and support mucosal healing, but this would likely need to occur in combination with other anti-inflammatory mediators, or by identifying selective strategies to boost ILC3s or prevent their loss from the inflamed intestine during IBD.
Methods
Mice.
Wild-type, Rag1−/−, Rorc−/−, Il10−/−, Cd4cre and Vav1cre mice were purchased from the Jackson Laboratory. Rag2−/−Il2rg−/− mice were purchased from Taconic Farms. Rorccre mice were provided by G. Eberl. Hbegff/f mice were generated by E. Mekada and provided by R. Harris (Vanderbilt University). The presence of spontaneous germ line mutations in the Hbegff/f mouse line was monitored by quantifying Hbegf transcripts in the heart and kidney, and by assessing for broad LacZ activity in the intestine. No germ line mutants were detected in our colony. Ptger2f/f were provided by K. Andreasson. Both male and female mice were utilized in this study. All mice were bred on a C57/BL6/J background and maintained in a 12-h light/dark cycle specific pathogen-free facility with an average ambient temperature of 21°C and an average humidity of 48% at Weill Cornell Medicine. Sex- and age-matched animals between 6 and 16 weeks of age were used for experiments if not otherwise indicated. No animals were excluded from the analyses unless clearly indicated. All animal experiments were approved and are in accordance with the Institutional Animal Care and Use Committee guidelines at Weill Cornell Medicine.
In vivo administration of neutralization antibodies and recombinant TNF.
Anti-CD90.2 monoclonal antibody (30H12, BioXCell) or anti-IL-22 monoclonal antibody (clone IL22-01 provided by Pfizer) were administered intraperitoneally once at a dose of 500 μg per mouse. Mice were injected with recombinant murine TNF (0.5 mg/kg, Biolegend) intraperitoneally and sacrificed 4 hours post injection.
Immunofluorescence microscopy and quantification.
Tissues were harvested as swiss roll and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2–4 hours at 4°C before washing 3 times in PBS. Tissues were then dehydrated overnight at 4°C in 30% sucrose dissolved in PBS. Dehydrated tissues were cryoprotected in OCT medium (Tissue-Tek) and stored at −80°C until sectioning at a thickness of 10 μm using a cryotome (Leica Instruments) and immobilization on Superfrost Plus slides (VWR). Immobilized tissues were then stored at −20°C until immunostaining. Slides were allowed to come to room temperature and excess OCT medium was washed off in PBS and tissue sections were then blocked in PBS with 10% normal donkey serum (Jackson Immunoresearch) and 0.1% Triton X-100 (Sigma) for 30 minutes. Tissue sections were then stained with the following primary antibodies diluted in blocking buffer overnight at 4°C: anti-cleaved-caspase 3 (1:300) or anti-cleaved-caspase 8 (1:300) (Cell Signaling Technology) and anti-E-cadherin 1:300 (Biolegend, DECMA-1). Sections were washed 3 times with PBS and then incubated with secondary antibodies (Rhodamine conjugated donkey anti-Rabbit IgG (H+L), Jackson Immunoresearch) diluted 1:500 in blocking buffer for 1 hour at room temperature. Tissue sections were then washed 3 times in PBS, incubated with DAPI (Invitrogen) for 5 minutes, and mounted with Prolong Gold antifade reagent (Invitrogen). TUNEL staining was performed by using In Situ Cell Death Detection Kit, Fluorescein (Roche), according to the manufacturer’s instructions. Images were captured using a Nikon Eclipse Ti microscope and NIS-Elements 4.30.02 software (Nikon). Comparable cell death was observed in multiple areas of the small intestine and approximately 6–8 images per mouse were captured throughout the tissue. For cleaved-caspase3 staining quantification, each dot in the figures represents the average CC3 signal in the epithelium (as determined by co-localization with E-cadherin) per villi. For TUNEL staining quantification, each dot in the figures represents the average TUNEL signal (co-localized with DAPI) per field. Immunofluorescence images were analyzed by Image J v2.0.
Isolation of cells from the intestinal lamina propria.
Intestines were removed, cleaned from remaining fat tissue and washed in ice-cold PBS (Corning). Peyer’s patches on the small intestine were identified and completely eliminated. Intestines were opened longitudinally and washed in ice-cold PBS. Afterwards, mucus was gently removed by forceps and intestines were cut into approximately 0.5 cm sections. Dissociation of epithelial cells was performed by incubation on a shaker in HBSS (Sigma-Aldrich) containing 5 mM EDTA (Thermo Fisher Scientific), 1 mM DTT (Sigma-Aldrich) and 2% FBS two times for 20 min at 37 °C. After each step, samples were vortexed and the epithelial fraction discarded. Afterwards, samples were washed by PBS and enzymatic digestion was performed using dispase (0.4 U/mL; Thermo Fisher Scientific), collagenase III (1 mg/mL; Worthington) and DNase I (20 μg/mL; Sigma-Aldrich) in 10% FBS RPMI on a shaker for 45 minutes at 37°C. Leukocytes were further enriched by a 40%/80% Percoll gradient centrifugation (GE Healthcare).
Human samples.
For human tonsils and surgical resections from IBD patients, samples were provided by the Cooperative Human Tissue Network (CHTN), which is funded by the National Cancer Institute (www.chtn.org). Other investigators may have received specimens from the same subjects. Samples were provided as entirely de-identified human specimens with diagnoses were confirmed by medical records and trained pathologists. This protocol was reviewed by the Weill Cornell Medicine IRB and determined to meet the Exemption Category 4 of HHS 45 CFR 46.104(d). Additional oversight of the CHTN is outlined at www.chtn.org.
Isolation of cells from human tonsil and intestinal surgical resection samples.
Single cell suspensions were isolated from the tonsil by dissociating samples through a 70 μm cell strainer. Cells were viably cryopreserved at −150 °C in 90% FBS and 10% DMSO for future analyses. For the intestinal samples, inflamed and non-inflamed regions were isolated by a trained pathologist following surgical resection and confirmed diagnosis of IBD. Single cell suspensions from intestinal tissues were obtained by incubating tissues for 30 min at 37°C with shaking in stripping buffer (1 mM EDTA, 1 mM DTT and 5% FCS) to remove the epithelial layer. Supernatants were then discarded. Tissues were then mechanically dissociated with a sterile scalpel. The lamina propria fraction was obtained by incubating the dissociated tissues for 1 hour at 37°C with shaking in 2 mg/ml collagenase D (Roche), 0.1 mg/mL DNase I (Sigma) and 1 mg/mL of Trypsin Inhibitor (Gibco) digestion solution. Remaining tissues were then filtered through a 70 μm cell strainer. All cells were then viably cryopreserved in 90% FBS and 10% DMSO for side-by-side analysis at a later time point. Following thawing and filtering through a 70 μm cell strainer, cells were stained with antibodies for flow cytometry acquisition.
Flow cytometry and cell sorting.
Single cell suspensions were incubated on ice with conjugated antibodies in PBS containing 2% FBS and 1 mM EDTA. Unlabeled anti-CD16/32 (clone 2.4G2, BD biosciences) was used to block Fc receptors when analyzing myeloid cells. Dead cells were excluded with Fixable Aqua Dead Cell Stain (Thermo Fisher Scientific). The staining antibodies for flow cytometry were mainly purchased from Thermo Fisher Scientific, Biolegend or BD Biosciences. For mouse cell-surface staining: B220 (RA3-6B2), CCR6 (29-2L17), CD3ε (145-2C11), CD4 (RM4-5), CD5 (53-7.3), CD8α (53-6.7), CD11b (M1/70), CD11c (N418), CD19 (eBio1D3), CD45 (30-F11), CD64 (X54-5/7.1), CD90.2 (30-H12), CD127 (A7R34), cleaved-caspase3 (C92-605), F4/80 (BM8), MHC-II (M5/114.15.2), NK1.1 (PK136). For mouse intracellular staining: FOXP3 (FJK-16S), GATA3 (L50-823), IL-17A (eBio 17B7), IL-22 (IL22JOP), IFN-γ (XMG1.2), Ki-67 (SolA15), RORγt (B2D) and T-bet (eBio4B10). COX-2 and mPGES-1 antibodies were purchased from Abcam and VWR-Bioss, respectively. Lineage markers for mouse are: CD3ε, CD5, CD8α, NK1.1, TCRγδ, CD11b, CD11c, B220. All mouse antibodies were used at 1:200, except for CCR6 was used at 1:100. Human samples were stained for CD3 (UCHT1), CD11b (CBRM1/5), CD11c (3.9), CD14 (TuK4), CD19 (HIB19), CD34 (581), CD45 (HI30), CD94 (DX22), CD117 (104D2), CD123 (6H6), CD127 (A019D5), FcεR1 (AER-37) and NKp44 (44.189). Human ILC3s were gated as CD45+CD3−CD11b−CD11c−CD14−CD19−CD34−CD94−CD123−FcεR1− CD127+CD117+NKp44+/−. All human antibodies were used at 1:200, except for CD34, CD94, CD117 and CD127 were used at 1:100.
For intracellular transcription factor staining, cells were stained for surface markers, followed by fixation and permeabilization before nuclear factor staining according to the manufacturer’s protocol (FoxP3 staining buffer set from Thermo Fisher Scientific). For intracellular cytokine staining, cells were incubated for 4 hours in RPMI with 10% FBS, phorbol 12-myristate 13-acetate (PMA) (50 ng/mL), ionomycin (750 ng/mL) and brefeldin A (10 μg/mL), all obtained from Sigma-Aldrich. Staining was then performed as above with transcription factors. LacZ was visualized by using the FluoReporter LacZ Flow Cytometry Kit according to the manufacture’s protocol (Thermo Fisher Scientific) and with a 3 min incubation time. Gating strategy to analyze innate lymphoid cells and CD4+ T cells in the small intestine was described previously18. All flow cytometry experiments were performed using a Fortessa flow cytometer and the FACS Diva software (BD Biosciences) and analyzed with FlowJo V10 software (TreeStar) or sort-purified by using a FACSAria cell sorter (BD Biosciences).
Single cell RNA-Sequencing.
scRNA-Seq libraries were generated from sorted ILCs (CD45+CD3ε−CD5−CD8α−NK1.1−TCRγδ−CD11b−CD11c−B220−CD127+) using the 10X Genomics Chromium system with 3’ version 3 chemistry. Reads were processed using 10X’s Cell Ranger version 3.0.2 using the mm10 reference genome, resulting in a filtered HDF5 file. Further processing and analysis of scRNA-Seq data were performed in R version 3.6.3 (R Core Team 2020) using the Seurat package version 3.2.151. Specifically, Cell Ranger output was imported using the Read10X_h5. Seurat objects were created using only genes appearing in at least 3 cells. Cells were further filtered to exclude those with fewer than 1000 genes detected, more than 5000 genes detected, or more than 10 percent mitochondrial reads. Read counts were then normalized using the SCTransform function. Data from PBS- and TNF-treated samples were then integrated using the functions SelectIntegrationFeatures (with 3000 integration features), PrepSCTIntegration, FindIntegrationAnchors, and IntegrateData. Further analysis using the Seurat package was performed on the integrated data. Namely, the graph representing cells with similar expression patterns was generated with the FindNeighbors function using the 20 largest principal components. Cell clusters were generated using the Louvain algorithm implemented by the FindClusters function with resolution parameter equal to 0.2. Marker genes for each cluster were determined using the Wilcoxon test on the raw counts, implemented by the function FindConservedMarkers with TNF/PBS as the grouping variable, and including only positive marker genes with log fold changes greater than 0.25 and Bonferroni-corrected p values less than 0.05. Likewise, the Wilcoxon test implemented by the FindMarkers function was used to determine genes differentially expressed according to PBS/TNF treatment within each cluster. Cluster names were assigned by manual inspection of the selectively enriched gene lists. Dimensionality reduction by Uniform Manifold Approximation and Projection was performed using the RunUMAP function with the 30 largest principal components. All visualizations of scRNA-Seq data were generated using the Seurat package as well as ggplot2 version 3.3.2.
Stimulation of ILC3s and cell lines.
Sort-purified ILC3s (CD45+Lin−CD90.2+CD127+KLRG1− CD45dimCD90.2hi) from small intestine of wild type mice were plated in a 96-well plate (0.5–1×104 cells per well) and incubated in DMEM with high glucose supplemented with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, non-essential amino acids, 80 μM 2-mercaptoethanol, 2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (all from Gibco) in the presence of recombinant mouse IL-1β (20 ng/mL, Thermo Fisher Scientific), IL-23 (20 ng/mL, Thermo Fisher Scientific), TNF-α (20 ng/mL, Thermo Fisher Scientific) or FICZ (500 nM, Sigma-Aldrich) for 4 hours at 37 °C.
For PGE2, agonist or retinoic acid treatment, cells were stimulated with PGE2 (500 nM, Cayman Chemical), EP2 agonist (Butaprost, 200 nM, Cayman Chemical), EP4 agonist (CAY10684, 200 nM, Cayman Chemical) or retinoic acid (1 μM, Sigma-Aldrich) for 12 hours with the presence of recombinant mouse or human IL-7 and IL-2 (10 ng/mL, Thermo Fisher Scientific). For antagonist assay, cells were pre-treated with EP2 antagonist (PF-04418948, 1 μM, Cayman Chemical) or EP4 antagonist (GW 627368X, 1 μM, Cayman Chemical) for 2 hours, followed by PGE2 stimulation. For in vivo treatment, RorccreHbegff/− mice were intraperitoneally injected with corn oil with or without EP2 agonist (10 μg per mouse) daily for 4 consecutive days.
For in vitro TNF treatment of intestinal epithelial cells, the murine rectum epithelial cell line CMT-93 (ATCC, CCL-223, were tested to be mycoplasma-negative by the standard PCR method) was treated with recombinant TNF (20 ng/mL) and a cIAP inhibitor (100 nM, Birinapant, Cayman Chemical) for 6 hours, followed by flow cytometric analysis of cleaved-caspase 3. Recombinant HB-EGF (Novus) was also included in some conditions. For inhibition assay, cells were pre-treated with ErbB1 inhibitor (100 nM, Erlotinib HCl (OSI-744), MedChemExpress), ErbB4 blockade antibody (20 μg/mL, H4.72.8 (Ab72), Thermo Fisher), ErbB1&4 inhibitor (100 nM, Afatinib (BIBW2992), Selleckchem) or Akt inhibitor (100 nM, MK-2206 (hydrochloride), Cayman Chemical) for 1 hour before inducing cell death.
Quantitative PCR.
Sort-purified cells were lysed in Buffer RLT (QIAGEN). RNA was extracted via RNeasy mini kits (QIAGEN) as per the manufacturer’s instructions. Reverse transcription of RNA was performed using Superscript reverse transcription according to the protocol provided by the manufacturer (Thermo Fisher Scientific). Real-time PCR was performed on cDNA using SYBR green chemistry (Applied Biosystems). Reactions were run on a real-time PCR system (ABI7500; Applied Biosystems). Samples were normalized to Hprt or Actb (for mouse samples) or ACTB (for human samples).
ELISA.
Sort-purified ILC3s (4–5×104 per well) from small intestine of wild type mice were treated with or without IL-1β (100 ng/mL) for 4 hours at 37°C. Supernatants were then harvested and detected by ELISA (PGE2 ELISA Kit-Monoclonal, Cayman Chemical) according to the manufacturer’s instructions.
Western Blot analysis.
Rag1−/− mice were treated with or without 10 μg EP2 agonist Butaprost for four consecutive days, 1 million ILC3s or ILC2s were then sort-purified and lysed in RIPA buffer (Cell Signaling). For detection, the following primary antibodies were used: anti-HB-EGF antibody (AF8239, R&D systems), anti-β-actin (AC-15, Sigma-Aldrich), and secondary HRP conjugated anti-sheep antibody (R&D systems).
DSS administration and histological scoring.
Colitis-grade dextran sulfate sodium salt with average MW of 36,000–50,000 Da (MP Biomedicals) was added to drinking water at 3% w/v starting on day 0. Mice were weighed at the same time of day at indicated time points. Tissue samples from the intestines of mice were fixed with 4% paraformaldehyde, embedded in paraffin, and 5 μm sections were stained with haematoxylin and eosin. Histopathological scoring of DSS-induced intestinal inflammation was performed in a blind manner according to a previous publication52. Briefly, inflammation was graded on a scale from 0 to 3, including the following criteria: (i) inflammatory cell infiltration, which can be graded as mild, moderate or marked (ii) intestinal architecture, which ban be graded as focal erosions, erosions and or focal ulcerations, extended ulcerations and or granulation. Scores for individual criteria were added up for an overall inflammation score between 0 and 6.
Helicobacter hepaticus-induced chronic intestinal inflammation.
Helicobacter hepaticus was originally purchased from ATCC (#51449). Frozen stock was stored at −80°C in Brucella broth (BD) with 20% glycerol (Sigma-Aldrich). For maintenance and infection, the bacteria were grown on Brucella agar plates (BD) supplemented with 5% sheep blood (Thermo Scientific) in anaerobic jars with Oxoid CampyGen sachet (Thermo Scientific) at 37°C for 5 days. Mice were oral infected with 108 CFU Helicobacter hepaticus on day 0 and 5, and 800 μg of anti-IL-10R antibody (BioXcell, clone 1B1.3A) was administered intraperitoneally per week. Tissue samples from the intestines of mice were fixed with 4% paraformaldehyde, embedded in paraffin, and 5 μm sections were stained with haematoxylin and eosin. Histopathological scoring of intestinal inflammation was performed in a blind manner according to a previous publication53. Briefly, inflammation was graded on a scale from 0 to 3, including the following criteria: (i) epithelial hyperplasia and goblet cell depletion, (ii) lamina propria leukocyte infiltration, (iii) area of tissue affected, (iv) markers of severe inflammation, including crypt abscesses, sub-mucosal inflammation, and ulceration. Scores for individual criteria were added up for an overall inflammation score between 0 and 12.
Statistical analysis.
P value of data set was determined by one-way analysis of variance (ANOVA), paired or unpaired two-tailed Student’s t-test with 95% confidence interval. Variance was analyzed using F-test. Welch’s correction was performed in case of unequal variance. Where appropriate, Mann–Whitney U-test or Wilcoxon matched-pairs test were performed. All statistical tests were performed with Graph Pad Prism V9 software. P values less than 0.05 were considered significant.
Extended Data
Extended Data Fig. 1. The role of ILC3s and IL-22 in TNF-induced intestinal epithelial cell death.
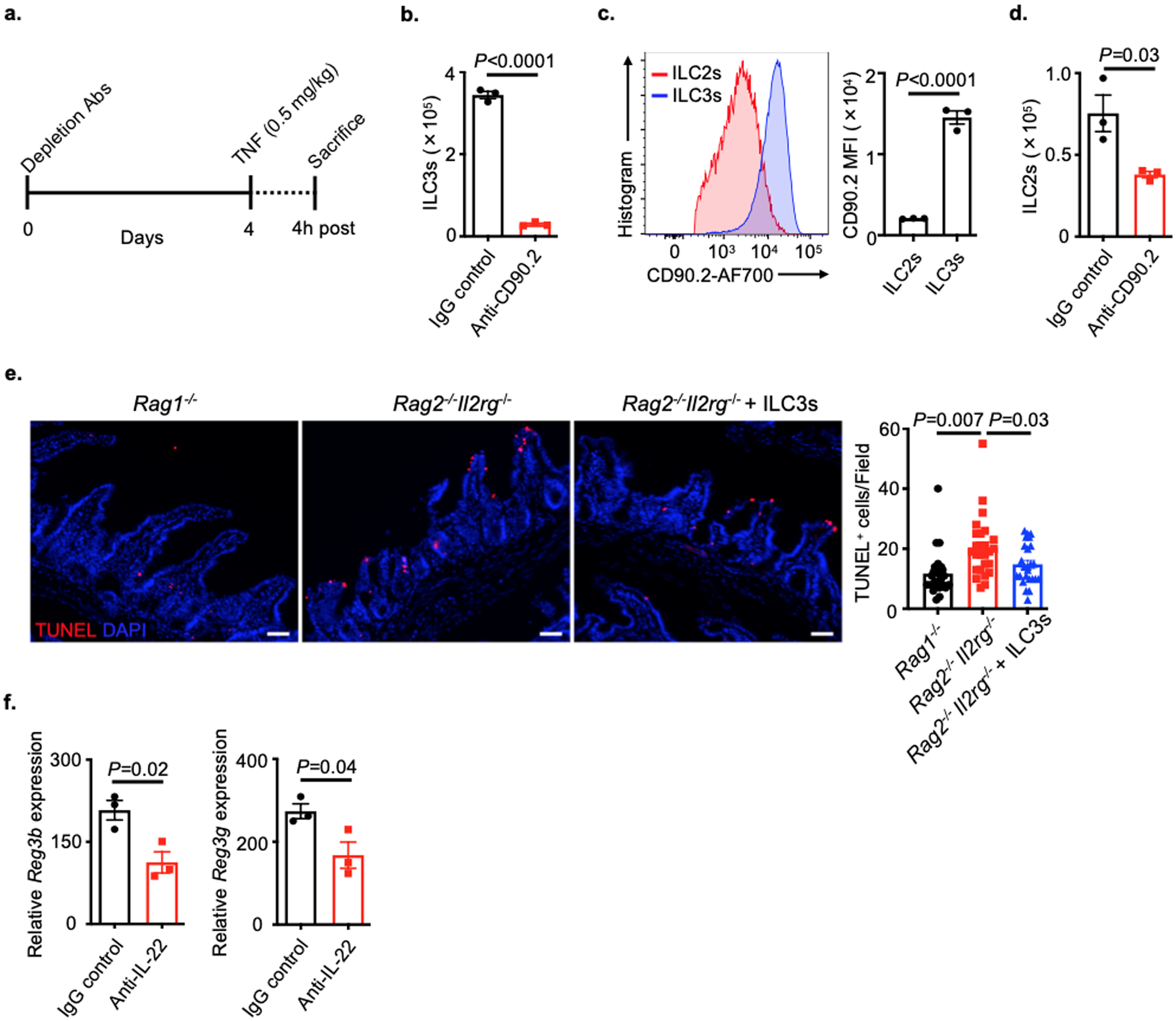
a. Experimental design of the assay. b. Mice in (a) were examined for the depletion efficiency of ILC3s in the small intestine. (n=3). c. CD90.2 levels in ILC2s or ILC3s were determined in IgG control mice. (n=3 individual mice). d. Mice in (a) were examined for the depletion efficiency of ILC2s in the small intestine. (n=3). e. Representative immunofluorescence sections and quantifications of TUNEL staining of the small intestine from noted mice 4 hours post recombinant TNF injection (N=3 mice per group, n=24 fields quantified per group). f. Mice in (a) were examined for the neutralization efficiency of endogenous IL-22 (n=3 individual mice). Data are representative of two independent experiments, shown as the means ± S.E.M. Statistics are calculated by unpaired two-tailed Student’s t-test (b-d and f) or one-way ANOVA (e). Scale bars=50 μm.
Extended Data Fig. 2. Signature gene expression of ILC clusters in the scRNA-Seq data set.

Small intestinal ILCs were sort-purified from PBS- or TNF-treated mice and determined by scRNA-Seq. UMAP shows the relative expression of signature genes in all clusters of each treatment condition.
Extended Data Fig. 3. ILC3s express HB-EGF in the large intestine.
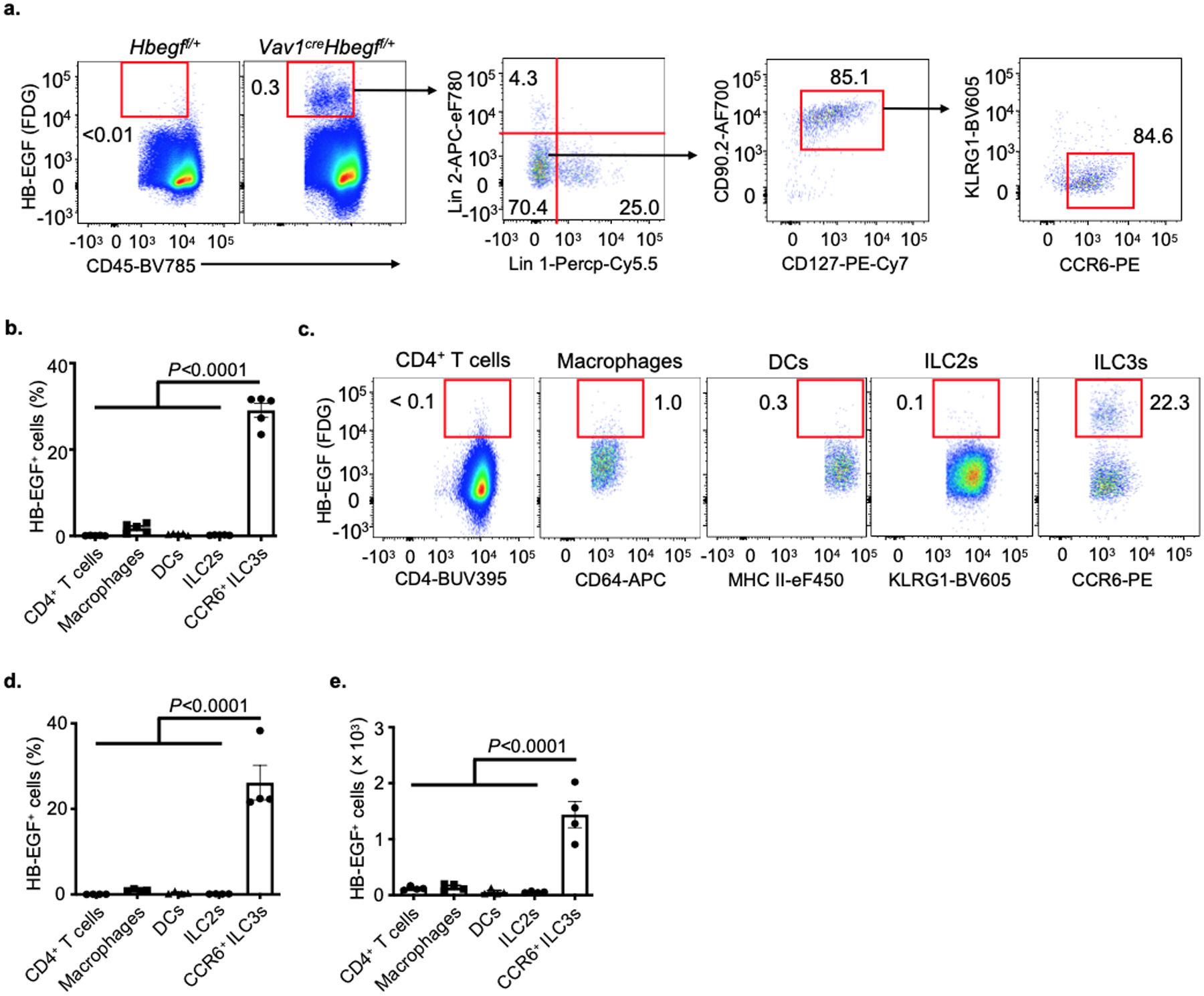
a. Flow cytometry plots show HB-EGF expression in large intestine lamina propria (LI-LP) of noted mice as measured by conversion of the fluorescent LacZ substrate fluorescein di-β-d-galactopyranoside (FDG). b. Graph of frequency of HB-EGF+ cells in SI-LP of noted mice as measured by LacZ activity (n=5 individual mice). c-e. Flow cytometry plots (c), graph of frequency (d) and quantification of cell number (e) of HB-EGF+ cells in LI-LP of noted mice as measured by LacZ activity (n=4 individual mice). In both small and large intestines, CD4+ T cells were gated on CD45+CD3ε+CD4+, macrophages were gated on CD45+CD11b+CD11c+MHC II+CD64+, dendritic cells were gated on CD45+CD11b+CD11c+MHCII+CD64−, ILC2s were gated on CD45+Lin− CD127+CD90.2+KLRG1+, ILC3s were gated on CD45+Lin−CD127+CD90.2+KLRG1−CD45dim CCR6+. Lineage 1: CD3ε, CD5, CD8α, NK1.1, TCRγδ. Lineage 2: CD11b, CD11c, B220. Data in a-e are representative of two or three independent experiments, shown as the means ± S.E.M. Statistics are calculated by one-way ANOVA.
Extended Data Fig. 4. HB-EGF protects from TNF-induced intestinal epithelial cell death.

a-c. Cell death was induced in the murine rectum epithelial cell line CMT-93 by TNF and a cIAP inhibitor. Cell death was determined by representative flow cytometric analysis of cleaved-caspase 3 (a) or cleaved-caspase 8 (c) in the presence or absence of recombinant HB-EGF. Cell death was determined by representative flow cytometric analysis of cleaved-caspase 3 in the presence of various inhibitors (b). Data in a and b are representative of three independent experiments, data in c are pooled from three independent experiments, shown as the means ± S.E.M. Statistics are calculated by one-way ANOVA.
Extended Data Fig. 5. A PGE2-EP2 axis controls HB-EGF expression in ILC3s.

a. qPCR analysis of Hbegf expression in sort-purified ILC3s cultured with or without TNF treatment, relative to Hprt (n=4 individual mice). b and c. UMAP show the expression of Ptgs2 (b) or Ptges (c) gene in all clusters of each treatment condition. The dotted line indicates CCR6+ ILC3 clusters. d. qPCR analysis of Hbegf expression in sort-purified ILC3s cultured with or without retinoic acid, relative to Hprt (n=6). e. Bar graph of the frequencies of IL-17A, IL-22, IFN-γ or GM-CSF in ILC3s in the presence or absence of PGE2 stimulation ex vivo (n=6). f. qPCR examination of Hbegf transcript in sort-purified ILC3s in the presence of various stimulus, relative to Hprt (n=4 individual mice). g. Flow cytometry plots with graph of the frequencies of HB-EGF+ immune cells in small intestine of RorccreHbegff/− mice with or without EP2 agonist in vivo treatment (n=3 individual mice of Mock and n=4 individual mice of EP2 agonist). h. Western blot analysis of pro-HB-EGF in sort-purified ILC2s or ILC3s from small intestine of Rag1−/− mice with or without EP2 agonist in vivo treatment. i. Sex- and age- matched Ptger2f/f and RorccrePtger2f/f mice were examined for the deletion efficiency of EP2 in ILC3s in the small intestines by qPCR, relative to Hprt (n=6 individual mice). j. Sort-purified ILC3s were stimulated with IL-1β in the presence or absence of EP2 antagonist, Hbegf expression was then examined by qPCR (n=6 individual mice). Data in a and f-h are representative of two independent experiments, data in d, e, i and j are pooled from two independent experiments, shown as the means ± S.E.M. Statistics are calculated by unpaired (a, g and i) or paired (d and e) two-tailed Student’s t-test or one-way ANOVA (f and j). *, non-specific band.
Extended Data Fig. 6. ILC3-derived HB-EGF does not impact immune homeostasis in the healthy gut.
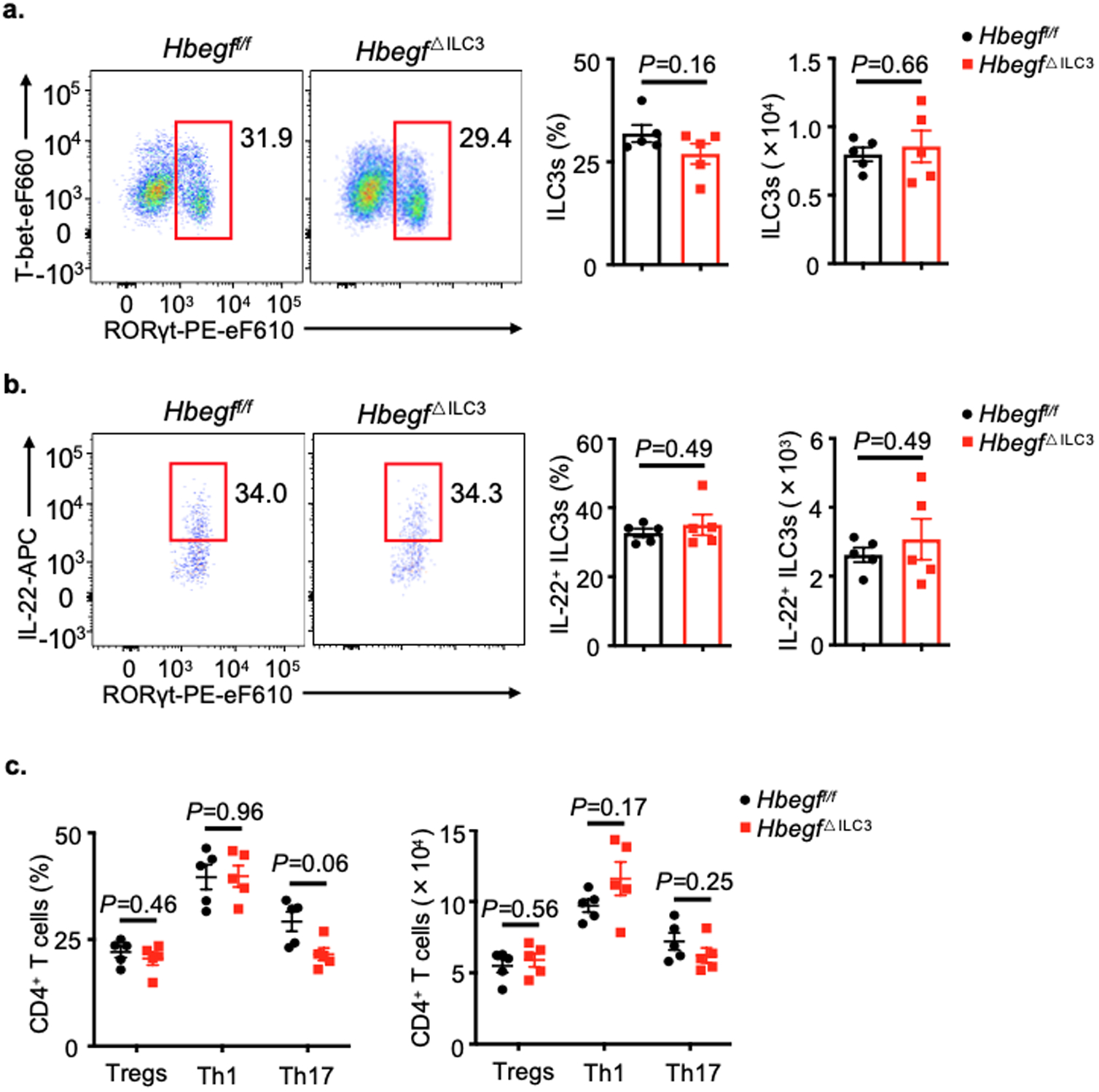
a and b. Flow cytometry plots with graph of frequencies and quantification of cell numbers of ILC3s (a) or IL-22+ ILC3s (b) in the large intestine of Hbegff/f and Hbegf△ILC3 mice at steady state (n=5 individual mice). c. Graph of frequencies and quantification of cell numbers of T cell subsets in the large intestine of Hbegff/f and Hbegf△ILC3 mice at steady state (n=5 individual mice). Data in a-c are representative of two independent experiments, shown as the means ± S.E.M. Statistics are calculated by unpaired two-tailed Student’s t-test.
Extended Data Fig. 7. Loss of T cell-derived HB-EGF does not alter susceptibility to experimental intestinal inflammation.
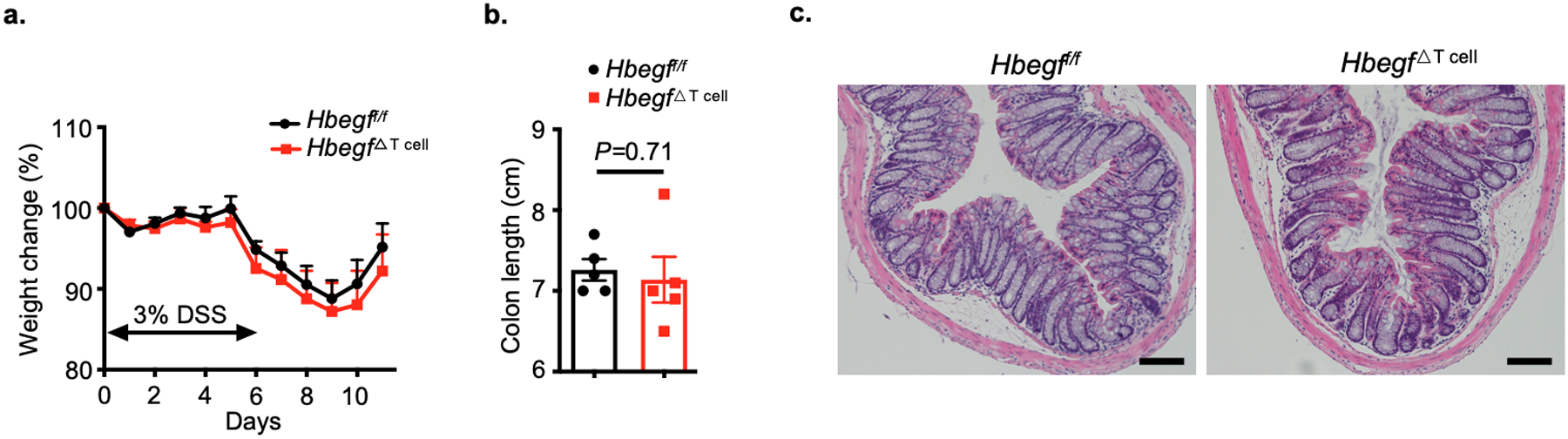
a-c. Hbegff/f and Hbegf△T cell mice were given 3% DSS for 6 days, disease and recovery were monitored by weight loss (a), colon shortening (b) and H&E staining of the distal colon (c) at day 11 (n=5 individual mice). Data in a-c are representative of two independent experiments, shown as the means ± S.E.M. Statistics are calculated by unpaired two-tailed Student’s t-test. Scale bars=100 μm.
Extended Data Fig. 8. Intestinal epithelial cell responses in the absence of ILC3-specific HB-EGF.
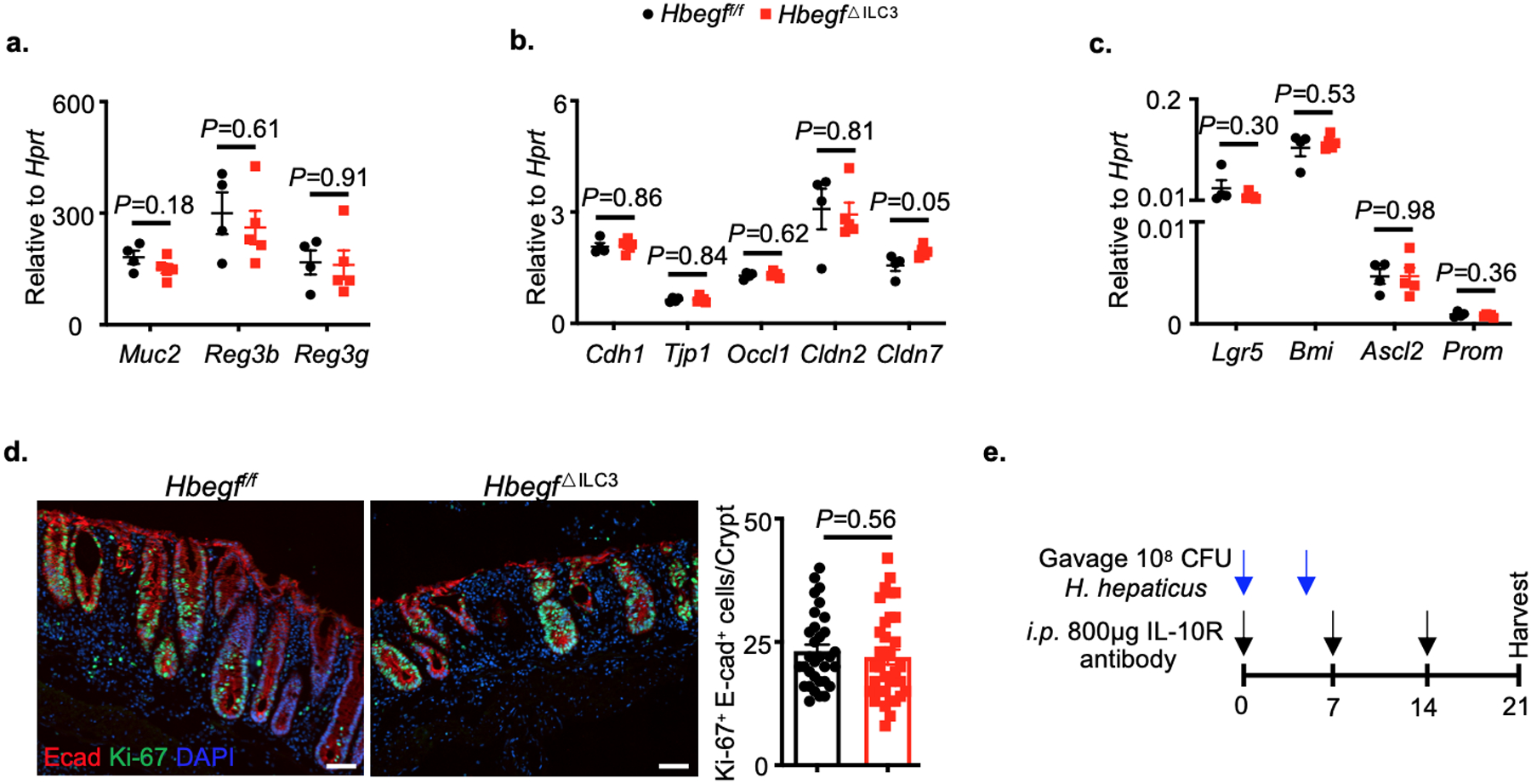
a-d. Hbegff/f and Hbegf△ILC3 mice were challenged with 3% DSS for 6 days (n=6 individual mice). The functional ability of intestinal epithelium was analyzed, including anti-microbial peptides production (a), tight junction proteins expression (b), stem cell composition (c) and proliferative ability (d) (N=4 individual mice per group, n=27 crypts quantified per group). e. Schematic model of chronic colitis induction. Data in a-d are representative of two independent experiments, shown as the means ± S.E.M. Statistics are calculated by unpaired two-tailed Student’s t-test. Scale bars=50 μm.
Extended Data Fig. 9. ILC3s produce HB-EGF to protect the intestine from TNF.
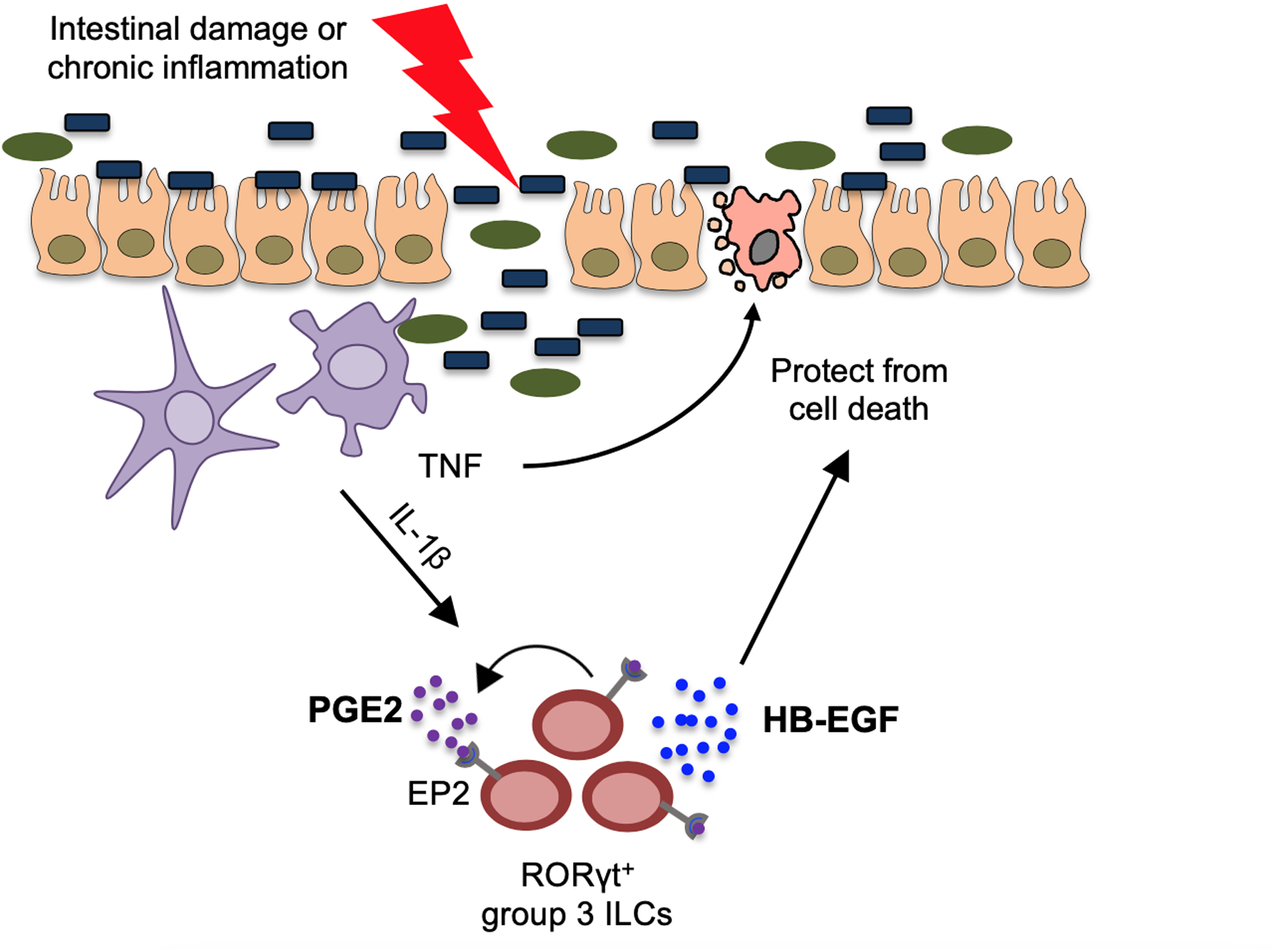
Here we define a novel pathway of tissue protection in the mammalian intestine. Briefly, ILC3s sense intestinal damage or inflammation through IL-1β release, which amplifies ILC3 responses through a PGE2-EP2 axis, subsequently promoting HB-EGF production and protection against TNF-induced cell death in the intestinal epithelium.
Supplementary Material
Acknowledgements
We thank members of the Sonnenberg Laboratory for discussions and critical reading of the manuscript, the Epigenomics Core of Weill Cornell Medicine, and Dr. Raymond C. Harris for sharing critical mouse lines. Research in the Sonnenberg Laboratory is supported by the National Institutes of Health (R01AI143842, R01AI123368, R01AI145989, U01AI095608, R21CA249274 and R01AI162936), the NIAID Mucosal Immunology Studies Team (MIST), the Searle Scholars Program, the American Asthma Foundation Scholar Award, an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, a Wade F.B. Thompson/Cancer Research Institute (CRI) CLIP Investigator grant, the Meyer Cancer Center Collaborative Research Initiative, Linda and Glenn Greenberg, the Dalton Family Foundation, and the Roberts Institute for Research in IBD. L.Z. and W.Z. are supported by fellowship from the Crohn’s and Colitis Foundation (608975) and (831404), respectively. C.C. is supported by the Sackler Brain and Spine Institute Research Grant. A.M.J is supported by T32DK116970. G.F.S. is a CRI Lloyd J. Old STAR.
Footnotes
Competing interests
The authors declare no competing interests.
Data availability.
scRNA-Seq sequencing data are available at Gene Expression Omnibus under accession number GSE166266. All datasets generated and/or analyzed during the current study are presented in this published article, the accompanying Source Data or Supplementary Information, or are available from the corresponding author upon reasonable request.
References
- 1.Kalliolias GD & Ivashkiv LB TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nature reviews. Rheumatology 12, 49–62, doi: 10.1038/nrrheum.2015.169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tracey KJ & Cerami A Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annual review of medicine 45, 491–503, doi: 10.1146/annurev.med.45.1.491 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Friedrich M, Pohin M & Powrie F Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 50, 992–1006, doi: 10.1016/j.immuni.2019.03.017 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Graham DB & Xavier RJ Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 578, 527–539, doi: 10.1038/s41586-020-2025-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neurath MF Current and emerging therapeutic targets for IBD. Nature reviews. Gastroenterology & hepatology 14, 269–278, doi: 10.1038/nrgastro.2016.208 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Patankar JV & Becker C Cell death in the gut epithelium and implications for chronic inflammation. Nature reviews. Gastroenterology & hepatology 17, 543–556, doi: 10.1038/s41575-020-0326-4 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Croft M, Benedict CA & Ware CF Clinical targeting of the TNF and TNFR superfamilies. Nature reviews. Drug discovery 12, 147–168, doi: 10.1038/nrd3930 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artis D & Spits H The biology of innate lymphoid cells. Nature 517, 293–301, doi: 10.1038/nature14189 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Vivier E et al. Innate Lymphoid Cells: 10 Years On. Cell 174, 1054–1066, doi: 10.1016/j.cell.2018.07.017 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Spits H et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews. Immunology 13, 145–149, doi: 10.1038/nri3365 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Monticelli LA et al. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proceedings of the National Academy of Sciences of the United States of America 112, 10762–10767, doi: 10.1073/pnas.1509070112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monticelli LA et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature immunology 12, 1045–1054, doi: 10.1031/ni.2131 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA & Artis D CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134, doi: 10.1016/j.immuni.2010.12.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouyang W & O’Garra A IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity 50, 871–891, doi: 10.1016/j.immuni.2019.03.020 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Zhou L & Sonnenberg GF Essential immunologic orchestrators of intestinal homeostasis. Science immunology 3, doi: 10.1126/sciimmunol.aao1605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernink JH et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature immunology 14, 221–229, doi: 10.1038/ni.2534 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Teng F et al. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Science immunology 4, doi: 10.1126/sciimmunol.aax1215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 568, 405–409, doi: 10.1038/s41586-019-1082-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goc J et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell, doi: 10.1016/j.cell.2021.07.029 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker A et al. Elevated apoptosis impairs epithelial cell turnover and shortens villi in TNF-driven intestinal inflammation. Cell death & disease 10, 108, doi: 10.1038/s41419-018-1275-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piguet PF, Vesin C, Guo J, Donati Y & Barazzone C TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. European journal of immunology 28, 3499–3505, doi: (1998). [DOI] [PubMed] [Google Scholar]
- 22.Van Hauwermeiren F et al. TNFR1-induced lethal inflammation is mediated by goblet and Paneth cell dysfunction. Mucosal immunology 8, 828–840, doi: 10.1038/mi.2014.112 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Eberl G RORgammat, a multitask nuclear receptor at mucosal surfaces. Mucosal immunology 10, 27–34, doi: 10.1038/mi.2016.86 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Singh B, Carpenter G & Coffey RJ EGF receptor ligands: recent advances. F1000Research 5, doi: 10.12688/f1000research.9025.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J et al. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiological reviews 96, 1025–1069, doi: 10.1152/physrev.00030.2015 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Dao DT, Anez-Bustillos L, Adam RM, Puder M & Bielenberg DR Heparin-Binding Epidermal Growth Factor-Like Growth Factor as a Critical Mediator of Tissue Repair and Regeneration. The American journal of pathology 188, 2446–2456, doi: 10.1016/j.ajpath.2018.07.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh B & Coffey RJ Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annual review of physiology 76, 275–300, doi: 10.1146/annurev-physiol-021113-170406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamoto R et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proceedings of the National Academy of Sciences of the United States of America 100, 3221–3226, doi: 10.1073/pnas.0537588100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michalsky MP, Kuhn A, Mehta V & Besner GE Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. Journal of pediatric surgery 36, 1130–1135, doi: 10.1053/jpsu.2001.25730 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Hilliard VC, Frey MR, Dempsey PJ, Peek RM Jr. & Polk DB TNF-alpha converting enzyme-mediated ErbB4 transactivation by TNF promotes colonic epithelial cell survival. American journal of physiology. Gastrointestinal and liver physiology 301, G338–346, doi: 10.1152/ajpgi.00057.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Assal ON & Besner GE HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 129, 609–625, doi: 10.1016/j.gastro.2005.05.054 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Elenius K, Paul S, Allison G, Sun J & Klagsbrun M Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. The EMBO journal 16, 1268–1278, doi: 10.1093/emboj/16.6.1268 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higashiyama S, Abraham JA, Miller J, Fiddes JC & Klagsbrun M A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251, 936–939, doi: 10.1126/science.1840698 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Kalinski P Regulation of immune responses by prostaglandin E2. Journal of immunology 188, 21–28, doi: 10.4049/jimmunol.1101029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennis EA & Norris PC Eicosanoid storm in infection and inflammation. Nature reviews. Immunology 15, 511–523, doi: 10.1038/nri3859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins DJ et al. Autocrine-paracrine prostaglandin E2 signaling restricts TLR4 internalization and TRIF signaling. Nature immunology 19, 1309–1318, doi: 10.1038/s41590-018-0243-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugimoto Y & Narumiya S Prostaglandin E receptors. The Journal of biological chemistry 282, 11613–11617, doi: 10.1074/jbc.R600038200 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Johansson JU et al. Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 16016–16032, doi: 10.1523/JNEUROSCI.2203-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunther C, Neumann H, Neurath MF & Becker C Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 62, 1062–1071, doi: 10.1136/gutjnl-2011-301364 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Leppkes M, Roulis M, Neurath MF, Kollias G & Becker C Pleiotropic functions of TNF-alpha in the regulation of the intestinal epithelial response to inflammation. International immunology 26, 509–515, doi: 10.1093/intimm/dxu051 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Gunasekera DC et al. The development of colitis in Il10(−/−) mice is dependent on IL-22. Mucosal immunology 13, 493–506, doi: 10.1038/s41385-019-0252-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell N et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut 69, 578–590, doi: 10.1136/gutjnl-2019-318483 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouskra D et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510, doi: 10.1038/nature07450 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Higashiyama S & Nanba D ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochimica et biophysica acta 1751, 110–117, doi: 10.1016/j.bbapap.2004.11.009 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Takenobu H, Yamazaki A, Hirata M, Umata T & Mekada E The stress- and inflammatory cytokine-induced ectodomain shedding of heparin-binding epidermal growth factor-like growth factor is mediated by p38 MAPK, distinct from the 12-O-tetradecanoylphorbol-13-acetate- and lysophosphatidic acid-induced signaling cascades. The Journal of biological chemistry 278, 17255–17262, doi: 10.1074/jbc.M211835200 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Ongusaha PP et al. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer research 64, 5283–5290, doi: 10.1158/0008-5472.CAN-04-0925 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Nakanishi M & Rosenberg DW Multifaceted roles of PGE2 in inflammation and cancer. Seminars in immunopathology 35, 123–137, doi: 10.1007/s00281-012-0342-8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyoshi H et al. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. The EMBO journal 36, 5–24, doi: 10.15252/embj.201694660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patankar JV et al. E-type prostanoid receptor 4 drives resolution of intestinal inflammation by blocking epithelial necroptosis. Nature cell biology 23, 796–807, doi: 10.1038/s41556-021-00708-8 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Kabashima K et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. The Journal of clinical investigation 109, 883–893, doi: 10.1172/JCI14459 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuart T et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902 e1821, doi: 10.1016/j.cell.2019.05.031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erben U et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. International journal of clinical and experimental pathology 7, 4557–4576 (2014). [PMC free article] [PubMed] [Google Scholar]
- 53.Song-Zhao GX & Maloy KJ Experimental mouse models of T cell-dependent inflammatory bowel disease. Methods in molecular biology 1193, 199–211, doi: 10.1007/978-1-4939-1212-4_18 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
scRNA-Seq sequencing data are available at Gene Expression Omnibus under accession number GSE166266. All datasets generated and/or analyzed during the current study are presented in this published article, the accompanying Source Data or Supplementary Information, or are available from the corresponding author upon reasonable request.


