Abstract
Jasmonic acid (JA) and salicylic acid (SA) regulate stomatal closure, preventing pathogen invasion into plants. However, to what extent abscisic acid (ABA), SA and JA interact, and what the roles of SA and JA are in stomatal responses to environmental cues, remains unclear. Here, by using intact plant gas-exchange measurements in JA and SA single and double mutants, we show that stomatal responsiveness to CO2, light intensity, ABA, high vapor pressure deficit and ozone either did not or, for some stimuli only, very slightly depended upon JA and SA biosynthesis and signaling mutants, including dde2, sid2, coi1, jai1, myc2 and npr1 alleles. Although the stomata in the mutants studied clearly responded to ABA, CO2, light and ozone, ABA-triggered stomatal closure in npr1–1 was slightly accelerated compared with the wild type. Stomatal reopening after ozone pulses was quicker in the coi1–16 mutant than in the wild type. In intact Arabidopsis plants, spraying with methyl-JA led to only a modest reduction in stomatal conductance 80 min after treatment, whereas ABA and CO2 induced pronounced stomatal closure within minutes. We could not document a reduction of stomatal conductance after spraying with SA. Coronatine-induced stomatal opening was initiated slowly after 1.5–2.0 h, and reached a maximum by 3 h after spraying intact plants. Our results suggest that ABA, CO2 and light are major regulators of rapid guard cell signaling, whereas JA and SA could play only minor roles in the whole-plant stomatal response to environmental cues in Arabidopsis and Solanum lycopersicum (tomato).
Keywords: jasmonic acid, salicylic acid, abscisic acid, stomata, carbon dioxide, ozone, Arabidopsis thaliana, Solanum lycopersicum
INTRODUCTION
The colonization of dry land required vascular plants to reduce excessive water loss from plant tissues. Stomatal pores actively control transpiration as well as the uptake of CO2 for photosynthesis in mesophyll cells. Guard cells respond to many environmental and endogenous cues and regulate ion channels and solute transporters in the guard cell membranes (Assmann and Jegla, 2016; Kollist et al., 2014; Sussmilch et al., 2019). The resulting reversible changes in guard cell turgor and volume lead to stomatal opening or closure, in accordance with light conditions, intercellular CO2 concentration, air humidity and soil water availability. Stomatal closure is also triggered by pathogen-associated molecular patterns and elicitors to prevent an invasion of pathogenic microorganisms into plants (Melotto et al., 2006; Sawinski et al., 2013). As part of a complex multicellular organism, stomata should be coordinated with processes and events occurring in distant plant organs and tissues. Indeed, guard cells are able to recognize long-distance endogenous stimuli of different nature, including hormones (Jia and Zhang, 2008; Marten et al., 1991). Stomatal responsiveness to plant hormones has been known for a long time (Acharya and Assmann, 2009), although certain aspects of this regulation remain unresolved, e.g. details of the interplay between hormones in stomatal aperture regulation (Murata et al., 2015). Abscisic acid (ABA) efficiently induces rapid stomatal closure and modulates stomatal regulation by environmental factors (Brandt et al., 2015; Chater et al., 2015; Hsu et al., 2018; Merilo et al., 2013). In addition, other hormones, including jasmonic acid (JA) and salicylic acid (SA), were suggested to control stomatal aperture (Khokon et al., 2010; Melotto et al., 2006; Munemasa et al., 2007). However, their potential to mediate stomatal regulation in response to changes in the environment requires further research.
Signaling events in guard cells during ABA-induced stomatal closure have been well characterized (Kim et al., 2010; Munemasa et al., 2015). The binding of ABA by PYR1/PYL/RCAR receptors results in the inhibition of type-2C protein phosphatases (PP2Cs) (Ma et al., 2009; Park et al., 2009). This leads to the activation of the protein kinase OST1 (Park et al., 2009; Takahashi et al., 2020; Umezawa et al., 2009; Vlad et al., 2009), calcium-dependent protein kinases (Brandt et al., 2012, 2015; Geiger et al., 2010) and the receptor-like protein GHR1 (Hua et al., 2012; Sierla et al., 2018), promoting anion currents through the anion channel SLAC1 (Geiger et al., 2009; Lee et al., 2009; Negi et al., 2008; Vahisalu et al., 2008). These events trigger the efflux of anions, potassium and water from guard cells, eventually leading to stomatal closure. Notably, ABA signaling in guard cells is also involved in the regulation of stomatal closure triggered by elevated CO2, periods of darkness, reduced air humidity (high vapor pressure deficit, VPD) and ozone (Chater et al., 2015; Hsu et al., 2018; Merilo et al., 2013, 2018; Sierla et al., 2018; Xue et al., 2011). Low air humidity has been reported to activate ABA biosynthesis in the guard cells or whole leaves (Bauer et al., 2013; McAdam et al., 2016), whereas elevated CO2 concentrations do not trigger rapid ABA accumulation in guard cells (Hsu et al., 2018; Zhang et al., 2020). Furthermore, longer 24- and 48-h exposures to elevated CO2 did not enhance ABA-induced reporter gene expression in guard cells, in contrast to ABA controls (Hsu et al., 2018).
Both JA and its derivatives regulate vegetative and reproductive plant growth as well as defense responses to abiotic stress and pathogen attack (Katsir, Chung, et al., 2008; Song et al., 2014). Methyl jasmonate (MeJA) was shown to trigger stomatal closure in epidermal peels by the activation of slow-type anion channels through a process that requires calcium channels, NO accumulation and reactive oxygen species (ROS) production by NADPH oxidases (RBOH D and F) (Hua et al., 2012; Munemasa et al., 2007; Suhita et al., 2004; Yan et al., 2015). MeJA-triggered stomatal closure involves the JA receptor CORONATINE INSENSITIVE 1 (COI1), as the stomatal apertures were not reduced by MeJA in the epidermal peels of the coi1 mutant (Munemasa et al., 2007). However, other research groups could not confirm the MeJA-triggered stomatal closure in Arabidopsis (Montillet et al., 2013) or found that MeJA had a significantly lower potency in promoting stomatal closure compared with ABA or 12-oxo-phytodienoic acid, the precursor of JA (Savchenko et al., 2014). In contrast, yet other research suggested that the pathogen-produced JA mimetic coronatine (COR) opens stomata (Melotto et al., 2006). In JA signaling, MeJA is converted to the biologically active isoleucine-JA conjugate that is bound by the JA co-receptor comprising the JASMONATE ZIM DOMAIN (JAZ) proteins and COI1 (Katsir, Schilmiller, et al., 2008). Isoleucine-JA and COR that mimics isoleucine-JA promote stomatal reopening and suppress the stomatal closure triggered by pathogen-associated molecular patterns (Melotto et al., 2006; Okada et al., 2009; Toum et al., 2016). Recently, the quantification of metabolites in guard cells during high CO2-induced stomata closure indicated a role for JA in CO2 signaling. Furthermore, Arabidopsis JA-signaling and -biosynthesis mutants displayed impaired stomatal responses to elevated CO2 in mesophyll-free leaf disks (Geng et al., 2016). The involvement of COI1-dependent JA signaling in the regulation of stomatal apertures and responses to the changing environment should be further confirmed in intact plants.
Salicylic acid (SA) has been extensively studied in plant–pathogen interactions, and its role in the regulation of plant development and response to abiotic stress has also been shown (Miura and Tada, 2014). SA is important for stomatal immunity against pathogens based on impaired pathogen-triggered stomatal closure in mutants defective in SA biosynthesis and signaling (Melotto et al., 2006; Zeng and He, 2010). The ability of SA to induce stomatal closure was directly demonstrated in experiments with SA-treated epidermal peels and detached leaves (Khokon et al., 2010; Mori et al., 2001; Panchal et al., 2016). Furthermore, SA over-accumulating mutants display reduced stomatal aperture and elevated drought tolerance (Miura et al., 2013). SA signaling in guard cells is mediated through the SA receptor NPR1 (Ding et al., 2018; Zeng and He, 2010) and components of ABA signaling, including calcium-dependent protein kinases, but not OST1 (Prodhan et al., 2018). The involvement of ethylene biosynthesis and signaling in SA-induced stomatal closure was recently suggested (Wang et al., 2020). Although ABA and MeJA induce ROS production by RBOH D and F in guard cells (Kwak et al., 2003; Suhita et al., 2004), ROS in SA-triggered stomatal closure could be produced by cell wall-bound peroxidases (Khokon et al., 2010; Mori et al., 2001) and, as recently indicated, by RBOH D and F (Wang et al., 2020). SA activates anion currents in guard cells through the slow-type anion channel SLAC1 (Prodhan et al., 2018).
Although it has been demonstrated that ABA signaling is involved in stomatal closure in response to many environmental cues (Chater et al., 2015; Hsu et al., 2018; Merilo et al., 2013), the roles of JA and SA signaling in stomatal responsiveness to environmental stimuli have not been studied thoroughly. Here we used a genetic approach in Arabidopsis and Solanum lycopersicum (tomato) to address the impacts of JA and SA biosynthesis and signaling on stomatal function under changing environmental conditions. As JA and SA display mutually antagonistic interactions (Bürger and Chory, 2019), we additionally studied double mutants with impaired responses to both JA and SA to explore possible interactions between these hormones in stomatal regulation. These experiments with plants defective in both SA and JA signaling, carried out in two independent laboratories, highlight that disruption of JA and SA signaling does not considerably modulate stomatal responsiveness to environmental factors. Furthermore, experiments with intact plants treated with increasing concentrations of MeJA and SA indicate that these hormones had a limited effect on stomatal conductance in intact Arabidopsis plants, and thus the direct effect of these hormones to induce stomatal closure is significantly lower than that of ABA when these hormones are applied exogenously.
RESULTS AND DISCUSSION
Plants defective in JA and SA signaling and biosynthesis display stomatal responses to elevated CO2, darkness and low air humidity
Numerous reports have demonstrated the role for ABA signaling in the regulation of stomatal closure in response to low air humidity, darkness and higher-than-ambient CO2 (Bauer et al., 2013; Chater et al., 2015; Hsu et al., 2018; Merilo et al., 2013, 2018; Webb and Hetherington, 1997; Yaaran et al., 2019; Zhang et al., 2020). MeJA and SA, hormones crucial for plant defense responses, have also been reported to affect stomatal aperture (Khokon et al., 2010; Melotto et al., 2006; Munemasa et al., 2007; Suhita et al., 2004; Yan et al., 2015); however, their impact on stomatal responsiveness to environmental cues is less understood. To address this question, we used plant lines with mutations in JA and SA signaling and biosynthesis to monitor their stomatal responses to high/low CO2, artificial darkness period and low air humidity.
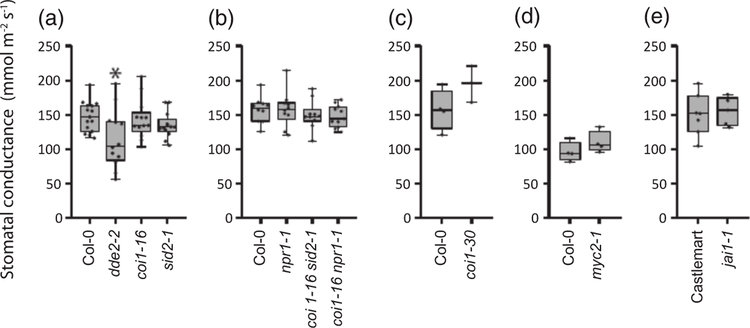
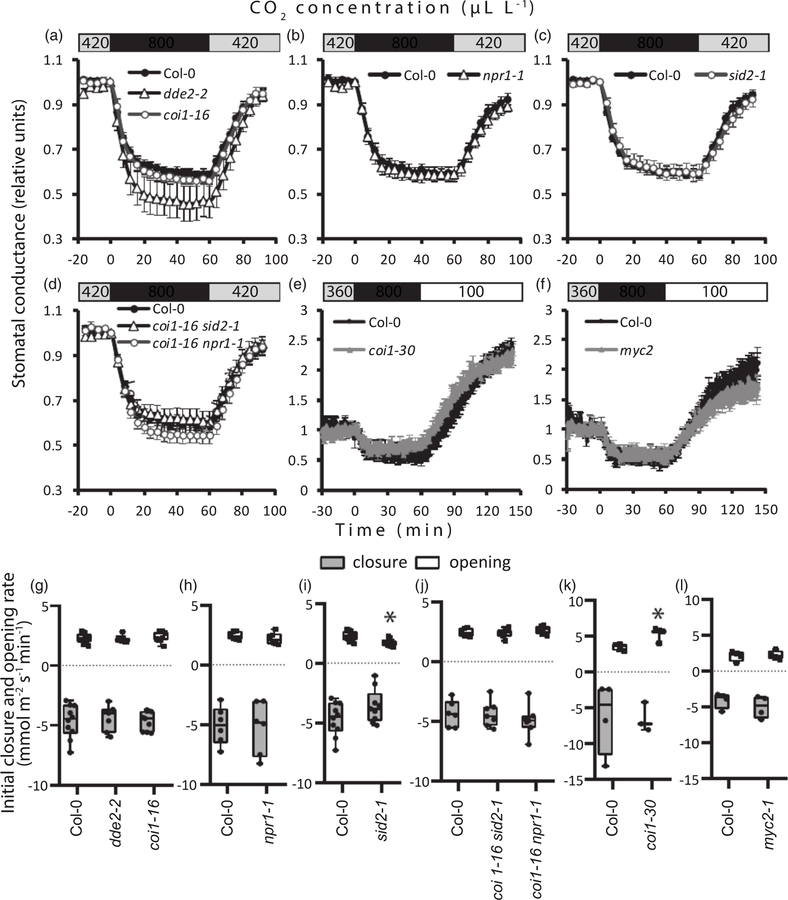
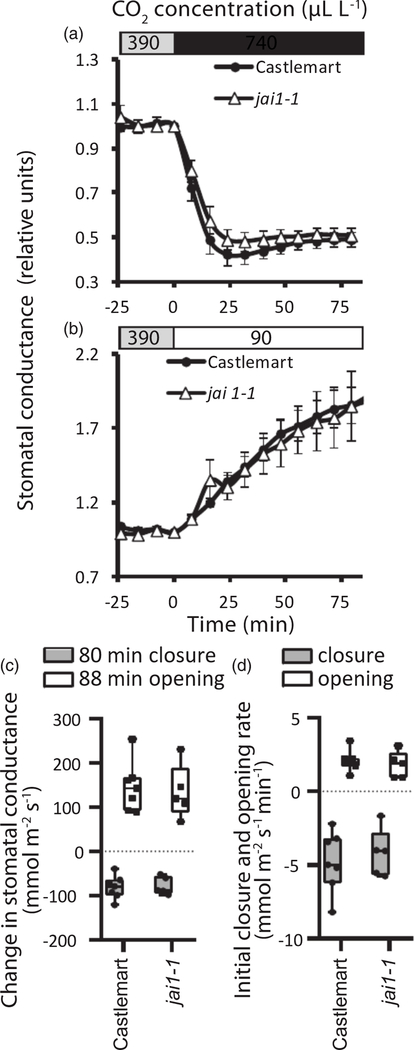
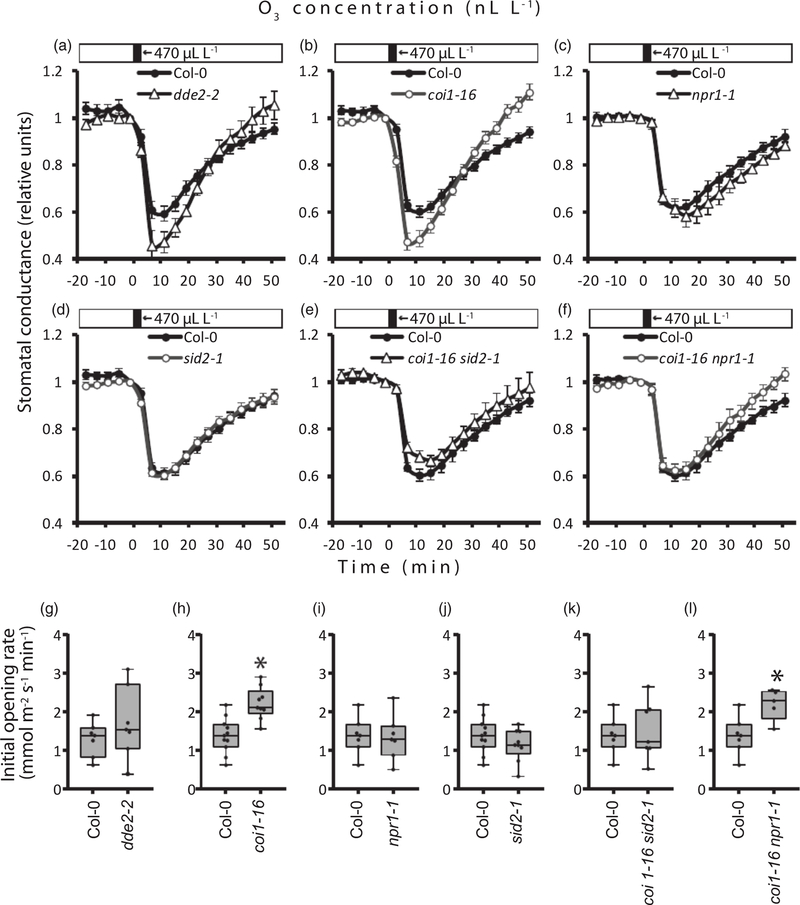
Throughout the gas-exchange experiments, steady-state whole-plant stomatal conductance in the Arabidopsis and tomato mutants studied did not vary dramatically, although this trait was 20% reduced in the JA-deficient dde2–2 mutant compared with wild type Col-0 (Figure 1). When the intact rosettes of Arabidopsis plants were subjected to elevated CO2, effective stomatal closure was observed in wild-type Col-0 as well as in all SA- and JArelated mutant plants (Figures 2a–d and S1). Elevated CO2 levels reduced stomatal conductance in plants with impaired JA and SA biosynthesis (dde2–2 and sid2–1, respectively) or in plants lacking the receptors to JA and SA (coi1–16 and npr1–1, respectively), as well as in the coi1–16 sid2–1 and coi1–16 npr1–1 double mutants that combine the impairment of JA and SA signaling and biosynthesis. The magnitudes and rates of stomatal closure induced by high levels of CO2 did not differ between the Col-0 plants and the mutant lines (Figures 2g–j and S1). Stomatal reopening in ambient CO2 was possibly very slightly delayed in sid2–1, according to the reduced initial rate of stomatal conductance recovery observed in some experiments (Figure 2i). To confirm these results, we carried out parallel experiments in the laboratory of JIS (University of California, San Diego, UCSD) with another experimental approach where we monitored the stomatal conductance of individual Arabidopsis leaves upon changes in CO2 concentration (Hu et al., 2015). The myc2–1 mutant with inactive MYC2 transcription factor regulating diverse JA-dependent processes (Lorenzo et al., 2004) and the coi1–30 mutant without active COI1 were studied. In accordance with the whole-plant responses, intact leaves of the myc2–1 and coi1–30 plants effectively closed and opened their stomata under higher and lower than ambient CO2 concentrations (Figures 2e,f,k,l and S1). The initial rate of stomatal opening in coi1–30 was potentially very slightly enhanced compared with Col-0 in some of the experiments (Figure 1k). An intact responsiveness to CO2 was observed in the top leaves of the tomato jai1–1 mutant, defective in the JA receptor COI1 homolog (Li et al., 2004). This mutant did not differ from the corresponding wild-type line in the experiments with high and low CO2 treatments (Figures 3 and S2). Thus, the results obtained by two independent laboratories using different experimental set-ups demonstrate that JA and SA do not play a significant role in high-CO2-induced stomatal closure. Stomatal opening in response to low CO2 and the recovery of stomatal conductance after elevated CO2 levels might be only very slightly modulated by JA and SA signaling in Arabidopsis.
Figure 1.
Steady-state stomatal conductance in the jasmonic acid (JA) and salicylic acid (SA) biosynthesis and signaling mutants studied.
Whole-plant stomatal conductance was monitored in Arabidopsis plants at the age of 3–4 weeks (a, b). Stomatal conductance was also measured in individual leaves of intact 4–5-weeks-old Arabidopsis plants (c, d) and in top leaves of 3–4-weeks-old Solanum lycopersicum (tomato) plants (e). The boxes extend from the 25th to the 75th percentiles, with the horizontal lines plotted at the median values. The individual data points are shown as dots whereas the whiskers are the minimum and maximum values (n = 4–10). Asterisks show significant differences between mutants and the wild type (one-way ANOVA followed by Dunnett’s post hoc test; P < 0.05).
Figure 2.
CO2-triggered regulation of stomatal conductance in Arabidopsis jasmonic acid (JA) and salicylic acid (SA) biosynthesis and signaling mutants.
Time courses for stomatal conductance in the mutants and corresponding wild-type Col-0 plants studied are shown (average ± SE). Stomatal conductance is shown in relative values calculated from the data presented in Figure S1. At time 0, elevated CO2 (approx. 800 μl L−1) was applied for 60 min, followed by ambient C2O (approx. 420 μl L−1) (a–d) or reduced C2O (approx. 100 μl L−1) (e, f). Experiments with intact plants included the single (a–c) and double (d) mutants with impaired JA and SA biosynthesis and signaling (n = 6–10). Stomatal responses to elevated and reduced C2O levels were studied in the leaves of the coi1–30 (e) and myc2–1 (f) Arabidopsis mutants (n = 3–4). Changes in C2O concentrations are indicated in the bars above the graphs. (g–j) Initial rates of stomatal closure and stomatal opening were calculated as linear slopes of the stomatal conductance curve within 8 and 20 min after the application of high and ambient CO2, respectively. (k, l) Initial rates of stomatal closure and stomatal opening in individual leaves were calculated within 10 and 20 min after application of high and low CO2, respectively. The boxes extend from the 25th to the 75th percentiles, with the horizontal lines plotted at the median values. The individual data points are shown as dots, whereas the whiskers are the minimum and maximum values (n = 3–10). Asterisks show significant differences between mutant lines and Col-0 (one-way ANOVA, followed by Dunnett’s post hoc test; P < 0.05).
Figure 3.
Stomatal responsiveness to elevated (a) or reduced (b) CO2 concentrations in leaves of the jai1–1 Solanum lycopersicum (tomato) mutant and the corresponding wild type (Castlemart).
The treatments were started at time 0. Time courses for stomatal conductance are shown (average ± SE). Stomatal conductance is shown in relative values calculated from the data presented in Figure S2 (n = 5 for jai1–1; n = 7 for Castlemart). (c) Changes in stomatal conductance in leaves of jai1–1 and Castlemart at 80 and 88 min with elevated and reduced CO2, respectively. (d) The rates of stomatal closure and stomatal opening in elevated and reduced CO2 were calculated as linear slopes of the stomatal conductance curves within 16 and 56 min after application of elevated and reduced CO2, respectively. The boxes extend from the 25th to the 75th percentiles, with the horizontal lines plotted at the median values. The individual data points are shown as dots, whereas the whiskers are the minimum and maximum values. No statistically significant differences were detected between mutant lines and Castlemart with one-way ANOVA (P < 0.05).
A recent study of the guard cell metabolome in Brassica napus demonstrated the activation of the JA biosynthesis pathway by elevated CO2 (Geng et al., 2016). This suggested that JA biosynthesis might play a role in CO2-triggered stomatal closure, and further stomatal aperture assays carried out with epidermal peels showed reduced stomatal CO2 sensitivity in the Arabidopsis JA-insensitive mutants (coi1, jar1 and jin1/myc2) (Geng et al., 2016). It is possible that the CO2 insensitivity phenotype that was observed by Geng et al. in the coi1, jar1 and jin1/myc2 mutants could be related to the disruption of the contacts between stomata and mesophyll cells in epidermal peels. Involvement of mesophyll-driven signals in the CO2-induced regulation of stomatal apertures was suggested by a number of studies, although the nature of these signals remains elusive (Lawson et al., 2014). Apoplastic malate promotes high CO2-triggered stomatal closure by enhancement of the activity of the malate-sensitive R-type anion channel QUAC1 (Hedrich and Marten, 1993; Lee et al., 2008; Meyer et al., 2010). Sucrose and glucose in the apoplastic space provide another link between mesophyll and guard cells, connecting photosynthesis with the regulation of stomatal apertures (Flütsch et al., 2020; Lawson et al., 2014; Santelia and Lawson, 2016). Rates of high-CO2-triggered stomatal closure can be modulated by a balance between foliar sucrose and malate in ferns and angiosperms (Lima et al., 2019).
Darkness-induced stomatal closure recruits several independent signaling pathways in guard cells. The lack of CO2 assimilation by photosynthesis during darkness and respiration leads to an increase of intercellular CO2 that contributes to darkness-induced stomatal closure through the activation of CO2 signaling in guard cells (Roelfsema et al., 2002). Furthermore, the absence of blue light prevents activation of phototropins and plasma-membrane H+-ATPases in guard cell membranes (Shimazaki et al., 2007). Darkness-induced stomatal closure depends on ABA signaling, as it is partially impaired in the mutants lacking six ABA receptors or OST1 protein kinase (Merilo et al., 2013). The receptor-like protein GHR1 that mediates ABA and CO2 signaling in guard cells is also important for stomatal closure in darkness (Sierla et al., 2018). In contrast, the mutants with interrupted JA and SA biosynthesis and signaling (coi1–16, dde2–2, npr1–1 and sid2–1) as well as mutants combining these defects (coi1–16 sid2–1 and coi1–16 npr1–1) demonstrated unaffected stomatal closure during the artificially imposed periods of darkness (Figures S3 and S4). Similar to the gas-exchange experiments with changing CO2, the sid2–1 mutant demonstrated slightly slower recovery of stomatal conductance upon re-illumination than the wild-type plants (Figure S4). These experiments indicate that JA and SA are not involved in stomatal closure triggered by darkness, whereas the recovery of stomatal conductance after darkness can depend on SA biosynthesis.
Both ABA and basal ABA concentrations in guard cells have a paramount role in determining the overall stomatal conductance in a plant (Gonzalez-Guzman et al., 2012; Merilo et al., 2013, 2018). However, ABA accumulation in guard cells during rapid stomatal closure induced by abrupt changes in VPD, i.e. reduced air humidity, is still under debate because of conflicting results, probably associated with the experimental set-ups and species studied. Some reports show that high-VPD-induced stomatal closure is controlled by ABA biosynthesis and signaling (Bauer et al., 2013; McAdam et al., 2016; Merilo et al., 2013; Xie et al., 2006). However, a recent study demonstrated that the impaired ABA biosynthesis did not affect high-VPD-induced stomatal closure, whereas OST1, one of the central regulators of the ABA response in guard cells, was of high importance for stomatal closure in response to reduced air humidity (Merilo et al., 2018; Xie et al., 2006). Apparently, the rapid stomatal closure triggered by high VPD is controlled by OST1, which might be activated independently of ABA signaling by Raf-like kinases (Katsuta et al., 2020; Soma et al., 2020). The long-term stomatal adaptation to dry air involves the modulation of ABA levels, further decreasing stomatal conductance (Yaaran et al., 2019). In our experiments, single and double JA and SA biosynthesis and signaling mutant plants showed pronounced responses to the increase in VPD (Figure S5). The sid2–1 mutant demonstrated a slightly lower magnitude of stomatal closure 15 and 60 min after the increase in VPD than the wild-type Col-0 plants (Figure S6). Our results do not exclude that JA/SA-activated signaling can affect stomatal opening under elevated relative air humidity (>95%), as indicated by previous studies (Panchal et al., 2016; Panchal and Melotto, 2017). Stomatal opening under these conditions is associated with the activation of JA signaling and the simultaneous downregulation of SA-responsive genes in guard cells (Panchal et al., 2016; Panchal and Melotto, 2017).
In summary, our results indicate that the SA and JA biosynthesis and signaling mutants maintain stomatal responsiveness to CO2 levels, light intensity and low air humidity. At the same time, the rates and magnitudes of stomatal movements induced by these stimuli can be partly modulated by JA and SA.
JA and SA biosynthesis and signaling have a minor influence on ABA-induced stomatal closure
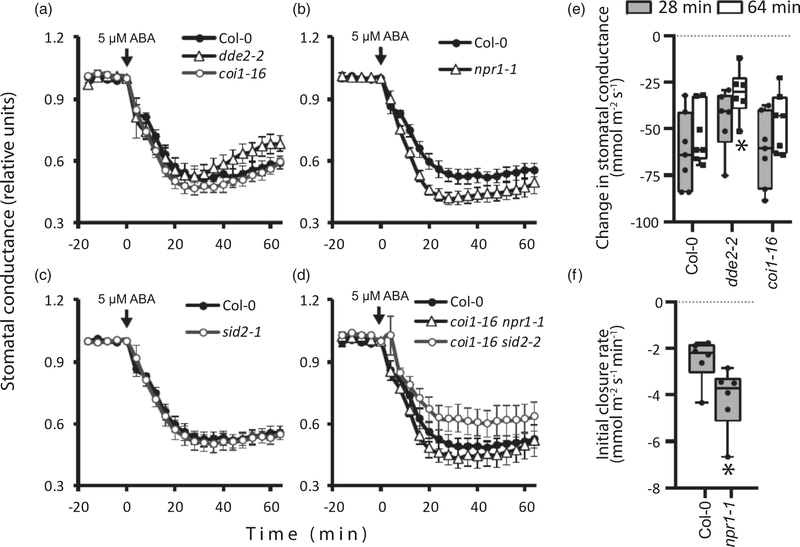
As plant hormones frequently interact in physiological processes, we aimed to study whether JA and SA would influence ABA-induced stomatal closure in intact plants. We sprayed the rosettes of wild-type plants with 5 μM ABA, which induced a fast and robust reduction in stomatal conductance (Figures 4 and S7). Stomata in leaves of the coi1–16, dde2–2, npr1–1, sid2–1, coi1–16 sid2–1 and coi1–16 npr1–1 mutants displayed rapid stomatal closure in response to 5 μM ABA, which was comparable with wild-type plants in magnitude (Figures 4 and S7). Interestingly, the stomata in ABA-treated dde2–2 plants partially reopened by the end of the experiments and the reduction of stomatal conductance was lower in dde2–2 than that in the wild-type plants 64 min after spraying with ABA (Figure 4e). The JA biosynthesis in dde2–2 is interrupted before the formation of 12-oxo-phytodienoic acid that is able to induce stomatal closure by itself, most efficiently in combination with ABA (Montillet et al., 2013; Savchenko et al., 2014). Seemingly, the duration of ABA effect on stomata depends on the level of 12-oxo-phytodienoic acid, as the coi1–16 mutant demonstrated the same stomatal responsiveness to ABA as the wild-type plants.
Figure 4.
Arabidopsis plants impaired in jasmonic acid (JA) and salicylic acid (SA) biosynthesis and signaling demonstrate pronounced abscisic acid (ABA)-induced stomatal closure.
Plants 3–4 weeks old were sprayed with 5 μM ABA at time 0 (marked with the arrows). Single mutants with impaired JA (a) and SA (b, c) signaling and biosynthesis, as well as double mutants (d), were studied. Time courses for stomatal conductance in the mutants and the corresponding wild-type Col-0 plants studied are shown (average ± SE, n = 6–10). Stomatal conductance is shown in relative values calculated from the data presented in Figure S7. (e) Reduction of stomatal conductance at 28 and 64 min after ABA spraying. (f) The initial rates of ABA-induced stomatal closure in npr1–1 and Col-0 were calculated as slopes of the stomatal conductance curve within 12 min after spraying with ABA. The boxes extend from the 25th to the 75th percentiles, with the horizontal lines plotted at the median values. The individual data points are shown as dots, whereas the whiskers are the minimum and maximum values (n = 6–10). Asterisks show significant differences between mutant lines and the wild type (one-way ANOVA, followed by Dunnett’s post hoc test; P < 0.05).
The antagonistic interaction between ABA and SA signaling has been suggested in studies of systemic acquired resistance (Ton et al., 2009; Yasuda et al., 2008). We found that the lack of NPR1 in the npr1–1 mutant resulted in faster ABA-induced stomatal closure than that in the wild-type plants, although the magnitude of the reduction in stomatal conductance did not differ between npr1–1 and Col-0 at 28 and 64 min after ABA spraying (Figures 4f and S7e). The sid2–1 mutant displayed the same changes in stomatal conductance as the wild-type plants (Figure S7f,i), probably through a residual level of SA in this mutant (Wildermuth et al., 2001). Thus, our results suggest that NPR1-dependent SA signaling influences ABA-triggered stomatal closure.
Previous research indicated that stomatal closure induced by MeJA and SA depends on both ABA basal levels and ABA signaling. An impaired stomatal responsiveness to MeJA and SA was observed in ABA-deficient mutants (Hossain et al., 2011; Zeng and He, 2010). Studies of ABA-insensitive mutants showed that MeJA-induced stomatal closure involves ABI1, ABI2 and OST1, and does not require PYR1, PYL1, PYL2 and PYL4 ABA receptors (Hossain et al., 2011; Munemasa et al., 2007; Yin et al., 2016). A recent study using a real-time SnRK2/OST1 protein kinase Förster resonance energy transfer (FRET) reporter showed strong SnRK2/OST1 activation by ABA, but no activation by MeJA or elevated CO2 (Zhang et al., 2020). SA was shown to trigger the phosphorylation of SLAC1 in guard cells via calcium-dependent protein kinases, but not by OST1 (Prodhan et al., 2018). This mechanism is possibly involved in the regulation of stomatal closure by pathogens. The dependence of ABA-induced stomatal closure kinetics on JA and SA should be studied additionally.
Disruption of SA and JA biosynthesis and signaling does not influence ozone-triggered stomatal closure
As an air pollutant, ozone damages plants and can lead to substantial losses in crop yield (Ainsworth et al., 2012). As ozone enters plants through stomata and degrades to ROS in the apoplast, ozone exposure became instrumental in studies of apoplastic ROS signaling in relation to cell death and stomatal functioning (Kangasjärvi et al., 2005; Kollist et al., 2007; Vahisalu et al., 2010; Xu et al., 2015). Importantly, a short-term ozone pulse induces stomatal closure through the activation of ROS-dependent signaling pathways in guard cells (Kollist et al., 2007; Vahisalu et al., 2010). As the apoplastic ROS formed from ozone resembles the ROS burst during pathogen infection (Vaahtera et al., 2013), we studied whether stomatal responsiveness to apoplastic ROS/ozone depends on JA and SA levels or signaling. The JA and SA biosynthesis and signaling mutants were exposed to a 3-min pulse of approx. 470 nl L−1 ozone, and changes in stomatal conductance were monitored. All of the mutants analyzed closed their stomata effectively after the 3-min ozone pulses, similar to the wild type (Figures 5 and S8). The coi1–16 sid2–1 double mutant showed a slightly reduced magnitude of ozone-induced stomatal closure (Figure S8). Although the recovery of stomatal conductance after ozone-induced stomatal closure has not been studied completely (Moldau et al., 2011), our data indicate that the coi1–16 mutant plants may show accelerated stomatal reopening (Figure 5h). Additionally, the coi1–16 npr1–1 double mutant demonstrated an enhanced rate of stomatal conductance recovery compared with Col-0 (Figures 5l and S8).
Figure 5.
Ozone-induced stomatal closure in Arabidopsis mutants with disrupted jasmonic acid (JA) and salicylic acid (SA) biosynthesis and signaling.
Plants at the age of 3–4 weeks were exposed to a pulse of approx. 470 nl L −1 ozone for 3 min at time 0. Single mutants with impaired JA (a, b) and SA (c, d) signaling and biosynthesis, as well as double mutants (e, f), were studied. Time courses for stomatal conductance in the mutants and the corresponding wild-type Col-0 plants studied are shown (average ± SE, n = 5–10). Stomatal conductance is shown in relative values calculated from the data presented in Figure S8. (g–j) The rates of stomatal conductance recovery were calculated as the linear slopes of the stomatal conductance curve within 23–35 min after ozone exposure. The boxes extend from the 25th to the 75th percentiles, with the horizontal lines plotted at the median values. The individual data points are shown as dots, whereas the whiskers are the minimum and maximum values. Asterisks show significant differences between mutant lines and the wild type (one-way ANOVA, followed by Dunnett’s post hoc test; P < 0.05).
In general, although SA and JA can induce apoplastic ROS formation (Khokon et al., 2010; Mori et al., 2001; Suhita et al., 2004), stomatal responsiveness to apoplastic ROS induced by ozone does not depend on SA and JA levels or signaling. However, the recovery of stomatal conductance after a brief pulse of ozone might depend on COI1.
MeJA and SA are considerably less effective in inducing stomatal closure than ABA
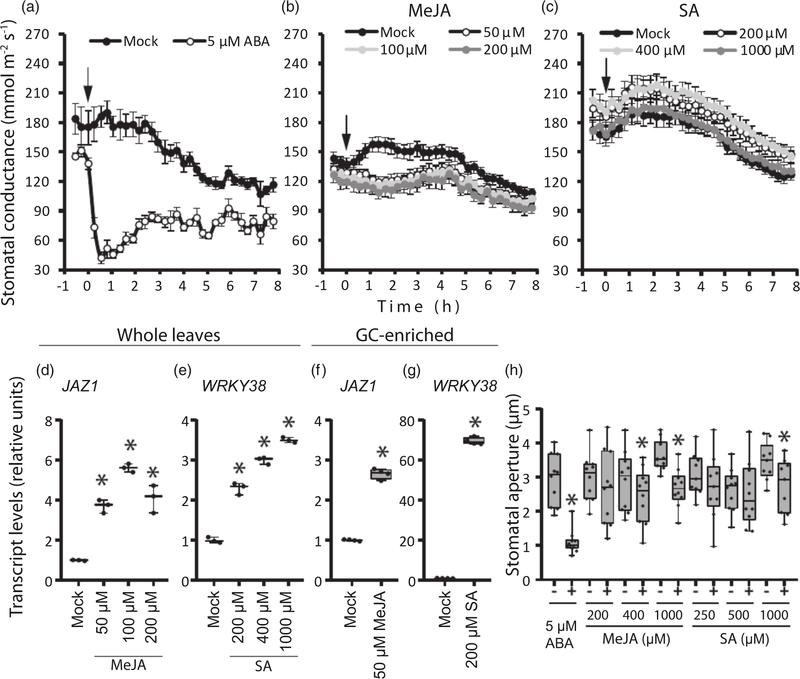
Stomatal closure induced by MeJA and SA was reported in several studies, typically by measuring stomatal apertures in epidermal peels or detached leaves (Khokon et al., 2010; Melotto et al., 2006; Munemasa et al., 2007; Suhita et al., 2004; Yan et al., 2015). Here, we compared stomatal responses to MeJA and SA with that to ABA in intact plants. We sprayed whole Arabidopsis plants with ABA, MeJA or SA and monitored whole-plant stomatal conductance for 23 h (Figures 6a–c and S9). Stomatal conductance in the mock-treated plants was slightly increased in response to sprays and then gradually reduced before the night-time, according to the diurnal stomatal rhythm (Sierla et al., 2018). As expected, spraying plants with 5 μM ABA resulted in rapid, pronounced and prolonged stomatal closure (Figures 6a and S9) that was still clearly detectable after 23 h (Figure S9). At the same time, MeJA and SA in concentrations up to 200 and 1000 μM, respectively, did not induce a prominent reduction of stomatal conductance, which would be comparable with that in plants treated with 5 μM ABA (Figures 6b,c and Figure S9). However, MeJA suppressed the stomatal opening induced by brief leaf wetting/high humidity in the chambers (Panchal et al., 2016; Yokoyama et al., 2019), and further induced detectable slight stomatal closure. In 80 min after spraying, stomatal conductance in MeJA-treated plants was significantly reduced by 5–10% of the initial values (Figure S9). Stomatal conductance in SA-treated plants was indistinguishable from that in the corresponding mock plants. These results demonstrate the ability of MeJA to suppress stomatal opening and to induce detectable stomatal closure in the sprayed intact Arabidopsis plants, although this response is not comparable with ABA-induced stomatal closing. Although a role for JA in stomatal opening requires further research, a recent publication demonstrates that JA signaling suppresses high temperature-triggered stomatal opening in tomato plants damaged by insects or mechanical wounding (Havko et al., 2020).
Figure 6.
Effects of abscisic acid (ABA), methyl jasmonate (MeJA) and salicylic acid (SA) treatments on stomatal functioning in Arabidopsis.
Arabidopsis plants (Col-0) at the age of 3–4 weeks were sprayed with ABA (a), MeJA (b) or SA (c), in various concentrations. Time courses are shown for stomatal conductance (average ± SE, n = 4 for ABA, n = 16–24 for MeJA and SA). Relative expression of JAZ1 and WRKY38 after MeJA (d) and SA (e) treatments, respectively, in plants analyzed in (b) and (c) (n = 3). The same transcripts were quantified in guard-cell-enriched epidermal fractions (GC-enriched) collected from MeJA- and SA-treated plants (n = 4) (f and g). Asterisks show significant differences between mock and hormonal treatments (one-way ANOVA, followed by Dunnett’s post hoc test; P < 0.05). (h) ABA demonstrates a significantly higher potency to induce stomatal closure in stomatal assays than MeJA and SA. Epidermal peels were collected from plants at age of 4–5 weeks and incubated in stomatal opening buffer supplemented with ethanol (mock), ABA, MeJA or SA. The width of stomatal apertures was measured after exposure for 3 h (n = 9–10 individual plants). The boxes extend from the 25th to the 75th percentiles, with the horizontal lines plotted at the median values. The individual data points are shown as dots, whereas the whiskers are the minimum and maximum values. Asterisks show statistically significant differences between the mock (-) and the hormonal treatment (+) by repeated-measures ANOVA (P < 0.05).
Both MeJA and SA can enter guard cells via diffusion across plasma membranes (Maruri-López et al., 2019; Seo et al., 2001) and via specific transporters, which should still be identified in guard cells. To confirm that the hormones penetrated the sprayed plants and guard cells, we measured transcript levels of hormone-responsive marker genes (Figure 6d–g). Leaves of MeJA- and SA-treated plants demonstrated an accumulation of JAZ1 and WRKY38 transcripts (Figure 6d,e), respectively, as it has been shown for comparable plant treatments in other studies (Chung et al., 2008; Kim et al., 2008). Spraying with 5 μM ABA similarly induced elevated ABA-responsive HAI1 transcripts (Figure S9). The uptake of the sprayed hormones by guard cells was confirmed by the upregulation of JAZ1 and WRKY38 in guard cell-enriched epidermal fractions collected from JA- and SA-treated plants, respectively (Figure 6f,g). In another study, concentrations of JA and SA were estimated on the levels of approximately 9 and 115 pg mg−1 fresh weight, respectively (corresponding to approx. 40 pmol g−1 JA and approx. 0.83 nmol g−1 SA) in guard-cell-enriched epidermal peels collected from Arabidopsis plants (David et al., 2020). The quantification of JA and SA in Arabidopsis leaves revealed similar ranges of the hormones (Forcat et al., 2008; Pan et al., 2010; Trapp et al., 2014). Thus, spraying with MeJA and SA solutions in the concentrations used in this study led to a significant increase of these hormones in guard cells and were efficient regarding the induction of biological effects in guard cells.
As a complementary method to study the role of MeJA and SA in the modulation of stomatal apertures, we collected epidermal peels from Arabidopsis plants and incubated them with ABA, MeJA or SA for 3 h. The application of 400 and 1000 μM MeJA induced 15 and 26% stomatal closure in epidermal peels, respectively. In these experiments, SA induced noticeable stomatal closure only when applied at higher concentrations (1000 μM; Figure 6h). In comparison, 5 μM ABA triggered a 2.7-fold reduction in stomatal aperture.
Taken together, our gas-exchange results and direct stomatal aperture assays indicate that stomata are much more sensitive to ABA than to MeJA or SA. It is also possible that a long-term accumulation of MeJA or SA in guard cells is required to induce stomatal closure, whereas stomata respond to ABA almost immediately (Figures 4, 6a and S7).
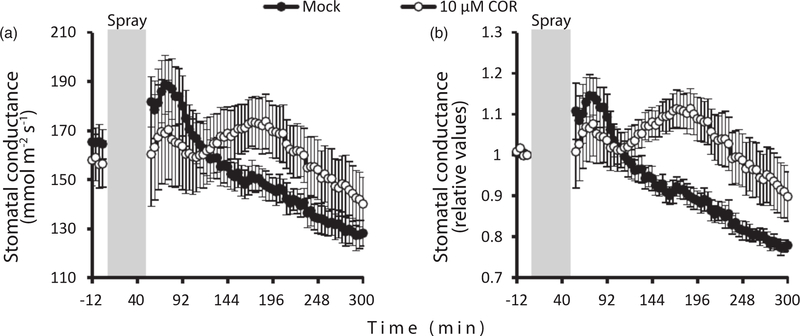
Coronatine induces stomatal opening in intact Arabidopsis plants
Like the endogenous jasmonates, the phytotoxin coronatine (COR) activates JA signaling through COI1 in plants (Katsir, Schilmiller, et al., 2008). COR has been reported to trigger stomatal opening and suppress stomatal closure to promote pathogen invasion (Toum et al., 2016). As MeJA showed only a weak effect on stomata in intact plants, we decided to investigate whether COR, also acting through COI1, would enhance stomatal conductance under the same conditions. Intact Arabidopsis plants were sprayed with 10 μM COR and stomatal conductance was monitored for 6 h. As observed in other experiments with plant spraying, a transient increase in stomatal conductance was observed for approximately 90 min after both mock and COR spraying. Although stomatal conductance was not altered after a single spray with 10 μM COR in a preliminary experiment, four consecutive rounds of sprays with 10 μM COR, which provided a long-term treatment for guard cells, resulted in a delayed but significantly increased stomatal conductance compared with mock treatments, which were performed in parallel (Figures 7 and S10). Stomatal opening in COR-treated plants started approximately 110 min after spraying and reached maximal values 3 h after spraying before the stomata closed again. The mock-treated plants displayed a continuous decrease in stomatal conductance, attributed to the diurnal stomatal rhythm.
Figure 7.
Stomatal opening induced by coronatine (COR) in intact Arabidopsis plants.
(a) Plants at the age of 3–4 weeks were sprayed with 10 μM COR four rounds with an interval of 12 min (shown as the gray box). For comparison, other plants were sprayed with mock solution (0.1% methanol, 0.012% Silwet L-77) in parallel with the COR treatments. Time courses for stomatal conductance are shown (average ± SE, n = 4–5). (b) The same data as (a), expressed as relative values to time 0.
Methyl jasmonate (MeJA) and COR have opposing roles in modulating the stomatal response. MeJA induces stomatal closure, as demonstrated in numerous studies and also in this work by using different experimental approaches (Hua et al., 2012; Munemasa et al., 2007; Suhita et al., 2004; Figures 6b and S9). At the same time, COR triggers stomatal opening even in low concentrations delivered by spraying plants (Figure 7). The different effects of COR and MeJA on stomatal functioning could be explained by alternative signaling pathways activated by these substances in addition to the canonical COI1–JAZ1 pathway (Devoto et al., 2005; Liu et al., 2009; Zhou et al., 2015). Thus, COR-induced stomatal opening can be mediated by RPM1-INTERACTING PROTEIN 4, which activates AHA1 and AHA2 plasma membrane H+-ATPases (Liu et al., 2009).
CONCLUSION
The gas-exchange experiments with JA and SA biosynthesis and signaling mutants showed that JA and SA did not directly mediate stomatal responses to CO2, light–darkness transitions, low air humidity, ABA and ozone in intact plants (Figures 2–5 and S1–S8). Only small differences between the wild-type plants and the mutants studied were observed in the rates of stomatal opening and closing. JA signaling tends to restrict stomatal opening as the lack of COI1 accelerated the recovery of stomatal conductance after ozone pulses and very slightly enhanced the low-CO2-induced stomatal opening (Figures 2k and 5h). Furthermore, the MeJA-treated plants did not exhibit the stomatal opening triggered by high humidity (Figure 6b). At the same time, ABA-induced stomatal closure was accelerated in the SA-insensitive npr1–1 mutants, compared with the wild type. Although the influence of JA and SA on stomatal responsiveness to environmental stimuli was weak, the modulation of stomatal opening and closing rates by these hormones deserves further attention.
Although stomata respond rapidly and robustly to ABA, CO2, darkness and ozone, stomatal closure triggered by MeJA and SA is substantially less efficient when the hormones were sprayed on intact plants (Figures 6a–c and S9). Although the uptake kinetics by guard cells for ABA, MeJA and SA might be significantly different, all of these hormones were delivered to guard cells by the same spraying method (Figures 6d–g and S9). The time-resolved analysis of whole-plant stomatal conductance demonstrates that stomata in intact plants respond to MeJA much slower and in a much smaller extent compared with ABA (Figure 6a,b). Furthermore, the biological effect of COR on guard cells develops with a lag, as only a partial stomatal opening was initiated at 1.5–2.0 h and reached the maximum 3 h after spraying with COR (Figure 7), in contrast to the rapid stomatal opening induced by low CO2 and light. As spraying with SA did not decrease stomatal conductance, SA-induced stomatal closure apparently requires high concentrations and/or prolonged SA treatments, which can be achieved by soaking epidermal peels in SA solutions (Figure 6h). Our results highlight the different roles of the MeJA and SA defense hormones from that of ABA in the regulation of stomatal movement. ABA is a central regulator of many aspects in stomatal function, including stomatal responsiveness to environmental cues (Chater et al., 2015; Hsu et al., 2018; Merilo et al., 2013; Xue et al., 2011). JA and SA, which regulate plant defenses against biotic stresses, induce partial stomatal closure, as previously reported under specific conditions (e.g. herbivore or pathogen attack), and might modulate rates of stomatal reactions to some environmental cues. Further investigations of stomatal responses under diverse conditions could best be accomplished using gas-exchange analyses of whole intact plants and in intact leaves, which enables time-dependent kinetic analysis.
EXPERIMENTAL PROCEDURES
Plant material and growth conditions
Arabidopsis plants were grown in soil containing 2:1 (v:v) peat: vermiculite. Arabidopsis plants for whole-plant measurements of stomatal conductance were cultivated in growth chambers (AR-66LX and AR-22L; Percival Scientific, https://www.percival-scientific.com) with a 12-h photoperiod, 23°C day/18°C night, 150 μmol m−2 sec−1 light and 70% relative humidity. Soil moisture was kept at approximately 80% of maximum water capacity. The plants for stomatal aperture assays were grown in growth rooms under the same conditions. For gas-exchange experiments, plants were grown through a hole in a glass plate covering the growth pot as described by Kollist et al. (2007). The Arabidopsis plants for studies with individual leaves were grown on soil in a Conviron E7/2 plant growth chamber (Conviron, https://www.conviron.com) with a 12-h photoperiod, 21°C day/19°C night, relative humidity of 70–80% and photosynthetic photon flux density of approximately 150 μmol m−2 sec −1. The plants were watered twice per week.
The JA and SA mutants in this study have been well characterized before. The following Arabidopsis mutants were used: dde2–2 (also known as aos, allene oxide synthase) and sid2–1, which fail to synthesize JA and SA, respectively (Malek et al., 2002; Wildermuth et al., 2001); coi1–16 and coi1–30, defective in the JA receptor COI1 (Ellis et al., 2002; Xu et al., 2015); and npr1–1, lacking the SA receptor (Ding et al., 2018). Additionally, we used the myc2–1 (also known as jin1) mutant with inactive MYC2 transcription factor regulating diverse JA-dependent processes (Lorenzo et al., 2004). To acquire more information about the interactions of defense hormones in stomatal regulation, we used the double mutants coi1–16 sid2–1 and coi1–16 npr1–1 (Xu et al., 2015). All Arabidopsis mutants were in the Col-0 genetic background and their homozygosity was verified routinely.
The tomato jai1–1 mutant (Li et al., 2004; the jai1 mutant is equivalent to the coi1 mutant in Arabidopsis) and the corresponding wild-type line (cultivar Castlemart) were germinated on wet filter paper in darkness and were transferred into the soil at the age of 7–8 days. Plants were grown in growth chambers with a 12-h photoperiod, 23°C day/18°C night, 200 μmol m−2 sec−1 light and 70% relative humidity. As the jai1–1 mutation impairs seed production (Li et al., 2004), homozygous jai1–1 plan ts were selected from the progeny of heterozygous jai1–1 plants (Bosch et al., 2014). At the age of 14–16 days, cotyledons from the tomato plants were collected and used for DNA isolation. The plants were genotyped by using pairs of primers to the genomic DNA with and without the deletion in the tomato COI1 analog (Table S1). The homozygous jai1–1 mutants were employed for the gas-exchange experiments at the age of 3–4 weeks.
Gas-exchange experiments
The whole-plant stomatal conductance in the single (coi1–16, sid2–1, dde2–2 and npr1–1) and double (coi1–16 sid2–1 and coi1–16 npr1–1) Arabidopsis mutants was measured in the eight-chamber gas-exchange devices, which have been described before (Kollist et al., 2007). Arabidopsis plants at the age of 3–4 weeks were inserted into the device and incubated for about 1 h for the stabilization of stomatal conductance. The standard conditions in the chambers were as follows: ambient CO2 (423.9 ± 4.8 μl L−1, here and thereafter average ± SD), 150 μmol m−2 sec−1 light, 69.8 ± 2.2% relative air humidity and 24.4 ± 0.15°C. In order to characterize the stomatal responses, the following stimuli promoting stomatal closure were applied: elevated CO2 (798.8 ± 11.5 μl L−1 CO2); light-to-dark transition; spraying with 5 μM ABA; a reduction of relative air humidity (from 68.9 ± 1.7 to 27.5 ± 4.85%, increasing the VPD from 0.97 ± 0.05 to 2.22 ± 0.16 kPa); and a 3-min pulse of 467.3 ± 80.7 nl L −1 ozone. For ABA treatments, the plants were sprayed with 5 μM ABA (in 0.012% aqueous Silwet L-77 solution) three times from different sides. Stomatal conductance in the JA and SA mutants and the wild-type plants was monitored for 1 h. In some experiments, the recovery of stomatal conductance after the treatments was recorded.
Stomatal responses to ABA, COR, MeJA and SA were also studied in the eight-chamber gas-exchange devices. The applied solutions of these hormones were prepared from 10 or 100 mM stock solutions (in 96% ethanol for ABA, MeJA and SA; in 100% methanol for COR) and were supplemented with 0.012% aqueous Silwet L-77. The working solutions were used during a single day. The Arabidopsis Col-0 plants at the age of 3–4 weeks were stabilized for 1 h under standard conditions in the eight-chamber gas-exchange device. For hormonal treatments or the mock, the plants were taken from the chambers and sprayed three times from different sides. In some experiments, the sprays with COR were repeated four times with 12-min intervals. Then, the plants were put back into the chamber and stomatal conductance was recorded every 4 or 16 min. For the mock, a solution with the corresponding concentrations of the solvent and Silwet L-77 were used to spray plants in parallel with the treatments with ABA, COR, MeJA and SA. All experiments were started at the same time and followed the day/night regime of the plants, if needed.
Photographs of plants were taken before the experiments and leaf areas were calculated using IMAGEJ 1.37 (https://imagej.nih.gov/ij). Stomatal conductance for water vapor was calculated according to von Caemmerer and Farquhar (1981). The energy budget equation was used to calculate the temperatures of the Arabidopsis rosettes in cuvettes of the gas-exchange device (Parkinson, 1985).
Stomatal responsiveness to CO2 was studied in individual leaves of the coi1–30 and myc2–1 Arabidopsis mutants attached to intact plants. Intact leaves of plants at 4–5 weeks old were analyzed using the LI-6400 infrared gas analysis (IRGA) gas-exchange analyzer system with a leaf chamber (LI-6400–40; LI-COR Biosciences, https://www.licor.com). The clamped leaves were equilibrated and stabilized at 150 μmol m−2 sec−1 light intensity (LED light source), 58–65% relative humidity, 21°C, 360 μl L−1 CO2 with an incoming air flow of 200 μmol sec −1 for 40 min. Stomatal conductance was recorded for 30 min at 360 μl L−1 CO2, followed by 60 min at 800 μl L−1 CO2 and 60 min at 100 μl L−1 CO2.
Stomatal conductance in tomato plants was monitored using a thermostated four-chamber, custom-built, flow-through gas-exchange device (Hõrak et al., 2017). The measurements were performed on two or three intact top leaves of the homozygous jai1–1 mutant and the wild-type plants. The leaves were hermetically sealed in the chambers of the device and stabilized at 394.3 ± 20.4 μl L −1 CO2, 150 μmol m−2 sec−1 light, 69.0 ± 9.5% relative air humidity. Stomatal closure and opening were triggered by 740.9 ± 35.6 and 93.5 ± 6.0 μl L −1 CO2, respectively. 2
Measurements of stomatal apertures
Leaves for stomatal aperture assays were collected from Arabidopsis plants at 4–5 weeks old. From the same leaf, abaxial epidermal peels for a mock and a hormonal treatment were collected. The epidermal peels were immediately transferred cuticle-side up into 6-cm Petri dishes filled with 10 mM 2-(Nmorpholine)-ethanesulphonic acid (MES), pH 6.15, 50 mM KCl. The buffer was supplemented with ethanol (mock) or hormones in various concentrations. The Petri dishes were incubated in a thermostatic water bath at a temperature of 22°C with light of 150 μmol m−2 sec−1 for 3 h. Stomata in the epidermal peels were examined with a Zeiss Axio Examiner D1 microscope with an 509 objective (Zeiss, https://www.zeiss.com). Pictures of stomata were collected with VISIVIEW 2.0 (Visitron, https://www.visitron.de) and processed with IMAGEJ 1.37 to measure stomatal aperture width. For each of the samples, the average width of stomatal apertures was calculated for the treatment and the corresponding mock, based on an examination of 15–30 stomata.
Transcript quantification
To confirm the entry of MeJA and SA into the sprayed leaves, we quantified transcripts that are known to be induced by these hormones. Leaf samples were collected from the hormone-treated plants: 2 h after spraying for MeJA, 4 h after spraying for SA and 2 h after spraying for ABA. DNA-free total RNA was isolated from the samples using the Spectrum Plant Total RNA kit (Sigma-Aldrich, https://www.sigmaaldrich.com), according to the manufacturer’s recommendations. cDNA was synthesized with the RevertAid Premium Reverse Transcriptase (ThermoFisher Scientific, https://www.thermofisher.com) and was used for real-time quantitative PCR (qPCR), with the conditions described before (Kaurilind and Brosché, 2017). Sequences of the primers used for qPCR are listed in Table S1. Three reference genes, TIP41, YLS8, and SAND, were used to normalize the qPCR data in QBASE 2.0 (Biogazelle, https://services.biogazelle.com). Guard-cell-enriched epidermal fractions were collected according to a method described previously (Bauer et al., 2013; Jalakas et al., 2017).
Statistical analysis
All experiments were repeated at least twice with similar results. One-way analysis of variance (ANOVA) was used to determine whether there were any statistically significant differences between groups in experiments. If the ANOVA models were statistically significant, post hoc tests were applied to identify the significantly different groups. Tukey’s honestly significant difference (HSD) test was used to compare multiple groups of samples (STATISTICA 7.1). Differences between wild-type plants and mutant lines were estimated by using Dunnett’s post hoc test. Effects of hormones on stomatal apertures and stomatal conductance were estimated using the paired Student’s t-test (STATISTICA 7.1). All effects were considered significant at P < 0.05. The ANOVA models, the numbers of biological repeats as well as the results of the Student’s t-tests and the post hoc tests are shown in Data S1.
The gas-exchange results are presented in absolute and relative values. To characterize stomatal movements in response to changing environmental conditions and ABA, additional parameters were calculated. Changes in stomatal conductance were computed as a difference between stomatal conductance values before and at a certain time after a stimulus was applied. The initial rates of change in stomatal conductance were calculated as linear slopes of the curve, reflecting short-time changes in stomatal conductance after the application of a treatment.
Supplementary Material
Figure S1. Changes in stomatal conductance driven by various CO2 concentrations in Arabidopsis JA- and SA-biosynthesis and signaling mutants.
Figure S2. Changes in stomatal conductance induced by elevated (a) or reduced (b) CO2 concentrations in leaves of the jai1–1 tomato mutant and the corresponding wild type (Castlemart).
Figure S3. Stomatal responsiveness to a period of darkness in single (a–d, g–j) and double (e, f, k, l) Arabidopsis mutants with disturbed JA and SA biosynthesis and signaling.
Figure S4. Characterization of stomatal movements induced by darkness and re-illumination in JA- and SA-biosynthesis and -signaling mutants.
Figure S5. Reduction of stomatal conductance under elevated vapor pressure deficit in JA- and SA-biosynthesis and -signaling mutants.
Figure S6. Changes in stomatal conductance of JA- and SA-biosynthesis and -signaling mutants in response to elevated vapor pressure deficit.
Figure S7. ABA-induced reduction of stomatal conductance in JA-and SA-biosynthesis and -signaling mutants.
Figure S8. Ozone-induced changes in stomatal conductance of Arabidopsis plants defective in JA and SA biosynthesis and signaling.
Figure S9. Plant responses to ABA, MeJA and SA sprays.
Figure S10. Changes in stomatal conductance after spraying with coronatine (COR).
Table S1. Primers used in this work.
Data S1. ANOVA tables and results of Student’s t-tests and post hoc tests.
ACKNOWLEDGEMENTS
We thank Prof. Gregg Howe (Michigan State University) for sending us the jai1–1 tomato seeds. This research was funded by the Estonian Research Council (grants PRG433 and PRG719) and the European Regional Development Fund (Center of Excellence in Molecular Cell Engineering, CEMCE), and by grants from the National Science Foundation (NSF; MCB-1900567) and the National Institutes of Health (GM060396) to JIS and a Deutsche Forschungsgemeinschaft fellowship (SCHU 3186/1-1:1) to SS.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest associated with this work.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
DATA AVAILABILITY STATEMENT
The results of gas-exchange measurements and stomatal assays included in this study are available upon request from the corresponding author.
REFERENCES
- Acharya BR & Assmann SM (2009) Hormone interactions in stomatal function. Plant Molecular Biology, 69, 451–462. 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Yendrek CR, Sitch S, Collins WJ & Emberson LD (2012) The Effects of tropospheric ozone on net primary productivity and implications for climate change. Annual Review of Plant Biology, 63, 637–661. 10.1146/annurev-arplant-042110-103829. [DOI] [PubMed] [Google Scholar]
- Assmann SM & Jegla T (2016) Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2. Current Opinion in Plant Biology, 33, 157–167. 10.1016/j.pbi.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid K et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Current Biology, 23, 53–57. 10.1016/j.cub.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Bosch M, Wright LP, Gershenzon J, Wasternack C, Hause B, Schaller A et al. (2014) Jasmonic acid and its precursor 12-oxophytodienoic acid control different aspects of constitutive and induced herbivore defenses in tomato. Plant Physiology, 166, 396–410. 10.1104/pp.114.237388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjarvi J et al. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proceedings of the National Academy of Sciences, 109, 10593–10598. 10.1073/pnas.1116590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B, Munemasa S, Wang C, Nguyen D, Yong T, Yang PG et al. (2015) Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife, 4. 10.7554/eLife.03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger M & Chory J (2019) Stressed out about hormones: how plants orchestrate immunity. Cell Host & Microbe, 26, 163–172. 10.1016/j.chom.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Peng K, Movahedi M, Dunn J, Walker H, Liang Y-K et al. (2015) Elevated CO2-induced responses in stomata require ABA and ABA signaling. Current Biology, 25, 2709–2716. 10.1016/j.cub.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Koo AJK, Gao X, Jayanty S, Thines B, Jones AD et al. (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiology, 146, 952–964. 10.1104/pp.107.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L, Kang J & Chen S (2020) Targeted metabolomics of plant hormones and redox metabolites in stomatal immunity. In: Champion A & Laplaze L (Eds.) Jasmonate in plant biology: methods and protocols. Springer, pp. 79–92. 10.1007/978-1-0716-0142-6_6. [DOI] [PubMed] [Google Scholar]
- Devoto A, Ellis C, Magusin A, Chang H-S, Chilcott C, Zhu T et al. (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Molecular Biology, 58, 497–513. 10.1007/s11103-005-7306-5. [DOI] [PubMed] [Google Scholar]
- Ding Y, Sun T, Ao K, Peng Y, Zhang Y, Li X et al. (2018) Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell, 173, 1454–1467.e15. 10.1016/j.cell.2018.03.044. [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I & Turner JG (2002) Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. MPMI, 15, 1025–1030. 10.1094/MPMI.2002.15.10.1025. [DOI] [PubMed] [Google Scholar]
- Flüutsch S, Nigro A, Conci F, Fajkus J, Thalmann M, Trtílek M et al. (2020) Glucose uptake to guard cells via STP transporters provides carbon sources for stomatal opening and plant growth. EMBO Reports, 21, e49719. 10.15252/embr.201949719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcat S, Bennett MH, Mansfield JW & Grant MR (2008) A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods, 4, 16. 10.1186/1746-4811-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S et al. (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proceedings of the National Academy of Sciences, 107, 8023–8028. 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proceedings of the National Academy of Sciences, 106, 21425–21430. 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S, Misra BB, de Armas E, Huhman DV, Alborn HT, Sumner LW et al. (2016) Jasmonate-mediated stomatal closure under elevated CO2 revealed by time-resolved metabolomics. The Plant Journal, 88, 947–962. 10.1111/tpj.13296. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW et al. (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. The Plant Cell, 24, 2483–2496. 10.1105/tpc.112.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havko NE, Das MR, McClain AM, Kapali G, Sharkey TD & Howe GA (2020) Insect herbivory antagonizes leaf cooling responses to elevated temperature in tomato. Proceedings of the National Academy of Sciences, 117, 2211–2217. 10.1073/pnas.1913885117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R & Marten I (1993) Malate-induced feedback regulation of plasma membrane anion channels could provide a CO2 sensor to guard cells. EMBO Journal, 12, 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hõrak H, Kollist H & Merilo E (2017) Fern stomatal responses to ABA and CO2 depend on species and growth conditions. Plant Physiology, 10.1104/pp.17.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC & Murata Y (2011) Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiology, 156, 430–438. 10.1104/pp.111.172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P-K, Takahashi Y, Munemasa S, Merilo E, Laanemets K, Waadt R et al. (2018) Abscisic acid-independent stomatal CO2 signal transduction pathway and convergence of CO2 and ABA signaling downstream of OST1 kinase. Proceedings of the National Academy of Sciences, 115, E9971–E9980. 10.1073/pnas.1809204115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Rappel W-J, Occhipinti R, Ries A, Böhmer M, You L et al. (2015) Distinct cellular locations of carbonic anhydrases mediate carbon dioxide control of stomatal movements. Plant Physiology, 169, 1168–1178. 10.1104/pp.15.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z et al. (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. The Plant Cell, 24, 2546–2561. 10.1105/tpc.112.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalakas P, Yarmolinsky D, Kollist H & Brosché M (2017) Isolation of guard-cell enriched tissue for RNA extraction. Bio-Protocol, 7, 10.21769/BioProtoc.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W & Zhang J (2008) Stomatal movements and long-distance signaling in plants. Plant Signaling & Behavior, 3, 772–777. 10.4161/psb.3.10.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangasjärvi J, Jaspers P & Kollist H (2005) Signalling and cell death in ozone-exposed plants. Plant, Cell & Environment, 28, 1021–1036. 10.1111/j.1365-3040.2005.01325.x. [DOI] [Google Scholar]
- Katsir L, Chung HS, Koo AJK & Howe GA (2008a) Jasmonate signaling: a conserved mechanism of hormone sensing. Current Opinion in Plant Biology, 11, 428–435. 10.1016/j.pbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY & Howe GA (2008b) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proceedings of the National Academy of Sciences USA, 105, 7100–7105. 10.1073/pnas.0802332105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuta S, Masuda G, Bak H, Shinozawa A, Kamiyama Y, Umezawa T et al. (2020) Arabidopsis Raf-like kinases act as positive regulators of subclass III SnRK2 in osmostress signaling. The Plant Journal, 103, 634–644. 10.1111/tpj.14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaurilind E & Brosché M (2017) Stress marker signatures in lesion mimic single and double mutants identify a crucial leaf age-dependent salicylic acid related defense signal. PLoS One, 12, e0170532. 10.1371/journal.pone.0170532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon MAR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y et al. (2010) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant, Cell & Environment, 34, 434–443. 10.1111/j.1365-3040.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- Kim K-C, Lai Z, Fan B & Chen Z (2008) Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. The Plant Cell, 20, 2357–2371. 10.1105/tpc.107.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-H, Böhmer M, Hu H, Nishimura N & Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology, 61, 561–591. 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M & Roelfsema MRG (2014) Closing gaps: linking elements that control stomatal movement. New Phytologist, 203, 44–62. 10.1111/nph.12832. [DOI] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Rämma H, Hüve K et al. (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiologia Plantarum, 129, 796–803. 10.1111/j.1399-3054.2006.00851.x. [DOI] [Google Scholar]
- Kwak JM, Mori IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL et al. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. The EMBO Journal, 22, 2623–2633. 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Simkin AJ, Kelly G & Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytologist, 203, 1064–1081. 10.1111/nph.12945. [DOI] [PubMed] [Google Scholar]
- Lee M, Choi Y, Burla B, Kim Y-Y, Jeon B, Maeshima M et al. (2008) The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nature Cell Biology, 10, 1217–1223. 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB & Luan S (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proceedings of the National Academy of Sciences of the United States of America, 106, 21419–21424. 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME et al. (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell, 16, 126–143. 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VF, dos Anjos L, Medeiros DB, Cândido-Sobrinho SA, Souza LP, Gago J et al. (2019) The sucrose-to-malate ratio correlates with the faster CO2 and light stomatal responses of angiosperms compared to ferns. New Phytologist, 223, 1873–1887. 10.1111/nph.15927. [DOI] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ & Coaker G (2009) RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biology, 7, 10.1371/journal.pbio.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sánchez-Serrano JJ & Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. The Plant Cell, 16, 1938–1950. 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A et al. (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science, 324, 1064–1068. 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- Marten I, Lohse G & Hedrich R (1991) Plant growth hormones control voltage-dependent activity of anion channels in plasma membrane of guard cells. Nature, 353, 758–762. 10.1038/353758a0. [DOI] [Google Scholar]
- Maruri-López I, Aviles-Baltazar NY, Buchala A & Serrano M (2019) Intra and extracellular journey of the phytohormone salicylic acid. Frontiers in Plant Science, 10, 10.3389/fpls.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC & Brodribb TJ (2016) Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant, Cell & Environment, 39, 485–491. 10.1111/pce.12633. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K & He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell, 126, 969–980. 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, Tulva I et al. (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiology, 162, 1652–1668. 10.1104/pp.113.220608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Yarmolinsky D, Jalakas P, Parik H, Tulva I, Rasulov B et al. (2018) Stomatal VPD response: there is more to the story than ABA. Plant Physiology, 176, 851–864. 10.1104/pp.17.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS et al. (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. The Plant Journal, 63, 1054–1062. 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM et al. (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. The Plant Journal, 73, 91–104. 10.1111/tpj.12014. [DOI] [PubMed] [Google Scholar]
- Miura K & Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Frontiers in Plant Science, 5, 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldau H, Vahisalu T & Kollist H (2011) Rapid stomatal closure triggered by a short ozone pulse is followed by reopening to overshooting values. Plant Signaling & Behavior, 6, 311–313. 10.4161/psb.6.2.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montillet J-L, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLOS Biology, 11, e1001513. 10.1371/journal.pbio.1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Pinontoan R, Kawano T & Muto S (2001) Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant and Cell Physiology, 42, 1383–1388. [DOI] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B & Schroeder JI (2015) Mechanisms of abscisic acid-mediated control of stomatal aperture. Current Opinion in Plant Biology, 28, 154–162. 10.1016/j.pbi.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y & Murata Y (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiology, 143, 1398–1407. 10.1104/pp.106.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Mori IC & Munemasa S (2015) Diverse stomatal signaling and the signal integration mechanism. Annual Review of Plant Biology, 66, 369–392. 10.1146/annurev-arplant-043014-114707. [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M et al. (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature, 452, 483–486. 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- Okada M, Ito S, Matsubara A, Iwakura I, Egoshi S & Ueda M (2009) Total syntheses of coronatines by exo-selective Diels-Alder reaction and their biological activities on stomatal opening. Organic & Biomolecular Chemistry, 7, 3065–3073. 10.1039/B905159G. [DOI] [Google Scholar]
- Pan X, Welti R & Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nature Protocols, 5, 986–992. 10.1038/nprot.2010.37. [DOI] [PubMed] [Google Scholar]
- Panchal S, Chitrakar R, Thompson BK, Obulareddy N, Roy D, Hambright WS et al. (2016) Regulation of stomatal defense by air relative humidity. Plant Physiology, 172, 2021–2032. 10.1104/pp.16.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S & Melotto M (2017) Stomate-based defense and environmental cues. Plant Signaling & Behavior, 12, 10.1080/15592324.2017.1362517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y et al. (2009) Abscisic acid inhibits PP2Cs via the PYR/PYL family of ABA-binding START proteins. Science, 324, 1068–1071. 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson KJ (1985) A simple method for determining the boundary layer resistance in leaf cuvettes. Plant, Cell & Environment, 8, 223–226. 10.1111/1365-3040.ep11604618. [DOI] [Google Scholar]
- Prodhan MY, Munemasa S, Nahar M-N-E-N, Nakamura Y & Murata Y (2018) Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+ /CPK-dependent pathway. Plant Physiology, 178, 441–450. 10.1104/pp.18.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle HH & Hedrich R (2002) CO2 provides an intermediate link in the red light response of guard cells. The Plant Journal, 32, 65–75. 10.1046/j.1365-313X.2002.01403.x. [DOI] [PubMed] [Google Scholar]
- Santelia D & Lawson T (2016) Rethinking guard cell metabolism. Plant Physiology, 172, 1371–1392. 10.1104/pp.16.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko T, Kolla VA, Wang C-Q, Nasafi Z, Hicks DR, Phadungchob B et al. (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiology, 164, 1151–1160. 10.1104/pp.113.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawinski K, Mersmann S, Robatzek S & Böhmer M (2013) Guarding the green: pathways to stomatal immunity. Molecular Plant-Microbe Interactions®, 26, 626–632. 10.1094/MPMI-12-12-0288-CR. [DOI] [PubMed] [Google Scholar]
- Seo HS, Song JT, Cheong J-J, Lee Y-H, Lee Y-W, Hwang I et al. (2001) Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proceedings of the National Academy of Sciences USA, 98, 4788–4793. 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K-I, Doi M, Assmann SM & Kinoshita T (2007) Light regulation of stomatal movement. Annual Review of Plant Biology, 58, 219–247. 10.1146/annurev.arplant.57.032905.105434. [DOI] [PubMed] [Google Scholar]
- Sierla M, Hõrak H, Overmyer K, Waszczak C, Yarmolinsky D, Maierhofer T et al. (2018) The receptor-like pseudokinase GHR1 Is required for stomatal closure. The Plant Cell, 30, 2813–2837. 10.1105/tpc.18.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma F, Takahashi F, Suzuki T, Shinozaki K & Yamaguchi-Shinozaki K (2020) Plant Raf-like kinases regulate the mRNA population upstream of ABA-unresponsive SnRK2 kinases under drought stress. Nature Communications, 11, 1373. 10.1038/s41467-020-15239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Qi T, Wasternack C & Xie D (2014) Jasmonate signaling and crosstalk with gibberellin and ethylene. Current Opinion in Plant Biology, 21, 112–119. 10.1016/j.pbi.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM & Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology, 134, 1536–1545. 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmilch FC, Schultz J, Hedrich R & Roelfsema MRG (2019) Acquiring control: the evolution of stomatal signalling pathways. Trends in Plant Science, 24, 342–351. 10.1016/j.tplants.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Zhang J, Hsu P-K, Ceciliato PHO, Zhang L.i., Dubeaux G et al. (2020) MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nature Communications, 11, 12. 10.1038/s41467-019-13875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Flors V & Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends in Plant Science, 14, 310–317. 10.1016/j.tplants.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Toum L, Torres PS, Gallego SM, Benavídes MP, Vojnov AA & Gudesblat GE (2016) Coronatine inhibits stomatal closure through guard cell-specific inhibition of NADPH oxidase-dependent ROS production. Frontiers in Plant Science, 7, 10.3389/fpls.2016.01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp MA, De Souza GD, Rodrigues-Filho E, Boland W & Mithöfer A (2014) Validated method for phytohormone quantification in plants. Frontiers in Plant Science, 5, 10.3389/fpls.2014.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K et al. (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proceedings of the National Academy of Sciences, 106, 17588–17593. 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaahtera L, Brosché M, Wrzaczek M & Kangasjärvi J (2013) Specificity in ROS signaling and transcript signatures. Antioxidants & Redox Signaling, 21, 1422–1441. 10.1089/ars.2013.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang Y-F, Nishimura N, Chan W-Y, Valerio G et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature, 452, 487–491. 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H et al. (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. The Plant Journal, 62, 442–453. 10.1111/j.1365-313X.2010.04159.x. [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Vlad F et al. (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. The Plant Cell, 21, 3170–3184. 10.1105/tpc.109.069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S & Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta, 153, 376–387. 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- von Malek B, van der Graaff E, Schneitz K & Keller B (2002) The Arabidopsis male-sterile mutant dde2–2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta, 216, 187–192. 10.1007/s00425-002-0906-2. [DOI] [PubMed] [Google Scholar]
- Wang H-Q, Sun L-P, Wang L-X, Fang X-W, Li Z-Q, Zhang F-F et al. (2020) Ethylene mediates salicylic-acid-induced stomatal closure by controlling reactive oxygen species and nitric oxide production in Arabidopsis. Plant Science, 294, 110464. 10.1016/j.plantsci.2020.110464. [DOI] [PubMed] [Google Scholar]
- Webb A & Hetherington AM (1997) Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiology, 114, 1557–1560. 10.1104/pp.114.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G & Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature, 414, 562–565. 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Xie X, Wang Y, Williamson L, Holroyd GH, Tagliavia C, Murchie E et al. (2006) The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Current Biology, 16, 882–887. 10.1016/j.cub.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Xu E, Vaahtera L & Brosché M (2015) Roles of defense hormones in the regulation of ozone-induced changes in gene expression and cell death. Molecular Plant, 8, 1776–1794. 10.1016/j.molp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H & Schroeder JI (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO Journal, 30, 1645–1658. 10.1038/emboj.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaaran A, Negin B & Moshelion M (2019) Role of guard-cell ABA in determining steady-state stomatal aperture and prompt vapor-pressure-deficit response. Plant Science, 281, 31–40. 10.1016/j.plantsci.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Yan S, McLamore ES, Dong S, Gao H, Taguchi M, Wang N et al. (2015) The role of plasma membrane H+-ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. The Plant Journal, 83, 638–649. 10.1111/tpj.12915. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T et al. (2008) Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. The Plant Cell, 20, 1678–1692. 10.1105/tpc.107.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Adachi Y, Nakamura Y, Munemasa S, Mori IC & Murata Y (2016) Involvement of OST1 protein kinase and PYR/PYL/RCAR receptors in methyl jasmonate-induced stomatal closure in Arabidopsis guard cells. Plant and Cell Physiology, 57, 1779–1790. 10.1093/pcp/pcw102. [DOI] [PubMed] [Google Scholar]
- Yokoyama G, Yasutake D, Tanizaki T & Kitano M (2019) Leaf wetting mitigates midday depression of photosynthesis in tomato plants. Photosynthetica, 57, 740–747. 10.32615/ps.2019.088. [DOI] [Google Scholar]
- Zeng W & He SY (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiology, 153, 1188–1198. 10.1104/pp.110.157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Takahashi Y, Hsu P-K, Kollist H, Merilo E, Krysan PJ & Schroeder JI (2020) FRET kinase sensor development reveals SnRK2/OST1 activation by ABA but not by MeJA and high CO2 during stomatal closure. eLife, 9, 10.7554/eLife.56351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wu Y, Yang Y, Du M, Zhang X, Guo Y et al. (2015) An Arabidopsis plasma membrane proton ATPase modulates JA signaling and is exploited by the Pseudomonas syringae effector protein AvrB for stomatal invasion. The Plant Cell, 27, 2032–2041. 10.1105/tpc.15.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Changes in stomatal conductance driven by various CO2 concentrations in Arabidopsis JA- and SA-biosynthesis and signaling mutants.
Figure S2. Changes in stomatal conductance induced by elevated (a) or reduced (b) CO2 concentrations in leaves of the jai1–1 tomato mutant and the corresponding wild type (Castlemart).
Figure S3. Stomatal responsiveness to a period of darkness in single (a–d, g–j) and double (e, f, k, l) Arabidopsis mutants with disturbed JA and SA biosynthesis and signaling.
Figure S4. Characterization of stomatal movements induced by darkness and re-illumination in JA- and SA-biosynthesis and -signaling mutants.
Figure S5. Reduction of stomatal conductance under elevated vapor pressure deficit in JA- and SA-biosynthesis and -signaling mutants.
Figure S6. Changes in stomatal conductance of JA- and SA-biosynthesis and -signaling mutants in response to elevated vapor pressure deficit.
Figure S7. ABA-induced reduction of stomatal conductance in JA-and SA-biosynthesis and -signaling mutants.
Figure S8. Ozone-induced changes in stomatal conductance of Arabidopsis plants defective in JA and SA biosynthesis and signaling.
Figure S9. Plant responses to ABA, MeJA and SA sprays.
Figure S10. Changes in stomatal conductance after spraying with coronatine (COR).
Table S1. Primers used in this work.
Data S1. ANOVA tables and results of Student’s t-tests and post hoc tests.
Data Availability Statement
The results of gas-exchange measurements and stomatal assays included in this study are available upon request from the corresponding author.