Abstract
Objective:
In the present study we investigate the therapeutic potential of a small molecule inhibitor of PAI-1, TM5441, in reversing diet-induced obesity in mice.
Methods:
Wild-type C57BL/6J mice were fed a high-fat, high-sugar diet (HFHS) for 8 weeks to induce obesity at which time TM5441 was added to the diet for an additional 8 weeks. To determine the efficacy of PAI-1 inhibition in conjunction with dietary modification, mice were fed a HFHS diet for 8 weeks to induce obesity and were then switched to a low-fat diet with or without TM5441 for an additional 2 to 8 weeks.
Results:
Obese mice showed weight reduction and significant improvement in hepatic steatosis when TM5441 was added to the HFHS diet. Obese mice that were treated with TM5441 in conjunction with dietary modification showed enhanced weight loss and more rapid reversal of hepatic steatosis compared to obese mice treated with dietary modification alone. The enhanced weight loss among mice treated with TM5441 was associated with increased adipose tissue expression of adipose triglyceride lipase (ATGL), phosphorylated hormone sensitive lipase, and phosphorylated perilipin 1, and induction of adipose tissue lipolysis.
Conclusion:
Pharmacologic PAI-1 inhibition stimulates adipose tissue lipolysis and enhances weight loss in obese mice.
Keywords: obesity, nonalcoholic fatty liver disease, steatohepatitis, TM5441, metabolic syndrome
Introduction
Obesity has become a major public health crisis in the United States. It is now estimated that over two-thirds of the US population have overweight or obesity, and it has been projected that by 2030 nearly half of adults in the US will have obesity (1, 2). Unfortunately there is no truly effective strategy in place to reverse this trend. The prevalence of nonalcoholic fatty liver disease (NAFLD), a major complication of obesity, is also rapidly rising as a consequence of the obesity epidemic (3, 4). It is estimated that up to 30% of the US population has hepatic steatosis (5). While only a small proportion of these patients progress to develop clinically significant liver disease, it is now estimated that over 4 million people in the United States have advanced fibrosis or cirrhosis secondary to NAFLD (5). Currently there are limited pharmacologic therapies for NAFLD owing, in part, to an incomplete understanding of the molecular pathogenesis of this disease.
Plasminogen activator inhibitor 1 (PAI-1) is increasingly implicated as a mediator of metabolic diseases such as obesity and NAFLD, a function that is distinct from its classic role in the fibrinolytic cascade (6, 7, 8, 9, 10, 11). Over a decade ago, it was discovered that mice bearing a global deletion of Pai-1 are protected from high-fat diet-induced obesity and resultant insulin resistance (12, 13). Furthermore, numerous studies have linked elevated circulating PAI-1 levels to human obesity and NAFLD (7, 8, 9, 14, 15, 16). We and others have shown that targeted inhibition of PAI-1 attenuates the development of obesity and NAFLD in mice (17, 18, 19). While it is becoming clear that PAI-1 inhibition may be an effective intervention to prevent the development of obesity and its sequelae, a pharmacologic therapy for human metabolic disease should ideally have efficacy in existing disease. Therefore, in the present study we test the efficacy of a small molecule inhibitor of PAI-1, TM5441, in reversing obesity in mice.
Methods
Animals and Diets
Wild-type C57BL/6J male mice (n=16) (Jackson Laboratories, Bar Harbor, ME) were fed a high-fat, high-cholesterol, high-sugar diet (HFHS) for 8 weeks to induce obesity and NAFLD. The HFHS diet is composed of 40% of energy as fat (milk fat, 12% saturated) with 2% cholesterol (AIN-76 Western Diet, Test Diet) and drinking water supplemented with 42 g/l of 55% fructose/45% glucose by weight. After induction of obesity, a small molecule inhibitor of PAI-1, TM5441, was added to the HFHS diet in half of the mice (n=8). Mice were continued on a HFHS supplemented with a PAI-1 inhibitor or a HFHS diet alone for an additional 8 weeks. TM5441 was mixed into the diet to achieve a final dosing of 20 mg/kg/day. Details regarding the development and validation of TM5441 and related chemical analogues have been previously reported (20, 21). To determine the effect of a PAI-1 inhibitor as an adjunctive therapy to dietary modification, wild-type C57BL/6J male mice were fed a HFHS diet for 8 weeks to induce obesity (n=36) and were subsequently switched to either a low-fat diet (LFD) or a low-fat diet supplemented with TM5441 (LFD+TM) for an additional 2, 4, or 8 weeks. As part of the dietary intervention, mice fed a LFD or LFD+TM were switched to sugar-free standard drinking water. Mice were maintained in social housing (up to 5 mice per cage) receiving environmental enrichment in specific-pathogen-free (SPF) barrier facilities with 14/10-hour light/dark cycling and were given free access to food and water. At the end of the feeding protocols, mice were euthanized by CO2 inhalation. Blood was collected by cardiac puncture and centrifuged to collect the plasma. The livers and gonadal and inguinal fat pad masses were rapidly excised, weighed, and flushed with ice-cold saline. An aliquot was fixed in 10% formalin for histologic analysis. The remainder of the tissue was sectioned and snap-frozen in liquid nitrogen. All animal protocols were approved by the Northwestern University Institutional Animal Care and Use Committee (IACUC).
Histologic analysis
Liver sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome stain and adipose sections were stained with H&E and F4/80 at the Northwestern University Mouse Histology and Phenotyping Laboratory (MHPL, Chicago, IL). Liver sections were blindly scored for grade of steatosis using the following criteria. Steatosis: grade 0 = steatosis <5% of liver parenchyma, grade 1 = steatosis 5-25% of liver parenchyma, grade 2 = steatosis 26-50% of liver parenchyma, grade 3 = steatosis 51-75% of liver parenchyma, grade 4 = steatosis >75% of liver parenchyma.
Hepatic Lipid Analysis
Hepatic triglyceride levels in liver homogenate were measured using an Infinity spectrophotometric assay per the manufacturer’s protocol (Thermo Electron Corporation, Melbourne, Australia).
Adipose tissue lipolysis and lipase activity assays
Lipase activity in freshly isolated adipose tissue was measured using a Lipase Activity Kit (Sigma-Aldrich) per manufacturer’s protocol. Isoproterenol stimulated glycerol release from freshly isolated adipose tissue was performed using a High-Sensitivity Lipolysis Assay Kit (Sigma-Aldrich) per manufacturer’s protocol. Plasma free fatty acid level was measured used a Free Fatty Acid Quantitation Kit (Sigma-Aldrich).
Analysis of Gene and Protein Expression
Total RNA from frozen liver samples was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA was used for reverse transcription PCR using a qScript cDNA Synthesis Kit (Quanta BioSciences, Gaithersburg, MD). Real-time quantitative PCR was performed using Quantitect SYBR Green PCR Mastermix (Qiagen, Valencia, CA) along with primers specific for the gene of interest. GAPDH was employed as a housekeeping gene. Amplification was performed on an ABI 7300 sequence detector (Applied Biosystems, Foster City, CA). Gene expression was calculated relative to respective age and sex matched controls using the comparative threshold cycle method. Total protein was isolated from frozen liver and adipose samples and Western blotting was performed as previously described.(22, 23) Protein detection was performed using polyclonal antibodies to adipose tissue triglyceride lipase (ProteinTech, Rosemont, IL), total and phosphorylated hormone sensitive lipase (Cell Signaling Technology, Danvers, MA), phosphorylated perilipin 1 (Valasciences, San Diego, CA), total perilipin 1 (Cell Signaling Technology), and GAPDH (ProteinTech). Bound antibody was detected using goat anti-rabbit (Cell Signaling Technology) or goat anti-mouse (Protein Tech) polyclonal HRP antibody and developed using ECL Western Blotting Substrate (Cell Signaling Technology). Representative western blots of pooled samples are shown. Density was performed on blots run in singlet using ImageJ software (imagej.nih.gov/ij/).
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Comparisons between groups were performed using a two-tailed Student’s t-test analysis, one-way ANOVA, or two-way ANOVA with repeated measures. p<0.05 was considered statistically significant.
Results
PAI-1 inhibition attenuates weight gain and NAFLD in obese mice
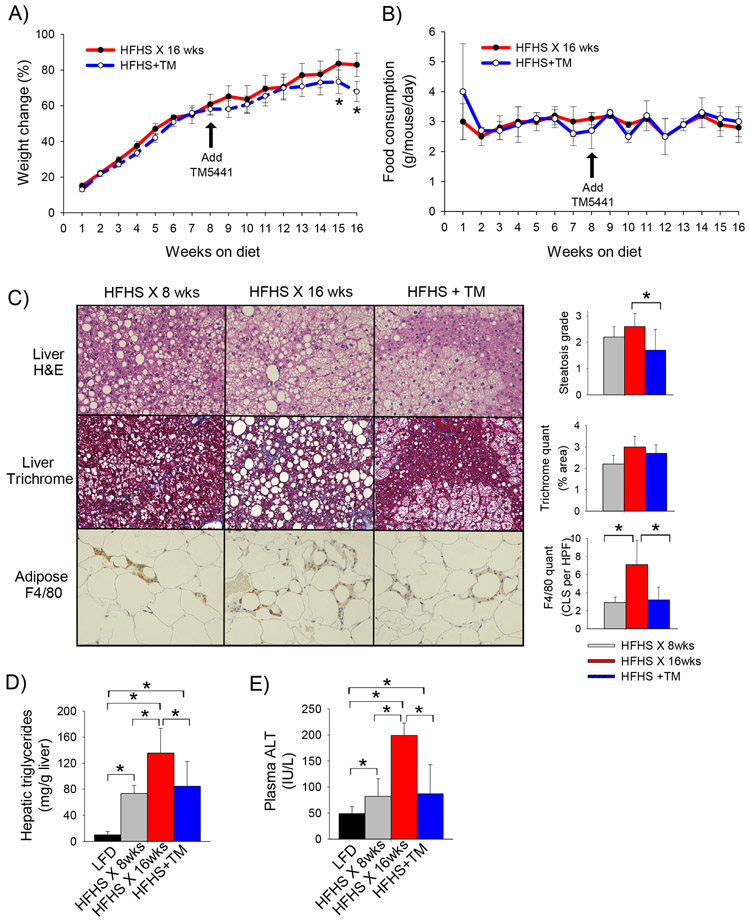
We have recently shown that TM5441 attenuates the development of diet-induced obesity and NAFLD mice (18). We now examined the role of PAI-1-inhibition in treating existing diet-induced obesity in mice. Wild-type C57BL/6J mice were fed a HFHS diet for 8 weeks at which time TM5441 was initiated in half of the mice (n=10). Mice were continued on a HFHS diet supplemented with TM5441 (HFHS+TM) or a HFHS alone for an additional 8 weeks. Mice fed the HFHS diet alone showed steady weight gain over the course of 16 weeks (Figure 1A). The addition of TM5441 at week 8 resulted in modestly attenuated weight gain that reached statistical significance at weeks 15 and 16. There was no difference in food consumption to explain the divergence in body weight (Figure 1B).
Figure 1: TM5441 attenuates diet-induced obesity and hepatic steatosis in mice.
A) Body weight change (%), B) average daily food consumption (g/mouse/day), C) representative H&E and trichrome-stained liver sections and F4/80-stained adipose tissue sections with associated quantification, D) hepatic triglyceride content (mg trig per gram liver), and E) plasma ALT level (U/L) in C57BL/6J mice fed a HFHS diet for 16 weeks or a HFHS diet for 8 weeks followed by a HFHS diet supplemented with TM5441 for an additional 8 weeks (HFHS+TM). Values expressed as mean (n=8) ± SD, *p<0.05.
Mice fed the HFHS diet for 16 weeks showed grade 2-3 hepatic steatosis (2.6 ± 0.5) and an elevated hepatic triglyceride content (Figure 1 C,D). The addition of TM5441 at week 8 resulted in significant improvement in hepatic steatosis (1.7 ± 0.8) and reduction in hepatic triglyceride content by week 16. Plasma ALT, a marker of hepatic inflammation, was also markedly attenuated in response to PAI-1 inhibition (Figure 1E). The addition of TM5441 at week 8 did not, however, significantly reverse NASH-related hepatic fibrosis (Figure 1C).
Adipose tissue inflammation is increasingly recognized as the critical pathophysiologic process driving the complications of obesity, such as NAFLD and insulin resistance. Adipose tissue macrophages, in particular, are postulated to play a critical role in promoting the chronic inflammatory state characteristic of the obese phenotype. Mice fed a HFHS diet demonstrated progressive accumulation of adipose tissue macrophages and formation of “crown-like structures” (CLS) from 8 to 16 weeks of HFHS feeding (Figure 1C). The number of CLS’s was significantly reduced in mice treated with TM5441, indicating that PAI-1 inhibition attenuates adipose tissue inflammation.
PAI-1 inhibition enhances weight loss in obese mice treated with dietary modification
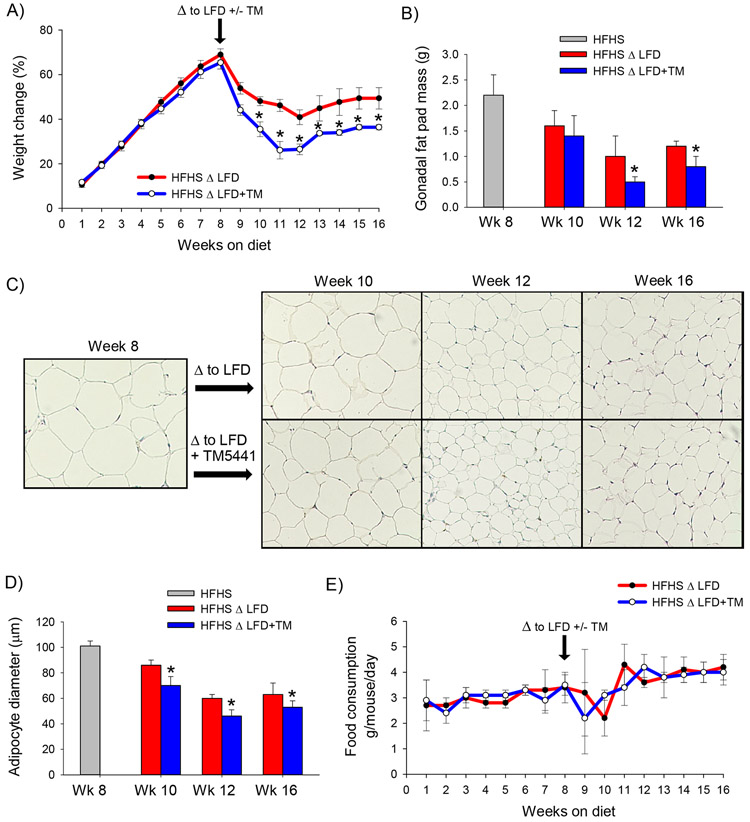
Lifestyle modification is the first-line therapy for obesity and NAFLD, and in clinical practice, a drug targeting obesity should ideally be used in conjunction with dietary modification. We, therefore, tested the efficacy of a PAI-1 inhibitor as an adjunctive therapy to dietary modification in mice. Mice were fed a HFHS diet for 8 weeks to induce the obesity and were subsequently switched to either a low-fat diet (LFD) or a low-fat diet supplemented with TM5441 (LFD+TM) for an additional 2, 4 or 8 weeks (n=6 for per diet at each time point). Switching obese mice to a LFD resulted in rapid weight loss from weeks 8 through 12 at which time a new steady baseline was achieved (Figure 2A). Obese mice that were switched to a low-fat diet supplemented with TM5441 demonstrated more rapid and greater absolute weight loss compared to mice switched to a LFD alone. Weight loss among mice fed a LFD was associated with a significant reduction in gonadal fat pad mass, an effect that was enhanced by TM5441 (Figure 2B). Consistent with a reduction in fat pad mass, mice treated with TM5441 showed a greater reduction in adipocyte size compared to mice fed a LFD alone (Figure 2 C,D). There was no difference in food intake between LFD and LFD+TM groups to explain the divergence in weight loss (Figure 2E).
Figure 2: TM5441 enhances weight loss and rapidly reverses hepatic steatosis in conjunction with dietary modification in mice.
A) Weight change (%), B) Gonadal fat pad mass (g), C) H&E stained adipose tissue sections, D) average adipocyte diameter (μm), and E) average daily food consumption (g/mouse/day) in C57BL/6J mice fed a high-fat, high-sugar diet for 8 weeks (HFHS) then either a low-fat diet (LFD) or low-fat diet supplemented with TM5441 (LFD+TM) for an additional 2, 4, or 8 weeks (i.e. week 10, 12, or 16, respectively.) Values are expressed as mean (n=6) ± SD, * p<0.05. Additionally, there was a statistically significant difference (p<0.05) in gonadal fat pad mass and adipocyte diameter (panels B,D) among mice fed a HFHS for 8 weeks compared to all other cohorts.
PAI-1 inhibition promotes adipose tissue lipolysis
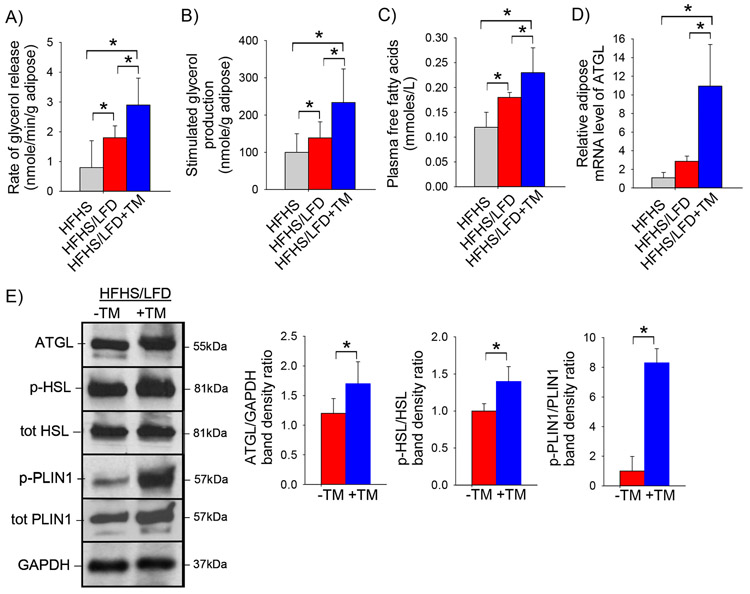
The caloric reduction associated with switching mice from a high-fat to low-fat diet is a potent stimulus of adipose tissue lipolysis. We therefore considered whether the enhanced weight loss among mice treated with LFD+TM is associated with enhanced induction of adipose tissue lipolysis. As expected, experimental markers of adipose tissue lipolysis were induced by switching from a HFHS diet to a LFD (Figure 3). Importantly, the lipase activity of gonadal adipose tissue was greater among obese mice treated with LFD+TM compared to LFD alone (Figure 3A). Similarly, isoproterenol-stimulated glycerol release was enhanced by the addition of TM5441 to the LFD (Figure 3B). Consistent with enhanced lipolysis, the circulating level of free fatty acids was increased among mice treated with TM5441 (Figure 3C). The major enzyme controlling adipose tissue lipolysis is adipose triglyceride lipase (ATGL) (24). Obese mice treated with a LFD+TM showed greater induction of adipose tissue Atgl mRNA and ATGL protein compared to obese mice treated with LFD alone (Figure 3D,E). Similarly, mice treated with TM5441 showed enhanced activation of hormone sensitive lipase (HSL) in response to switching to a low-fat diet (Figure 3E). Induction of ATGL activity is mediated, in part, by phosphorylation of perilipin 1 (PLIN1), a lipid droplet protein in adipocytes (25, 26). Treatment with TM5441 resulted in massive induction of phosphorylated PLIN1 (Figure 3E).
Figure 3: PAI-1 inhibition promotes adipose tissue lipolysis.
A) Rate of glycerol release from adipose tissue (nmole/min/g), B) isoproterenol-stimulated adipose tissue glycerol production (nmole/g adipose), C) plasma total free fatty acid concentration (nmole/L), D) relative adipose tissue mRNA expression of Atgl, and E) representative western blot of ATGL, phosphorylated and total HSL and phosphorylated and total PLIN1 protein level in pooled adipose tissue protein samples with corresponding densitometric analysis in C57BL/6J mice fed a high-fat, high-sugar diet for 8 weeks (HFHS) then either a low-fat diet (LFD) or low-fat diet supplemented with TM5441 (LFD+TM) for an additional 2 weeks. Values are expressed as mean (n=6) ± SD, * p<0.05.
PAI-1 inhibition rapidly reverses hepatic steatosis in obese mice treated with dietary modification
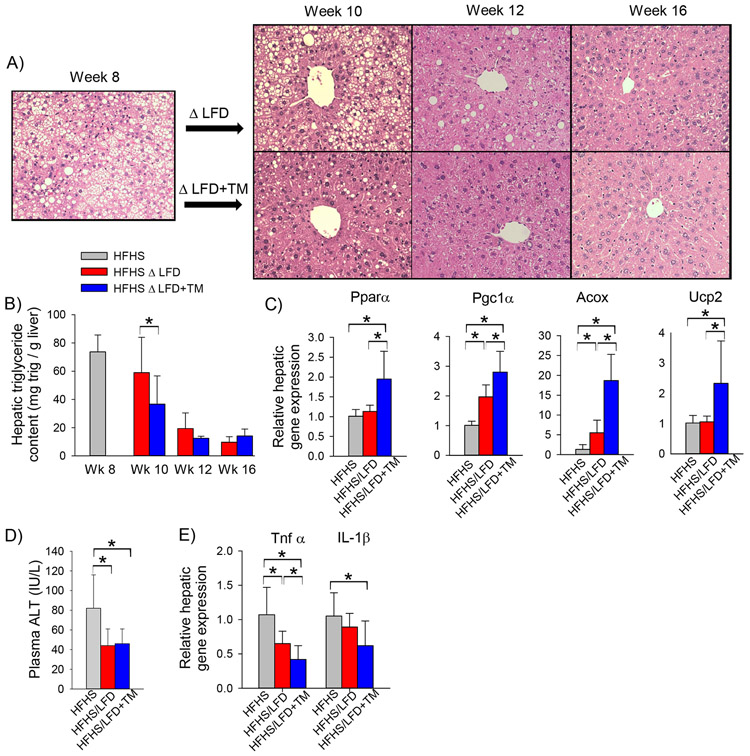
We have previously shown that PAI-1 inhibition protects against high-fat diet induced hepatic steatosis (18); however, enhanced adipose tissue lipolysis resulting from rapid weight can lead to ectopic fat deposition, raising the possibility that PAI-1 inhibition may worsen hepatic steatosis in a model of rapid weight loss. We therefore examined the effect of PAI-1 inhibition on the hepatic steatosis in obese mice subjected to dietary modification. Obese mice switched to a LFD showed persistent, yet modestly improving, hepatic steatosis at week 10 (week 2 of dietary intervention) (Figure 4A). Mice switched to a LFD supplemented with a PAI-1 inhibitor showed markedly improved hepatic steatosis at week 10 compared to mice treated with dietary intervention alone. Mirroring the histologic findings, the hepatic triglyceride content was lower in mice treated with a LFD+TM compared to mice treated with LFD alone at week 10 (Figure 4B). At week 12 (week 4 of dietary intervention), scant residual hepatic steatosis was present in the LFD cohort, whereas mice fed a LFD supplemented with TM5441 showed complete resolution of hepatic steatosis (Figure 4A). By week 16, hepatic steatosis had resolved histologically and hepatic triglyceride content had normalized in all mice regardless of the intervention (Figure 4A,B). To explain the rapid reversal of hepatic steatosis, the expression of genes regulating hepatic fatty acid oxidation was measured two weeks after switching mice to a LFD (Figure 4C). Mice fed a LFD+TM showed enhanced induction of key fatty acid oxidation genes, consistent with a significant reduction in hepatic steatosis at this time point.
Figure 4: TM5441 accelerates the reversal of NAFLD in conjunction with dietary modification in mice.
A) Representative H&E stained liver section, B) hepatic triglyceride content (mg trig per gram liver) in C57BL/6J mice fed a high-fat, high-sugar diet for 8 weeks (HFHS) then either a low-fat diet (LFD) or low-fat diet supplemented with TM5441 (LFD+TM) for an additional 2, 4, or 8 weeks (i.e. week 10, 12, or 16, respectively), C) hepatic mRNA expression of fatty acid oxidation genes, D) Plasma ALT (IU/L) and E) hepatic mRNA expression of pro-inflammatory genes in C57BL/6J mice fed a HFHS diet for 8 weeks then either LFD or LFD+TM for an additional 2 weeks. Values are expressed as mean (n=6) ± SD, * p<0.05.
Obese mice showed rapid improvement in hepatic inflammatory markers after initiation of a low-fat diet. Plasma ALT had normalized by week 2 after switching to either a LFD or LFD+TM (Figure 4C). By week two of dietary intervention there was also suppressed hepatic expression of proinflammatory cytokines, Tnfα and IL-1β, an effect that was enhanced by TM5441 (Figure 4D). In summary, despite enhancing adipose tissue lipolysis, PAI-1 inhibition did not worsen hepatic steatosis but instead resulted in more rapid resolution of fatty liver disease in obese mice treated with dietary modification.
Discussion
PAI-1 is increasingly recognized as a mediator of metabolic disease, a function distinct from its classic role in the plasminogen activator (i.e. fibrinolytic) system. Although initially conceptualized as a treatment for human thrombotic disease, PAI-1 antagonism is emerging as a highly promising therapeutic strategy for the metabolic syndrome (17, 18, 27). In the present study, we demonstrate that PAI-1 inhibition is an effective strategy to treat existing diet-induced obesity in mice.
Lifestyle modification is the first-line treatment for obesity and any pharmacologic therapy for this condition should ideally serve as an adjunct to dietary intervention. Importantly, we demonstrate that PAI-1 inhibition not only attenuates diet-induced metabolic disease in mice but enhances the reversal of metabolic disease when used as an adjunctive therapy to dietary modification. Unfortunately, strict adherence to a low-fat diet can be challenging in clinical practice. We therefore also examined the effect of PAI-1 inhibition in mice allowed to continue consuming an obesogenic diet. Interestingly, supplementing an obesogenic diet with a PAI-1 inhibitor results in pronounced improvement in NAFLD despite only modest improvement in obesity. Although this may suggest a direct effect of PAI-1 inhibition on hepatic steatosis, the degree of observed weight loss among TM5441-treated mice may be sufficient to cause the observed reduction in steatosis. Moreover, in humans, moderate weight reduction (~10%) has been associated with reversal of hepatic steatosis and insulin resistance (28, 29, 30). As such, the observed improvement in NAFLD with PAI-1 inhibition may be entirely secondary to improvement in obesity.
While it is becoming increasing evident that disruption of PAI-1 protects against obesity, in part, via enhanced energy expenditure (12, 31, 32), in the present work we demonstrate that inhibition of PAI-1 also enhances adipose tissue lipolysis, thus identifying a critical interrelated mechanism by which targeting PAI-1 reverses obesity. Treatment with TM5441 resulted in profound induction of phosphorylated perilipin 1, a gatekeeper of adipose tissue lipolysis that enhances the activity of ATGL. Although we hypothesize that PAI-1 inhibition directly induces lipolysis via p-PLIN and ATGL, we acknowledge the possibility that the enhanced lipolysis observed in response to TM5441 may be a consequence of rapid weight loss. Additional studies are warranted to determine whether PAI-1 directly regulates PLIN1 and/or ATGL.
We have shown that PAI-1 inhibition promotes adipose tissue lipolysis and weight loss, however, enhanced adipose tissue lipolysis alone cannot explain an overall reduction in body fat. Instead, adipose tissue lipolysis must function in conjunction with enhanced fatty acid oxidation and increased energy expenditure to promote weight loss and prevent ectopic deposition of fatty acids released from lipolysis. Moreover, adipose tissue lipolysis is a tightly regulated process that has a complex interplay with obesity and its sequelae. While caloric restriction, a stimulus of lipolysis, promotes weight loss and improves metabolic parameters, the enhanced adipose tissue lipolysis associated with extreme or rapid weight loss can actually precipitate hepatic steatosis in the absence of compensatory upregulation of fatty acid oxidation. Importantly, PAI-1 inhibition in our model of caloric reduction did not aggravate hepatic steatosis, and, in fact, resulted in more rapid reversal of fatty liver disease associated with induction of hepatic fatty acid oxidation genes. Further studies are warranted to determine whether this beneficial effect may be replicated in human disease.
Obesity is an epidemic for which no truly effective pharmacologic therapy currently exists. The present work demonstrates the efficacy of PAI-1 inhibition in reversing diet-induced obesity and NAFLD in mice, and lays the foundation to study PAI-1 inhibition as a pharmacologic intervention in patients with obesity.
Study Importance.
What is already known?
Obesity is associated with elevated circulating levels of PAI-1
Small molecule inhibitors of PAI-1 have demonstrated efficacy in preventing diet-induced obesity in mice
What does this study add?
We now show that a PAI-1 inhibitor reverses existing obesity in mice
We identify adipose tissue lipolysis as a novel mechanism targeted by PAI-1 inhibition
How might these results change the direction of research or the focus of clinical practice?
These data provide the foundation to study this therapeutic strategy in human obesity.
Acknowledgments
Financial support: This work was supported by National Institutes of Health grant 1K08DK095992 (AH), a VA Merit Award BX003854-01 (AH), the Endocrine Fellows Foundation (JAL), and the Irving S. Cutter Endowment (DV). JAL was supported by the Ruth L. Kirschstein National Research Service Award T32 DK007169 from NIDDK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Disclosure: TM is an employee of RenaScience. JAL, TM, and DEV hold provisional patent number 47460-64 (NU2019-041-01). JAL is an employee and holds equity at Eli Lilly and Company.
References:
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307: 491–497. [DOI] [PubMed] [Google Scholar]
- 2.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med 2019;381: 2440–2450. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003;37: 1202–1219. [DOI] [PubMed] [Google Scholar]
- 5.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol 2017;112: 581–587. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost 2005;3: 1879–1883. [DOI] [PubMed] [Google Scholar]
- 7.De Taeye B, Smith LH, Vaughan DE. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol 2005;5: 149–154. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Scala L, Zenari L, Lippi G, Franchini M, et al. Plasma PAI-1 levels are increased in patients with nonalcoholic steatohepatitis. Diabetes Care 2007;30: e31–32. [DOI] [PubMed] [Google Scholar]
- 9.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 2008;138: 1452–1455. [DOI] [PubMed] [Google Scholar]
- 10.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11: 303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coudriet GM, Stoops J, Orr AV, Bhushan B, Koral K, Lee S, et al. A Noncanonical Role for Plasminogen Activator Inhibitor Type 1 in Obesity-Induced Diabetes. Am J Pathol 2019;189: 1413–1422. [DOI] [PubMed] [Google Scholar]
- 12.Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 2004;53: 336–346. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Chen L, Liu Z, Liu Y, Luo M, Chen N, et al. PAI-1 Exacerbates White Adipose Tissue Dysfunction and Metabolic Dysregulation in High Fat Diet-Induced Obesity. Front Pharmacol 2018;9: 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring) 2008;16: 1394–1399. [DOI] [PubMed] [Google Scholar]
- 15.Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, Hubens G, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2014;59: 121–129. [DOI] [PubMed] [Google Scholar]
- 16.Sookoian S, Castano GO, Burgueno AL, Rosselli MS, Gianotti TF, Mallardi P, et al. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis 2010;209: 585–591. [DOI] [PubMed] [Google Scholar]
- 17.Piao L, Jung I, Huh JY, Miyata T, Ha H. A novel plasminogen activator inhibitor-1 inhibitor, TM5441, protects against high-fat diet-induced obesity and adipocyte injury in mice. Br J Pharmacol 2016;173: 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkel AS, Khan SS, Olivares S, Miyata T, Vaughan DE. Inhibition of Plasminogen Activator Inhibitor 1 Attenuates Hepatic Steatosis but Does Not Prevent Progressive Nonalcoholic Steatohepatitis in Mice. Hepatol Commun 2018;2: 1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoukaz HB, Ji Y, Braet DJ, Vadali M, Abdelhamid AA, Emal CD, et al. Drug Targeting of Plasminogen Activator Inhibitor-1 Inhibits Metabolic Dysfunction and Atherosclerosis in a Murine Model of Metabolic Syndrome. Arterioscler Thromb Vasc Biol 2020;40: 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, et al. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proceedings of the National Academy of Sciences of the United States of America 2014;111: 7090–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D, et al. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nomega-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 2013;128: 2318–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkel AS, Elias MS, Green RM. Homocysteine supplementation attenuates the unfolded protein response in a murine nutritional model of steatohepatitis. J Biol Chem 2009;284: 31807–31816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henkel AS, Anderson KA, Dewey AM, Kavesh MH, Green RM. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J Lipid Res 2011;52: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004;306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 25.Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, et al. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab 2012;15: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sztalryd C, Brasaemle DL. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids 2017;1862: 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaughan DE, De Taeye BM, Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets 2007;8: 962–970. [DOI] [PubMed] [Google Scholar]
- 28.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004;53: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149: 367–378 e365; quiz e314-365. [DOI] [PubMed] [Google Scholar]
- 31.Khan SS, Mackie A, Beussink-Nelson L, Kamide CE, Henkel AS, Place AT, et al. Targeted Inhibition of Plasminogen Activator Inhibitor-1 Attenuates Weight Gain and Prevents Vascular Dysfunction Following a High Fat Diet. Circulation Research 2015;117: A179. [Google Scholar]
- 32.Hosaka S, Yamada T, Takahashi K, Dan T, Kaneko K, Kodama S, et al. Inhibition of Plasminogen Activator Inhibitor-1 Activation Suppresses High Fat Diet-Induced Weight Gain via Alleviation of Hypothalamic Leptin Resistance. Front Pharmacol 2020;11: 943. [DOI] [PMC free article] [PubMed] [Google Scholar]