Abstract
Objectives:
Oxidative stress is hypothesized to contribute to age-related somatic deterioration. Both reproductive and ecological context may necessitate tradeoffs that influence this outcome. We examined whether measures of lifetime reproductive effort were related to levels of oxidative stress biomarkers in peri- and post-menopausal women and whether associations were moderated by rural or urban residence.
Methods:
We surveyed 263 healthy women (age 62.1 ± 10.0 SD) from rural (N=161) and urban Poland (N=102), collecting sociodemographic data and urine samples to analyze biomarkers of oxidative stress (8-oxo-2’-deoxyguanosine, 8-OHdG) and antioxidative defense (copper-zinc superoxide dismutase, Cu-Zn SOD). Linear regression models, adjusted for residence, were used to test for associations between reproductive effort and 8-OHdG and Cu-Zn SOD.
Results:
Univariate models demonstrated significant associations between gravidity and the biomarkers of oxidative stress (8-OHdG: R2 = 0.042, p = <0.001 ; Cu-Zn SOD: R2 = 0.123, p = <0.001). Multivariate models incorporating potential confounding variables, as well as cross-product interaction terms, indicated that gravidity was associated with 8-OHdG (p < 0.01, R2adj = 0.067) and Cu-Zn SOD (p = 0.01, R2adj = 0.159). Residence (i.e urban v. rural) did not significantly moderate the associations between the biomarkers and reproductive effort.
Conclusions:
Higher lifetime reproductive effort contributes to increases in oxidative stress and antioxidative defenses. Our results provide evidence of potential mechanisms underlying the physiological tradeoffs influencing senescence for women with high reproductive effort. We illustrate the value of applying an evolutionary perspective to elucidate variation in human health and senescence.
Keywords: Senescence, tradeoffs, oxidative stress, costs of reproduction, evolutionary theory
Background and objectives
A central conceptual framework in evolutionary biology is life history theory, stating that natural selection favors organisms that allocate their finite somatic resources over the course of their lives to optimize fitness (Bogin and Smith, 1996; Gadgil and Bossert, 1970; Partridge and Barton, 1996; Stearns, 1989). When resources are limited, organisms may face tradeoffs resulting from allocation challenges between reproductive effort and somatic maintenance (Ellison, 2003; Jasienska, 2009; Prentice and Goldberg, 2000). Reproduction exacts costs, as finite energetic resources can be diverted away from genetic, cellular, and tissue repair, potentially contributing to somatic deterioration (Bell, 1980; Bell and Koufopanou, 1986; Calow, 1979; Ellison, 2003; Harshman and Zera, 2007; Reznick, 1985; Williams, 1966). In human females, the metabolic costs of reproduction are particularly evident during pregnancy and lactation (Durnin, 1991; Goldberg and others, 1993; Jasienska, 2020; Prentice and Prentice, 1988). The manifestation of these costs has been investigated in various ways, mostly by examining the relationship between lifetime investment in reproductive effort and lifespan (Beise and Voland, 2002; Helle and others, 2002; Helle and others, 2010; Jasienska and others, 2006) or disease risk (Prentice and Goldberg, 2000; Prentice and others, 1983).
Predicted tradeoffs between reproductive effort and lifespan have been the focus of considerable discussion (Kirkwood and Rose, 1991; Partridge and others, 2005). Expanding on the Disposable Soma Theory, Kirkwood and colleagues proposed this tradeoff should exist in humans, although the relationship between parity and lifespan in humans was noted as early as 1900 (Beeton and others, 1900; Kirkwood and Rose, 1991; Westendorp and Kirkwood, 1998). Using church records from the Mogielica Human Ecology Study Site in rural Poland, Jasienska and colleagues (2006) reported results that were consistent with predicted trade-offs, noting that women with higher parity had shorter lifespans.
With respect to tradeoffs, there are, however, mixed results in the literature. Among the Sami of Finland as well as rural Ethiopians, maternal longevity was reported to be shorter with number of sons but not daughters, implying that offspring sex may be an important variable affecting maternal lifespan (Gibson and Mace, 2003; Helle and others, 2002). In contrast, a study of two pre-modern populations of Germany (1720–1874) and Canada (1608–1760) reported no significant effect of parity on lifespan (Beise and Voland, 2002). Similarly, no parity effect on maternal lifespan was found among Swedish Sami women (Cesarini and others, 2007). They argued that the earlier results of Helle et al. (2002) were a false positive due to small sample size (Cesarini and others, 2009). They also suggested that the Helle results were unique to the Sami from Finland and not generalizable to other populations. However, supplementary analyses of the original Sami dataset from Finland with increased sample sizes and the addition of non-Sami Finnish women support the original conclusions of the Helle group (Helle and others, 2010).
Jasienska (2009) argued that these varying results are due to methodological and population differences. She suggested, for example that the energetic costs of lactation, which are higher than gestation, are not fully incorporated into studies of reproduction and lifespan. She also added that maternal energetic status needs to be taken into consideration. Lifespan variation is subject to numerous factors outside of reproductive effort, including gene/environment interactions, socioeconomic factors, and disease challenges (Bae and others, 2017; Dato and others, 2017). While associations between lifespan and reproductive effort may be a useful test of the predicted life history tradeoff in semelparous and short-lived organisms (Oakwood and others, 2001; Partridge and Farquhar, 1981; Partridge and others, 2005), variation in lifespan in association with reproductive effort may simply be too blunt of a measure to test the expected tradeoffs between reproductive effort and aging, especially in a long-lived iteroparous species such as Homo sapiens. A more nuanced and targeted physiological assessment may be merited and more informative. Examining a physiological cost that directly results from metabolic investment in reproduction such as oxidative stress would be clarifying.
This study seeks to advance our understanding of the long-term costs of reproduction by examining associations between reproductive effort and biomarkers of oxidative stress and antioxidant response. This is because limited empirical research has investigated the physiological mechanisms underlying the costs of reproduction during the lifetime of individual women. We draw from a dataset collected in peri- and post-menopausal women living in two distinct ecologies in Poland.
Oxidative stress
An established physiological contributor to senescence is oxidative stress (OS), which refers to the imbalance between the production of damaging reactive oxidative species (ROS), their utilization, and the detoxification of these agents via antioxidant defenses (Finkel and Holbrook, 2000; Liguori and others, 2018). ROS results from electrons escaping the mitochondrial transport chain during oxidative phosphorylation. Though they are an inevitable aspect of aerobic metabolism and other vital cellular processes, excessive concentrations of ROS can incur significant damage to cellular lipids, nucleic acids, and proteins (Agarwal and others, 2005; Aruoma, 1998; Block and others, 2002; Finkel and Holbrook, 2000).
To avoid and alleviate OS, organisms have the ability to neutralize ROS and repair their damage via enzymatic and non-enzymatic agents (Beckman and Ames, 1997). Intracellular antioxidant defense is provided largely by specific enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (Bannister and others, 1987). The effectiveness of these protective mechanisms, and levels of ROS generated, determine whether there is an imbalance in the body, and the extent of OS.
Physical activity levels also contribute to metabolic rates since energy conversion and mobilization will vary depending on the performance of different tasks. Variation in physical activity is therefore expected to influence the rates of ROS production and OS. Compelling evidence supports the link between activity, and OS (Alessio, 1993; Alessio and others, 2000; Finaud and others, 2006; Powers and Jackson, 2008). For example, studies have shown that acute bouts of vigorous physical activity can increase ROS production and oxidative damage, while moderate increases in metabolic rate can reduce or have no significant effect on OS (Alessio and others, 2000; Campbell and others, 2010; Devries and others, 2008; Elosua and others, 2003; Mori and others, 1999; Samjoo and others, 2013). Thus, it is hypothesized that people differing in the level of vigorous activity may also differ in their levels of OS.
Oxidative stress & senescence
OS is an appropriate biomarker of senescence due to its role as a key physiological contributor to age-related somatic deterioration. While the impact and effects of OS on aging can vary across species (Martinez-Moral and Kannan, 2019; Metcalfe and Monaghan, 2013; Pérez and others, 2009), the effects of oxidative stress on age-associated cellular, genetic, and tissue damage are well-established. The link between OS and senescence is supported by studies demonstrating increasing ROS-induced damage to DNA, lipids, and proteins, with increasing age in many organisms, including humans, mice, Drosophila melanogaster, and C. elegans (Beckman and Ames, 1997; Hamilton and others, 2001). There is also well established evidence that oxidative damage is implicated in the pathogenesis of several age-related diseases, including cardiovascular disease, cancer, diabetes, hypertension, neurodegenerative and hepatic diseases, and chronic inflammation (Aruoma, 1998; Frijhoff and others, 2015). Despite ongoing debate regarding the ultimate relevance of this connection, together these studies suggest that oxidative stress is related to physiological changes associated with senescence, including age-related pathologies and mortality risk (Kasapoglu and Özben, 2001). However, the ultimate relevance of this connection - in terms of its effect on lifespan - is still under debate.
Oxidative stress & reproduction
OS varies between and within individuals across the course of their lives, as external conditions, behaviors, and life events influence the production of ROS and antioxidative agents. For women, reproduction affects the generation of ROS, as well as protective capacities through antioxidant defenses (Agarwal and others, 2005; Agarwal and others, 2003; Alonso-Alvarez and others, 2004; Speakman and Garratt, 2014). One reason for this shift is the high energetic and aerobic requirements of pregnancy and lactation, increases in the generation of ROS and potentially overtaxing physiological defense mechanisms, shifting the balance of the system toward the accumulation of OS (Hung and others, 2010; Sharma and others, 2011; Zheng and others, 2015). Pregnancy additionally alters lipid metabolism and typically increases circulating cholesterol levels, conditions associated with increased ROS production (Brizzi and others, 1999; Toescu and others, 2002). Increases in body fat that are often associated with pregnancy weight-gain, particularly abdominal fat, are further expected to drive an increase in the production of ROS during this period (Martinez-Moral and Kannan, 2019). The systemic inflammatory response associated with normal pregnancy has also been demonstrated to lead to increased ROS production, and this condition has been observed to an even greater degree in preeclamptic pregnancies (Brizzi and others, 1999; Toescu and others, 2002).
Though the production of ROS is expected to increase as a result of the physiological changes associated with investment in reproduction, there is limited evidence about whether this alteration in the generation of ROS translates into an accumulated or long-lasting increase in OS levels, representing a potential lifetime cost of reproduction (Ziomkiewicz and others, 2017; Ziomkiewicz and others, 2016). Some studies have suggested both that biomarkers of OS return to normal in the post-partum period, while others implied that these indicators remain elevated long after gestation is complete (Mutlu and others, 2012; Toescu and others, 2002). As aging results in the decline of physiological function, including decreases in the efficiency of the management of oxidative stress, senescence may contribute to variation in this persistence of OS. The accumulation of OS biomarkers such as 8-OHdG with age has been well documented in rat models and humans (Kaneo and others, 1996; Sakano and others, 2009a). In contrast, 8-OHdG levels were negatively associated with age in female rhesus macaques (Georgiev and others, 2015). While it is thought that age-related increases in mitochondrial and cellular ROS production are likely to contribute to the persistence and presence of elevated biomarkers of OS, additional research is necessary to parse out the impact of phylogenetic, environmental, and life history variables. This was one of the motivating factors for the current investigation.
Furthermore, the degree to which OS accumulates with consecutive pregnancies appears to vary across studies. For example, previous analyses of OS levels and reproductive investment in rural Polish women have shown significant associations between OS biomarkers and a composite measure of reproductive investment (Ziomkiewicz and others, 2016). However, follow-up research that broadened the ecological context of these studies with the investigation of urban American participants demonstrated no significant association between biomarkers of OS and reproductive investment for post-menopausal women (Ziomkiewicz and others, 2017). This revealed that ecological and lifestyle conditions that contribute to women’s energetic status may contribute to variation in vulnerability to OS in association with reproductive effort (Jasienska, 2009). This investigation builds on these studies by interrogating the influence of ecological variation and comparing OS biomarkers between rural and urban Polish women.
Study goals
This study examines, for a sample of Polish women, whether reproductive effort is associated with oxidative stress biomarkers and antioxidant levels. We hypothesize that gravidity is positively associated with greater exposure to oxidative stress, whereby greater OS would possibly contribute to accelerated senescence and greater somatic deterioration. We further ask whether this association is moderated by lifestyle factors, represented here via residence in rural or urban locales.
We tested relationships between reproductive effort, indicated by gravidity, and two urinary biomarkers: 8-hydroxy-2’-deoxyguanosine (8-OHdG) - an indicator of oxidative DNA damage - and copper-zinc superoxide dismutase (Cu-Zn SOD) - an endogenous antioxidant enzyme that catalyzes the breakdown of ROS. Our biomarkers were chosen because they are easily measured from urine samples, which can be collected non-invasively and stored safely under field conditions. These methods have also been validated by commercial ELISAs, and are largely unaffected by potential confounding factors such as dietary composition, specifically foods that contain antioxidants (Kashino and others, 2018; Kim and others, 2011; Zanolin and others, 2015). Our biomarkers are also widely used in the studies as representing a direct assessment of excised and excreted guanine base-pair lesions resulting from ROS within the whole body (Kim and others, 2011; Matsumoto and others, 2008; Sakano and others, 2009b; Valavanidis and others, 2009; Zanolin and others, 2015).
Methods
Study participants were healthy women (n = 263; mean age: 62.2, SD = 10.0) living in rural (N = 161) and urban Poland (N = 102). Rural women were recruited from the Mogielica Human Ecology Study Site. This field site consists of several villages in the Carpathian Mountains where, due to the mountainous terrain, women typically engaged in intense physical activity associated with seasonally demanding agricultural work during most of their lives (Jasienska, 2013; Jasienska and Ellison, 2004). The urban study site consisted of Krakow (population: 761,000) and Gliwice (population: 183,000), two cities 70 km and 175 km from the rural field site (respectively) (Glówny Urzad Statystyczny, 2015). Participants were recruited from the general population via door-to-door visits to their homes or via referral through community groups.
Sample size for each sub-sample was determined a priori using a power analysis of 2013 data from 100 post-menopausal women residing at the Mogielica Human Ecology Study Site, results that were published in Ziomkiewicz et al (2016). A power analysis indicated a necessary sample size of 114 participants to demonstrate statistically significant correlative results with a 1- β set at 0.80, using G*Power software (version 3.0.10). All sample and data collection for the present study occurred in the summers between July 2014 and August 2016, and laboratory analyses in 2017–18. The study protocol was reviewed and approved by the Bioethical Committee of Jagiellonian University and the Institutional Review Board of Yale University (HIC/HSC Protocol # 1505015877).
Data collection
Upon obtaining written consent, female research assistants, trained by experienced researchers from Jagiellonian University, collected sociodemographic and anthropometric data in participants’ homes. Interview data included information about age, level of education, marital status, current and previous employment, physical workload, long-term health problems, medical history, use of medication, lifestyle factors such as smoking and alcohol consumption, and reproductive history. Anthropometric measurements included height, weight, body fat percentage, and waist circumference. Body height and sitting height were measured using a stadiometer. Body mass, body fat percentage, and abdominal fat were assessed by bioelectrical impedance analysis using a TANITA scale (Model BC 545), measured to the nearest 0.1 kg (for body mass) and 0.1% (for body fat).
Menopausal status was established based on information from self-reports of menopausal age and timing of participants’ most recent menstruation. Women were determined to be postmenopausal if they reported a year or more since their last menstruation, or had undergone procedures that caused the cessation of regular menstrual cycles (Kaufert and others, 1987). Previous research has demonstrated that cessation of menstruation of 12 or more months is associated with hormonal indicators of post-menopausal status, and that self-report data on menopausal status have high reproducibility (Bertone-Johnson and others, 2018; Colditz and others, 1987; Rodstrom and others, 2005; Sievert, 2005). Women who had menstruation patterns that were irregular or who had their last menstrual period within a year of data collection were classified as peri-menopausal.
Reproductive effort was quantified on the basis of total lifetime number of pregnancies (gravidity), including number of live births, still births, and miscarriages. We did not analyze recall data on lactation duration. Previous research with this population has shown that number of pregnancies is highly correlated with time spent on lactation, suggesting that the inclusion of this variable was not likely to improve statistical models (Ziomkiewicz and others, 2016).
Participants collected a single first morning urine sample in sterile specimen containers on the day following interviews. Samples were retrieved from participants at their homes on the same morning, aliquoted into separate vials, and frozen at −80C within an hour of collection according to the assay manufacturer and other published validated protocols (Matsumoto and others, 2008).
The urine samples remained in Poland for the duration of data collection and were transported on dry ice to the Yale Reproductive Ecology Laboratory (YREL) in the Department of Anthropology at Yale University. Samples remained frozen at −80C until laboratory analyses were conducted. These protocols were carried out in accordance with recommendations and protocols from previous studies on the collection, storage, and shipping protocols for urinary oxidative stress measurements (Matsumoto and others, 2008; Ziomkiewicz and others, 2016).
Lab analyses
Two urinary biomarkers of OS were measured in these samples. Directly measuring ROS levels is difficult due to their short half-life within the body (Halliwell and Whiteman, 2004; Poljšak and Jamnik, 2010). Consequently, human studies have typically used indirect biomarkers to quantify ROS, particularly indicators of antioxidants, and of DNA and lipid repair (Halliwell and Whiteman, 2004). We analyzed two biomarkers of OS: (1) repaired oxidative damage to DNA via measurements of 8-hydroxy-2-deoxyguanosine (8-OHdG), and (2) antioxidant defense via measurements of copper-zinc superoxide dismutase, an antioxidant enzyme (Cu-Zn SOD).
8-OHdG is a modified nucleotide base and a byproduct of guanine base pair damage caused by ROS, excreted in the urine upon repair (Chiou and others, 2003). Measurements of 8-OHdG are frequently utilized to indirectly quantify OS levels, as it is one of the predominant forms of free radical-induced oxidative lesions (Chiou and others, 2003; Valavanidis and others, 2009). 8-OHdG has been used widely both for the measurement of endogenous oxidative DNA damage, and as a tool of risk assessment of various aging-related degenerative diseases and cancers (Chiou and others, 2003; Valavanidis and others, 2009).
Cu-Zn superoxide dismutase (Cu-Zn SOD) is an endogenously produced antioxidant enzyme that catalyzes the breakdown of toxic superoxide radicals to free oxygen and hydrogen peroxide (Bannister and others, 1987). Three major superoxide dismutase families exist, and these enzymes are classified based on their protein structure and the metal cofactor with which it must bind to enable the enzyme’s activity (Sheng and others, 2014). Cu-Zn SOD, which binds to both copper and zinc and is the most common form of the enzyme in eukaryotes, has been demonstrated to be induced by ROS production and detoxify ROS, affecting lifespan in multiple organisms (Sheng and others, 2014).
Urine concentration was determined using specific gravity measurements using an Atago 4410 PAL-10S digital pocket refractometer (range: 1.000–1.060). All samples were then analyzed for levels of 8-OHdG and Cu-Zn SOD using commercial enzyme-linked immunosorbent assay (ELISA) kits in accordance with manufacturer directions and procedures at the Yale Reproductive Ecology Laboratory at Yale University (YREL). A DNA damage EIA kit (Enzo Life Sciences, catalogue number ADI-EKS-350) was used to measure levels of 8-OHdG. The sensitivity of this assay kit reported by the manufacturer is 0.59 ng/mL. Levels of the antioxidant enzyme Cu-Zn superoxide dismutase were measured using the human Cu-Zn SOD EIA (Enzo Life Sciences, catalog number ALX-850–033), which has a manufacturer-reported sensitivity level of 0.04 ng/mL. All samples were run in duplicates and the mean measurements of the biomarkers were calculated. Biomarker measurements were corrected for urine concentration based on urine sample specific gravity. The average quality control inter-assay variability was 17.1 % for 8-OHdG and 13.7% for Cu-Zn-SOD, and the average intra-assay variability for 8-OHdG was 5.9% and 10.3% for Cu-Zn-SOD.
Statistical analyses
All analyses were conducted using R Statistical Software (R Core Team, 2018). Alpha was set at 0.05. Though there are well-documented effects of smoking on oxidative stress levels, the 17 current smokers in this study did not demonstrate significantly different biomarker levels than non-smoking participants of similar ages and reproductive histories, so were included in all analyses (results not shown). Because of the advanced age of much of the study sample, 61% of participants reported some sort of long-term health problem (e.g. high cholesterol or cancer). 19% of participants had undergone hormone replacement therapy at some point in their lives, for an average of 3.17 years.
Linear regression models were used to separately test for associations between reproductive effort (defined based on gravidity levels) and levels of each biomarker of oxidative stress (8-OHdG) and antioxidative defense (Cu-Zn SOD). Univariate linear regression models were employed to assess the associations between the biomarkers of oxidative damage (8-OHdG) and antioxidative defense (Cu-Zn SOD). Further, we utilized polynomial regression models to test whether there was a U-shaped association between oxidative stress biomarkers and gravidity.
Additionally, a range of variables considered a priori as potential confounders were added to the models. These variables were included in the analyses because they have been shown to independently influence oxidative stress levels (Martinez-Moral and Kannan, 2019). Models were used to assess the contributions of independent variables, as well as cross-product interaction terms for gravidity and rural/urban location, on outcome variables reflecting oxidative stress.
Results
Sample characteristics
Our total sample population included 263 women (Table 1), namely 161 rural and 102 urban participants. Average age was 62.1 (SD = 10.0). Number of pregnancies ranged from zero to fourteen. A high average body mass index (30.0 kg/m2; SD = 5.6) indicates that participants were not likely energetically challenged at the time of sampling.
Table 1.
Anthropometric, demographic, and reproductive characteristics of Polish women in the study group.
| N = 263 | Rural N = 161 | Urban N = 102 | Total | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | Mean | SD | |
| Age (years) | 62.5 | 10.1 | 61.3 | 10.3 | 0.29 | 62.1 | 10.0 |
| Age at menopause (years) | 50.6 | 4.3 | 49.3 | 4.8 | 0.04 | 50.1 | 4.5 |
| Height (cm) | 157.8 | 6.0 | 159.7 | 5.7 | 0.01 | 158.5 | 6.0 |
| Weight (kg) | 76.7 | 15.1 | 73.6 | 13.3 | 0.11 | 75.4 | 14.6 |
| BMI (kg/m2) | 30.8 | 5.7 | 28.9 | 5.1 | 0.01 | 30.0 | 5.6 |
| Body fat (%) | 37.7 | 7.0 | 37.3 | 6.4 | 0.89 | 37.4 | 7.0 |
| Abdominal fat (%) | 10.3 | 3.5 | 9.3 | 3.0 | 0.03 | 9.9 | 3.4 |
| Education (years) | 10.1 | 3.2 | 13.3 | 3.0 | 13.3 | 11.4 | 3.5 |
| Gravidity | 4.1 | 2.1 | 1.9 | 1.2 | ≤0.01 | 3.4 | 2.2 |
| Parity | 4.1 | 2.0 | 2.0 | 0.9 | ≤0.01 | 3.3 | 2.0 |
| Lifetime number of months spent pregnant | 37.7 | 17.9 | 16.8 | 11.9 | ≤0.01 | 29.6 | 18.9 |
|
8-OHdG
†
(ng/mL) Biomarker of oxidative damage |
53.1 | 30.2 | 42.0 | 21.6 | ≤0.01 | 48.8 | 27.7 |
|
Cu-Zn SOD
‡
(ng/mL) Antioxidant enzyme |
4.0 | 4.1 | 1.3 | 1.5 | ≤0.01 | 2.8 | 3.5 |
8-Oxo-2’-deoxyguanosine;
Copper-zinc superoxide dismutase
Of this sample, 217 women were post-menopausal, and 46 peri-menopausal. Biomarker levels did not significantly differ between post- and peri-menopausal women (p = 0.12 for 8-OHdG; p = 0.88 for Cu-Zn SOD), thus, samples from all women were included in subsequent analyses.
Of the 263 urine samples, 252 provided reliable within-range measurements detectable by the ELISA kit for the measurement of DNA damage caused by ROS, 8-OHdG, and 204 samples provided within-range measurements via the ELISA kit for the antioxidant enzyme Cu-Zn SOD, and 201 provided within-range measurements for both. Average levels of oxidative stress biomarkers in the sample populations were 49.04 ng/ml (SD = 27.79) for 8-OHdG and 2.82 ng/ml (SD = 3.49) for Cu/Zn SOD.
The two biomarkers (8-OHdG and Cu-Zn SOD) were significantly positively associated (R2=0.10, p < 0.001).
Oxidative stress & reproductive effort
Univariate models demonstrated significant associations between gravidity and the biomarkers of oxidative stress (8-OHdG: β Estimate = 0.21, R2 = 0.042, p = <0.001 ; Cu-Zn SOD: β Estimate = 0.36, R2 = 0.123, p = <0.001) (Figure 1a & 1b). These results indicate that women with higher gravidity had higher levels of oxidative stress biomarker and antioxidant enzyme levels than women with lower gravidity.
Figure 1.
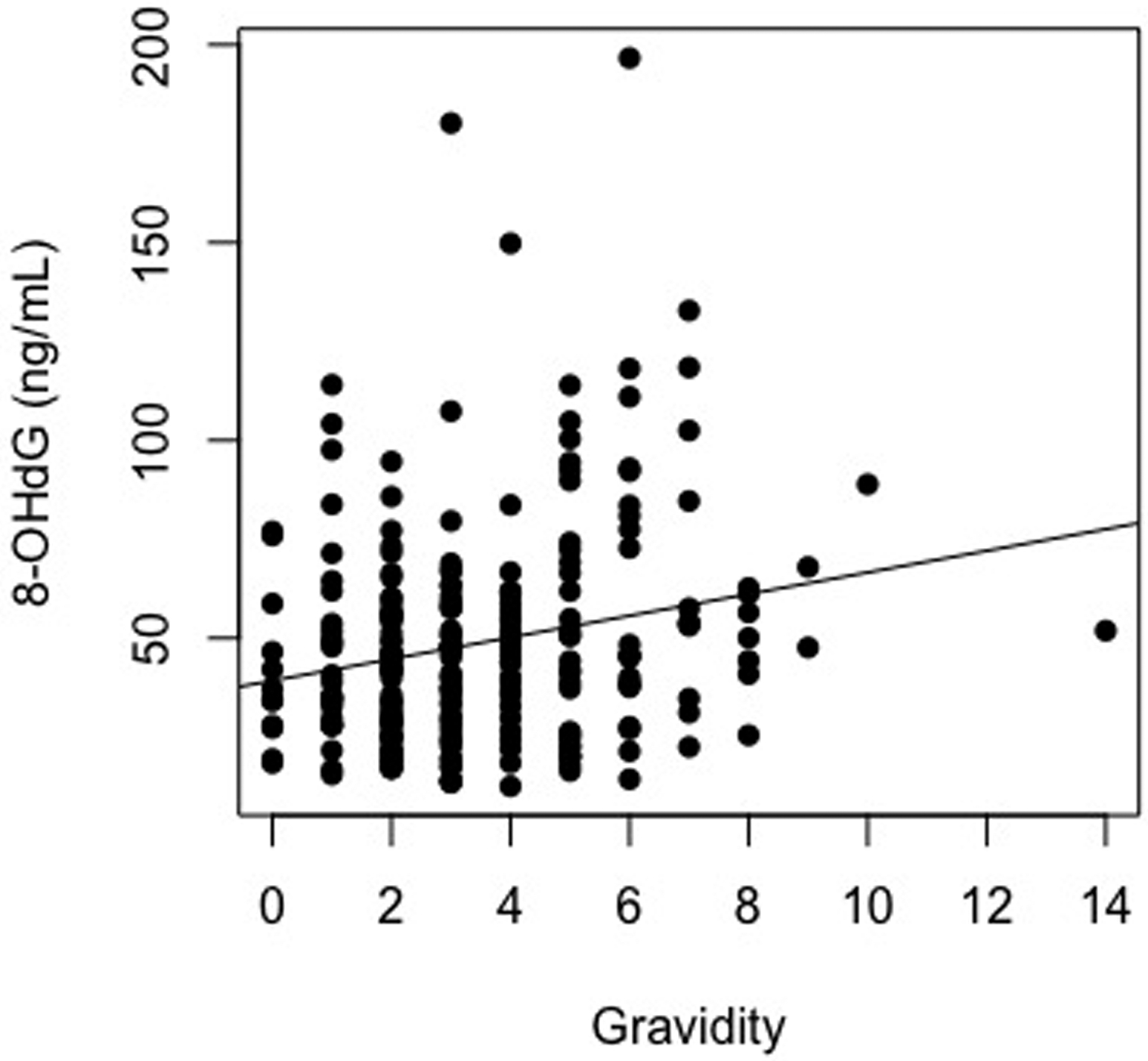
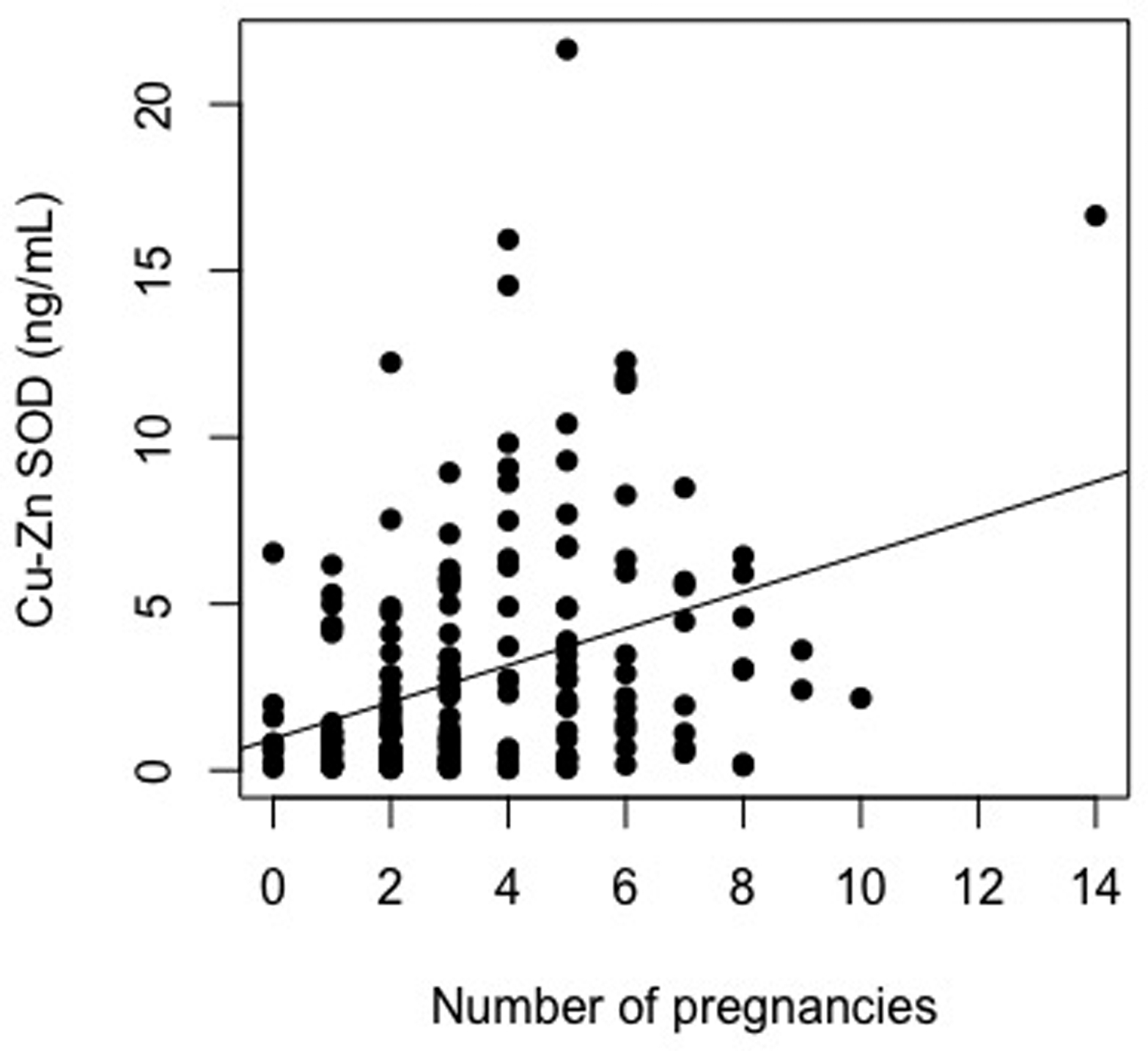
Scatter plots depicting the association between gravidity and 8-Oxo-2′-deoxyguanosine, A, (y = 39.25 + 2.73(gravidity)) and copper-zinc superoxide dismutase, B, (y = 0.95 + 0.55(gravidity))
Expanded models (including additional age, BMI, marital status, education, menopausal status, smoking status, long-term health concerns, and alcohol use) also demonstrated significant association between gravidity and the biomarkers of oxidative stress. Sensitivity analyses indicated that the results of these expanded models did not significantly differ from the univariate models (p > 0.05 for both 8-OHdG and Cu-Zn SOD). Robustness checks confirmed this significance when below-curve out-of-range samples were assigned low values and included in analyses. Second order polynomial regression analyses demonstrated no evidence for U-shaped associations between gravidity and levels of oxidative stress biomarkers.
Oxidative stress, reproductive effort, & residence location
For the overall sample, there were statistically significant associations between gravidity and the biomarkers of oxidative stress when models included the interaction term (residence and gravidity) and additional potential confounding variables. Controlling for additional independent variables, 8-OHdG (β = 0.23, p < 0.01, R2adj = 0.067) and Cu-Zn SOD (β = 0.26, p < 0.01, R2adj = 0.159) were associated with gravidity (Table 2). However, though means of 8-OHdG and Cu-Zn SOD differed between urban/rural women (Table 1), neither the interaction term nor residence were associated with 8-OHdG or Cu-Zn SOD (Table 2).
Table 2.
Regression analyses for gravidity and biomarkers (oxidative stress and antioxidant defense, 8-OHdG and Cu-Zn SOD).
| 8-OHdG† | β Coeff (SE) | P value | Model R2 |
|---|---|---|---|
| Intercept | 0 | 0.004 | 0.067 |
| Age | −0.076 (0.23) | 0.387 | |
| BMI | −0.096 (0.32) | 0.140 | |
| Marital status | −0.038 (4.15) | 0.595 | |
| Education (years) | −0.047 (2.19) | 0.572 | |
| Menopausal status | −0.108 (5.50) | 0.155 | |
| Smoking | 0.029 (5.18) | 0.660 | |
| Long-term Health Problems | −0.048 (3.78) | 0.474 | |
| Gravidity | 0.231 (1.10) | 0.008 | |
| Rural/urban sample | −0.027 (7.61) | 0.841 | |
| Alcohol use | −0.092 (3.77) | 0.156 | |
| Gravidity * Rural/urban sample | −0.120 (2.47) | 0.278 | |
| Cu-Zn SOD‡ | β Coeff (SE) | P value | Model R2 |
| Intercept | 0 | <0.001 | 0.159 |
| Age | 0.027 (0.03) | 0.766 | |
| BMI | 0.006 (0.042) | 0.925 | |
| Marital status | −0.070 (0.54) | 0.361 | |
| Education (years) | −0.052 (0.29) | 0.560 | |
| Menopausal status | −0.117 (0.76) | 0.146 | |
| Smoking | −0.050 (0.72) | 0.468 | |
| Long-term Health Problems | −0.004 (0.49) | 0.954 | |
| Alcohol use | −0.071 (0.51) | 0.303 | |
| Gravidity | 0.263 (0.15) | 0.006 | |
| Rural/urban sample | −0.094 (0.97) | 0.499 | |
| Gravidity * Rural/urban sample | −0.188 (0.31) | 0.091 |
8-Oxo-2’-deoxyguanosine;
Copper-zinc superoxide dismutase Presents association between biomarkers and gravidity, possible confounding variables, and residence location. N = 263.
Discussion
This study demonstrated modest positive associations between measures of reproductive effort and biomarkers of oxidative damage (8-OHdG) and antioxidative defense (Cu-Zn SOD) in a sample of peri- and post-menopausal Polish women. Women residing in rural areas had, on average, higher levels of 8-OHdG and SOD than urban women. Residence (i.e. urban v. rural) was not significantly associated with the biomarkers, nor did it significantly moderate the associations between the biomarkers and reproductive effort.
Positive associations between gravidity and levels of oxidative damage lend support to the hypothesis that, for women, increased reproductive effort contributes to long-lasting OS due to increased ROS production; however, the fraction of the variance explained in the data is small. In turn, higher reproductive effort was associated with higher levels of protective factors, as signaled by positive associations with antioxidant enzyme, even after menopause. Taken together, these results indicate that an imbalance between damaging ROS and protective mechanisms remains, even though levels of antioxidant enzymes increase to help minimize the accumulation of somatic damage associated with reproduction. This imbalance leads to the accumulation of oxidative damage in association with increased reproductive effort.
The biological significance of positive associations between OS levels and reproductive effort remains undetermined. Notably, previous research has demonstrated that increased OS levels are associated with increased mortality and the development of many age-related diseases, independent of baseline health status (Schöttker and others, 2014). These findings suggest that the increased oxidative stress biomarkers may represent costs of reproduction, potentially leading to accelerated senescence, and therefore decreased lifespan through accumulation of cellular and tissue damage.
By measuring two complementary biomarkers, this study elucidated ways in which these potential trade-offs might be mitigated. They allowed us to investigate whether the tradeoffs between reproductive effort and somatic deterioration have occurred in this population, as predicted by life history theory (Bell, 1980; Calow, 1979; Dowling and Simmons, 2009; Ellison, 2003; Jasienska, 2009). We demonstrated an increase in oxidative damage in association with reproductive effort, as well as an increase in protective factors, indicating that women may have been able to simultaneously devote resources to both reproduction and somatic maintenance. The combined biomarker results indicate that women may have minimized the costs of increased investment in reproductive effort, mitigating potential physiological tradeoffs, while still experiencing some associated damage.
Results of this nature have prompted a potentially complementary hypothesis to explain the association between reproductive effort and biomarkers of antioxidative defenses, termed the ‘oxidative shielding hypothesis’ (Blount and others, 2016). This hypothesis states that women actively protect gametes and their offspring during pregnancy by increasing their antioxidative defense levels in order to lessen the potential costs of reproduction that can cause physiological harm to themselves and their offspring (Blount and others, 2016). Research demonstrating significantly lower levels of oxidative stress in females during reproductive periods, relative to females in non-reproductive states, is cited as support for this hypothesis (Blount and others, 2016). Our findings indicate that antioxidative defenses are positively associated with reproductive effort, consistent with predictions stemming from this hypothesis. However, the demonstrated imbalance between damaging and protective factors indicates that the costs of reproductive effort have not been fully mitigated within this population.
We demonstrate these costs using urinary biomarkers of OS and antioxidant enzymes, which were measured because they allow for whole-body assessment of damage and defense. Excised damage and antioxidant enzyme levels in urine reflect circulating levels of these biomarkers (Halliwell and Whiteman, 2004). Urinary 8-OHdG and Cu-Zn SOD were also chosen because the collection and analysis of urine is relatively non-invasive and field-friendly. This facilitated the collection of samples and data in this population of interest, despite its relatively remote location to laboratory where analyses were conducted (Poljšak and Jamnik, 2010). Despite its advantages, the use of biomarkers limits our ability to make statements about the long-lasting physiological effects of reproductive effort on the areas of the body that are most likely to be affected by the process of pregnancy, as urinary biomarkers employed here are not tissue- or system-specific. Moreover, while the metabolic clearance pathway and assessment of urinary 8-OHdG is well established and widely deployed, measurements of Cu Zn SOD in urine should be interpreted with caution since the clearance mechanism and association between urinary, serum, and cellular assessments of Cu Zn SOD are less well understood (Adachi and others, 1992; Adachi and others, 1988; Adachi and others, 1999; Gyuraszova and others, 2018).
Another study limitation is that research with a post-menopausal sample population is unable to determine how consecutive pregnancies affected women’s oxidative stress levels in the short-term, and therefore assess the costs of their reproductive effort during their reproductive lifespans. However, from an evolutionary perspective, research with post-menopausal women provides the ability to assess variation in the long-term accumulated costs that have resulted from tradeoffs women have made across the course of their lives (Vitzthum, 2008). Future research with pre-menopausal or pregnant women – and, ideally, longitudinal research across the reproductive episodes through menopause – would help to elucidate the shorter-term costs of potential tradeoffs and complement the work we present here.
These results replicate and build upon previous research conducted in post-menopausal rural Polish women (Ziomkiewicz and others, 2016). The present study includes urban women and thus allow to generalized conclusion on the whole population of postmenopausal women regardless of place of residence. It also has a larger sample size than our 2016 study, which facilitated the use of linear regression models that incorporated more confounding variables than the previous study, and were able to directly demonstrate the associations between the continuous variables of reproductive effort and the biomarkers of oxidative stress and defense. It also used an analytical method that could better capture variation in gravidity data, improving on the 2016 study which integrated gravidity into linear models as a categorical variable of “high” and “low” groups (< 4 and ≥ 4 pregnancies). Because the variation in the measurements of reproductive effort employed here is not categorized into high and low gravidity groups, we are able to demonstrate the degree to which oxidative stress and defense levels might accrue with increased number or months of pregnancy. Given the results obtained with a larger sample collected in two locales and a more sensitive analysis of data, the present study lends further support to the primary finding of the 2016 study, that reproductive effort is significantly associated with biomarkers of oxidative stress and defense.
Other previous studies examining OS as a physiological cost of reproductive effort have typically pointed to the high metabolic costs of pregnancy and lactation as a likely driving force contributing to the imbalance between the production and protection from ROS (Alonso-Alvarez and others, 2004; Butte and King, 2005). The sustained increase in metabolic rate associated with these processes may necessitate that energetic resources are allocated away from somatic maintenance and toward reproductive effort, particularly in environmental contexts of limited resources (Ellison, 2003). Limited energy intake and energy stores are unlikely to be the primary factors eliciting a tradeoff between OS and reproductive investment among women in this sample, as this and previous studies have indicated that women at this field site have relatively high and stable amounts of energy stored in their bodies (Jasienska and Ellison, 1998). Rather, factors such as high physical activity demands during their reproductive periods, which are expected to influence ROS production, are likely to have more saliently contributed to the ways in which reproductive costs have been manifested within this sample (Powers and Jackson, 2008).
Though our results did not indicate that residence location moderated the association between OS and gravidity, continued research should investigate how lifestyle factors, including habitual levels of energy expenditure, influence women’s tradeoffs during their reproductive lifetime. Future work should intentionally incorporate and analyze data on physical activity level as a potential moderator of the association between reproductive effort and oxidative stress. These data might be collected retrospectively with post-reproductive individuals, or directly, as an aspect of research with pre-menopausal women. Meta-analyses on this topic have also called for increased research with model organisms in laboratory settings in which reproductive environments are manipulated so that energetic resources are limited, necessitating life history tradeoffs within these artificial settings (Speakman and Garratt, 2014). These approaches would amplify and elucidate the allocation decisions faced by individuals during their reproductive lives.
This study lends support to the hypothesis that increased reproductive effort contributes to increased oxidative stress in women. Our hypotheses were informed by life history theory and based on the expectation that the investment in reproductive effort would necessitate tradeoffs requiring the allocation of resources away from somatic maintenance. Reproduction exacts costs, as finite energetic resources are diverted away from the soma, resulting in tradeoffs with consequences such as increased risks of morbidity and mortality. Our results contribute to understanding of occurrence of tradeoffs by providing evidence of potential mechanisms through which the physiological conditions of women with high reproductive effort is influenced. We illustrate the value of applying an evolutionary perspective to elucidate variation in human health and senescence.
Acknowledgements
We thank our study assistants and the women who participated in this study.
We also thank our funding sources: the National Science Foundation Doctoral Dissertation Research Improvement Grant (Award Number: 1613433), the Yale University MacMillan Center Dissertation Research Grant, the Yale Institute for Biospheric Studies - Program in Reproductive Ecology, and the Yale Institute for Biospheric Studies Dissertation Research Grant.
References
- Adachi T, Ohta H, Yamada H, Futenma A, Kato K, Hirano K. 1992. Quantitative analysis of extracellular-superoxide dismutase in serum and urine by ELISA with monoclonal antibody. Clin Chim Acta 212(3):89–102. [DOI] [PubMed] [Google Scholar]
- Adachi T, Usami Y, Kishi T, Hirano K, Hayashi K. 1988. An enzyme immunoassay for cuprozinc superoxide dismutase using monoclonal antibodies. Application for pharmacokinetic study. J Immunol Methods 109(1):93–101. [DOI] [PubMed] [Google Scholar]
- Adachi T, Yamada H, Hara H, Futenma A, Kakumu S. 1999. Increase of urinary extracellular-superoxide dismutase level correlated with cyclic adenosine monophosphate. FEBS Lett 458(3):370–374. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma RK. 2005. Role of oxidative stress in female reproduction. Reproductive biology and endocrinology : RB&E 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Saleh RA, Bedaiwy MA. 2003. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79(4):829–843. [DOI] [PubMed] [Google Scholar]
- Alessio HM. 1993. Exercise-induced oxidative stress. Medicine and science in sports and exercise 25(2):218–224. [PubMed] [Google Scholar]
- Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. 2000. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Medicine and science in sports and exercise 32(9):1576–1581. [DOI] [PubMed] [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. 2004. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecology Letters 7(5):363–368. [Google Scholar]
- Aruoma O 1998. Free radicals, oxidative stress, and antioxidants in human health and disease. Journal of the American Oil Chemists’ Society 75(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Kim YY, Lee JS. 2017. Factors Associated With Subjective Life Expectancy: Comparison With Actuarial Life Expectancy. J Prev Med Public Health 50(4):240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister JV, Bannister WH, Rotilio G. 1987. Aspects of the structure, function, and applications of superoxide dismutas. Critical Reviews in Biochemistry 22(2):111–180. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. 1997. Oxidative decay of DNA. Journal of Biological Chemistry 272(32):19633–19636. [DOI] [PubMed] [Google Scholar]
- Beeton M, Yule GU, Pearson K. 1900. Data for the problem of evolution in man. V. On the correlation between duration of life and the number of offspring. Proc R Soc Lond 67:159–179. [Google Scholar]
- Beise J, Voland E. 2002. Effect of producing sons on maternal longevity in premodern populations. Science 298(5592):317; author reply 317. [DOI] [PubMed] [Google Scholar]
- Bell G 1980. The costs of reproduction and their consequences. American Naturalist 116:45–76. [Google Scholar]
- Bell G, Koufopanou V. 1986. The cost of reproduction. In: Ridley R, editor. Oxford Surveys in Evolutionary Biology. Oxford: Oxford University Press. p 83–131. [Google Scholar]
- Bertone-Johnson ER, Manson JE, Purdue-Smithe AC, Steiner AZ, Eliassen AH, Hankinson SE, Rosner BA, Whitcomb BW. 2018. Anti-Mullerian hormone levels and incidence of early natural menopause in a prospective study. Hum Reprod 33(6):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. 2002. Factors associated with oxidative stress in human populations. American Journal of Epidemiology 156(3):274–285. [DOI] [PubMed] [Google Scholar]
- Blount JD, Vitikainen EI, Stott I, Cant MA. 2016. Oxidative shielding and the cost of reproduction. Biological Reviews 91(2):483–497. [DOI] [PubMed] [Google Scholar]
- Bogin B, Smith BH. 1996. Evolution of the human life cycle. American Journal of Human Biology: The Official Journal of the Human Biology Association 8(6):703–716. [DOI] [PubMed] [Google Scholar]
- Brizzi P, Tonolo G, Esposito F, Puddu L, Dessole S, Maioli M, Milia S. 1999. Lipoprotein metabolism during normal pregnancy. American journal of obstetrics and gynecology 181(2):430–434. [DOI] [PubMed] [Google Scholar]
- Butte NF, King JC. 2005. Energy requirements during pregnancy and lactation. Public health nutrition 8(7a):1010–1027. [DOI] [PubMed] [Google Scholar]
- Calow P 1979. The cost of reproduction - a physiological approach. Biological Reviews 54(1):23–40. [DOI] [PubMed] [Google Scholar]
- Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. 2010. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Medicine and science in sports and exercise 42(8):1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarini D, Lindqvist E, Wallace B. 2009. Is there an adverse effect of sons on maternal longevity? Proceedings of the Royal Society B: Biological Sciences 276(1664):2081–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarini D, Lindqvist E, Wallace Br. 2007. Maternal longevity and the sex of offspring in preindustrial Sweden. Annals of Human Biology 34(5):535–546. [DOI] [PubMed] [Google Scholar]
- Chiou C-C, Chang P-Y, Chan E-C, Wu T-L, Tsao K-C, Wu JT. 2003. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clinica chimica acta 334(1–2):87–94. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. 1987. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. American journal of epidemiology 126(2):319–325. [DOI] [PubMed] [Google Scholar]
- Dato S, Rose G, Crocco P, Monti D, Garagnani P, Franceschi C, Passarino G. 2017. The genetics of human longevity: an intricacy of genes, environment, culture and microbiome. Mech Ageing Dev 165(Pt B):147–155. [DOI] [PubMed] [Google Scholar]
- Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA. 2008. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free radical biology and medicine 45(4):503–511. [DOI] [PubMed] [Google Scholar]
- Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proceedings of the Royal Society B: Biological Sciences 276(1663):1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin JV. 1991. Energy requirements of pregnancy. Acta Paediatr Scand Suppl 373:33–42. [DOI] [PubMed] [Google Scholar]
- Ellison P 2003. Energetics and reproductive effort. American Journal of Human Biology 15(3):342–351. [DOI] [PubMed] [Google Scholar]
- Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada J, Covas M, Ordonez-Llanos J, Marrugat J. 2003. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis 167(2):327–334. [DOI] [PubMed] [Google Scholar]
- Finaud J, Lac G, Filaire E. 2006. Oxidative stress. Sports medicine 36(4):327–358. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408(6809):239. [DOI] [PubMed] [Google Scholar]
- Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T. 2015. Clinical relevance of biomarkers of oxidative stress. Antioxidants & redox signaling 23(14):1144–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil M, Bossert WH. 1970. Life historical consequences of natural selection. The American Naturalist 104(935):1–24. [Google Scholar]
- Georgiev AV, Thompson ME, Mandalaywala TM, Maestripieri D. 2015. Oxidative stress as an indicator of the costs of reproduction among free-ranging rhesus macaques. Journal of Experimental Biology 218(13):1981–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MA, Mace R. 2003. Strong mothers bear more sons in rural Ethiopia. Proceedings of the Royal Society of London Series B: Biological Sciences 270(Suppl 1):S108–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg GR, Prentice AM, Coward WA, Davies HL, Murgatroyd PR, Wensing C, Black AE, Harding M, Sawyer M. 1993. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am J Clin Nutr 57(4):494–505. [DOI] [PubMed] [Google Scholar]
- Gyuraszova M, Kovalcikova A, Jansakova K, Sebekova K, Celec P, Tothova L. 2018. Markers of oxidative stress and antioxidant status in the plasma, urine and saliva of healthy mice. Physiol Res 67(6):921–934. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. 2004. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British journal of pharmacology 142(2):231–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. 2001. Does oxidative damage to DNA increase with age? Proceedings of the National Academy of Sciences 98(18):10469–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman LG, Zera AJ. 2007. The cost of reproduction: the devil in the details. Trends in ecology & evolution 22(2):80–86. [DOI] [PubMed] [Google Scholar]
- Helle S, Lummaa V, Jokela J. 2002. Sons reduced maternal longevity in preindustrial humans. Science 296(5570):1085. [DOI] [PubMed] [Google Scholar]
- Helle S, Lummaa V, Jokela J. 2010. On the number of sons born and shorter lifespan in historical Sami mothers. Proceedings of the Royal Society B: Biological Sciences 277(1696):2909–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Lo LM, Chiu TH, Li MJ, Yeh YL, Chen SF, Hsieh TT. 2010. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod Sci 17(4):401–409. [DOI] [PubMed] [Google Scholar]
- Jasienska G 2009. Reproduction and lifespan: Trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. American Journal of Human Biology: The Official Journal of the Human Biology Association 21(4):524–532. [DOI] [PubMed] [Google Scholar]
- Jasienska G 2013. The fragile wisdom: an evolutionary view on women’s biology and health: Harvard University Press. [Google Scholar]
- Jasienska G 2020. Costs of reproduction and ageing in the human female. Philosophical Transactions of the Royal Society B 375(1811):20190615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Ellison P. 1998. Physical work causes suppression of ovarian function in women. Proceedings of the Royal Society of London B: Biological Sciences 265(1408):1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Ellison P. 2004. Energetic factors and seasonal changes in ovarian function in women from rural Poland. American Journal of Human Biology 16(5):563–580. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Nenko I, Jasienski M. 2006. Daughters increase longevity of fathers, but daughters and sons equally reduce longevity of mothers. American Journal of Human Biology 18(3):422–425. [DOI] [PubMed] [Google Scholar]
- Kaneo T, Tahara S, Matsuo M. 1996. Non-linear accumulation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidized DNA damage, during aging. Mutation Research/DNAging 316(5–6):277–285. [DOI] [PubMed] [Google Scholar]
- Kasapoglu M, Özben T. 2001. Alterations of antioxidant enzymes and oxidative stress markers in aging. Experimental gerontology 36(2):209–220. [DOI] [PubMed] [Google Scholar]
- Kashino I, Li YS, Kawai K, Nanri A, Miki T, Akter S, Kobayashi S, Kasai H, Mizoue T. 2018. Dietary non-enzymatic antioxidant capacity and DNA damage in a working population. Nutrition 47:63–68. [DOI] [PubMed] [Google Scholar]
- Kaufert PA, Gilbert P, Tate R. 1987. Defining menopausal status: the impact of longitudinal data. Maturitas 9(3):217–226. [DOI] [PubMed] [Google Scholar]
- Kim JY, Yang YJ, Yang YK, Oh SY, Hong YC, Lee EK, Kwon O. 2011. Diet quality scores and oxidative stress in Korean adults. Eur J Clin Nutr 65(12):1271–1278. [DOI] [PubMed] [Google Scholar]
- Kirkwood JK, Rose M. 1991. Evolution of senescence: late survivial sacrificed for reproduction. In: Southwood L, editor. The evolution of reproductive strategies. Cambridge: Cambridge University Press. p 15–24. [DOI] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D. 2018. Oxidative stress, aging, and diseases. Clinical interventions in aging 13:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moral M-P, Kannan K 2019. How stable is oxidative stress level? An observational study of intra-and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environment international 123:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Ogawa Y, Yoshida R, Shimamori A, Kasai H, Ohta H. 2008. The stability of the oxidative stress marker, urinary 8-hydroxy-2’- deoxyguanosine (8-OHdG), when stored at room temperature. J Occup Health 50(4):366–372. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P. 2013. Does reproduction cause oxidative stress? An open question. Trends Ecol Evol 28(6):347–350. [DOI] [PubMed] [Google Scholar]
- Mori TA, Dunstan DW, Burke V, Croft KD, Rivera JH, Beilin LJ, Puddey IB. 1999. Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non—insulin-dependent diabetic patients. Metabolism 48(11):1402–1408. [DOI] [PubMed] [Google Scholar]
- Mutlu B, Bas AY, Aksoy N, Taskin A. 2012. The effect of maternal number of births on oxidative and antioxidative systems in cord blood. Journal of Maternal-Fetal and Neonatal Medicine 25(6):802–805. [DOI] [PubMed] [Google Scholar]
- Oakwood M, Bradley AJ, Cockburn A. 2001. Semelparity in a large marsupial. Proceedings: Biological Sciences 268(1465):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Barton NH. 1996. On measuring the rate of ageing. Proceedings of the Royal Society of London Series B: Biological Sciences 263(1375):1365–1371. [Google Scholar]
- Partridge L, Farquhar M. 1981. Sexual activity reduces lifespan of male fruitflies. Nature 294:580–582. [Google Scholar]
- Partridge L, Gems D, Withers DJ. 2005. Sex and death: what is the connection? Cell 120(4):461–472. [DOI] [PubMed] [Google Scholar]
- Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. 2009. Is the oxidative stress theory of aging dead? Biochimica et Biophysica Acta (BBA)-General Subjects 1790(10):1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljšak B, Jamnik P. 2010. Methodology for oxidative state detection in biological systems.
- Powers SK, Jackson MJ. 2008. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological reviews 88(4):1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, Goldberg GR. 2000. Energy adaptations in human pregnancy: limits and long-term consequences. Am J Clin Nutr 71(5 Suppl):1226S–1232S. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Lunn PG, Watkinson M, Whitehead RG. 1983. Dietary supplementation of lactating Gambian women. II. Effect on maternal health, nutritional status and biochemistry. Human Nutrition: Clinical Nutrition 37C:65–74. [PubMed] [Google Scholar]
- Prentice AM, Prentice A. 1988. Energy costs of lactation. Annual review of nutrition 8:63–79. [DOI] [PubMed] [Google Scholar]
- Reznick D. 1985. Costs of reproduction: an evaluation of the empirical evidence. Oikos 44(2):257–267. [Google Scholar]
- Rodstrom K, Bengtsson C, Lissner L, Bjorkelund C. 2005. Reproducibility of self-reported menopause age at the 24-year follow-up of a population study of women in Goteborg, Sweden. Menopause (New York, NY 12(3):275–280. [DOI] [PubMed] [Google Scholar]
- Sakano N, Takahashi N, Wang D-H, Sauriasari R, Takemoto K, Kanbara S, Sato Y, Takigawa T, Takaki J, Ogino K. 2009a. Plasma 3-nitrotyrosine, urinary 8-isoprostane and 8-OHdG among healthy Japanese people. Free radical research 43(2):183–192. [DOI] [PubMed] [Google Scholar]
- Sakano N, Wang DH, Takahashi N, Wang B, Sauriasari R, Kanbara S, Sato Y, Takigawa T, Takaki J, Ogino K. 2009b. Oxidative stress biomarkers and lifestyles in Japanese healthy people. J Clin Biochem Nutr 44(2):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samjoo I, Safdar A, Hamadeh M, Raha S, Tarnopolsky M. 2013. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutrition & diabetes 3(9):e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttker B, Saum K-U, Jansen EH, Boffetta P, Trichopoulou A, Holleczek B, Dieffenbach AK, Brenner H. 2014. Oxidative stress markers and all-cause mortality at older age: a population-based cohort study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 70(4):518–524. [DOI] [PubMed] [Google Scholar]
- Sharma N, Singh NK, Singh OP, Pandley V, Verma PK. 2011. Oxidative stress and antioxidant status during transition period in dairy cows. Asian-Australian Journal of Animal Science 24(4):24–58. [Google Scholar]
- Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller A-F, Teixeira M, Valentine JS. 2014. Superoxide dismutases and superoxide reductases. Chemical reviews 114(7):3854–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert LL. 2005. Recalling age at menopause. Menopause (New York, NY 12(3):248–249. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Garratt M. 2014. Oxidative stress as a cost of reproduction: Beyond the simplistic trade-off model. Bioessays 36(1):93–106. [DOI] [PubMed] [Google Scholar]
- Statystyczny GU. 2015. Population by sex and city. 9/12/2015 ed.
- Stearns S. 1989. Trade-offs in life-history evolution. Functional ecology 3(3):259–268. [Google Scholar]
- Team RC. 2018. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Toescu V, Nuttall S, Martin U, Kendall M, Dunne F. 2002. Oxidative stress and normal pregnancy. Clinical endocrinology 57(5):609–613. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 2009. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. Journal of environmental science and health Part C 27(2):120–139. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. 2008. Evolutionary models of women’s reproductive functioning. Annual Review of Anthropology 37. [Google Scholar]
- Westendorp RG, Kirkwood TB. 1998. Human longevity at the cost of reproductive success. Nature 396(6713):743–746. [DOI] [PubMed] [Google Scholar]
- Williams GC. 1966. Natural selection, the cost of reproduction, and a refinement of Lack’s principle. American Naturalist 100:687–690. [Google Scholar]
- Zanolin ME, Girardi P, Degan P, Rava M, Olivieri M, Di Gennaro G, Nicolis M, De Marco R. 2015. Measurement of a urinary marker (8-hydroxydeoxy-guanosine, 8-OHdG) of DNA oxidative stress in epidemiological surveys: a pilot study. Int J Biol Markers 30(3):e341–345. [DOI] [PubMed] [Google Scholar]
- Zheng GX, Lin JT, Zheng WH, Cao J, Zhao ZJ. 2015. Energy intake, oxidative stress and antioxidant in mice during lactation. Dongwuxue Yanjiu 36(2):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziomkiewicz A, Frumkin A, Zhang Y, Sancilio A, Bribiescas RG. 2017. The cost of reproduction in women: Reproductive effort and oxidative stress in premenopausal and postmenopausal American women. Amer J Hum Biol. [DOI] [PubMed] [Google Scholar]
- Ziomkiewicz A, Sancilio A, Galbarczyk A, Klimek M, Jasienska G, Bribiescas RG. 2016. Evidence for the Cost of Reproduction in Humans: High Lifetime Reproductive Effort Is Associated with Greater Oxidative Stress in Post-Menopausal Women. PLoS One 11(1):e0145753. [DOI] [PMC free article] [PubMed] [Google Scholar]


