Abstract
Viral infections during pregnancy are associated with adverse pregnancy outcomes, including maternal and fetal mortality, pregnancy loss, premature labor, and congenital anomalies. Mammalian gestation encounters an immunological paradox wherein the placenta balances the tolerance of an allogeneic fetus with protection against pathogens. Viruses cannot easily transmit from mother to fetus due to physical and immunological barriers at the maternal-fetal interface posing a restricted threat to the fetus and newborns. Despite this, the unknown strategies utilized by certain viruses could weaken the placental barrier to trigger severe maternal and fetal health issues especially through vertical transmission, which was not fully understood until now. In this review, we summarize diverse aspects of the major viral infections relevant to pregnancy, including the characteristics of pathogenesis, related maternal-fetal complications, and the underlying molecular and cellular mechanisms of vertical transmission. We highlight the fundamental signatures of complex placental defense mechanisms, which will prepare us to fight the next emerging and re-emerging infectious disease in the pregnancy population.
Keywords: Trophoblasts, Congenital infection, Hepatitis B virus, Human immunodeficiency virus, Influenza A virus, Severe acute respiratory syndrome coronavirus 2, Zika virus
Introduction
The recent outbreaks of emerging viruses, like Zika virus (ZIKV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), repeatedly raise concerns about the impacts of viral infections during pregnancy on maternal and fetal health.1,2 In general, a series of physiological adaptations to pregnancy, especially immunological and endocrinological changes, make the mother and fetus more susceptible to certain viral and bacterial infections, which is associated with greater risk for severe complications caused by infectious diseases.3 The poor outcomes triggered by viral infections during pregnancy include maternal morbidity, pregnancy loss, stillbirth, intrauterine growth restriction (IUGR), preterm birth, neonatal death, and congenital abnormalities.3,4 However, given the potential safety and toxicity issues regarding the usage of antiviral treatments and vaccines during pregnancy, pregnant women are believed to be even more vulnerable to devastating consequences induced by viral infections.5
The human placenta, a specialized and complex organ that is formed exclusively during pregnancy, is indispensable for sustaining fetal growth and development normally.6 The placenta presents an immunological paradox since it simultaneously bears the responsibility of immunologic tolerance to the fetus and retaining immunity against potential infections. Without complicated pathogen defense strategies at the maternal-fetal surface, fetal survival and development cannot be preserved due to the constant onslaught of microorganisms in our environment. However, several underappreciated mechanisms could be utilized by selective pathogens to escape from the monitoring of placenta resulting in maternal-fetal transmission. The pathogenesis of typical TORCH pathogens (refers to Toxoplasma, Others, Rubella, Cytomegalovirus, and Herpes simplex virus) causing vertical transmission has been reviewed in detail elsewhere.3,7,8 In this review, we mainly focus on the classic and emerging virus causing desperate maternal-fetal outcomes, including the hepatitis B virus (HBV), human immunodeficiency virus (HIV), influenza A virus (IAV), ZIKV, and SARS-CoV-2. We discuss the basic biology of viral infections during pregnancy, their pathogenesis characteristics, maternal-fetal complications they cause (Fig. 1), and the underlying molecular and cellular mechanisms of vertical transmission from the evidence available to date. Moreover, we elaborate a concise description of placental defense mechanisms in response to viral insults for a better understanding of strategies utilized to restrict the maternal-fetal transmission of pathogens, which could be also targeted as potential antiviral therapies.
Figure 1.
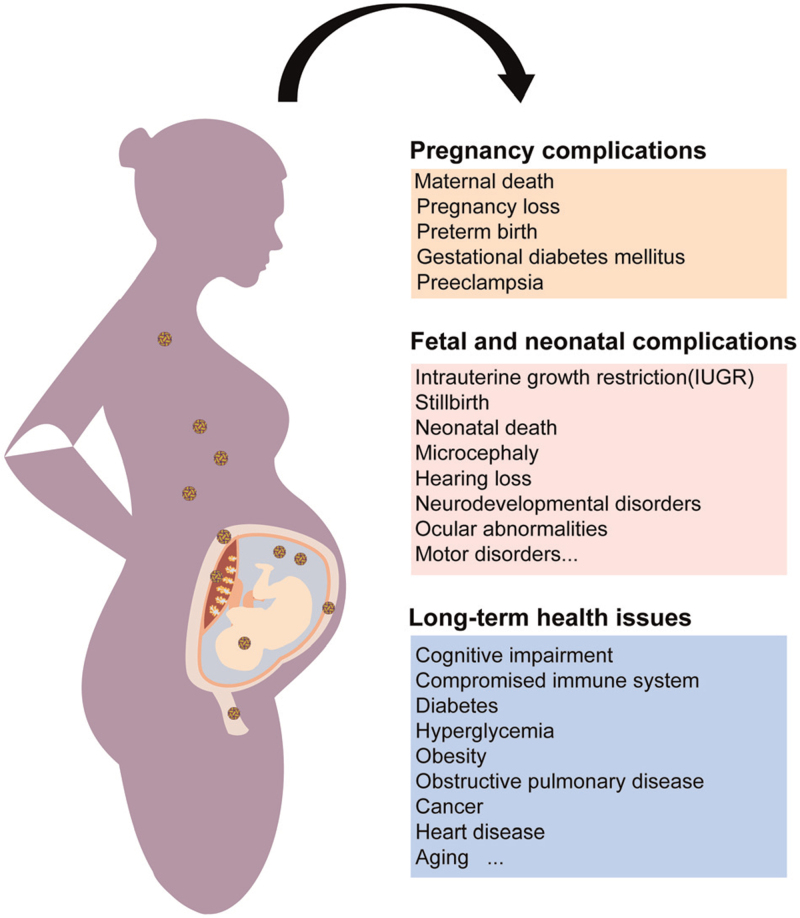
Viral infections during pregnancy and the corresponding maternal-fetal outcomes. Viral infections throughout the entire course of pregnancy can lead to diverse pregnancy complications such as maternal death, pregnancy loss, and preterm birth. Fetal and neonatal anomalies relevant to viral infections during pregnancy, especially the ones causing vertical transmission, can cause IUGR, stillbirth, microcephaly, motor disorders, and other neurodevelopmental disorders. Additionally, the offspring with prenatal exposure to maternal infectious diseases also encounter lifelong health issues.
Viral infections during pregnancy
HBV
HBV, a member of the Hepadnaviridae family, is the most common blood-borne pathogen globally, which could lead to acute and chronic hepatitis in humans. The transmission routes of HBV are predominantly through blood and bodily fluids, vertical transmission, as well as sexual and parenteral contacts.9 Perinatal transmission from the mother to fetus or newborns is still responsible for the most chronic HBV infections in adults who are more prone to severe liver diseases and poor responses to antiviral therapies.10,11 The risk of perinatal transmission in case of mothers with positive HBV e antigen or high viral loads has been estimated to be as high as 90% if the newborns accept no immunoprophylaxis treatment (includes HBV vaccine and immune globulin).12 Therefore, to prevent the vertical transmission of HBV in advance, universal maternal screenings for the HBV surface antigen, HBV e antigen, viral load, and alanine aminotransferase level during pregnancy are priorities to be adopted.12 Although immunoprophylaxis at birth together with antiviral treatments for mothers in endemic areas currently are the common and effective strategies for global elimination and preventive interventions of HBV, vertical transmission of HBV occurs with high prevalence and should be taken seriously due to uneven coverage of vaccine globally and/or prophylaxis failure.13–15
Although pregnancy complications related to HBV infection if any are minimal, clinical evidence has indicated that chronic HBV infection may be vaguely associated with gestational diabetes, preterm labor, antepartum hemorrhage, and preeclampsia.16–18 For preterm birth, several meta-analyses have confirmed that seropositivity for HBV surface antigen in pregnant women could increase the risk of preterm labor, while another study involving 6781 prematurity cases inconsistently revealed no association with HBV infection in the preterm birth group.17,19–22 Worthy of note, in the above-mentioned studies, half of the enrolled pregnant women exhibited abnormal liver functions such as non-alcoholic fatty liver disease, which may be an independent risk factor for preterm labor rather than the virus per se. Interestingly, women with HBV infection were observed to develop 2.18-fold higher antepartum hemorrhage, probably due to placenta previa and placental abruption, which is attributed to coinfection of HBV with other viruses.21 Unexpectedly, a negative association or protective effect of HBV infection on preeclampsia was demonstrated in a meta-analysis involving 11,566 cases.23 Nonetheless, the explicit causes underlying the above adverse pregnancy outcomes have not been extensively evaluated until now, placental inflammation, insulin resistance, increased immunotolerance, or impaired immune response upon HBV infection were proposed as suspected mechanisms.18,24
Furthermore, the hint of fetal and neonatal anomalies was also observed in pregnant women with chronic infection. It was noticed that 60% increased non-reassuring fetal heart rate patterns and 80% increase in asphyxia referring to 7600 pregnant HBV carriers from 18 studies by meta-analysis suggested fetal distress conditions related to HBV infection.25,26 Additionally, 25.8% increased low birth weight and small infants were reported to be associated with HBV infection, while abnormally enhanced fetal growth and macrosomia were also found in a series of researches.26–28 Viral genotypes, co-existing hepatic disorders, coinfections with other pathogenic organisms, synergism with pregnancy complications, and the phase of chronic HBV infection probably led to contradictory phenotypes of fetal growth.29,30
The path of HBV vertical transmission includes intrauterine transmission, labor, and delivery, as well as breastfeeding. The primary risk period for infant HBV infection is the peripartum period. Most cases of infection occur during delivery when the mucosa of newborns is easily contaminated by maternal blood and secretions that contain high viral loads. Alternatively, some researchers suggested that different placental compartments from the maternal side to the fetal side, such as trophoblasts and endothelial cells of villous vessels, can be infected by HBV, indicating a potential mechanism of HBV transplacental infection.31,32 Moreover, clinical studies have shown that mother-to-child transmission of HBV can be almost completely blocked by maternal antiviral therapy during pregnancy, supporting the plausibility of intrauterine infection.33,34 However, the presence of intrauterine HBV transmission is still debatable as shown by some controversial evidence.35 Therefore, the possibility and mechanisms underpinning HBV intrauterine transmission require more comprehensive in-depth clinical and basic research, which would help clinicians to improve prophylactic measures preventing mother-to-child transmission of HBV.36 At present, exploring the reasons of prophylaxis failure will be one of the big challenges in clinical practice to successfully eliminate HBV vertical transmission. Meanwhile, a comprehensive long-term safety profile of antiviral prophylaxis also needs to be assessed in the future.37,38
HIV
HIV (including two types HIV-1 and HIV-2) destroys the immune system and eventually causes acquired immunodeficiency syndrome.39 HIV-1 has stronger transmission capabilities and pathogenicity than HIV-2, herein we discuss HIV-1 primarily.40 Since the beginning of this century, >1.5 million childbearing age women have been annually threatened by HIV-1 infection to varying extents.41 Maternal HIV-1 infection accounts for adverse pregnancy outcomes, such as premature labor, miscarriage, and IUGR, which deteriorates in women with advanced HIV disease or immunosuppression.41–43 The vertical transmission of HIV could be archived through intrauterine, intrapartum (contacting with mother's blood, cervix, and vaginal secretions), and postpartum transmission. Maternal viral load, immune and nutritional status, and fetal birth mode will directly affect the likelihood and efficiency of HIV-1 perinatal transmission. Fortunately, the risk of HIV-1 vertical transmission could be decreased from 40% to <1% if the appropriate clinical management including cesarean section, combination antiretroviral therapy (cART), neonatal antiretroviral administration, and bottle-feeding were implemented in HIV-1-infected mothers during pregnancy.44–47
The definitive mechanisms of HIV vertical transmission remain largely unknown, especially the ones contributing to intrauterine transmission. Despite a large number of in utero transmission cases being documented in the third trimester, detectable HIV in aborted fetuses as early as 8 weeks implicates that HIV could disseminate to the fetus prior to a mature placental barrier.48 It has been originally suggested that HIV-1 circulating in the amniotic cavity could pass through the mucosal surface of fetal oropharynx or gastrointestinal tract, while recently published studies have not observed HIV-1 in the amniotic fluid even if systemic viral titers of some participants in plasma exceeded 105 copies/mL.49–51 Additionally, fetal HIV infection in utero is hypothesized primarily to occur by the transplacental route, which is supported by the presence of HIV-1 in placentas from infected mothers.52 Although HIV could infect trophoblasts straight by a syncytin-dependent mechanism, the virus enters the trophoblasts with much lower efficacy compared to CD4+ cells.53–55 Several in vivo studies have also confirmed that HIV-1 resides within placental Hofbauer cells (HBCs).56,57 Indeed, these unique macrophages express the HIV-1 (co)-receptors such as CD4, C-X-C motif chemokine receptor 4, and DC-SIGN, along with fragment crystallizable (Fc) γ receptors which may sequester HIV-1-antibody (Ab) immune complexes for in utero transmission of HIV-1.58 Therefore, transcytosis transmission of Ab-associated virions is another hypothesis of HIV vertical transmission.
It is not clear how HIV infection causes specific adverse pregnancy outcomes. Therein, recognizing the alternations of placental structural and functional features upon HIV infection could provide mechanistic insights into fetal and neonatal health. Gross pathological assessments of placental specimens have identified low placental weight, maternal vascular malperfusion, chorioamnionitis, and placental inflammation relative to HIV infection.59,60 More recently, Kalk et al.61 reported HIV-infection induced a 2.21-fold higher frequency of maternal vascular malperfusion, which can significantly boost the risk of IUGR and fetal demise. Crucially, excessive inflammatory response, disordered immune cell composition, and performance in HIV-1 infected placenta could also directly affect the fetal growth and development leading to babies with low birth weight. For instance, several observations have shown that the functions and subsets of tissue-resident T cells were changed, specifically a growing tendency of activated tumor necrosis factor-α-producing T cells in HIV-1-infected pregnant women.62 Besides T cells, other studies have shown that HIV-1 infection could impair natural killer (NK) cell effector functions and reduce anergic NK cells accumulation, which may cause the insufficient formation of placental vasculatures and nutrient supply for the infant, thereupon then, fetal growth is impacted.63
Vertical transmission of HIV has been effectively reduced in the last two decades mainly attributing to the highly active antiretroviral therapy. The anti-HIV interventions include strategies either reducing maternal viral exposure and load or prophylaxis in the infants by antiretroviral treatment. The pharmacological nature of antiretroviral reagents covers the antagonists of viral enzymes such as reverse transcriptase, protease, and integrase, as well as HIV co-receptor inhibitors.63,64 However, cART could simultaneously increase the risk of pregnancy complications, particularly preterm birth.65 A study from Europe first reported the association of cART and preterm birth in 1998, with subsequent studies complementing this phenomenon and speculating on the possible mechanisms: (1) Certain antiviral medicine, Ritonavir for example, may directly reduce progesterone and estradiol levels and consequently lead to IUGR, which provides a potential mechanistic link between protease inhibitors-based antiviral treatment and greater risk of preterm birth.66 (2) Antiretroviral agents could cross the blood-placenta barrier to varying degrees, thus the toxicity of specific drugs in adults could be observed theoretically in the exposed fetuses and newborns as well.67 Moreover, fetuses may be more sensitive than adults to certain toxic antiviral medicines, such as zidovudine and stavudine, leading to mitochondrial dysfunction and inflammasome activation in placentas. (3) Usage of cART may dysregulate immune response systemically and locally at the maternal-fetal interface, which could cause a shifted immunological feature during pregnancy to favor preterm birth.68 Overall, assessing the potential health threats of such antiretrovirals exposures are quite challenging but critical for optimizing the cART treatment on HIV-1-infected pregnant women.
IAV
Influenza viruses could cause acute respiratory symptoms including fever, malaise, and coughing. Although influenza viruses are classified into A, B, C, and D types, only type A viruses, such as swine influenza (H1N1), are recognized to have a wide range of hosts including mankind and become a major driver of human infectious diseases.69 Airborne droplets are the main route of IAV transmission from human to human, potentially, active virus particles can also spread to the eyes or nose via contacting with other body fluids and contaminated inanimate objects.69 The current epidemiological data show that the symptoms of H1N1 infection in the general population are mild and self-healing, while people with chronic diseases and from the age group of >60 years hold the highest mortality rate, indicating that IAV poses a greater threat to high-risk groups.70,71 Pregnant women have been found 4∼5 times more susceptible to IAV infections and increased risk for extrapulmonary complications than nonpregnant women.72,73 During the 2009 pandemic of IAV, pregnant women were more frequently hospitalized than individuals in the general population.74 In addition, the maternal mortality rate of IAV infection was significantly higher in the United States, which is in line with 27%–45% maternal mortality and 52% pregnancy loss rate during the 1918 pandemic.75 It should be noted that the possibility of adverse maternal outcomes increases with the advancing gestational age.75 Women with full-term pregnancy are five times more likely to be hospitalized with IAV infection than the ones at postpartum or early pregnancy stage.76 Reviewing the characteristics of patients in the past influenza epidemic, it was found that the proportion of hospitalized pregnant women with heart and lung damages increased significantly in the later trimester comparing early-to-middle trimester.74 Furthermore, pregnant women who are complicated with obesity, metabolic diseases, smoking history, and heart and respiratory diseases should be given extra attention to avoid severe or even life-threatening symptoms.77,78
Thus far, mechanisms underlying the high occurrence and severity of IAV infection have not been fully clarified. Pregnant women are particularly vulnerable to IAV infection, which may partially result from pregnancy-induced immunological changes, including suppressed cell-mediated immunity, alterations in NK cell activity, by contrast, enhanced humoral immunity.79–81 For example, several studies have suggested the significantly attenuated interferon (IFN) response in peripheral mononuclear cells isolated from pregnant women with IAV infection, indicating a possible mechanism underlying increased susceptibility to viral infections during pregnancy.82 Furthermore, others suggested that inactivated lung dendritic cells and virus-specific CD8 T cells deficiency in the airway contributed to the inability to control the virus in pregnancy.83,84 Besides, anatomical and physiological changes of a pregnant woman could also contribute to the high-risk adverse pregnancy outcomes in the setting of IAV infection.85 Functional changes of the cardiopulmonary system during pregnancy, critical for meeting the metabolic demands of the mother and fetus, have been recognized as a risk factor for severe influenza infection. During pregnancy, increased maternal oxygen consumption and lung tidal volumes, with reduced residual volume, expiratory reserve volume, and functional residual capacity, could compromise the compensatory capacity of the respiratory system to meet the IAV challenge and result in worse outcomes.86,87
IAV infection during pregnancy causes severe fetal and neonatal complications as well, including IUGR, preterm birth, neonatal death, and neurological disorders. The reasons for fetal mortality and morbidity upon IAV infection during pregnancy are currently unclear. Different from TORCH pathogens, vertical transmission of IAV appears to be rare because of infrequent viremia, despite a recent study reported that the highly pathogenic strain H5N1 could be detected in the placental trophoblasts and fetal respiratory tract.88,89 Maternal symptoms of IAV infection, such as fever, hypoxia, and septic shock, could exert influence on fetal health in the absence of direct fetal infection. Recently, Liong et al.90 demonstrated virus dissemination to major maternal blood vessels leads to a peripheral “vascular storm”, featured as profoundly elevated proinflammatory and antiviral mediators in the maternal vascular system, which could subsequently induce hypoxia in the placenta and fetal brain. Moreover, accumulating evidence suggests that offspring born to prenatally IAV-infected mothers exposed high risk of chronic diseases in later life, among which neurocognitive disorders, autism for instance, are the best described ones.91–93 Animal models corroborate this link and suggest that IAV infection can affect lifelong neuropathology and altered behaviors in offspring, which may be due to oxygen deprivation, placental transmission of cytokines, and antibodies or dysregulated hormone signaling after infection with influenza virus.94,95 Moreover, Jacobsen et al.92 proposed that neonates from IAV-infected pregnant dame exhibited increased susceptibility to viral and bacterial infections due to reduced hematopoietic development and immune responses, which further highlights the importance of fundamental mechanistic study regarding IAV induced poor maternal-fetal outcomes in the future.
ZIKV
ZIKV is a single-stranded RNA virus of the Flaviviridae family, which was originally isolated from a rhesus monkey residing in Uganda. It spread globally from 2015 to 2016 and led to a Public Health Emergency of International Concern declared by the World Health Organization due to emerging neuropathogenicity such as the Guillain-Barré syndrome in adults and microcephaly in neonates, respectively.96 Although ZIKV is initially recognized as a mosquito-borne virus predominately transmitted by Aedes, there has been a great accumulation of new evidence that ZIKV can be transmitted in many new routes, especially through sexual contact, blood transfusion, and most important of all, vertical transmission. About 80% of ZIKV infection cases in the general population are asymptomatic like other arbovirus infections, and only a few patients develop symptoms such as fever, joint pain, and malaise. It is estimated that the mortality rate of ZIKV infection is as low as 0.01%, while a limited number of death cases occurred in individuals with immunocompromised complications.2 The clinical manifestations of systemic ZIKV infections during pregnancy present similar symptoms like those described in nonpregnant individuals, usually mild and self-limiting diseases, suggesting no specific maternal clinical features and increased pathogenicity in general. Nevertheless, clinical and basic investigations have shown that congenital ZIKV infection could dramatically provoke pregnancy diseases, such as miscarriage, IUGR, premature delivery, and fetal death.97,98
Moreover, the fetal congenital Zika syndrome (CZS), a unique pattern of birth defects and disabilities found among fetuses and babies born to mothers exposed to ZIKV during pregnancy, has attracted extensive attention since the most recent epidemics in the Americas.99 Until now, >4000 infants worldwide have laboratory-confirmed CZS.96,100 Typical clinical manifestations of CZS mainly include severe microcephaly, severe brain abnormalities (subcortical calcifications, ventriculomegaly), ocular abnormalities, etc.101 A study of Brazilian infants with microcephaly caused by ZIKV infection revealed that 93% of infants had brain calcification, 69% encountered cortical developmental malformations such as lissencephaly and pachygyria, and 66% developed ventriculomegaly due to brain atrophy.102 Following studies have demonstrated that 35%–70% of infants with microcephaly and cerebral calcification are accompanied by a spectrum of ocular alterations such as optic nerve hypoplasia and double-ring sign, foveal reflex loss, chorioretinal macular atrophy, and cataracts.101,103,104 Mechanistically, Li et al.105 reported a direct link between ZIKV infection in the embryonic brain and microcephaly by disrupting neural progenitor development in a mouse model, which could provide valuable resources for further exploration of the underlying mechanisms and management of ZIKV-related pathological effects. Besides the above disorders, musculoskeletal, genitourinary, and pulmonary abnormalities are also observed in CZS babies, indicating phenotypic diversity caused by congenital ZIKV infection.106–108
Sadly, up to now, very little is understood about the pathogenic mechanisms underlying the vertical transmission of ZIKV. It has been reported that ZIKV can infect human and mouse blastocysts in vitro, especially in trophectoderm which gives rise to various trophoblast lineages in the mature placenta. This result indicates that ZIKV infection may directly lead to the developmental restriction of blastocysts and trophoblast stem cells through apoptosis and necrosis pathway, and eventually cause the occurrence of severe CZS.109 However, whether ZIKV could infect blastocysts and induce any embryonic abnormality in vivo remains unknown. If it is true, it could explain at least partially why ZIKV infection in early pregnancy causes more serious adverse pregnancy outcomes observed in human and mouse models.106,110,111 Another explanation for different vulnerabilities to ZIKV infection at different pregnancy stages is due to the gradually gained placental defense mechanisms along with the progress of pregnancy, which will be discussed in detail below. In addition, the transplacental transmission routines of ZIKV are still debatable. Several cellular mechanisms may mediate the vertical transmission of ZIKV, including cell-to-cell spread within trophoblasts, para-placental routes (traversing the amino-chorion), autophagy-mediated placental transmission, ZIKV dissemination seeded from infected HBCs, and Ab dependent enhancement of ZIKV infection.112,113 Each of those mechanisms is unique somehow, indicating the complexity and heterogeneity of vertical transmitted ZIKV infection, which need to be further explored in future in-depth mechanistic studies. It is worthy of note that although some molecules are proposed as potential receptors or host factors for ZIKV infections in different organs, such as Axl, Tyro3, and Mertk, none of them has been validated in the setting of transplacental infection, which impedes the mechanisms underpinning strong placental tropism of ZIKV.114,115
Extensive researches have focused on how ZIKV infection targeting the developing brain independently causes neurological damages in fetuses. Nonetheless, maternal ZIKV infection in human and multiple animal models resulting in microcephaly was accompanied by substantial placental insufficiency and abnormalities, where few studies keep a watchful eye on how placental structure and function are modified upon ZIKV infection and the contribution of placental defects to CZS.116,117 In fact, malfunction of placenta alone could induce CZS-like diseases. Thus, whether CZS is caused by direct ZIKV infection or placental insufficiency indirectly (or combined effects) is another aspect worth pondering. Moreover, whether ZIKV infection during pregnancy could affect neonatal development and even long-term health issues especially in those who had no obvious CZS at birth? There is evidence that some normal-appearing newborns born to the mother with ZIKV exposure during pregnancy developed a microcephaly phenotype during postnatal development, such as head growth restrictions and brain neuroimaging abnormalities. Furthermore, motor disorders and neurodevelopmental delay in the language function in infants can also be observed after birth in some ZIKV-exposed cases in utero.118 In line with those, Paul et al.119 did not observe significant morphological alterations and ZIKV existence in perinatal mice after intraperitoneal ZIKV infection on pregnant dams, while manifested postnatal growth impediments and neurobehavioral deficits appeared later in postnatal life. Therefore, a transient ZIKV infection during pregnancy is likely to affect future development and health of the neonates and even adulthood health in a long run, which still needs in-depth mechanistic studies to address.
SARS-CoV-2
Coronaviruses belonging to the family Coronaviridae are positive-sense RNA viruses.120 To date, the Middle East respiratory syndrome coronavirus, SARS-CoV, and SARS-CoV-2 are deadly pathogens with high transmissibility.120 The outbreak of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 seriously threatens the health of people all over the world.121 Clinical manifestations of COVID-19 range from asymptomatic, through mildly symptomatic with cough, fever, myalgia, and malaise to full-blown viral pneumonia.121 Acute respiratory distress syndrome belonging to serious COVID-19 can progress to multi-organ failure that figured the main cause of death worldwide in infected patients.121 As new evidence accumulated exponentially due to the increasing number of infected pregnant women, a picture of how COVID-19 impacts pregnant women and their infants has been crystallized.122–125
At the beginning of the current SARS-CoV-2 epidemic, a substantially higher hospitalization percentages in pregnant women (31.5%) than non-pregnant women (5.8%) were reported based on the limited cases.126,127 However, whether the higher hospitalization rate is due to special cautions given to the pregnant population rather than more serious COVID-19 is still controversial.127,128 In addition, a few maternal death cases were reported in pregnant women infected with SARS-CoV-2, while most pregnant women positive for SARS-CoV-2 showed overall only mild to moderate symptoms of COVID-19.129–134 On the contrary, a systematic review discovered that pregnant women even have a lower incidence of cough, sore throat, fatigue, headache, and diarrhea. Notably, risk factors such as race, nutritional status, age, and chronic diseases including obesity, diabetes, and hypertension could aggravate COVID-19 related poor outcomes.127,135–137
In general, viral infections on the maternal respiratory track were associated with higher rates of several adverse fetal-neonatal consequences, including IUGR, preterm birth, in some serious cases, even intrauterine fetal demise and neonatal death.74,137–141 Thus far, most larger cohort studies surely confirmed the intense relationship between COVID-19 during pregnancy and iatrogenic preterm birth.142–144 Interestingly, the increased preterm labor may be related to a higher rate of cesarean delivery, which might be the optimal scheme to ensure the safety of both mother and fetus under the scenario of SARS-CoV-2 infection.145,146 Fortunately, although certain neonatal abnormalities were observed, the overall frequency of adverse outcomes in the neonates from women with COVID-19 showed no significant difference in general.145 Systematic reviews reported that the rates of stillbirth and neonatal death in pregnancy with COVID-19 were <2.5% and 0.6%,147,148 respectively, which is comparable with healthy pregnant women. Despite this, SARS-CoV-2 was proven to cross the blood-brain barrier, proposing the possibility that it could attack brain tissues leading to the potential pathogenesis of neural tube defects.149 Given the importance of placenta throughout the entire episodes of pregnancy, information on pathological changes of placentas from mothers with COVID-19 is rapidly increasing. The most common pathological signature of SARS-CoV-2 infected placentas includes perivillous fibrin diffusion, maternal and fetal vascular malperfusion, intervillous thrombi, multi-focal infarctions, and chronic inflammatory lesions.1,150 However, whether the pathological changes of placenta are directly caused by the viral infection at the maternal-fetal interface or secondary to systemic infection needs further confirmation.
A significant concern regarding COVID-19 during pregnancy is whether vertical transmission of SARS-CoV-2 exists and what are the corresponding impacts on fetal-neonatal outcomes. Recent investigations demonstrated the presence of SARS-CoV-2 in infected placentas at different gestation stages through reverse transcriptase polymerase chain reaction, RNA in situ hybridization, immunohistochemistry, and electron microscopy, which suggested potential transplacental infection caused by the SARS-CoV-2.151–156 For example, Hosier et al.151 found that the placenta from a pregnant woman with symptomatic COVID-19 infection at the second trimester complicated by severe preeclampsia showed SARS-CoV-2 infection localized predominantly to syncytiotrophoblasts (STBs). Mechanistically, the transplacental transmission of SARS-CoV-2 is also implicated by extrapolating placental transcriptomics. The molecular basis of SARS-CoV-2 infection is highly dependent on the angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2).123 Blockade of ACE2 or TMPRSS2 protein function can significantly reduce the sensitivity of cells to the virus, suggesting their expression dynamics may be responsible for SARS-CoV-2 infectivity, tropism, and pathogenicity.123,157,158 Single-cell RNA sequencing on maternal-fetal interface revealed the heterogenous expression patterns of ACE2 and TMPRSS2 in different cell types, which suggests that placentas might be vulnerable to SARS-CoV-2 floating in the maternal blood and seed the dissemination of the virus to fetus.159–161
Although the presence of virus in placental cells raises the possibility of transplacental transmission of SARS-CoV-2, the evidence supporting intrauterine fetal infection is controversial. Original studies at the beginning of the pandemic implicated that the SARS-CoV-2 could spread vertically based on the detection of immunoglobulin M Ab specific to SARS-CoV-2 from the neonatal blood following birth, as well as aggravated immunoglobulin G (IgG) and inflammatory cytokines.162,163 However, with the increase of cases and the improvement of supervision system, the majority of studies believed that vertical transmission of SARS-CoV-2 should be rare.164–166 No neonatal infection with SARS-CoV-2 was proved in a recent study involved in 43 pregnant cases with COVID-19 on the first day of life.167 Perinatal transmission of SARS-CoV-2 still needs further validation owing to several unaddressed technical issues. First, for most congenital viral infections, immunoglobulin M is not a reliable criterion to diagnose an intrauterine infection due to low sensitivity and specificity, which may cause an unpredicted high rate of false positive.168 Additionally, a solid proof of vertical transmission of pathogens such as ZIKV requires the isolation of viral RNA in fetal or placental tissues within the sterile intrauterine environment and/or in the newborn.169,170 To exclude horizontal transmission during vaginal delivery and potential virions contamination due to intimate contact, an adequate range of biological samples from both mother and neonate (including neonatal nasopharyngeal or rectal swabs, serum, placenta, amniotic fluid, umbilical cord blood, and vaginal secretions) should be included and tested, which could reflect the authenticity of vertical transmission more precisely. Unfortunately, according to the transplacental transmission classification system, there has been no report showing the virus particles can be isolated from the fetus so far.170 Therefore, the direct and convincing evidence of SARS-CoV-2 transplacental infection is still lacking.
Other emerging viruses
Some other emerging viruses, such as Ebola virus, Rift Valley fever virus (RVFV), and West Nile virus (WNV), may also threaten maternal and fetal health through underappreciated mechanisms. For instance, RVFV, as an arbovirus, is strongly associated with fetal loss and/or stillbirth in pregnant domesticated animals, where some same outcomes have been observed in pregnant women infected by RVFV.171–173 In humans, RVFV could infect placental cytotrophoblasts (CTBs) and STBs in ex vivo experiment, which could highlight the possibility of vertical transmission.174 Indeed, the maternal-fetal transmission of RVFV in the third trimester was reported in a case study, while the specific transplacental route of RVFV is still unclear.172 Additionally, several studies have demonstrated the maternal-fetal transmission of WNV, and the corresponding abnormalities in the central nervous system like CZS induced by ZIKV.175,176 Human placental extravillous trophoblasts (EVTs) are permissive to WNV infection, which may disseminate the virus to fetus.175 In general, the emerging virus infection during pregnancy brings great challenges to a healthy pregnancy, which should be a prioritized research area we need to show solicitude for in the future.
Placental defense mechanisms
The nosogenesis and substantial consequences resulting from viral infections during pregnancy still remain largely uncharacterized, in particular, relatively little is known about the fundamental biology behind the vertical transmission of viruses. Although it has been widely accepted that the placenta acts as a defensive barrier against viral insults, the molecular mechanisms underlying control of placental infection are still poorly understood. Several hypotheses have been proposed to explain how the placenta avoids viral infections under normal conditions based on human and mouse studies, which include but not limited to the physical or anatomical defenses, intrinsic cellular mechanisms, the constitutive release of antimicrobial immunomodulators, and so on8 (Fig. 2). Moreover, great progress has been achieved regarding the transplacental transmission and pathogenesis of TORCH pathogens, especially those advanced insights obtained in animal models.3,4,177,178 In this section, we will highlight molecular and cellular signatures of the placenta barrier function, which not only illustrate the etiology of vertical transmission but also shed light on the foundation of possible new therapeutic approaches to mitigate viral infection-related diseases during pregnancy.
Figure 2.
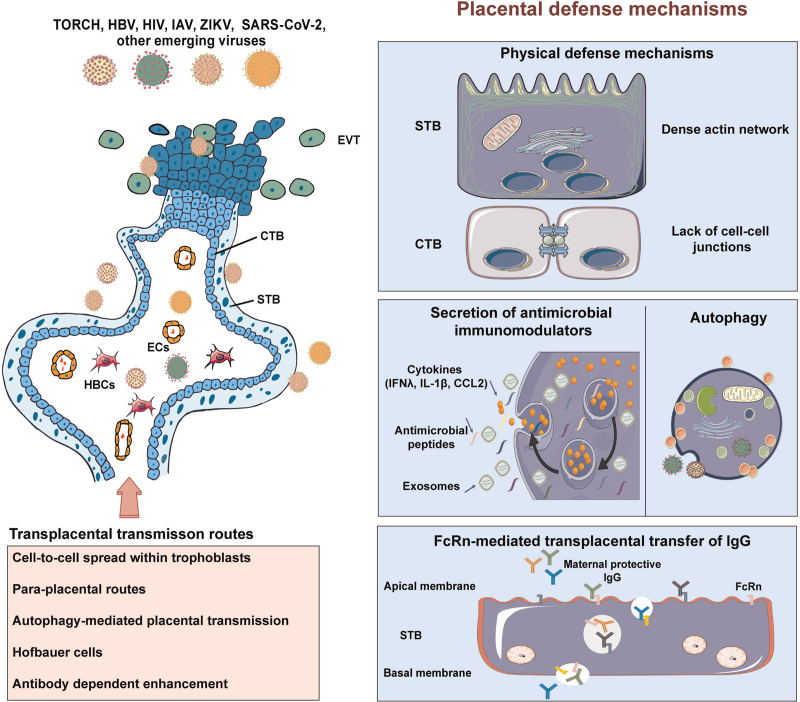
Placental defense mechanisms against viral infections. The human placenta exploits disparate cellular and molecular mechanisms of antiviral defense, which encompasses the physical or anatomical defenses, constitutive release of antimicrobial immunomodulators, autophagy and FcRn mediated antibodies transplacental transfer. CCL2: C-C motif chemokine ligand 2; CTB: Cytotrophoblast; ECs: Endothelial cells; EVT: Extravillous trophoblast; FcRn: The neonatal fragment crystallizable receptor; HBCs: Hofbauer cells; HBV: Hepatitis B virus; HIV: Human immunodeficiency virus; IAV: Influenza A virus; IFNλ: Interferon λ; IgG: Immunoglobulin G; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; STB: Syncytiotrophoblast; TORCH: Toxoplasma, Others, Rubella, Cytomegalovirus, and Herpes simplex virus; ZIKV: Zika virus.
Cellular structure of human placenta and the corresponding susceptibility to viral infections
The structure of human placenta consists of both floating villi and anchoring villi. A continuous single layer of multinucleated STBs sets up the outer lining of floating villous tree that is in direct contact with maternal blood flowing into the intervillous space, which is critical to facilitate maternal/fetal exchange of gases, waste products, and nutrients. Furthermore, the STBs are also a major cell type undertaking the endocrine function of human placenta to drive the physiological and metabolic adaptations to pregnancy. Underneath the STB layer is the undifferentiated, mononucleated CTBs, which are anchored to a basement membrane within the placental villus. The CTBs have high proliferative capacity and form a monolayer of polarized stem cells, which eventually differentiate via cell-cell fusion into STBs that cover the entire villous surface. Another subtype of differentiated CTBs can invade and remodel the decidualized endometrium, where they are termed EVTs.6,179–182 Moreover, EVTs are unique in their immune-privileged status as they are coated with self-antigens including the major histocompatibility antigen, human leukocyte antigen G, which is expressed almost exclusively in EVTs and is implicated in the maintenance of immune tolerance.183
Different subtypes of trophoblasts exhibit differential susceptibilities to pathogenic infections. Although the mechanisms are still poorly understood, the STBs have been shown to resist infections by diverse pathogens. For instance, in human placental explant cultures, cytomegaloviruses could preferentially infect CTBs verse STBs.184,185 Nevertheless, in the recent pandemic, it was proposed that SRAS-CoV-2 prefers to target STBs probably due to high abundance of viral receptors on their apical surface.161,186 EVTs are known to be more sensitive to bacterial and viral infections than the CTBs and STBs, but the mechanisms underlying this observation are not well defined.187 Indeed, in naturally infected human term placentas, most cytomegalovirus is predominantly found in the EVTs.188,189 Moreover, chorionic villus explants from first-trimester placentas have confirmed that ZIKV appears to bypass the STBs to reproducibly replicate in EVTs.113,190 The are the most plausible hosts to pathogens maybe due to the fact that these cells express human leukocyte antigen G and may provide a protected niche for viral reservoirs.191 In addition to trophoblasts, leukocytes derived from maternal compartments, including decidual NK cells, macrophages, T cell subsets, and placenta-specific macrophages-the HBCs, not only play a key role in maintaining the immune tolerance feature of normal pregnancy but also have some complex mechanisms for removing pathogens or as a “Trojan Horse” for viral vertical transmission. For example, decidual NK cells could limit HIV infection in decidual macrophages by the contact-dependent cytolysis and IFN release. On the contrary, a higher permission of HBCs to ZIKV infection could promote the dissemination of ZIKV within fetal compartments.116,192,193
Physical defense mechanism
By term, owing to extensive branching morphogenesis of placenta villi during 9 months of gestation, the overall epithelial surface area of STB layer reaches approximately 12–14 m2, which forms the frontline of placental defense restricting the hematogenous spread of pathogens.177 Physical characteristics of placenta syncytium that naturally confers microbial resistance cover the brush border formed at the apical surface of STB layer, the diminished cell–cell junctions, and condensed cortical actin network.194,195 Cell–cell junctions, intercellular seal structures formed by various transmembrane proteins coupled with cytoplasmic adaptors, are essential components of the epithelial fence, which were weakened or exploited by many pathogens in the process of traversing epithelial barriers.195 Therefore, lack of cell–cell junctions in STBs may be beneficial for blocking pathogens from a gateway to fetus in general. The unique cytoskeletal organization of the STB (such as disordered mesh of actin microfilament) conduces to its elasticity, while obstruction of this feature promotes microbial infections. It should be noted that pathogens accessing to fetus also should overcome the defensive functions of the cells embedding in villous stroma like microvasculature of fetal blood vessels.8
Autophagy
Autophagy is established to be a vital part of the host immune response to microbial infection and is considered to directly eliminate intracellular pathogens by mediating their delivery to lysosomes.196 Trophoblast cells exhibit a high basal autophagy level at term, which could be used as a pan-antimicrobial strategy to limit the replication and transplacental transmission of various pathogens.197,198 Consistent with this idea, dysregulated autophagy has been characterized in malaria- and bacteria-infected human placenta samples.199,200 However, major pathogens, known to induce transplacental infection, can evade or subvert autophagic cellular machinery to survive or replicate intracellularly. Recently, we have demonstrated that vertical transmission of ZIKV was significantly reduced in autophagy-deficient mouse models, consistently, treatment of autophagy inhibitors also hindered placental and fetal ZIKV infection and rescued the corresponding poor pregnancy outcomes.201 In summary, the effects of autophagy in terms of transplacental infection might be highly dependent on the pathogens involved.
Secretion of antimicrobial immunomodulators
Aside from the protective architecture of human placental, trophoblasts possess the robust innate immune activity and secrete antimicrobial molecules to limit infection. We and others proved that trophoblast-derived IFNs confer complex protection from viral infections. IFN-α receptor knockout mice recapitulate the maternal and fetal phenotypes of transplacental ZIKV infection.117 However, hyperactivated type I IFN signaling in response to live virus or viral mimics could result in detrimental outcomes to fetus including fetal demise and IUGR, which is at least partly due to deficient trophoblast syncytialization caused by IFN induced trans-membrane proteins.202–204 Therefore, the activation of type I IFNs in placenta in response to infectious signals is a double-edged sword with regards to pregnancy outcomes. Type III IFNs (including interferon λ (IFNλ1), IFNλ2, and IFNλ3) also confer resistance to ZIKV infection in both mice and human placenta, which may partially explain distinct vulnerability to ZIKV infection in placentas from different gestational ages. Exogenous prophylactical and therapeutic IFN-λ treatment has been proposed against vertical transmission in the setting of ZIKV as well.205
The Toll-like receptors, Retinoic acid-inducible gene I-like receptors, and nucleotide-binding oligomerization domain-like receptors are pattern recognition receptors expressed at the maternal-fetal interface throughout pregnancy, whose expression exhibit both temporal and cell type specific fluctuations. The activation of antiviral signals downstream of pattern recognition receptors triggers a potent defense mechanism utilized by placenta cells to effectively protect the fetus from pathogen attacks through producing large amounts of pro-inflammatory cytokines and chemokines such as tumor necrosis factor-α, interleukin-1β, and C-C motif chemokine ligand 2.206,207 Indeed, the inhibition of inflammasome signaling could robustly sensitize trophoblasts to the infection of Listeria monocytogenes in human placental explants, highlighting cytokines secreted from placenta could limit infections. However, detrimental actions of interleukin-1β and other pro-inflammatory cytokines in infected placentas are involved in adverse neonatal outcomes, suggesting that tightly regulated immune responses are crucial to sustain placenta homeostasis.208 Besides, human trophoblasts-associated antiviral microRNAs, such as chromosome 19 miRNA cluster that are packaged into placental exosomes, were also systemically isolated from pregnant women, which operate in a paracrine or autocrine manner to resist infection.209,210 Mechanistically, primary human trophoblast-derived chromosome 19 miRNA cluster family members drastically limited both RNA and DNA viral infections in non-placental cells by inducing autophagy, demonstrating a unique placenta-secreted effector for shielding virus-sensitive cells in placenta from infections.198,211
The neonatal fragment crystallizable receptor (FcRn) mediated antibodies transplacental transfer
Transplacental passage of IgG begins in the first trimester of pregnancy and lasts until labor, which sets up another layer of fetal protection from viral infections.212 Generally, endocytosis of IgG from maternal blood was initiated by binding to a canonical IgG shuttle receptor, FcRn, on the apical side of STBs. Furthermore, a successful transplacental transfer of IgG must cross other two barriers: the villous stroma and the fetal endothelium. However, fibroblasts, HBCs, and fetal endothelial cells do not express FcRn, which remains somewhat a knowledge gap regarding IgG transfer.213 Some noncanonical Fc receptors, such as FcγRIII and FcγRII, may be engaged in transplacental IgG transfer as well.213 Recent findings revealed that the selective transfer of maternal IgG traversing placenta involved a panel of risks, including antigen, IgG subclass, and glycan profile, which simultaneously determine the efficient placental transfer of maternal antigen-specific IgG.214 For example, Jennewein et al.215 demonstrated that di-glycosylated Fc-glycans of antigen-specific antibodies, selectively binding to FcRn and FCGR3A, were preferentially passed across the placenta. Future studies defining the determinants and mechanisms of placental IgG transportation will generate possible new strategies to improve the transfer of maternal IgGs to the vulnerable fetus and fundamentally mitigate viral infection-related diseases.
Conclusions and prospects
The mechanisms contributing to maternal and fetal damages due to viral infections are complex and highly depend on various pathogenesis factors such as the infectious power of certain viruses, tissue and cellular tropism, and host–pathogen interactions in the placenta niche. Sustained research efforts on understanding how maternal physiological adaptations to pregnancy govern different susceptibility to certain viral infections are needed. Besides, much remains to be learned about the fundamental and unique features of placental defense mechanisms that can help us to cope better with emerging viruses to avoid congenital diseases during pregnancy, which is especially critical in the context of vertical transmission. Comprehensive clinical and basic studies uncovering the etiological nature of viral infections during pregnancy can empower us with countermeasures to face new viral epidemics that cause known and unanticipated maternal and fetal complications. Foundational research on the development of antiviral treatments and vaccines effective in the pregnant population with no safety concern should be prioritized as a powerful weapon to fight the next epidemics and pandemics.
Funding
This work was supported by the grants from the National Key Research and Development Program of China (2018YFC1004400 to BC), the National Natural Sciences Foundation in China (81971414 and 82130047 to BC), and the Natural Sciences Foundation of Fujian Province of China (2020J06003 to BC).
Author Contributions
Wenzhe Yu, Xiaoqian Hu, and Bin Cao prepared the figures and drafted the manuscript. All authors reviewed the final manuscript and had no dissent on the submission.
Conflicts of Interest
None.
References
- [1].Aghaamoo S, Ghods K, Rahmanian M. Pregnant women with COVID-19: the placental involvement and consequences. J Mol Histol 2021;52(3):427–435. doi:10.1007/s10735-021-09970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baud D, Gubler DJ, Schaub B, et al. An update on Zika virus infection. Lancet 2017;390(10107):2099–2109. doi:10.1016/S0140-6736(17)31450-2. [DOI] [PubMed] [Google Scholar]
- [3].Yong HEJ, Chan SY, Chakraborty A, et al. Significance of the placental barrier in antenatal viral infections. Biochim Biophys Acta Mol Basis Dis 2021;1867(12):166244. doi:10.1016/j.bbadis.2021.166244. [DOI] [PubMed] [Google Scholar]
- [4].Silasi M, Cardenas I, Kwon JY, et al. Viral infections during pregnancy. Am J Reprod Immunol 2015;73(3):199–213. doi:10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol 2014;70(3):401.e1–401.e14. doi:10.1016/j.jaad.2013.09.010. [DOI] [PubMed] [Google Scholar]
- [6].Turco MY, Moffett A. Development of the human placenta. Development 2019;146(22):dev163428. doi:10.1242/dev.163428. [DOI] [PubMed] [Google Scholar]
- [7].Espino A, El Costa H, Tabiasco J, et al. Innate immune response to viral infections at the maternal-fetal interface in human pregnancy. Front Med (Lausanne) 2021;8:674645. doi:10.3389/fmed.2021.674645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Megli CJ, Coyne CB. Infections at the maternal-fetal interface: an overview of pathogenesis and defence [published online ahead of print, 2021 Aug 25]. Nat Rev Microbiol 2021;1–16. doi:10.1038/s41579-021-00610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yuen MF, Chen DS, Dusheiko GM, et al. Hepatitis B virus infection. Nat Rev Dis Primers 2018;4:18035. doi:10.1038/nrdp.2018.35. [DOI] [PubMed] [Google Scholar]
- [10].Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386(10003):1546–1555. doi:10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- [11].Li J, Dong XQ, Wu Z, et al. Unsatisfying antiviral therapeutic effect in patients with mother-to-child transmissed chronic hepatitis B virus infection: a prospective multi-center clinical study. Chin Med J (Engl) 2019;132(22):2647–2656. doi:10.1097/CM9.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Patton H, Tran TT. Management of hepatitis B during pregnancy. Nat Rev Gastroenterol Hepatol 2014;11(7):402–409. doi:10.1038/nrgastro.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lo CM, Liu CL, Chan SC, et al. Failure of hepatitis B vaccination in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B. J Hepatol 2005;43(2):283–287. doi:10.1016/j.jhep.2005.03.013. [DOI] [PubMed] [Google Scholar]
- [14].Tsai KN, Kuo CF, Ou JJ. Mechanisms of hepatitis B virus persistence. Trends Microbiol 2018;26(1):33–42. doi:10.1016/j.tim.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cheung KW, Lao TT. Hepatitis B - vertical transmission and the prevention of mother-to-child transmission. Best Pract Res Clin Obstet Gynaecol 2020;68:78–88. doi:10.1016/j.bpobgyn.2020.02.014. [DOI] [PubMed] [Google Scholar]
- [16].Tan J, Mao X, Zhang G, et al. Hepatitis B surface antigen positivity during pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. J Viral Hepat 2018;25(11):1372–1383. doi:10.1111/jvh.12964. [DOI] [PubMed] [Google Scholar]
- [17].Tse KY, Ho LF, Lao T. The impact of maternal HBsAg carrier status on pregnancy outcomes: a case-control study. J Hepatol 2005;43(5):771–775. doi:10.1016/j.jhep.2005.05.023. [DOI] [PubMed] [Google Scholar]
- [18].Kong D, Liu H, Wei S, et al. A meta-analysis of the association between gestational diabetes mellitus and chronic hepatitis B infection during pregnancy. BMC Res Notes 2014;7:139. doi:10.1186/1756-0500-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu J, Zhang S, Liu M, et al. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. Lancet Glob Health 2017;5(6):e624–e632. doi:10.1016/S2214-109X(17)30142-0. [DOI] [PubMed] [Google Scholar]
- [20].Huang QT, Zhong M. Maternal hepatitis B virus infection and risk of preterm birth in China. Lancet Glob Health 2017;5(6):e563–e564. doi:10.1016/S2214-109X(17)30175-4. [DOI] [PubMed] [Google Scholar]
- [21].Lao TT. Hepatitis B - chronic carrier status and pregnancy outcomes: an obstetric perspective. Best Pract Res Clin Obstet Gynaecol 2020;68:66–77. doi:10.1016/j.bpobgyn.2020.03.006. [DOI] [PubMed] [Google Scholar]
- [22].Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385(9966):430–440. doi:10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- [23].Huang QT, Chen JH, Zhong M, et al. Chronic hepatitis B infection is associated with decreased risk of preeclampsia: a meta-analysis of observational studies. Cell Physiol Biochem 2016;38(5):1860–1868. doi:10.1159/000445548. [DOI] [PubMed] [Google Scholar]
- [24].Huang QT, Chen JH, Zhong M, et al. The risk of placental abruption and placenta previa in pregnant women with chronic hepatitis B viral infection: a systematic review and meta-analysis. Placenta 2014;35(8):539–545. doi:10.1016/j.placenta.2014.05.007. [DOI] [PubMed] [Google Scholar]
- [25].Safir A, Levy A, Sikuler E, et al. Maternal hepatitis B virus or hepatitis C virus carrier status as an independent risk factor for adverse perinatal outcome. Liver Int 2010;30(5):765–770. doi:10.1111/j.1478-3231.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- [26].Luo L, Wu J, Qu Y, et al. Association between maternal HBsAg carrier status and neonatal adverse outcomes: meta-analysis. J Matern Fetal Neonatal Med 2015;28(11):1308–1317. doi:10.3109/14767058.2014.953475. [DOI] [PubMed] [Google Scholar]
- [27].Wan Z, Zhou A, Zhu H, et al. Maternal hepatitis B virus infection and pregnancy outcomes: a hospital-based case-control study in Wuhan, China. J Clin Gastroenterol 2018;52(1):73–78. doi:10.1097/MCG.0000000000000842. [DOI] [PubMed] [Google Scholar]
- [28].Borgia G, Carleo MA, Gaeta GB, et al. Hepatitis B in pregnancy. World J Gastroenterol 2012;18(34):4677–4683. doi:10.3748/wjg.v18.i34.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yarrington CD, Cantonwine DE, Seely EW, et al. The association of early unexplained elevated alanine aminotransferase with large-for-gestational-age birthweight. Am J Obstet Gynecol 2016;215(4):474.e1–474.e5. doi:10.1016/j.ajog.2016.04.051. [DOI] [PubMed] [Google Scholar]
- [30].Kennedy P, Litwin S, Dolman GE, et al. Immune tolerant chronic hepatitis B: the unrecognized risks. Viruses 2017;9(5):96. doi:10.3390/v9050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002;67(1):20–26. doi:10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- [32].Bai H, Zhang L, Ma L, et al. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol 2007;13(26):3625–3630. doi:10.3748/wjg.v13.i26.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med 2018;378(10):911–923. doi:10.1056/NEJMoa1708131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen HL, Lee CN, Chang CH, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology 2015;62(2):375–386. doi:10.1002/hep.27837. [DOI] [PubMed] [Google Scholar]
- [35].Zhou YH. Evidence against in utero transmission of hepatitis B virus. Nat Rev Gastroenterol Hepatol 2021;18(6):445. doi:10.1038/s41575-021-00455-z. [DOI] [PubMed] [Google Scholar]
- [36].Xu DZ, Yan YP, Zou S, et al. Role of placental tissues in the intrauterine transmission of hepatitis B virus. Am J Obstet Gynecol 2001;185(4):981–987. doi:10.1067/mob.2001.117968. [DOI] [PubMed] [Google Scholar]
- [37].Shih YF, Liu CJ. Mother-to-infant transmission of hepatitis B virus: challenges and perspectives. Hepatol Int 2017;11(6):481–484. doi:10.1007/s12072-017-9831-0. [DOI] [PubMed] [Google Scholar]
- [38].Mavilia MG, Wu GY. Mechanisms and prevention of vertical transmission in chronic viral hepatitis. J Clin Transl Hepatol 2017;5(2):119–129. doi:10.14218/JCTH.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sauter D, Kirchhoff F. Key viral adaptations preceding the AIDS pandemic. Cell Host Microbe 2019;25(1):27–38. doi:10.1016/j.chom.2018.12.002. [DOI] [PubMed] [Google Scholar]
- [40].Nyamweya S, Hegedus A, Jaye A, et al. Comparing HIV-1 and HIV-2 infection: lessons for viral immunopathogenesis. Rev Med Virol 2013;23(4):221–240. doi:10.1002/rmv.1739. [DOI] [PubMed] [Google Scholar]
- [41].Wedi CO, Kirtley S, Hopewell S, et al. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV 2016;3(1):e33–e48. doi:10.1016/S2352-3018(15)00207-6. [DOI] [PubMed] [Google Scholar]
- [42].Kreitchmann R, Li SX, Melo VH, et al. Predictors of adverse pregnancy outcomes in women infected with HIV in Latin America and the Caribbean: a cohort study. BJOG 2014;121(12):1501–1508. doi:10.1111/1471-0528.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tukei VJ, Hoffman HJ, Greenberg L, et al. Adverse pregnancy outcomes among HIV-positive women in the era of universal antiretroviral therapy remain elevated compared with HIV-negative women. Pediatr Infect Dis J 2021;40(9):821–826. doi:10.1097/INF.0000000000003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol 2007;17(6):381–403. doi:10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- [45].Rimawi BH, Haddad L, Badell ML, et al. Management of HIV infection during pregnancy in the United States: updated evidence-based recommendations and future potential practices. Infect Dis Obstet Gynecol 2016;2016:7594306. doi:10.1155/2016/7594306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cai CW, Sereti I. Residual immune dysfunction under antiretroviral therapy. Semin Immunol 2021;51:101471. doi:10.1016/j.smim.2021.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Atta MG, De Seigneux S, Lucas GM. Clinical pharmacology in HIV therapy. Clin J Am Soc Nephrol 2019;14(3):435–444. doi:10.2215/CJN.02240218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lewis SH, Reynolds-Kohler C, Fox HE, et al. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological precursors in eight-week fetuses. Lancet 1990;335(8689):565–568. doi:10.1016/0140-6736(90)90349-a. [DOI] [PubMed] [Google Scholar]
- [49].Weinberg A, Naglik JR, Kohli A, et al. Innate immunity including epithelial and nonspecific host factors: workshop 1B. Adv Dent Res 2011;23(1):122–129. doi:10.1177/0022034511399917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Maiques V, García-Tejedor A, Perales A, et al. HIV detection in amniotic fluid samples. Amniocentesis can be performed in HIV pregnant women. Eur J Obstet Gynecol Reprod Biol 2003;108(2):137–141. doi:10.1016/s0301-2115(02)00405-0. [DOI] [PubMed] [Google Scholar]
- [51].Mohlala BK, Tucker TJ, Besser MJ, et al. Investigation of HIV in amniotic fluid from HIV-infected pregnant women at full term. J Infect Dis 2005;192(3):488–491. doi:10.1086/431604. [DOI] [PubMed] [Google Scholar]
- [52].Johnson EL, Chakraborty R. HIV-1 at the placenta: immune correlates of protection and infection. Curr Opin Infect Dis 2016;29(3):248–255. doi:10.1097/QCO.0000000000000267. [DOI] [PubMed] [Google Scholar]
- [53].Tang Y, Woodward BO, Pastor L, et al. Endogenous retroviral envelope syncytin induces HIV-1 spreading and establishes HIV reservoirs in placenta. Cell Rep 2020;30(13):4528–4539.e4. doi:10.1016/j.celrep.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lagaye S, Derrien M, Menu E, et al. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J Virol 2001;75(10):4780–4791. doi:10.1128/JVI.75.10.4780-4791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Menu E, Mbopi-Keou FX, Lagaye S, et al. Selection of maternal human immunodeficiency virus type 1 variants in human placenta. European network for in utero transmission of HIV-1. J Infect Dis 1999;179(1):44–51. doi:10.1086/314542. [DOI] [PubMed] [Google Scholar]
- [56].Johnson EL, Chu H, Byrareddy SN, et al. Placental Hofbauer cells assemble and sequester HIV-1 in tetraspanin-positive compartments that are accessible to broadly neutralizing antibodies. J Int AIDS Soc 2015;18:19385. doi:10.7448/IAS.18.1.19385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Johnson EL, Chakraborty R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 2012;9:101. doi:10.1186/1742-4690-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zulu MZ, Martinez FO, Gordon S, et al. The elusive role of placental macrophages: the Hofbauer cell. J Innate Immun 2019;11(6):447–456. doi:10.1159/000497416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rongkavilit C, Asmar BI. Advances in prevention of mother-to-child HIV transmission: the international perspectives. Indian J Pediatr 2011;78(2):192–204. doi:10.1007/s12098-010-0258-z. [DOI] [PubMed] [Google Scholar]
- [60].Ikumi NM, Matjila M, Gray CM, et al. Placental pathology in women with HIV. Placenta 2021;115:27–36. doi:10.1016/j.placenta.2021.09.006. [DOI] [PubMed] [Google Scholar]
- [61].Kalk E, Schubert P, Bettinger JA, et al. Placental pathology in HIV infection at term: a comparison with HIV-uninfected women. Trop Med Int Health 2017;22(5):604–613. doi:10.1111/tmi.12858. [DOI] [PubMed] [Google Scholar]
- [62].Hygino J, Vieira MM, Kasahara TM, et al. The impact of pregnancy on the HIV-1-specific T cell function in infected pregnant women. Clin Immunol 2012;145(3):177–188. doi:10.1016/j.clim.2012.10.001. [DOI] [PubMed] [Google Scholar]
- [63].Slyker JA, Lohman-Payne B, John-Stewart GC, et al. The impact of HIV-1 infection and exposure on natural killer (NK) cell phenotype in Kenyan infants during the first year of life. Front Immunol 2012;3:399. doi:10.3389/fimmu.2012.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin North Am 2014;28(3):371–402. doi:10.1016/j.idc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Townsend CL, Tookey PA, Newell ML, et al. Antiretroviral therapy in pregnancy: balancing the risk of preterm delivery with prevention of mother-to-child HIV transmission. Antivir Ther 2010;15(5):775–783. doi:10.3851/IMP1613. [DOI] [PubMed] [Google Scholar]
- [66].Rough K, Seage GR, 3rd, Williams PL, et al. Birth outcomes for pregnant women with HIV using tenofovir-emtricitabine. N Engl J Med 2018;378(17):1593–1603. doi:10.1056/NEJMoa1701666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].McCormack SA, Best BM. Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin Pharmacokinet 2014;53(11):989–1004. doi:10.1007/s40262-014-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Altfeld M, Bunders MJ. Impact of HIV-1 infection on the feto-maternal crosstalk and consequences for pregnancy outcome and infant health [published correction appears in Semin Immunopathol. 2016 Nov;38(6):783-784]. Semin Immunopathol 2016;38(6):727–738. doi:10.1007/s00281-016-0578-9. [DOI] [PubMed] [Google Scholar]
- [69].Petrova VN, Russell CA. The evolution of seasonal influenza viruses [published correction appears in Nat Rev Microbiol. 2017 Nov 07]. Nat Rev Microbiol 2018;16(1):47–60. doi:10.1038/nrmicro.2017.118. [DOI] [PubMed] [Google Scholar]
- [70].Van Kerkhove MD, Vandemaele KA, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 2011;8(7):e1001053. doi:10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 2013;347:f5061. doi:10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yupiana Y, de Vlas SJ, Adnan NM, et al. Risk factors of poultry outbreaks and human cases of H5N1 avian influenza virus infection in West Java Province, Indonesia. Int J Infect Dis 2010;14(9):e800–e805. doi:10.1016/j.ijid.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [73].Mauad T, Hajjar LA, Callegari GD, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 2010;181(1):72–79. doi:10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- [74].Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374(9688):451–458. doi:10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- [75].Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010;303(15):1517–1525. doi:10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Neuzil KM, Reed GW, Mitchel EF, et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998;148(11):1094–1102. doi:10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- [77].Flerlage T, Boyd DF, Meliopoulos V, et al. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat Rev Microbiol 2021;19(7):425–441. doi:10.1038/s41579-021-00542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Magdy Beshbishy A, Hetta HF, Hussein DE, et al. Factors associated with increased morbidity and mortality of obese and overweight COVID-19 patients. Biology (Basel) 2020;9(9):280. doi:10.3390/biology9090280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Littauer EQ, Skountzou I. Hormonal regulation of physiology, innate immunity and antibody response to H1N1 influenza virus infection during pregnancy. Front Immunol 2018;9:2455. doi:10.3389/fimmu.2018.02455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Le Gars M, Seiler C, Kay AW, et al. Pregnancy-induced alterations in NK cell phenotype and function. Front Immunol 2019;10:2469. doi:10.3389/fimmu.2019.02469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010;63(6):425–433. doi:10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Forbes RL, Wark PA, Murphy VE, et al. Pregnant women have attenuated innate interferon responses to 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 2012;206(5):646–653. doi:10.1093/infdis/jis377. [DOI] [PubMed] [Google Scholar]
- [83].Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol 2012;30:243–270. doi:10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- [84].Vanders RL, Murphy VE, Gibson PG, et al. CD8 T cells and dendritic cells: key players in the attenuated maternal immune response to influenza infection. J Reprod Immunol 2015;107:1–9. doi:10.1016/j.jri.2014.09.051. [DOI] [PubMed] [Google Scholar]
- [85].Somerville LK, Basile K, Dwyer DE, et al. The impact of influenza virus infection in pregnancy. Future Microbiol 2018;13:263–274. doi:10.2217/fmb-2017-0096. [DOI] [PubMed] [Google Scholar]
- [86].Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med 2005;33(10 Suppl):S390–S397. doi:10.1097/01.ccm.0000182483.24836.66. [DOI] [PubMed] [Google Scholar]
- [87].Yeomans ER, Gilstrap LC, 3rd. Physiologic changes in pregnancy and their impact on critical care. Crit Care Med 2005;33(10 Suppl):S256–S258. doi:10.1097/01.ccm.0000183540.69405.90. [DOI] [PubMed] [Google Scholar]
- [88].Shu Y, Yu H, Li D. Lethal avian influenza A (H5N1) infection in a pregnant woman in Anhui Province, China. N Engl J Med 2006;354(13):1421–1422. doi:10.1056/NEJMc053524. [DOI] [PubMed] [Google Scholar]
- [89].Gu J, Xie Z, Gao Z, et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 2007;370(9593):1137–1145. doi:10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Liong S, Oseghale O, To EE, et al. Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc Natl Acad Sci U S A 2020;117(40):24964–24973. doi:10.1073/pnas.2006905117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Al-Haddad B, Jacobsson B, Chabra S, et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry 2019;76(6):594–602. doi:10.1001/jamapsychiatry.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jacobsen H, Walendy-Gnirß K, Tekin-Bubenheim N, et al. Offspring born to influenza A virus infected pregnant mice have increased susceptibility to viral and bacterial infections in early life. Nat Commun 2021;12(1):4957. doi:10.1038/s41467-021-25220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shuid AN, Jayusman PA, Shuid N, et al. Association between viral infections and risk of autistic disorder: an overview. Int J Environ Res Public Health 2021;18(6):2817. doi:10.3390/ijerph18062817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Short SJ, Lubach GR, Karasin AI, et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry 2010;67(10):965–973. doi:10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci 2005;23(2–3):299–305. doi:10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [96].Lowe R, Barcellos C, Brasil P, et al. The Zika virus epidemic in Brazil: From discovery to future implications. Int J Environ Res Public Health 2018;15(1):96. doi:10.3390/ijerph15010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hcini N, Kugbe Y, Rafalimanana Z, et al. Association between confirmed congenital Zika infection at birth and outcomes up to 3 years of life. Nat Commun 2021;12(1):3270. doi:10.1038/s41467-021-23468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Britt WJ. Adverse outcomes of pregnancy-associated Zika virus infection. Semin Perinatol 2018;42(3):155–167. doi:10.1053/j.semperi.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Adibi JJ, Marques ETA, Jr, Cartus A, et al. Teratogenic effects of the Zika virus and the role of the placenta. Lancet 2016;387(10027):1587–1590. doi:10.1016/S0140-6736(16)00650-4. [DOI] [PubMed] [Google Scholar]
- [100].Broxmeyer L, Kanjhan R. Does Zika really have the capacity to affect the nervous system and cause microcephaly or intracranial calcifications? Mod Res Inflammation 2016;5(2):20–30. doi:10.4236/mri.2016.52003. [Google Scholar]
- [101].Robinson N, Mayorquin Galvan EE, Zavala Trujillo IG, et al. Congenital Zika syndrome: pitfalls in the placental barrier. Rev Med Virol 2018;28(5):e1985. doi:10.1002/rmv.1985. [DOI] [PubMed] [Google Scholar]
- [102].Microcephaly Epidemic Research Group. Microcephaly in infants, Pernambuco State, Brazil, 2015. Emerg Infect Dis 2016;22(6):1090–1093. doi:10.3201/eid2206.160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Del Campo M, Feitosa IM, Ribeiro EM, et al. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet A 2017;173(4):841–857. doi:10.1002/ajmg.a.38170. [DOI] [PubMed] [Google Scholar]
- [104].Valentine G, Marquez L, Pammi M. Zika virus-associated microcephaly and eye lesions in the newborn. J Pediatric Infect Dis Soc 2016;5(3):323–328. doi:10.1093/jpids/piw037. [DOI] [PubMed] [Google Scholar]
- [105].Li C, Xu D, Ye Q, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 2016;19(1):120–126. doi:10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- [106].Chan JF, Choi GK, Yip CC, et al. Zika fever and congenital Zika syndrome: an unexpected emerging arboviral disease. J Infect 2016;72(5):507–524. doi:10.1016/j.jinf.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ticconi C, Pietropolli A, Rezza G. Zika virus infection and pregnancy: what we do and do not know. Pathog Glob Health 2016;110(7–8):262–268. doi:10.1080/20477724.2016.1234804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].King N, Teixeira MM, Mahalingam S. Zika virus: mechanisms of infection during pregnancy. Trends Microbiol 2017;25(9):701–702. doi:10.1016/j.tim.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [109].Tan L, Lacko LA, Zhou T, et al. Pre- and peri-implantation Zika virus infection impairs fetal development by targeting trophectoderm cells. Nat Commun 2019;10(1):4155. doi:10.1038/s41467-019-12063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].de Noronha L, Zanluca C, Burger M, et al. Zika virus infection at different pregnancy stages: anatomopathological findings, target cells and viral persistence in placental tissues. Front Microbiol 2018;9:2266. doi:10.3389/fmicb.2018.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Jagger BW, Miner JJ, Cao B, et al. Gestational stage and IFN-lambda signaling regulate ZIKV infection in utero. Cell Host Microbe 2017;22(3):366–376e.3. doi:10.1016/j.chom.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Simoni MK, Jurado KA, Abrahams VM, et al. Zika virus infection of Hofbauer cells. Am J Reprod Immunol 2017;77(2):e12613. doi:10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tabata T, Petitt M, Puerta-Guardo H, et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016;20(2):155–166. doi:10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wells MF, Salick MR, Wiskow O, et al. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from Zika virus infection. Cell Stem Cell 2016;19(6):703–708. doi:10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- [115].Hastings AK, Yockey LJ, Jagger BW, et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep 2017;19(3):558–568. doi:10.1016/j.celrep.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rosenberg AZ, Yu W, Hill DA, et al. Placental pathology of Zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch Pathol Lab Med 2017;141(1):43–48. doi:10.5858/arpa.2016-0401-OA. [DOI] [PubMed] [Google Scholar]
- [117].Miner JJ, Cao B, Govero J, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016;165(5):1081–1091. doi:10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Raper J, Chahroudi A. Clinical and preclinical evidence for adverse neurodevelopment after postnatal Zika virus infection. Trop Med Infect Dis 2021;6(1):10. doi:10.3390/tropicalmed6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Paul AM, Acharya D, Neupane B, et al. Congenital Zika virus infection in immunocompetent mice causes postnatal growth impediment and neurobehavioral deficits. Front Microbiol 2018;9:2028. doi:10.3389/fmicb.2018.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].V’kovski P, Kratzel A, Steiner S, et al. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat Rev Microbiol 2021;19(3):155–170. doi:10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19(3):141–154. doi:10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy [published online ahead of print, 2021 Sep 14]. Am J Obstet Gynecol 2021;doi:10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Meyerowitz EA, Richterman A, Gandhi RT, et al. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med 2021;174(1):69–79. doi:10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Phoswa WN, Khaliq OP. Is pregnancy a risk factor of COVID-19? Eur J Obstet Gynecol Reprod Biol 2020;252:605–609. doi:10.1016/j.ejogrb.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Haitao T, Vermunt JV, Abeykoon J, et al. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc 2020;95(10):2189–2203. doi:10.1016/j.mayocp.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69(44):1641–1647. doi:10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020;369:m2107. doi:10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jardine J, Relph S, Magee LA, et al. Maternity services in the UK during the coronavirus disease 2019 pandemic: a national survey of modifications to standard care. BJOG 2021;128(5):880–889. doi:10.1111/1471-0528.16547. [DOI] [PubMed] [Google Scholar]
- [129].Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, et al. Maternal death due to COVID-19. Am J Obstet Gynecol 2020;223(1):109.e1–109.e16. doi:10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395(10226):809–815. doi:10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mullins E, Hudak ML, Banerjee J, et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol 2021;57(4):573–581. doi:10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Zamaniyan M, Ebadi A, Aghajanpoor S, et al. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn 2020;40(13):1759–1761. doi:10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM 2020;2(2):100118. doi:10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69(25):769–775. doi:10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. doi:10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Brandt JS, Hill J, Reddy A, et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol 2021;224(4):389.e1–389.e9. doi:10.1016/j.ajog.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yee J, Kim W, Han JM, et al. Clinical manifestations and perinatal outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Sci Rep 2020;10(1):18126. doi:10.1038/s41598-020-75096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kulaga SS, Miller C. Viral respiratory infections and psychosis: a review of the literature and the implications of COVID-19. Neurosci Biobehav Rev 2021;127:520–530. doi:10.1016/j.neubiorev.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Englund JA, Chu HY. Respiratory virus infection during pregnancy: does it matter? J Infect Dis 2018;218(4):512–515. doi:10.1093/infdis/jiy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chu HY, Katz J, Tielsch J, et al. Clinical presentation and birth outcomes associated with respiratory syncytial virus infection in pregnancy. PLoS One 2016;11(3):e0152015. doi:10.1371/journal.pone.0152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020;12(2):194. doi:10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hedermann G, Hedley PL, Bækvad-Hansen M, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed 2021;106(1):93–95. doi:10.1136/archdischild-2020-319990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yang R, Mei H, Zheng T, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med 2020;18(1):330. doi:10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Vousden N, Bunch K, Morris E, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One 2021;16(5):e0251123. doi:10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Jafari M, Pormohammad A, Sheikh Neshin SA, et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol 2021;31(5):1–16. doi:10.1002/rmv.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG 2020;127(12):1548–1556. doi:10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Papapanou M, Papaioannou M, Petta A, et al. Maternal and neonatal characteristics and outcomes of COVID-19 in pregnancy: an overview of systematic reviews. Int J Environ Res Public Health 2021;18(2):596. doi:10.3390/ijerph18020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Khalil A, Kalafat E, Benlioglu C, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020;25:100446. doi:10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Khan M, Nabeka H, Akbar S, et al. Risk of congenital birth defects during COVID-19 pandemic: draw attention to the physicians and policymakers. J Glob Health 2020;10(2):020378. doi:10.7189/jogh.10.020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Leal CRV, Maciel RAM, Corrêa Júnior MD. SARS-CoV-2 infection and placental pathology. Infecção por SARS-CoV-2 e patologia placentária. Rev Bras Ginecol Obstet 2021;43(6):474–479. doi:10.1055/s-0041-1730291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest 2020;130(9):4947–4953. doi:10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bouachba A, Allias F, Nadaud B, et al. Placental lesions and SARS-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta 2021;112:97–104. doi:10.1016/j.placenta.2021.07.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cribiù FM, Erra R, Pugni L, et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J Clin Invest 2021;131(6):e145427. doi:10.1172/JCI145427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM 2020;2(3):100145. doi:10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Algarroba GN, Hanna NN, Rekawek P, et al. Confirmatory evidence of the visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020;223(6):953–954. doi:10.1016/j.ajog.2020.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11(1):3572. doi:10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Xiu S, Dick A, Ju H, et al. Inhibitors of SARS-CoV-2 entry: current and future opportunities. J Med Chem 2020;63(21):12256–12274. doi:10.1021/acs.jmedchem.0c00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Davidson AM, Wysocki J, Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension 2020;76(5):1339–1349. doi:10.1161/HYPERTENSIONAHA.120.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2. Elife 2020;9:e58716. doi:10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Li M, Chen L, Zhang J, et al. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 2020;15(4):e0230295. doi:10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Cui D, Liu Y, Jiang X, et al. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet Gynecol 2021;57(2):248–256. doi:10.1002/uog.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020;323(18):1846–1848. doi:10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020;323(18):1848–1849. doi:10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Tang JY, Song WQ, Xu H, et al. No evidence for vertical transmission of SARS-CoV-2 in two neonates with mothers infected in the second trimester. Infect Dis (Lond) 2020;52(12):913–916. doi:10.1080/23744235.2020.1798499. [DOI] [PubMed] [Google Scholar]
- [165].Grimminck K, Santegoets L, Siemens FC, et al. No evidence of vertical transmission of SARS-CoV-2 after induction of labour in an immune-suppressed SARS-CoV-2-positive patient. BMJ Case Rep 2020;13(6):e235581. doi:10.1136/bcr-2020-235581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Joma M, Fovet CM, Seddiki N, et al. COVID-19 and pregnancy: vertical transmission and inflammation impact on newborns. Vaccines (Basel) 2021;9(4):391. doi:10.3390/vaccines9040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Delahoy MJ, Whitaker M, O’Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 States, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep 2020;69(38):1347–1354. doi:10.15585/mmwr.mm6938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kimberlin DW, Stagno S. Can SARS-CoV-2 infection be acquired in utero?: more definitive evidence is needed. JAMA 2020;323(18):1788–1789. doi:10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- [169].Mahyuddin AP, Kanneganti A, Wong J, et al. Mechanisms and evidence of vertical transmission of infections in pregnancy including SARS-CoV-2s. Prenat Diagn 2020;40(13):1655–1670. doi:10.1002/pd.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Shah PS, Diambomba Y, Acharya G, et al. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand 2020;99(5):565–568. doi:10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Baudin M, Jumaa AM, Jomma H, et al. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health 2016;4(11):e864–e871. doi:10.1016/S2214-109X(16)30176-0. [DOI] [PubMed] [Google Scholar]
- [172].Arishi HM, Aqeel AY, Al Hazmi MM. Vertical transmission of fatal Rift Valley fever in a newborn. Ann Trop Paediatr 2006;26(3):251–253. doi:10.1179/146532806X120363. [DOI] [PubMed] [Google Scholar]
- [173].McMillen CM, Hartman AL. Rift Valley fever: a threat to pregnant women hiding in plain sight. J Virol 2021;95(9):e01394–e01419. doi:10.1128/JVI.01394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Oymans J, Wichgers Schreur PJ, van Keulen L, et al. Rift Valley fever virus targets the maternal-foetal interface in ovine and human placentas. PLoS Negl Trop Dis 2020;14(1):e0007898. doi:10.1371/journal.pntd.0007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Julander JG, Winger QA, Rickords LF, et al. West Nile virus infection of the placenta. Virology 2006;347(1):175–182. doi:10.1016/j.virol.2005.11.040. [DOI] [PubMed] [Google Scholar]
- [176].O’Leary DR, Kuhn S, Kniss KL, et al. Birth outcomes following West Nile virus infection of pregnant women in the United States: 2003-2004. Pediatrics 2006;117(3):e537–545. doi:10.1542/peds.2005-2024. [DOI] [PubMed] [Google Scholar]
- [177].Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Annu Rev Virol 2014;1(1):133–146. doi:10.1146/annurev-virology-031413-085524. [DOI] [PubMed] [Google Scholar]
- [178].Caine EA, Jagger BW, Diamond MS. Animal models of Zika virus infection during pregnancy. Viruses 2018;10(11):598. doi:10.3390/v10110598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Aplin JD, Myers JE, Timms K, et al. Tracking placental development in health and disease. Nat Rev Endocrinol 2020;16(9):479–494. doi:10.1038/s41574-020-0372-6. [DOI] [PubMed] [Google Scholar]
- [180].Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta 2014;35(5):303–304. doi:10.1016/j.placenta.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev 2016;96(4):1509–1565. doi:10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Boss AL, Chamley LW, James JL. Placental formation in early pregnancy: how is the centre of the placenta made? Hum Reprod Update 2018;24(6):750–760. doi:10.1093/humupd/dmy030. [DOI] [PubMed] [Google Scholar]
- [183].Goldman-Wohl DS, Ariel I, Greenfield C, et al. HLA-G expression in extravillous trophoblasts is an intrinsic property of cell differentiation: a lesson learned from ectopic pregnancies. Mol Hum Reprod 2000;6(6):535–540. doi:10.1093/molehr/6.6.535. [DOI] [PubMed] [Google Scholar]
- [184].Amirhessami-Aghili N, Manalo P, Hall MR, et al. Human cytomegalovirus infection of human placental explants in culture: histologic and immunohistochemical studies. Am J Obstet Gynecol 1987;156(6):1365–1374. doi:10.1016/0002-9378(87)90002-0. [DOI] [PubMed] [Google Scholar]
- [185].Fisher S, Genbacev O, Maidji E, et al. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 2000;74(15):6808–6820. doi:10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y) 2021;2(5):591–610.e10. doi:10.1016/j.medj.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Robbins JR, Skrzypczynska KM, Zeldovich VB, et al. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog 2010;6(1):e1000732. doi:10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Liu T, Zheng X, Chen J, et al. Effect of human cytomegalovirus on invasive capability of early pregnant extravillous cytotrophoblasts. J Huazhong Univ Sci Technolog Med Sci 2011;31(6):819–823. doi:10.1007/s11596-011-0683-x. [DOI] [PubMed] [Google Scholar]
- [189].Chou D, Ma Y, Zhang J, et al. Cytomegalovirus infection of trophoblast cells elicits an inflammatory response: a possible mechanism of placental dysfunction. Am J Obstet Gynecol 2006;194(2):535–541. doi:10.1016/j.ajog.2005.07.073. [DOI] [PubMed] [Google Scholar]
- [190].Petitt M, Tabata T, Puerta-Guardo H, et al. Zika virus infection of first-trimester human placentas: utility of an explant model of replication to evaluate correlates of immune protection ex vivo. Curr Opin Virol 2017;27:48–56. doi:10.1016/j.coviro.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Tilburgs T, Crespo ÂC, van der Zwan A, et al. Human HLA-G+ extravillous trophoblasts: immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci U S A 2015;112(23):7219–7224. doi:10.1073/pnas.1507977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Quicke KM, Bowen JR, Johnson EL, et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016;20(1):83–90. doi:10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zimmerman MG, Quicke KM, O’Neal JT, et al. Cross-reactive dengue virus antibodies augment Zika virus infection of human placental macrophages. Cell Host Microbe 2018;24(5):731–742.e6. doi:10.1016/j.chom.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Zeldovich VB, Clausen CH, Bradford E, et al. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog 2013;9(12):e1003821. doi:10.1371/journal.ppat.1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta 2009;1788(4):832–841. doi:10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- [196].Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011;469(7330):323–335. doi:10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hung TH, Hsieh TT, Chen SF, et al. Autophagy in the human placenta throughout gestation. PLoS One 2013;8(12):e83475. doi:10.1371/journal.pone.0083475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Jackson WT. Autophagy as a broad antiviral at the placental interface. Autophagy 2013;9(12):1905–1907. doi:10.4161/auto.26819. [DOI] [PubMed] [Google Scholar]
- [199].Cao B, Macones C, Mysorekar IU. ATG16L1 governs placental infection risk and preterm birth in mice and women. JCI Insight 2016;1(21):e86654. doi:10.1172/jci.insight.86654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Dimasuay KG, Gong L, Rosario F, et al. Impaired placental autophagy in placental malaria. PLoS One 2017;12(11):e0187291. doi:10.1371/journal.pone.0187291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Cao B, Parnell LA, Diamond MS, et al. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J Exp Med 2017;214(8):2303–2313. doi:10.1084/jem.20170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Yockey LJ, Jurado KA, Arora N, et al. Type I interferons instigate fetal demise after Zika virus infection. Sci Immunol 2018;3(19):eaao1680. doi:10.1126/sciimmunol.aao1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Buchrieser J, Degrelle SA, Couderc T, et al. IFITM proteins inhibit placental syncytiotrophoblast formation and promote fetal demise. Science 2019;365(6449):176–180. doi:10.1126/science.aaw7733. [DOI] [PubMed] [Google Scholar]
- [204].Zani A, Zhang L, McMichael TM, et al. Interferon-induced transmembrane proteins inhibit cell fusion mediated by trophoblast syncytins. J Biol Chem 2019;294(52):19844–19851. doi:10.1074/jbc.AC119.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Bayer A, Lennemann NJ, Ouyang Y, et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016;19(5):705–712. doi:10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol 2010;63(6):587–600. doi:10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Cornish EF, Filipovic I, Åsenius F, et al. Innate immune responses to acute viral infection during pregnancy. Front Immunol 2020;11:572567. doi:10.3389/fimmu.2020.572567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Reis AS, Barboza R, Murillo O, et al. Inflammasome activation and IL-1 signaling during placental malaria induce poor pregnancy outcomes. Sci Adv 2020;6(10):eaax6346. doi:10.1126/sciadv.aax6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Bayer A, Lennemann NJ, Ouyang Y, et al. Chromosome 19 microRNAs exert antiviral activity independent from type III interferon signaling. Placenta 2018;61:33–38. doi:10.1016/j.placenta.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Donker RB, Mouillet JF, Chu T, et al. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod 2012;18(8):417–424. doi:10.1093/molehr/gas013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A 2013;110(29):12048–12053. doi:10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Marshall H, McMillan M, Andrews RM, et al. Vaccines in pregnancy: the dual benefit for pregnant women and infants. Hum Vaccin Immunother 2016;12(4):848–856. doi:10.1080/21645515.2015.1127485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Martinez DR, Fouda GG, Peng X, et al. Noncanonical placental Fc receptors: what is their role in modulating transplacental transfer of maternal IgG. PLoS Pathog 2018;14(8):e1007161. doi:10.1371/journal.ppat.1007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Palmeira P, Quinello C, Silveira-Lessa AL, et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012;2012:985646. doi:10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Jennewein MF, Goldfarb I, Dolatshahi S, et al. Fc glycan-mediated regulation of placental antibody transfer. Cell 2019;178(1):202–215.e14. doi:10.1016/j.cell.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]