Abstract
Background
Older age is associated with poorer outcomes of SARS-CoV-2 infection, although the heterogeneity of ageing results in some older adults being at greater risk than others. The objective of this study was to quantify the association of a novel geriatric risk index, comprising age, modified Charlson comorbidity index, and Eastern Cooperative Oncology Group performance status, with COVID-19 severity and 30-day mortality among older adults with cancer.
Methods
In this cohort study, we enrolled patients aged 60 years and older with a current or previous cancer diagnosis (excluding those with non-invasive cancers and premalignant or non-malignant conditions) and a current or previous laboratory-confirmed COVID-19 diagnosis who reported to the COVID-19 and Cancer Consortium (CCC19) multinational, multicentre, registry between March 17, 2020, and June 6, 2021. Patients were also excluded for unknown age, missing data resulting in unknown geriatric risk measure, inadequate data quality, or incomplete follow-up resulting in unknown COVID-19 severity. The exposure of interest was the CCC19 geriatric risk index. The primary outcome was COVID-19 severity and the secondary outcome was 30-day all-cause mortality; both were assessed in the full dataset. Adjusted odds ratios (ORs) and 95% CIs were estimated from ordinal and binary logistic regression models.
Findings
5671 patients with cancer and COVID-19 were included in the analysis. Median follow-up time was 56 days (IQR 22–120), and median age was 72 years (IQR 66–79). The CCC19 geriatric risk index identified 2365 (41·7%) patients as standard risk, 2217 (39·1%) patients as intermediate risk, and 1089 (19·2%) as high risk. 36 (0·6%) patients were excluded due to non-calculable geriatric risk index. Compared with standard-risk patients, high-risk patients had significantly higher COVID-19 severity (adjusted OR 7·24; 95% CI 6·20–8·45). 920 (16·2%) of 5671 patients died within 30 days of a COVID-19 diagnosis, including 161 (6·8%) of 2365 standard-risk patients, 409 (18·5%) of 2217 intermediate-risk patients, and 350 (32·1%) of 1089 high-risk patients. High-risk patients had higher adjusted odds of 30-day mortality (adjusted OR 10·7; 95% CI 8·54–13·5) than standard-risk patients.
Interpretation
The CCC19 geriatric risk index was strongly associated with COVID-19 severity and 30-day mortality. Our CCC19 geriatric risk index, based on readily available clinical factors, might provide clinicians with an easy-to-use risk stratification method to identify older adults most at risk for severe COVID-19 as well as mortality.
Funding
US National Institutes of Health National Cancer Institute Cancer Center.
Introduction
The COVID-19 pandemic, caused by SARS-CoV-2, has disproportionately affected older adults. Mortality and complication rates from the disease have been reported as higher among older adults than younger adults.1 Individuals with cancer who develop COVID-19 are at risk for more severe outcomes than those without cancer.2 Among those with cancer, age also increases the risk of adverse outcomes of infection.3
Because aging is heterogeneous, subgroups of older adults with cancer will probably be at greater risk than others for developing adverse outcomes of COVID-19, and identification of individuals at the highest risk is important in their clinical care. Studies in the general geriatric population have shown that the presence of comorbidities and frailty increase the risk of death and other complications of COVID-19, as do the presence of comorbidities and poor performance status in those with cancer.4, 5, 6
Research in context.
Evidence before this study
We searched PubMed for studies published from inception up to Oct 28, 2021, using the key terms “COVID-19” in combination with “cancer”, “frailty”, and “comorbidities”. Systematic reviews showed the increased risk of adverse outcomes of COVID-19 among people of older age, with cancer, comorbidities, and who were frail. Studies identifying older adults at greatest risk for adverse outcomes and mortality after SARS-CoV-2 infection are scarce.
Added value of this study
Among patients aged 60 years or older reported to the COVID-19 and Cancer Consortium, patients categorised as high risk—using a novel geriatric risk index comprising age, performance status, and comorbidities—had significantly higher COVID-19 severity, including hospitalisation, need for intensive care and mechanical ventilation, and death, within 30 days due to any cause than patients categorised as standard risk or intermediate risk.
Implications of all the available evidence
Patients with cancer identified as high risk are at higher risk for severe complications due to COVID-19 than patients categorised as standard risk or intermediate risk, highlighting the need for continued protective strategies for this vulnerable population.
Despite this heightened risk of mortality, there is insufficient understanding of which subpopulations are at high risk for adverse outcomes of COVID-19.7 The Cancer and Aging Research Group and the International Society of Geriatric Oncology released statements on older people with cancer, but noted that these statements were based on clinical consensus, not robust evidence.8, 9 As a result, there is an urgent need to determine the effect of COVID-19 in older adults with cancer and identify those most vulnerable for adverse outcomes. The primary objective of this study was to determine whether a measure of geriatric risk—combining age, comorbidities, and performance status—could capture risk of severe clinical outcomes among older patients with cancer and COVID-19. We also sought to describe the presentation, complications, and effect of COVID-19 on subsequent cancer care among older adults with cancer.
Methods
Study design and participants
This was a cohort study of patients reported to the COVID-19 and Cancer Consortium (CCC19) registry.10, 11 The CCC19 registry is accruing deidentified data, from contributing institutions (appendix pp 1–5), on patients aged 18 years or older with a current or past history of haematological malignancy or invasive solid tumour who have either a laboratory-confirmed SARS-CoV-2 infection or a presumptive diagnosis of COVID-19. Contributing institutions in the consortium independently identify patients and report data through the online REDCap data collection survey instruments developed by CCC19, described in detail previously.10 The mechanism of data collection can be retrospective (after the acute course of COVID-19) or concurrent, at the discretion of the respondent, and can be for outpatients or hospitalised patients.
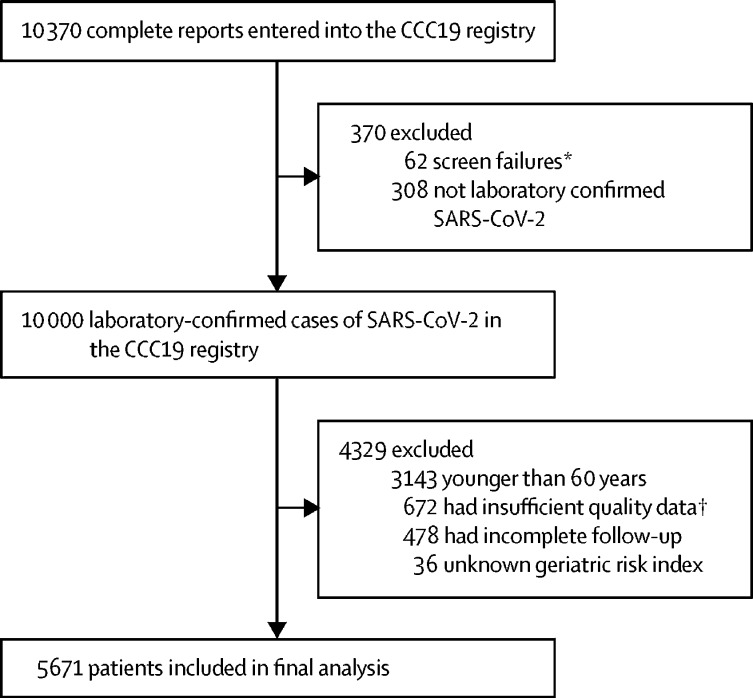
We describe patients who were reported to the registry between March 17, 2020, and June 6, 2021, who were 60 years or older at the time of COVID-19 diagnosis, with a current or previous diagnosis of invasive malignancy (regardless of date of cancer diagnosis), and a current or previous laboratory-confirmed diagnosis of SARS-CoV-2 (excluding those with a presumptive diagnosis). All variables used for the study are data abstracted from electronic health records by health-care professionals. We selected people 60 years or older because of the incidence of geriatric vulnerabilities, a potentially high risk for adverse outcomes in this age group, and the fact that previous work in this registry population did not show an inflection point in outcomes at commonly used but arbitrary cutoffs for age (such as ≥65 years or ≥70 years).6, 12 Patients with non-invasive cancers and premalignant or non-malignant conditions were excluded. Patients were also excluded due to unknown age, missing data resulting in unknown geriatric risk measure, inadequate data quality (quality score ≥5 according to our previously published metric11), or incomplete follow-up resulting in unknown COVID-19 severity (figure 1 ). The full CCC19 data dictionary is available online. No protected health information, as defined by the Health Insurance Portability and Accountability Act of 1996 is collected by this centralised registry, which was considered exempt from institutional review board review (VUMC institutional review board 200467) and was approved by local institutional review boards at participating sites per institutional policy, according to the principles of the Declaration of Helsinki. This study is registered on ClinicalTrials.gov, NCT04354701, and is ongoing.
Figure 1.
CONSORT diagram and cohort assembly
CCC19=COVID-19 and Cancer Consortium. *In-situ malignancy, precursor haematologic condition, benign haematologic condition, non-melanoma skin cancer, non-invasive malignancy, false-positive SARS-CoV-2 test, or non-CCC19 site. †Quality score ≥5.
Procedures
Presentation of SARS-CoV-2 infection was categorised as typical, atypical, or none of the listed symptoms.13 Patients considered to have typical presentations reported one or more typical symptom, as classified by the US Centers for Disease Control and Prevention: fever (subjective, >100·4°F, or >38°C), cough, dyspnoea, myalgia, arthralgia, headache, anosmia, ageusia, sore throat, rhinorrhoea, nausea, vomiting, diarrhoea, and abdominal pain.13 Patients considered to have atypical presentations reported one or more atypical symptoms only: fatigue, altered mental state, abdominal discomfort, conjunctivitis, and all other symptoms not previously categorised as typical. COVID-19 severity at presentation was categorised as follows: mild if no hospitalisation indicated, moderate if hospitalisation indicated, and severe if intensive care unit (ICU) admission indicated. Presentation of infection was categorised on the basis of the level of care medically indicated for COVID-19 as reported in the registry, rather than only on the level of care administered, to more accurately reflect the true severity of disease than categorisation on the basis of patient preference or institutional policies in the setting of resource limitation.
To define geriatric risk level, we a priori adapted the simplified International Myeloma Working Group (IMWG) frailty score, developed in non-COVID-19 patients, adding an intermediate group (appendix p 6).14 The CCC19 registry uses data generally available from electronic health records and was not specifically designed to examine frailty or the population of older adults. As such, no comprehensive geriatric assessment data or specific data encompassing most extant approaches to operationalising frailty (ie, Clinical Frailty Scale) are collected. Of the available registry variables that can be associated with ageing, performance status and comorbidities were the most complete and a priori expected to be prognostic, on the basis of existing literature. However, rather than simply examining each variable, we sought to aggregate these ageing-associated variables into a single index to provide a clinically useful measure. Adapting the IMWG measure into the CCC19 geriatric risk index allowed us to use a previously published approach to combining these variables. The calculation for CCC19 geriatric risk index was based on age, Charlson comorbidity index (CCI; modified to exclude cancer diagnosis as a comorbidity, not age-adjusted)15, and Eastern Cooperative Oncology Group (ECOG)16 performance status as follows: age (≤75 years, 0 points; 76–80 years, 1 point; >80 years, 2 points); CCI (zero, 0 points; one or two, 1 point; more than two, 2 points); and ECOG performance status (zero, 0 points; one, 1 point; two or more, 2 points). ECOG performance status was determined by the health-care professionals abstracting from the patient's chart, and they were instructed to enter the ECOG performance status at the time closest to COVID-19 diagnosis, or to note that no ECOG performance status was recorded within 3 months before COVID-19 diagnosis if applicable. Based on the sum of these data patients were categorised as standard risk (0 or 1 point), intermediate risk (2 or 3 points), or high risk (4–6 points). To maximise use of the available data, all patients had a geriatric risk point total calculated; patients with unknown ECOG performance status were initially categorised separately on the basis of the available age and comorbidity data and denoted as belonging to at least that category, recognising that their level of geriatric risk might be underestimated. For simplicity of clinical application and given similarity to the next lowest groups, these at least categories were consolidated (eg, at least standard risk was consolidated with standard risk). We analysed the non-consolidated measure in a sensitivity analysis. Variables used to define the CCC19 geriatric risk measure are summarised in the appendix (p 6).
Covariates
Covariates were defined a priori and were sex, race and ethnicity, smoking status, obesity, dementia, malignancy type (solid cancer or haematological neoplasm), cancer status (remission or no evidence of disease vs active or measurable disease, with active or measurable disease defined as responding to therapy, stable, or progressing), anti-cancer therapy within 3 months before COVID-19 diagnosis (any systemic therapy, radiotherapy, and excluding surgery), country of patient residence (USA or outside USA), and month of COVID-19 diagnosis (January to April, 2020; May to August, 2020; September to December, 2020; January to April, 2021; and May to June, 2021).
Outcomes
The primary outcome was a six-level ordinal scale of COVID-19 severity based on a patient's most severe reported disease status: none of the following complications; hospitalisation; hospitalisation with oxygen requirement; ICU admission; requirement for mechanical ventilation; and death due to any cause. This scale was similar to the five-level ordinal variable used in previous CCC19 analyses, but with the additional level of hospitalisation with oxygen requirement, to account for older adults with greater severity than those hospitalised without oxygen requirement, but not requiring ICU admission.6 These outcomes were assessed over the patient's total follow-up period. The secondary outcome was death from any cause within 30 days of COVID-19 diagnosis. Primary and secondary outcomes were assessed in the full dataset.
Predefined exploratory outcomes included symptoms and severity of COVID-19 at presentation, as well as receipt of anti-COVID-19 treatments (including remdesivir, hydroxychloroquine, and corticosteroids), major clinical complications (including cardiovascular, pulmonary, and gastrointestinal complications and acute kidney injury), and COVID-19 effect on subsequent cancer care (ie, therapy modifications).
Statistical analysis
All analysis approaches were specified in a statistical analysis plan before the initiation of the analysis.17 Standard descriptive statistics were used to summarise baseline demographic and clinical characteristics, symptoms and severity of COVID-19 at presentation, rates of anti-COVID-19 treatments, outcomes, clinical complications, and anti-cancer therapy modifications among geriatric risk subgroups. Intersections of COVID-19 symptoms were summarised overall and among patients aged 80 years or older. Multiple imputation (ten iterations; missingness rates were <5%) using additive regression, bootstrapping, and predictive mean matching was used to impute missing and unknown data, except unknown ECOG performance scores and unknown cancer status, which were included as unknown categories. Imputation was done on the full dataset before exclusions.
Unadjusted and adjusted odds ratios (ORs) quantifying the association of geriatric risk with COVID-19 severity were estimated from ordinal logistic regression models and 30-day mortality from binary logistic regression models. Models for COVID-19 severity included an offset for (log) follow-up time because severity was assessed throughout patients' total follow-up period. Covariates were selected for inclusion in adjusted models on the basis of the LASSO.18 Multivariable models that included all covariates (listed above) plus age, modified CCI, ECOG performance status, and dementia were fit to each imputed dataset, with the shrinkage penalty estimated from the first two datasets via cross-validation and averaged. Covariates with non-zero coefficients in all imputed datasets were retained; country of patient residence and month of COVID-19 diagnosis were included as design variables. Coefficients and SEs from unadjusted and adjusted models, variance inflation factors, and clinical judgement were used to assess model stability. Exploratory analyses with smoothing splines were used to determine the association of age (as a continuous variable) with outcomes, which appeared linear. All other covariates were categorical. The relative importance of each variable was quantified using its proportion of the model's χ2 statistic, obtained via analysis of variance. Analyses were done using R (version 4.0.2), including the Hmisc, rms, ordinalNet, and glmnet extension packages.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
In total, 5671 patients were included in the final analysis, with only 36 (0·6% of 5671) patients excluded from the cohort because of non-calculable geriatric risk index due to both missing or unknown performance scores and comorbidities (figure 1). The median age was 72 years (IQR 66–79); 1296 (22·9%) patients were 80 years or older. Median follow-up time was 56 days (IQR 22–120). 4659 (82·2%) patients had a solid tumour diagnosis. Regarding cancer status, 2730 (48·1%) patients had cancer in remission or no evidence of disease, and 2439 (43·0%) had active malignancy; the remainder had unknown or missing cancer status. When categorised using the CCC19 geriatric risk measure, 2365 (41·7%) patients were standard risk, 2217 (39·1%) were intermediate risk, and 1089 (19·2%) were high risk (table 1 ; appendix p 7). Smoking and dementia were substantially more common in high-risk patients than standard-risk patients (table 2 ); no other substantial differences were observed. Cancer types were distributed similarly across geriatric risk categories (appendix p 8).
Table 1.
COVID-19 symptoms, severity, treatments, outcomes, and complications among older adults with cancer, stratified by geriatric risk index
| Standard-risk patients | Intermediate-risk patients | High-risk patients | |||
|---|---|---|---|---|---|
| Outcomes | |||||
| Hospitalised | 1122/2365 (47·4%) | 1524/2217 (68·7%) | 925/1088 (85·0%) | ||
| Without oxygen requirement | 351/2358 (14·9%) | 406/2205 (18·4%) | 221/1080 (20·5%) | ||
| With oxygen requirement | 764/2358 (32·4%) | 1108/2205 (50·2%) | 699/1080 (64·7%) | ||
| Admitted to intensive care unit | 319/2350 (13·6%) | 478/2193 (21·8%) | 249/1060 (23·5%) | ||
| Received mechanical ventilation | 211/2357 (9·0%) | 305/2204 (13·8%) | 128/1076 (11·9%) | ||
| Died within 30 days | 161/2365 (6·8%) | 409/2216 (18·5%) | 350/1089 (32·1%) | ||
| Died during follow-up | 234/2365 (9·9%) | 523/2217 (23·6%) | 438/1089 (40·2%) | ||
| Presentation of infection* | |||||
| No symptoms | 332/2365 (14·0%) | 307/2217 (13·8%) | 125/1089 (11·5%) | ||
| Typical | 1926/2365 (81·4%) | 1788/2217 (80·6%) | 865/1089 (79·4%) | ||
| Atypical | 107/2365 (4·5%) | 122/2217 (5·5%) | 99/1089 (9·1%) | ||
| Severity at presentation† | |||||
| Mild | 1431/2360 (60·6%) | 958/2210 (43·3%) | 282/1084 (26·0%) | ||
| Moderate | 736/2360 (31·2%) | 964/2210 (43·6%) | 625/1084 (57·7%) | ||
| Severe | 193/2360 (8·2%) | 288/2210 (13·0%) | 177/1084 (16·3%) | ||
| Anti-COVID-19 treatments | |||||
| Remdesivir | 283/2268 (12·5%) | 362/2144 (16·9%) | 165/1063 (15·5%) | ||
| Hydroxychloroquine | 306/2268 (13·5%) | 384/2144 (17·9%) | 226/1063 (21·3%) | ||
| Corticosteroids | 471/2268 (20·8%) | 587/2144 (27·4%) | 270/1063 (25·4%) | ||
| Other | 554/2268 (24·4%) | 595/2144 (27·8%) | 296/1063 (27·8%) | ||
| None | 1339/2268 (59·0%) | 1059/2144 (49·4%) | 488/1063 (45·9%) | ||
| Complications | |||||
| Cardiovascular | 401/2354 (17%) | 649/2201 (29·5%) | 430/1079 (39·9%) | ||
| Pulmonary | 761/2353 (32·3%) | 1052/2207 (47·7%) | 663/1081 (61·3%) | ||
| Gastrointestinal | 88/2347 (3·7%) | 104/2195 (4·7%) | 49/1074 (4·6%) | ||
| Systemic | 293/2349 (12·5%) | 513/2199 (23·3%) | 309/1076 (28·7%) | ||
| Acute kidney injury | 268/2349 (11·4%) | 522/2199 (23·7%) | 382/1079 (35·4%) | ||
| Cancer therapy modification‡ | 290/640 (45·3%) | 207/462 (44·8%) | 77/157 (49·0%) | ||
| Delayed | 273/640 (42·7%) | 189/462 (40·9%) | 71/157 (45·2%) | ||
| Stopped | <5/640 (<1%) | 8/462 (1·7%) | <5/157 (<3%) | ||
| Continued at a lower dose | <5/640 (<1%) | <5/462 (<1%) | <5/157 (<3%) | ||
Data are n/N (%). Cells with less than five patients were masked (ie, <5) to minimise the risk of re-identification as per CCC19 policy. Number of patients does not include those with missing data. CCC19=COVID-19 and Cancer Consortium.
No symptoms: none of the following typical or atypical symptoms reported; typical: at least one typical symptom reported (fever [subjective, >100·4°F, or >38°C], cough, dyspnoea, myalgia, arthralgia, headache, anosmia, ageusia, sore throat, rhinorrhoea, nausea, vomiting, diarrhoea, or abdominal pain); atypical: only atypical symptoms reported (fatigue, altered mental state, abdominal discomfort, conjunctivitis, or all other symptoms).
Mild: no hospitalisation indicated; moderate: hospitalisation indicated, despite whether it occurred; severe: intensive care unit admission indicated, despite whether it occurred.
Among patients receiving anticancer therapy within 3 months before a COVID-19 diagnosis. Subsequent cancer therapy modification is only collected on the follow-up forms, such that patients with only baseline forms filled would not have this information.
Table 2.
Demographic and clinical characteristics at COVID-19 diagnosis of older adults with cancer, stratified by geriatric risk index
| Standard risk (n=2365) | Intermediate risk (n=2217) | High risk (n=1089) | ||
|---|---|---|---|---|
| Age, years* | 67 (63–72) | 73 (67–79) | 83 (78–87) | |
| 60–69 | 1516 (64·1%) | 750 (33·8%) | 70 (6·4%) | |
| 70–79 | 822 (34·8%) | 949 (42·8%) | 268 (24·6%) | |
| ≥80 | 27 (1·1%) | 518 (23·4%) | 751 (68·9%) | |
| Sex | ||||
| Female | 1199 (50·7%) | 1012 (45·6%) | 500 (45·9%) | |
| Male | 1165 (49·3%) | 1203 (54·3%) | 588 (54 ·0%) | |
| Missing data or unknown | 1 (<0·1%) | 2 (<0·1%) | 1 (<0·1%) | |
| Race and ethnicity | ||||
| Non-Hispanic White | 1356 (57·3%) | 1267 (57·1%) | 660 (60·6%) | |
| Non-Hispanic Black | 416 (17·6%) | 437 (19·7%) | 198 (18·2%) | |
| Hispanic | 303 (12·8%) | 234 (10·6%) | 86 (7·9%) | |
| Other | 247 (10·4%) | 240 (10·8%) | 130 (11·9%) | |
| Missing data or unknown | 43 (1·8%) | 39 (1·8%) | 15 (1·4%) | |
| Smoking status | ||||
| Ever | 1024 (43·3%) | 1213 (54·7%) | 625 (57·4%) | |
| Never | 1271 (53·7%) | 943 (42·5%) | 414 (38·0%) | |
| Missing data or unknown | 8 (0·3%) | 16 (0·7%) | 4 (0·4%) | |
| Obesity† | ||||
| Obese | 883 (37·3%) | 774 (34·9%) | 306 (28·1%) | |
| Not obese | 1482 (62·7%) | 1436 (64·8%) | 783 (71·9%) | |
| Missing data or unknown | 0 | 7 (0·3%) | 0 | |
| Has dementia | ||||
| Yes | 7 (0·3%) | 93 (4·2%) | 186 (17·1%) | |
| No | 2358 (99·7%) | 2115 (95·4%) | 903 (82·9%) | |
| Missing data or unknown | 0 | 9 (0·4%) | 0 | |
| Type of malignancy‡ | ||||
| Solid tumour | 1919 (81·1%) | 1844 (83·2%) | 896 (82·3%) | |
| Haematological neoplasm | 530 (22·4%) | 478 (21·6%) | 254 (23·3%) | |
| Cancer stage | ||||
| Localised | 1314 (55·6%) | 1131 (51·0%) | 583 (53·5%) | |
| Disseminated | 646 (27·3%) | 667 (30·1%) | 317 (29·1%) | |
| Missing data or unknown | 405 (17·1%) | 419 (18·9%) | 189 (17·4%) | |
| Cancer status | ||||
| Remission or no evidence of disease | 1194 (50·5%) | 1033 (46·6%) | 503 (46·2%) | |
| Active and responding | 271 (11·5%) | 237 (10·7%) | 111 (10·2%) | |
| Active and stable | 445 (18·8%) | 405 (18·3%) | 208 (19·1%) | |
| Active and progressing | 251 (10·6%) | 350 (15·8%) | 161 (14·8%) | |
| Unknown | 201 (8·5%) | 190 (8·6%) | 106 (9·7%) | |
| Missing data | 3 (0·1%) | 2 (0·1%) | 0 | |
| Timing of anti-cancer therapy | ||||
| Never or after COVID-19 diagnosis | 223 (9·4%) | 157 (7·1%) | 98 (9·0%) | |
| <2 weeks before COVID-19 diagnosis | 611 (25·8%) | 544 (24·5%) | 242 (22·2%) | |
| 2–4 weeks before COVID-19 diagnosis | 204 (8·6%) | 181 (8·2%) | 71 (6·5%) | |
| 1–3 months before COVID-19 diagnosis | 195 (8·2%) | 215 (9·7%) | 84 (7·7%) | |
| >3 months before COVID-19 diagnosis | 1055 (44·6%) | 1035 (46·7%) | 534 (49·0%) | |
| Missing data or unknown | 49 (2·1%) | 55 (2·5%) | 50 (4·6%) | |
| Intent of recent anti-cancer therapy§ | ||||
| No recent therapy | 1292 (54·6%) | 1223 (55·2%) | 676 (62·1%) | |
| Palliative | 511 (21·6%) | 554 (25·0%) | 264 (24·2%) | |
| Curative | 449 (19·0%) | 334 (15·1%) | 108 (9·9%) | |
| Missing data or unknown | 50 (2·1%) | 52 (2·3%) | 25 (2·3%) | |
| Modality of recent anti-cancer therapyठ| ||||
| None | 1292 (54·6%) | 1223 (55·2%) | 676 (62·1%) | |
| Cytotoxic chemotherapy | 389 (16·4%) | 398 (18·0%) | 135 (12·4%) | |
| Targeted therapy | 320 (13·5%) | 269 (12·1%) | 118 (10·8%) | |
| Endocrine therapy | 250 (10·6%) | 191 (8·6%) | 113 (10·4%) | |
| Immunotherapy | 119 (5·0%) | 154 (6·9%) | 59 (5·4%) | |
| Locoregional therapy | 222 (9·4%) | 211 (9·5%) | 71 (6·5%) | |
| Other | 35 (1·5%) | 32 (1·4%) | 18 (1·7%) | |
| Missing data or unknown | 63 (2·7%) | 54 (2·4%) | 16 (1·5%) | |
| Country of residence | ||||
| USA | 2266 (95·8%) | 2132 (96·2%) | 1005 (92·3%) | |
| Outside USA | 99 (4·2%) | 85 (3·8%) | 84 (7·7%) | |
| Month of COVID-19 diagnosis | ||||
| January–April, 2020 | 558 (23·6%) | 571 (25·8%) | 377 (34·6%) | |
| May–August, 2020 | 980 (41·4%) | 886 (40·0%) | 403 (37·0%) | |
| September–December, 2020 | 493 (20·8%) | 401 (18·1%) | 154 (14·1%) | |
| January–April, 2021 | 320 (13·5%) | 338 (15·2%) | 142 (13%) | |
| May–June, 2021 | 10 (0·4%) | 14 (0·6%) | 9 (0·8%) | |
| Missing data or unknown | 4 (0·2%) | 7 (0·3%) | 4 (0·4%) | |
Data are n (%) or median (IQR). The missing data or unknown category indicates either missingness due to non-response to optional survey questions or a response of unknown; an unknown category was provided for all survey questions.
For patients older than 89 years, age was truncated to 90 years. Truncation was done in concordance with the Health Insurance Portability and Accountability Act of 1996 and to reduce the risk of re-identifiability.
Patient reported to be obese or to have a body-mass index ≥30 kg/m2.
Percentages could sum to >100% because categories are not mutually exclusive.
Within 3 months before COVID-19 diagnosis.
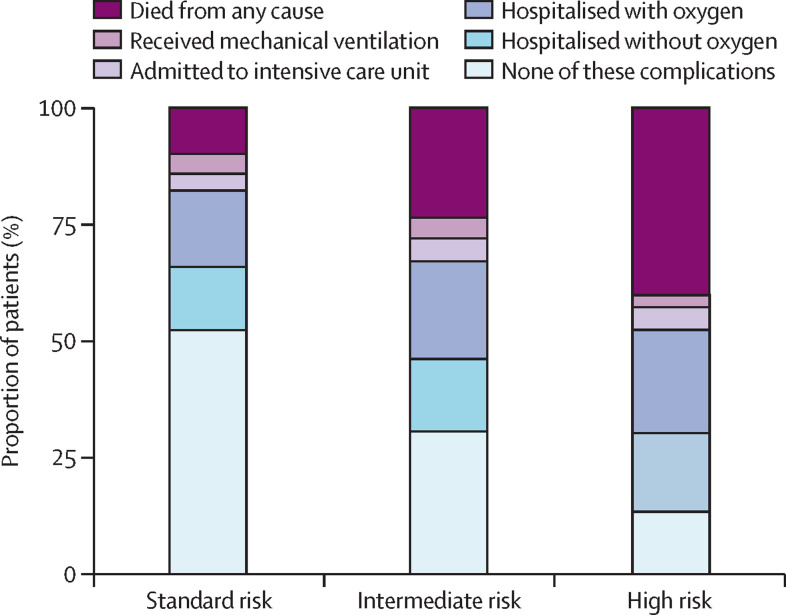
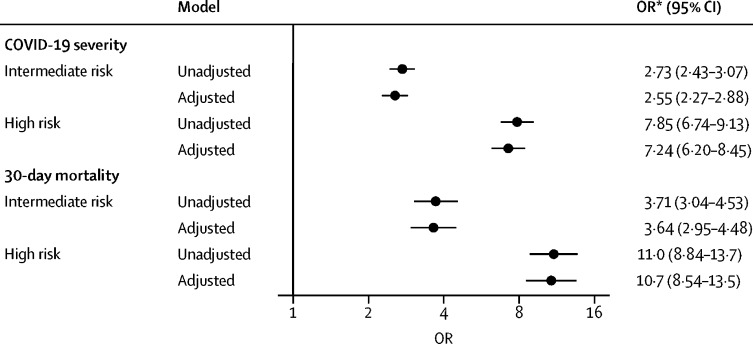
The primary objective of our study was to examine COVID-19 severity across the different risk groups. High-risk patients had higher COVID-19 severity as indicated by a greater burden of hospitalisation with and without supplemental oxygen, ICU admission, mechanical ventilation, and death (figure 2 ) than standard-risk patients (table 1). 920 (16·2%) of 5671 patients died within 30 days of a COVID-19 diagnosis, including 161 (6·8%) of 2365 standard-risk patients, 409 (18·5%) of 2216 intermediate-risk patients, and 350 (32·1%) of 1089 high-risk patients (table 1). After adjustment for other factors associated with COVID-19 severity and 30-day mortality, there was a strong significant association between the high CCC19 geriatric risk index and higher COVID-19 severity and higher odds of 30-day mortality compared to standard-risk patients (adjusted OR for COVID-19 severity: 7·24, 95% CI 6·20–8·45; adjusted OR for 30-day mortality: 10·7, 95% CI 8·54–13·5; figure 3 ). In a sensitivity analysis, we examined the subgroups of patients who were initially categorised by available data into at least a member of the associated group (eg, at least intermediate risk if they met criteria for intermediate risk by age and modified CCI but had unknown ECOG performance scores). The estimated ORs for the primary outcome among these subgroups were similar to that among those with complete geriatric risk measure data, supporting the consolidation of these adjacent categories for clinical use (appendix p 9). Associations of other risk factors with COVID-19 severity and 30-day mortality are provided in the appendix (p 10). The CCC19 geriatric risk index showed the largest proportion of the model's χ2 statistic for both COVID-19 severity (48%) and 30-day mortality (52%; appendix p 12).
Figure 2.
Distribution of the primary ordinal COVID-19 severity outcome in older adults with cancer, stratified by COVID-19 and Cancer Consortium geriatric risk index
Figure 3.
Association of COVID-19 and Cancer Consortium geriatric risk index with COVID-19 severity and 30-day all-cause mortality among older adults with cancer
Model for COVID-19 severity adjusted for sex, race and ethnicity, smoking status, obesity, type of malignancy, cancer status, recent (within 3 months) anti-cancer therapies (cytotoxic chemotherapy, targeted therapy, endocrine therapy, immunotherapy, locoregional therapy), country of patient residence, and month of COVID-19 diagnosis; all variance inflation factors <1·6. Model for 30-day all-cause mortality adjusted for sex, race and ethnicity, smoking status, obesity, type of malignancy, cancer status, recent (within 3 months) anti-cancer therapies (cytotoxic chemotherapy, endocrine therapy, immunotherapy, locoregional therapy), country of patient residence, and month of COVID-19 diagnosis; all variance inflation factors <1·7. OR=odds ratio. *ORs relative to standard-risk patients.
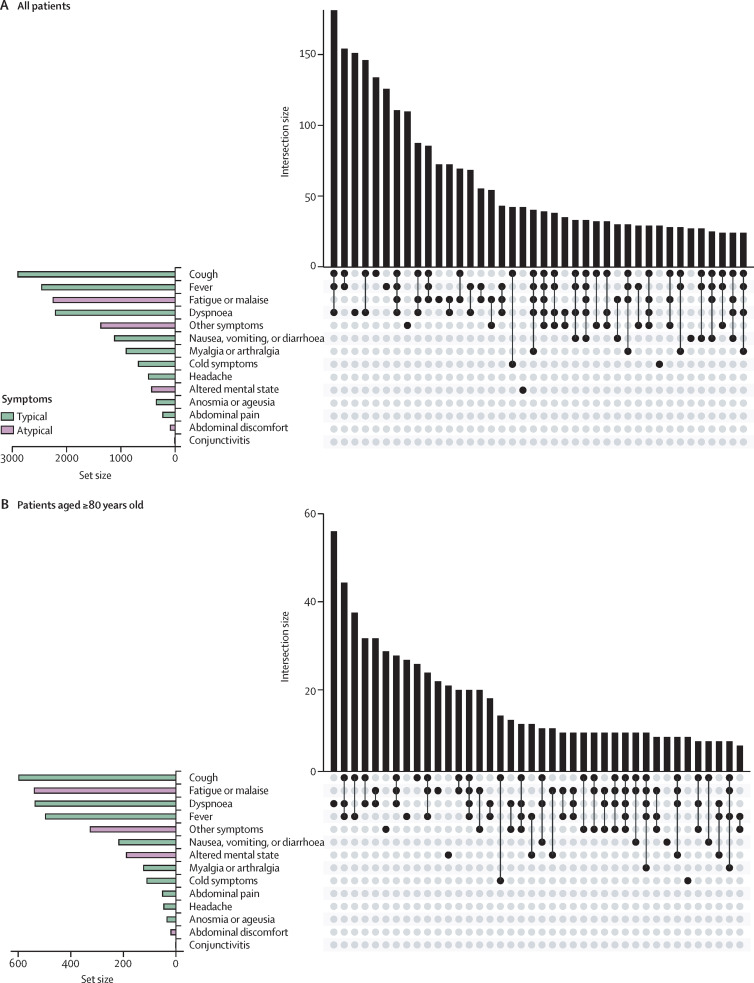
Among our secondary objectives was to examine COVID-19 presentation. Few patients presented with only atypical symptoms (table 1). Cough, fever, fatigue or malaise, and dyspnoea were the most common symptoms (figure 4A ). Notably, altered mental state was more common among patients aged 80 years and older compared to younger patients (figure 4B). Severity of COVID-19 at presentation varied across geriatric risk groups, with only 193 (8·2%) of 2360 standard-risk patients presenting with severe disease, compared with 288 (13·0%) of 2210 intermediate-risk patients and 177 (16·3%) of 1084 high-risk patients. Most patients did not receive any specific treatment for COVID-19, and treatments were similar across geriatric risk groups (table 1).
Figure 4.
Presenting symptoms of COVID-19 among older adults with cancer
UpSet plot indicating intersection between symptoms in older adults with cancer in a matrix layout. The bar graph in the lower left corner depicts symptom-level distribution across each category (typical or atypical). Each row in the dot graph represents a symptom classification; solid dots represent each symptom part of the intersecting sets. The centre bar graph depicts the number of symptoms in each intersection.
Complications from COVID-19 were common, with the most common being pulmonary complications. High-risk patients had more complications reported than intermediate and standard risk groups (table 1). Of the patients for whom cancer therapy status was known and who were on active anti-cancer therapy within 3 months before COVID diagnosis, approximately half had subsequent modification of their therapy (table 1). The most common cancer therapy modification for those with available data was delay in cancer therapy, and this was similar across all risk groups (table 1).
Discussion
In this large study of 5671 older adults with COVID-19 and cancer, we found that the CCC19 geriatric risk index was associated with poorer outcomes, including clinical complications, hospitalisation, and mortality. A higher CCC19 geriatric risk index was significantly associated with higher COVID-19 severity and 30-day all-cause mortality after adjustment of major prognostic confounding factors and explained the greatest amount of variation in these outcomes compared with all other risk factors. Our results suggest that the CCC19 geriatric risk index, using a combination of previously studied poor prognostic factors for COVID-19 mortality,6, 10, 19, 20 can effectively risk stratify this vulnerable patient population.
Our study examines the relationship between our novel geriatric risk index and adverse outcomes from COVID-19 among older adults with cancer, as well as the frequency of atypical presentations in this population. With this geriatric risk index, we identified that individuals categorised as high risk had a 10 times greater odds of death within 30 days than standard-risk patients. Although data on frailty specifically are not available in this dataset, frailty is a commonly used perspective to capture age-associated vulnerability. In a meta-analysis of studies of frailty and COVID-19, of 23 944 patients with COVID-19 in the general population, frailty was present in 51% of patients and was strongly associated with higher mortality than non-frail patients, with a pooled OR of 2·48 (95% CI 1·78–3·46), regardless of frailty tool used or geographical location.5 However, operationalising frailty requires specific data, such as comprehensive geriatric assessment or objective measures that are rarely available in routine clinical practice. Thus, the applicability of frailty in real-world clinical care is limited. The CCC19 geriatric risk index, in contrast, uses information readily available from routine oncology care (age, comorbidities, and ECOG performance status) to identify a group with an extremely high risk for severe outcomes of COVID-19, lending credence to our approach of combining known risk factors into a single geriatric risk index.
Given the adverse COVID-19 outcomes observed in this high-risk population, our study reinforces the need to focus efforts on SARS-CoV-2 infection prevention strategies among older adults with cancer, including vaccination.21, 22, 23 Given concerns about vaccine efficacy among older adults and those with cancer, vaccination is only one part of a broader mitigation strategy for this extremely vulnerable population, which must also include public health measures: social distancing, mask wearing, and aggressive vaccination of household contacts. Unfortunately, these public health measures have their own serious adverse consequences, as older adults with or without cancer are at risk of poor outcomes from social isolation, loneliness, physical deconditioning, and loss of autonomy, which all increase the risk of depression and anxiety.24, 25, 26
Clinicians should remain cognisant of atypical presentations of COVID-19. Nearly one in ten patients aged 80 years and older in our cohort presented with only atypical symptoms. The recognition that older adults might present with fewer and atypical symptoms of infection predates the pandemic; in a study of the presentation of community-acquired pneumonia in older adults, 639 (20·5%) of 3124 patients older than 65 years presented with atypical symptoms, and 175 (4·8%) of 3630 presented with altered mental state.27 In our study, altered mental state was the sixth most common symptom present at diagnosis. Of note, our study probably underestimated the prevalence of delirium, because it is under-recognised and under-reported in clinical practice. In a study that used a standardised method for identifying acute confusion as a proxy for delirium in patients with COVID-19, the prevalence of delirium was 34·6% (45 of 130 patients) in the subgroup of frail older adults.28
The high prevalence of complications in the high-risk group in our study is noteworthy and might inform simple changes in care to prevent complications. For instance, one in three (35·4%) high-risk older adults in our cohort developed acute kidney injury (compared with 23·7% in the intermediate-risk group and only 11·4% in the standard-risk group). Although renal function naturally declines with age, the acute illness presents a number of potential threats to renal function, including haemodynamic and nephrotoxic medications. Adopting a nephroprotective mindset for these patients, such as avoiding nephrotoxic medications and contrast for computed tomography imaging if possible, could be a simple step to reduce this complication.29
Despite being a large multicentre study examining a comprehensive cohort of older adults with cancer and COVID-19, our study has several limitations that are inherent to its retrospective registry nature. First, the CCC19 registry was designed to report COVID-19 outcomes with time intervals rather than with precise timing of COVID-19 diagnosis and cancer-related outcomes, to meet Health Insurance Portability and Accountability Act (HIPPA) and general data protection regulation requirements. Second, COVID-19-specific death is underspecified, as this variable was included several months after the inception of the registry and would lead to high rates of missingness if included in our study, and this is an important limitation. Given the diverse mechanisms of pathophysiology resulting from SARS-CoV-2, accurate attributable cause of death is a limitation common to most COVID-19 studies. Patients aged 89 years or older were masked per HIPAA de-identification requirements, such that we cannot examine subpopulations of patients aged 90 years or older; notably, 272 (5%) of the patients in our analysis fall into this category. Moreover, as with any registry-based study, there might be selection bias toward hospitalised patients.30 Similarly, although adjusted analyses were done, observational studies are subject to selection bias and unmeasured confounding. Additionally, we used a simplified geriatric risk measure adapted from a measure validated in myeloma patients.14 This measure does not fully approximate the existing approaches to operationalising frailty, such as the phenotypic frailty approach, which requires assessment of grip strength or gait speed31 and which are rarely obtained in routine practice and are not included in the CCC19 registry. Another key limitation is that the IMWG score was validated in patients with haematologic malignancies, and our cohort was primarily a cohort of patients with solid tumours; however, the proportion of patients with solid tumours versus haematologic malignancies in each risk group was similar, lending face validity to its application in this study. Another limitation is possible misclassification of ECOG in older adults when compared with geriatric assessment, as performance scores have been shown repeatedly to underestimate the level of vulnerability in older adults, as even individuals with good performance scores might have impairments in functional status and other important domains.32, 33 Consequently, the CCC19 geriatric risk index might not identify at-risk patients who have ageing-associated vulnerabilities other than impaired performance scores or comorbidities. Lastly, the CCC19 geriatric risk index will require external validation. Despite these limitations, our large study generates important data and sets the foundation for further investigation in this large vulnerable population.
Future research questions include the extent to which vaccines will reduce incidence and severity of COVID-19 in this vulnerable population who might not mount as robust an immune response to vaccination; these studies are ongoing, with encouraging initial results with regard to older adults with cancer.34 Older adults with cancer should be prioritised for vaccination roll-out.30 It is unknown how modifications of anti-cancer therapy affect cancer control, and whether functional decline due to COVID-19 affects subsequent ability to tolerate cancer therapy in a group who might already have been at high risk for toxicity of therapy.
Patients with high-risk geriatric profiles present with more severe initial presentations and are at greater risk for death and other adverse sequelae of COVID-19 than those with standard-risk profiles. Our CCC19 geriatric risk index, based on readily available clinical factors, might provide clinicians with an easy-to-use risk stratification method to identify older adults most at risk for severe COVID-19 as well as mortality.
Data sharing
The statistical analysis plan and data dictionary used for this study are included in the appendix. The full CCC19 data dictionary and the code to create all derived variables is publicly available. Aggregate deidentified patient data with site identifiers removed and geographical region of patient residence masked to a level no smaller than USA census divisions will be made publicly available for any purpose beginning from 6 months and ending a minimum of 36 months after publication of this Article through the CCC19 website. Individual deidentified patient data with site identifiers removed and geographical region of patient residence masked to a level no smaller than USA census divisions will be made available to researchers who provide a methodologically sound proposal, whose proposed use of the data has been approved by an independent review committee identified for this purpose. Proposals can be submitted beginning 6 months and up to 36 months after publication of this Article through the following REDCap form: https://redcap.link/CCC19-data-request. To gain access, approved data requestors will need to sign a data access agreement.
Declaration of interests
The following authors declare competing interests not related to the current work: ZB reports grants from Genentech/imCORE, non-financial support from Bristol Myers Squibb, and personal fees from UpToDate. AE reports salary support from the Canadian Institute of Health Research, the Detweiler Travelling Fellowship (Royal College of Physicians and Surgeons of Canada), and the Henry R Shibata Fellowship (Cedar's Cancer Foundation). CRF reports grants from Merck Foundation, NCCN/Pfizer, and National Cancer Institute, and other support from National Cancer Institute and the Patient-Centered Outcomes Research Institute. PGri reports personal fees and non-financial support from AstraZeneca, personal fees from Astellas Pharma, personal fees from Bayer, grants and personal fees from Bristol Myers Squibb, grants and non-financial support from Clovis Oncology, personal fees from Dyania Health, grants and personal fees from EMD Serono, personal fees from Exelixis, personal fees from Foundation Medicine, personal fees from Genentech/Roche, personal fees from Genzyme, grants and personal fees from GlaxoSmithKline, personal fees from Guardant Health, grants and personal fees from Immunomedics/Gilead, personal fees from Infinity Pharmaceuticals, personal fees from Janssen, grants and personal fees from Merck, grants and personal fees from Mirati Therapeutics, grants and personal fees from Pfizer, grants and personal fees from QED Therapeutics, personal fees from Regeneron Pharmaceuticals, personal fees from Seattle Genetics, personal fees from 4D Pharma, personal fees from UroGen, grants from Bavarian Nordic, and grants from Debiopharm. SGup reports grants and personal fees from Bristol Myers Squibb, personal fees from Merck, Janssen, Seattle Genetis, EMD Sorono, and Pfizer, and grants from Astellas and BMS. CHw reports grants from Merck, Bayer, and AstraZeneca, and personal fees from Tempus and EMD Sorono, and other support from Johnson and Johnson. AK reports other support from TESARO, Fibrogen, Geistlich Pharma, Astellas Pharma, Rafael Pharmaceuticals, and Novocure. ARK reports other support from Merck and Sanofi, personal fees from OncLive, grants from ASCO Conquer Cancer Foundation, and grants from Bladder Cancer Advocacy Network. HK reports position on advisory board of Sanofi Genzyme NSCLC Northeast. NMK reports personal fees from BMS, Janssen, Seattle Genetics, Celldex, Sandoz, Invitae, Beyond Spring, Spectrum G1 Therapeutics, and Total Health, and grants from Amgen, Jazz Therapeutics, G1 Therapeutics, and Samsung. CL reports grants from imCORE/Genentech. RRM reports serving on advisory board or as a consultant for Astrazeneca, Aveo, Bayer, BMS, Caris, Dendreon, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, Sorrento Therapeutics, and Tempus. RRM received institutional research funding from Pfizer, Bayer, Tempus. SM reports personal fees from National Geographic. OAP reports grants from National Institutes of Health (NIH) and Agency for Healthcare Research and Quality, and personal fees from International Consulting Associates. NAP reports personal fees from AstraZeneca, Merck, Pfizer, Eli Lilly, Genentech, BMS, Amgen, Inivata, G1 Therapeutics, Xencor, Mirati, Janssen, Boehringer Ingelheim, and Sanofi-Genzyme-Regeneron. RPR reports grants from BMS and Janssen, and personal fees from BMS, Janssen, Dova, and Inari. MAT reports personal fees from VIA Oncology, GSK, and Adaptive Advisory Board, other support from Syapse, UpToDate, Takeda, Celgene, Doximity, AbbVie, BMS, CRAB CTC, Denovo, Hoosier Research Network, Lilly, LynxBio, Strata Oncology, and TG Therapeutics. JLW reports personal fees from Roche, Westat, Flatiron Health, Melax Tech, and IBM Watson Health, other support from HemOnc and Janssen, and grants from AACR. TMW reports personal fees from Carevive, and personal fees from Sanofi, and Seattle Genetics. TMW-D reports grants from BMS, Merck, Janssen, and GSK/Tesaro, personal fees from Exicure, Shattuck Labs, Merck, Caris Life Science, and SITC, and other support from High Enroll. EW-B reports grants from Pfizer Global Medical Grants, personal fees from Astellas, Aveo Oncology Bristol Myers Squibb, Exelixis, and Janssen, and other support from Immunomedics and Nektar. The following authors declare competing interests during the conduct of the study: SM reports grants and other support from National Cancer Institute and from the International Association for the Study of Lung Cancer. DPS reports grants from American Cancer Society and Hope Foundation for Cancer Research and from NIH. LT reports grants from NIH. JLW reports grants from NIH. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank all members of the CCC19 steering committee: Toni Choueiri, Petros Grivas, Gilberto Lopes, Corrie Painter, Solange Peters, Brian Rini, Dimpy Shah, Mike Thompson, Dimitrios Farmakiotis, Narjust Duma, and Jeremy Warner for their invaluable guidance of the CCC19 consortium. We also thank Gary Lyman for important contributions to the study design. The CCC19 Research Coordinating Center (CHe, SM, BF, JLW, and KV-L) is supported by the NIH National Cancer Institute (NCI) Cancer Center Support Grants P30 CA068485. AE is supported by the Canadian Institute of Health Research, the Detweiler Travelling Fellowship, and the Henry R Shibata Fellowship. AS was supported in part by the Beatriz and Samuel Seaver Foundation, the Memorial Sloan Kettering Cancer and Aging Program, and NIH NCI Cancer Center Support Grant P30 CA008748. CRF was supported by T32 CA 236621 and P30 CA 046592. OAP was supported by P01 AG027296 and R01 AG053307. PGri was supported by P30CA015704–45. REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH).
Contributors
AE, JLW, NMK, OAP, and TMW conceived and designed the study and reviewed and edited the manuscript. AE, JLW, and TMW did the formal analysis and methodology, and wrote the original draft. BF and CHe designed the study, did the formal analysis, visualisation, and methodology, wrote the original draft, and reviewed and edited the manuscript. SM did project administration and coordination and reviewed and edited the manuscript. KV-L did the data analysis (for treatment change) and reviewed and edited the manuscript. SMR, EW-B, RPR, MAT, AD, DRR, ARK, LT, RCL, CS, RE, GB, AK, and DPS designed the study and reviewed and edited the manuscript. ZB, AC, JCl, JCr, BD, CRF, SLF, PGri, PGro, SGul, SGup, CHw, HK, SJK, EJK, CL, RRM, AN, NAP, MP, ALS, AS, JAS, CTS, SW, NW, and TMW-D contributed substantial patient cases to the CCC19 and were responsible for reading and editing the manuscript. BF, CHe, and JLW accessed and verified the data. BF, CHe, and JLW had full access to data in the study. All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35:1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ofori-Asenso R, Ogundipe O, Agyeman AA, et al. Cancer is associated with severe disease in COVID-19 patients: a systematic review and meta-analysis. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19-infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113:371–380. doi: 10.1093/jnci/djaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fathi M, Vakili K, Sayehmiri F, et al. The prognostic value of comorbidity for the severity of COVID-19: a systematic review and meta-analysis study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X-M, Jiao J, Cao J, et al. Frailty as a predictor of mortality among patients with COVID-19: a systematic review and meta-analysis. BMC Geriatr. 2021;21:186–211. doi: 10.1186/s12877-021-02138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balducci L, Colloca G. Natural disaster and rationing of care. J Geriatr Oncol. 2020;11:750–752. doi: 10.1016/j.jgo.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohile S, Dumontier C, Mian H, et al. Perspectives from the cancer and aging research group: caring for the vulnerable older patient with cancer and their caregivers during the COVID-19 crisis in the United States. J Geriatr Oncol. 2020;11:753–760. doi: 10.1016/j.jgo.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battisti NML, Mislang AR, Cooper L, et al. Adapting care for older cancer patients during the COVID-19 pandemic: recommendations from the International Society of Geriatric Oncology (SIOG) COVID-19 working group. J Geriatr Oncol. 2020;11:1190–1198. doi: 10.1016/j.jgo.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abidi M, Aboulafia DM, Accordino MK, et al. A systematic framework to rapidly obtain data on patients with cancer and COVID-19: CCC19 governance, protocol, and quality assurance. Cancer Cell. 2020;38:761–766. doi: 10.1016/j.ccell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limpawattana P, Phimson K, Sookprasert A, Sirithanaphol W, Chindaprasirt J. Prevalence of geriatric syndromes in elderly cancer patients receiving chemotherapy. Curr Gerontol Geriatr Res. 2020;2020 doi: 10.1155/2020/9347804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34:224–233. doi: 10.1038/s41375-019-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 17.Shyr Y, Berry LD, Hsu C-Y. Scientific rigor in the age of COVID-19. JAMA Oncol. 2021;7:171–172. doi: 10.1001/jamaoncol.2020.6639. [DOI] [PubMed] [Google Scholar]
- 18.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc B. 1996;1:267–288. [Google Scholar]
- 19.Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garassino MC, Vyas M, de Vries EGE, Kanesvaran R, Giuliani R, Peters S. The ESMO call to action on COVID-19 vaccinations and patients with cancer: vaccinate. Monitor. Educate. Ann Oncol. 2021;32:579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network Cancer and COVID-19 vaccination version 5. Jan 4, 2022. https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v5-0.pdf?sfvrsn=b483da2b_74
- 23.Cavanna L, Citterio C, Toscani I. COVID-19 vaccines in cancer patients. Seropositivity and safety. Systematic review and meta-analysis. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groarke JM, Berry E, Graham-Wisener L, McKenna-Plumley PE, McGlinchey E, Armour C. Loneliness in the UK during the COVID-19 pandemic: cross-sectional results from the COVID-19 Psychological Wellbeing Study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domènech-Abella J, Mundó J, Haro JM, Rubio-Valera M. Anxiety, depression, loneliness, and social network in the elderly: longitudinal associations from The Irish Longitudinal Study on Ageing (TILDA) J Affect Disord. 2019;246:82–88. doi: 10.1016/j.jad.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 26.Tomaka J, Thompson S, Palacios R. The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. J Aging Health. 2006;18:359–384. doi: 10.1177/0898264305280993. [DOI] [PubMed] [Google Scholar]
- 27.Jung YJ, Yoon JL, Kim HS, Lee AY, Kim MY, Cho JJ. Atypical clinical presentation of geriatric syndrome in elderly patients with pneumonia or coronary artery disease. Ann Geriatr Med Res. 2017;21:158–163. [Google Scholar]
- 28.Poco PCE, Aliberti MJR, Dias MB, et al. Divergent: age, frailty, and atypical presentations of COVID-19 in hospitalized patients. J Gerontol A Biol Sci Med Sci. 2021;76:e46–e51. doi: 10.1093/gerona/glaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chronopoulos A, Cruz DN, Ronco C. Hospital-acquired acute kidney injury in the elderly. Nat Rev Nephrol. 2010;6:141–149. doi: 10.1038/nrneph.2009.234. [DOI] [PubMed] [Google Scholar]
- 30.Desai A, Mohammed TJ, Duma N, et al. COVID-19 and cancer: a review of the registry-based pandemic response. JAMA Oncol. 2021;7:1882–1890. doi: 10.1001/jamaoncol.2021.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 32.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 33.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iacono D, Cerbone L, Palombi L, et al. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol. 2021;12:1253–1255. doi: 10.1016/j.jgo.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The statistical analysis plan and data dictionary used for this study are included in the appendix. The full CCC19 data dictionary and the code to create all derived variables is publicly available. Aggregate deidentified patient data with site identifiers removed and geographical region of patient residence masked to a level no smaller than USA census divisions will be made publicly available for any purpose beginning from 6 months and ending a minimum of 36 months after publication of this Article through the CCC19 website. Individual deidentified patient data with site identifiers removed and geographical region of patient residence masked to a level no smaller than USA census divisions will be made available to researchers who provide a methodologically sound proposal, whose proposed use of the data has been approved by an independent review committee identified for this purpose. Proposals can be submitted beginning 6 months and up to 36 months after publication of this Article through the following REDCap form: https://redcap.link/CCC19-data-request. To gain access, approved data requestors will need to sign a data access agreement.