Abstract
Background
Cancer is one of the leading causes of death worldwide. More than two-thirds of deaths due to cancers occur in low- and middle-income countries where Zambia belongs. This study, therefore, sought to assess the epidemiology of various types of cancers in Zambia.
Methods
We conducted a retrospective observational study using the Zambia National Cancer Registry (ZNCR) population based data from 2007 to 2014. Zambia Central Statistics Office (CSO) demographic data were used to determine catchment area denominator used to calculate prevalence and incidence rates of cancers. Age-adjusted rates and case fatality rates were estimated using standard methods. We used a Poisson Approximation for calculating 95% confidence intervals (CI).
Results
The seven most cancer prevalent districts in Zambia were Luangwa, Kabwe, Lusaka, Monze, Mongu, Katete and Chipata. Cervical cancer, prostate cancer, breast cancer and Kaposi's sarcoma were the four most prevalent cancers as well as major causes of cancer related deaths in Zambia. Age adjusted rates and 95% CI for these cancers were: cervix uteri (186.3; CI = 181.77 – 190.83), prostate (60.03; CI = 57.03 – 63.03), breast (38.08; CI = 36.0 – 40.16) and Kaposi's sarcoma (26.18; CI = 25.14 – 27.22). CFR were: Leukaemia (38.1%); pancreatic cancer (36.3%); lung cancer (33.3%); and brain, nervous system (30.2%). The cancer population was associated with HIV with p-value of 0.000 and a Pearson correlation coefficient of 0.818.
Conclusions
The widespread distribution of cancers with high prevalence observed in the southern zone may have been perpetrated by lifestyle and sexual culture (traditional male circumcision known to prevent STIs is practiced in the northern belt) as well as geography. Intensifying cancer screening and early detection countrywide as well as changing the lifestyle and sexual culture would greatly help in the reduction of cancer cases in Zambia.
Keywords: Cancer epidemiology, Cervical cancer, Prostate cancer, Breast cancer, Kaposi's sarcoma, Cancers in Zambia
Introduction
The global burden of cancer has been on the increase over the past few decades despite some remarkable advances in treatment and prevention1. Cancer continues to be one of the leading causes of death worldwide. This has driven scientists to make advancements in conventional medicine as well as anticancer nanomedicine and nanotechnology to broaden the spectrum of combating the scourge2-4. In the year 2017, cancers caused over 9.6 million deaths worldwide and moved from the third leading cause of death in 1990 to the second leading cause behind cardiovascular diseases5-7. More than two - thirds of deaths due to cancers occur in low- and middle-income countries8. Low-income countries reported approximately 51% of all cancers globally in the year 1975 but this proportion steadily increased to 55% in 2007. It is estimated that by 2050, low-income countries will account for 61% of all cancers globally9-11.
The global increase in the number of cancer cases is due to multiple factors, which include: lifestyle factors (smoking, alcohol, lack of physical exercise, poor/unhealthy diet, environmental factors (exposure to carcinogens), socioeconomic status and infectious agents12-15. Developing countries have 26% cancers attributable to infection while developed countries have 8% which is a third of those in developing countries16. The oncogenic infections that have been linked to these cancers are Human Papilloma Virus (HPV)17, Hepatitis B Virus (HBV)18, Helicobacter pylori (H. pylori)19, Human Herpes Virus (HHV8)20, and Epstein Barr Virus (EBV)21.
Although cancer incidence has been increasing in every part of the world, there are huge inequalities between developed and developing countries. Age adjusted incidence rates remain highest in developed countries and mortality is relatively much higher in developing countries due to lack of early detection and access to treatment facilities8. Infections due to human papillomavirus and hepatitis B and C viruses significantly contribute to the burden of cervical and liver cancers respectively on the African Continent8.The most common cancers in the African region are cervical, breast, liver and prostate as well as Kaposi's sarcoma and non-Hodgkin's lymphoma8.
Zambia is one of the sub-Saharan African countries that has not been spared by the increasing burden of cancers. Many lives can be saved if appropriate investment is made in raising public awareness on the early signs and symptoms of common cancers as well as implementation of early detection strategies8. This study therefore sought to assess the epidemiology of cancers in Zambia.
Materials and Methods
Study site
Zambia is located in the southern zone of the African continent and lies between latitudes 8° and 18° south, and longitudes 22° and 34° east. The country has a total geographical area of 753,612km2,22.
Study design
We conducted a retrospective observational study using the Zambia National Cancer Registry (ZNCR) population based data from 2007 to 2014. ZNCR collects and keeps population based cancer data in Zambia. Sources of data include Health Management Information System (HMIS), Government and private Hospital Registries, which are also linked from grass root level by health centres to ensure that all suspected cancer cases are referred to the hospitals. Other sources include death records, Community Health Workers (CHW) and Churches Health Organisation (CHAZ). The collected information include patient's personal details (names, age, sex, date of birth and residential address at diagnosis). Hospital details (hospital, consultant patient unit number), diagnostics, tumour and treatment details (site of primary, morphology, laterality, stage, grade of tumour, basis of diagnosis, date of diagnosis, treatment indicators) as well as death details (alive or dead, date of death, cause(s) and place of death).
ZNCR ensures that cancer registration data is stored securely, backed up and only accessible to authorised cancer registry staff and researchers. All cancer notification forms or books are filed and locked in a secure place. Information request for data on cancers are made in writing to the Registrar and the Permanent Secretary at the Ministry of Health for approval. This study got formal administrative approval from the Cancer registry for data access, approved by both the University of Zambia Biomedical Research Ethics Committee (UNZABREC) and the National Health Research Authority of Zambia with reference number 003-08-17.
Zambia Central Statistics Office (CSO) provided us with shapes files for provinces and districts as well as demographic data, which we used to determine prevalence and Age-specific rates while World Standard Population23 was used to determine Standardised Incidence Rates (SIR). Case fatality rate (CFR) was calculated as:. We used a Poisson Approximation for calculating 95% confidence intervals. The 95% confidence interval for the age-standardised rate was calculated as:
Where the variance for the age-standardised rate was given
di is the number of events in age group i in the study population
ri is the incidence rate in the study population for the persons in age group i
Pi is the number of persons in age group i in the standard population.
Mapping of the distribution of cancers at district and provincial levels were done using Geographical Information System (ArcGIS) version 10.3.1, Redlands, CA. Some big districts in Zambia were split to form new districts for effective administrative purposes. New population figures for new districts were not available hence population figures for original districts were considered. For instance, districts such as Ikelenge, Zimba and Mafinga were still part of the original districts namely, Mwinilunga, Kalomo and Isoka respectively during the study period hence; we considered cancer rates of original districts in these new districts.
Sampling
Since our study was registry based, we considered all cancer cases in the Zambia National Cancer Registry database from 2007 to 2014. We ,however, excluded cancer cases not belonging to any of the ten provinces in Zambia (i.e. not coded cases from any region in Zambia) for the purpose of GIS mapping which required that cancer cases be linked to districts and provinces of origin. In addition, we sampled and surveyed ten districts across Zambia to assess the incidence and prevalence of cancer and determine risk factors contributing to escalating cancer cases.
Statistical Analyses
We used SPSS version 21 in our data analyses. Logistic regression model was used to determine the association between cancer and HIV while Pearson Correlation was used to determine the correlation coefficient. We used a Poisson Approximation for calculating 95% confidence intervals.
Results
Distribution of cancers by region and province
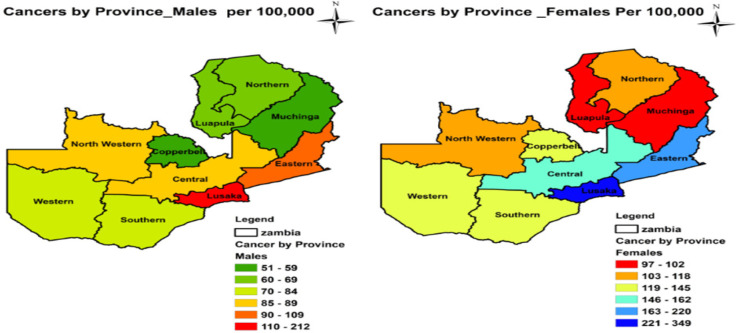
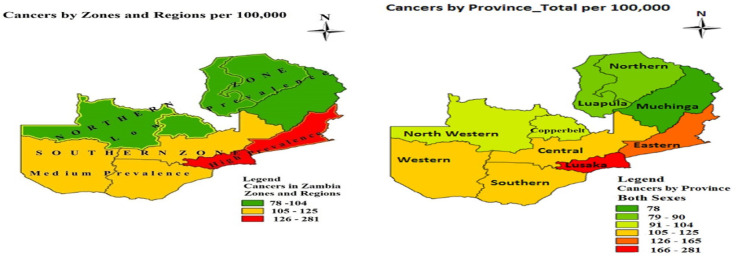
During our study period from 2007 to 2014, a total of 21,512 cancer cases were notified to ZNCR of which 7,560 (35.14%) were males and 13,952 (64.86%) were females. The four most prevalent types of cancers were cervical (97.1/10000), prostate (22.1/100,000), breast (19.3/100,000) and Kaposis sarcoma (19.2/100,000). Cancers were widely distributed in Zambia with high prevalence (105-281/100,000) concentrated in the southern zone comprising Eastern, Central, Lusaka, Western and Southern Provinces. The southern zone was divided into high prevalence and medium prevalence regions. Eastern and Lusaka Provinces formed the high prevalence region (126-281/100,000) while Central, Southern and Western Provinces formed the medium prevalence region (105-125/100,000). The northern zone comprising North Western, Copperbelt, Luapula, Northern and Muchinga Provinces had relatively low cancer prevalence of 78-104/100,000 population.
By gender, cancers were more prevalent in females than in males in all the ten provinces with mean sex ratio F/M = 1.85. Table 1 shows the prevalence of cancers by province and sex. The prevalence of cancer in both males and females were more in Lusaka, Eastern and Central Provinces. However, for males, the prevalence was also more in Western Province. In addition, Southern, Copperbelt and Western Provinces had more prevalence of cancer in females
Table 1.
Distribution of cancers (all types) in Zambia by Province
| Prevalence Rate Per 100,000 Population | |||
| Province | Total | Males | Females |
| Central | 125 | 87 | 162 |
| Copperbelt | 97 | 51 | 143 |
| Eastern | 165 | 109 | 220 |
| Luapula | 84 | 66 | 102 |
| Lusaka | 281 | 212 | 349 |
| Northern | 90 | 69 | 118 |
| Muchinga | 78 | 59 | 97 |
| North West | 104 | 89 | 118 |
| Southern | 115 | 83 | 145 |
| Western | 114 | 84 | 142 |
Figure 2 shows details of provincial distribution of cancers by sex.
Figure 2.
Provincial distribution of cancers (all types) by sex
Distribution of cancers by district
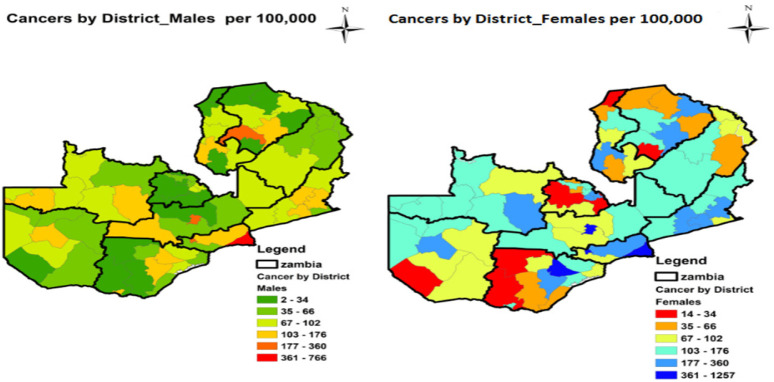
The distribution of cancers at district level was similar to the zonal pattern at provincial level. Figure 3 shows the geographical distribution of cancers by district in Zambia. Some districts such as Mafinga, Ikelenge and Zimba were still part of the original districts namely Isoka, Mwinilunga and Kalomo respectively at the beginning of our research period hence: are shown as combined districts in Table 2. Some big districts in Zambia were split to form new districts for effective administrative purposes. The prevalence of cancers in the original districts reflects cancer prevalence in the new districts.
Fig 3.
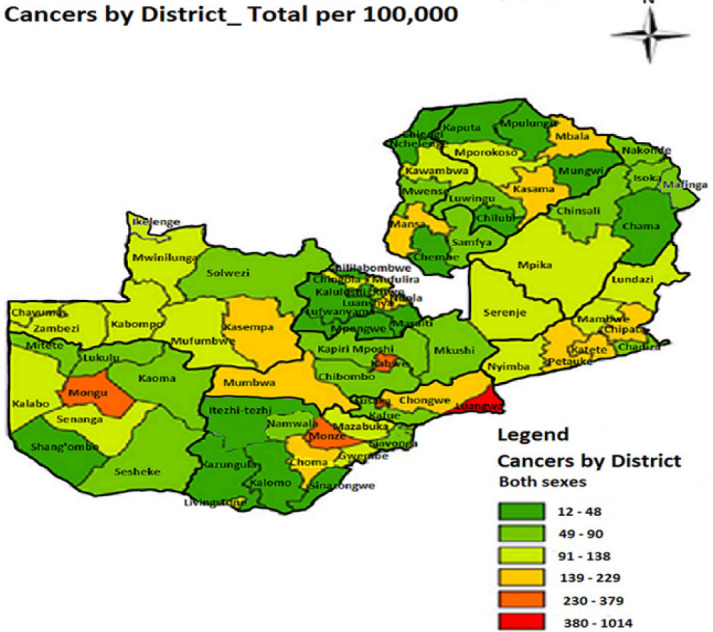
Distribution of cancers (all types) by district
Table 2.
Ranked prevalence of cancers (all types) by district per 100,000 population
| District | Total | Males | Females |
| Luangwa | 1014 | 766 | 1257 |
| Kabwe | 379 | 251 | 499 |
| Lusaka | 311 | 233 | 387 |
| Monze | 282 | 157 | 404 |
| Mongu | 260 | 176 | 337 |
| Katete | 229 | 142 | 313 |
| Chipata | 213 | 133 | 291 |
| Kasempa | 193 | 176 | 210 |
| Kasama | 168 | 118 | 217 |
| Ndola | 165 | 76 | 252 |
| Mansa | 165 | 110 | 217 |
| Choma | 158 | 126 | 189 |
| Luanshya | 156 | 77 | 234 |
| Chongwe | 155 | 123 | 187 |
| Petauke | 153 | 102 | 202 |
| Mumbwa | 144 | 116 | 171 |
| Mbala | 142 | 96 | 187 |
| Livingstone | 138 | 118 | 157 |
| Zambezi | 135 | 116 | 155 |
| Mazabuka | 126 | 86 | 165 |
| Lundazi | 120 | 86 | 153 |
| Gwembe | 113 | 80 | 145 |
| Mambwe | 112 | 110 | 113 |
| Kalabo | 111 | 91 | 130 |
| Mufumbwe | 110 | 97 | 124 |
| Mwinilunga/Ikelenge | 107 | 73 | 141 |
| Nyimba | 107 | 72 | 141 |
| Kabompo | 106 | 97 | 116 |
| Serenje | 105 | 78 | 132 |
| Chavuma | 105 | 102 | 108 |
| Mufulira | 104 | 60 | 148 |
| Mpika | 102 | 82 | 122 |
| Mporokoso | 100 | 77 | 123 |
| Kawambwa | 95 | 78 | 110 |
| Senanga | 93 | 84 | 101 |
| Chingola | 93 | 53 | 133 |
| Chinsali | 90 | 52 | 127 |
| Mkushi | 88 | 57 | 120 |
| Mwense | 88 | 76 | 99 |
| Luwingu | 87 | 360 | 114 |
| Kitwe | 82 | 52 | 113 |
| Kaoma | 80 | 62 | 96 |
| Sesheke | 78 | 64 | 91 |
| Isoka/ Mafinga | 77 | 62 | 91 |
| Kafue | 77 | 66 | 88 |
| Lukulu | 77 | 37 | 114 |
| Chadiza | 74 | 49 | 99 |
| Samfya | 70 | 74 | 67 |
| Siavonga | 70 | 49 | 90 |
| Namwala | 69 | 66 | 71 |
| Solwezi | 65 | 60 | 71 |
| Kapiri Mposhi | 61 | 32 | 89 |
| Chibombo | 56 | 45 | 68 |
| Nakonde | 53 | 36 | 70 |
| Sinazongwe | 48 | 49 | 47 |
| Chama | 48 | 47 | 48 |
| Kalomo/ Zimba | 45 | 34 | 47 |
| Mpongwe | 44 | 19 | 70 |
| Nchelenge | 43 | 30 | 55 |
| Chililabombwe | 40 | 19 | 61 |
| Kalulushi | 35 | 13 | 57 |
| Milenge | 29 | 20 | 38 |
| Mpulungu | 29 | 14 | 43 |
| Mungwi | 27 | 19 | 35 |
| Kaputa | 26 | 16 | 35 |
| Shang'ombo | 19 | 18 | 20 |
| Chilubi | 18 | 14 | 22 |
| Masaiti | 17 | 17 | 17 |
| Lufwanyama | 16 | 2 | 31 |
| Itezhi tezhi | 16 | 17 | 14 |
| Kazungula | 14 | 13 | 16 |
| Chienge | 12 | 9 | 15 |
Luangwa was the most cancer prevalent district in Zambia with the rate of 1,014 per 100,000 population, followed by Kabwe with the prevalence rate of 379 per 100,000 population. Lusaka was the third district with prevalence rate of 311 per 100,000, Monze ranked fourth with the rate of 282 per 100,000, Mongu ranked fifth with prevalence rate of 260 per 100,000, Katete ranked sixth with prevalence rate of 229 per 100,000 population and Chipata district ranked seventh with prevalence of 213 per 100,000 population. All these seven districts are from the southern zone.
The four most cancer prevalent districts in the northern zone were Kasempa (193 / 100,000), Kasama (168 / 100,000), Ndola (165 /100,000) and Mansa (165 / 100,000 population). The least four cancer prevalent districts in Zambia were Lufwanyama (16 / 100,000), Itezhi tezhi (16 /100,000), Kazungula (14 / 100,000) and Chienge (12 / 100,000 population) during the study period. Table 2 shows details of ranked prevalence of cancers by district per 100,000 population.
With few exceptions of districts such as Luwingu, Sinazongwe and Samfya where cancers were more prevalent in males than in females, most districts in Zambia had high cancer prevalence in females than in males. The trend in the total district pattern (see Figure 3) remained the same as cancer distribution by sex at district level (see Figure 4). The detailed geographical distribution of cancers by sex at district level is shown in Figure 4.
Figure 4.
Distribution of cancers all types by sex
Morbidity and mortality of cancers in Zambia
Cervical cancer was the most prevalent cancer in Zambia followed by prostate cancer, breast cancer and Kaposi's sarcoma. The prevalence rate of cervical cancer was 97.1 per 100,000 females and represented 34.3% of all cancers in Zambia. In proportional terms, Kaposi's sarcoma ranked second and represented 13.3%, prostate cancer ranked third and represented 7.7% while breast cancer ranked fourth and represented 6.8%. Myeloma and Other Pharynx were the least prevalent among other cancers; each represented 0.2% of all cancers in Zambia. Table 3 shows details of prevalence and proportions of cancers in Zambia from 2007 to 2014.
Table 3.
The Prevalence and proportions of cancers in Zambia, 2007 – 2014
| Cancer Type | Cases Both Sexes |
Prevalence per 100, 000 pop. |
Male | Female | Both Sexes Proportion (%) |
||
| Cases | Proportion (%) | Cases | Proportion (%) | ||||
| Cervix Uteri | 7389 | 97.1 | 7389 | 53 | 34.3 | ||
| Kaposi's Sarcoma | 2891 | 19.2 | 1759 | 23.3 | 1132 | 8.1 | 13.4 |
| Prostate | 1651 | 22.1 | 1651 | 21.8 | 7.7 | ||
| Breast | 1469 | 19.3 | 41 | 0.5 | 1428 | 10.2 | 6.8 |
| Eye | 909 | 6.0 | 431 | 5.7 | 478 | 3.4 | 4.2 |
| Non-Hodgkin Lympoma | 665 | 4.4 | 358 | 4.7 | 307 | 2.2 | 3.1 |
| Oesophagus | 581 | 3.9 | 365 | 4.8 | 216 | 1.5 | 2.7 |
| Large Bowel | 539 | 3.6 | 278 | 3.7 | 261 | 1.9 | 2.5 |
| Bladder | 478 | 3.2 | 261 | 3.5 | 217 | 1.6 | 2.2 |
| Liver | 460 | 3.1 | 283 | 3.7 | 177 | 1.3 | 2.1 |
| Other Skin | 406 | 2.7 | 192 | 2.5 | 214 | 1.5 | 1.9 |
| Stomach | 395 | 2.6 | 210 | 2.8 | 185 | 1.3 | 1.8 |
| Bone | 252 | 1.7 | 130 | 1.7 | 122 | 0.9 | 1.2 |
| Vulva/Vagina | 246 | 3.2 | 246 | 1.8 | 1.1 | ||
| Ovary | 238 | 3.1 | 238 | 1.7 | 1.1 | ||
| Kidney | 171 | 1.1 | 80 | 1.1 | 91 | 0.7 | 0.8 |
| Oral Cavity | 168 | 1.1 | 95 | 1.3 | 73 | 0.5 | 0.8 |
| Penis | 166 | 2.2 | 166 | 2.2 | 0.8 | ||
| Hodgkin Disease | 151 | 1.0 | 99 | 1.3 | 52 | 0.4 | 0.7 |
| Melanoma of the skin | 145 | 1.0 | 56 | 0.7 | 89 | 0.6 | 0.7 |
| Lung | 135 | 0.9 | 90 | 1.2 | 45 | 0.3 | 0.6 |
| Brain, Nervous System | 126 | 0.8 | 69 | 0.9 | 57 | 0.4 | 0.6 |
| Leukaemia | 126 | 0.8 | 79 | 1 | 47 | 0.3 | 0.6 |
| Larynx | 125 | 0.8 | 102 | 1.3 | 23 | 0.2 | 0.6 |
| Uterus | 115 | 1.5 | 115 | 0.8 | 0.5 | ||
| Pancreas | 102 | 0.7 | 61 | 0.8 | 41 | 0.3 | 0.5 |
| Corpus Uteri | 95 | 0.6 | 95 | 0.7 | 0.4 | ||
| Thyroid | 92 | 0.6 | 30 | 0.4 | 62 | 0.4 | 0.4 |
| Nasopharynx | 85 | 0.6 | 51 | 0.7 | 34 | 0.2 | 0.4 |
| Myeloma | 45 | 0.3 | 27 | 0.4 | 18 | 0.1 | 0.2 |
| Other Pharynx | 42 | 0.3 | 29 | 0.4 | 13 | 0.1 | 0.2 |
| Others | 1054 | 7.0 | 567 | 7.5 | 487 | 3.5 | 4.9 |
| Totals | 21512 | 142.8 | 7560 | 100 | 13952 | 100 | 100 |
Age specific rates, Standardised Incidence Rates (SIR) and the 95% confidence intervals (CI) for all cancers in Zambia are shown in Table 4. Rates were adjusted using the world standard population [23]. The standardised incidence rates and 95% confidence intervals for the top four cancers in Zambia were: cervix uteri (186.3; 95% CI = 181.77 – 190.83), prostate (60.03; 95% CI = 57.03 – 63.03), breast (38.08; 95% CI = 36.0 – 40.16) and Kaposi's sarcoma (26.18; 95% CI = 25.14 – 27.22). Peaks of age specific rates were in the age range 40 – 49 years for cervix uteri, 60 – 69 years for prostate cancer, and 40 – 49 years for breast cancer and 30 – 39 years for Kaposi's sarcoma.
Table 4.
Standardised Incidence Rates (SIR) for all cancers in Zambia, 2007 – 2014
| CANCER TYPE | Age-specific Incidence Rate per 100,000 population | SIR all ages |
95% CI | |||||||||
| 0–9yr | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Lower | Upper | ||
| Cervix Uteri | 0 | 0.07 | 5.5 | 23.41 | 49.68 | 46.11 | 39.78 | 14.09 | 7.67 | 186.3 | 181.77 | 190.83 |
| Prostate | 0 | 0 | 0 | 0.07 | 0.89 | 5.52 | 22.83 | 21.92 | 8.8 | 60.03 | 57.03 | 63.03 |
| Breast | 0 | 0.03 | 1.29 | 4.03 | 9.46 | 8.53 | 9.09 | 4.38 | 1.26 | 38.08 | 36 | 40.16 |
| Kaposi's Sarcoma | 0.35 | 0.65 | 3.58 | 7.66 | 7.42 | 3.17 | 1.82 | 0.64 | 0.88 | 26.18 | 25.14 | 27.22 |
| Oesophagus | 0.01 | 0.01 | 0.11 | 0.64 | 1.6 | 1.67 | 2.49 | 1.45 | 0.4 | 8.38 | 7.66 | 8.38 |
| Eye | 0.7 | 0.1 | 0.57 | 1.97 | 2.27 | 1.06 | 0.85 | 0.25 | 0.52 | 8.29 | 7.69 | 8.89 |
| Bladder | 0.01 | 0.02 | 0.12 | 0.34 | 0.78 | 1.36 | 2.94 | 1.41 | 0.46 | 7.45 | 6.74 | 8.16 |
| Large Bowel | 0 | 0.03 | 0.32 | 0.64 | 1.63 | 1.53 | 1.93 | 0.87 | 0.24 | 7.19 | 6.54 | 7.84 |
| Non-Hodgkin Lympoma | 0.31 | 0.45 | 0.51 | 0.8 | 1.46 | 1.33 | 1.28 | 0.48 | 0.2 | 6.82 | 6.23 | 7.41 |
| Vulva/Vagina | 0 | 0.01 | 0.31 | 0.8 | 1.61 | 1.22 | 1.23 | 0.46 | 0.3 | 5.94 | 5.14 | 6.74 |
| Liver | 0.02 | 0.09 | 0.27 | 0.56 | 1.21 | 1.22 | 1.3 | 0.83 | 0.36 | 5.86 | 5.28 | 6.44 |
| Ovary | 0.01 | 0.14 | 0.41 | 0.47 | 1.56 | 1.34 | 0.98 | 0.62 | 0.14 | 5.66 | 4.88 | 6.44 |
| Stomach | 0.01 | 0.02 | 0.12 | 0.41 | 0.93 | 1.23 | 1.53 | 1.04 | 0.32 | 5.61 | 5.02 | 6.2 |
| Other Skin | 0.05 | 0.08 | 0.28 | 0.53 | 0.81 | 1 | 1.26 | 0.69 | 0.38 | 5.07 | 4.53 | 5.61 |
| Penis | 0.02 | 0 | 0.03 | 0.26 | 0.98 | 1.28 | 1.38 | 0.58 | 0.41 | 4.93 | 4.13 | 5.73 |
| Uterus | 0 | 0.01 | 0.12 | 0.26 | 1.04 | 0.61 | 0.64 | 0.1 | 0.11 | 2.89 | 2.33 | 3.45 |
| Corpus Uteri | 0 | 0 | 0.06 | 0.12 | 0.42 | 0.85 | 1.15 | 0.2 | 0.07 | 2.87 | 2.26 | 3.48 |
| Melanoma of the skin | 0 | 0.02 | 0.02 | 0.1 | 0.28 | 0.48 | 0.88 | 0.39 | 0.09 | 2.26 | 1.87 | 2.65 |
| Bone | 0.1 | 0.38 | 0.25 | 0.22 | 0.36 | 0.22 | 0.52 | 0.12 | 0.07 | 2.25 | 1.92 | 2.58 |
| Oral Cavity | 0.04 | 0.05 | 0.08 | 0.13 | 0.36 | 0.55 | 0.63 | 0.34 | 0.07 | 2.24 | 1.87 | 2.61 |
| Larynx | 0.01 | 0 | 0.01 | 0.01 | 0.24 | 0.45 | 0.99 | 0.35 | 0.07 | 2.14 | 1.75 | 2.53 |
| Lung | 0 | 0 | 0.03 | 0.07 | 0.2 | 0.55 | 0.72 | 0.39 | 0.16 | 2.12 | 1.75 | 2.49 |
| Pancreas | 0 | 0 | 0.02 | 0.06 | 0.34 | 0.5 | 0.27 | 0.19 | 0.06 | 1.45 | 1.16 | 1.75 |
| Hodgkin Disease | 0.08 | 0.19 | 0.13 | 0.15 | 0.32 | 0.19 | 0.2 | 0.05 | 0.04 | 1.34 | 1.09 | 1.59 |
| Thyroid | 0 | 0.04 | 0.07 | 0.06 | 0.2 | 0.27 | 0.38 | 0.11 | 0.09 | 1.22 | 0.95 | 1.49 |
| Kidney | 0.45 | 0.09 | 0.01 | 0.1 | 0.13 | 0.12 | 0.11 | 0.07 | 0.07 | 1.16 | 0.95 | 1.37 |
| Brain, Nervous System | 0.16 | 0.08 | 0.1 | 0.08 | 0.23 | 0.2 | 0.18 | 0.09 | 0.02 | 1.13 | 0.9 | 1.36 |
| Nasopharynx | 0.03 | 0.05 | 0.04 | 0.11 | 0.21 | 0.25 | 0.29 | 0.02 | 0 | 0.99 | 0.75 | 1.23 |
| Leukaemia | 0.24 | 0.21 | 0.04 | 0.04 | 0.09 | 0.06 | 0.04 | 0.05 | 0 | 0.78 | 0.62 | 0.94 |
| Other Pharynx | 0 | 0.01 | 0.01 | 0.01 | 0.07 | 0.11 | 0.29 | 0.09 | 0.06 | 0.65 | 0.58 | 0.72 |
| Myeloma | 0 | 0.01 | 0.01 | 0.04 | 0.07 | 0.28 | 0.16 | 0.04 | 0.02 | 0.63 | 0.43 | 0.83 |
| Others | 0.35 | 0.52 | 0.84 | 1.07 | 1.98 | 2.47 | 2.88 | 1.32 | 0.68 | 12.11 | 11.29 | 12.93 |
Like other diseases, various forms of cancers have been classified in the International Classification of Diseases known as ICD10.
Table 5 shows details of ICD10 codes for cancers, their mortality and case fatality rates (CFR). Leukaemia (38.1%) and pancreatic cancer (36.3%) have the highest CFR among cancers in Zambia followed by lung cancer (33.3%) and brain, nervous system (30.2%).
Table 5.
Cancer classification and their mortality and CFR in Zambia, 2007 – 2014
| Cancer Type |
International Classification of Diseases (ICD10) |
Cases | Deaths | Case Fatality Rate (%) |
| Leukaemia | C91-C95 | 126 | 48 | 38.1 |
| Pancreas | C25 | 102 | 37 | 36.3 |
| Lung | C33-C34 | 135 | 45 | 33.3 |
| Brain, Nervous System | C70-C72 | 126 | 38 | 30.2 |
| Kidney | C64 | 171 | 38 | 22.2 |
| Liver | C22 | 460 | 91 | 19.8 |
| Stomach | C16 | 395 | 78 | 19.7 |
| Oesophagus | C15 | 581 | 113 | 19.4 |
| Bladder | C67 | 478 | 90 | 18.8 |
| Ovary | C56 | 238 | 44 | 18.5 |
| Myeloma | C90 | 45 | 8 | 17.8 |
| Nasopharynx | C11 | 85 | 15 | 17.6 |
| Corpus Uteri | C54 | 95 | 16 | 16.8 |
| Bone | C40-C41 | 252 | 40 | 15.9 |
| Melanoma of the Skin | C43 | 145 | 21 | 14.5 |
| Non-Hodgkin Lympoma | C82,C85,C96 | 665 | 89 | 13.4 |
| Prostate | C61 | 1651 | 215 | 13.0 |
| Large Bowel | C18-C21 | 539 | 68 | 12.6 |
| Kaposi's Sarcoma | C46 | 2891 | 332 | 11.5 |
| Thyroid | C73 | 92 | 10 | 10.9 |
| Larynx | C32 | 125 | 12 | 9.6 |
| Hodgkin Disease | C81 | 151 | 14 | 9.3 |
| Uterus | C55 | 115 | 9 | 7.8 |
| Breast | C50 | 1469 | 113 | 7.7 |
| Cervix Uteri | C53 | 7389 | 543 | 7.3 |
| Penis | C60 | 166 | 12 | 7.2 |
| Oral Cavity | C00-C06 | 169 | 12 | 7.1 |
| Other Skin | C44 | 406 | 25 | 6.2 |
| Eye | C69 | 909 | 53 | 5.8 |
| Vulva/Vagina | C51-C52 | 246 | 12 | 4.9 |
| Other Pharynx | C09-C10, C12-C14 | 42 | 2 | 4.8 |
Although CFR has been very high in Leukaemia, pancreatic cancer, lung cancer and cancers of the brain and nervous system, cancer morbidity rates were high in cervical cancer, prostate cancer, Kaposi's sarcoma, breast cancer and oesophageal cancer. During our study period 2007 to 2014, there were 543 cervical cancer deaths (CFR =7.3). Prostate cancer ranked second with 215 deaths (CFR=13) followed by Kaposis' sarcoma with 332 deaths (CFR=11.5), then breast cancer with 113 deaths (CFR=7.7) and oesophagus cancer with 113 deaths (CFR=19.4).
Association between cancers and HIV in Zambia
The cancer population in Zambia has high HIV prevalence. Results of the logistic regression analysis and Pearson correlation indicated a strong association between cancer and HIV with p-value of 0.000 and correlation coefficient of 0.818. Cancer cases, HIV positive cases and percentage of HIV positive cancer cases are shown in Table 6.
Table 6.
Association between cancers and HIV in Zambia, 2007 – 2014
| ICD10 | Cancer Type | Cases | HIV positive |
Percentage of HIV + |
r | p-value |
| C00-C06 | Oral Cavity | 169 | 20 | 12 | ||
| C09-C10, C12-C14 | Other Pharynx | 42 | 4 | 10 | ||
| C11 | Nasopharynx | 85 | 22 | 26 | ||
| C15 | Oesophagus | 581 | 71 | 12 | ||
| C16 | Stomach | 395 | 41 | 10 | ||
| C18-C21 | Large Bowel | 539 | 60 | 11 | ||
| C22 | Liver | 460 | 43 | 9 | ||
| C25 | Pancreas | 102 | 6 | 6 | ||
| C32 | Larynx | 125 | 14 | 11 | ||
| C33-C34 | Lung | 135 | 12 | 9 | ||
| C40-C41 | Bone | 252 | 6 | 2 | ||
| C43 | Melanoma of the skin | 145 | 11 | 8 | ||
| C44 | Other Skin | 406 | 65 | 16 | ||
| C46 | Kaposi's Sarcoma | 2891 | 1734 | 60 | ||
| C50 | Breast | 1469 | 169 | 12 | ||
| C51-C52 | Vulva/Vagina | 246 | 58 | 24 | ||
| C53 | Cervix Uteri | 7389 | 1331 | 18 | 0.818 | 0.000 |
| C54 | Corpus Uteri | 95 | 8 | 8 | ||
| C55 | Uterus | 115 | 14 | 12 | ||
| C56 | Ovary | 238 | 25 | 11 | ||
| C60 | Penis | 166 | 29 | 17 | ||
| C61 | Prostate | 1651 | 99 | 6 | ||
| C64 | Kidney | 171 | 4 | 2 | ||
| C67 | Bladder | 478 | 22 | 5 | ||
| C69 | Eye | 909 | 154 | 17 | ||
| C70-C72 | Brain, Nervous System | 126 | 10 | 8 | ||
| C73 | Thyroid | 92 | 8 | 9 | ||
| C81 | Hodgkin Disease | 151 | 31 | 21 | ||
| C82,C85,C96 | Non-Hodgkin Lympoma | 665 | 161 | 24 | ||
| C90 | Myeloma | 45 | 3 | 7 | ||
| C91-C95 | Leukaemia | 126 | 5 | 4 | ||
| C07-C08, C17,... | Others | 1054 | 97 | 9 | ||
| Totals | 21512 | 4337 | 20 |
Discussion
This study assessed the incidence and prevalence of major cancers in the Zambian population as well as the associated risk factors. The study makes an important contribution in showing the incidence and prevalence of cancers in Zambia, which will be important for primary and secondary prevention methods.
Although cancers were widely distributed in Zambia, this study established that cancers were more prevalent in the southern zone than the northern zone. The southern zone comprised the high and medium prevalence regions. Lusaka and Eastern provinces with very high cancer prevalent districts such as Luangwa, Katete, Chipata and Lusaka formed the belt of the high prevalence region while Central, Southern and Western provinces with high cancer prevalent districts such as Kabwe, Mongu and Monze among others formed the medium prevalence region. Although the northern zone had relatively low prevalence of cancers, there were notable districts such as Kasempa, Kasama, Ndola and Mansa with relatively high prevalence rates.
Some studies argue that the observed geographic variation in cancer distribution is due to differences in the availability of cancer screening and detection facilities as well as health seeking behaviour24–26. Our study however has established that geographic location played a big role in the pattern of cancer distribution in Zambia. This observation was made after mapping cancer cases based on patients' residential addresses. Distinct patterns were geographically displayed implying that geographic distribution played a role.
This study also observed that the northern belt of Zambia practices childhood male circumcision unlike the southern belt. Previous studies have shown that male circumcision reduces the spread of HPV, which causes cervical cancer29. Low uptake of childhood circumcision in the southern region could therefore be linked with the observed high prevalence of cervical cancer in that region. Furthermore, lack of awareness and education of cervical cancer and HPV coupled with low screening uptake led to the spread of human papilloma virus (HPV) which causes cervical cancer24.
Sex disaggregated data showed that cancers in Zambia affected more females than males in the ratio 64.86% to 35.14% (F/M = 1.85). This is mostly because of the high prevalence of cervical cancer in Zambian women. This study identified cervical cancer, prostate cancer, breast cancer and Kaposi's sarcoma as the top four most prevalent cancers in Zambia. Cervical cancer, with standardised incidence rate of 186.3 per 100,000 females was the leading cause of morbidity and mortality among cancers in Zambian women. This is contrary to other studies, which established that cervical cancer was the third leading cause of cancer-related deaths in developing countries, which is not the case with Zambia30. Age standardized incidence rates (Table 4) show that cervical cancer rates peak around the age range of 40–49 years implying that this age group is the high-risk group for cervical cancer in Zambia.
The prevalence of HPV infection was 5% in North America and 21% in Africa. This is as a result of high screening uptake and vaccine which are more available in the west compared to Africa24,27. In sub-Saharan Africa, cervical cancer incidence has been influenced by the high prevalence of HIV infection, which has been found to promote progression of cancerous lesions30. Cervical cancer incidence rates decreased by as much as 4% annually, and 70% overall in developed countries where screening programs were introduced several decades ago32,33. The observed adjusted incidence rates of cervical cancer in Zambia is similar to observed trends in Zimbabwe and Uganda27,4,35.
Prostate cancer was the second most prevalent cancer after cervical cancer and number one cancer in Zambian men with adjusted standardised incidence rate of 60.03 per 100,000 males. These results are similar to the results of other studies, which indicated that prostate cancer was the most prevalent cancer in men30. In this study, prostate cancer peak ranged from 60 – 69 years. These results indicate that prostate cancer is more prevalent in ageing men.
Breast cancer ranked third among cancers in Zambia and second in women after cervical cancer. It had an adjusted standardised incidence rate of 38.08 per 100,000 females. Breast cancer peak ranged from 40 – 49 years, which is the productive age group for women. On the global scale, breast cancer is the leading cause of cancer-related deaths among females. It is highest in the United States and Western Europe while Africa and Asia have relatively low rates30,36. Risk factors for breast cancer include weight gain after age 18 years, excess body weight (for postmenopausal breast cancer), use of menopausal hormone therapy (MHT), physical inactivity, alcohol consumption, and reproductive and hormonal factors, such as a long menstrual history, and nulliparity or later age at first birth. Risk factors for breast cancer also include inherited changes in BRCA1 and BRCA2 genes37,38. On the other hand, breastfeeding decreases the risk of breast cancer38.
The distribution of some cancers in Zambia followed the trend of HIV epidemiology. This study established a strong positive association between cancers and HIV with p-value of 0.000. These results indicate that HIV is a risk factor of cancers in Zambia. Some cancers such as Kaposi's sarcoma are HIV defining malignancies. Kaposi's sarcoma ranked fourth among cancers in Zambia with adjusted incidence rate of 26.18 per 100,000 population. Kaposi's sarcoma peak ranged from 30 – 39 years. This study observed that Kaposi's sarcoma was more prevalent in males than in females (see Table 3). The incidence of Kaposi's sarcoma is higher in Sub-Saharan Africa than in developed countries. The endemic African form of Kaposi's sarcoma was reported in the 1960s39, but with the emergence of HIV/AIDS, an atypical aggressive type was reported in most African countries40,41,42. Although morbidity rate was high in cervical, prostate and breast cancers as well as oesophageal cancer and Kaposi's sarcoma, case fatality rate was moderate in these cancers compared to Leukaemia, pancreatic cancer, lung cancer and cancers of the brain and nervous system where case fatality rates were high.
This study had some limitations, firstly, the missing information in the database due to challenges in active cancer surveillance made us leave some patients' files, as they could not be linked to districts of origin. Secondly, the Zambia National Cancer Registry had huge backlog of data due to understaffing. This brought about the adjustment of our study from 2007 – 2017 to 2007 – 2014 period. Lastly, registry based studies have challenges to provide precise data as registries data capturing system do not always suit all study designs. ZNCR did not fully capture risk factors for all cancers hence; we could not critically determine all risk factors in this study.
In conclusion, the widespread distribution of cancers with high prevalence in the southern zone was perpetrated by lifestyle and sexual culture as well as geography. Traditional male circumcision known to prevent STIs is practiced in the northern belt (particularly North Western Province) and not the southern belt. The seven most cancer prevalent districts in Zambia were Luangwa, Kabwe, Lusaka, Monze, Mongu, Katete and Chipata. Cervical cancer, prostate cancer, breast cancer and Kaposi's sarcoma were the four most prevalent cancers as well as major causes of cancer related deaths in Zambia; although Leukaemia and pancreatic cancer had, the highest case fatality rate. Most cancers in Zambia are HIV defining malignancies and their upward trend is due to the increase in HIV cases. Changing the lifestyle and sexual culture (multiple sexual partners) would greatly help in the prevention of rampant spread of HPV, HIV, HBV and EBV among others which would result in the reduction of cancer cases in Zambia. Furthermore, intensifying cancer screening and early detection countrywide would help to drastically reduce cancer prevalence in Zambia over time.
Figure 1.
Regional and provincial distribution of cancers in Zambia
Acknowledgements
Compliments go to the Research Ethics Committee of Institute of Urban Environment, Chinese Academy of Sciences, the University of Zambia Biomedical Research Ethics Committee and the National Health Research Authority of the Republic of Zambia, for allowing the researchers to conduct a study in Zambia. Sincere gratitude extends to the Zambia National Cancer Registry for availing the data used in this study. The principal researcher was a PhD candidate at the Institute of Urban Environment, university of Chinese Academy of Sciences, 1799 Jimei Road, Xiamen, 361021, PR China under the scholarship of UCAS.
Competing Interests
Authors declare that they have no competing interests.
Authors' Contributions
MK designed the study, developed and programmed the model and drafted the manuscript. HS and LL approved the model and provided technical support in analyses. All authors read and approved the manuscript.
References
- 1.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020 Feb;8(2):e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 2.Fitzmaurice C, Allen C, Baber RM, et al. Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.James SL, Abate D, Abate KH, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Cancer Collaboration, author. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicker D, Nguyen G, Abate D, et al. GBD 2017 Mortality Collaborators Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1684–1735. doi: 10.1016/S0140-6736(18)31891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyu HH, Abate D, Abate KH, et al. GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vorobiof DA, Abratt R. The cancer burden in Africa. S Afr Med J. 2007;97:937–939. [PubMed] [Google Scholar]
- 10.Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases--where worlds meet. N Engl J Med. 2010;363:1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- 11.Bray F, Moller B. Predicting the future burden of cancer. Nat Rev Cancer. 2006;6:63–74. doi: 10.1038/nrc1781. [DOI] [PubMed] [Google Scholar]
- 12.Pisani P, Parkin DM, Muñoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- 13.Ngoma T. World Health Organization cancer priorities in developing countries. Ann Oncol. 2006;17(Suppl 8):viii9–viii14. doi: 10.1093/annonc/mdl982. [DOI] [PubMed] [Google Scholar]
- 14.Reeler AV, Mellstedt H. Cancer in developing countries: challenges and solutions. Ann Oncol. 2006;17(Suppl 8):viii7–viii8. doi: 10.1093/annonc/mdl981. [DOI] [PubMed] [Google Scholar]
- 15.Fontham ET. Infectious diseases and global cancer control. CA Cancer J Clin. 2009;59:5–7. doi: 10.3322/caac.20000. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 17.IARC Working Group, author. Proceedings on the Evaluation of Carcinogenic Risks to Humans. Epstein - Barr virus and Kaposi's Sarcoma Herpesvirus/Human Herpesvirus 8. Lyon, France, 17-24. 100 J. Public Health Epidemiol. June 1997. IARC Monogr Eval Carcinog Risks Hum. 1997;70:1–492. [Google Scholar]
- 18.Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, Hainaut P, Hall AJ, Whittle H, Montesano R. The Gambia liver cancer study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatol. 2004;39:211–219. doi: 10.1002/hep.20027. [DOI] [PubMed] [Google Scholar]
- 19.Blankfield RP. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2002;346:65–67. doi: 10.1056/NEJM200201033460115. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 21.Knowles DM. Etiology and pathogenesis of AIDS-related nonHodgkin's lymphoma. Hematol Oncol Clin North Am. 2003;17:785–820. doi: 10.1016/s0889-8588(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 22.Zambia latitude and longitude map. Source: http//www.mapsofworld. com/lat_long/zZambialat-long.html.
- 23.World (WHO 2000–2025) Standard available at: https://seer.cancer.gov/stdpopulations/world.who.html.
- 24.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 25.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 26.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Bray F, Lortet-Tieulent J, Znaor A, Brotons M, Poljak M, Arbyn M. Patterns and trends in human papillomavirus-related diseases in Central and Eastern Europe and Central Asia. Vaccine. 2013;31(Suppl 7):H32–H45. doi: 10.1016/j.vaccine.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 28.Maver PJ, Seme K, Korac T, Dimitrov G, Dobrossy L, Engele L, et al. Cervical cancer screening practices in central and eastern Europe in 2012. Acta Dermatovenerol Alp Pannonica Adriat. 2013;22:7–19. [PubMed] [Google Scholar]
- 29.Wawer Maria J, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet (London, England) 2011;377(9761):209–218. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] [cited 2015 July 30]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 31.De Vuyst H, Alemany L, Lacey C, Chibwesha CJ, Sahasrabuddhe V, Banura C, et al. The burden of human papillomavirus infections and related diseases in sub-saharan Africa. Vaccine. 2013;31(Suppl 5):F32–F46. doi: 10.1016/j.vaccine.2012.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bray F, Loos AH, McCarron P, Weiderpass E, Arbyn M, Moller H, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev. 2005;14:677–686. doi: 10.1158/1055-9965.EPI-04-0569. [DOI] [PubMed] [Google Scholar]
- 33.Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8:755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 34.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chokunonga E, Borok M, Chirenje Z, Nyakabau A, Parkin D. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer. 2013;133:721–729. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 36.Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol. 2005;34:405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 37.Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK, Stefanick ML, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study. J Natl Cancer Inst. 2013;105:526–535. doi: 10.1093/jnci/djt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schottenfeld D, Fraumeni JF, Jr, Colditz GA, Baer HJ, Tamimi RM. Breast cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. 3rd ed. New York: Oxford University Press; 2006. pp. 995–1012. [Google Scholar]
- 39.Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi's sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78:1521–1528. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wabinga HR, Parkin DM, Wabwire-Mangen F, Mugerwa JW. Cancer in Kampala, Uganda, in 1989–91: changes in incidence in the era of AIDS. Int J Cancer. 1993;54:26–36. doi: 10.1002/ijc.2910540106. [DOI] [PubMed] [Google Scholar]
- 41.Bassett MT, Chokunonga E, Mauchaza B, Levy L, Ferlay J, Parkin DM. Cancer in the African population of Harare, Zimbabwe, 1990–1992. Int J Cancer. 1995;63:29–36. doi: 10.1002/ijc.2910630107. [DOI] [PubMed] [Google Scholar]
- 42.Chintu C, Athale UH, Patil PS. Childhood cancers in Zambia before and after the HIV epidemic. Arch Dis Child. 1995;73:100–104. doi: 10.1136/adc.73.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]