Abstract
Background
Interstitial lung disease (ILD) is associated with substantial morbidity and mortality, which is one of the key systematic manifestations of connective tissue disease (CTD). Tripterygium wilfordii, known as Leigongteng in Chinese, has been applied to treat connective tissue disease-related interstitial lung disease (CTD-ILD) for many years. Triptolide is a key effective component from Tripterygium wilfordii. But the molecular mechanism of Triptolide for treating CTD-ILD is not yet clear.
Methods
Gaining insight into the molecular mechanism of Triptolide intervention CTD-ILD, we used the method of network pharmacology. And then we conducted drug-target networks to analyse the potential protein targets between Triptolide and CTD-ILD. Finally, AutoDock Vina was selected for molecular docking.
Results
By analysing the interaction genes between Triptolide and CTD-ILD, 242 genes were obtained. The top 10 targets of the highest enrichment scores were STAT3, AKT1, MAPK1, IL6, TP53, MAPK3, RELA, TNF, JUN, JAK2. GO and KEGG enrichment analysis exhibited that multiple signalling pathways were involved. PI3K-Akt, multiple virus infections, cancer signalling, chemokine, and apoptosis signalling pathway are the main pathways for Triptolide intervention CTD-ILD. And it is related to various biological processes such as inflammation, infection, cell apoptosis, and cancer. Molecular docking shows Triptolide can bind with its target protein in a good bond by intermolecular force.
Conclusions
This study preliminarily reveals the internal molecular mechanism of Triptolide interfere with CTD-ILD through multiple targets, multiple access, validated through molecular docking.
KEY MESSAGES
Triptolide intervention CTD-ILD, which are related to various biological processes such as inflammation, infection, cell apoptosis, and cancer.
PI3K-Akt, multiple virus infections, and apoptosis signalling pathway are the main pathways for Triptolide intervention CTD-ILD.
Triptolide can bind with related target protein in a good bond by Intermolecular force, exhibiting a good docking activity.
Keywords: Tripterygium wilfordii, Triptolide, CTD-ILD, Network pharmacology, Molecular docking
1. Introduction
Interstitial lung disease (ILD) [1], associated with significant morbidity and mortality, is a common manifestation and amongst the leading causes of morbidity and mortality in patients with connective tissue disease (CTD) [2]. CTD-ILD, which is characterized by immune-mediated tissue injury that can involve the lungs, has a mix of inflammatory and fibrosis [2]. ILD along with multicompartment lung involvement including airways, pleural and pulmonary vascular disease, may contribute to the aetiology of their respiratory impairment and potential responses to therapy [3]. Both the innate and adaptive immune systems can induce the development of fibrosis [4]. Following the injury, wound-healing responses are accompanied. If sustained and deregulated, pathological fibrogenesis then occurs, whereby the rate of new collagen synthesis exceeds the rate of collagen degradation, culminating in the accumulation of collagen over time [5]. During the development and evolution of this disease, and immune imbalance is the main pathological factor, and a variety of immune cells, such as T cells, B cells, macrophages, etc. are involved in the occurrence and development of this disease [6,7]. Controlling a variety of pro-inflammatory factors and restoring immune homeostasis is the main direction of inhibiting the progression of this disease from inflammation to fibrosis [8]. Among them, FGF, VEGF, TGF, PDGF were thought the critical pro-inflammatory factors [8]. But there is currently little high-level evidence to guide the management of CTD-ILD. Novel approaches involving biological agents, antifibrotic drugs, and even stem cell transplants have been introduced for CTD-ILD treatment, although specific pulmonary benefit has not been conclusive. Thus, further efforts are still urgently needed to develop novel strategies to prevent this refractory respiratory disease.
Tripterygium wilfordii is a clinically common Chinese herbal medicine, which has been used to treat clinical diseases for more than 2000 years in China. Because of its good immunosuppressive effect in the fields of metabolic diseases [9], kidney diseases [10], rheumatic diseases [11], inflammatory bowel diseases [12], and so on [13], Tripterygium wilfordii hance has been paid much attention by scholars at home and abroad. Triptolide is a major active natural product isolated from the medicinal plant Tripterygium wilfordii, which has exhibited to have good anti-inflammatory, immunosuppressive and anti-fibrosis therapeutic effects [9], but its molecular mechanism of action is still unknown. Network pharmacology is an emerging discipline based on the network of disease-gene-drug targets [14], based on network interaction to study the basic biological knowledge of TCM can provide a deep insight or scientific evidence for the discovery of TCM, and help us to clarify the pharmacological mechanism of active ingredients of TCM at the level of biomolecule [15]. Network pharmacology is gradually becoming a holistic and efficient tool to describe the complex interactions between drugs and biological systems including the human organs, diseases, metabolic pathways, and target proteins from a network perspective [16].
In this study, we constructed a network pharmacological model of Triptolide and systematically analysed the potential anti-CTD-ILD mechanism of Triptolide. Our studies revealed the potential mechanisms which may contribute to the occur of CTD-ILD. To confirm the predicted result, we provide a molecular docking model with potential targets and known targets. The detailed procedures can be seen in Figure 1.
Figure 1.
Workflow of the network pharmacology to identify Triptolide targets in CTD-ILD.
2. Materials and methods
2.1. Screening of triptolide-related disease targets
All targets of Triptolide were gathered by using the following five database: Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (http://lsp.nwu.edu.cn/index.php) [17], the Comparative Toxicogenomics Database (CTD) [18] (http://ctdbase.org/), GeneCards database (https://www.genecards.org) [19,20], STITCH (http://stitch.embl.de/) [21]and SymMap database (https://www.symmap.org/) [22]. Organism equal to Homo sapiens was limited. After removing the duplicated genes, related genes were returned.
2.2. Gene screening of CTD-ILD related targets
Using “Connective Tissue Disease-associated with Interstitial Lung Diseases” as the search keywords, we searched OMIM database (http://www.omim.org), Genebank database (https://www.ncbi.nlm.nih.gov/genbank), GeneCards database (https://www.genecards.org) to excavate potential targets associated with CTD-ILD. Access the DrugBank database (https://www.drugbank.ca) to find drug targets for approved intervention CTD-ILD [23]. The 4 disease database targets were combined and the repeat value was deleted to get the CTD-ILD related targets.
2.3. Construction of protein-protein interaction (PPI) network
Using R X 64 4.0.2 software, the intersection of the Triptolide target and related CTD-ILD target was obtained, then the Venn diagram was drawn. Intersection targets were extracted and submitted to STRING database (https://string-db.org) to build a protein-protein interaction (PPI) network [24]. The species type was set to “Homo sapiens”, the minimum interaction threshold was set to “highest confidence” (>0.9), and the rest were set as the default. Finally, the PPI result was imported into Cytoscape 3.8.0 software to construct a PPI network.
2.4. Hub gene analysis
Hub gene of PPI network of Triptolide against CTD-ILD was calculated by MCC algorithm in Cytohubba plugin of CytoScape [25], then the related protein targets network was constructed. Finally, the core targets of the top 10 were also exhibited.
2.5. Go and KEGG enrichment analysis
To clarify the role of target proteins interacting with Triptolide target genes in gene function and signalling pathways, we conducted GO and KEGG enrichment analysis of potential targets of Triptolide intervention CTD-ILD by R Software (R 4.0.2 for Windows). Save the data results and use R software for visual analysis.
2.6. Molecular docking
Molecular docking was performed among the top 5 potential target proteins and known 4 relative proteins of ILD with Triptolide. The 3D structure of the target protein was downloaded from the PDB database (https://www.rcsb.org). The water molecules and the original ligands were removed from the target protein through PyMOL, Later, the target proteins were imported into AutoDock Tools 1.5.6 for hydrogenation, charge calculation, and non-polar hydrogen combination, and then the result was stored in PDBQT format. Set the size of Grid Box to 40 × 40 × 40. Finally, run AutoDock Vina using CMD command characters for molecular docking [26], and use PyMOL to visualize the results.
3. Result
3.1. Acquisition of the main protein targets of triptolide
By searching the following databases: TCMSP, CTD, GeneCards, STITCH, and SymMap database, confining the result to “Homo sapiens”, 702 genes related with Triptolide were collected by using the term “Connective Tissue Disease-associated Interstitial Lung Diseases”.
3.2. Acquisition of CTD-ILD related targets
In Genecards database, the higher the Score value is, the closer the relationship is to the disease. When there are too many targets, the target with a score greater than the median is a CTD-ILD-related target. The maximum score of the target obtained by GeneCards is 166.33, the minimum score is 0.38, and the median is 17.64. Therefore, the genetic target with a Score of >17.64 is considered a CTD-ILD-related target. Then 1764 targets are obtained after screening. After searching the GenBank database, we found 86 related genes. A total of 1294 targets were obtained from the Genemap database. The DrugBank database search is complemented, and about 12 targets for Nidanib were obtained. Results were merged and the duplicate genes were eliminated, then 2860 CTD-ILD related targets were obtained.
3.3. Venn diagram and PPI network construction
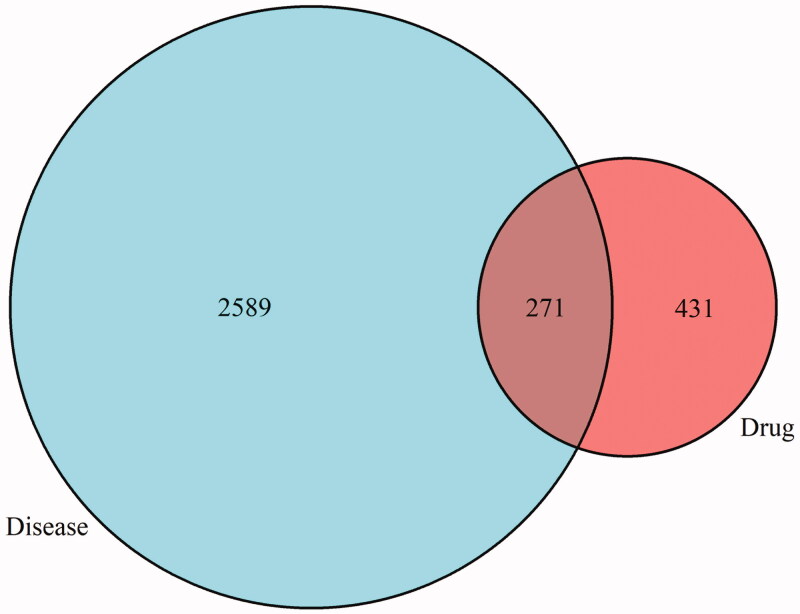
The intersection of Triptolide targets and CTD-ILD disease targets was taken, and Venn diagram was drawn by R software to obtain 271 intersection targets (Figure 2). The target proteins that act with their corresponding ingredients were submitted to STRING version 11.0 (http://string-db.org/) for PPI network construction, and high confidence of protein interaction data with a score >0.9 was selected. Removing free proteins that do not interact, There were 242 shared proteins between Triptolide and CTD-ILD. The protein-protein interactions networks suggested that 242 proteins and 1628 interactions (edges) could potentially interact in the intersection targets between Triptolide and CTD-ILD (Figure 3(A)).
Figure 2.
The Venn diagram of Triptolide and CTD-ILD targets.
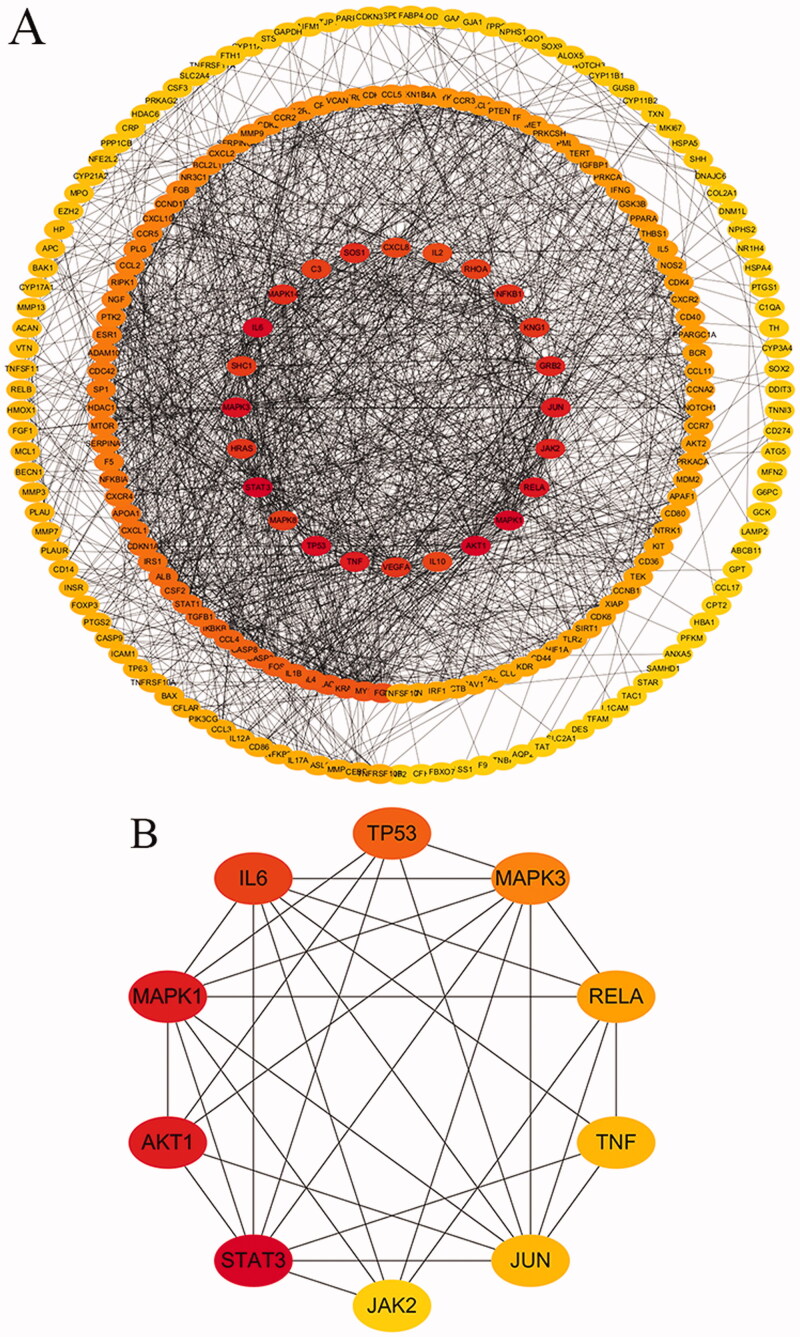
Figure 3.
Against CTD-ILD targets network of Triptolide, the target genes are sorted according to a degree. (A) The degree of the outermost circle is 0–20, the middle circle is 21–40 , the smallest circle is 41–100. (B) Cytohubba, the plug-in of Cytoscape, was used to analyse the top 10 hub gene network of target proteins by MCC algorithm, in which gray value represents the importance in the network.
3.4. Screening of hub gene and topological network analysis
Based on Cytohubba, the plug-in of Cytoscape, the Hub gene was screened in the interaction network. We used the MCC algorithm to find out the top 10 Hub genes of target proteins, and the Hub gene network diagram was constructed. Based on network topological analysis, the average betweenness centrality was 0.01 and the average degree of freedom was 13.45, signalling hub connectivity between genes. A total of 98 genes produced betweenness centrality and degree of freedom values above the mean, but only the top 10 genes are exhibited (Table 1). We can conclude that STAT3, AKT1, MAPK1, IL6, TP53, MAPK3, RELA, TNF, JUN, JAK2 are the important targets for Triptolide intervention CTD-ILD (Figure 3(B)).
Table 1.
Target proteins with potentially critical roles in Triptolide treatment of CTD-ILD.
| NO. | Gene abbreviation | Betweenness centrality | Degree |
|---|---|---|---|
| 1 | STAT3 | 0.093621689 | 63 |
| 2 | MAPK1 | 0.068443793 | 54 |
| 3 | AKT1 | 0.065861966 | 54 |
| 4 | IL6 | 0.080875215 | 52 |
| 5 | TP53 | 0.078781834 | 51 |
| 6 | MAPK3 | 0.034771135 | 50 |
| 7 | RELA | 0.042217185 | 46 |
| 8 | JUN | 0.035870028 | 42 |
| 9 | TNF | 0.031940891 | 42 |
| 10 | JAK2 | 0.028176151 | 38 |
3.5. Go and KEGG analysis
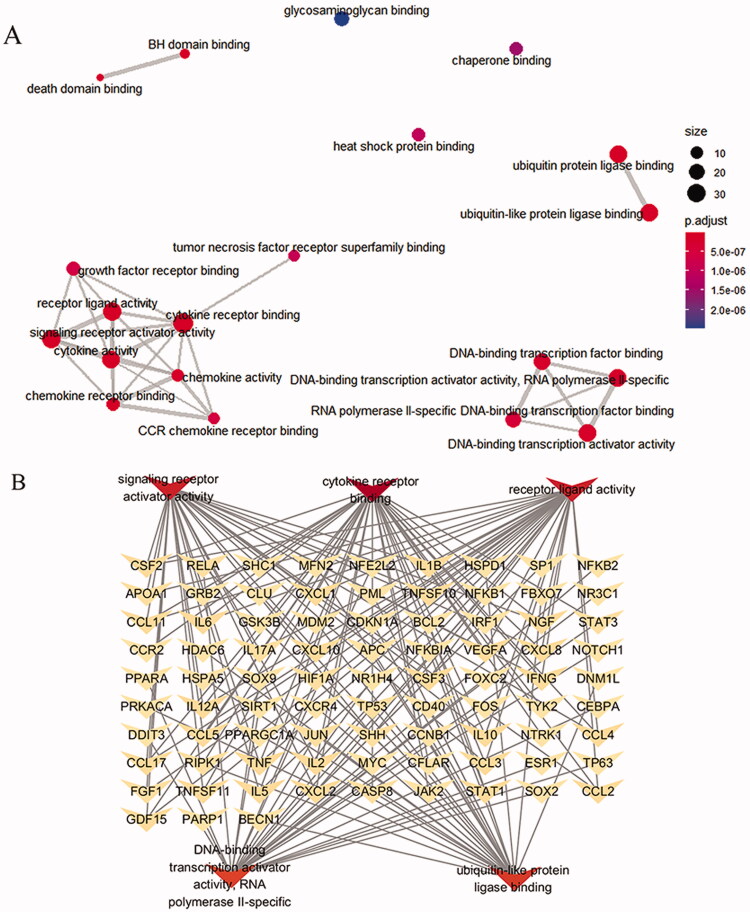
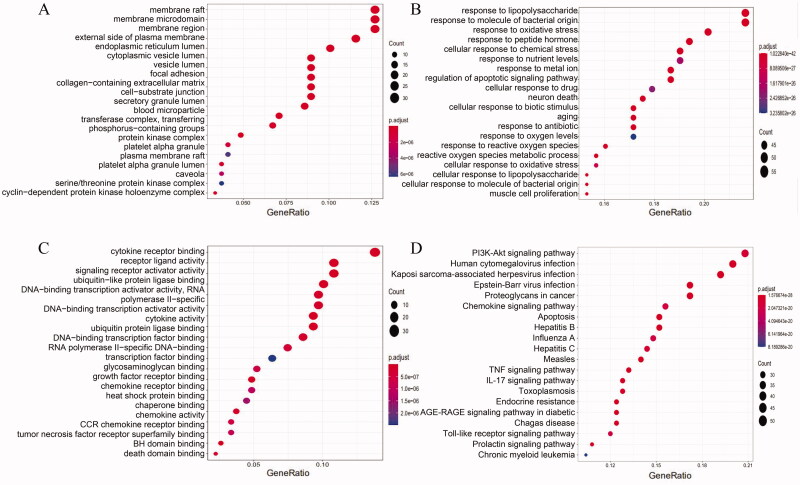
The GO and KEGG enrichment analysis of Triptolide intervention CTD-ILD related targets was carried out with R software and the results were visualized. The topological network schematic of target proteins in enriched molecular functions analysed by enrichMap in the clusterProfiler package is listed (Figure 4).
Figure 4.
Topological network schematic of proteins targeted by Triptolide and associated with CTD-ILD. (A) Interaction networks between enriched molecular functions are analysed by enrichMap in the clusterProfiler package. The scales indicated different thresholds of adjusted p-values, and the sizes of the dots represent the gene count of each term. (B) Sub-network showing important genes in the top 5 GO terms. The subnetwork depicts the relationships among 5 GO terms and CTD-ILD associated genes. Abbreviations: GO, gene ontology.
The GO enrichment analysis results obtained a total of 3464 items, The top 20 significantly enriched terms in BP, MF, and CC categories were selected, according to p < .05, p-values were corrected using the Benjamini–Hochberg procedure. In the biological processes category, the target proteins were mainly involved in Apoptosis, cell oxidative stress, inflammatory response, etc. In the MF category, the target proteins were mainly involved in Receptor ligand binding activity, Ubiquitination of proteins, transcription factor binding, Cytokine activity. In the CC category, the target proteins were classified into the plasma membrane and cell surface, endoplasmic reticulum lumen, etc.
There were 173 KEGG enrichment items in total, and the top 20 items were screened according to the KEGG analysis with BH-corrected p-values < .05, mainly included PI3K-Akt signalling pathway, multiple virus infections, chemokine signalling pathway, and apoptosis signalling pathway et al. The first 20 related enrichment results were visualized based on GO and KEGG enrichment results (Figure 5).
Figure 5.
GO and KEGG analysis of genes encoding proteins targeted by Triptolide. GO enrichment analysis identified genes involved in (A) GO-CC analysis, (B) GO-BP analysis, (C) GO-MF Analysis, (D) KEGG analysis. (B) KEGG pathway analyses from bioinformatics data for the molecular signal pathway of Triptolide against CTD-ILD.
3.6. Molecular docking
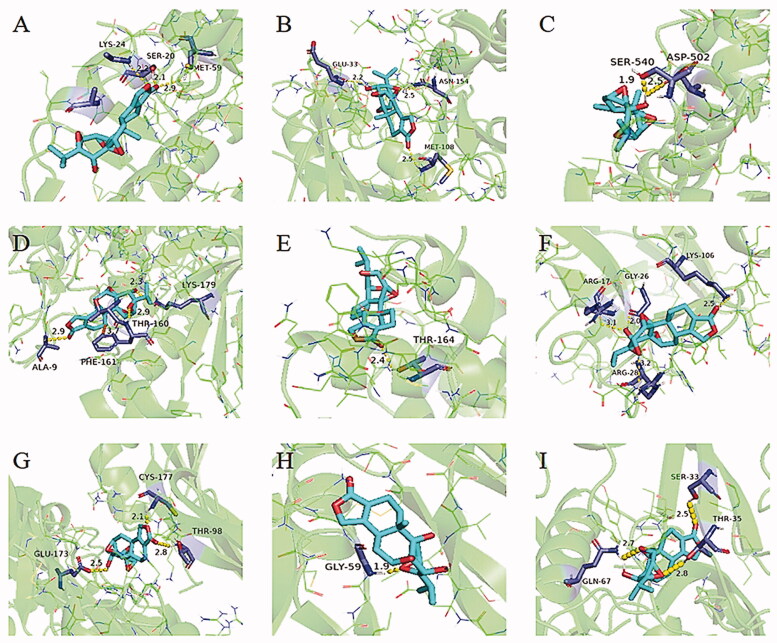
Molecular docking was conducted between Triptolide, the core components of Tripterygium wilfordii and the key targets. Then the docking results showed that Triptolide could be bound into the docking pocket, with good docking activity between the target proteins. As we tested the following potential target proteins (Figure 6): TP53(PDB: 3DAB), MAPK1(PDB: 3W55), STAT3 (PDB: 6NUQ), AKT1(PDB: 6NPZ), and IL6(PDB: 2IL6), which were high-degree nodes in the interaction network, suggesting they play a critical role in the response to Triptolide in CTD-ILD. Triptolide bound to TP53 by forming hydrogen bonds with SER-20 (length:2.1 Å), MET-59(2.9 Å), and LYS-24 (2.2 Å, Figure 6(A)). There are also 3 hydrogen bonds, GLU-33(2.2 Å), MET108(2.5 Å), and ASN-154(2.5 Å) were predicted between Triptolide and MAPK1(Figure 6(B)). Similarly, Triptolide was predicted to dock into the binding pocket of STAT3 via hydrogen bonds SER-540(1.9 Å), ASP-502(2.5 Å, Figure 6(C)). Triptolide was also predicted to dock in the binding pocket of AKT1 via multiple hydrogen bonds with THR-160(2.6 Å), LYS-179(2.3 Å), ALA-9(2.9 Å), and π–π interactions with PHE-161 (3.7 Å, Figure 6(D)). Lastly, Triptolide was predicted to dock into the pocket of IL-6 via a single hydrogen bond with THR-164 at a distance of 2.4 Å (Figure 6(C)). Furthermore, known protein targets of ILD (FGF, PDGF, VEGF, TGF) was also conducted molecule docking with Triptolide, of whom binding energies were all less than −5kcal·mol−1. It is suggested that Triptolide may affect its function by competitively inhibiting the binding of the docking pocket to the target receptor and play an important role in the treatment of CTD-ILD.
Figure 6.
Molecular models of Triptolide binding to its predicted protein targets. Proteins (A)TP53(3DAB), (B) MAPK1(3W55), (C)STAT3(6NUQ), (D)AKT1(6NPZ), (E)IL6(2IL6), (F)FGF(2K8R), (G)PDGF(3MJK), (H) VEGF(1VPF), (I)TGF(3KFD) are shown interacting with a Triptolide molecule, represented by a blue stick model. Lines represent residues in the binding sites. The light dashed lines represent hydrogen bonds, the dark dashed lines demarcate π-π interactions, and the interaction distances are indicated next to the bonds (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4. Discussion
Network pharmacology, as a cutting-edge approach provides a full or partial understanding of the principles of network theory and systems biology [27]. This approach has been used to study the pathway “compound-proteins/genes-disease” in a way that captures the complexities among biological systems, drugs, and diseases from a network perspective. Therefore, the network pharmacology research method is used to predict the interrelationship network between drugs and diseases in the study of different fields such as discovering new drugs [28], elaborating pharmacological mechanisms [29], and exploring new targets [30]. In our study, topological analysis of the drug-disease network was conducted, and corresponding molecular docking studies were conducted to enhance the reliability of target prediction conclusions.
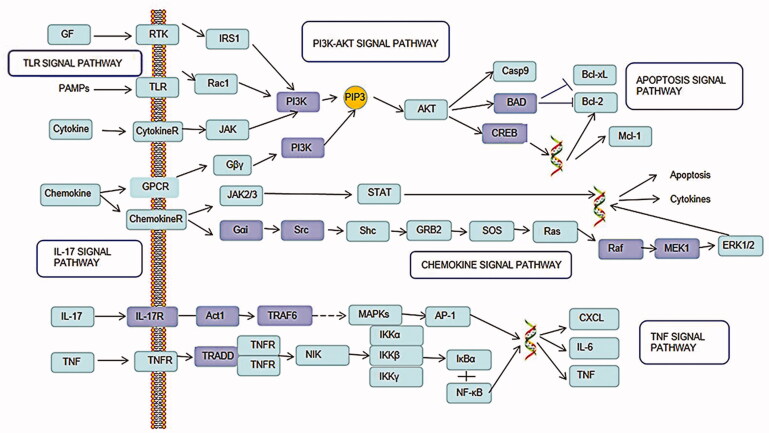
In the present report, we predict interactions between Triptolide and its potential protein targets by integrating information from publicly available databases about CTD-ILD, as well as elucidating the numerous signalling pathways and networks in which Triptolide targets participate. We also performed docking studies to predict specific interactions between Triptolide and its predicted protein targets. The molecular docking results of Triptolide showed that hydrogen bonding and π-π stacking were the main forms of interaction. Pathway analysis suggested that Triptolide regulates the activation of mainly included PI3K-Akt signalling pathway, multiple virus infections, chemokine signalling pathway, apoptosis signalling pathway, TNF signalling pathway, and IL-17 signalling pathway in CTD-ILD (Figure 7). The results proved that Triptolide can interfere with the process of CTD-ILD, through multiple targets, multiple access. Among them, the top 10 targets are STAT3, AKT1, MAPK1, IL6, TP53, MAPK3, RELA, TNF, JUN, JAK2. By analysis of the hub genes and main significant KEGG pathways, the potential mechanisms of Triptolide in the treatment of CTD-ILD might be attributed to the following aspects.
Figure 7.
Distribution of the target proteins of Triptolide on the predicted pathway. The dark nodes are potential target proteins of Triptolide, while the light nodes are relevant targets in the pathway.
First of all, These potential targets are closely associated with the progression of pulmonary fibrosis. For example, STAT3 controls crosstalk between epithelial cells and fibroblasts which in turn contributes to disease progression [31]. In fibrogenesis, IL-6 can promote the phosphorylation of STAT3 and its translocation to the nucleus for further gene transcription [32]. JAK2 and STAT3 were verified as a hub pathway to modulating oxidative stress, inflammation, and cell apoptosis in a model of bleomycin-induced acute lung injury rat [33]. c-Jun-N-terminal kinase 1 (JNK1) [34] signalling pathways play an essential role in various physiological and pathological processes such as cell cycle, reproduction, apoptosis, and cellular stress. Recently, it has been found that the transcription factor JUN is highly expressed in pulmonary fibrosis, and its overexpression in mice can induce pulmonary fibrosis. During BLM injury, the pro-inflammatory cytokine such as IL-17A will be up-regulated and mediate the inflammation in the alveolar epithelial cell and also brings about recruitment of certain inflammatory cells in the alveolar surface. IL-17A-mediated p53-fibrinolytic aspects are involved in the regulation of pathogenic progression of acute lung injury (ALI) and pulmonary fibrosis [35].
Second, these targets also participate in the process of epithelial-mesenchymal transition (EMT). EMT is a process in which epithelial cells gradually transform into mesenchymal like cells, losing epithelial function and characteristics. EMT is thought to be involved in the pathogenesis of many lung diseases, ranging from developmental disorders, fibrous tissue remodelling to lung cancer [36]. MAPK3(ERK1), MAPK1(ERK2) belong to ERK signal pathway. In vitro and in vivo studies have shown that activation of ERK1/2 signalling pathway is involved in TGF-β1-induced EMT [37]. Besides, it’s also necessary for TGF-β1-induced tight junction dissociation and cell migration. Then the expression of downstream fibrosis-related genes were regulated [38]. RELA is one of the major members of the NF-κB family, involved in the transcription and regulation of multiple inflammatory factors. One study confirmed that NF-κB/RelA signalling network plays an important role in type II epithelial mesenchymal transformation in primary airway epithelial cells [39].
Then, the PI3K-Akt signalling pathway, TNF signalling pathway, and IL-17 signalling pathway are known as inflammation-related signalling pathways. In addition, By activating the PI3K-Akt and the mammalian target of rapamycin (mTOR) signalling pathway can inhibit autophagy of bronchial epithelial cells and exacerbate lung damage and fibrosis [40]. Accordingly, inhibiting this pathway can attenuate mitochondrial-dependent apoptosis, endoplasmic reticulum stress, and inflammation in acute lung injury [41]. TNF signalling pathway plays an important role in a variety of connective tissue diseases. Tumour necrosis factor receptors and their corresponding cytokine ligands are involved in many aspects of immune function [42]. Activation of the TNF signalling pathway has been found to induce interstitial lung disease [43]. Interleukin (IL)-17 signal pathway has demonstrated pro-inflammatory effects in chronic inflammation and autoimmune diseases and showed pathogenicity in pulmonary fibrosis are potential therapeutic targets for the treatment of fibroproliferative lung diseases [44]. Interestingly, Signalling pathways are not isolated from each other, such as the IL-17 and Akt pathways have a mutual promoting effect. One study confirmed that IL-17A can inhibit autophagy in keratinocytes by activating the PI3K/Akt/mTOR signalling pathway [45]. Multiple studies have found that viral infection can induce the formation of hyaluronic acid [46] and contribute to a variety of inflammatory cytokines release [47], associated with acute lung interstitial injury [48]. All of them are a costimulatory factors in the progression of pulmonary interstitial disease [49]. Apoptosis was thought to be the only regulated cell death mechanism in the past years [50]. Regulation of cell death is the main mechanism for eliminating damaged, infected, or excess cells, which is also found to be related to the occurrence of ILD [51].
Experimental studies have found that Triptolide has shown good anti-inflammatory and immunosuppressive effects in the treatment of many diseases and verified that Triptolide can attenuate inflammatory response, lung injury, and kidney damage in multiple autoimmune diseases by inhibiting NF-κB, vascular cell adhesion molecule-1, IL-1, IL-6, IL-17, and TNF-α, through interfering NF-κB [10,52], PI3K-AKT-mTOR, and apoptosis signal pathway et al. [53]. So we think that Triptolide may play a vital role in the treatment of CTD-ILD through the above pathway.
Given the uncertainty of the benefits in the present treatment of CTD-ILD, it remains an urgent need for new treatment strategies. we provide several potential targets for CTD-ILD treatment, which could contribute to the development of new therapeutic strategies. Although the therapeutic effect of tripterysium glycosides in the treatment of CTD-ILD has been satisfactory during the past several years. But its mechanism has been rarely studied. This paper is the first study based on the network pharmacology, and molecular docking technology discussed the molecular mechanism of Triptolide, an element of tripterysium glycosides, intervene CTD-ILD. Further vitro and vivo studies are required to explore the mechanism of tripterygium wilfordii and its bioactive constituents in the treatment of CTD-ILD. But, the current research on the treatment of CTD-ILD by Tripterygium wilfordii and its bioactive ingredients is still in the preliminary stage. So, up to date, it’s still a lack of research models to simulate the pathology of CTD-ILD. Last but not least, It is worth noting that tripterygium wilfordii and its active ingredients are toxic to the liver, kidney, or reproductive system. So, how to reduce its toxicity and increase the curative effects should be also taken into consideration.
Conclusion
The present study analysed the mechanisms underlying the therapeutic effect of Triptolide in CTD-ILD using network pharmacology. Our findings revealed that Triptolide exerts pharmacological effects in CTD-ILD in a multicomponent–multitarget–multipathway manner, including Apoptosis, cell oxidative stress, inflammatory response, and so on. Our findings offer a reference for further investigation of the mechanism underlying the therapeutic effect of Triptolide in CTD-ILD.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant 81774274, grant 81973769].
Author contributions
WZ and YL designed this study and performed the online database search. YW and JZ all contributed to the data collection and data analysis. YL and WZ prepared the original draft. YW finished the revision of the manuscript. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
References
- 1.Gao Y, Moua T.. Treatment of the connective tissue disease-related interstitial lung diseases: a narrative review. Mayo Clinic Proceedings. 2020;95(3):554–573. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, Decramer M, Wedzicha JA, et al. An official american thoracic society/european respiratory society statement: research questions in COPD. Eur Respir J. 2015;45(4):879–905. [DOI] [PubMed] [Google Scholar]
- 3.Gutsche M, Rosen GD, Swigris JJ.. Connective tissue disease-associated interstitial lung disease: a review. Curr Respir Care Rep. 2012;1:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spagnolo P, Distler O, Ryerson CJ, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann Rheum Dis. 2021;80(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolahian S, Fernandez IE, Eickelberg O, et al. Immune mechanisms in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2016;55(3):309–322. [DOI] [PubMed] [Google Scholar]
- 7.Todd NW, Scheraga RG, Galvin JR, et al. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J Inflamm Res. 2013;6:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann-Vold AM, Weigt SS, Saggar R, et al. Endotype-phenotyping may predict a treatment response in progressive fibrosing interstitial lung disease. EBioMedicine. 2019;50:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SR, Dai Y, Zhao J, et al. A mechanistic overview of triptolide and celastrol, natural products from tripterygium wilfordii hook F. Front Pharmacol. 2018;9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Hong Y, Huang H.. Triptolide attenuates inflammatory response in membranous Glomerulo-Nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press Res. 2016;41(6):901–910. [DOI] [PubMed] [Google Scholar]
- 11.Fan D, He X, Bian Y, et al. Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis. Int J Mol Sci. 2016;17(4):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Yu C, Zhu WM, et al. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol Immunol. 2010;47(15):2467–2474. [DOI] [PubMed] [Google Scholar]
- 13.Yuan K, Li X, Lu Q, et al. Application and mechanisms of triptolide in the treatment of inflammatory diseases-A review. Front Pharmacol. 2019;10:1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang-Xiao , Liu , Rui , et al. Network pharmacology bridges traditional application and modern development of traditional Chinese medicine. Chin Herb Med. 2015;7(1):3–17. [Google Scholar]
- 15.Yang M, Chen J, Xu L, et al. A network pharmacology approach to uncover the molecular mechanisms of herbal formula Ban-Xia-Xie-Xin-Tang. Evid Based Complement Alternat Med. 2018;2018:4050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang YQ, Xia M, Guo QY, et al. Network pharmacology-based approaches capture essence of chinese herbal medicines. Chin Herbal Med. 2016;8(2):107–116. [Google Scholar]
- 17.Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis AP, Murphy CG, Saraceni-Richards CA, et al. Comparative toxicogenomics database: a knowledgebase and discovery tool for chemical-gene-disease networks. Nucleic Acids Res. 2009;37(Database issue):D786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safran M, Solomon I, Shmueli O, et al. GeneCards 2002: towards a complete, object-oriented, human gene compendium. Bioinformatics. 2002;18(11):1542–1543. [DOI] [PubMed] [Google Scholar]
- 20.Safran M, Dalah I, Alexander J, et al. GeneCards version 3: the human gene integrator. Database. 2010;2010(0):baq020–baq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn M, Szklarczyk D, Franceschini A, et al. STITCH 2: an interaction network database for small molecules and proteins. Nucleic Acids Res. 2010;38(Database issue):D552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Zhang F, Yang K, et al. SymMap: an integrative database of traditional chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019;47(D1):D1110–d1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–d1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin CH, Chen SH, Wu HH, et al. cytoHubba: identifying hub objects and Sub-networks from complex interactome. BMC Syst Biol. 2014;(Suppl 4):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trott O, Olson AJ.. AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XJ, Jiang ZZ, Zhang LY.. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 2014;155(1):67–79. [DOI] [PubMed] [Google Scholar]
- 28.Zhou W, Wang Y, Lu A, et al. Systems pharmacology in small molecular drug discovery. Int J Mol Sci. 2016;17(2):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding M, Ma W, Wang X, et al. A network pharmacology integrated pharmacokinetics strategy for uncovering pharmacological mechanism of compounds absorbed into the blood of Dan-Lou tablet on coronary heart disease. J Ethnopharmacol. 2019;242:112055. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Nie Q, MacLean AL, et al. Multiscale modeling of Inflammation-Induced tumorigenesis reveals competing oncogenic and oncoprotective roles for inflammation. Cancer Res. 2017;77(22):6429–6441. [DOI] [PubMed] [Google Scholar]
- 31.Chakraborty D, Šumová B, Mallano T, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8(1):1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulivendala G, Bale S, Godugu C.. Honokiol: a polyphenol neolignan ameliorates pulmonary fibrosis by inhibiting TGF-β/Smad signaling, matrix proteins and IL-6/CD44/STAT3 axis both in vitro and in vivo. Toxicol Appl Pharmacol. 2020;391:114913. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Zhao B, Zhao Y, et al. Protective effect of anisodamine on bleomycin-induced acute lung injury in immature rats via modulating oxidative stress, inflammation, and cell apoptosis by inhibiting the JAK2/STAT3 pathway. Ann Transl Med. 2021;9(10):859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Velden JL, Alcorn JF, Chapman DG, et al. Airway epithelial specific deletion of Jun-N-terminal kinase 1 attenuates pulmonary fibrosis in two independent mouse models. PLoS One. 2020;15(1):e0226904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouda MM, Bhandary YP.. Acute lung injury: IL-17A-Mediated inflammatory pathway and its regulation by curcumin. Inflammation. 2019;42(4):1160–1169. [DOI] [PubMed] [Google Scholar]
- 36.Bartis D, Mise N, Mahida RY, et al. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69(8):760–765. [DOI] [PubMed] [Google Scholar]
- 37.Davies M, Robinson M, Smith E, et al. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, smad and AP-1 signalling pathways. J Cell Biochem. 2005;95(5):918–931. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Cheng L, Li J, et al. Peptide DR8 analogs alleviate pulmonary fibrosis via suppressing TGF-β1 mediated epithelial-mesenchymal transition and ERK1/2 pathway in vivo and in vitro. Eur J Pharm Sci. 2021;167:106009. [DOI] [PubMed] [Google Scholar]
- 39.Tian B, Li X, Kalita M, et al. Analysis of the TGFβ-induced program in primary airway epithelial cells shows essential role of NF-κB/RelA signaling network in type II epithelial mesenchymal transition. BMC Genomics. 2015;16(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cong LH, Li T, Wang H, et al. IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/AKT/mTOR-mediated autophagy. J Cell Mol Med. 2020;24(15):8532–8544. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Luo X, Lin B, Gao Y, et al. Genipin attenuates mitochondrial-dependent apoptosis, endoplasmic reticulum stress, and inflammation via the PI3K/AKT pathway in acute lung injury. Int Immunopharmacol. 2019;76:105842. [DOI] [PubMed] [Google Scholar]
- 42.Meylan F, Siegel RM.. TNF superfamily cytokines in the promotion of Th9 differentiation and immunopathology. Semin Immunopathol. 2017;39(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu EK, Henkes ZI, McGowan B, et al. TNF-Induced interstitial lung disease in a murine arthritis model: Accumulation of activated monocytes, conventional dendritic cells, and CD21+/CD23– B Cell follicles is prevented with Anti-TNF therapy. J Immunol. 2019;203(11):2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mi S, Li Z, Yang HZ, et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol. 2011;187(6):3003–3014. [DOI] [PubMed] [Google Scholar]
- 45.Varshney P, Saini N.. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim Biophys Acta Mol Basis Dis. 2018;1864(5 Pt A):1795–1803. [DOI] [PubMed] [Google Scholar]
- 46.Reeves SR, Barrow KA, Rich LM, et al. Respiratory syncytial virus infection of human lung fibroblasts induces a hyaluronan-enriched extracellular matrix that binds mast cells and enhances expression of mast cell proteases. Front Immunol. 2019;10:3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seki E, Yoshizumi M, Tanaka R, et al. Cytokine profiles, signalling pathways and effects of fluticasone propionate in respiratory syncytial virus-infected human foetal lung fibroblasts. Cell Biol Int. 2013;37(4):326–339. [DOI] [PubMed] [Google Scholar]
- 48.Wang K, Lai C, Li T, et al. Basic fibroblast growth factor protects against influenza a virus-induced acute lung injury by recruiting neutrophils. J Mol Cell Biol. 2018;10(6):573–585. [DOI] [PubMed] [Google Scholar]
- 49.Vannella KM, Moore BB.. Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogenesis Tissue Repair. 2008;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sauler M, Bazan IS, Lee PJ.. Cell death in the lung: the apoptosis-necroptosis axis. Annu Rev Physiol. 2019;81:375–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahavadi P, Korfei M, Henneke I, et al. Epithelial stress and apoptosis underlie Hermansky-Pudlak syndrome-associated interstitial pneumonia. Am J Respir Crit Care Med. 2010;182(2):207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang J, Zhou L, Wu H, et al. Triptolide inhibits osteoclast formation, bone resorption, RANKL-mediated NF-κB activation and titanium particle-induced osteolysis in a mouse model. Mol Cell Endocrinol. 2015;399:346–353. [DOI] [PubMed] [Google Scholar]
- 53.Dai J, Sun Y, Chen D, et al. Negative regulation of PI3K/AKT/mTOR axis regulates fibroblast proliferation, apoptosis and autophagy play a vital role in triptolide-induced epidural fibrosis reduction. Eur J Pharmacol. 2019;864:172724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.