Abstract
We describe a patient with methicillin-resistant Staphylococcus aureus (MRSA) colonizing the pharynx. The MIC of mupirocin was 0.25 μg/ml before treatment and increased after treatment to 8 μg/ml. Using pulsed-field gel electrophoresis, we confirmed that the genotypes of MRSA that colonized the pharynx before and after the use of mupirocin were identical. We measured the delivery of mupirocin to the pharynx in three normal volunteers and two patients. Low concentrations of mupirocin were present in the pharynx in all cases 10 min to 3 days after intranasal application. Our data suggested that low concentrations of the drug in the pharynx after intranasal application of mupirocin ointment might explain the selection of mupirocin resistance in MRSA.
Methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen and a major cause of nosocomial infection (19). MRSA strains easily colonize a host, particularly immunodeficient patients, and can cause a variety of serious infections that are often difficult to control, such as septicemia, endocarditis, meningitis, and postoperative intra-abdominal infection (3, 12, 13). Mupirocin is the most effective antibiotic for elimination of MRSA from the nasal passages (6). Moreover, intranasal application of mupirocin ointment is effective in reducing surgical-site infections and the likelihood of bronchopulmonary tract infection (5, 15). However, cases of mupirocin-resistant MRSA have already been reported (11). In Japan, mupirocin has been used only for the eradication of nasal colonization by MRSA since September 1996, and low-level mupirocin-resistant MRSA appeared in the early stages (17). We had a patient in whom the MRSA that colonized in the pharynx changed to low-level mupirocin-resistant MRSA after intranasal application of mupirocin ointment. We examined the genotypes of MRSA that colonized the pharynx before and after the use of mupirocin in the same patient and the delivery of mupirocin to the pharynx after intranasal application in three healthy volunteers and two patients with MRSA colonization of the pharynx.
All studies described herein were approved by the Human Ethics Review Boards of our institutions, and a signed consent form was obtained from each subject. The patient with low-level mupirocin-resistant MRSA was a 78-year-old male who had an old cerebral infarction. Spread of low-level mupirocin-resistant MRSA had been reported in the hospital to which the patient was admitted (17). MRSA colonized only the nasal cavity and pharynx, and MRSA strains in both were mupirocin susceptible (MIC, 0.25 μg/ml). MIC was determined by the agar dilution method according to the guidelines of the National Committee for Clinical Laboratory Standards (8). Specimens were obtained by swabs from the anterior nares and the pharynx and then were cultured in TSA II medium supplemented with 5% rabbit blood agar and on selective medium for MRSA (OPA-Staphylococcus agar) (both from Becton Dickinson Microbiology Systems) for 24 h at 37°C. Identification of S. aureus was based on the morphology of colonies and use of specific kits (Staphylo-LA [slide latex agglutination]; Denka Seiken, Tokyo, Japan). MRSA was evaluated by the oxacillin disk diffusion method (Kirby-Bauer) according to the guidelines of the National Committee for Clinical Laboratory Standards (9). Intranasal application of mupirocin ointment resulted in the eradication of MRSA strains that colonized the nasal cavity but not those that colonized the pharynx. The latter were transformed into low-level mupirocin-resistant MRSA (MIC, 8 μg/ml). The genotypes of MRSA that colonized the pharynx were examined before and after intranasal application of mupirocin by using pulsed-field gel electrophoresis (PFGE) as described previously (18) and were found to be identical (Fig. 1). The DNA was digested with 10 U of SmaI (Takara Shuzo Co., Shiga, Japan) at 30°C overnight. A contour-clamped homogeneous electric field pulsed-field electrophoresis system (CHEF Mapper; Bio-Rad Life Science) was used for electrophoresis, with the potential set at 6 V/cm, switch times set at 0.47 and 63 s, and the run time set at 20 h 18 min. After staining with ethidium bromide, the band patterns were compared according to the criteria for bacterial strain typing described by Tenover et al. (16). Since no other antibiotics were used during this period apart from mupirocin, we suspected that application of mupirocin nasal ointment was the possible cause of the selection of mupirocin-resistant MRSA that colonized the pharynx.
FIG. 1.
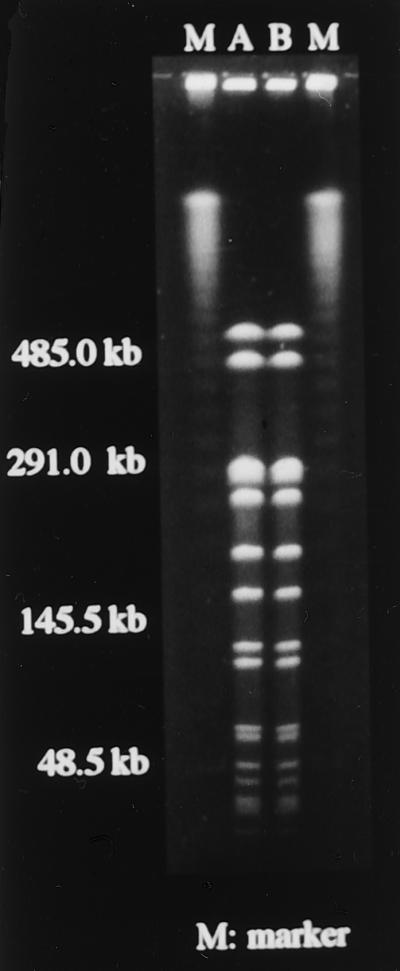
Mupirocin-susceptible MRSA (A; MIC, 0.25 μg/ml) that colonized the pharynx of our patient was transformed into low-level mupirocin-resistant MRSA (B; MIC, 8 μg/ml) after intranasal application of mupirocin ointment. The genotypes of MRSA that colonized the pharynx before and after the use of mupirocin in the same patient were identical by PFGE with SmaI.
To confirm this hypothesis, we measured in another series of studies the concentrations of mupirocin in the pharynges of three healthy volunteers and two patients with MRSA colonization in the pharynx (one in only the pharynx and the other in both the nasal cavity and pharynx) using a simple in vitro bioassay. For the bioassay, a standard curve was constructed by twofold serial dilutions of mupirocin-lithium phosphate buffer at pH 6.5. The bioassay was performed in brain heart infusion agar seeded with S. aureus ATCC 6538P. The detection limit of the assay was 0.05 μg. Two patients had bronchiectasis and allergic rhinitis or chronic sinusitis as the underlying disease. The MICs of MRSA against mupirocin were 4.0 and 0.25 μg/ml for the two patients. Mupirocin was applied to the anterior nares for 3 days. Cotton-tipped swab sticks were used to obtain pharyngeal secretions transorally, and the collected samples adhering to the cotton were dissolved in 6 ml of methanol. Subsequently, the dry-fixed samples were dissolved in 0.1 M phosphate buffer and analyzed. The concentration of mupirocin in the pharynx ranged from 0.13 to 27.7 μg/sample, although the local concentration of mupirocin was 20,000 μg/ml. At these concentrations, MRSA colonization in the pharynges of the two patients could not be eradicated, although MRSA colonizing the nasal cavity of one patient was eradicated. The presence of low concentrations of mupirocin in the pharynx throughout the entire observation period of 3 days was confirmed in all cases, although the numerical value was different in each case.
Cilia in the nasopharynx are known to move particles from the nasal cavity into the pharynx, and such a process might be involved in the transport of mupirocin nasal ointment to the pharynx, resulting in low concentrations of the antibiotic in the pharynx, thus enhancing the selection of mupirocin resistance. Low-level and high-level mupirocin-resistant MRSA strains have been described previously (1, 4). Mupirocin resistance in MRSA results from changes in the target enzyme, isoleucyl-tRNA synthetase, while high-level mupirocin resistance is plasmid encoded. Mupirocin-resistant MRSA was first isolated from the skin of patients who were treated with mupirocin for long periods (11, 14), and several reports have cautioned against the long-term use of mupirocin in patients with skin infection (2, 10). Other studies have reported the development of mupirocin-resistant MRSA after widespread use of nasal mupirocin ointment (7). On the basis of these studies, it could be speculated that the cause of such resistance is related to our results, i.e., low concentrations of the antibiotic in the pharynx.
In conclusion, nasal application of mupirocin at clinically effective concentrations may result in low levels of the antibiotic in the pharynx, which could consequently induce or select for the emergence of mupirocin-resistant MRSA.
Acknowledgments
We thank Akihiro Wada (Department of Bacteriology, Institute of Tropical Medicine, Nagasaki University), Chieko Shimauchi (Miyazaki Prefectural Nursing University), and Matsuhisa Inoue (Kitasato University School of Medicine) for their help in completion of PFGE studies. We also thank Glaxo SmithKline K.K. for measuring the concentration of mupirocin.
REFERENCES
- 1.Bastos M C, Mondino P J, Azevedo M L, Santos K R, Giambiagi deMarval M. Molecular characterization and transfer among Staphylococcus strains of a plasmid conferring high-level resistance to mupirocin. Eur J Clin Microbiol Infect Dis. 1999;18:393–398. doi: 10.1007/s100960050306. [DOI] [PubMed] [Google Scholar]
- 2.Cookson B D, Lacey R W, Noble W C, Reeves D S, Wise R, Redhead R J. Mupirocin-resistant Staphylococcus aureus. Lancet. 1990;335:1095–1096. doi: 10.1016/0140-6736(90)92667-7. [DOI] [PubMed] [Google Scholar]
- 3.Fierobe L, Decre D, Muller C, Lucet J C, Marmuse J P, Mantz J, Desmonts J M. Methicillin-resistant Staphylococcus aureus as a causative agent of postoperative intra-abdominal infection: relation to nasal colonization. Clin Infect Dis. 1999;29:1231–1238. doi: 10.1086/313454. [DOI] [PubMed] [Google Scholar]
- 4.Gilbart J, Perry C R, Slocombe B. High-level mupirocin resistance in Staphylococcus aureus: evidence for two distinct isoleucyl-tRNA synthetases. Antimicrob Agents Chemother. 1993;37:32–38. doi: 10.1128/aac.37.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbarth S, Dharan S, Liassine N, Herrault P, Auckenthaler R, Pittet D. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1412–1416. doi: 10.1128/aac.43.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill R L R, Duckworth G J, Casewell M W. Elimination of nasal carriage of methicillin-resistant Staphylococcus aureus with mupirocin during a hospital outbreak. J Antimicrob Chemother. 1988;22:377–384. doi: 10.1093/jac/22.3.377. [DOI] [PubMed] [Google Scholar]
- 7.Miller M A, Dascal A, Portnoy J, Mendelson J. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect Control Hosp Epidemiol. 1996;17:811–813. doi: 10.1086/647242. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2–A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 10.Neu H C. The use of mupirocin in controlling methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 1990;11:11–12. doi: 10.1086/646071. [DOI] [PubMed] [Google Scholar]
- 11.Rahman M, Noble W C, Cookson B. Mupirocin-resistant Staphylococcus aureus. Lancet. 1987;ii:387. doi: 10.1016/0140-6736(90)92667-7. [DOI] [PubMed] [Google Scholar]
- 12.Rubio M, Romero J, Corral O, Roca V, Picazo J J. Bacteremia by Staphylococcus aureus: analysis of 311 episodes. Enferm Infec Microbiol Clin. 1999;17:56–64. [PubMed] [Google Scholar]
- 13.Sakamoto T, Kikuchi K, Mineura K, Kowada M, Nakagomi O. MRSA meningitis in postoperative patients. Report of 4 cases. Jpn J Antibiot. 1990;43:1137–1142. [PubMed] [Google Scholar]
- 14.Smith G E, Kennedy C T C. Staphylococcus aureus resistant tomupirocin. J Antimicrob Chemother. 1988;21:141–142. doi: 10.1093/jac/21.1.141. [DOI] [PubMed] [Google Scholar]
- 15.Talon D, Rouget C, Cailleaux V, Bailly P, Thouverez M, Barale F, Michel-Briand Y. Nasal carriage of Staphylococcus aureus and cross-contamination in a surgical intensive care unit: efficacy of mupirocin ointment. J Hosp Infect. 1995;30:39–49. doi: 10.1016/0195-6701(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 16.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe H, Masaki H, Asoh N, Watanabe K, Oishi K, Furumoto A, Kobayashi S, Sato A, Nagatake T. Emergence and spread of low-level mupirocin resistance in methicillin-resistant Staphylococcus aureus isolated from a community hospital in Japan. J Hosp Infect. 2001;47:294–300. doi: 10.1053/jhin.2000.0931. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Masaki H, Asoh N, Watanabe K, Oishi K, Kobayashi S, Sato A, Nagatake T. Molecular analysis of methicillin-resistant Staphylococcus aureus as a causative agent of bronchopulmonary infection: relation to colonization in the upper respiratory tract. J Clin Microbiol. 2000;38:3867–3869. doi: 10.1128/jcm.38.10.3867-3869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzel R P, Nettleman M D, Jones R N, Pfaller M A. Methicillin-resistant Staphylococcus aureus: implications for the 1990s and effective control measures. Am J Med. 1991;91(Suppl. 3B):221S–227S. doi: 10.1016/0002-9343(91)90372-5. [DOI] [PubMed] [Google Scholar]


