Abstract
Background:
Intracoronary epinephrine has been effectively used in treating refractory no-reflow, but there is a dearth of data on its use as a first-line drug in normotensive patients in comparison to the widely used adenosine.
Methods:
In this open-labeled randomized clinical trial, 201 patients with no-reflow were randomized 1:1 into intracoronary epinephrine as the treatment group and intracoronary adenosine as the control group and followed for 1 month. The primary end points were improvement in coronary flow, as assessed by TIMI (Thrombolysis in Myocardial Infarction) flow, frame counts, and myocardial blush. Secondary end points were in-hospital and short-term mortality and major adverse cardiac events.
Results:
In all, 101 patients received intracoronary epinephrine and 100 patients received adenosine. Epinephrine was generally well tolerated with no immediate table death or ventricular fibrillation. No-reflow was more effectively improved with epinephrine with final TIMI III flow (90.1% versus 78%, P=0.019) and final corrected TIMI frame count (24±8.43 versus 26.63±9.22, P=0.036). However, no significant difference was observed in final grade III myocardial blush (55.4% versus 45%, P=0.139), mean reduction of corrected TIMI frame count (−25.71±11.79 versus −26.08±11.71, P=0.825), in-hospital and short-term mortality, and major adverse cardiac events.
Conclusions:
Epinephrine is relatively safe to use in no-reflow in normotensive patients. A significantly higher frequency of post-treatment TIMI III flow grade and lower final corrected TIMI frame count with relatively better achievement of myocardial blush grade III translate into it displaying relatively better efficacy than adenosine.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04699110.
Keywords: acute coronary syndrome, adenosine, epinephrine, myocardial infarction, percutaneous coronary intervention
What Is Known
Intracoronary epinephrine has been effectively used for treating refractory no-flow after percutaneous coronary intervention in acute coronary syndrome.
Data regarding its safety and efficacy with the widely used adenosine, especially in normotensive patients, is not well established.
This trial compares the safety and efficacy of epinephrine with adenosine.
What the Study Adds
Our results suggest that epinephrine is safe and has relatively better efficacy than adenosine. However, overall, in-hospital and short-term outcomes are comparable.
It can be used as an alternative first-line drug, especially where there is a fear of bradycardia or hypotension associated with adenosine in normotensive patients.
No-reflow is defined as deficient perfusion at myocardial level, despite patent epicardial coronary vessel in the setting of percutaneous coronary interventions (PCI), excluding the presence of dissection, underlying stenosis, thrombus, or spasm.1 No-reflow has been shown to be prevalent at a rate of up to 32% in patients with acute coronary syndrome (ACS).2 It is exceptionally frequent in venous grafts and during rotational atherectomy.3,4 Tools used to measure coronary flow for successful procedure after PCI are TIMI (Thrombolysis in Myocardial Infarction) flow grading, myocardial blush grading, and corrected TIMI frame count (cTFC).5
Several pharmacological therapies have been used via the intracoronary route—mainly adenosine, verapamil, nitroprusside, or nicardipine—for treatment of no-reflow and have shown improvement in coronary flow and better prognosis.6–8 Adenosine and nitroprusside are widely studied drugs in the treatment of no-reflow, with mixed and debatable results.9–15 As one of the major consequences of no-reflow is hypotension, where use of adenosine, nicardipine, verapamil, or nitroprusside will be questionable, in that scenario, the only useful option is epinephrine; however, it has limited evidence so far, but has shown to be effective in refractory cases in some studies.16,17 Nonetheless, Huang et al18 reported that nearly 98% of restoration of TIMI III flow in no-reflow patients without significant chronotropic or hemodynamic compromise with intracoronary nicardipine. However, this data is limited; therefore, in this study, epinephrine has been selected to determine if it would be safe to use as a first-line drug and as an alternative to adenosine (as this is widely used drug in our institution as well as in our region), especially where use of adenosine is limited by hypotension or bradycardia, either as its direct effect or as a consequence of no-reflow. However, in this trial, we targeted patients with normotension with systolic blood pressure of ≥100 mm Hg at baseline to obtain a comparative patient subset, where either epinephrine or adenosine could be used with equal proportions, removing the selection bias, based on the presence of hypotension. In a retrospective study, Aksu et al16 reported that intracoronary epinephrine may exhibit encouraging effects in patients with no-reflow refractory to one or more medicine during primary PCI and showed significant improvement with resumption of TIMI III flow in 9 out of 12 patients (75%). Intracoronary epinephrine was tolerated well and led to quick and drastic recovery of coronary flow in many cases.`
Furthermore, after a thorough review of the literature, it is also evident that there is a paucity of data comparing its effectiveness with widely used adenosine in ACS settings. Therefore, we proposed this trial to determine the effectiveness of intracoronary epinephrine and to compare its efficacy with intracoronary adenosine for the treatment of no-reflow, mainly in patients with normotensive ACS, with the hypothesis that intracoronary epinephrine might have better efficacy and could be a good alternative to adenosine.
Methods
Study Design
This was an open-labeled randomized-controlled trial and was registered after approval of the ethical review committee of the National Institute of Cardiovascular Disease, Karachi, Pakistan, with a reference number of ERC-76/2020. The data that support the findings of this study are available from the corresponding author upon reasonable request. A total of 201 patients who were admitted to National Institute of Cardiovascular Disease from December 2020 to May 2021, with ACS were recruited consecutively.
Study Population
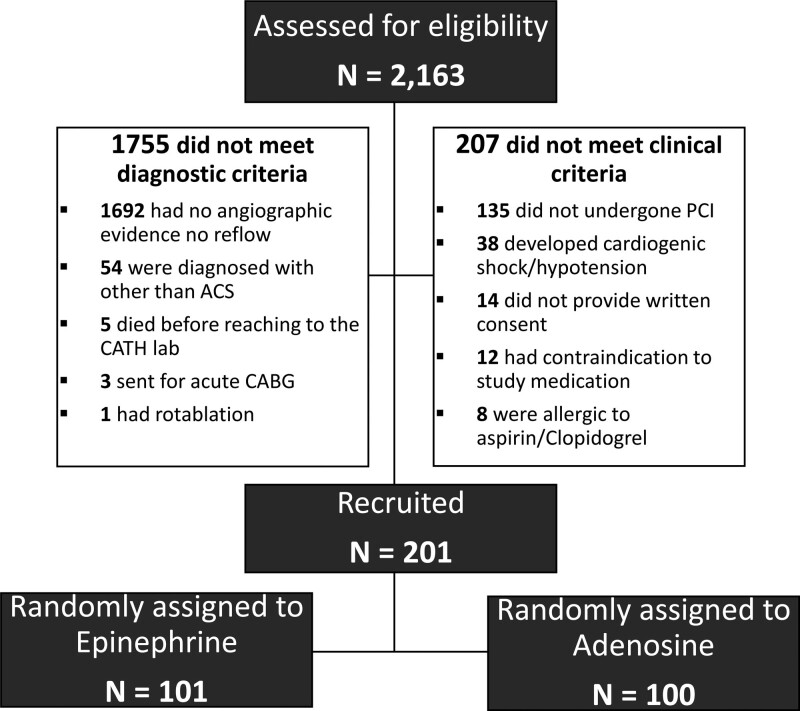
All of the included patients were ≥18 years of age with a baseline systolic blood pressure of ≥100 mm Hg and had an established diagnosis of no-reflow during PCI for ACS. No-reflow was defined as a poststenting TIMI flow grade of ≤2. Patients with baseline hypotension (systolic blood pressure <100 mm of Hg), valvular, or congenital heart disease, known allergic reaction to epinephrine or adenosine, contraindication to use of aspirin or clopidogrel, cardiomyopathy, pericarditis, myocarditis, and those who refused to give consent were excluded (Figure 1). As per the Declaration of Helsinki, written informed consent was obtained before reaching the Cath Lab.
Figure 1.
Study flow chart. ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; CATH, catheterization laboratory; and PCI, percutaneous coronary intervention.
Sample Size Calculation
Sample size for the study was calculated based on hypothesis testing of 2 independent population proportions using the World Health Organization sample size calculator, version 2.0. With the expected proportion of postadministration TIMI III flow as 90% versus 75% in the treatment and control group at the 5% level of significance and a power of 80%, the calculated sample size was 100 patients in each group.
Randomization and Allocation
All recruited patients were randomized to either the treatment or control group in a ratio of 1:1 using a computer-generated randomization schema in relation to the order of participation in the study. Random allocation schema was designed with the help of the random number generation tool from Microsoft Excel 2013 using the Bernoulli distribution with one trial and a probability of 0.50 of a given patient being assigned to the treatment group. The random number generation process was repeated until the perfect balance of 1:1 was achieved. However, even after the tenth iteration, perfect balance (1:1) could not be obtained; hence, a treatment to control ratio of 101:99 was finalized. To maintain the required sample size of 100 patients in the control group, an additional (nonrandomized) patient was added at 201st recruitment order. Hence, the final allocation ratio for the treatment to control group was 101:100 patients. A list of group allocations against the recruitment order was generated, which was accessible to the data monitoring team comprising of 2 members, a statistician, and an appointed member of institutional ethical review committee. The group allocation for every patient was communicated to the participant enrollment team by the data monitoring team at the time of recruitment.
Management
A total of 101 patients were randomized to the treatment group and received 100 to 600 µg intracoronary epinephrine (prepared after diluting 1:1000 [1 mg/mL] ampoule in 10 cc of normal saline so that 1 cc=100 µg and then each cc was further diluted in 9 cc so that 10 cc=100 µg) with or without intracoronary nitrates. The protocol was to start with a dose of 1 cc=10 µg and to escalate the dose at intervals of 1 to 2 minutes until improvement of TIMI flow, provided hemodynamic tolerance. In all, 100 patients randomized to the control group received 60 to 1000 µg intracoronary adenosine with or without intracoronary nitrates. The final dose selection in both groups was determined by either achievement of primary end point or hemodynamic tolerance. Baseline demographics, hemodynamic profile, comorbidity, culprit coronary vessel, and number of diseased vessels were documented. As mentioned earlier, intracoronary nitrates were given to both groups at an amount ranging between 100 and 1000 µg, as tolerated by patients. All patients were given oral aspirin (300 mg), clopidogrel (300 mg), and intravenous unfractionated heparin at 70 to 100 U/kg to maintain an activated clotting time of 250 to 300 s. GPIIa IIIb (glycoprotein IIb IIIa) inhibitors were used as per the American College of Cardiology and American Heart Association guidelines.19
Measurements
Standard right and left coronary angiograms were obtained in at least 2 projections at 15 frames/s, before and after administration of intracoronary medication. In both groups, poststenting, preadministration and postadministration TIMI flow grade, cTFC (which is the number of cine-frames needed for contrast to get to the distal coronary landmark, corrected for the vessel length), and myocardial blush grade (MBG) were assessed by at least 2 experienced interventional cardiologists blinded to the administered drug. The effects of both these medications were documented by improvement in TIMI flow grade, MBG, and reduction in final cTFC of the concerned coronary artery as the primary outcome. The time of cineangiogram from start of procedure to establishment of no-reflow and time from medication to the follow-up cineangiogram was also noted. Left ventricular (LV) angiogram was performed in the majority of patients at the time of the procedure, and a transthoracic echocardiogram was performed in almost all patients at follow-up by an expert echocardiographer, using a vivid 7 machine, blinded to the clinical insight of the patients.
Study Outcomes
The primary outcome of the study was achievement of postadministration TIMI III flow along with final cTFC and grade III MBG. Secondary outcomes of the study were the incidence of all-cause mortality and major adverse cardiovascular events, including arrhythmias, recurrent MI, repeat revascularization, cerebrovascular accident, and heart failure, which were observed at the time of discharge and 30 days after the procedure. LV ejection fraction was also documented by echocardiography at 30 days. LV ejection fraction was assessed by using the biplane method as recommended by the American Society of Echocardiography.18
Statistical Analysis
IBM SPSS version 21 was used for data analysis. Descriptive statistics, such as mean±SD or frequency and percentages, were calculated for quantitative and qualitative variables, respectively. Continuous response variables, such as cTFC, vessel size, dose of heparin, epinephrine, nitrates, adenosine, and duration of follow-up, were compared between the treatment and control groups with the help of an independent sample t test. The Pearson χ2 test or Fisher exact test were applied to compare the treatment and control groups for categorical response variables with binary response or 2 levels, such as primary outcome of achievement of postadministration TIMI III flow grade, postprocedure complications, and in-hospital and 30-day outcomes. Appropriate Pearson χ2 test or likelihood ratio test was applied to compare 2 groups for the categorical response variables with more than 2 levels. A 2-sided P value of ≤0.05 was taken as the criterion for significance.
Safety Concern
To ensure further safety of intracoronary epinephrine in patients with normotension, although its use in earlier studies16,17 has been documented with a good safety profile, all recruited patients were closely monitored by an independent safety monitoring team appointed by the ethical review committee of the institution. Interim data analysis was performed and presented to the ethical review committee of the institution at the completion of recruitment of the first 50 and 100 patients in the study. There were no specific rules for stopping the study besides significant increase in adverse complications, including mortality, in the treatment group.
Results
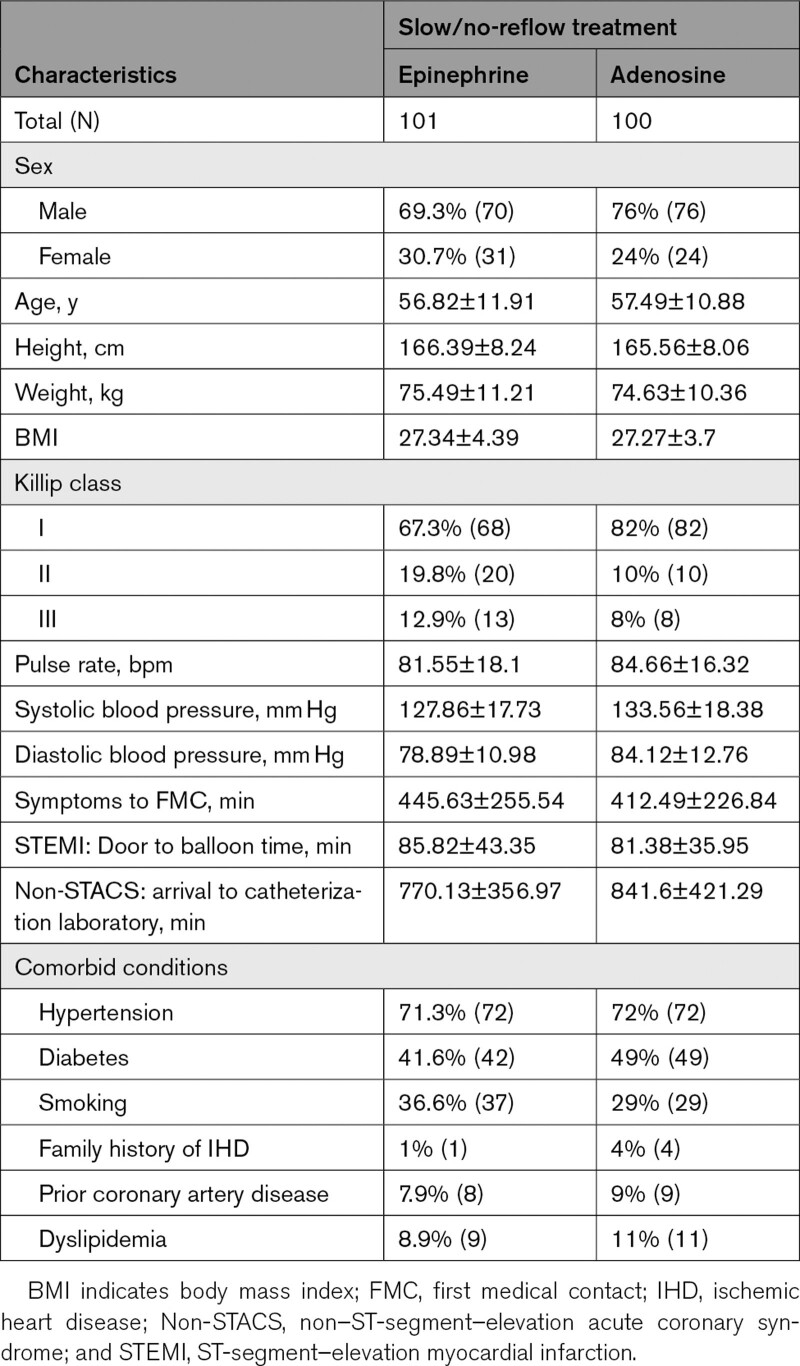
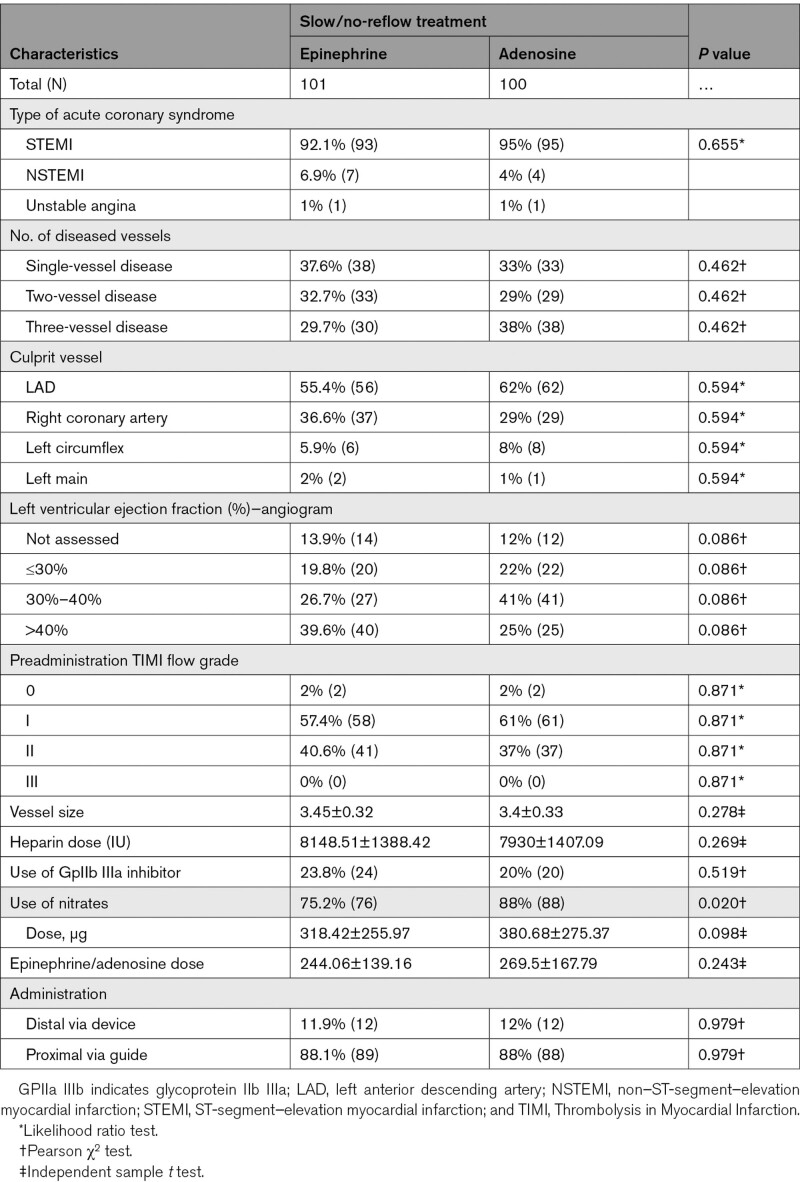
Baseline demographics, hemodynamic profile, comorbidity, culprit coronary vessel, and the number of diseased vessels were comparable in both groups, as shown in Tables 1 and 2. In the treatment and control groups, patients with ST-segment–elevation myocardial infarction were 92.1% versus 95%; non–ST-segment–elevation myocardial infarction was 6.9% versus 4%, and unstable angina was 1% versus 1%. Use of nitrates was 75.2% versus 88%, P=0.020, and use of GPIIa IIIb inhibitor was 23.8% versus 20%, P=0.519 for the treatment and control groups, respectively. The mean dose of epinephrine was 244.06±139.16 µg and adenosine was 269.5±167.79 µg. Nitrates were used at a mean dose of 318.42±255.97 versus 380.68±275.3 (P=0.098).
Table 1.
Comparisons of Baseline Demographic and Clinical Characteristics Between Treatment (Epinephrine) and Control (Adenosine) Group
Table 2.
Comparisons of Angiographic and Procedural Characteristics Between Treatment (Epinephrine) and Control (Adenosine) Group
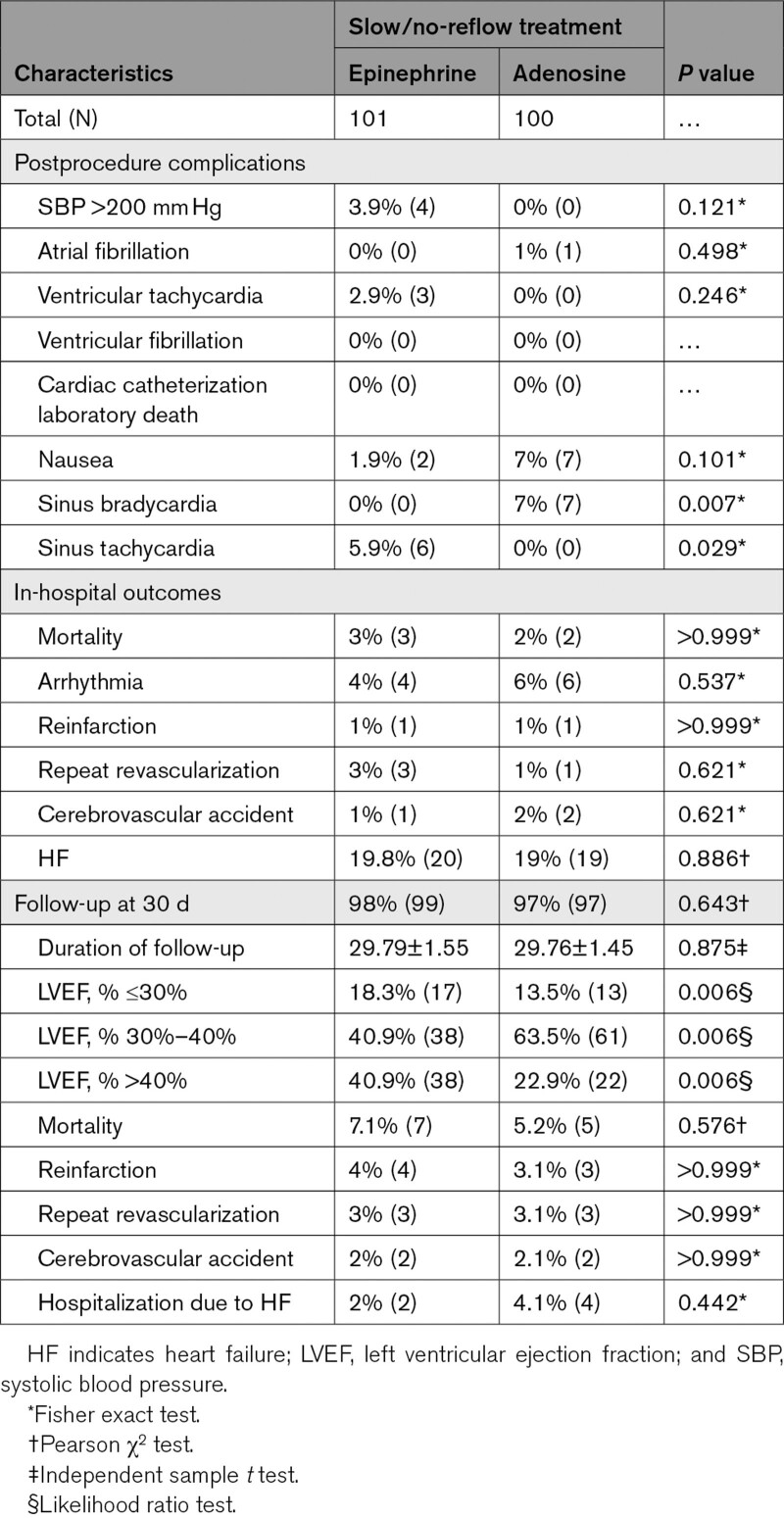
Some adverse reactions occurred in a few cases in both groups, but none of the patients in either group experienced ventricular fibrillation or cardiac catheterization laboratory death. In the epinephrine group, 5.94% (6) of the patients experienced a significant but tolerable and self-limiting increase in heart rate ≤160 b/min; 3.9% (4) showed a self-limiting rise in systolic blood pressure of ≥200 mm Hg. Less than 2% (3) patients developed ventricular tachycardia in which one patient reverted spontaneously to sinus rhythm, and 2 patients required direct current cardioversion. Two (1.9%) patients experienced nausea or vomiting. In the adenosine group, 7% (7) patients felt nausea or vomiting, and 7 (7%) developed self-limiting sinus bradycardia. During the procedure, transvenous pacing was required in 3% (6) overall.
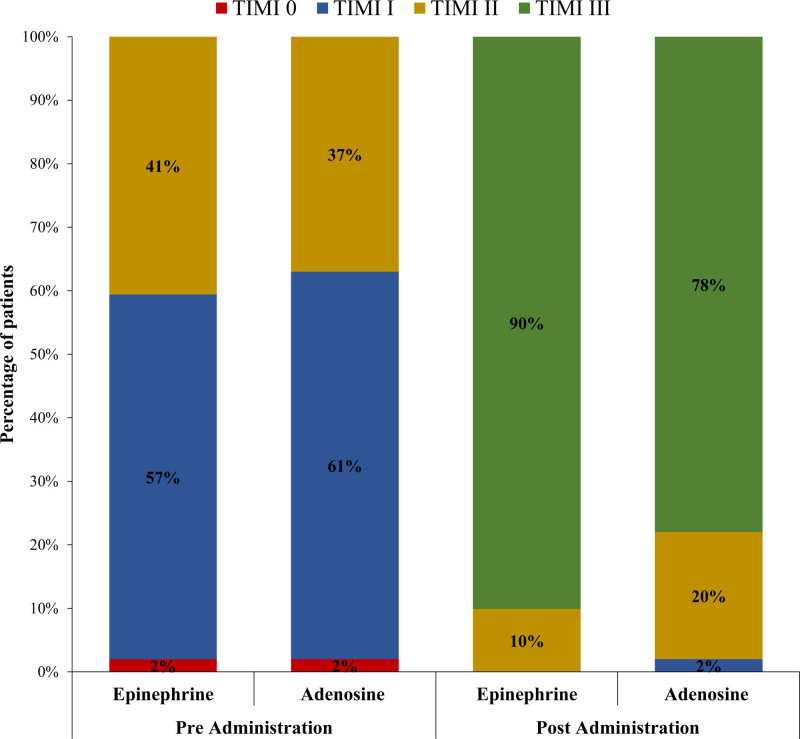
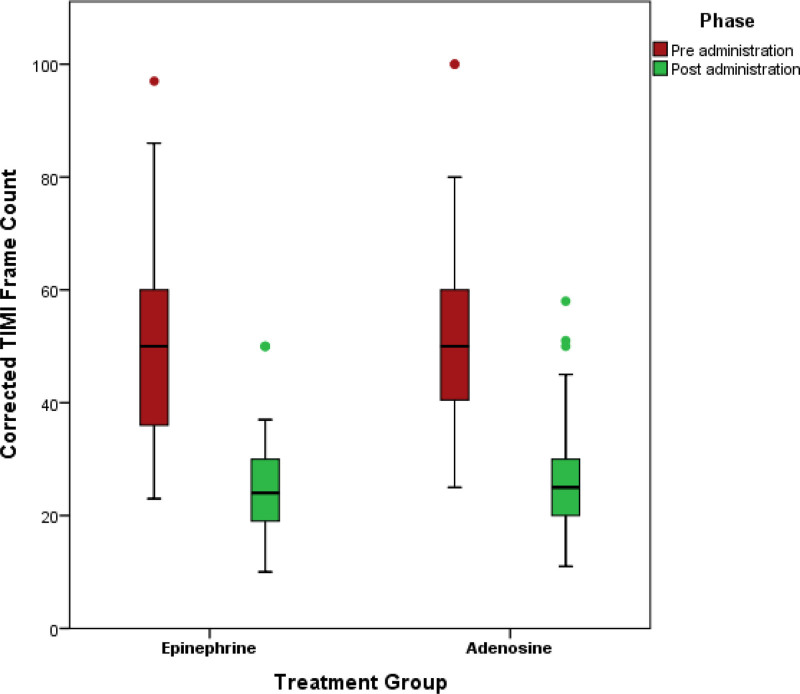
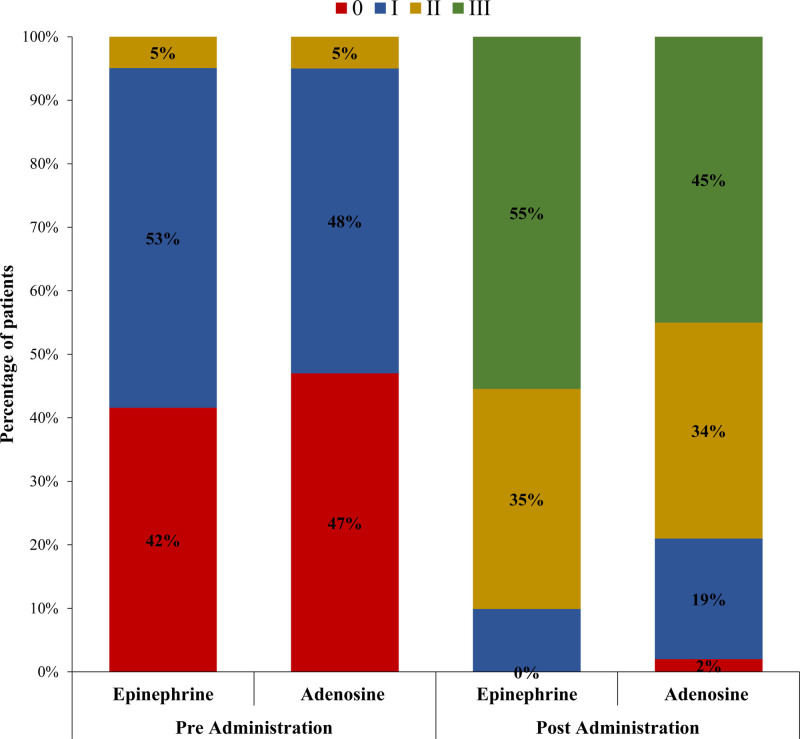
Resumption of final TIMI III flow in the treatment and control groups was 90.1% versus 78% (P=0.019), and final cTFC was 24±8.43 versus 26.63±9.22 (P=0.036); however, mean reduction of cTFC was −25.71±11.79 versus −26.08±11.71 (P=0.825), respectively (Figures 2 through 3). Final grade III myocardial blush was 55.4% versus 45% (P=0.139; Figure 4).
Figure 2.
Comparison of post stenting pre-administration and post-administration TIMI (Thrombolysis in Myocardial Infarction) flow grade between treatment (epinephrine) and control (adenosine) groups.
Figure 3.
Comparison of post stenting pre-administration and post-administration TIMI (Thrombolysis in Myocardial Infarction) frame count between treatment (epinephrine) and control (adenosine) groups.
Figure 4.
Comparison of post stenting pre-administration and post-administration myocardial blush grade between treatment (epinephrine) and control (adenosine) groups.
Secondary outcomes in the treatment and control groups were as follows: in-hospital mortality was 3% versus 2% (P>0.999) and major adverse cardiac events in terms of arrhythmia in 4% versus 6% (P=0.537), recurrent MI in 1% versus 1% (P>0.999), repeat revascularization in 3% versus 1% (P=0.621) cerebrovascular accident in 1% versus 2% (P=0.621), and heart failure in 19.8 % versus 19% (P=0.886) of the patients, respectively. At the 30-day follow-up, cumulative all-cause mortality was 7% versus 5.2% (P=0.576), recurrent MI was 4% versus 3.1% (P>0.999), repeat revascularization was 3% versus 3.1% (P>0.999), and cerebrovascular accident was 2% versus 2.1% (P>0.999) among patients in the treatment and control groups, respectively. Moreover, an echocardiographic LV ejection fraction of above 40% was 40.9% versus 22.9% in the treatment and control groups, respectively (Table 3).
Table 3.
Comparisons of In-Hospital and 30-Day Outcomes Between Treatment (Epinephrine) and Control (Adenosine) Group
Discussion
Generally, 93.4% (188 out of 201) of patients recruited in this trial had ST-segment–elevation myocardial infarction and enrolled during primary PCI, as this is the most common scenario for the development of no-reflow among ACS settings. There are other settings like rotational atherectomy or saphenous venous graft PCI where no-reflow is common. The saphenous venous graft PCI patients could not be enrolled in either group in this trial due to rare presentation or failure to meet the inclusion criteria during the study interim, whereas patients with rotational atherectomy were excluded due to their procedure in non-ACS setting. Epinephrine administration was generally well tolerated with occasional and controllable adverse effects, and none of the patients experienced ventricular fibrillation or table death, as observed in earlier studies such as Skelding et al17 in 2002, where intracoronary epinephrine was used in 29 patients at a mean dose of 139±189 µg, and Aksu et al16 in 2015, who utilized intracoronary epinephrine to treat refractory no-reflow in 12 patients, demonstrated the safety of its use with significant improvement of TIMI flow grade and attainment of TIMI 3 flow in 69% and 75% of patients, respectively, with no significant dysrhythmia except significant but controllable rise in heart and blood pressure. In this trial, mostly similar effects of epinephrine were translated as far as safety was concerned, where only 2 patients out of 101 (<2%) developed ventricular tachycardia and required direct current cardioversion, and one patient developed nonsustained ventricular tachycardia, whereas the remaining patients tolerated intracoronary epinephrine well with significant but self-limiting rise in heart rate and blood pressure in only 5.94% and 3.9%, respectively. Use of nitrates was more frequent in the control group (88% versus 75.2%, P=0.020), suggesting better tolerance in terms of blood pressure. However, the use of GpIIb IIIa inhibitor was similar in the treatment and control groups (23.8% versus 20%, P=0.519).
Estimation of coronary flow down the culprit/infarct-related vessel is commonly made using the TIMI flow grading system, although with some limitations in terms of interobserver variation or the presence of abnormal flow in the nonculprit vessel used for comparison. The cTFC, as introduced by Gibson et al,20 is a continuous and quantitative parameter. Therefore, it was used in this study to overcome the limitations of the TIMI flow grade assessment. Although TIMI flow grade and cTFC are good measures of epicardial flow, they remain indirect tools for assessment of myocardial perfusion, which is more properly assessed by MBG, which is the main target of reperfusion.21,22
No-reflow was improved significantly higher with absolute resumption of TIMI III flow in 90.1% versus 78% (P=0.019), along with significantly low final corrected TIMI frame count (cTFC) 24±8.43 versus 26.63±9.22 (P=0.036) in the epinephrine group. However, there was no significant difference in the mean reduction of cTFC (−25.71±11.79 versus −26.08±11.71, P=0.825) and attainment of grade III myocardial blush, although relatively better in the epinephrine group (55.4% versus 45%, P=0.139), suggesting comparable efficiency of epinephrine with adenosine in the studied subjects. Moreover, there was better recovery of myocardium, as assessed by echocardiography at follow-up in the epinephrine group, and results found an LV ejection fraction value of ≥ 40% in 40.9% versus 22.9%, supporting its use as a good alternative. However, no significant difference was found in the secondary outcomes as depicted by in-hospital and 30-day all-cause mortality and major adverse cardiac events in the 2 groups.
The present trial is the biggest open-labeled randomized clinical trial focusing on the usage, safety, and efficacy of intracoronary epinephrine in the management of no-reflow in comparison to widely used intracoronary adenosine in patients with normotensive ACS. None of the earlier studies were conducted on such a large number of patients and were retrospective in nature. Although it has been used for the treatment of refractory no-reflow in several past studies, its utilization in patients with normotension was debatable before the results of the present trial.
To the best of our knowledge, ours is the largest clinical trial where epinephrine and adenosine were compared head to head and that showed favorable results for epinephrine as a first-line medication for no-reflow with a good safety profile and also in contexts where there is a fear of hypotensive or bradycardic effect from adenosine.
Nonetheless, this trial has certain limitations, such as single-centered recruitment and exclusion of patients who refused to participate in the study, which may have induced certain selection bias. Hence, larger multicenter trials are warranted with wider geographic and demographic coverage.
Conclusions
Epinephrine is relatively safe for use in no-reflow complications in patients with normotensive ACS. A significantly higher frequency of post-treatment TIMI III flow grade and a significantly lower final cTFC with relatively better achievement of MBG III translate into its relatively better efficacy than adenosine. However, in-hospital and short-term outcomes in terms of all-cause mortality and major adverse cardiac events are comparable with adenosine.
Perspectives
Our findings suggest that epinephrine can be used as a proper and safe alternative first-line drug compared to adenosine. Moreover, in situations where there is a fear of hypotensive or bradycardic effects related to adenosine in patients with normotension, it can be a better choice.
Article Information
Acknowledgments
We wish to acknowledge the support of the staff members of the Clinical Research Department of the National Institute of Cardiovascular Diseases (NICVD) Karachi, Pakistan.
Sources of Funding
None.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ACS
- acute coronary syndrome
- cTFC
- corrected TIMI frame count
- MACE
- major adverse cardiovascular events
- PCI
- percutaneous coronary intervention
- TIMI
- Thrombolysis in Myocardial Infarction
For Sources of Funding and Disclosures, see page 171.
Contributor Information
Nadeem Qamar, Email: drnadeemqamar6@gmail.com.
Tahir Saghir, Email: tahirsaghir@gmail.com.
Jawaid Akbar Sial, Email: drjawaids@yahoo.com.
Dileep Kumar, Email: rajeshnarsoolal@gmail.com.
Rajesh Kumar, Email: rajeshnarsoolal@gmail.com.
Danish Qayyum, Email: drdanishqayyum@hotmail.com.
Umamah Yasin, Email: drayzul@gmail.com.
Musa Karim, Email: mkarim.nicvd@gmail.com.
References
- 1.Reffelmann T, Kloner RA. The “no-reflow” phenomenon: basic science and clinical correlates. Heart. 2002;87:162–168. doi: 10.1136/heart.87.2.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezkalla SH, Dharmashankar KC, Abdalrahman IB, Kloner RA. No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. J Interv Cardiol. 2010;23:429–436. doi: 10.1111/j.1540-8183.2010.00561.x [DOI] [PubMed] [Google Scholar]
- 3.Tesic MB, Stankovic G, Vukcevic V, Ostojic MC. The use of intracoronary sodium nitroprusside to treat no-reflow after primary percutaneous coronary intervention in acute myocardial infarction. Herz. 2010;35:114–118. doi: 10.1007/s00059-010-3243-4 [DOI] [PubMed] [Google Scholar]
- 4.Hong MK, Mehran R, Dangas G, Mintz GS, Lansky AJ, Pichard AD, Kent KM, Satler LF, Stone GW, Leon MB. Creatine kinase-MB enzyme elevation following successful saphenous vein graft intervention is associated with late mortality. Circulation. 1999;100:2400–2405. doi: 10.1161/01.cir.100.24.2400 [DOI] [PubMed] [Google Scholar]
- 5.Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879 [DOI] [PubMed] [Google Scholar]
- 6.Micari A, Belcik TA, Balcells EA, Powers E, Wei K, Kaul S, Lindner JR. Improvement in microvascular reflow and reduction of infarct size with adenosine in patients undergoing primary coronary stenting. Am J Cardiol. 2005;96:1410–1415. doi: 10.1016/j.amjcard.2005.06.090 [DOI] [PubMed] [Google Scholar]
- 7.Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101:2154–2159. doi: 10.1161/01.cir.101.18.2154 [DOI] [PubMed] [Google Scholar]
- 8.Claeys MJ, Bosmans J, De Ceuninck M, Beunis A, Vergauwen W, Vorlat A, Vrints CJ. Effect of intracoronary adenosine infusion during coronary intervention on myocardial reperfusion injury in patients with acute myocardial infarction. Am J Cardiol. 2004;94:9–13. doi: 10.1016/j.amjcard.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 9.Stoel MG, Marques KM, de Cock CC, Bronzwaer JG, von Birgelen C, Zijlstra F. High dose adenosine for suboptimal myocardial reperfusion after primary PCI: a randomized placebo-controlled pilot study. Catheter Cardiovasc Interv. 2008;71:283–289. doi: 10.1002/ccd.21334 [DOI] [PubMed] [Google Scholar]
- 10.Grygier M, Araszkiewicz A, Lesiak M, Janus M, Kowal J, Skorupski W, Pyda M, Mitkowski P, Grajek S. New method of intracoronary adenosine injection to prevent microvascular reperfusion injury in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am J Cardiol. 2011;107:1131–1135. doi: 10.1016/j.amjcard.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Fokkema ML, Vlaar PJ, Vogelzang M, Gu YL, Kampinga MA, de Smet BJ, Jessurun GA, Anthonio RL, van den Heuvel AF, Tan ES, et al. Effect of high-dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: a randomized controlled trial. Circ Cardiovasc Interv. 2009;2:323–329. doi: 10.1161/CIRCINTERVENTIONS.109.858977.109.858977 [DOI] [PubMed] [Google Scholar]
- 12.Desmet W, Bogaert J, Dubois C, Sinnaeve P, Adriaenssens T, Pappas C, Ganame J, Dymarkowski S, Janssens S, Belmans A, et al. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J. 2011;32:867–877. doi: 10.1093/eurheartj/ehq492 [DOI] [PubMed] [Google Scholar]
- 13.Wang HJ, Lo PH, Lin JJ, Lee H, Hung JS. Treatment of slow/no-reflow phenomenon with intracoronary nitroprusside injection in primary coronary intervention for acute myocardial infarction. Catheter Cardiovasc Interv. 2004;63:171–176. doi: 10.1002/ccd.20149 [DOI] [PubMed] [Google Scholar]
- 14.Pasceri V, Pristipino C, Pelliccia F, Granatelli A, Speciale G, Roncella A, Pironi B, Capasso M, Richichi G. Effects of the nitric oxide donor nitroprusside on no-reflow phenomenon during coronary interventions for acute myocardial infarction. Am J Cardiol. 2005;95:1358–1361. doi: 10.1016/j.amjcard.2005.01.082 [DOI] [PubMed] [Google Scholar]
- 15.Amit G, Cafri C, Yaroslavtsev S, Fuchs S, Paltiel O, Abu-Ful A, Weinstein JM, Wolak A, Ilia R, Zahger D. Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial. Am Heart J. 2006;152:887.e9–887.14. doi: 10.1016/j.ahj.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 16.Aksu T, Guler TE, Colak A, Baysal E, Durukan M, Sen T, Guray U. Intracoronary epinephrine in the treatment of refractory no-reflow after primary percutaneous coronary intervention: a retrospective study. BMC Cardiovasc Disord. 2015;15:10. doi: 10.1186/s12872-015-0004-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skelding KA, Goldstein JA, Mehta L, Pica MC, O’Neill WW. Resolution of refractory no-reflow with intracoronary epinephrine. Catheter Cardiovasc Interv. 2002;57:305–309. doi: 10.1002/ccd.10303 [DOI] [PubMed] [Google Scholar]
- 18.Huang RI, Patel P, Walinsky P, Fischman DL, Ogilby JD, Awar M, Frankil C, Savage MP. Efficacy of intracoronary nicardipine in the treatment of no-reflow during percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;68:671–676. doi: 10.1002/ccd.20885 [DOI] [PubMed] [Google Scholar]
- 19.Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davidson TW, Davis JL, Douglas PS, Gillam LD. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation. 1997;95:1686–1744. doi: 10.1161/01.cir.95.6.1686 [DOI] [PubMed] [Google Scholar]
- 20.Gibson CM, Dotani MI, Murphy SA, Marble SJ, Dauterman KW, Michaels AD, Dodge JT, Jr; RESTORE Investigators. Correlates of coronary blood flow before and after percutaneous coronary intervention and their relationship to angiographic and clinical outcomes in the RESTORE trial. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Am Heart J. 2002;144:130–135. doi: 10.1067/mhj.2002.123142 [DOI] [PubMed] [Google Scholar]
- 21.Kampinga MA, Nijsten MW, Gu YL, Dijk WA, de Smet BJ, van den Heuvel AF, Tan ES, Zijlstra F. Is the myocardial blush grade scored by the operator during primary percutaneous coronary intervention of prognostic value in patients with ST-elevation myocardial infarction in routine clinical practice? Circ Cardiovasc Interv. 2010;3:216–223. doi: 10.1161/CIRCINTERVENTIONS.109.916247 [DOI] [PubMed] [Google Scholar]
- 22.Arefin MM, Rahman A, Azam MG, Momen A, Rahman MA, Jahan J, Cader FA. TCTAP A-123 Impact of myocardial blush grade on in-hospital outcome after primary percutaneous coronary intervention. J Am Coll Cardiol. 2018;71:S14. [PubMed] [Google Scholar]