Supplemental Digital Content is available in the text.
Keywords: iliac vein, quality of life, stent, thromboembolism, thrombosis
Abstract
Background:
Iliofemoral venous obstruction is recognized with increasing frequency as the underlying cause of lower extremity symptoms including edema, pain, skin changes, and, in advanced cases, ulceration. This study sought to evaluate the safety and effectiveness of the Abre venous self-expanding stent system for the treatment of symptomatic iliofemoral venous outflow obstruction.
Methods:
The ABRE Study (A Multi-Center, Non-Randomized Study to Evaluate the Safety and Effectiveness of the Abre Venous Self-Expanding Stent System in Patients With Symptomatic Iliofemoral Venous Outflow Obstruction) is a single-arm, multicenter, prospective study that included 200 subjects from 24 global sites. The primary end points were 12-month primary patency and major adverse events within 30 days. Secondary end points included lesion and procedure success, primary-assisted and secondary patency, major adverse events, stent migration, stent fracture, and quality of life changes. End point-related adverse events and imaging studies were adjudicated by independent clinical events committee and core laboratories, respectively.
Results:
Venous obstruction cause was classified as acute deep vein thrombosis (16.5%, 33/200), post-thrombotic syndrome (47.5%, 95/200), or nonthrombotic iliac vein lesion (36.0%, 72/200). The common iliac and external iliac veins were stented in 96.0% (192/200), 80.5% (161/200) of subjects, respectively. Stent implant into the common femoral vein was required in 44.0% (88/200). Primary patency at 12 months was 88.0% (162/184). Four (2.0%) major adverse events occurred within 30 days. Twelve-month primary-assisted and secondary patency were 91.8% (169/184) and 92.9% (171/184), respectively. No stent fractures or migrations were reported. Mean target limb Villalta score decreased from 11.2±5.6 at baseline to 4.1±4.8 at 12 months, and the mean target limb revised Venous Clinical Severity Score decreased from 8.8±4.7 at baseline to 4.3±3.6 at 12 months. Clinically meaningful improvements in quality of life and venous functional assessment scores from baseline were demonstrated through 12 months in all measures.
Conclusions:
Symptomatic iliofemoral venous obstruction can be successfully treated with an Abre venous stent. Study outcomes demonstrated a high patency rate with a good safety profile. Patients demonstrated a significant reduction in clinical symptoms and improvement in quality of life that was maintained through 12-month follow-up.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03038438.
What Is Known
Iliofemoral venous stenting is a viable and increasingly common treatment for symptomatic iliofemoral venous outflow obstruction.
The venous anatomy requires stent attributes that differ from the arterial system which has led to the development of venous specific stents.
There is a paucity of overall data regarding the outcome of the new generation of stents both in respect of technical performance and patient outcomes.
What the Study Adds
The ABRE Study (A Multi-Center, Non-Randomized Study to Evaluate the Safety and Effectiveness of the Abre Venous Self-Expanding Stent System in Patients With Symptomatic Iliofemoral Venous Outflow Obstruction) is a prospective, multicenter, single-arm study of the Abre venous self-expanding stent system for the treatment of symptomatic iliofemoral outflow obstruction.
The 12-month safety and effectiveness performance goals were exceeded, and the study demonstrated meaningful clinical and quality of life improvements after venous stenting with no stent fractures occurring despite a high proportion of stents crossing the inguinal ligament.
Iliofemoral venous obstruction is a common problem with significant associated morbidity.1,2 Endovascular intervention has become more widespread as data for the safety and efficacy of stenting has been established.3–5 Stenting can reduce symptoms of venous hypertension and improve quality of life (QoL) in patients with nonthrombotic venous compression and post-thrombotic obstruction.1 In the setting of acute iliofemoral deep vein thrombosis (DVT), stenting may be utilized to treat underlying residual obstruction after successful early thrombus removal with the goals of accomplishing a rapid reduction in symptoms and to potentially reduce the incidence of recurrent DVT and post-thrombotic syndrome (PTS).6–8
Engineering challenges exist for venous stent design that are different from their arterial counterparts. Strength attributes of the ideal venous stent must include compression resistance and radial recoil resistance, which are beyond the standard radial outward forces required in arterial products. Venous stents must also be larger in diameter and be available in longer lengths. The stents must transverse the curvature of pelvic veins without kinking or distorting their course and often need to cross the inguinal ligament. These anatomic challenges mandate that venous stents be flexible and tolerate both deformations and repetitive loading, respectively.9–11 Patients are often younger than those with arterial disease, so durability is paramount. Delivery must be precise and accurate to allow stenting to the level of the confluences, cranially and caudally, as stents that do not extend from a diseased inflow vessel to a widely patent outflow vessel is a potential reason for stent failure.
The Abre (Medtronic, Inc, Minneapolis) venous self-expanding stent, made of high-purity nitinol, has a novel design intentionally tailored to perform in the iliofemoral veins.12 The primary objective of this study was to evaluate the safety and effectiveness of the Abre venous stent for the treatment of patients with symptomatic iliofemoral venous outflow obstruction.
Methods
Study Design
The ABRE Study (A Multi-Center, Non-Randomized Study to Evaluate the Safety and Effectiveness of the Abre Venous Self-Expanding Stent System in Patients With Symptomatic Iliofemoral Venous Outflow Obstruction) is a prospective, multicenter, single-arm, international study of the Abre venous stent for use in patients with symptomatic iliofemoral venous outflow obstruction. The study was conducted under an investigational device exemption from the FDA. Research procedures followed during this clinical study were prospectively registered. The uniform study protocol was approved by institutional review boards or by ethical committees of the respective participating centers. Study participants provided written informed consent before entering the study. An independent Clinical Events Committee reviewed and adjudicated end point-related adverse events (AEs), and independent core laboratories assessed duplex ultrasound (DUS), x-ray, intravascular ultrasound (IVUS), and venography. The data, analytic methods, and study materials may be available to other researchers on request from the sponsor.
Stent Design
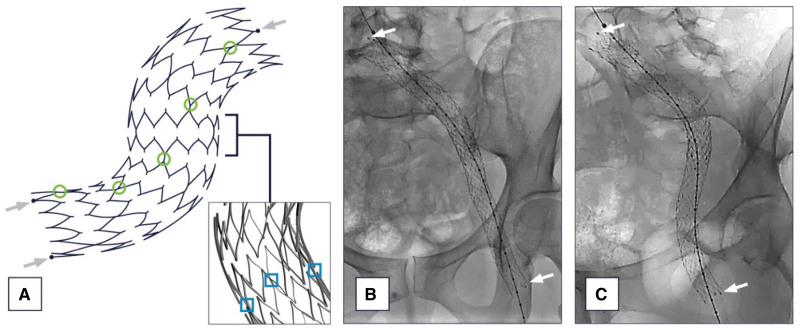
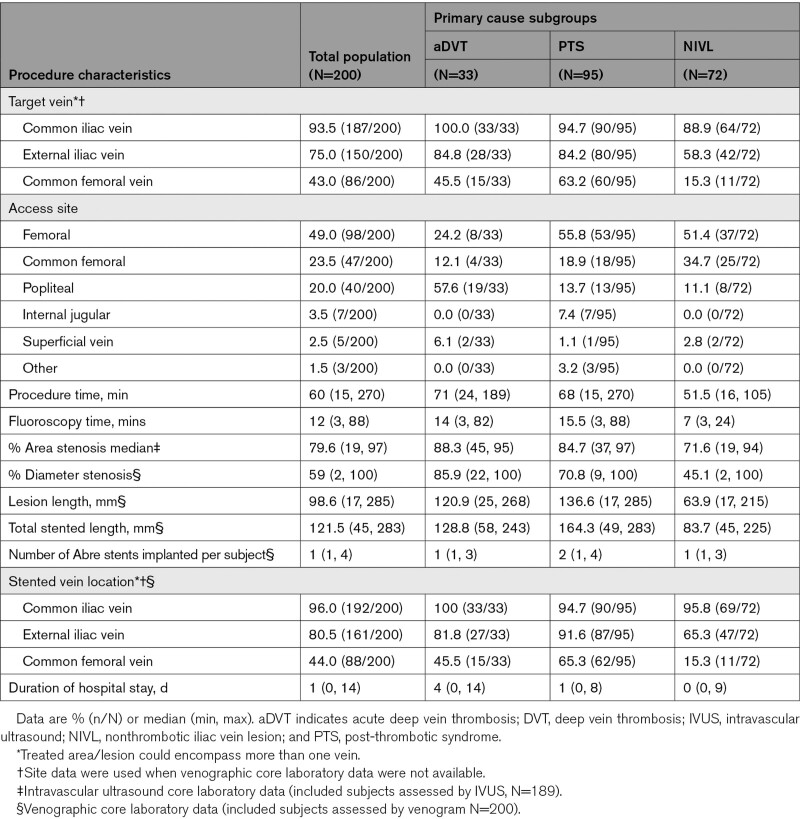
The Abre venous stent is indicated for use in iliofemoral veins. It consists of the stent and accompanying delivery system. The stent component is an open cell nitinol (nickel-titanium alloy) self-expanding design. It has 3 off-set connection points that spiral down the stent intentionally to maximize flexibility while minimizing any stent kinking or instability during deployment. The length and thickness of the stent struts vary according to stent diameter to create uniform stent strength across varying stent diameters.13 The openings of the strut radii are varied selectively (determined by a patented method) to improve stent durability and minimize stent fractures (Figure 1).13 The stent is currently available in lengths from 40 to 150 mm (40, 60, 80, 100, 120, 150 mm) and in diameters ranging from 10 to 20 mm (10, 12, 14, 16, 18, 20 mm).
Figure 1.
Abre venous stent design. The width and thickness of the struts on the Abre venous stent were altered for each stent diameter to achieve strength uniformity of all stent sizes, and the strut radii were opened selectively to achieve maximal fracture resistance. The stent has connection points that spiral down the stent (A, green circles). The stent cells are connected at 3 points around the circumference for maximum flexibility and kink resistance (A, blue squares). The Abre venous stent is easily seen under fluoroscopy (B, C) with 3 radiopaque markers on the cranial and caudal stent ends (see arrows on A, B, and C).
The accompanying delivery system utilizes a 9 Fr introducer sheath and is an over-the-wire system compatible with an 0.035-inch guidewire.
Study Enrollment
Enrollment occurred across 24 international study centers between December 2017 and November 2018. In total, 200 subjects were enrolled and were implanted with Abre venous stent, including 128 subjects from 16 sites in the United States and 72 subjects from the 8 sites in Europe.
Eligible patients included men and women between 18 and 80 years of age who presented with symptomatic lower extremity venous hypertension secondary to iliofemoral venous obstruction. Included patients were required to have one or more of the following: (1) Clinical Etiologic Anatomic Pathophysiologic (CEAP) score of ≥3; (2) moderate leg pain with revised Venous Clinical Severity Score (rVCSS) pain score of ≥2; or (3) suspected DVT. All patients were required to have a nonmalignant venous obstruction within the common iliac, external iliac, and/or common femoral vein (CFV) believed to be the cause of the presenting symptoms. Venous obstruction was defined as the presence of one or more of the following: (1) a reduction in diameter by ≥50% on venography or IVUS, (2) an area reduction by ≥50% with IVUS, or (3) venous occlusion. A venous occlusion was defined as the complete occlusion of one or more segments of the common iliofemoral vein, external iliac vein, or CFV. The implanting physician reported the cause for each subject’s venous obstruction as (1) nonthrombotic iliac vein lesion (NIVL), (2) PTS, or (3) acute DVT (aDVT). In the setting of aDVT, stenting was indicated for residual iliac vein obstruction after successful thrombectomy, thrombolysis, or both (detailed definitions are provided in Table S1). Interventions for aDVT and stent implantation had to have been completed within 14 days from symptom onset.
Patients were excluded if they had symptomatic peripheral arterial disease, prior venous stents in the ipsilateral vasculature, bilateral iliofemoral or inferior vena cava lesions, or a contraindication to anticoagulation. For more detailed information on the eligibility criteria, please refer to the summary of safety and effectiveness data at URL: https://clinicaltrials.gov/, unique identifier: NCT03038438.
Intervention and Follow-Up
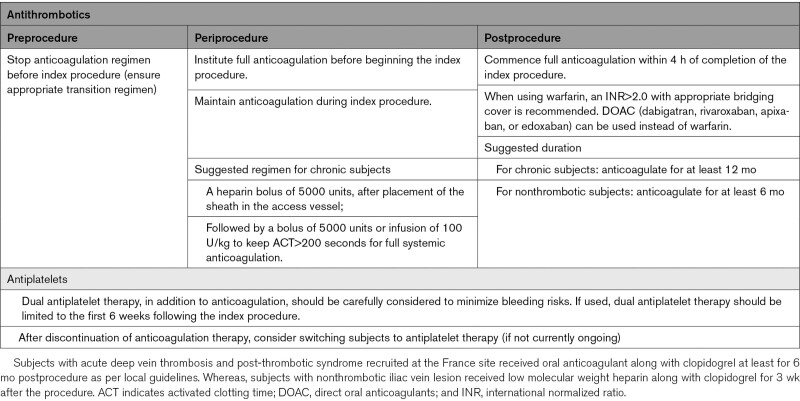
Ipsilateral femoral access was encouraged when feasible. To permit assessment of the entire CFV to the level of the profunda/femoral confluence, access from the ipsilateral CFV or greater saphenous vein was discouraged. Venography and IVUS were used to determine the extent of disease and were also utilized for procedural guidance. The study protocol recommended that subjects be anticoagulated during the index procedure (Table 1). The following recommendations were given for stent sizing and placement: oversize the stent by at least 2 mm more than the chosen reference vessel diameter to achieve good wall apposition; place the stent at least 1 cm cranial and 1 cm caudal to the target lesion(s); and when overlapping stents, overlap at least 1.5 cm without skip areas between stents. It was recommended that lesions were predilated and stents post-dilated to stent size with the recommendation to utilize a high-pressure balloon. Completion imaging was obtained. In cases of occlusion where normal reference segment is difficult to obtain, the IVUS/venogram core laboratory used the following reference vessel diameters: common iliofemoral vein: 16 mm, external iliac vein: 14 mm, and CFV: 12 mm.
Table 1.
Study Anticoagulation Regimen Recommendations
Postprocedure anticoagulation recommendations are provided in Table 1; however, anticoagulation decisions were ultimately at the discretion of the investigators.
Postprocedure follow-up assessments were completed at 30 days, 6, and 12 months. Assessments included an interim history with medication review, an assessment for AEs, physical examination, and DUS. QoL questionnaires and venous functional assessments were completed during the 6- and 12-month follow-up visits. At the 30-day follow-up, the first 30 subjects underwent a multi-planar pelvic x-ray to evaluate for stent fractures. Multi-planar pelvic x-rays were collected for all subjects at 12-, 24-, and 36-month study visits. A venogram was required at the 12-month visit if the DUS imaging indicated a greater than or equal to 50% stent obstruction, if DUS imaging was nondiagnostic, or if dictated by clinical symptoms. Further follow-up visits collecting the same data points were scheduled at 24 and 36 months.
Outcomes
Primary End Points
The primary effectiveness and safety end points were compared with performance goals derived from the literature.14–29 The primary effectiveness end point was primary patency at 12-month, defined as freedom from occlusion, freedom from restenosis ≥50% of the stented segment, and freedom from clinically driven target lesion revascularization (TLR) measured at 12 months postindex procedure. Primary patency was a subject-level assessment that used worst-case scenario where the assessment was conducted within the entire stented segment regardless of whether it was stented with a single stent or multiple stents. If a failure was identified, the subject was counted as a loss of primary patency. Clinically driven was defined as the recurrence of symptoms present at baseline or the onset of new symptoms, including but not limited to ipsilateral venous pain, swelling, stasis dermatitis, or ulceration. Subjects with nonclinically driven TLR were censored for primary patency end point reporting to eliminate bias caused by nonstudy device usage after the index procedure of subjects being intervened on for a stenosis percentage on ultrasound that did not correlate with subject symptoms. In the venous system, there has yet to be an identified percentage of stenosis that is considered to put the stent at risk; thus in the absence of data, we did not consider a re-intervention for asymptomatic stenosis as a reliable indication of stent failure. Nonetheless, to evaluate the impact of missing or unknown outcomes data on the primary effectiveness end point result, sensitivity analyses were performed by multiple imputation and tipping point analysis. In tipping point analysis, the worst-case scenario assumed all missing subjects failed the end point and best-case scenario assumed all missing subjects were patent. The lower limit of the 97.5% one-sided CI of the 12-month primary patency exceeded the literature-derived performance goal of 75.0% indicating the primary effectiveness objective was met. The primary safety end point was the rate of major AEs (MAEs) within 30 days of the index procedure. MAEs were defined as a composite of all-cause death, clinically significant pulmonary embolism, procedure-related major bleeding complications, and stent thrombosis or stent migration as confirmed by imaging within 30 days of the index procedure (Table S1). The primary safety objective was met with the upper limit of the 97.5% one-sided CI of the 30-day MAEs rate lower than the performance goal of 12.5%.
Secondary End Points
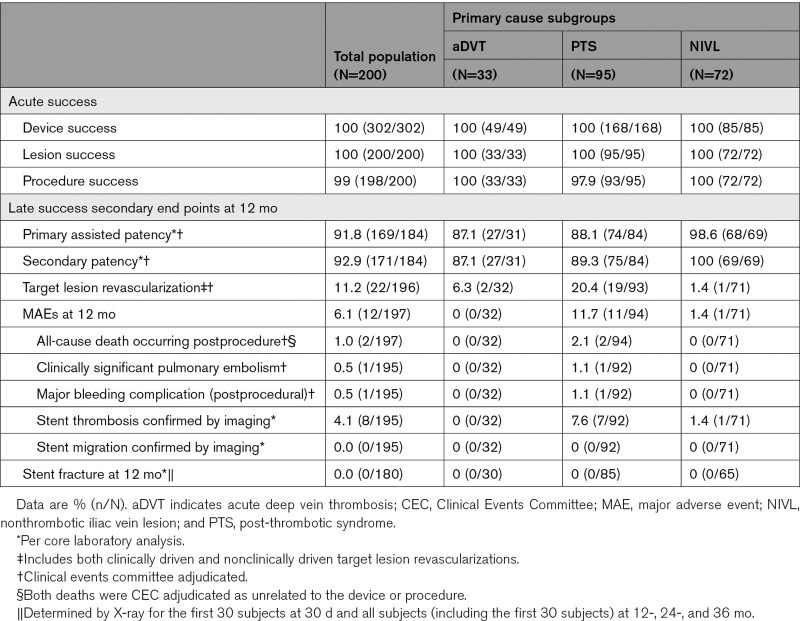
The secondary clinical end points were assessed at various time points. The detailed definitions of the important outcomes of this study are provided in Table S1. Acute success end points included device success and lesion success (assessed at the time of procedure); and procedure success (assessed within 30 days). Primary-assisted and secondary patency was assessed at 12 months.
Additional secondary end points evaluated at 12 months included TLR (including clinically driven and nonclinically driven), major bleeding complications, MAEs, stent migration, and stent fracture. These end points were Clinical Events Committee adjudicated or imaging core laboratory-confirmed. Quality of life and venous functional assessments were assessed using validated scoring systems.30–35 Quality of life was evaluated using EuroQoL Five Dimensions and VEINES-QoL/Sym (Venous Insufficiency Epidemiological and Economic Study Quality of Life/Symptoms. Clinical outcomes were measured before and after treatment using the rVCSS and the Villalta Scores measured from the target limb.
Statistical Analysis
The planned sample size of 200 subjects provided an overall power of at least 84.0% for the hypothesis testing of primary safety and primary effectiveness end points against the performance goal respectively. The performance goal of 75.0% was set for 12 months primary patency, and 12.5% was used for primary safety end point of MAE within 30 days. Primary patency is a subject-level assessment that uses worst-case scenario where the assessment is conducted within the entire stented segment regardless of whether it was stented with a single stent or multiple stents. If a failure is identified, the subject would be counted as loss of primary patency. Exact binomial test was used with a 1-sided type I error of 2.5%. All analyses were based on subjects with evaluable data. For baseline characteristics, continuous variables were described as mean±SD or median with the range (min, max) if not normally distributed; dichotomous and categorical variables were described as counts and proportions. For event rates expressed as proportions, the number of subjects with events within 360 days was the numerator and the total number of subjects with events or at least 330 days of clinical follow-up was the denominator. Statistical analyses were performed using SAS (SAS Institute, Cary, NC) version 9.4 or higher.
Results
Enrollment and Compliance
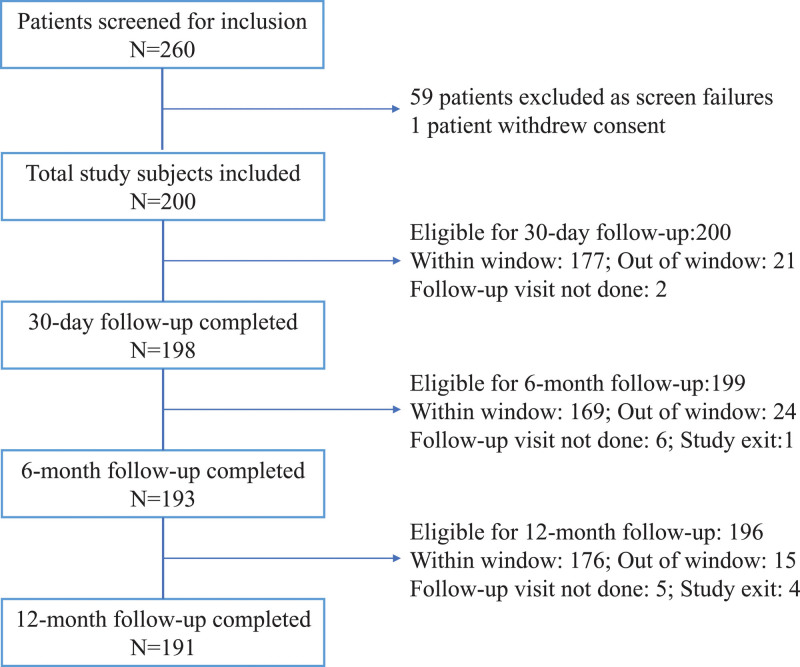
From December 2017 to November 2018, 260 subjects were screened for eligibility at 24 global sites. Of these, 59 subjects were excluded from study participation, and one subject withdrew consent before the index procedure. The most common reasons for study exclusion were minimal iliac vein disease (less than 50% stenosis of the involved segments, n=20) and extension of disease outside of the targeted iliofemoral segment with the involvement of the inferior vena cava, inflow vessels, or contralateral iliofemoral veins (n=19). Inability to cross occluded iliac segments (n=5) and medical comorbidities that would interfere with study compliance (n=5) were also cited as reasons for exclusion. Ultimately 200 subjects were included in the study. Overall, 191 (95.5%) subjects completed the 12-month follow-up visit, while one subject was lost-to follow-up, one subject exited due to incarceration, 2 subjects died (see Secondary End point section), and 5 subjects missed the 12-month follow-up visit (Figure 2).
Figure 2.
Subject compliance flowchart. The ABRE Study (A Multi-Center, Non-Randomized Study to Evaluate the Safety and Effectiveness of the Abre Venous Self-Expanding Stent System in Patients With Symptomatic Iliofemoral Venous Outflow Obstruction) included 200 subjects with 191 (95.5%) subjects completing the 12-mo follow-up.
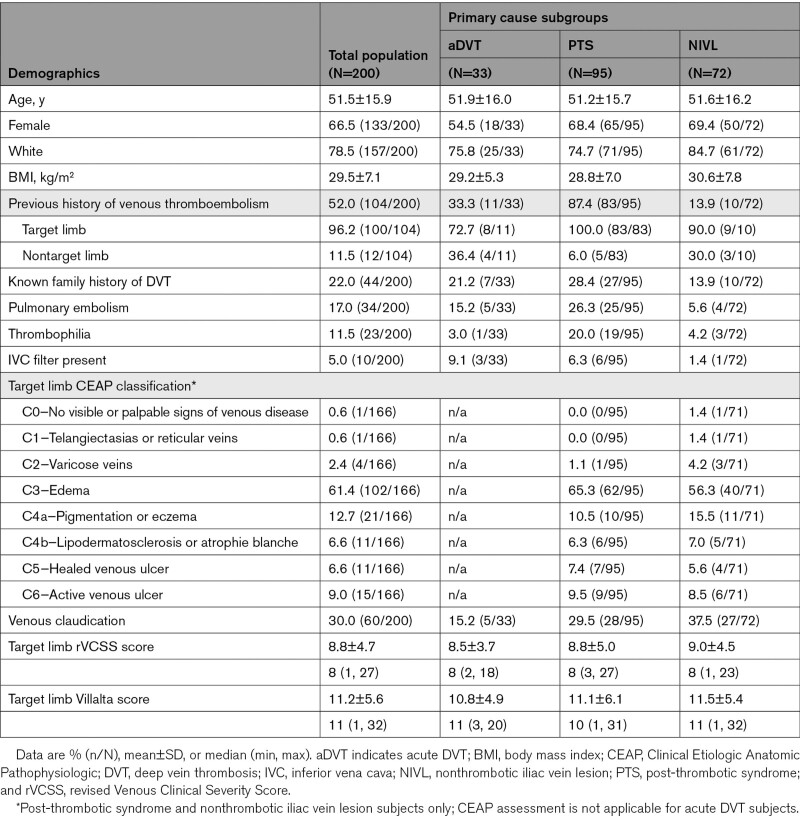
Demographic and Clinical Characteristics
Two-thirds (133/200) of the subjects were female, with a mean subject age of 51.5±15.9 years. The most common pertinent medical history was a personal history of venous thromboembolism (52.0%, 104/200, with 96.2%, 100/104 in target limb). Documented prior venous thromboembolism was specifically noted in 87.4% (83/95) of PTS subjects and 33.3% (11/33) of subjects presenting with aDVT. A family history of DVT was noted in 22.0% (44/200) of subjects or a known diagnosis of thrombophilia in 11.5% (23/200) of subjects (Table 2). The target limb was primarily the left leg (92.0%, 184/200). Subjects were categorized as PTS (47.5%, 95/200), NIVL (36.0%, 72/200), or aDVT (16.5%, 33/200).
Table 2.
Baseline Demographic and Clinical Characteristics
Sixty-one percent (102/166) of NIVL and PTS target limbs presented with predominantly edema (CEAP score C3). Thirty-three percent (55/167) of NIVL and PTS subjects reported venous claudication. Additional presenting signs of chronic venous disease included skin changes, such as pigmentation/eczema and lipodermatosclerosis (12.7%, 21/166 CEAP score C4a and 6.6%, 11/166 CEAP score C4b, respectively), as well as a healed or active venous ulcer (15.7%, 26/166 CEAP score C5/C6). Baseline demographics and clinical characteristics are listed in Table 2.
Procedural Characteristics
Procedure characteristics are reported in Table 3. The common iliac vein was the most common site for stent implantation (96.0%, 192/200). Stents were implanted in the external iliac vein in 80.5% (161/200) and the CFV in almost half of the subjects (44%, 88/200). Most of the PTS subjects required stent placement into the CFV (PTS: 65.3%, 62/95; aDVT: 45.5%, 15/33; NIVL: 15.3%, 11/72) as two-thirds (63.2%, 60/95) of the PTS group had long-segment disease involving >1 venous segment. One-third of PTS subjects had chronic total occlusions 35.8% (34/95).
Table 3.
Procedural Characteristics
Among the 302 Abre venous stents implanted, 46.0% (139/302) were 16 mm in diameter, and 36.4% (110/302) were 14 mm in diameter. Less frequently, smaller diameter (12 mm: 3.0%, 9/302) or larger diameter stents (18 and 20 mm: 14.6%, 44/302) were implanted. Predilation and postdilation were performed in 97% (194/200) of subjects. The median stented length was 121.5 mm, with a maximum stented length of 283 mm. The median total number of the stents implanted per subject was 1 (range, 1 to 4); 55.5% (111/200) of subjects received one stent; 38.5% (77/200) received 2 stents, and 6.0% (12/200) received 3 or more stents. The postprocedure residual diameter stenosis was 14.2%±8.2% by venography and 11.7%±8.1% by IVUS. The median procedure time was 60 (range, 15 to 270) minutes, and the median duration of hospital stay was 1 (range, 0 to 14) day (Table 3).
Primary End Points
Primary Effectiveness at 12 Months
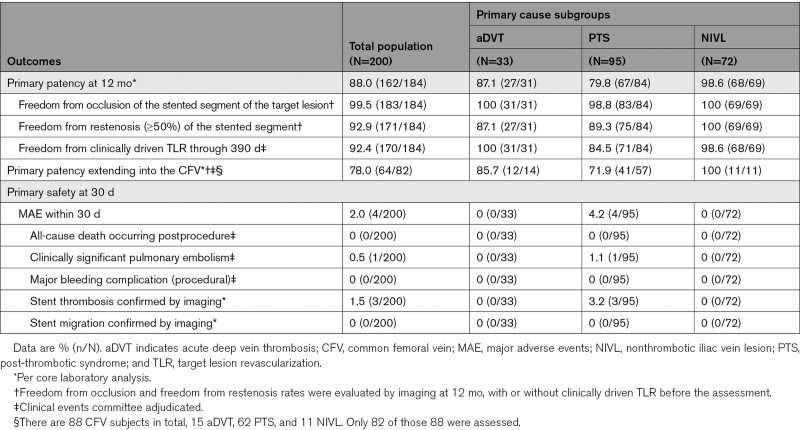
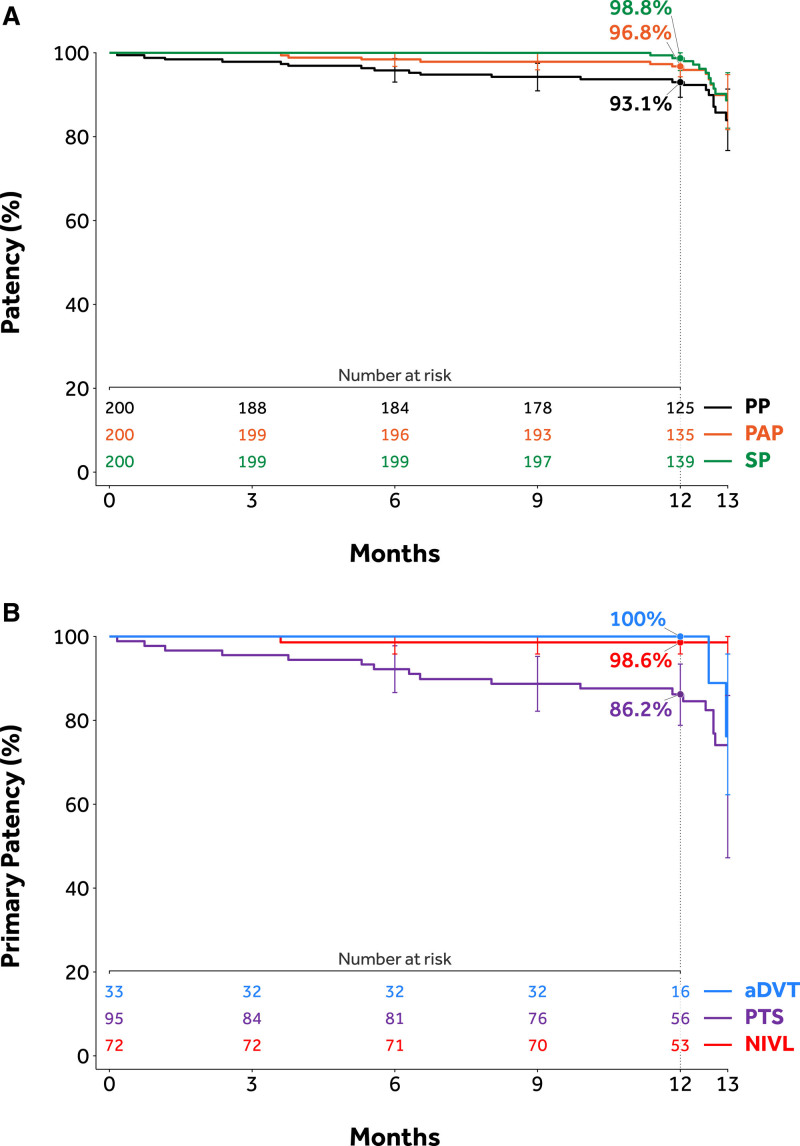
Overall, 184 out of 200 (92.0%) subjects were evaluable for the primary effectiveness end point; 17 subjects had imaging component of the primary patency determined by venogram, the remaining 167 subjects were analyzed with DUS. Sixteen subjects were excluded from the primary patency analysis. The reasons included: death in 2 subjects, 1 subject was lost-to follow-up, 5 subjects missed the 12-month follow-up visit, and censoring of data for 8 nonclinically driven TLR, as determined by the Clinical Events Committee. A total of 162 subjects, or 88.0%, among 184 assessable subjects achieved primary patency. The lower limit of the 97.5% one-sided CI was 82.5%, higher than the performance goal of 75%, signifying that the 12-month primary effectiveness end point (88%; P<0.0001) was met (Table 4). Twenty-two subjects (22/184, 12.0%) failed one or more components of the primary patency end point. Of these 22 subjects, 14 had a clinically driven TLR through 390 days, among them, 1 had total occlusion. An additional 8 subjects had restenosis (≥50%) based on their 12-month imaging by core laboratory assessment. Sensitivity analyses were performed to assess the impact of the missing data on the primary patency end point. In the tipping point sensitivity analysis, the primary patency end point was met when up to 15 missing data points were counted as loss of primary patency; it did not meet only under the worst-case scenario when all 16 missing were counted as loss of primary patency. Multiple imputation sensitivity analysis yield result consistent with the primary analysis. Subgroup analyses demonstrated primary patency of 87.1% (27/31) in the aDVT group 79.8% (67/84) in the PTS group, and 98.6% (68/69) in the NIVL group. Primary patency based on Kaplan-Meier estimates is shown in Figure 3. The overall primary patency rate of stents extending into the CFV in evaluable subjects was 78.0% (64/82; Table 4). There was no decline in patency noted with stent extension below the groin in subjects with nonthrombotic disease or aDVT, which were 100% (11/11) and 85.7% (12/14), respectively. In the PTS group, there was a decrease in patency when stents were taken into the CFV (71.9%, 41/57) compared with those that were not (96.3%, 26/27).
Table 4.
Primary Effectiveness and Safety End Points
Figure 3.
Kaplan-Meier estimates of patency. A, Primary patency rates through 12 mo across subgroups. B, Patency estimates by Kaplan-Meir estimate through 12 mo. aDVT indicates acute deep vein thrombosis; NIVL, nonthrombotic iliac vein lesion; PAP, primary assisted patency; PP, primary patency; PTS, post-thrombotic syndrome; and SP, secondary patency.
Safety Outcomes
All 200 subjects were evaluable for the MAE primary safety end point within 30 days. A total of 4 (2.0%) subjects experienced MAEs within 30 days: 1 subject presented with symptomatic pulmonary embolism (although not an occlusion, stent thrombosis was present) on postprocedure day 25, and 3 subjects presented with stent thrombosis on postprocedure days 1, 5, and 21, respectively. All 4 of these subjects with MAEs were from the PTS group. There were no reported all-cause deaths, major bleeding, or stent migrations within 30 days. The upper bound of the one-sided 97.5% CI was 5.0%, which was less than the 12.5% performance goal, signifying that the primary safety end point was met (P<0.0001; Table 4). Within 12 months, investigative sites reported in total 36 device- or procedure-related SAEs in 26 out of 200 (13.0%) subjects. Three subjects experienced bleeding complications within the first 30 days: 2 subjects with vascular access site hematoma and one with retroperitoneal hematoma. One subject (0.5%) had postoperative groin pain and 2 (1.0%) reported postoperative back pain (nonserious) within 30 days postindex procedure.
Secondary End Points
Acute and Late Success Results
Device success and lesion success were 100% (302/302 and 200/200, respectively), whereas procedure success was 99% (198/200); 2 subjects experienced MAEs before discharge. Of 184 subjects evaluable for the primary-assisted and secondary patency end points, 169 (91.8%) subjects achieved primary assisted patency, and 171 (92.9%) achieved secondary patency. Kaplan-Meier estimates for primary patency, primary assisted patency, and secondary patency are shown in Figure 3A. Primary patency Kaplan-Meier curves across subgroups are shown in Figure 3B. MAEs were evaluated in 197 out of 200 (98.5%) subjects at 12 months (3 subjects had no MAE and had less than 330 days of follow-up). Twelve (12/197, 6.1%) subjects experienced MAEs—1 (0.5%) clinically significant pulmonary embolism, 1 (0.5%) delayed major bleeding event (31 days postprocedure), 8 (4.1%) stent thrombosis, and 2 (1%) all-cause death (Table 5). Both deaths were Clinical Events Committee adjudicated as unrelated to the device or procedure. One subject died 8 months after iliac stent placement secondary to new and acute onset congestive heart failure with resultant cardiogenic shock and multiorgan failure in the absence of thrombotic events. The second subject developed acute cholecystitis and pneumonia 65 days after stenting. This subject’s course was then complicated by septic shock progressing to multiorgan failure resulting in death. Upon reviewing the 8 stent occlusions that occurred through 12 months, at least 7 had significant inflow disease below the stented venous segments that likely contributed to stent occlusion directly. All 7 of these subjects had post-thrombotic disease as the indication for stenting. The remaining subject was the only stent occlusion from the NIVL group. The subject’s stent occlusion was accompanied by multiple confounding factors including remaining inflow disease, medication noncompliance, and air travel. No stent fractures and no stent migrations were reported through 12-month follow-up.
Table 5.
Secondary End Points
Quality of Life and Functional Assessments
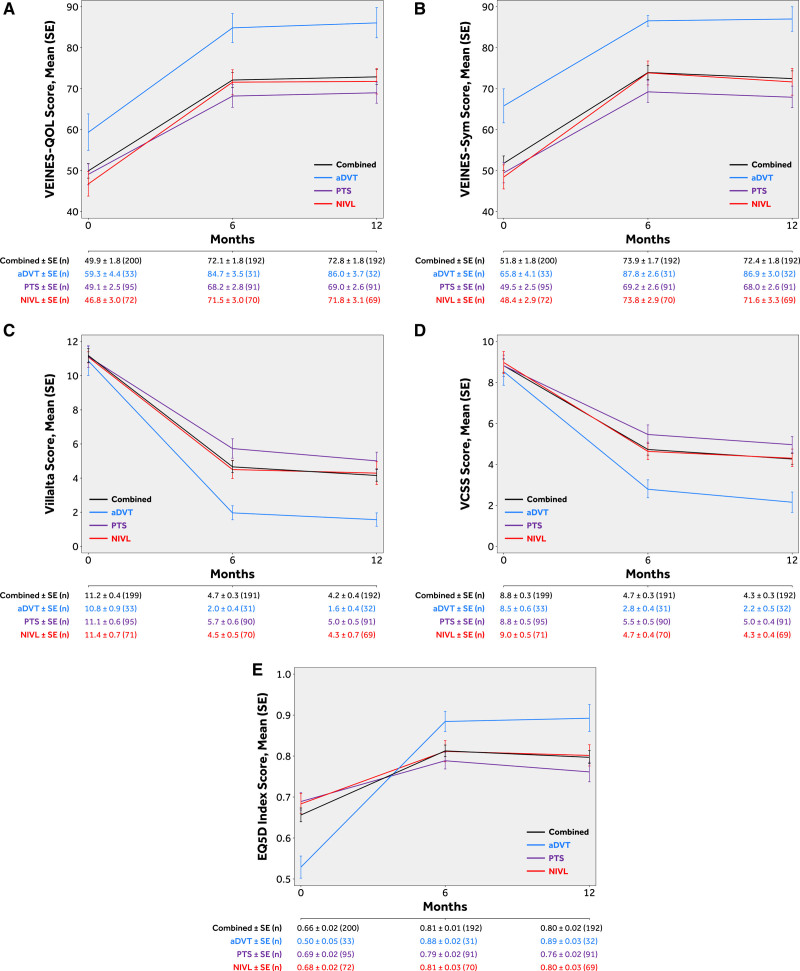
Sustained and clinically meaningful improvement in QoL and venous functional assessment scores were demonstrated by the VEINES-QoL/Sym, EuroQoL Five Dimensions (Index and VAS), Villalta, and rVCSS scores at 12 months compared with baseline (Figure 4). Specifically, the mean target limb Villalta score decreased from 11.2±5.6 at baseline to 4.1±4.8 at 12 months and the mean target limb rVCSS score decreased from 8.8±4.7 at baseline to 4.3±3.6 at 12 months. The number of subjects with active venous leg ulcers on the target limb decreased from 15 at baseline to 4 at 12 months.
Figure 4.
Summary of quality of life and functional assessments. Quality of life and functional assessments were taken at baseline (0 d), 6 mo, and 12 mo. Sustained and clinically meaningful improvement were demonstrated for all measures at 12 mo compared with baseline, despite a high proportion of stents crossing the inguinal ligament. A, Venous Insufficiency Epidemiological and Economics Study Quality of Life (VEINES-QoL). B, VEINES-Symptoms. C, Villalta score. D, Revised Venous Clinical Severity Score (VCSS). E, EuroQoL Five Dimensions (EQ-5D). aDVT indicates acute deep vein thrombosis; NIVL, nonthrombotic iliac vein lesion; and PTS, post-thrombotic syndrome.
Discussion
In this study, all prespecified performance goals, including both the primary effectiveness and primary safety performance goals, were met. The results indicate a near 90% primary patency rate with similar rates of primary-assisted and secondary-assisted patency rates, as well as only 2% MAEs reported within 30 days.
Importantly, successful endovenous stenting in this study translated into meaningful QoL improvements in EuroQoL Five Dimensions and VEINES-QoL scores observed from baseline and maintained through 12 months.
Overall, performance outcomes including patency rates, safety outcomes, and QoL improvement observed in this study are comparable to the results of other previously published investigational device exemption and cohort studies for other nitinol stents used in treating iliofemoral obstruction through a 12-month follow-up.36–39 The consistency of results across studies will add validity to the importance of interventional treatment for patients with symptomatic iliofemoral obstructive venous disease.
Unique to this study design was that all patient disease states causing iliofemoral venous obstruction, including nonthrombotic, aDVT, and post-thrombotic disease, were represented. Subjects with post-thrombotic disease, the largest included cohort (47.5%, 95/200) in this study, are understood to have the most complex disease.3 The lowest stent patency rates in the literature are seen in patients with advanced post-thrombotic obstruction, including those with long-segment disease, coincident inflow disease, and completely occluded iliac segments.3 Also unique to this study is the higher percentage of subjects with advanced, extensive PTS, demonstrated by the presence of total iliofemoral occlusions in 35.8% (34/95) of the PTS group. Similarly, long-segment disease was present in 65.3% (62/95) of the PTS group as evidenced by stenting in the CFV. The aDVT group was additionally complex as one-third of this group had prior DVT in the affected limb, most occurring in the iliofemoral segment.
Overall, the primary patency of the Abre venous stent in the PTS group was 79.8% (67/84), which was expectantly lower than the patency rates observed in the nonthrombotic and aDVT groups, which were 98.6% (68/69) and 87.1% (27/31), respectively. Nevertheless, the value of treating patients in the post-thrombotic group remains and is underscored by clinically meaningful QoL improvements in that group. When isolating the PTS group, there was a 56% reduction in the Villalta score observed from baseline to 12 months. In fact, after interventional treatment, over half of the subjects (54/91, 59.3%) were no longer classified as having PTS when PTS is defined by a Villalta score ≥5.
With the complexity of subjects included in this study came a high number of subjects requiring long-segment stenting spanning across the inguinal ligament. In this study, just under half of the subjects had stents crossing the inguinal ligament and extending into the CFV (44%, 88/200). The ABRE Study had the highest number of stents extending into the CFV compared with recent venous stent studies to date.36–38,40,41 The most closely comparable study was the VIRTUS study (VIRTUS Safety and Efficacy of the Veniti Vici Venous Stent System [Veniti, Inc] When Used to Treat Clinically Significant Chronic Non-Malignant Obstruction of the Iliofemoral Venous Segment) which reported that 45.7% of chronic post-thrombotic subjects (58/127) and 35.9% (61/170) of all subjects overall in their study required stent extension into the CFV.38 Extension of nonbraided stents into the CFV has previously raised concerns regarding stent durability because arterial stents crossing joints have been prone to both neointimal hyperplasia as well as stent fracture, both potentially resulting in premature stent failure.42 In the VIRTUS study, there was a small incidence of stent fractures (3.6% of all implanted stents), with most fractures occurring in the CFV of PTS subjects and none displaying clinical consequences through 1 year.38 In our study, despite the high number of stents extending into the CFV, there were no stent fractures or mechanical stent issues in any of the implanted stents (n=302) up to 1 year.
Although there were no stent fractures in the ABRE Study, a decrease in patency was observed when stents were extended across the inguinal ligament into the CFV. With further investigation, this was only observed in the post-thrombotic subgroup. Stent patency was not affected in subjects with nonthrombotic obstructions, nor in aDVT subjects, despite similar stent extensions. This suggests that it is not mechanical issues that arise from merely crossing into the groin but possibly a reflection of compromised and diseased inflow in extensive post-thrombotic disease. In fact, upon review of the 8 stent occlusions through 12 months in this study, all but one (7/8) occurred in post-thrombotic subjects. All 7 of the post-thrombotic subjects with stent occlusions appeared to have significant remaining inflow disease remaining after treatment which seemed to be the main reason for stent thrombosis. Improvements in classifying and managing complex inflow disease may improve outcomes from stenting, particularly in the post-thrombotic group. The remaining stent occlusion, occurring in a NIVL subject, appeared multifactorial. Although this subject had some remaining inflow disease, subject noncompliance likely played a significant role, including a failure to take anticoagulation medication as prescribed.
In summary, in this multicenter, prospective study we found that the Abre venous stent was a successful treatment option for iliofemoral outflow obstruction. Study outcomes are broadly comparable to previously completed venous stent studies with other devices demonstrating the benefits of intervention for venous obstruction. Subjects with post-thrombotic disease remain the most complex subject subset.
This study is limited by its single-arm, nonrandomized design. Study designs and subgroups widely differed among all available venous stent studies, mostly limiting detailed comparative analyses between available venous stents.34
Conclusions
Symptomatic iliofemoral venous obstruction can be successfully treated with an Abre venous stent. Study outcomes demonstrated a high patency rate with a good safety profile. Patients exhibited a significant reduction in clinical symptoms and improvement in QoL that was maintained through 12-month follow-up. Further long-term follow-up trials and prospective studies with a comparator arm are needed to assess the durability of the stent and patient outcomes.
Article Information
Acknowledgments
We thank the staff at the ABRE Core Labs: Venogram, and X-Ray Core Labs (Syntactx, Inc), Duplex Ultrasound Core Lab (Massachusetts General Physicians Organization, Inc, Vascore). In addition, thank you Pei Li, PhD (Medtronic) for validation of statistical analysis; Sue Kim, DPM (Medtronic), Stephanie Brucato, BS (Medtronic) and Jeffrey Vogel, PhD (Medtronic) for critical review and Ashwini Atre, PhD and Ramu Periyasamy, PhD of Indegene Pvt. Ltd. Bangalore, India for medical writing support that was funded by Medtronic, Inc (Minneapolis, MN).
Sources of Funding
This work was supported by Medtronic USA, Inc.
Disclosures
Co-principal investigator, Dr Murphy serves as an advisor and consultant for Medtronic, Philips, Boston Scientific, Cook, Gore, and Vesper Medical. Prof Black serves with Dr Murphy as co-principal investigator of this study, and as an advisor and consultant for Medtronic, Boston Scientific, Vetex, Philips, Cook, Bard, and Veryan. Dr Gibson reports consulting, speaking, and research funding from Medtronic. Prof Sapoval has no relevant conflicts to report. Dr Dexter has an advisory and consulting relationship with Medtronic, Bard, and Boston Scientific. R. Kolluri reports noncompensated, consulting relationship with Medtronic. Dr Razavi has an advisory role with Medtronic, Abbot Vascular, Boston Scientific, and Philips.
Supplemental Material
Table S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- aDVT
- acute DVT
- AE
- adverse event
- CEAP
- Clinical Etiologic Anatomic Pathophysiologic
- CFV
- common femoral vein
- DUS
- duplex ultrasound
- DVT
- deep vein thrombosis
- IVUS
- intravascular ultrasound
- MAE
- major adverse event
- NIVL
- nonthrombotic iliac vein lesion
- PTS
- post-thrombotic syndrome
- QoL
- quality of life
- rVCSS
- revised Venous Clinical Severity Score
- TLR
- target lesion revascularization
- VEINES-QoL/Sym
- Venous Insufficiency Epidemiological and Economic Study Quality of Life/Symptoms
- VTE
- venous thromboembolism
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.121.010960.
For Sources of Funding and Disclosures, see page 203.
Contributor Information
Kathleen Gibson, Email: drgibson@lkwv.com.
Marc Sapoval, Email: marc.sapoval2@aphp.fr.
David J. Dexter, Email: ddextermd@gmail.com.
Raghu Kolluri, Email: kolluri.raghu@gmail.com.
Mahmood Razavi, Email: mrazavi@pacbell.net.
Stephen Black, Email: Stephen.Black@gstt.nhs.uk.
References
- 1.Mahnken AH, Thomson K, de Haan M, O’Sullivan GJ. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37:889–897. doi: 10.1007/s00270-014-0875-4 [DOI] [PubMed] [Google Scholar]
- 2.Esposito A, Charisis N, Kantarovsky A, Uhl JF, Labropoulos N. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur J Vasc Endovasc Surg. 2020;60:118–125. doi: 10.1016/j.ejvs.2020.03.020 [DOI] [PubMed] [Google Scholar]
- 3.Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046 [DOI] [PubMed] [Google Scholar]
- 4.Raju S, Darcey R, Neglén P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51:401–8. discussion 408. doi: 10.1016/j.jvs.2009.08.032 [DOI] [PubMed] [Google Scholar]
- 5.Rossi FH, Kambara AM, Izukawa NM, Rodrigues TO, Rossi CB, Sousa AG, Metzger PB, Thorpe PE. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2018;6:183–191. doi: 10.1016/j.jvsv.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, Gupta DK, Prandoni P, Vedantham S, Walsh ME, et al. ; American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636–1661. doi: 10.1161/CIR.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Peterson E, Dooner J, Baerlocher M, Zypchen L, Gagnon J, Delorme M, Sing CK, Wong J, Guzman R, et al. ; Interdisciplinary Expert Panel on Iliofemoral Deep Vein Thrombosis (InterEPID). Diagnosis and management of iliofemoral deep vein thrombosis: clinical practice guideline. CMAJ. 2015;187:1288–1296. doi: 10.1503/cmaj.141614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meissner MH, Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Lohr JM, McLafferty RB, Murad MH, Padberg F, et al. ; Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012;55:1449–1462. doi: 10.1016/j.jvs.2011.12.081 [DOI] [PubMed] [Google Scholar]
- 9.Badesha AS, Bains PRS, Bains BRS, Khan T. A systematic review and meta-analysis of the treatment of obstructive chronic deep venous disease using dedicated venous stents. J Vasc Surg Venous Lymphat Disord. 2022;10:267–282.e4. doi: 10.1016/j.jvsv.2021.04.014 [DOI] [PubMed] [Google Scholar]
- 10.Dabir D, Feisst A, Thomas D, Luetkens JA, Meyer C, Kardulovic A, Menne M, Steinseifer U, Schild HH, Kuetting DLR. Physical properties of venous stents: an experimental comparison. Cardiovasc Intervent Radiol. 2018;41:942–950. doi: 10.1007/s00270-018-1916-1 [DOI] [PubMed] [Google Scholar]
- 11.Taha MAH, Busuttil A, Bootun R, Thabet BAH, Badawy AEH, Hassan HA, Shalhoub J, Davies AH. A clinical guide to deep venous stenting for chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2022;10:258–266.e1. doi: 10.1016/j.jvsv.2020.12.087 [DOI] [PubMed] [Google Scholar]
- 12.Robertson SW, Launey M, Shelley O, Ong I, Vien L, Senthilnathan K, Saffari P, Schlegel S, Pelton AR. A statistical approach to understand the role of inclusions on the fatigue resistance of superelastic Nitinol wire and tubing. J Mech Behav Biomed Mater. 2015;51:119–131. doi: 10.1016/j.jmbbm.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 13.Vogel JH, Noffke P, Zhang Z, Inventors; Covidien LP, Assignee. Stent with varying radius of curvature between struts. US Patent 10,022,253. 2018. [Google Scholar]
- 14.Alhalbouni S, Hingorani A, Shiferson A, Gopal K, Jung D, Novak D, Marks N, Ascher E. Iliac-femoral venous stenting for lower extremity venous stasis symptoms. Ann Vasc Surg. 2012;26:185–189. doi: 10.1016/j.avsg.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 15.de Wolf MA, de Graaf R, Kurstjens RL, Penninx S, Jalaie H, Wittens CH. Short-term clinical experience with a dedicated venous nitinol stent: initial results with the sinus-venous stent. Eur J Vasc Endovasc Surg. 2015;50:518–526. doi: 10.1016/j.ejvs.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 16.Ganelin A, Hingorani A, Ascher E, Kheyson B, Iadgarova E, Marks N, Ostrozhynskyy Y. Complications with office-based venoplasties and stenting and their clinical correlation. J Vasc Surg Venous Lymphat Disord. 2015;3:376–379. doi: 10.1016/j.jvsv.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 17.Hartung O, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Management of pregnancy in women with previous left ilio-caval stenting. J Vasc Surg. 2009;50:355–359. doi: 10.1016/j.jvs.2009.01.034 [DOI] [PubMed] [Google Scholar]
- 18.Kölbel T, Lindh M, Akesson M, Wassèlius J, Gottsäter A, Ivancev K. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther. 2009;16:483–491. doi: 10.1583/09-2719.1 [DOI] [PubMed] [Google Scholar]
- 19.Kurklinsky AK, Bjarnason H, Friese JL, Wysokinski WE, McBane RD, Misselt A, Moller SM, Gloviczki P. Outcomes of venoplasty with stent placement for chronic thrombosis of the iliac and femoral veins: single-center experience. J Vasc Interv Radiol. 2012;23:1009–1015. doi: 10.1016/j.jvir.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JY, Ahn JH, Jeon YS, Cho SG, Kim JY, Hong KC. Iliac vein stenting as a durable option for residual stenosis after catheter-directed thrombolysis and angioplasty of iliofemoral deep vein thrombosis secondary to May-Thurner syndrome. Phlebology. 2014;29:461–470. doi: 10.1177/0268355513491724 [DOI] [PubMed] [Google Scholar]
- 21.Park SI, Lee M, Lee MS, Kim MD, Won JY, Lee DY. Single-session aspiration thrombectomy of lower extremity deep vein thrombosis using large-size catheter without pharmacologic thrombolysis. Cardiovasc Intervent Radiol. 2014;37:412–419. doi: 10.1007/s00270-013-0676-1 [DOI] [PubMed] [Google Scholar]
- 22.Raju S, Ward M, Jr, Kirk O. A modification of iliac vein stent technique. Ann Vasc Surg. 2014;28:1485–1492. doi: 10.1016/j.avsg.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 23.Sang H, Li X, Qian A, Meng Q. Outcome of endovascular treatment in postthrombotic syndrome. Ann Vasc Surg. 2014;28:1493–1500. doi: 10.1016/j.avsg.2014.03.031 [DOI] [PubMed] [Google Scholar]
- 24.Sarici IS, Yanar F, Agcaoglu O, Ucar A, Poyanli A, Cakir S, Aksoy SM, Kurtoglu M. Our early experience with iliofemoral vein stenting in patients with post-thrombotic syndrome. Phlebology. 2014;29:298–303. doi: 10.1177/0268355513477641 [DOI] [PubMed] [Google Scholar]
- 25.Shi WY, Gu JP, Liu CJ, He X, Lou WS. Endovascular treatment for iliac vein compression syndrome with or without lower extremity deep vein thrombosis: a retrospective study on mid-term in-stent patency from a single center. Eur J Radiol. 2016;85:7–14. doi: 10.1016/j.ejrad.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 26.Xue GH, Huang XZ, Ye M, Liang W, Zhang H, Zhang JW, Zhang BG. Catheter-directed thrombolysis and stenting in the treatment of iliac vein compression syndrome with acute iliofemoral deep vein thrombosis: outcome and follow-up. Ann Vasc Surg. 2014;28:957–963. doi: 10.1016/j.avsg.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 27.Ye K, Lu X, Jiang M, Yang X, Li W, Huang Y, Huang X, Lu M. Technical details and clinical outcomes of transpopliteal venous stent placement for postthrombotic chronic total occlusion of the iliofemoral vein. J Vasc Interv Radiol. 2014;25:925–932. doi: 10.1016/j.jvir.2014.02.031 [DOI] [PubMed] [Google Scholar]
- 28.Ye K, Lu X, Li W, Huang Y, Huang X, Lu M, Jiang M. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol. 2012;23:497–502. doi: 10.1016/j.jvir.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 29.Yin M, Shi H, Ye K, Lu X, Li W, Huang X, Lu M, Jiang M. Clinical assessment of endovascular stenting compared with compression therapy alone in post-thrombotic patients with iliofemoral obstruction. Eur J Vasc Endovasc Surg. 2015;50:101–107. doi: 10.1016/j.ejvs.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Dumville JC, Ashby RL, Gabe R, Stubbs N, Adderley U, Kang’ombe AR, Cullum NA. Validation of the VEINES-QOL quality of life instrument in venous leg ulcers: repeatability and validity study embedded in a randomised clinical trial. BMC Cardiovasc Disord. 2015;15:85. doi: 10.1186/s12872-015-0080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn SR, Lamping DL, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, et al. ; VETO Study. Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049–1056. doi: 10.1016/j.jclinepi.2005.10.016 [DOI] [PubMed] [Google Scholar]
- 33.Lamping DL, Schroter S, Kurz X, Kahn SR, Abenhaim L. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg. 2003;37:410–419. doi: 10.1067/mva.2003.152 [DOI] [PubMed] [Google Scholar]
- 34.Strijkers RH, Wittens CH, Kahn SR. Villalta scale: goals and limitations. Phlebology. 2012;27(Suppl 1):130–135. doi: 10.1258/phleb.2011.012s02 [DOI] [PubMed] [Google Scholar]
- 35.Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, Meissner MH, Rutherford RB; American Venous Forum Ad Hoc Outcomes Working Group. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working group. J Vasc Surg. 2010;52:1387–1396. doi: 10.1016/j.jvs.2010.06.161 [DOI] [PubMed] [Google Scholar]
- 36.Lichtenberg M, Breuckmann F, Stahlhoff WF, Neglén P, Rick G. Placement of closed-cell designed venous stents in a mixed cohort of patients with chronic venous outflow obstructions - short-term safety, patency, and clinical outcomes. Vasa. 2018;47:475–481. doi: 10.1024/0301-1526/a000731 [DOI] [PubMed] [Google Scholar]
- 37.Lichtenberg M, de Graaf R, Stahlhoff WF, Özkapi A, Simon M, Breuckmann F. Patency rates, safety and clinical results of the sinus-Obliquus venous stent in the treatment of chronic ilio-femoral venous outflow obstruction - data from the Arnsberg venous registry. Vasa. 2019;48:270–275. doi: 10.1024/0301-1526/a000772 [DOI] [PubMed] [Google Scholar]
- 38.Razavi MK, Black S, Gagne P, Chiacchierini R, Nicolini P, Marston W; VIRTUS Investigators. Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv. 2019;12:e008268. doi: 10.1161/CIRCINTERVENTIONS.119.008268 [DOI] [PubMed] [Google Scholar]
- 39.Dake M, O'Sullivan G. A prospective, multicenter evaluation of the Venovo self-expanding vascular stent used to treat obstructive lesions of the iliac and femoral veins: 12-month results from the VERNACULAR trial. J Vasc Interv Radiol. 2019;30:3. [Google Scholar]
- 40.Guillen K, Falvo N, Nakai M, Chevallier O, Aho-Glélé S, Galland C, Demaistre E, Pescatori L, Samson M, Audia S, et al. Endovascular stenting for chronic femoro-iliac venous obstructive disease: clinical efficacy and short-term outcomes. Diagn Interv Imaging. 2020;101:15–23. doi: 10.1016/j.diii.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 41.Murphy EH. Surveying the 2019 venous stent landscape. Endovasc Today. 2019;18:53–64. https://evtoday.com/articles/2019-july/surveying-the-2019-venous-stent-landscape. [Google Scholar]
- 42.Moeri L, Lichtenberg M, Gnanapiragasam S, Barco S, Sebastian T. Braided or laser-cut self-expanding nitinol stents for the common femoral vein in patients with post-thrombotic syndrome. J Vasc Surg Venous Lymphat Disord. 2021;9:760–769. doi: 10.1016/j.jvsv.2020.08.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.