Abstract
Background:
Coronary microvascular dysfunction results in angina and adverse outcomes in patients with evidence of ischemia and nonobstructive coronary artery disease; however, no specific therapy exists. CD34+ cell therapy increases microvasculature in preclinical models and improves symptoms, exercise tolerance, and mortality in refractory angina patients with obstructive coronary artery disease. The objective of this research was to evaluate the safety, tolerability, and efficacy of intracoronary CD34+ cell therapy in patients with coronary microvascular dysfunction.
Methods:
We conducted a 2-center, 20-participant trial of autologous CD34+ cell therapy (protocol CLBS16-P01; NCT03508609) in patients with ischemia and nonobstructive coronary artery disease with persistent angina and coronary flow reserve ≤2.5. Efficacy measures included coronary flow reserve, angina frequency, Canadian Cardiovascular Society angina class, Seattle Angina Questionnaire, SF-36, and modified Bruce exercise treadmill test obtained at baseline and 6 months after treatment. Autologous CD34+ cells (CLBS16) were mobilized by administration of granulocyte-colony stimulating factor 5µg/kg/day for 5 days and collected by leukapheresis. Participants received a single intracoronary left anterior descending infusion of isolated CD34+ cells in medium that enhances cell function.
Results:
Coronary flow reserve improved from 2.08±0.32 at baseline to 2.68±0.79 at 6 months after treatment (P<0.005). Angina frequency decreased (P<0.004), Canadian Cardiovascular Society class improved (P<0.001), and quality of life improved as assessed by the Seattle Angina Questionnaire (P≤0.03, all scales) and SF-36 (P≤0.04, all scales). There were no cell-related serious adverse events.
Conclusions:
In this pilot clinical trial of microvascular angina, patients with ischemia and nonobstructive coronary artery disease receiving intracoronary infusion of CD34+ cell therapy had higher coronary flow reserve, less severe angina, and better quality of life at 6 months. The current study supports a potential therapeutic role for CD34+ cells in patients with microvascular angina.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03508609.
Keywords: cell therapy, exercise tolerance, ischemia, leukapheresis, microvascular angina
What Is Known
Coronary microvascular dysfunction is an ischemic disease that affects the small vessels of the heart and is associated with reduced coronary flow reserve
In patients with coronary microvascular dysfunction, coronary flow reserve is a strong and independent predictor of major adverse cardiovascular events
Autologous CD34+ cells have been shown to be proangiogenic in preclinical models and in human studies for patients with refractory angina
What the Study Adds
This is the first study in which patients with coronary microvascular dysfunction and documented coronary flow reserve<2.5 were treated with GCSF-mobilized autologous CD34+ cells
Treatment of patients with coronary microvascular dysfunction with autologous CD34+ cells led to an improvement in coronary flow reserve, quality of life, and angina
Patients with signs and symptoms of ischemia but nonobstructive coronary artery disease (INOCA) are increasingly recognized, with an estimated prevalence of 3 to 4 million and occurs more frequently in women.1,2 The original Women’s Ischemia Syndrome Evaluation cohort (NCT00832702) and other studies have documented a high prevalence (47%–64%) of coronary microvascular dysfunction (CMD) in patients with INOCA.3–6 Endothelial-independent CMD is identified by impaired hyperemic coronary flow reserve (CFR) in response to intracoronary adenosine on invasive coronary function testing.7 In symptomatic individuals with INOCA, the diagnosis of CMD is associated with increased risk of all-cause mortality, and major adverse cardiovascular events, including myocardial infarction, hospitalization for heart failure, and cardiac death compared with INOCA without evidence of CMD.5,8–16 Persistent angina is common in INOCA and associated with worse outcomes, however no specific therapy exists for these patients.
Cell therapy utilizing autologous CD34+ (auto-CD34+) cells is a promising therapy for patients with INOCA. Endothelial progenitor cells, identified and isolated using the CD34+ cell-surface marker, have potent angiogenic capabilities in vitro and in vivo.17,18 CD34+ cells induce capillary growth to regenerate damaged microcirculation in preclinical models. Three consecutive randomized double-blind controlled trials in patients with class 3-4 refractory angina from obstructive coronary artery disease in the United States established the feasibility and dose-response of intramyocardial delivered auto-CD34+ cells.19–21 A patient-level pooled analysis from these 3 randomized double-blinded trials showed that treatment with auto-CD34+ in these refractory angina patients resulted in statistically significant improvements in total exercise time, angina frequency, and a reduction in mortality.22 PreSERVE-AMI, a large clinical trial using CD34+ cell therapy for left ventricular dysfunction post-ST-segment elevation myocardial infarction demonstrated safety and potential efficacy for intracoronary infusion of CD34+ cell therapy in patients post–myocardial infarction.23
The primary objective of this study was to evaluate the initial safety, tolerability, and efficacy of intracoronary autologous CD34+ cells (CLBS16) in patients with INOCA with documented CMD and persistent angina. The primary outcome measure was change from baseline in CFR.
Methods
The data that support the findings of this study may be made available to noncommercial entities upon reasonable request. Requests for data may be sent to jwang@caladrius.com.
Study Population
The study population consisted of patients with a diagnosis of CMD documented by CFR≤2.5. Key inclusion and exclusion criteria included men and women 18 years of age and over, with effort-induced anginal symptoms and currently experiencing 3 or more episodes of angina each week, no obstructive coronary artery disease (defined by angiographic lesion stenosis ≤40% in all coronary arteries) on clinically indicated angiogram within 6 months before consent, and invasively confirmed endothelial-independent CMD (defined as CFR≤2.5 to intracoronary adenosine) at screening or within 6 months before screening. Participants needed to be able to give written informed consent and be on stable cardiovascular medical therapy for 30 days at time of consent, which included standard medical therapy (unless not effective or tolerated) at the treating physician’s discretion (statin, angiotensin converting enzyme inhibitor, beta blocker, calcium channel blocker, and/or ranolazine). Included participants were expected to remain on stable medications throughout the study duration. Participants with myocardial infarction within 90 days before consent, history of coronary artery bypass surgery, severe valvular heart disease, left ventricular ejection fraction <40%, advanced kidney disease (GFR <30 mL/minute per 1.73 m2), on chronic treatment with coumadin or anticipated need for coumadin therapy during the trial time frame, serious hypersensitivity or a history of adverse reactions to GCSF (granulocyte colony-stimulating factor) or apheresis, positive for HIV, hepatitis B or hepatitis C, malignant neoplasms within 5 years before consent, and pregnant or lactating at time of consent were excluded.
The study was conducted in accordance with the United States Code of Federal Regulations Title 21; the principles of Good Clinical Practice outlined in the International Council for Harmonisation’s Guideline for Good Clinical Practice E6(R2) dated November 9, 2016; and the Declaration of Helsinki (July 9, 2018). Institutional review boards of both clinical sites, Cedars-Sinai Medical Center, Los Angeles, CA and Mayo Clinic, Rochester, MN, approved the study, and all participants provided informed consent.
Coronary Function Testing
Participants withheld coronary vasoreactivity medications for at least 48 hours before coronary angiography and coronary functional testing (CFT). These medications included calcium channel blockers, beta blockers, long acting nitrates, and phosphodiesterase inhibitors. Short-acting sublingual nitroglycerin for chest pain was allowed >6 hours prior procedure. Arterial access was obtained through the right or left femoral artery. After diagnostic angiography and exclusion of patients with obstructive coronary artery disease, endothelium-dependent and -independent coronary functions were assessed as previously described.8 In brief, a Doppler guidewire (Flowire, Volcano Therapeutics Inc, Rancho Cordova, CA) was positioned into the proximal to mid-left anterior descending (LAD) coronary artery. Incremental intracoronary bolus injections of adenosine (18–72 µg) were administered until maximal hyperemia was achieved. Coronary endothelium-independent microvascular function was assessed by CFR, calculated as hyperemic flow velocity/baseline flow velocity. Subsequently, coronary epicardial and microvascular endothelial function were assessed by selective intracoronary infusion of escalating doses of acetylcholine (ACH) (10−6, 10−5, and 10−4 mol/L for 3 minutes at each concentration) into the LAD for total ACH doses of 0.364 ug, 36.4 ug, and 108 ug. Hemodynamic data, doppler measurements, and coronary angiograms were obtained after each infusion. In response to ACH, coronary artery diameter was measured by an independent investigator in the segment 5 mm distal to the tip of the Doppler wire offline using a quantitative coronary angiography program (Medis Corp, Leiden, the Netherlands), and blood flow was calculated from the Doppler derived time velocity integral and vessel diameter, where coronary blood flow (CBF)=π x (coronary artery diameter/2)2 x (average peak velocity/2).14,24–26 Patients with baseline endothelial-independent CMD (CFR≤2.5) were considered for enrollment irrespective of coronary endothelial function testing. CFT was repeated at 6 months post-CD34+ cell infusion for evaluation of CFR.
CD34+ Cell Mobilization, Isolation, Preparation, and Intracoronary Infusion
Following screening procedures and verification of patient eligibility, participants were placed on a dual-antiplatelet regimen and mobilization of CD34+ cells from the bone marrow was initiated with 5 daily injections of GCSF (filgrastim) at a dose of 5 µg/kg per day. On the fifth day of GCSF, participants underwent apheresis to isolate an enriched fraction of mononuclear cells.27 The mononuclear cells collected by apheresis were shipped overnight to a central manufacturing facility where, on the next day, CD34+ cells were isolated with a magnetic selection process (Miltenyi Biotec, Bergisch Gladbach, Germany), suspended in a proprietary formulation, and shipped overnight back to the originating clinical site.27 On the day after manufacturing, arterial access was obtained either through radial or femoral arteries in the catheterization laboratory, and intracoronary infusion of CD34+ cells preparation (CLBS16) was administered (10 mL of investigational product over 10 minute) in the proximal or mid LAD using an over the wire balloon catheter without balloon inflation (Abbott Trek balloon). Each participant received the maximum manufactured dose, up to a limit of 300×106 CD34+ cells. The safety of this dose was previously established in studies of intracoronary administration of CD34+ cells in subjects with refractory angina and nonischemic dilated cardiomyopathy.27–31 The dose received by each subject was influenced by the number of cells mobilized into the peripheral blood by GCSF and the number of cells harvested during the apheresis procedure. Each patient was dosed with as many cells as available. The average dose administered was 111±63×106 cells, and the minimum and maximum doses were 12 and 293×106 cells, respectively.
Follow-Up and Efficacy Measures
As described above, CFT was performed at baseline and 6 months post-CLBS16 treatment. Daily angina frequency and nitroglycerin use were assessed by paper diary at baseline, 3 months and 6 months after treatment. Canadian Cardiovascular Society angina class was assessed at baseline and 6 months after treatment according to the criteria originally proposed by Campeau.32 Total exercise time (TET) was assessed from an exercise tolerance test (ETT) conducted according to the modified Bruce protocol at baseline and 6 months after treatment.33 Time to angina, time to ST depression, peak metabolic equivalents (METs), and proportion of participants experiencing angina as a major symptom or as a reason for stopping were also assessed from the ETT data. Health-related quality of life was assessed at baseline, 3 months and 6 months after treatment by the Seattle Angina Questionnaire34 and the SF-36 V2.35
Safety Measures
Patients were monitored for 6 hours after CD34+ cell infusion, with vital signs recorded every 2 hours and 12-lead electrocardiography performed at 2 and 6 hours. Vitals signs and 12-lead electrocardiography were assessed on the day after cell infusion procedure. Adverse events were collected at each study visit. Hematology and clinical chemistry laboratory investigations were conducted at baseline, 1 day after treatment, and 1 month and 6 months after treatment. A lipid panel was assessed at baseline and 6 months after treatment. Electrocardiograms were collected at baseline, during treatment, and 1 day and 6 months after treatment. Physical examinations were conducted at baseline and 1 day and 6 months after treatment. Vital signs were assessed at each participant encounter.
Statistical Methods
The power estimate was modeled based on 2 prior studies, one which examined the effect of spironolactone on CFR in patients with diabetes and reported a change in CFR of 0.33±0.83 in the treated group36 and the second was the study of Quinapril in Women With Chest Pain, Coronary Flow Reserve Limitations and Evidence of Myocardial Ischemia (QWISE), which reported a change in CFR of 0.55±0.56 among treated patients with low baseline CFR.37 With a SD of 0.56, a study with 20 patients was predicted to have 80% power to detect a change in CFR of 0.37 or greater after treatment using a 2-sided paired t test with a type I error rate of 0.05. Assuming a larger SD of 0.83, 20 patients would have 80% power to detect a change in CFR of 0.55 or greater after treatment using the same kind of test and type I error rate.
For the safety analysis, all data from patients who received GCSF or had undergone apheresis or CFT or received a dose of CLBS16 were evaluated.
For the efficacy analysis, data from participants who received treatment with CLBS16 were evaluated. Continuous variables were analyzed using paired t test using change from baseline data. The change from baseline and confidence intervals were estimated. If the assumption of normal distribution was not warranted, Wilcoxon rank-sum test was conducted. For paired nominal data (proportions), McNemar test was used.
Results
The first participant was consented on April 19, 2018 and the last participant on May 15, 2019. Twenty-eight participants signed a consent form and 20 met inclusion and exclusion criteria, completed screening, and received CLBS16. One participant withdrew for personal reasons before the month 6 assessments. Baseline demographic information is shown in the Table.
Table 1.
Baseline Demographics
Safety Results
There were no cell-related adverse events. No participant had a spontaneous myocardial infarction, stroke, or died. One participant had a focal dissection during the CLBS16 infusion procedure requiring stent placement with elevation in troponin and subsequently withdrew from the trial. The cause of the dissection was felt by the investigator (A.L.) to be related to the distal tip of the balloon infusion catheter during an injection while the wire was removed.
Efficacy Results
Peak CFR to adenosine increased from 2.08±0.32 at baseline to 2.68±0.79 at 6 months after treatment (P=0.005; Figure 1). Fifteen of the 19 participants experienced an increase in CFR (78.9%), while 4/19 (21.1%) experienced a decrease. The percent change in CBF to ACH was 43.64±64.84 at baseline and 64.08±68.82 at 6 months (P=0.74). The percent change in coronary artery diameter to ACH was −11.35±27.9 at baseline and −3.83±11.41 at 6 months (P=0.44). The percent change in coronary artery diameter to nitroglycerin was 13.2±13.8 at baseline and 10.6±20.3 at 6 months (P=0.86).
Figure 1.
Coronary flow reserve at baseline and 6 mo post-CLBS16 treatment. Left, individual paired values of all subjects at baseline and 6 mo. Right, individual values showing change from baseline to 6 mo; horizontal black line indicates the mean change.
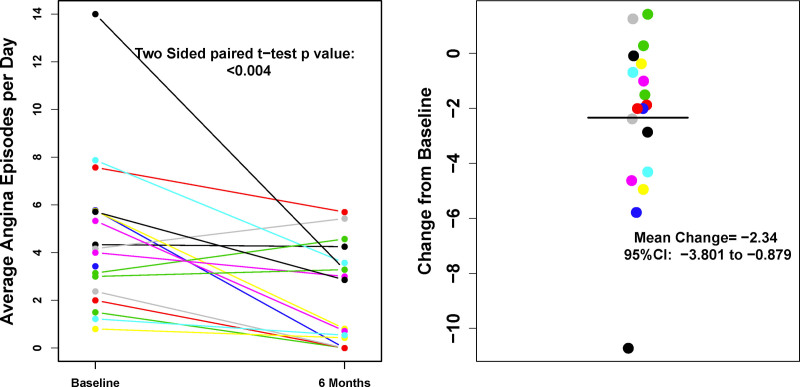
Angina frequency decreased from an average of 4.42±3.10 episodes/day at baseline to 2.41±2.23 at 3 months and 2.02±2.06 at 6 months after treatment (P=0.004; Figure 2).
Figure 2.
Angina frequency at baseline and 6 mo post-CLBS16 treatment. Left, individual paired values of all subjects at baseline and 6 mo. Right, individual values showing change from baseline to 6 mo; horizontal black line indicates the mean change.
Canadian Cardiovascular Society class improved from an average of 3.20±0.95 at baseline to 2.05±1.18 at 6 months after treatment (P<0.001; Figure 3).
Figure 3.
Canadian Cardiovascular Society (CCS) angina class changes from baseline to 6 mo post-CLBS16 treatment. Left panel is a stacked bar chart showing the number of subjects categorized as having CCS angina classes I, II, III, and IV. Right panel shows individual changes in CCS angina class from baseline to month 6.
Seattle Angina Questionnaire results showed improvements from baseline to 6 months in all scales (P≤0.03; Figure 4). The average physical component score of the SF-36 improved from 29.71±7.44 at baseline to 36.03±11.11 at 6 months (P=0.001) and reflected improvements in each of the sub-scores. Similarly, the average mental component scores improved from 47.60±10.35 at baseline to 53.85±9.41 at 6 months (P=0.01) and reflected improvements in each of the sub-scores.
Figure 4.
Seattle Angina Questionnaire (SAQ) changes from baseline to 6 mo post-CLBS16 treatment.
Total exercise time was 624±217 seconds at baseline and 685±194 seconds at 6 months after treatment (P=0.41). The time to angina analysis had 7 participants experiencing angina during the baseline test with an average of 315±243 seconds in contrast to 3 patients during the test at 6 months after treatment with an average time 404±39 seconds. The proportion of participants who reported angina as a primary symptom or a reason for stopping decreased from 68.4% at baseline to 27.8% at 6 months after treatment (P=0.005). As an ad hoc analysis, the peak METs achieved by patients during exercise testing was 7.6±3.5 at baseline and 8.8±3.4 at 6 months (P=0.14).
The use of sublingual nitroglycerin in tablets/day was 0.99±1.22 at baseline, 0.51±0.78 at 3 months (P=0.06) and 0.87±1.56 at 6 months (P=0.69).
Discussion
This first trial of autologous CD34+ cell therapy in INOCA participants with endothelial-independent CMD and persistent angina demonstrates that a single infusion of CLBS16 improved CFR at 6 months. There were no serious cell-related adverse events demonstrating excellent safety and tolerability of CLBS16 infusion. In addition, we found improvements in angina frequency, Canadian Cardiovascular Society angina class, and all Seattle Angina Questionnaire and SF-36 domains including quality of life. Our findings support further exploration of CD34+ a cell therapy as a strategy in INOCA patients with CMD and persistent angina.
This trial establishes the feasibility and safety of intracoronary administration of CD34+ cell therapy in patients with INOCA with CMD and persistent angina. Our findings are consistent with prior trials demonstrating safety and efficacy of CD34+ cell therapy in patients with refractory angina, acute myocardial infarction, and heart failure.19–23,27–31 This study extends the prior findings from pooled analysis of trials in patients with obstructive coronary artery disease and refractory angina to patients with INOCA with CMD and persistent angina.22 Thus, a prospective randomized study should be able to establish a potential therapeutic role for CD34+ cells in patients with microvascular angina.
CMD is characterized by coronary microvascular endothelial or nonendothelial dysfunction that limits myocardial perfusion. CD34+ cells can differentiate into endothelial cells that migrate to areas of ischemic injury and preserve or restore microcirculatory integrity.27 CD34+ cells incorporate into the sites of active angiogenesis and stimulate neovascularization and vascular repair at the site of the ischemic tissue.17,38,39 Data from animal models indicate that CD34+ cells promote vascular repair and enhance angiogenesis in the microvasculature, which restores the microcirculation and improves myocardial tissue perfusion in tissues damaged by acute and chronic ischemia.38
Improvement in microvascular function as measured by CFR after CD34+ cell therapy leads us to hypothesize that CD34+ cells repair the microvasculature in patients with CMD similarly to ischemic tissue. These results provided the support for the ongoing double-blind placebo-controlled FREEDOM trial (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease; NCT0414467).
Our study has strengths and limitations. Invasive CFT, a rigorous method for diagnosis of CMD, was used to measure CFR at both initial visit and follow-up. A placebo effect is possible given the absence of a control group,40 but the improvement in CFR tracked with improvement in angina frequency, all Seattle Angina Questionnaire domains, quality of life scores, and Canadian Cardiovascular Society angina class. Given the small number of patients, we were not powered to detect differences in outcomes in this feasibility trial. The current study did not demonstrate any improvement in coronary endothelial function. The reason for this lack of effect may be secondary to the preexisting exclusion criteria that did not require abnormal endothelial function for entry into the study. It is important to note there is natural variation in the quantity of CD34+ cells delivered because of difference in each patient’s response to mobilization and CD34+ cell count, but there was no evidence for a dose-response curve. We will continue to evaluate dose-response in larger ongoing trials.
Conclusions
The evidence generated in this clinical trial indicates that patients with INOCA receiving intracoronary infusion of CD34+ cell therapy had higher CFR, less severe angina, and better quality of life at 6 months. The improvement in CFR is consistent with the mechanism of action of CD34+ cells, that is, improvement in the microcirculation shown in preclinical trials. Further investigation beyond this proof of concept trial in humans is ongoing with the randomized placebo-controlled FREEDOM trial.
Article Information
Sources of Funding
This work was supported by Caladrius Biosciences, Inc. and by the National Heart, Lung, and Blood Institute at the NIH under award numbers R44HL135889 and K23HL151867 (OQ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
Dr Lerman serves as a consultant to Phillps/Volcano and Itamar Medical. Dr Bairey Merz reports consulting for Abbott, Sanofi and serves on the board of iRhthym. Dr Henry serves as a consultant to Caladrius Biosciences, Inc. Dr Sietsema, Dr Wang, Dr Takagi, Dr Lee, Dr Losordo, Ms. Kotynski, Ms. Lewis, Ms. Schumacher, Ms. Bartel, and Mr Shah all reports grants from NIH- National Heart, Lung, and Blood institute during the conduct of the study; other from Caladrius Biosciences, Inc., outside the submitted work. Dr Corban, Dr Quesada, Dr Lerman, Dr Wei and Ms. Juong have nothing to disclose.
Nonstandard Abbreviations and Acronyms
- CCS
- Canadian Cardiovascular Society
- CFR
- coronary flow reserve
- CFT
- coronary functional testing
- CMD
- coronary microvascular dysfunction
- GCSF
- granulocyte colony-stimulating factor
- INOCA
- ischemia and nonobstructive coronary artery disease
- LAD
- left anterior descending
- MACE
- major adverse cardiovascular event
For Sources of Funding and Disclosures, see page 136.
Contributor Information
C. Noel Bairey Merz, Email: noel.baireymerz@cshs.org.
Janet Wei, Email: janet.wei@cshs.org.
Michel T. Corban, Email: mtcorban@arizona.edu.
Odayme Quesada, Email: odayme.quesada@gmail.com.
Sandy Joung, Email: joung.sandy@gmail.com.
Christine L. Kotynski, Email: ckotynski@caladrius.com.
Jian Wang, Email: jwang@caladrius.com.
Michelle Lewis, Email: michelewis@gmail.com.
Ann M. Schumacher, Email: aschumacher@caladrius.com.
Ronnda L. Bartel, Email: rbartel@caladrius.com.
Hiroshi Takagi, Email: htakagi@caladrius.com.
Vishal Shah, Email: Shahvishal85@gmail.com.
Anna Lee, Email: anna.newyork@gmail.com.
William K. Sietsema, Email: bsietsema@caladrius.com.
Douglas W. Losordo, Email: dlosordo@gmail.com.
Amir Lerman, Email: lerman.amir@mayo.edu.
References
- 1.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, et al. ; American College of Cardiology-National Cardiovascular Data Registry Investigators. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562 [DOI] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ; WISE Investigators. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198 [DOI] [PubMed] [Google Scholar]
- 4.Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–1475. doi: 10.1016/s0735-1097(99)00072-8 [DOI] [PubMed] [Google Scholar]
- 5.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. doi: 10.1016/j.jcin.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 7.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889 [DOI] [PubMed] [Google Scholar]
- 8.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. doi: 10.1016/j.jacc.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Wei J, AlBadri A, Zarrini P, Bairey Merz CN. Coronary microvascular dysfunction - epidemiology, pathogenesis, prognosis, diagnosis, risk factors and therapy. Circ J. 2016;81:3–11. doi: 10.1253/circj.CJ-16-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukushima K, Javadi MS, Higuchi T, Lautamäki R, Merrill J, Nekolla SG, Bengel FM. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52:726–732. doi: 10.2967/jnumed.110.081828 [DOI] [PubMed] [Google Scholar]
- 11.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. doi: 10.1001/jama.2014.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948 [DOI] [PubMed] [Google Scholar]
- 15.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc. 2020;9:e014954. doi: 10.1161/JAHA.119.014954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518 [DOI] [PubMed] [Google Scholar]
- 19.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, et al. ; ACT34-CMI Investigators. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376 [DOI] [PubMed] [Google Scholar]
- 21.Povsic TJ, Henry TD, Traverse JH, Fortuin FD, Schaer GL, Kereiakes DJ, Schatz RA, Zeiher AM, White CJ, Stewart DJ, et al. ; RENEW Investigators. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34(+) Cell Administration in Patients With Refractory Angina. JACC Cardiovasc Interv. 2016;9:1576–1585. doi: 10.1016/j.jcin.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 22.Henry TD, Losordo DW, Traverse JH, Schatz RA, Jolicoeur EM, Schaer GL, Clare R, Chiswell K, White CJ, Fortuin FD, et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur Heart J. 2018;39:2208–2216. doi: 10.1093/eurheartj/ehx764 [DOI] [PubMed] [Google Scholar]
- 23.Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, Frohwein S, Henry TD, Schatz RA, Dib N, et al. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ Res. 2017;120:324–331. doi: 10.1161/CIRCRESAHA.115.308165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE [DOI] [PubMed] [Google Scholar]
- 25.Corban MT, Godo S, Burczak DR, Noseworthy PA, Toya T, Lewis BR, Lerman LO, Gulati R, Lerman A. Coronary endothelial dysfunction is associated with increased risk of incident atrial fibrillation. J Am Heart Assoc. 2020;9:e014850. doi: 10.1161/JAHA.119.014850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0 [DOI] [PubMed] [Google Scholar]
- 27.Sietsema WK, Kawamoto A, Takagi H, Losordo DW. Autologous CD34+ cell therapy for ischemic tissue repair. Circ J. 2019;83:1422–1430. doi: 10.1253/circj.CJ-19-0240 [DOI] [PubMed] [Google Scholar]
- 28.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519 [DOI] [PubMed] [Google Scholar]
- 29.Lezaic L, Socan A, Poglajen G, Peitl PK, Sever M, Cukjati M, Cernelc P, Wu JC, Haddad F, Vrtovec B. Intracoronary transplantation of CD34(+) cells is associated with improved myocardial perfusion in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2015;21:145–152. doi: 10.1016/j.cardfail.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Cui J, Peng W, Lu M. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology. 2010;117:140–147. doi: 10.1159/000320217 [DOI] [PubMed] [Google Scholar]
- 31.Prasad M, Corban MT, Henry TD, Dietz AB, Lerman LO, Lerman A. Promise of autologous CD34+ stem/progenitor cell therapy for treatment of cardiovascular disease. Cardiovasc Res. 2020;116:1424–1433. doi: 10.1093/cvr/cvaa027 [DOI] [PubMed] [Google Scholar]
- 32.Campeau L. Letter: Grading of angina pectoris. Circulation. 1976;54:522–523. [PubMed] [Google Scholar]
- 33.McInnis KJ, Balady GJ. Comparison of submaximal exercise responses using the Bruce vs modified Bruce protocols. Med Sci Sports Exerc. 1994;26:103–107. [PubMed] [Google Scholar]
- 34.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9 [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson C, Stewart-Brown S, Petersen S, Paice C. Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health. 1999;53:46–50. doi: 10.1136/jech.53.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, Jerosch-Herold M, Kwong RY, Di Carli MF, Adler GK. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64:236–242. doi: 10.2337/db14-0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–684. doi: 10.1016/j.ahj.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- 39.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 40.Jolicoeur EM, Henry TD. The cost of angina: how do we measure it? How do we improve it? Eur Heart J Qual Care Clin Outcomes. 2020;6:5–6. doi: 10.1093/ehjqcco/qcz058 [DOI] [PubMed] [Google Scholar]