Abstract
This study was conducted to assess the relationship of vitamin D deficiency (VDD) with various demographic characteristics, laboratory parameters, and predictors of mortality. This prospective observational study was performed at pediatric intensive care unit (PICU) of a tertiary care hospital situated in north India. A total of 125 children admitted in PICU with age from 2 months to 14 years were analyzed. The subjects were classified as Vitamin D deficient (≤20 ng/mL) and nondeficient (>20 ng/mL). The relationship between VDD and predictors of mortality were analyzed using correlation and multivariate analysis. Respiratory system (40%) was most commonly involved. VDD was seen in 72% of the patients. There was statistically significant correlation of VDD with age ( p = 0.019), season ( p = 0.018), height ( p = 0.005), and weight ( p = 0.003). On multivariate analysis factors associated with VDD were age (odds ratio [OR] = 1.01, 95% confidence interval [CI] 1.00–1.03, p = 0.006), season (OR = 3.98, 95% CI 1.09–14.50, p = 0.036). VDD was also correlated to bacteriuria ( p = 0.033), cardiovascular sequential sepsis-related organ failure assessment score (CV-SOFA score) ( p = 0.001), and mechanical ventilation ( p = 0.043). On multivariate analysis, factors associated with VDD were bacteriuria (OR = 4.88, 95% CI 1.04–22.89, p = 0.04), mechanical ventilation requirement (OR = 2.95, 95% CI 1.12–7.85, p = 0.029), and CV-SOFA score (OR = 2.33, 95% CI 1.14–4.76, p = 0.021). Median (interquartile range) duration of PICU stay in VDD patients was (3–7) days while in nondeficient patients it was (2–6) days ( p = 0.107). VDD was a significant risk factor for the need of mechanical ventilation, bacteriuria, and mortality among patients in our cohort.
Keywords: vitamin D deficiency, PRISM III score, CV-SOFA score, mechanical ventilation, mortality, bacteriuria
Introduction
Critical illness in children is a major cause of significant health care utilization and mortality around the world. The concern is more so in developing countries with limited resources. Nutritional status of the child is an important determinant of his/her health. 1
Various nutritional deficiencies make the child susceptible to a variety of illnesses which can be determined by several biochemical parameters. Identification of previously unrecognized, modifiable risk factors can help to guide new preventive or therapeutic strategies for pediatric critical illness. One of these parameters is vitamin D. 1
Vitamin D which was thought to have a role in bone health and calcium homeostasis only, is now known to be involved in many extraskeletal pathways. 2 3 4 5 6
Vitamin D also has potential benefits on innate immunity and potentiates antimicrobial action through a variety of mechanisms. Vitamin D also plays a significant role in proliferation, maturation, and death of cells. 7
Vitamin D deficiency (VDD) prevails in epidemic proportions all over the Indian subcontinent, with a prevalence of 70 to 100% in the general population despite abundant sunshine. 8 9 Most studies adopt the definition of vitamin D insufficiency as 25 hydroxy vitamin D [25(OH)D] concentrations below 30 ng/mL, and VDD as concentrations below 20 ng/mL. 10 11 Patients with poor nutrition or chronic illness are potentially at higher risk for both greater illness severity and VDD. For example, preexisting illness could lead to reduced vitamin D status through abnormal diets, altered metabolism, or reduced environmental ultraviolet radiation exposure. 12 Higher weight for age ratio is also found to be associated with lower 25 (OH)D concentration. 13 14
Data on the prevalence of VDD in critically ill children and its association with severity of illness is scarce and is derived mostly from research in the developed countries. However, information is lacking from resource limited settings, especially India. It is lacking from developing countries, especially India. We conducted this study to evaluate the prevalence of VDD in critically ill children and to assess its association with severity of illness (PRISM III score and CV-SOFA score) and other outcomes associated with critical illness in PICU.
Materials and Methods
The present study was conducted on patients admitted in pediatric intensive care unit (PICU) at Guru Gobind Singh Medical College, Faridkot.
Sample Size
Sample size was calculated using the formula:
X = Z α /2 2 × p × (1 − p )/ e 2
where,
Z α/2 is 1.96 (95% confidence interval), e is the margin of error (5%), p is the sample proportion (0.69), and N is the population size (200).
n = N × X /[ X + ( N − 1)], (finite population correction)
finite population was taken as 200 considering the previous year admission rate in the PICU.
Using the formula above, the derived sample size was 125. So, a sample size of 125 was taken for the purpose of this study.
Inclusion Criteria
All children admitted to PICU with medical conditions.
Age: 2 months to 14 years.
PICU stay >24 hours.
Consent given by parents/guardians.
Exclusion Criteria for Patients Admitted in PICU
Chronic renal disease.
Chronic liver disease.
Malabsorption syndrome.
Parathyroid disorders.
History of intake of vitamin D/calcium within last 3 months.
History of intake of steroids for >10 days within last 1 month.
Treatment with antitubercular or antiepileptic drugs within last 3 months.
Death within 24 hours of admission.
Discharged/shifted from the PICU within 24 hours of admission.
Lack of consent.
Refusal to consent for participation in study
Various investigations which were performed were complete blood count, platelet count, prothrombin time, renal function tests, liver function tests, random blood sugar, serum albumin, serum calcium and phosphorus, serum electrolytes (sodium, potassium), arterial blood gas, blood culture, urine culture, any other culture, 25(OH)D levels, and any other investigation relevant to the disease process.
Being a tertiary care center, we mostly receive referred patients who are sick with a prior history of hospital stay elsewhere. To rule out systemic infections, cultures which can help in identifying the causative organism are taken.
The midstream clean catch method was used to collect urine samples at admission from toilet trained children and urinary catheters for infants, young children, and sick catheterized patients. Bacteriuria (positive urine culture) was labeled if there was more than 10 5 CFU/mL of a single pathogen in a midstream urine sample or 10 4 CFU/mL of a single pathogen via urinary catheterization.
Study Design
This was a hospital-based prospective observational study. Proper history of all children admitted in PICU was taken and thorough clinical examination of the child was done. Demographic variables were recorded using structured proforma. Socioeconomic status of the family was recorded using modified Kuppuswamy scale. 15
Anthropometric parameters (weight, height, Body mass index (BMI)) of the child were recorded. Vitals (blood pressure, heart rate, respiratory rate (RR), and temperature), mental status (Glasgow coma scale), and pupillary response were documented. Relevant and routine investigations were sent.
Severity of illness was assessed using pediatric risk of mortality (PRISM III) score within 12 hours of admission. PRISM III has 17 physiological variables subdivided into 26 ranges. It includes four subscores, i.e., cardiovascular and neurological, acid base and blood gas, chemistry, and hematology. Maximum total PRISM III score is 74. The higher the total score, the worse the prognosis. A rising score indicates deterioration. Presence of hypotension, use of fluid boluses and inotropes, respiratory failure (ventilator support), organ dysfunction, and use of blood products was noted. Cardiovascular sequential sepsis-related organ failure assessment score [CV-SOFA score] was calculated based on type and dose of inotrope used. 16 17
Children were managed according to standard guidelines for given disease. Samples for vitamin D levels were taken soon after admission. Five milliliters of blood was taken from a peripheral vein as soon as patient was admitted. 25(OH)D was measured using chemiluminescent immunoassay method. Patients were divided into two groups based on vitamin D levels:
Vitamin D nondeficient: >20 ng/mL
Vitamin D deficient: ≤20 ng/mL
The final outcome of the patient, i.e., death or discharged was recorded. The length of stay in PICU and total stay in hospital were noted.
Ethical Clearance
The protocol was submitted to ethical and thesis committee of college and approval was received duly.
Statistical Analysis
The data were entered in the form of a data matrix in Microsoft Excel and was analyzed using IBM SPSS v. 20.0.0. The descriptive statistics for categorical variables were represented in the form of frequencies and percentages and as means and standard deviations for continuous variables. The association between categorical variables was explored using Pearson's chi-square test/Fisher's exact test. The comparison of normally distributed continuous variables across groups was done using independent samples t -test while for non-normally distributed data, independent samples Mann-Whitney U test was used. Correlation among the continuous variables was plotted using scatter plot and was assessed using Pearson's product moment correlation/Spearman's rank correlation as appropriate. A p -value of <0.05 was considered statistically significant for the purpose of this study. Correlation coefficient ( r ) was classified into following categories:
<0.2 weak correlation.
0.2 to 0.4 moderate correlation.
0.4 strong correlation.
Multivariate regression analysis was done for patient characteristics associated with VDD in univariate analysis ( p < 0.05) to assess the degree to which dependent variables were related to VDD.
Association of subdivisions of PRISMIII score at admission and the four-level CV-SOFA score during PICU stay and VDD was also assessed using multinomial logistic regression.
Results
During the period of study 250 children were admitted in PICU from October 2017 to September 2018, out of which 125 were excluded and finally 125 were enrolled for the analysis. Maximum number of patients (40%) were admitted with respiratory system involvement. There were 90 patients (72%) who were deficient in vitamin D with mean vitamin D levels of 14.62 ± 3.74 ng/ml while patients who were nondeficient (28%) had mean vitamin D levels of 27.68 ± 8.06 ng/ml. Majority of the patients (57.6%) were females with male: female ratio being 1:1.3. The median (interquartile range) duration of PICU stay in vitamin D deficient patients was 5.0(3–7) days and in nondeficient patients was 3.0(2–6) days.
Table 1 depicts the correlation of VDD with various demographic parameters. Significant moderate correlation was observed between VDD and increasing age, weight, height of the patients, and summer season.
Table 1. Correlation of vitamin D insufficiency with various demographic parameters.
| Characteristic | Number | 25(OH)D level | Test of significance | p -Value | |||
|---|---|---|---|---|---|---|---|
| N (90) | (ng/ml) % | N (35) | % | ||||
| <20 | >20 | ||||||
| Age group (mo) 2–12 |
42 | 28 | 31.10 | 14 | 40.00 | 51.46 b | 0.05 * |
| 13–48 | 34 | 19 | 21.10 | 15 | 42.85 | ||
| 49–144 | 40 | 35 | 38.90 | 5 | 14.30 | ||
| 145–168 | 9 | 8 | 8.90 | 1 | 2.85 | ||
| Gender Male |
53 | 40 | 44.40 | 13 | 37.10 | 0.255 b | 0.382 |
| Female | 72 | 50 | 55.60 | 22 | 62.90 | ||
| Season Winter |
93 | 62 | 66.70 | 31 | 33.30 | 5.57 b | 0.013 * |
| Summer | 32 | 28 | 87.50 | 4 | 12.50 | ||
| Weight | 125 | 90 | 72 | 35 | 28 | 0.260 a | 0.003 * |
| Height | 125 | 90 | 72 | 35 | 28 | 0.252 a | 0.005 * |
| BMI | 125 | 90 | 72 | 35 | 28 | 0.078 a | 0.386 |
Abbreviation: BMI, body mass index.
Denotes Spearman correlation coefficient.
Denotes Chi-square test.
Note: * p < 0.05 is significant.
Table 2 depicts the correlation of VDD with various clinical parameters and weak correlation was observed between VDD and positive urine culture (bacteriuria) and need of mechanical ventilation.
Table 2. Correlation of vitamin D insufficiency with various clinical parameters.
| Characteristic | Number | 25(OH)D level | Test of significance | p -Value | |||
|---|---|---|---|---|---|---|---|
| N (90) | (ng/ml) % | N (35) | % | ||||
| <20 | >20 | ||||||
| S. Calcium (mg/dL) <8 | 52 | 37 | 41.10 | 15 | 42.90 | 28.82 | 0.527 |
| >8 | 73 | 53 | 58.90 | 20 | 57.10 | ||
| Mechanical ventilation (Y) | 41 | 35 | 38.90 | 6 | 17.10 | 4.092 | 0.033 * |
| (N) | 84 | 55 | 61.10 | 29 | 82.90 | ||
| Fluid bolus (Y) | 77 | 58 | 64.40 | 19 | 54.20 | 0.781 | 0.247 |
| (N) | 48 | 32 | 35.60 | 16 | 45.80 | ||
| Inotrope use (Y) | 100 | 74 | 82.20 | 26 | 74.30 | 0.79 | 0.257 |
| (N) | 25 | 16 | 17.80 | 09 | 25.70 | ||
| Blood components use (Y) | 44 | 34 | 37.80 | 10 | 28.60 | 1.647 | 0.439 |
| (N) | 81 | 56 | 62.20 | 25 | 71.40 | ||
| Blood culture positivity (Y) | 28 | 21 | 23.30 | 7 | 20.00 | 0.254 | 0.402 |
| (N) | 97 | 69 | 76.70 | 28 | 80.00 | ||
| Bacteriuria (urine culture positivity) (Y) | 21 | 19 | 21.11 | 2 | 5.71 | 4.57 | 0.024 * |
| (N) | 104 | ||||||
| PICU length of stay (d) >1 | 31 | 21 | 67.7 | 10 | 32.3 | 10.185 | 0.679 |
| 3 | 19 | 11 | 57.9 | 8 | 42.10 | ||
| 4+ | 75 | 57 | 76 | 18 | 24 | ||
Abbreviation: PICU, pediatric intensive care unit.
Denotes Spearman correlation coefficient.
Note: * p < 0.05 is significant.
No significant correlation was observed between VDD and gender, BMI, need of inotropes, blood component administration, fluid bolus, positive blood culture, serum calcium levels, C-reactive protein levels, and duration of stay in PICU.
No significant relationship of VDD was observed with various laboratory parameters which are part of PRISM III score.
Table 3 shows the correlation of VDD with various predictors of mortality at the time of admission. Significant correlation was observed between VDD and different quartiles of CV-SOFA score while no statistical significant correlation was found between VDD and PRISM score and VDD and outcome.
Table 3. Correlation of vitamin D insufficiency and various mortality predictors.
| Characteristic | Number | 25(OH)D level | Test of significance | p -Value | |||
|---|---|---|---|---|---|---|---|
| n | (ng/ml) % | n | % | ||||
| <20 | >20 | ||||||
| PRISM score 0–9 |
8 | 4 | 4.50 | 4 | 11.40 | 5.03 a | 0.081 b |
| 10–19 | 93 | 65 | 72.20 | 28 | 80.00 | ||
| ≥ 20 | 24 | 21 | 23.30 | 3 | 8.60 | ||
| CV-SOFA score 0–4 |
9 | 5 | 5.60 | 4 | 11.40 | 7.68 a | 0.05 * b |
| 5–9 | 74 | 49 | 54.40 | 25 | 71.40 | ||
| 10–14 | 41 | 35 | 38.90 | 6 | 17.20 | ||
| ≥ 15 | 1 | 1.10 | 0 | ||||
| Outcome nonsurvivors |
35 | 28 | 80 | 7 | 20 | 0.27 c | 0.214 d |
| Survivors | 90 | 62 | 68.90 | 28 | 31.10 | ||
Abbreviation: CV-SOFA, cardiovascular sequential sepsis-related organ failure assessment.
p value < 0.05 is significant.
Denotes Pearson Chi-square.
Denotes p -Value for Pearson Chi-square.
Denotes Fischer's exact test.
Denotes * p -Value for Fischer's exact test.
Multivariate regression analysis ( Table 4 ) was used to assess the degree to which various parameters were affected by VDD. Increase in age (odds ratio [OR] 1.017, 95% confidence interval [CI] 1.00–1.03, p -value 0.006), summer season (OR 3.981, 95% CI 1.09–14.50, p -value 0.036), bacteriuria (OR 4.881, 95% CI 1.04–22.89, p -value 0.044), requirement of mechanical ventilation (OR 2.958, 95% CI 1.12–7.85, p -value 0.029), and worsening CV-SOFA score (OR 2.331, 95% CI 1.14–4.76, p -value 0.021) were the parameters significantly influenced by VDD.
Table 4. Multivariate logistic regression model studying the risk of vitamin D deficiency (yes/no) based on the baseline characteristics of participating patients.
| Independent variables | Adjusted OR | 95% CI for OR | p -Value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Age (mo) | 1.017 | 1.00 | 1.03 | 0.006 * |
| Season (summer vs. winter) |
3.981 | 1.09 | 14.50 | 0.036 * |
| Urine culture positivity | 4.881 | 1.04 | 22.89 | 0.044 * |
| CV-SOFA score | 2.331 | 1.14 | 4.76 | 0.021 * |
| Mechanical ventilation requirement |
2.958 | 1.12 | 7.85 | 0.029 * |
Abbreviations: CI, confidence interval; CV-SOFA, cardiovascular sequential sepsis-related organ failure assessment; OR, odds ratio.
*p -Value < 0.05 is significant.
Discussion
This study evaluated the association of VDD with various demographic, clinical and laboratory parameters and predictors of mortality in the patients admitted to PICU in our tertiary care center.
In Indian pediatric population, prevalence of VDD is significantly high which can be seen in our study as 72% of the patients were vitamin D deficient. Our results are in concordance with the results obtained by Prasad et al, Sankar et al, and McNally et al, who reported a prevalence of 83.8, 74, and 69.0%, respectively. 18 19 20 The reason for higher prevalence of VDD in our study could be due to the poor oral intake or lack of vitamin D supplements.
In the present study, majority of the patients (40%) had respiratory system involvement while 18.40% had central nervous system (CNS) involvement ( Fig. 1 ). Our results were in concordance with the results obtained by Ebenezer et al, who reported that 21.2 and 23.1% of their patients had respiratory system and CNS involvement, respectively. 17 Majority of the patients (22.9%) of the study conducted by Madden et al also had respiratory system involvement. 22 However, in another study conducted by García-Soler et al, 36 and 29% of the patients had hemodynamic and neurological involvement. 23
Fig. 1.
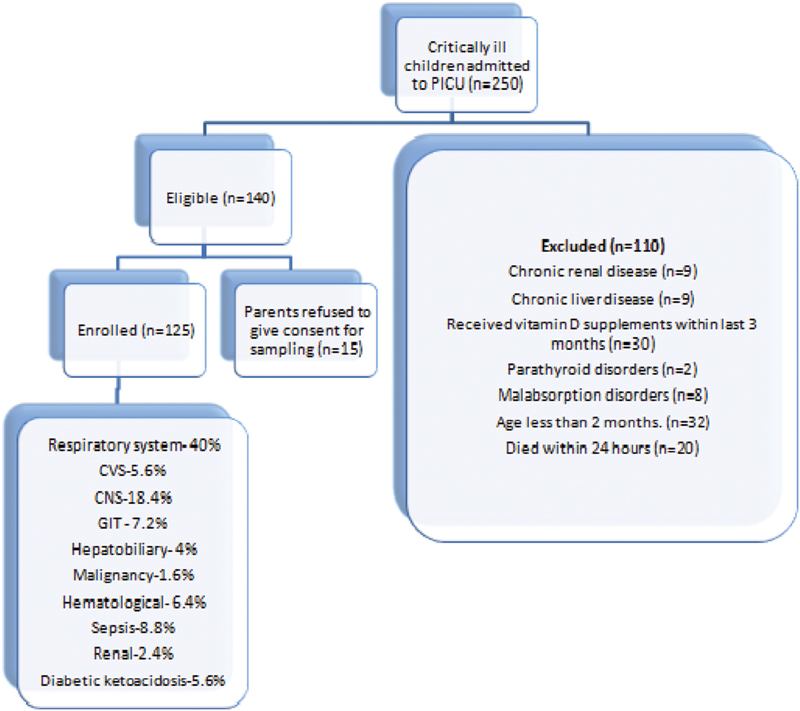
Trial flow of study.
In previous studies it was reported that the risk of VDD increases with the increasing age. 24 Our study supports this finding as statistically significant correlation was observed between VDD and increasing age ( Table 1 ) (symbol of chi square 51.46, p -value = 0.05) and this was further proved by multivariate analysis where VDD increased proportionally to increasing age. Madden et al also observed a highly significant correlation between age and vitamin D levels ( p < 0.0001). 22
In the present study, a significant correlation was observed while correlating VDD with weight ( r = 0.260, p = 0.003) and height of the patients ( r = 0.252, p = 0.005) ( Table 1 ). In the literature reviewed, we could not find any comparison of VDD with height. However, with respect to weight, our results are in concordance with the results obtained by Rey et al ( p < 0.001) and García-Soler et al ( p = 0.041). 10 23
In the present study, out of 93 patients reported during winter, 66.7% were vitamin D deficient whereas out of 32 patients reported in summer season, 87.5% were vitamin D deficient. As per our study, more of vitamin D deficient patients were admitted in winter but risk of VDD was higher in summer ( r s = 0.211, p = 0.018) and risk of VDD was 3.9 times higher in summer season which is in contrast to study by Garcia where risk of VDD was higher in winters (OR = 2.93, p = 0.04). 23 However, McNally et al did not find a significant correlation of percentage of vitamin-deficient patients with season ( p = 0.09). 12
Risk of mechanical ventilation ( Table 2 ) was correlated to VDD ( r = 4.092, p = 0.033). On multivariate analysis, ( Table 4 ) risk of mechanical ventilation increases with VDD (OR = 2.958, p = 0.029) which is in contrast to studies by McNally( p -value = 0.08), Prasad ( p -value = 0.13), Madden ( p -value = 0.1), Ponnarmeni ( p -value = 0.15), who could not observe any statistically significant difference in the risk of mechanical ventilation in patients with VDD. 12 18 22 25
In the present study, a statistically significant correlation was found between VDD and CV-SOFA Score ( Table 3 ; Fig. 2 ) (χ 2 = 7.68, p -value 0.05). This was further proved by multivariate analysis ( Table 4 ) as VDD was associated with a 2.3 times increased risk of CV-SOFA score being in higher quartile. Our results are in concordance with the study by Ebenezer et al where increasing vasopressor use correlated significantly with decreasing serum 25(OH) D levels ( r s = −0.29, p = 0.05). Our results are also supported by a study by Madden et al as lower admission 25(OH)D levels were associated with higher CV-SOFA scores, a 5 ng/mL decrease corresponding to a 1.13-fold increase in odds of being to the next higher category of CV-SOFA score (95% CI 1.01–1.27, p -value = 0.03). 21 22
Fig. 2.
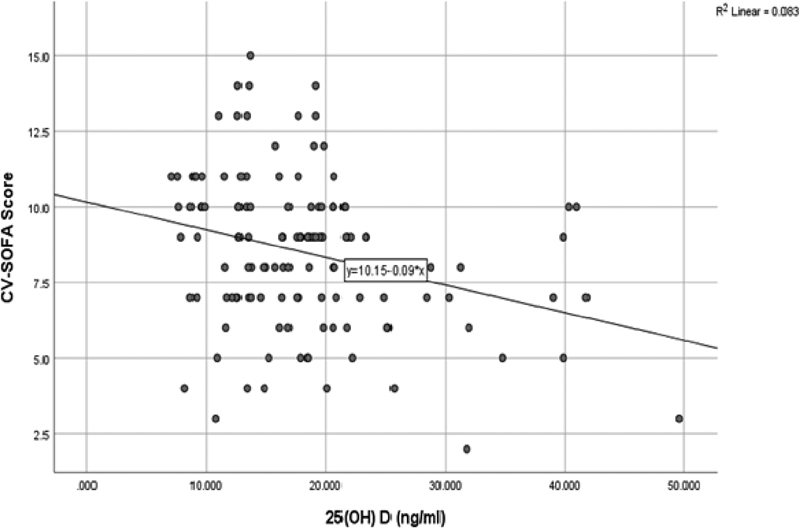
Scatter plot showing correlation of 25(OH) D levels with CV SOFA score.
In our study, bacteriuria ( Table 2 ; Table 4 ) was significantly correlated with VDD ( r = 4.57, p = 0.024) and this was further proved on multivariate analysis (OR = 4.881, p -value = 0.044). Our results are in concordance with studies by Shalaby et al (OR = 3.5, p = 0.001) 26 and Tekin et al (OR = 3.503, p = 0.001). 27 The reason for increased bacteriuria may be due to the role of urinary cathelicidin levels which are not upregulated in VDD. Cathelicidin is an endogenous antimicrobial peptide active against a broad spectrum of infectious agents including gram negative and positive bacteria, fungi, and mycobacteria. Cathelicidin is highly expressed at barrier sites including respiratory, colonic epithelium, urinary epithelium, saliva, and skin and thus provides an important first line defense mechanism for the innate immune system to respond to infectious insults. Antibacterial cathelicidin and autophagy then combine to enhance bacterial killing. 28
VDD on one hand leads to UTI while increased intake of vitamin D supplements can lead to UTI as was reported by a retrospective study conducted at Ochsner Clinic Foundation/Ochsner Medical Center, New Orleans, Louisiana, from the calendar year of 2001 to 2006. The study included both breastfed and bottle-fed infants <3 months. It was found that vitamin D supplementation significantly increased the risk of urinary tract infection (UTI), with an RR of 1.76 (1.07–2.91, p < 0.05) and only the formula-fed infants showed an increased risk of UTI after vitamin D supplementation (RR = 2.24 [1.29–3.90], p < 0.05). This finding could be due to the fact that vitamin D supplementation leads to a milder form of nephrocalcinosis seen with hypervitaminosis D, which could in turn serve as a nidus for bacterial seeding. 1,25 (OH)D is also an immune modulator which tends to decrease the immune response and activation of 25(OH) D to 1,25 (OH)D occurs locally at the site of infection. An excess of 25(OH) D after vitamin D supplementation may by mass action result in more 1,25 (OH)D production, overly suppressing the immune response and allowing the infection to spread.
In the present study ( Table 2 ), while correlating the VDD with length of PICU stay, nonsignificant results were obtained ( r = 10.185, p = 0.679). Our results are in concordance with the results obtained by Ponnarmeni et al ( p = 0.73), who also reported similar findings in their studies. 25 However; our results are in contrast to their results obtained by García-Soler et al ( p = 0.001), who reported a positive significant correlation between VDD and length of PICU stay. 23 So, as per our study, Duration of the hospital stay Hospital stay in PICU is related to the underlying illness and its severity and not predicted by VDD.
Conclusion
Our study revealed a high prevalence of VDD among critically ill children admitted to the PICU. VDD was positively correlated with age, summer season, bacteriuria, CV-SOFA score, and need of mechanical ventilation. No statistically significant correlation could be established between VDD and variables predicting clinical severity like length of stay and mortality as was previously reported.
Strengths
Our study reported risk of bacteriuria with VDD and very few studies have been done on association of bacteriuria with VDD.
Limitations
Patient's preadmission vitamin D levels were not available.
Comparison between patients and healthy controls in terms of vitamin D levels could not be done.
Parathormone could not be done in many patients due to financial constraints.
More multicentric studies are needed to ascertain the role of VDD in mortality before we can generalize the recommendations of vitamin D in therapeutic doses to improve the outcome in PICU in critically ill children.
Funding Statement
Funding None.
Conflict of Interest None declared.
Authors' Contributions
G.K. and G.G. designed the study. A.K. and S.B. drafted the article and did the statistical analysis. S.B. helped in acquisition of data. J.S., S.R., and J.K.S. helped in preparing the draft and revising it. All the authors approved the final version of study.
References
- 1.Million Death Study Collaborators Bassani D G, Kumar R, Awasthi S.Causes of neonatal and child mortality in India: a nationally representative mortality survey Lancet 2010376(9755):1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L, Ma J, Zhang X, Fan Y, Wang L. Protective role of the vitamin D receptor. Cell Immunol. 2012;279(02):160–166. doi: 10.1016/j.cellimm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Gallieni M, Cozzolino M, Fallabrino G, Pasho S, Olivi L, Brancaccio D. Vitamin D: physiology and pathophysiology. Int J Artif Organs. 2009;32(02):87–94. doi: 10.1177/039139880903200205. [DOI] [PubMed] [Google Scholar]
- 4.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(01):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yadav J, Madaan P, Jain V. Brown tumor due to vitamin D deficiency in a child with cerebral palsy. Indian J Pediatr. 2014;81(12):1419. doi: 10.1007/s12098-014-1517-1. [DOI] [PubMed] [Google Scholar]
- 6.Khadilkar V V, Khadilkar A V. Use of vitamin D in various disorders. Indian J Pediatr. 2013;80(03):215–218. doi: 10.1007/s12098-012-0877-7. [DOI] [PubMed] [Google Scholar]
- 7.Rippel C, South M, Butt W W, Shekerdemian L S. Vitamin D status in critically ill children. Intensive Care Med. 2012;38(12):2055–2062. doi: 10.1007/s00134-012-2718-6. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A. Vitamin D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6(02):729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi K, Bhatia V. Vitamin D deficiency in a tropical country—treatment and prevention in children. Indian J Pediatr. 2014;81(01):84–89. doi: 10.1007/s12098-013-1241-2. [DOI] [PubMed] [Google Scholar]
- 10.Rey C, Sánchez-Arango D, López-Herce J. Vitamin D deficiency at pediatric intensive care admission. J Pediatr (Rio J) 2014;90(02):135–142. doi: 10.1016/j.jped.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Lee J Y, So T-Y, Thackray J. A review on vitamin d deficiency treatment in pediatric patients. J Pediatr Pharmacol Ther. 2013;18(04):277–291. doi: 10.5863/1551-6776-18.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNally J D, Leis K, Matheson L A, Karuananyake C, Sankaran K, Rosenberg A M. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44(10):981–988. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 13.Wortsman J, Matsuoka L Y, Chen T C, Lu Z, Holick M F. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(03):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 14.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57(02):183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Singh T, Sharma S, Nagesh S. Socio-economic status scales updated for 2017. Int J Res Med Sci. 2017;5:3264–3267. [Google Scholar]
- 16.Pollack M M, Patel K M, Ruttimann U E. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(05):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Vincent J L, Moreno R, Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(07):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Prasad S, Raj D, Warsi S, Chowdhary S. Vitamin D deficiency and critical illness. Indian J Pediatr. 2015;82(11):991–995. doi: 10.1007/s12098-015-1778-3. [DOI] [PubMed] [Google Scholar]
- 19.Sankar J, Lotha W, Ismail J. Vitamin D deficiency and length of PICU stay: a prospective observational study. Ann Intensive Care. 2016;6:3. doi: 10.1186/s13613-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canadian Critical Care Trials Group . McNally J D, Menon K, Chakraborty P. The association of vitamin D status with pediatric critical illness. Pediatrics. 2012;130(03):429–436. doi: 10.1542/peds.2011-3059. [DOI] [PubMed] [Google Scholar]
- 21.Ebenezer K, Job V, Antonisamy B, Dawodu A, Manivachagan M N, Steinhoff M. Serum vitamin D status and outcome among critically ill children admitted to the pediatric intensive care unit in South India. Indian J Pediatr. 2016;83(02):120–125. doi: 10.1007/s12098-015-1833-0. [DOI] [PubMed] [Google Scholar]
- 22.Madden K, Feldman H A, Smith E M. Vitamin D deficiency in critically ill children. Pediatrics. 2012;130(03):421–428. doi: 10.1542/peds.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Soler P, Morales-Martínez A, Rosa-Camacho V, Lillo-Muñoz J A, Milano-Manso G. Déficit de vitamina D y morbimortalidad en pacientes críticos pediátricos. An Pediatr (Barc) 2017;87(02):95–103. doi: 10.1016/j.anpedi.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Hebbar K B, Wittkamp M, Alvarez J A, McCracken C E, Tangpricha V. Vitamin D deficiency in pediatric critical illness. J Clin Transl Endocrinol. 2014;1(04):170–175. doi: 10.1016/j.jcte.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponnarmeni S, Kumar Angurana S, Singhi S. Vitamin D deficiency in critically ill children with sepsis. Paediatr Int Child Health. 2016;36(01):15–21. doi: 10.1179/2046905515Y.0000000042. [DOI] [PubMed] [Google Scholar]
- 26.Shalaby S A, Handoka N M, Amin R E. Vitamin D deficiency is associated with urinary tract infection in children. Arch Med Sci. 2018;14(01):115–121. doi: 10.5114/aoms.2016.63262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekin M, Konca C, Celik V. The association between vitamin D levels and urinary tract infection in children. Horm Res Paediatr. 2015;83(03):198–203. doi: 10.1159/000370046. [DOI] [PubMed] [Google Scholar]
- 28.Dürr U H, Sudheendra U S, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(09):1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]


