Abstract
Purpose
Plant polyphenols possess beneficial functions against various diseases. This study aimed to identify phenolic ingredients in Camellia fascicularis (C. fascicularis) and investigate its possible underlying anti-inflammatory mechanism in lipopolysaccharide (LPS)-induced human monocytes (THP-1) macrophages.
Methods
C. fascicularis polyphenols (CFP) were characterized by ultra-performance liquid chromatography (UPLC) combined with quadrupole-time-of-flight mass/mass spectrometry (Q-TOF-MS/MS). The THP-1 cells were differentiated into macrophages under the stimulation of phorbol 12-myristate 13-acetate (PMA) and then treated with LPS to build a cellular inflammation model. The cell viability was detected by CCK-8 assay. The levels of reactive oxygen species (ROS) were assessed by flow cytometry. The secretion and expression of inflammatory cytokines were tested by enzyme-linked immunosorbent assay (ELISA) and real-time polymerase chain reaction (RT-PCR). In addition, the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were analyzed by Western blotting.
Results
Twelve phenolic constituents including (–)-epicatechin, casuariin, agastachoside, etc. in CFP were identified. The CCK-8 assay showed that CFP exhibited no significant cytotoxicity between 100 and 300 μg/mL. After treated with CFP, the release of ROS was significantly suppressed. CFP inhibited inflammation in macrophages by attenuating the polarization of LPS-induced THP-1 macrophages, down-regulating the expression of the pro-inflammatory cytokines IL-6, IL-1β and TNF-α, and up-regulating the expression of the anti-inflammatory cytokine IL-10. Western blotting experiments manifested that CFP could markedly inhibit the phosphorylation of p65, ERK and JNK, thereby suppressing the activation of NF-κB and MAPK signaling pathways.
Conclusion
These findings indicated that CFP exerted anti-inflammatory activity by inhibiting the activation NF-κB and MAPK pathways which may induce the secretion of pro-inflammatory cytokines. This study offers a reference for C. fascicularis as the source of developing natural, safe anti-inflammatory agents in the future.
Keywords: Camellia fascicularis, polyphenols, anti-inflammatory activity, NF-κB, MAPK
Introduction
Inflammation is a widespread, complex biological behavior and is a stress response of the organism to harmful stimuli.1,2 However, persistent and excessive inflammation is harmful to the host and is closely associated with diseases, such as rheumatoid arthritis,3 septic shock,4 inflammatory bowel disease,5 coronary artery disease,6 Parkinson’s disease,7 cancer,8 and even COVID-19, which is currently sweeping the world.9 Inflammation produces toxic effects on the body with pro-inflammatory cytokines, caused by activating the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways.10 The administration of exogenous anti-inflammatory ingredients is essential in curing inflammation diseases. Nevertheless, drug resistance and other side effects will occur due to the overuse of synthetic anti-inflammatory agents.11 Hence, it is important to search for and exploit natural anti-inflammatory drugs with low toxicity and no side effects from medicinal and edible biomaterials. Recently, studies have shown that polyphenols from tea plants and some tea products, such as Camellia sinensis,12 Camellia sinensis var. assamica,13 Camellia ptilophylla,14 Camellia nitidissima,15 oolong tea,16 green tea17 and black tea,18 possess significant anti-inflammatory activity.
Plant polyphenols have exhibited valuable biological functions, containing antioxidant,19 prevention of cardiovascular diseases,20 anti-inflammatory,21 anti-tumor,22 hypoglycemic23 and intestinal flora regulation.24 The activities of phenolic components in inflammation response generally arise from restricting downstream pro-inflammatory cytokine synthesis such as interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor-α (TNF-α), as well as promoting anti-inflammatory cytokine synthesis like IL-10, thereby interfering with relevant signaling pathways as NF-κB and MAPK.12,25 It has been shown that blue honeysuckle polyphenols could decrease the inflammatory responses and macrophage apoptosis by suppressing the phosphorylation of phosphatidylinositol 3-kinase (p38) and c-Jun N-terminal kinase (JNK).26 Chlorogenic acid, which is abundant in the blue honeysuckle polyphenols, may constrain inflammatory responses through regulation of NF-κB pathway.27 Tiliroside separated from Rubus chingii displayed anti-inflammatory effect via restraining MAPK pathway and pro-inflammatory cytokines released in macrophages.28
Camellia fascicularis (C. fascicularis) H. T. Chang, a Camellia plant resource unique to Yunnan, belongs to the genus Camellia29 and has been used traditionally as food and medicine. C. fascicularis leaves are rich in nutrients, including amino acids, unsaturated fatty acids and mineral elements, among which polyphenols are present in higher levels than other active components, such as polysaccharides and saponins.30 Optimization of the extraction process, purification and antioxidant activity assays of polyphenols were carried out, and it was found that the polyphenols in C. fascicularis leaves exhibited obvious in vitro antioxidant activities.31 Nevertheless, to our knowledge, there are no studies on the activities of C. fascicularis polyphenols (CFP) in inflammation response and their action mechanism. Hence, it is important to investigate the anti-inflammatory activities of CFP. In the present study, CFP were obtained and their main active ingredients were identified and characterized. Furthermore, the activity of CFP in inflammation and their possible mechanism were explored with human monocytes (THP-1) stimulated by lipopolysaccharide (LPS).
Materials and Methods
Materials and Chemicals
C. fascicularis leaves were collected in Daweishan Nature Reserve in Yunnan (103.95°N, 22.66°E), where the climate of the north is tropical and that of the south is subtropical, and its average annual rainfall is 1700–2200 mm. C. fascicularis were dried at 45°C, crushed using a 60-mesh sieve and kept dry in the shade until use. Acetonitrile with chromatographical purity (>99.9%) was purchased from Merck (Darmstadt, HD, Germany). Phorbol 12-myristate 13-acetate (PMA) and LPS were purchased from Gibco (Grand Island, NY, USA). Phosphate buffer solution (PBS) was purchased from Hyclon (Logan, UT, USA). (–)-Epicatechin was purchased from Tianjin Fengchuan Chemical Reagent Co., Ltd. (Tianjin, China). Ethanol and formic acid were purchased from Chengdu Desite Biotechnology Co., Ltd. (Chengdu, China). Other chemicals were presented in particular experimental approach.
Preparation of CFP
The extraction and purification process of polyphenols from C. fascicularis was described in our earlier research.31 Firstly, polyphenols were extracted using a microwave apparatus (Ningbo Xinzhi Biotechnology Co., Ltd, Zhejiang, China) under optimized conditions. The liquid-solid ratio was 32 mL/g, ethanol concentration was 50%, microwave power was 160 W and microwave time was 20s. Secondly, the extract was filtered, concentrated and lyophilized. Finally, crude polyphenols were purified through HP-20 macroporous adsorbent resin. After adsorption, the sample was eluted with ethanol at 2 mL/min. The eluate was collected, concentrated and lyophilized to yield CFP, which was stored near to 4°C before use.
Component Identification
A method based on a previous study with slight modifications was used to characterize the phenolic acid compounds and flavonoids of CFP.32 Ten milligrams of CFP were dissolved in 10 mL of methanol, filtered, and further analyzed using ultra-performance liquid chromatography (UPLC; Agilent Technologies Inc., CA, USA) combined with quadrupole-time-of-flight mass/mass spectrometry (Q-TOF-MS/MS) coupled with electrospray ionization (ESI). A Waters BEH C18 column (50 mm × 2.1 mm, 1.7 μm) was used at 30°C. The mobile phase consisted of 0.1% formic acid aqueous solution (A) and acetonitrile (B). A gradient elution procedure was shown below: 0–1 min, 5% B; 1–9 min, 5–95% B; 9–12 min, 95% B; 12–12.1 min, 95–5% B; 12.1–13 min, 5% B. The injection volume was 2 µL with the mobile phase at 0.3 mL/min, as well as flow rate sheath gas of 12 L/min at 350°C. The full-scan mass spectra were performed between 50 and 1000 m/z at a voltage of 3200 V under negative ion mode.
Cell Culture
THP-1 cells, provided by Cell Bank of Typical Cultures Preservation Committee in Shanghai, China, were cultivated using the previously published method with slightly modified.33 THP-1 cells were hatched at 37°C and 5% CO2 using Dulbecco’s Modified Eagle Medium with 10% (v/v) fetal bovine serum. Before experimental treatment, the differentiation process of THP-1 cells to macrophages was performed in stimulation for 48 h by 5 ng/mL of PMA.
Cell Viability
The assay was carried out using cell counting kits (CCK-8, Wanleibio Co., Ltd., Shenyang, China) to estimate the cytotoxicity of CFP against the THP-1 macrophages. After inoculation in a 96-well microplate at densities of 1 × 105/mL, the macrophages were incubated with 100 μL CFP (100–500 μg/mL) for 24 h. Incubation was continued with the addition of 10 μL CCK-8 solution. Four hours later, the cell viability, expressed as absorbance percentage, was determined at 450 nm by a microplate reader (BioTek, Winooski, VT, USA), compared against the untreated control macrophages. Furthermore, the morphological images of THP-1 macrophages including control, LPS and LPS+CFP group were viewed under a microscope (Nikon, Tokyo, Japan).
Inflammatory Stimulation
The inflammatory stimulation assay was carried out based on Zhao’s method with slight modifications.33 THP-1 macrophages were inoculated in a 96-well microplate with 1 × 105/mL cell density and cultivated until cells adhered to the wall. Then cells were pretreated with CFP (100, 200, 300 μg/mL), stimulated by LPS (1.0 μg/mL) and hatched for 24 h. Sample group included macrophages treated with CFP alone. LPS group included macrophages treated with LPS alone. Control group included macrophages not receiving CFP or LPS treatment.
Detection of Reactive Oxygen Species Levels
This assay was performed following Wang’s method with slightly modified.34 After inflammatory stimulation, THP-1 macrophages were resuspended in a culture medium. After hatched 30 min at constant temperature and 5% CO2, with shaking several times during the period, the macrophages were collected and centrifuged. Then the macrophages were resuspended with the same amount of PBS after discarding the supernatant. Finally, the fluorescence intensities were measured using flow cytometry (Becton, Dickinson and Company, Franklin Lake, NJ, USA), and the reactive oxygen species (ROS) values were represented as mean fluorescence intensity values (MFI).
Determination of Inflammatory Cytokines
After inflammatory stimulation, the cell cultures of each well were centrifuged and the production of IL-6, IL-1β, TNF-α and IL-10 in supernatants was tested using enzyme-linked immunosorbent assay (ELISA). Results were presented as concentration changes of the inflammatory cytokines compared to the control group.
Real-Time Polymerase Chain Reaction
The assay was carried out based on Zhao’s method with slight modifications.33 After inflammatory stimulation, the total cellular RNA in THP-1 macrophages was separated using the RNAzol RT RNA Isolation Reagent, following the manufacturer’s protocol. And cDNA was synthesized using Eastep® RT Master Mix kits (Shanghai Promega Biological Products, Ltd., Shanghai, China), following the manufacturer’s protocol. Expression levels of inflammatory cytokines were standardized with GAPDH selected to be the reference gene, which was detected using SYBR® Premix Ex Taq™ II kit (TaKaRa Biotechnology Co., Ltd., Dalian, China). Real-time polymerase chain reaction (RT-PCR) primers are presented in Table 1. The results were expressed as changes in the gene expression versus control group.
Table 1.
RT-PCR Primer Sequences
| Genes | Forward (5’- 3’) | Reverse (5’- 3’) |
|---|---|---|
| IL-6 | TACTCGGCAAACCTAGTGCG | GTGTCCCAACATTCATATTGTCATT |
| IL-1β | CCATGGAATCCGTGTCTTCCT | GTCTTGGCCGAGGACTAAGG |
| TNF-α | GGGGATTATGGCTCAGGGTC | CGAGGCTCCAGTGAATTCGG |
| IL-10 | ACCAAGACCCAGACATCA | TTCACAGGGAAGAAATCG |
| GAPDH | TTTGTCAAGCTCATTTCCTGGTATG | TGGCATAGGGCCTCTCTTGC |
Western Blotting
The assay was carried out based on Zhao’s method with slightly modified.33 Briefly, after inflammatory stimulation, THP-1 macrophages were treated with trypsin, phenylmethylsulfonyl fluoride, phosphatase inhibitor cocktail and RIPA lysis buffer, and then homogenized with a Vortex Mixer (DLAB Scientific Inc., Locust St, CA, USA) twice. The proteins in cell lysate were gathered by centrifugation, segregated using the SDS-PAGE Gel Kits (Solarbio Science & Technology Co., Ltd., Beijing, China), and transferred onto PVDF membranes. After obstructed by tris-buffered saline with bovine serum albumin and Tween 20, 1:2000 dilution of primary and secondary antibodies (Cell Signaling Technology, Danvers, USA) were added successively onto the membranes for incubation. Subsequently, the Western blots were visualized using the Super ECL Plus kits (Suzhou Yuheng Biotechnology Co., Ltd., Suzhou, China) and quantified using a gel image analysis system (Tanon 2500R, Tanon Science & Technology Co., Ltd., Shanghai, China). The expression levels of the target protein were determined as protein band intensity ratio relative to GAPDH and expressed as changes compared to control group.
Statistical Analysis
Data analyses were performed using GraphPad Prism 8.3.0 software (GraphPad Software, San Diego, CA, USA). The concentration of inflammatory cytokines and expression of target gene and protein were presented with mean ± standard deviation. One-way ANOVA was used for statistical differences with significant differences indicated as p < 0.05.
Results
Identification of Bioactive Constituents of CFP
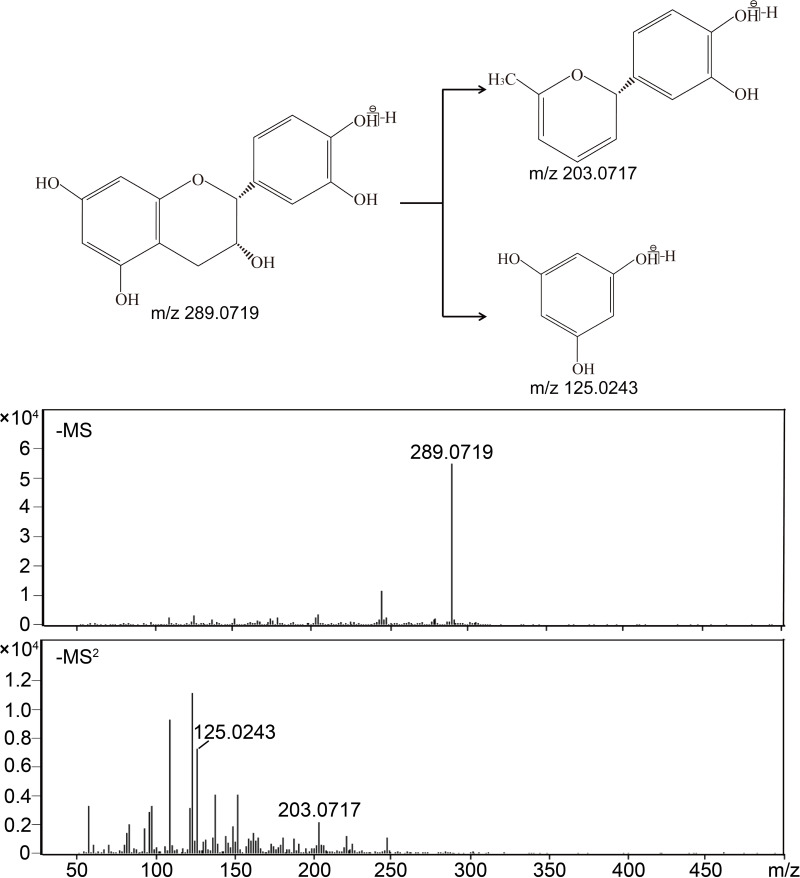
The possible structures of phenolic components were identified and characterized through retention times, molecular weight, as well as MS/MS fragmentation compared with literature information. The phenolic components in CFP, identified under negative ion modes, are listed in Table 2. The possible fragmentation pathway of (–)-epicatechin in negative ion modes was taken as a representative example for identifying phenolic constituents based on MS/MS fragmentation pattern. As shown in Figure 1, one molecular ion with 289.0719 m/z and two debris ions with 125.0243 m/z [C6H6O3]– and 203.0717 m/z [C12H12O3]− were displayed in the MS and MS/MS data, which represented the two possible fragmentation pathways of (–)-epicatechin, respectively. In comparison to the standard substance, the constituent was identified as (–)-epicatechin.
Table 2.
The Identification of Phenolic Constituents by UPLC-Q-TOF-MS/MS in CFP
| No | RT (Min) | Proposed Formula | [M-H]– (m/z) | MS/MS Fragments (m/z) | Identified Compounds | Ref |
|---|---|---|---|---|---|---|
| 1 | 1.569 | C34H24O22 | 783.0704 | 300.9993 | Casuariin | [35] |
| 2 | 2.358 | C7H6O3 | 137.0246 | 108.0205 | 3,4-Dihydroxybenzyl aldehyde | [36] |
| 3 | 3.233 | C30H26O12 | 577.1353 | 579.1396, 161.0247 | Apigenin-7-O-(6”-(E)-p-coumaroyl)-β-D-galactopyranoside | [37] |
| 4 | 3.609 | C45H38O18 | 865.1975 | 289.0714 | Robinetinidol-(4α->8)-catechin-(6->4α)-robinetinidol | [38] |
| 5 | 3.693 | C15H14O6 | 289.0719 | 203.0717, 125.0243 | (–)-Epicatechin | [39] |
| 6 | 3.749 | C15H16O6 | 337.0925 | 173.0461, 93.0361 | 8-Acetyl goniofufurone | [40] |
| 7 | 4.132 | C27H30O15 | 593.1500 | 473.1088 | Vitexin Glucoside | [41] |
| 8 | 4.133 | C27H30O15 | 593.1517 | 473.1061, 383.0749, 353.0636 | Apigenin-6,8-di-C-glucoside | [42] |
| 9 | 4.842 | C21H18O13 | 477.0689 | 300.9939 | Mingjinianuronide A | [43] |
| 10 | 4.882 | C32H38O20 | 741.1889 | 301.0349 | Saluenin | [44] |
| 11 | 4.965 | C24H24O11 | 533.1317 | 353.0670 | Agastachoside | [45] |
| 12 | 5.038 | C21H20O12 | 463.0886 | 300.0245 | 6-Hydroxykaempferol-7-O-glucoside | [46] |
Figure 1.
Mass spectra and possible fragmentation pathway of (–)-epicatechin.
Cell Viability
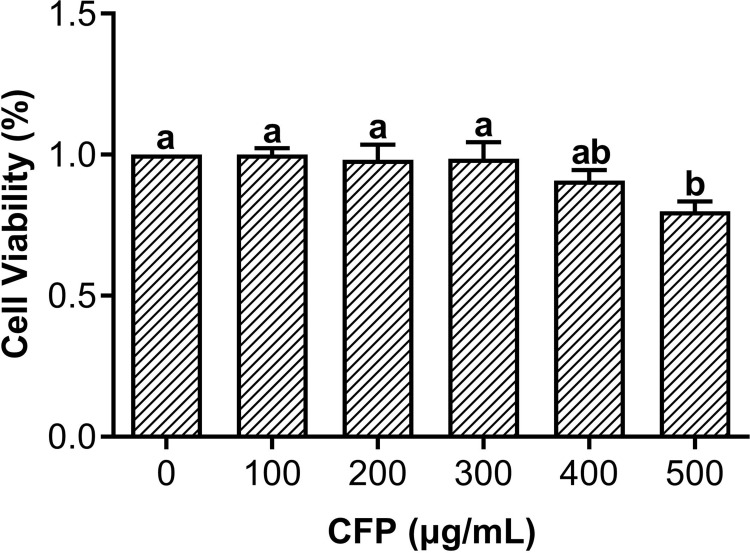
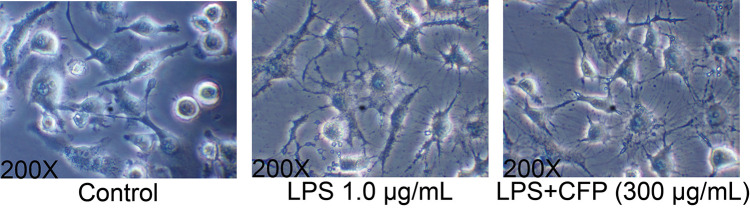
The potential cytotoxicity of the CFP to THP-1 macrophages was evaluated using CCK-8 assays after 24 h pretreatment with CFP (100–500 μg/mL). As shown in Figure 2, no important differences in viability were detected when CFP treatment concentration was 100–300 μg/mL. In addition, the morphological alterations of macrophages after LPS and CFP treatment are displayed in Figure 3. Normal cultured macrophages were round with clear borders. After LPS stimulation, macrophages exhibited obvious morphological alterations, such as increased volume, irregular roundness, extended pseudopods and indistinct borders. Nevertheless, the morphological changes in THP-1 macrophages were suppressed with CFP pretreatment, demonstrating that CFP treatment could attenuate the cell polarization induced by LPS.
Figure 2.
Effects of CFP on cell viability. THP-1 macrophages were pretreated by CFP at various concentrations. a,ab,bValues with different lowercase letters are significantly different between groups at P < 0.05.
Figure 3.
Effects of CFP on cell morphology of THP-1 macrophages. Representative morphological images are shown in each group (200X magnification). The treatment for cells was performed with control, LPS and LPS+CFP group.
CFP Suppresses ROS Production in THP-1 Macrophages
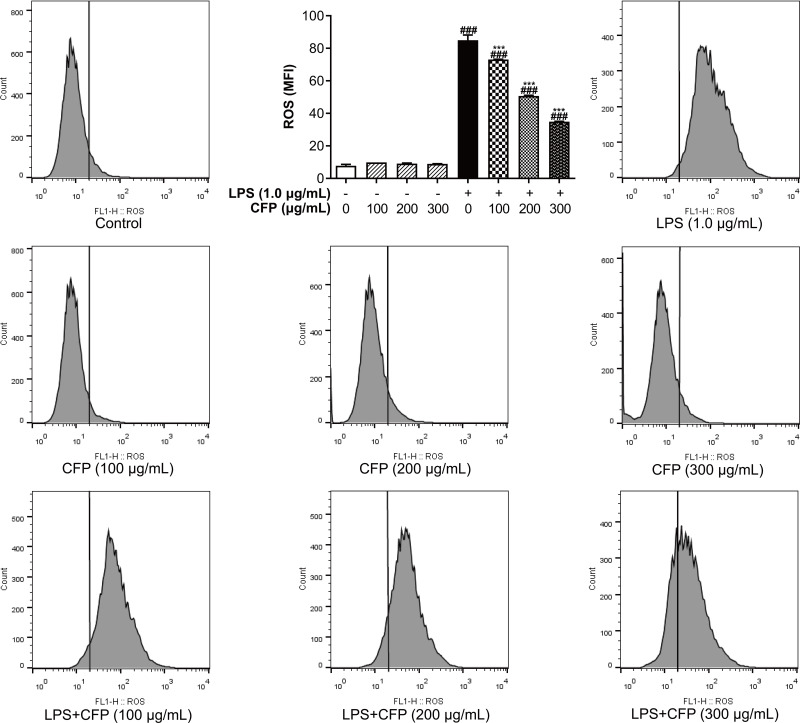
Influences of CFP on ROS production were detected using flow cytometry. As shown in Figure 4, the sample group did not have increased ROS release versus control group, yet ROS release in THP-1 macrophages stimulated with LPS increased significantly (P < 0.001), possibly relevant to an increase with IL-6 and IL-1β.34 Nonetheless, CFP pretreatment observably and dose-dependently inhibited this increase (P < 0.001).
Figure 4.
Inhibitory effects of CFP on ROS release in THP-1 macrophages. After inflammatory stimulation, ROS levels in THP-1 macrophages were visualized using fluorescence microscope and represented by mean fluorescence intensity values (MFI). Data represent the average of the three replicates. ###P < 0.001 vs control group; ***P < 0.001 vs LPS group.
Effects of CFP on Production and Gene Expression of Inflammatory Cytokines
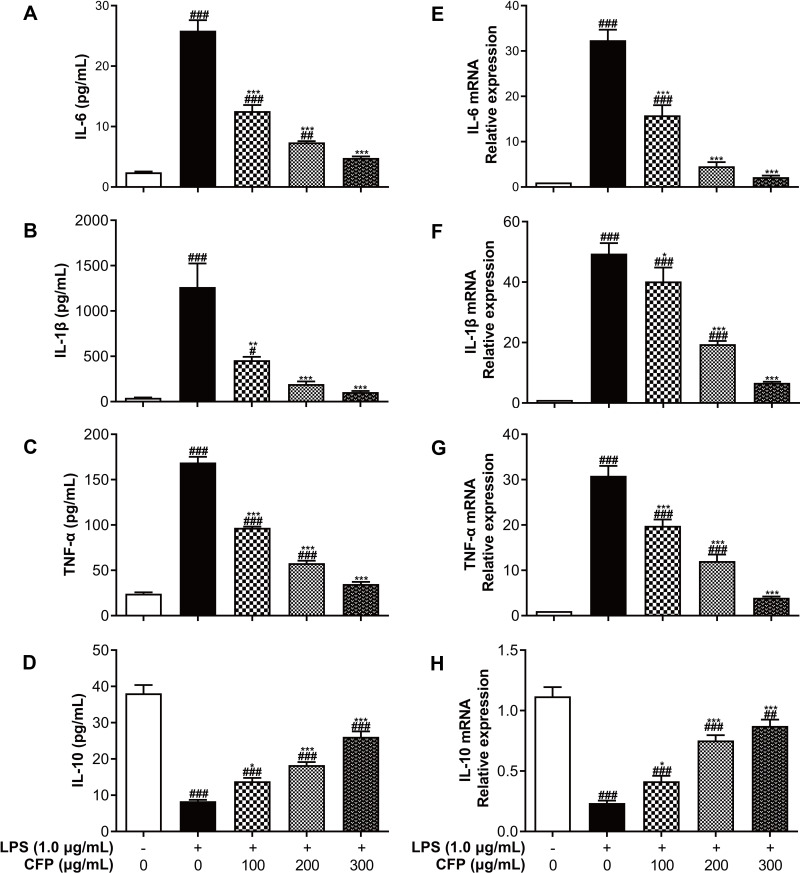
The anti-inflammatory effect of CFP was appraised using the inflammation model of THP-1 macrophage stimulated with LPS. ELISA and RT-PCR were utilized for the detection of production and gene expression of inflammatory cytokines, respectively. LPS-induced overproduction of IL-6, IL-1β and TNF-α was dramatically and dose-dependently suppressed by CFP compared to control group. Simultaneously, the low production of IL-10 was greatly ameliorated and dose-dependent by CFP (Figure 5A–D). With 300 μg/mL of CFP treatment, the liberation of IL-6, IL-1β and TNF-α was restored similar to those of control group. The secretion of inflammatory cytokines might undoubtedly be affected by their gene levels. As shown in Figure 5E–H, mRNA overexpression of IL-6, IL-1β and TNF-α was down-regulated, and low-expression of IL-10 was up-regulated by CFP. These results demonstrated that inflammation might be inhibited by affecting the gene levels of inflammatory cytokines. Under the treatment of CFP (300 μg/mL), the secretion of IL-6, IL-1β and TNF-α was completely restrained, on account of their expression levels presenting not much difference from control group.
Figure 5.
Effects of CFP on the production and gene expression of inflammatory cytokines. After LPS stimulation, the contents of IL-6 (A), IL-1β (B), TNF-α (C) and IL-10 (D) were performed using ELISA, and mRNA levels of IL-6 (E), IL-1β (F), TNF-α (G) and IL-10 (H) were measured by RT-PCR. Data represent the average of the three replicates. #P < 0.05, ##P < 0.01 and ###P < 0.001 vs control group; *P < 0.05, **P < 0.01 and ***P < 0.001 vs LPS group.
Effects of CFP on LPS-Induced Activation of NF-κB and MAPK Pathways
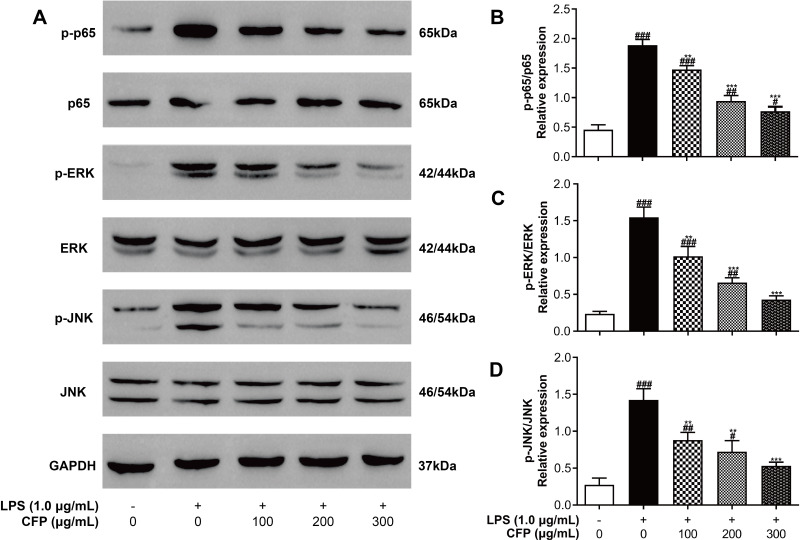
The relative expression of p-p65/p65, p-ERK/ERK and p-JNK/JNK were detected by Western blot in THP-1 macrophages to investigate the inhibition of NF-κB and MAPK Pathways by CFP. As shown in Figure 6, LPS stimulation resulted in a remarkable enhancement of p65 phosphorylation compared to control group (P < 0.001), whereas CFP significantly and dose-dependently suppressed the phosphorylation of p65 (P < 0.01), and thus may restrain the translocation of p65. Of note, the promotion of p65 expression levels by LPS was restored near to control group by 300 μg/mL of CFP treatment. In addition, both ERK and JNK phosphorylation stimulated by LPS were remarkably enhanced versus control group (P < 0.001). Nevertheless, CFP treatment could dramatically and dose-dependently inhibit the ERK phosphorylation (P < 0.01), and also strongly and dose-dependently inhibit the JNK phosphorylation (P < 0.01). Also noteworthy is that treated with 300 μg/mL of CFP could restore the expression level promotion of ERK and JNK.
Figure 6.
Effects of CFP on the LPS-induced activation of NF-κB and MAPK pathways. (A) Represents the Western immunoblots for p-p65, p65, p-ERK, ERK, p-JNK, JNK and GAPDH. After inflammatory stimulation, relative expression of p-p65/p65 (B), p-ERK/ERK (C) and p-JNK/JNK (D) were tested by Western blotting in THP-1 macrophages. Data represent the average of the three replicates. #P < 0.05, ##P < 0.01 and ###P < 0.001 vs control group; **P < 0.01 and ***P < 0.001 vs LPS group.
Discussion
As an endotoxin related to pathogens and infections, LPS could trigger NF-κB and cause inflammation.33 In this study, the model of LPS-stimulated THP-1 macrophages was chosen for the determination of the anti-inflammatory capacity of CFP. The influence of CFP on NF-κB and MAPK pathways was investigated.
In order to characterize the molecular basis of the anti-inflammatory effects exerted by CFP, the phenolic components of CFP were identified by UPLC-Q-TOF-MS/MS. As the result exhibited, twelve phenolic constituents in CFP were identified by combining the MS, MS/MS data, and literature information.35–46 The phenolic constituents detected in CFP, such as (–)-epicatechin, casuariin and agastachoside, have been considered as potential medicinal constituents.47–49 This is the first identification of the phenolic constituents in C. fascicularis using UPLC-Q-TOF-MS/MS. This approach could be applied for obtaining potentially active substances more quickly than other analytical methods.50 The results indicated that C. fascicularis possesses potentially medicinal constituents and offered a basis and orientation for further studies of the monomer constituents of C. fascicularis.
It is necessary to ensure that CFP produces no harmful effects on cell metabolism before conducting bioactivity studies. According to the toxicology assays, three dose groups of 100, 200 and 300 μg/mL CFP were selected for the subsequent study. Moreover, the polarization of inflammatory macrophages was found to be involved in the inflammatory response by observing the morphological changes of LPS-stimulated THP-1 macrophages, which was consistent with previous findings.51 Therefore, the debilitation effect of CFP on cell polarization may be of considerable significance in treating inflammatory diseases.
ROS are chemical substances that possess an unpaired electron generated by molecular oxygen.52 Metabolism dysregulation of ROS would lead to inflammation.53 Former study demonstrated that JNK signaling related to pro-inflammatory cytokines release could be activated by ROS.26 In this study, CFP mediated the metabolism of LPS-induced intracellular ROS. The results indicated that CFP pretreatment could regulate LPS-induced ROS metabolism under an oxidative stressful physiological environment, and might further modulate the inflammatory response.
Previous studies have shown that pro-inflammatory cytokines, which are rapidly released under inflammatory conditions, are closely related to multiple inflammatory processes. These increased pro-inflammatory cytokine levels were strongly related to multiple diseases, including septic shock, coronary artery disease, chronic liver diseases, cancer and diabetes.26,51,54 Therefore, the restraint of pro-inflammatory cytokines would be essential to reduce the inflammatory response. IL-10, released primarily from T cells, is one of the anti-inflammatory cytokines and conducive to inhibiting overactive inflammatory responses.33,55 IL-10 could inhibit NF-κB activation by suppressing IκB kinase (IKK) activity.56,57 Fortunately, CFP could inhibit the LPS-induced IKK activation through up-regulating IL-10 production and gene expression. Due to their ability to interfere with the release of inflammatory cytokines, natural ingredients possess considerable potential as anti-inflammatory drugs for treating inflammatory responses. In the present study, CFP suppressed the LPS-induced gene expression of IL-6, IL-1β and TNF-α, as well as enhanced the gene expression of IL-10. These results indicated that CFP possesses the potential used as bioactive ingredients for treating inflammation.
Inflammation has been recognized by the research community as a hallmark and cause of multiple diseases. The underlying molecular regulatory mechanisms of inflammation are complex and diverse.58 NF-κB, composed of the p50 subunit and trans-activating subunit (p65), is an influential nuclear transcription regulatory factor closely related to the modulation of inflammation and a major signaling pathway associated with pro-inflammatory cytokine regulation.51,59 Under normal conditions, NF-κB and IκB exist as a compound in the form of p65–p50 heterodimer tangled with IκB in cytoplasm. Upon stimulation by agents, such as LPS, inhibitor of NF-κB alpha (IκBα) will be phosphorylated and degraded by IKK under ubiquitination. Simultaneously, phosphorylated (p)-p65 enters nucleus from cytoplasm along with NF-κB liberation, thus activates gene transcription and induces the secretion of pro-inflammatory cytokines.57 The p65 release is intimately associated with IκBα degradation, and p-p65 levels are vital for p65 translocation. Hence, the prevention of p-p65 formation is essential for blocking NF-κB pathway. Herein, the results suggested that CFP might suppress the pro-inflammatory cytokine secretion via attenuating the p65 phosphorylation in NF-κB pathway. Meanwhile, MAPK is also a critical signaling pathway that regulates pro-inflammatory cytokines60 and also associated with cell proliferation, differentiation, apoptosis and other physiological processes. The MAPKs, containing extracellular regulated protein kinase (ERK), JNK and p38 pathways, are vital in regulating inflammatory cytokine production in macrophages stimulated by LPS.33 Upon stimulation by agents, such as LPS, the MAPK signaling pathway is activated and phosphorylated, which could be contributed as transcriptional activators for NF-κB.61 In the present study, the findings suggested that CFP may suppress MAPK pathway by blocking both ERK and JNK phosphorylation, thereby inhibiting pro-inflammatory cytokines release. Taken together, CFP could exert anti-inflammatory effects by remarkably suppressing the activations of NF-κB and MAPK.
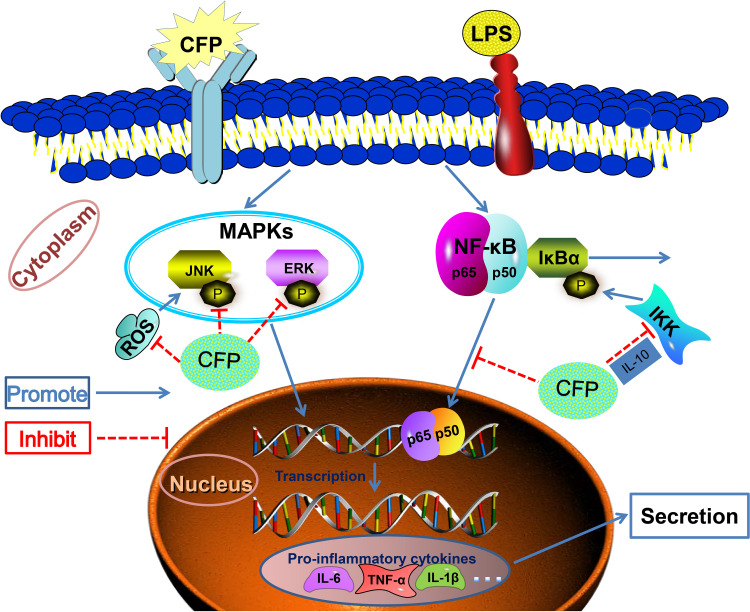
The inflammation signaling pathways are complex and diverse. NF-κB and MAPK are the main pathways controlling liberation and expression of inflammatory cytokines in inflammatory response.60 Previous studies have revealed that paeonol restrained pro-inflammatory cytokines release in mice macrophages and microglia by blocking NF-κB and MAPK pathways.62,63 Inflammatory cytokine regulation by CFP in THP-1 macrophages stimulated with LPS is probably related to obstructing the MAPK and NF-κB pathways. The suggested anti-inflammatory mechanism by CFP in LPS-induced THP-1 macrophages is illustrated in Figure 7.
Figure 7.
A suggested anti-inflammatory mechanism by CFP in LPS-induced THP-1 macrophages. CFP exhibited anti-inflammatory properties through attenuating NF-κB and MAPK pathways.
Conclusion
CFP were obtained by microwave-assisted extraction and HP-20 macroporous resin purification. Twelve phenolic constituents of CFP were identified using UPLC-Q-TOF-MS/MS. CFP exhibited beneficial regulation effects on the secretion and gene expression of inflammatory cytokines in LPS-induced THP-1 macrophages. These findings were further demonstrated to achieve anti-inflammatory activity, CFP suppressed NF-κB pathway by restriction of p65 phosphorylation and inhibited MAPK pathway through restraint of both ERK and JNK phosphorylation. This study offers a reference for C. fascicularis as the source of developing natural, safe anti-inflammatory agents in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 32060107), and the Agricultural Basic Research Joint Project of Yunnan Province (Grant No. 2017FG001-084).
Disclosure
The authors declare no conflicts of interest.
References
- 1.Jones MA, MacCuaig WM, Frickenstein AN, et al. Molecular imaging of inflammatory disease. Biomedicines. 2021;9(2):152. doi: 10.3390/biomedicines9020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JN, Liu ZY, Su J, Pan N, Song QL. Anti-inflammatory activity of 3β-hydroxycholest-5-en- 7-one isolated from Hippocampus trimaculatus leach via inhibiting iNOS, TNF-α, and 1L-1β of LPS induced RAW 264.7 macrophage cells. Food Funct. 2017;8(2):788–795. doi: 10.1039/c6fo01154c [DOI] [PubMed] [Google Scholar]
- 3.Bakheet SA, Ansari MA, Nadeem A, et al. CXCR3 antagonist AMG487 suppresses rheumatoid arthritis pathogenesis and progression by shifting the Th17/Treg cell balance. Cell Signal. 2019;64:109395. doi: 10.1016/j.cellsig.2019.109395 [DOI] [PubMed] [Google Scholar]
- 4.Zhang QQ, Sun H, Zhuang SG, et al. Novel pharmacological inhibition of EZH2 attenuates septic shock by altering innate inflammatory responses to sepsis. Int Immunopharmacol. 2019;76:105899. doi: 10.1016/j.cellsig.2019.109395 [DOI] [PubMed] [Google Scholar]
- 5.Xie Z, Wang Y, Yang G, et al. The role of the Hippo pathway in the pathogenesis of inflammatory bowel disease. Cell Death Dis. 2021;12(1):79. doi: 10.1038/s41419-021-03395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye S, Matthan NR, Lamon-Fava S, et al. Western and heart healthy dietary patterns differentially affect the expression of genes associated with lipid metabolism, interferon signaling and inflammation in the jejunum of Ossabaw pigs. J Nutr Biochem. 2021;90:108577. doi: 10.1016/j.jnutbio.2020.108577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romano S, Savva GM, Bedarf JR, Charles LG, Hildebrand F, Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi: 10.1038/s41531-021-00156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu CH, Yeh DW, Lai CY, et al. USP17 mediates macrophage-promoted inflammation and stemness in lung cancer cells by regulating TRAF2/TRAF3 complex formation. Oncogene. 2018;37(49):6327–6340. doi: 10.1038/s41388-019-0831-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12(5):1466. doi: 10.3390/nu12051466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo YH, Park HC, Choi S, et al. Metabolomic analysis reveals cyanidins in black raspberry as candidates for suppression of lipopolysaccharide-induced inflammation in murine macrophages. J Agric Food Chem. 2015;63(22):5449–5458. doi: 10.1021/acs.jafc.5b00560 [DOI] [PubMed] [Google Scholar]
- 11.Sun YL, Liu J, Jiang XX, et al. One-step synthesis of chiral oxindole-type analogues with potent anti-inflammatory and analgesic activities. Sci Rep. 2015;5:13699. doi: 10.1038/srep13699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Wang XH, Chen QB, et al. Camellia sinensis and Litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol Nutr Food Res. 2020;64(6):e1900943. doi: 10.1002/mnfr.201900943 [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Ho CT, Li XF, et al. Phytochemicals, anti-inflammatory, antiproliferative, and methylglyoxal trapping properties of zijuan tea. J Food Sci. 2018;83(2):517–524. doi: 10.1111/1750-3841.14029 [DOI] [PubMed] [Google Scholar]
- 14.Peng JM, Jia Y, Hu TY, et al. GC-(4→8)-GCG, a proanthocyanidin dimer from Camellia ptilophylla, modulates obesity and adipose tissue inflammation in high-fat diet induced obese mice. Mol Nutr Food Res. 2019;63(11):e1900082. doi: 10.1002/mnfr.201900082 [DOI] [PubMed] [Google Scholar]
- 15.Wang ZN, Guan Y, Yang R, Li JJ, Wang JS, Jia AQ. Anti-inflammatory activity of 3-cinnamoyltribuloside and its metabolomic analysis in LPS-activated RAW 264.7 cells. BMC Complement Med Ther. 2020;20(1):329. doi: 10.1186/s12906-020-03115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hisanaga A, Ishida H, Sakao K, et al. Anti-inflammatory activity and molecular mechanism of Oolong tea theasinensin. Food Funct. 2014;5(8):1891–1897. doi: 10.1039/c4fo00152d [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Rahman SU, Huang YY, et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J Nutr Biochem. 2020;78:108324. doi: 10.1016/j.jnutbio.2019.108324 [DOI] [PubMed] [Google Scholar]
- 18.Hudlikar RR, Venkadakrishnan V, Kaushal RK, et al. Polymeric black tea polyphenols (PBPs) inhibit benzo (a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced lung carcinogenesis potentially through down-regulation of p38 and Akt phosphorylation in A/J mice. Mol Carcinog. 2017;56(2):625–640. doi: 10.1016/j.jnutbio.2019.108324 [DOI] [PubMed] [Google Scholar]
- 19.Wang HY, Zhao MM, Yang B, Jiang YM, Rao GH. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008;107(4):1399–1406. doi: 10.1016/j.foodchem.2007.09.068 [DOI] [Google Scholar]
- 20.Xie H, Sun JQ, Chen YQ, Zong M, Li SJ, Wang Y. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxid Med Cell Longev. 2015;2015:214836. doi: 10.1155/2015/214836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duarte LJ, Chaves VC, dos Santos Nascimento MVP, et al. Molecular mechanism of action of pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018;247:56–65. doi: 10.1016/j.foodchem.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 22.Hajiaghaalipour F, Kanthimathi MS, Sanusi J, Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015;169:401–410. doi: 10.1016/j.foodchem.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 23.Oboh G, Ademiluyi AO, Akinyemi AJ, Henle T, Saliu JA, Schwarzenbolz U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J Funct Foods. 2012;4(2):450–458. doi: 10.1016/j.jff.2012.02.003 [DOI] [Google Scholar]
- 24.Etxeberria U, Fernández-Quintela A, Milagro FI, Aguirre L, Martínez JA, Portillo MP. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J Agric Food Chem. 2013;61(40):9517–9533. doi: 10.1021/jf402506c [DOI] [PubMed] [Google Scholar]
- 25.Chuang CC, McIntosh MK. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr. 2011;31:155–176. doi: 10.1146/annurev-nutr-072610-145149 [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Lin Y, Zhao YB, et al. Polyphenol-rich blue honeysuckle extract alleviates silica particle-induced inflammatory responses and macrophage apoptosis via NRF2/HO-1 and MAPK signaling. J Funct Foods. 2018;46:463–474. doi: 10.1016/j.jff.2018.05.024 [DOI] [Google Scholar]
- 27.Bao LP, Li JS, Zha DQ, et al. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the NRF2/HO-1 and NF-κB pathways. Int Immunopharmacol. 2018;54:245–253. doi: 10.1016/j.intimp.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 28.Zhang TT, Wang M, Yang L, Jiang JG, Zhao JW, Zhu W. Flavonoid glycosides from Rubus chingii Hu fruits display anti-inflammatory activity through suppressing MAPKs activation in macrophages. J Funct Foods. 2015;18:235–243. doi: 10.1016/j.jff.2015.07.006 [DOI] [Google Scholar]
- 29.Min TL, Bartholomew B. Theaceae, Camellia. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. Beijing: Science Press & St. Louis: Missouri Botanical Garden Press; 2007:367–372. Available from: http://flora.huh.harvard.edu/china/mss/volume12/Theaceae.pdf. Accessed January 24, 2022. [Google Scholar]
- 30.Liu Y, Zhao P, Bian JT, et al. Nutritional analysis and evaluation of Camellia fascicularis leaves. J Northwest A&F Univ. 2021;49(5):146–154. doi: 10.13207/j.cnki.jnwafu.2021.05.019 [DOI] [Google Scholar]
- 31.Liu Y, Kan H, Fan FY, Tang JR, Zhao P. Microwave-assisted extraction and antioxidant activities of polyphenols from Camellia fascicularis leaves. Curr Top Nutraceut Res. 2019;17(2):164–171. doi: 10.37290/ctnr2641-452X.17:164-171 [DOI] [Google Scholar]
- 32.Xu MJ, Shao QS, Ye SY, et al. Simultaneous extraction and identification of phenolic compounds in Anoectochilus roxburghii using microwave-assisted extraction combined with UPLC-Q-TOF-MS/MS and their antioxidant activities. Front Plant Sci. 2017;8:1474. doi: 10.3389/fpls.2017.01474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao DR, Jiang YS, Sun JY, Li HH, Luo XL, Zhao MM. Anti-inflammatory mechanism involved in 4-ethylguaiacol-mediated inhibition of LPS-induced inflammation in THP-1 cells. J Agric Food Chem. 2019;67(4):1230–1243. doi: 10.1021/acs.jafc.8b06263 [DOI] [PubMed] [Google Scholar]
- 34.Wang YH, Li B, Ma Y, et al. Lonicera caerulea berry extract attenuates lipopolysaccharide induced inflammation in BRL-3A cells: Oxidative stress, energy metabolism, hepatic function. J Funct Foods. 2016;24:1–10. doi: 10.1016/j.jff.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 35.Su JD, Osawa T, Kawakishi S, Namiki M. Tannin antioxidants from Osbeckia chinensis. Phytochemistry. 1988;27(5):1315–1319. doi: 10.1016/0031-9422(88)80184-5 [DOI] [Google Scholar]
- 36.Shao YP, Zhang W, Tong L, et al. Simultaneous determination of eight bioactive components of Qishen Yiqi Dripping Pills in rat plasma using UFLC–MS/MS and its application to a pharmacokinetic study. Biomed Chromatogr. 2017;31(8):e3941. doi: 10.1002/bmc.3941 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Chen YT. Two flavonoids from Lagopsis supina. Acta Pharm Sin. 2002;37(3):186–188. doi: 10.1007/978-3-662-04835-1_146 [DOI] [PubMed] [Google Scholar]
- 38.Noreljaleel AEM, Kemp G, Wilhelm A, van der Westhuizen JH, Bonneta SL. Analysis of commercial proanthocyanidins. part 5: a high resolution mass spectrometry investigation of the chemical composition of sulfited wattle (Acacia mearnsii De Wild.) bark extract. Phytochemistry. 2019;162:109–120. doi: 10.1016/j.phytochem.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 39.Liu GQ, Dong J, Wang H, Wan YR, Duan YS, Chen SZ. ESI fragmentation studies of four tea catechins. Chem J Chin Univ. 2009;30(8):1566–1570. doi: 10.3321/j.issn:0251-0790.2009.08.017 [DOI] [Google Scholar]
- 40.Fang XP, Anderson JE, Chang CJ, Fanwick PE, McLaughlin JL. Novel bioactive styryl-lactones: goniofufurone, goniopypyrone, and 8-acetylgoniotriol from Goniothalamus giganteus (Annonaceae). X-ray molecular structure of goniofufurone and of goniopyptrone. J Chem Soc Perkin Trans. 1990;1(6):1655–1661. doi: 10.1039/p19900001655 [DOI] [Google Scholar]
- 41.Huang B, Hu YZ, Li X, Wu XH, Wang YM, Chen L. Analysis of chemical constituents in Odontosoria chinensis based on UPLC-Q-TOF-MS. Pract Clin J Integr Tradit Chin West Med. 2021;21(9):155–159. doi: 10.13638/j.issn.1671-4040.2021.09.077 [DOI] [Google Scholar]
- 42.Zhang GQ, Yang YS, Xu YM, Luo HL, Yao JX, Lin ZX. Identify the chemical composition of Perilla frutescens L. by high resolution ion mobility liquid chromatography-mass spectrometry. J Fujian Agric Forest Univ. 2019;48(1):123–130. doi: 10.13323/j.cnki.j.fafu(nat.sci.).2019.01.020 [DOI] [Google Scholar]
- 43.Tan GS, Zuo CX. Studies on the chemical constituents of Hylotelephium mingjinianum (S. H. FU) H. Ohba. Acta Pharm Sin. 1994;29(7):519–525. doi: 10.16438/j.0513-4870.1994.07.008 [DOI] [Google Scholar]
- 44.Zhou ZH, Zhang YJ, Yang CR. Saluenin, a new flavonol glycoside from Camellia saluenensis. Plant Diversity. 2000;22(1):90–96. doi: 10.3969/j.issn.2095-0845.2000.01.014 [DOI] [Google Scholar]
- 45.Zakharova OI, Zakharov AM, Glyzin VI. Flavonoids of Agastache rugose. Chem Nat Compd. 1979;15(5):561–564. doi: 10.1007/BF00565924 [DOI] [Google Scholar]
- 46.Li YM, Che QM. Studies on chemical components of Carthamus tinctorius petals. Acta Pharm Sin. 1998;33(8):626–628. doi: 10.16438/j.0513-4870.1998.08.013 [DOI] [PubMed] [Google Scholar]
- 47.Dong H, Chen SX, Kini RM, Xu HX. Effects of Tannins from Geum japonicum on the catalytic activity of thrombin and factor Xa of blood coagulation cascade. J Nat Prod. 1998;61(11):1356–1360. doi: 10.1021/np9801458 [DOI] [PubMed] [Google Scholar]
- 48.Fraga CG, Oteiza PI, Galleano M. Plant bioactives and redox signaling: (–)-Epicatechin as a paradigm. Mol Aspects Med. 2018;61:31–40. doi: 10.1016/j.mam.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 49.Zielińska S, Matkowski A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem Rev. 2014;13(2):391–416. doi: 10.1007/s11101-014-9349-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh G, Passsari AK, Leo VV, et al. Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Front Plant Sci. 2016;7:407. doi: 10.3389/fpls.2016.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Xiao CM, Xu HS, et al. Anti-inflammatory effects of Ganoderma lucidum sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 264.7 macrophages. Food Chem Toxicol. 2021;150:112073. doi: 10.1016/j.fct.2021.112073 [DOI] [PubMed] [Google Scholar]
- 52.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernabé J, Mulero J, Cerdá B, et al. Effects of a citrus based juice on biomarkers of oxidative stress in metabolic syndrome patients. J Funct Foods. 2013;5(3):1031–1038. doi: 10.1016/j.jff.2013.02.003 [DOI] [Google Scholar]
- 54.Wang YH, Li B, Zhu JY, et al. Lonicera caerulea berry extract suppresses lipopolysaccharide-induced inflammation via Toll-like receptor and oxidative stress-associated mitogen-activated protein kinase signaling. Food Funct. 2016;7(10):4267–4277. doi: 10.1039/c6fo00627b [DOI] [PubMed] [Google Scholar]
- 55.Hung TV, Suzuki T. Short-chain fatty acids suppress inflammatory reactions in Caco-2 cells and mouse colons. J Agric Food Chem. 2018;66(1):108–117. doi: 10.1021/acs.jafc.7b04233 [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS Jr, Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IκB kinase activity. Blood. 2004;104(4):1100–1109. doi: 10.1182/blood-2003-12-4302 [DOI] [PubMed] [Google Scholar]
- 57.Fang J, Muto T, Kleppe M, et al. TRAF6 mediates basal activation of NF-κB necessary for hematopoietic stem cell homeostasis. Cell Rep. 2018;22(5):1250–1262. doi: 10.1016/j.celrep.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeung YT, Aziz F, Guerrero-Castilla A, Arguelles S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr Pharm Des. 2018;24(14):1449–1484. doi: 10.2174/1381612824666180327165604 [DOI] [PubMed] [Google Scholar]
- 59.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142 [DOI] [PubMed] [Google Scholar]
- 60.Cicero AFG, Fogacci F, Colletti A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br J Pharmacol. 2017;174(11):1378–1394. doi: 10.1111/bph.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan B, Zhao LY, Rakariyatham K, et al. Isolation of a novel bioactive protein from an edible mushroom Pleurotus eryngii and its anti-inflammatory potential. Food Funct. 2017;8(6):2175–2183. doi: 10.1039/c7fo00244k [DOI] [PubMed] [Google Scholar]
- 62.Himaya SWA, Ryu B, Qian ZJ, Kim SK. Paeonol from Hippocampus kuda Bleeler suppressed the neuro-inflammatory responses in vitro via NF-κB and MAPK signaling pathways. Toxicol in vitro. 2012;26(6):878–887. doi: 10.1016/j.tiv.2012.04.022 [DOI] [PubMed] [Google Scholar]
- 63.Pan LL, Dai M. Paeonol from Paeonia suffruticosa prevents TNF-α-induced monocytic cell adhesion to rat aortic endothelial cells by suppression of VCAM-1 expression. Phytomedicine. 2009;16(11):1027–1032. doi: 10.1016/j.phymed.2009.04.003 [DOI] [PubMed] [Google Scholar]