Abstract
Deregulated Wnt/β-catenin signaling is one of the main genetic alterations in human hepatocellular carcinoma (HCC). Comprehensive genomic analyses have revealed that gain-of-function mutation of CTNNB1, which encodes β-catenin, and loss-of-function mutation of AXIN1 occur in approximately 35% of human HCC samples. Human HCCs with activation of the Wnt/β-catenin pathway demonstrate unique gene expression patterns and pathological features. Activated Wnt/β-catenin synergizes with multiple signaling cascades to drive HCC formation, and it functions through its downstream effectors. Therefore, strategies targeting Wnt/β-catenin have been pursued as possible therapeutics against HCC. Here, we review the genetic alterations and oncogenic roles of aberrant Wnt/β-catenin signaling during hepatocarcinogenesis. In addition, we discuss the implication of this pathway in HCC diagnosis, classification, and personalized treatment.
Introduction
Hepatocellular carcinoma (HCC) is the most common form of liver cancer, representing the third leading cause of cancer-related death (1). The incidence of HCC ranks sixth among all tumor types worldwide. Increased HCC occurrence in this decade reflects persistent hepatitis B and C virus infection and the increase of nonalcoholic steatohepatitis (NASH) since 2000 (2). A projection study indicated that the age-standardized incidence rates per 100,000 person-years for primary liver cancer would increase in both men and women by the year 2030 in most countries as a result of increased NAFLD and/or NASH (3). During the past decade, multitargeted tyrosine kinase inhibitors (TKIs), such as sorafenib, lenvatinib, regorafenib, and cabozantinib, have been used as first- or second-line drugs for patients with unresectable HCC (4). However, these agents provide limited survival benefits and are associated with considerable toxicities and poor quality-of-life outcomes. Immune checkpoint inhibitors (ICIs) have been approved for HCC treatment and show a similar response rate (15%–30%) compared with TKI therapies (5). For example, HCC patients who received the CTLA4-blocking ICI tremelimumab showed a partial response rate of 18% and a disease control rate of 76% (6). PD-1 and PD-L1 blockade showed higher objective response rates, which could reach 20% in advanced HCC patients (7). Recently, the phase III IMbrave150 trial results showed that combining an anti–PD-L1 antibody with an anti–VEGF-A antibody leads to promising efficacy for advanced HCC patients (8). Currently, this combination immunotherapy has become the first-line treatment strategy against HCC (9). Nevertheless, most patients eventually progress under this regimen. Therefore, studies to elucidate the molecular mechanisms underlying HCC pathogenesis are imperative to develop additional and more effective drugs for precision medicine.
Molecular mechanisms of Wnt/β-catenin activation in HCC
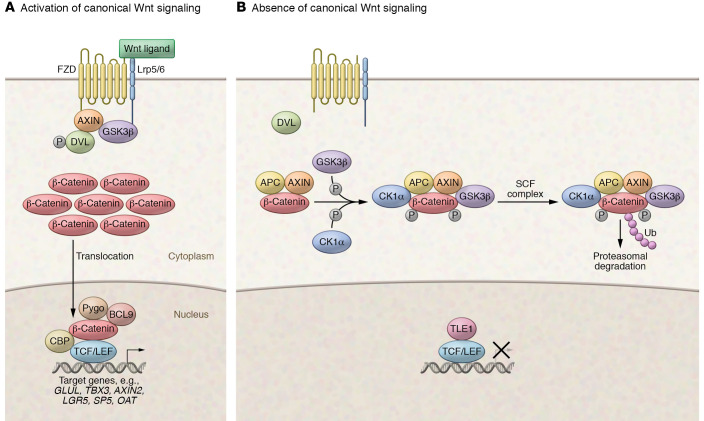
The Wnt/β-catenin cascade is one of the major signaling pathways regulating liver homeostasis, regeneration, and tumorigenesis (10), which has been extensively reviewed (11, 12). In brief, in the absence of Wnt ligands, most cellular β-catenin is sequestered in the adherens junctions at the plasma membrane (Figure 1). Cytosolic β-catenin associates in a complex with adenomatous polyposis coli (APC) and AXIN1 proteins, which mediate the N-terminal phosphorylation of β-catenin. This event leads to the ubiquitination of β-catenin by the E3 ubiquitin ligase β-transducin repeat–containing protein (β-TRCP) and subsequent proteasomal degradation. When Wnt ligands bind to the Frizzled receptors, Dvl/Dsh is phosphorylated and, in turn, recruits AXIN1 and GSK3β adjacent to the plasma membrane, thus preventing the formation of the degradation complex. As a result, unphosphorylated β-catenin escapes recognition by β-TRCP and translocates into the nucleus, where it binds to the T cell factor (TCF) and lymphoid enhancer–binding protein family (LEF) transcription factors. The activated β-catenin/TCF/LEF complex induces the transcription of genes regulating cell proliferation and survival (Figure 1).
Figure 1. Canonical Wnt/β-catenin signaling pathway in HCC.
(A) When Wnt ligands are present, Wnt/FZD signaling activation leads to the phosphorylation of mammalian homolog of dishevelled (DVL). Phosphorylated DVL recruits AXIN and GSK3β to the plasma membrane, hence blocking the degradation complex’s formation. Subsequently, β-catenin accumulates in the cytoplasm and then translocates into the nucleus. Nuclear β-catenin binds to TCF/LEF transcription factors and promotes the transcription of target genes. (B) When Wnt ligands are absent, soluble β-catenin is phosphorylated by the GSK3β-CK1α-APC-AXIN1 complex. Once phosphorylated, β-catenin is degraded by the proteasome after ubiquitination by the Skp-, Cullin-, and F-box–containing (SCF) protein complex. When β-catenin is absent in the nucleus, the TCF/LEF transcription factors are repressed by TLE-1. CTNNB1 (encoding β-catenin), AXIN1, and APC are mutated in 27%, 8%, and 3% of human HCCs, respectively.
In the normal liver, β-catenin is membrane-localized in hepatocytes, and the Wnt/β-catenin pathway is activated in pericentral hepatocytes, which is demonstrated by β-catenin–dependent glutamine synthetase (GS) staining in these cells (13, 14). In HCC, recent genomic studies revealed that 30% to 40% of tumors demonstrate aberrant activation of the Wnt/β-catenin cascade (15). The activation of this pathway could be subdivided into somatic genetic events and nongenetic events. For somatic mutations leading to Wnt/β-catenin activations, The Cancer Genome Atlas (TCGA) analysis reveals that gain-of-function (GOF) mutations of CTNNB1, which encodes β-catenin, occur in 27% of HCC patients (Figure 1). Most CTNNB1 missense mutations arise at the serine/threonine sites of exon 3 or adjacent amino acids, which prevents the β-catenin protein from phosphorylation and degradation, leading to its stabilization and unrestrained transcriptional activity (14). In addition, mutations in armadillo repeat domains 5 and 6 of the β-catenin protein are also frequently observed in human HCCs (16). Studies have suggested that these amino acid substitutions have reduced binding to APC, leading to increased Wnt/β-catenin signaling (16). Mutations have also been observed in APC and AXIN1, encoding critical components of the β-catenin degradation complex. Mutations in APC and AXIN1 are found in 3% and 8% of HCC, respectively (Figure 1). APC and AXIN1 mutations are mostly missense, deleted, and/or truncated mutations, resulting in loss of protein expression and function, a characteristic of tumor suppressors (17). Importantly, mutations of CTNNB1, APC, or AXIN1 rarely co-occur in the same HCC, suggesting that these mutations lead to common downstream effectors. Notably, HCC patients harboring GOF CTNNB1 mutations demonstrate robust upregulation of canonical Wnt target genes, including GLUL, TBX3, AXIN2, LGR5, SP5, and OAT (Figure 1).
Studies have also revealed multiple nongenetic mechanisms leading to Wnt/β-catenin activation. These include promoter hypermethylation and related silencing of the secreted Frizzled-related protein 1 gene (SFRP1), a Wnt/β-catenin antagonist (18); overexpression of Frizzled (FZD) membrane receptor and Wnt ligands (19); and deregulated expression of microRNAs (20) and long noncoding RNAs (21) that regulate Wnt/β-catenin signaling.
Unique features of HCC with Wnt/β-catenin activation
Studies have illustrated that human HCCs with aberrant Wnt/β-catenin activation have distinct clinical, pathological, and molecular features. Multiple investigations suggest that overexpression and mutations of β-catenin occur more frequently in HCV-related HCCs than in HBV-related HCCs (22–24) and are commonly observed in HCC with noncirrhotic liver in the absence of usual HCC risk factors (25, 26). Activation of the Wnt/β-catenin cascade has been linked to early-stage HCC (24, 27), but also tumor progression (28). Association between β-catenin activation and HCC patient survival remains controversial, with most studies suggesting that CTNNB1 mutation is a favorable prognostic marker. For instance, using meta-analysis, Wang et al. reported that HCC patients with CTNNB1 mutations demonstrate a more prolonged overall survival (29). Similar results came from a study by Ding et al. (30). However, Lu and colleagues reported that CTNNB1 mutations are not associated with prognosis in advanced HCC (31).
The histopathological features of human HCC lesions with β-catenin activation have also been extensively investigated, providing conflicting results. For instance, Hsu et al. showed that β-catenin mutations are associated with grade I HCC (22). In addition, Wong et al. found that HCCs with a non-nuclear type of β-catenin overexpression have poorer cellular differentiation (32). In contrast, there were no significant differences in HCC tumor grade between β-catenin–positive and –negative tumors in two other investigations (33, 34). These discrepancies remain to be addressed and might be due to the different analyses conducted (using either HCCs with β-catenin mutations or nuclear accumulation of the protein for the comparisons) or the lack of a standard and specific delineation of β-catenin–“positive” tumors based on the staining patterns (i.e., the percentage of cells positive for nuclear β-catenin defining an HCC as either β-catenin positive or negative). Finally, Audard et al. were the first to try to outline macroscopic and microscopic features of CTNNB1-mutated HCCs (25). They demonstrated that CTNNB1-mutated HCCs are usually large (>6 cm in diameter) and solitary lesions. Typical, albeit non-pathognomonic, microscopic features of CTNNB1-mutated HCCs are microtrabecular and acinar growth, a high degree of differentiation (Edmondson grade G1–G2), homogeneous microscopic appearance, prominent cholestasis, and lack of steatosis and inflammation. Interestingly, they showed that robust and uniform immunohistochemical expression of glutamine synthetase (GS), a target of the Wnt/β-catenin pathway, was more sensitive (90%) than cytoplasmic/nuclear β-catenin positivity (63%) in identifying CTNNB1-mutated HCCs, though with equal specificity (both 98%). Indeed, based on TCGA analysis, the upregulation of GLUL, which encodes GS, and other canonical Wnt/β-catenin target genes is strongly associated with CTNNB1 mutation status in HCC (Figure 1). These results were confirmed by Calderaro et al. in a large study comparing the correlation of morphology and molecular features in a large cohort of HCCs (35).
Overall, human HCCs can be subdivided into two major groups: a proliferation group and a nonproliferation group (36, 37). Each of these groups accounts for approximately 50% of human HCCs and consists of several subgroups identified in various genomic studies (Figure 1B). In addition, based on TCGA studies, HCC could be classified into clusters 1, 2, and 3 (38). Clusters 1 and 3 belong to the proliferation group and cluster 2 to the nonproliferation group. Boyault et al. further defined human HCCs into G1 to G6 subgroups (39). Among them, G1, G2, and G3 are classified as proliferation group, whereas G4, G5, and G6 are defined as nonproliferation group. The proliferation group and the nonproliferation group show different molecular, genetic, epigenetic, and clinical features. The proliferation group is associated with chromosomal instability, DNA hypomethylation, alcohol- or HCV-related HCC, low serum α-fetoprotein levels, and low frequency of vascular invasion. In contrast, the nonproliferation group is characterized by chromosomal stability, promoter hypermethylation, frequent HBV infection, more aggressive phenotype, poor tumor differentiation, high serum α-fetoprotein levels, and increased vascular invasion (40). Intriguingly, GOF CTNNB1 mutations are frequently found in the nonproliferation group, and are associated with cluster 2 and G5/G6 subgroups (Figure 1B). In contrast, HCCs with AXIN1 mutations belong to the proliferation group, and are associated with cluster 1 and G1 subgroups (Figure 1B).
Induction of hepatocarcinogenesis by Wnt/β-catenin
Activated Wnt/β-catenin signaling has been considered an early signaling event in HCC pathogenesis (41, 42). Importantly, studies have shown that CTNNB1 mutation is one of the significant key genetic events in human HCCs (43, 44). Furthermore, Wnt/β-catenin has also been implicated in HCC stemness, progression, metastasis, and drug resistance (45–49). For instance, this pathway has been identified as the prominent signaling that causes the proliferation of cancer stem cells (CSCs). Indeed, overexpression of β-catenin increases self-renewal and in vivo tumorigenicity of HCC CSCs (50–52). Furthermore, activated Wnt/β-catenin has also been associated with resistance to sorafenib and regorafenib in HCC patients (51, 53). All these data support the critical roles of Wnt/β-catenin in various steps of hepatocarcinogenesis.
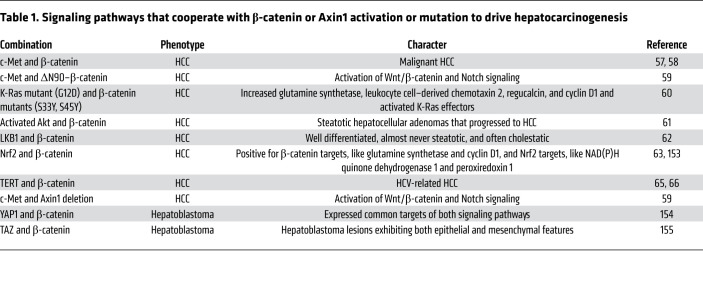
The oncogenic role of Wnt/β-catenin mutations in HCC was first investigated in transgenic mice. Importantly, transgenic mice overexpressing activated mutant forms of β-catenin develop hepatomegaly, but not HCC (54, 55). These results indicate that activation of Wnt/β-catenin alone may not be sufficient to drive hepatocarcinogenesis. Instead, a second signal is required to cooperate with activated β-catenin to induce HCC development. Consistent with this hypothesis, recent studies using hydrodynamic transfection (56) have demonstrated that oncogenic forms of β-catenin cooperate with other proto-oncogenes such as c-Met (57–59), K-RasV12 (60), activated Akt (61), LKB1 (62), and Nrf2 (63) to induce HCC formation in mice (Table 1). In human HCCs, coordinated activation of c-Met and β-catenin was found in approximately 10% of samples (64). While overexpression of c-Met or the activated mutant form of β-catenin via hydrodynamic injection alone cannot promote HCC formation in mice, coexpression of c-Met and activated β-catenin induces liver tumor development within 6–8 weeks after injection (58). Concomitant CTNNB1 mutations and NFE2L2/KEAP1 mutations, which lead to action of the Nrf2 pathway, occur in approximately 9% of human HCCs (63). Coexpression of activated forms of β-catenin with mutant NFE2L2, but not the wild-type form of NFE2L2, can induce HCC development in mice (63). Loss-of-function AXIN1 mutations and c-Met activation were detected in approximately 4% of human HCC, and coexpression of c-Met together with CRISPR/Cas9–based targeting of Axin1 (sgAxin1) in the mouse liver triggers HCC formation (59). Consequent RNA-Seq studies have demonstrated that these murine HCCs share similar gene expression patterns to the subset of human HCCs harboring similar genetic events. In addition, TERT promoter mutations are found in many HCC tissues with CTNNB1 mutations, indicating a possible synergistic effect of these two genes (65, 66).
Table 1. Signaling pathways that cooperate with β-catenin or Axin1 activation or mutation to drive hepatocarcinogenesis.
Once activated, β-catenin triggers the induction of downstream target expression via the TCF/LEF1 family of transcription factors. Many of these target genes are implicated in hepatocarcinogenesis. c-MYC is one of the best-characterized downstream effectors of β-catenin. However, c-MYC is also regulated by many other mechanisms, such as amplification of the c-MYC locus, increased protein stability, and activation of estrogen receptor, Ras/Raf, and IFN-γ pathways (67–69). c-MYC was first identified as a Wnt/β-catenin target gene in the human HT29 colorectal cancer cell line harboring mutant APC alleles (70). Subsequently, multiple Wnt response elements were identified in the c-MYC promoter (71). Furthermore, in human HCC, c-MYC could be induced by β-catenin activation (72, 73), and this pathway plays a critical role in gankyrin-driven increased glycolysis and glutaminolysis (74) as well as in sorafenib responsiveness (75).
Cyclin D1 is another direct target of β-catenin and might be a key molecule by which activated β-catenin promotes tumor cell proliferation (76, 77). Numerous studies have demonstrated that activated Wnt/β-catenin induces cyclin D1 expression in mouse and human HCC (78, 79). However, it is worth mentioning that cyclin D1 is not an exclusive effector of the Wnt/β-catenin signaling pathway. Indeed, other molecular cascades could regulate its expression, such as the NF-κB and MAPK pathways (80, 81). Studies conducted in vivo have also illustrated the critical role of cyclin D1 in HCC development (82). Specifically, the coexpression of c-Met and activated mutant forms of β-catenin rapidly induces HCC formation in mice; overexpression of c-Met and cyclin D1 also induces liver tumor development in mice, albeit with longer latency (58). Nevertheless, using Ccnd1-knockout mice, Patil et al. showed that cyclin D1 expression is not essential for liver tumor development induced by c-Met and activated mutant forms of β-catenin (58). Mechanistically, cyclin D2 expression in the liver is compensatorily upregulated upon cyclin D1 loss (58). Intriguingly, overexpression of cyclin D1 has also been shown to indirectly enhance the Wnt/β-catenin pathway, leading to increased HCC metastasis (83). Altogether, these studies suggest the interconnected and feedback mechanisms between cyclin D1 and Wnt/β-catenin cascades during hepatocarcinogenesis.
GS, which promotes glutamine synthesis in cells, is a liver-specific Wnt/β-catenin target (84). In normal liver, GS is expressed in a layer of pericentral hepatocytes. Liver-specific knockout of β-catenin in mice leads to complete loss of the pericentral expression of GS (85). As we discussed above, immunostaining of GS may represent a pathological marker for human HCCs with GOF CTNNB1 mutations (86), although GS expression could also be induced by other factors (87). Studies have shown that GS regulates autophagy downstream of activated β-catenin, which confers sensitivity to sorafenib. Notably, GS-mediated glutamine synthesis is required for CTNNB1-mutated HCC growth, since glutamine deprivation inhibits CTNNB1-mutated HCC growth in vitro and in vivo (88). Amino acids, including glutamine, are major regulators of mTOR activity in cells (89). Recently, it has been discovered that GS-mediated increased glutamine synthesis leads to mTORC1 activation (90). Accordingly, a strong correlation between activated β-catenin and positive expression of phosphorylated mTOR-S2448 (p–mTOR-S2448) characterizes human HCCs. In addition, CTNNB1-mutated HCCs are mTORC1-addicted, owing to the GS/glutamine/p–mTOR-S2448 axis. These studies suggest that mTORC1 inhibitors could be effective for treating CTNNB1-mutant and GS-positive human HCCs.
In addition to the genes mentioned above, activated Wnt/β-catenin drives the expression of hundreds of other genes, thus architecting a network of molecules that contributes to tumorigenesis (91, 92). For example, activated Wnt/β-catenin induces the expression of AXIN2, which functions as a negative-feedback mechanism to inhibit β-catenin, perhaps avoiding the harmful effects of a completely uncontrolled β-catenin activity (93). TBX3 is another liver-specific Wnt/β-catenin target gene that can contribute to specific pathological phenotypes via inhibition of the YAP cascade (94). Kinesin family member 2C (KIF2C) is also a direct target of the activated Wnt/β-catenin pathway (95). Its expression is upregulated in HCC and is associated with a poor prognosis. Furthermore, KIF2C enhances mTORC1 activation, providing another link between activated β-catenin and the mTOR cascade in HCC (95). In addition, Wnt/β-catenin is known to induce the expression of multiple matrix metalloproteinases (MMPs), such as MMP2 and MMP9, which contribute to tumor metastasis (96). VEGF-A and VEGF-C, key molecules promoting angiogenesis, are induced by Wnt/β-catenin (97). Moreover, Wnt/β-catenin positively regulates MCL1 expression, associated with sorafenib sensitivity in HCC (98). In addition to activating genes or pathways, Wnt/β-catenin negatively regulates signaling cascades. In the intestine, Wnt inhibits the MAPK pathway (99), whereas, in the liver, it suppresses the NF-κB cascade (100). In mice with liver-specific knockout of Ctnnb1, there is increased RelA expression and LPS-induced NF-κB activation (101). However, the inhibitory activities of the Wnt/β-catenin cascade in hepatocarcinogenesis have not been well characterized and require further investigation.
Targeting Wnt/β-catenin for HCC treatment
Since Wnt pathway activation promotes HCC cell proliferation, migration, and invasion, targeting this signaling cascade is an attractive therapeutic approach for human HCC treatment. Several agents have been screened and investigated for targeting the Wnt pathway in cancer, and some of them are under development. Those agents include small-molecule inhibitors that block the interaction of β-catenin with TCF, such as the fungal derivatives PKF115–854 and CGP049090 (102–106), or the binding of β-catenin to cAMP response element–binding protein (CREB)–binding protein (CBP), such as ICG-001 (107–109). Both PKF115–854 and CGP049090 have shown inhibitory effects against HCC cell growth (45, 106). Therapeutic monoclonal antibodies against Wnts were also developed to block the binding of Wnts to Frizzled (FZ/FZD) receptors, such as anti-Wnt2 monoclonal antibodies (110) and the anti-FZD monoclonal antibody OMP-18R5 (111). Moreover, several approved drugs currently in clinical use have been shown to possess activity against the Wnt pathway (112, 113). These include indomethacin (114, 115), pyrvinium (116), sulindac (117), aspirin (114), celecoxib, and rofecoxib (118). Unfortunately, the antitumor potency of these repurposed drugs has not been established clinically.
In addition to Wnt/TCF inhibitors, agents targeting porcupine (PORCN) or tankyrase (TNKS) have also been developed to block Wnt/β-catenin signaling in cancer cells. PORCN is an O-acyltransferase essential for Wnt ligand secretion (119). The PORCN inhibitors, such as LGK-974 (WNT-974) and ETC-159, may inhibit tumor growth via suppression of Wnt signaling. Indeed, studies have shown that LGK-974 can enhance the radiosensitivity of HepG2 cells by modulating Nrf2 signaling (120), and it is investigated in clinical trials for treating various solid tumors (121). TNKS targets AXIN protein for degradation, whereas TNKS inhibition can stabilize AXIN, thus antagonizing Wnt signaling (122). Several TNKS inhibitors with promising therapeutic effects have been developed, including XAV939, G007-LK, G244-LM, RK-287107, JW55, K-756, IWR-1, MSC2504877, AZ1366, JW74, and NVP-TNKS656 (123–132). Preclinical studies have shown that TNKS inhibitors, such as XAV939, can potently inhibit HCC growth in culture (133). However, PORCN and TNKS inhibitors target pathways upstream of β-catenin; therefore, they are unlikely to possess any efficacy against HCCs with GOF CTNNB1 mutations.
Interfering RNA– or antisense RNA–based therapy is another approach to inhibit the Wnt/β-catenin pathway. In particular, siRNAs targeting Wnts have been shown to suppress HCC cell growth in vitro (134–136). In a GOF Ctnnb1-mutant mouse HCC model induced by diethylnitrosamine (DEN) and phenobarbital, use of locked nucleic acid (LNA) antisense oligonucleotides against β-catenin strongly impaired HCC progression (137). In contrast, in the non–Ctnnb1-mutant HCC model, induced by DEN only, LNA-si-β-catenin demonstrated no efficacy (137). The therapeutic efficacy of LNA-si-β-catenin has been further validated in vivo in mouse HCCs induced by hydrodynamic transfection of activated forms of K-Ras and β-catenin oncogenes (60).
In summary, various strategies targeting the Wnt/β-catenin cascade have been developed in recent decades. Preclinical studies have provided evidence to support targeting this pathway against cancers, including HCCs. Nevertheless, considerable challenges remain, especially concerning the toxicity of these inhibitors, which suppress the Wnt/β-catenin pathway in normal tissues as well. Thus, the clinical development of these molecules has been somewhat limited to date.
Wnt/β-catenin as a biomarker for resistance to immunotherapy
Immunotherapy has become the first-line treatment strategy against advanced HCC (9). As we discussed above, in the IMbrave150 phase III clinical trial for advanced-stage HCC patients, the combination of the anti–PD-L1 antibody atezolizumab and the anti-VEGF antibody bevacizumab demonstrated an objective response rate of 36% (8). Unfortunately, ICIs have limited efficacy as monotherapy against HCC. For instance, the anti–PD-1 monoclonal nivolumab failed to improve HCC patient survival versus sorafenib in the phase III CheckMate 459 trial (9). One of the primary reasons for the failure of these clinical trials is that no biomarker-based patient selection has been implemented. Therefore, it is plausible to hypothesize that some patients harbor genetic events that confer resistance to ICIs. In this regard, aberrant activation of Wnt/β-catenin has emerged as an important pathway mediating ICI resistance (138, 139). Harding et al. reported that in HCC patients treated with ICIs, activation of the Wnt/β-catenin pathway correlates with lower disease control rate and lower progression-free and overall survival rates (140). Furthermore, studies using mouse HCC models confirmed that upregulated Wnt/β-catenin signaling in HCC promotes immune evasion and confers resistance to anti–PD-1 therapy (141). Mechanistically, it was found that activated β-catenin inhibits CCL5 expression, leading to impaired dendritic cell recruitment. Likewise, activated β-catenin in melanoma cells enhances ATF3 expression and subsequently represses CCL4 expression, leading to reduced recruitment of dendritic cells and consequently T cells into the tumor tissues (142). These findings suggest that CTNNB1 mutational status could represent a novel biomarker for HCC patient exclusion for ICI treatment. Nevertheless, more studies are required to address the roles of the Wnt/β-catenin pathway in immunotherapy. For example, what is the Wnt/β-catenin mutation status in the IMbrave150 phase III clinical trial? Does the mutation status correspond to insensitivity to the combination immunotherapy or eventual progression over the treatment? Studies have suggested that NASH-related HCCs are particularly resistant to immunotherapies (143). Because the status of the Wnt/β-catenin pathway in NASH-related HCCs has not been well characterized, this question should be addressed using human HCC tissues and preclinical approaches.
Challenges and future directions
Despite extensive studies on the Wnt/β-catenin cascade during hepatocarcinogenesis, our understanding of the molecular pathways deregulated by activated Wnt/β-catenin and how we can effectively target Wnt/β-catenin remains quite limited. Here, we discuss several key issues that need to be addressed to guide us for precision medicine.
GOF CTNNB1 mutations and LOF AXIN1 mutations: same or different?
As we discussed above, both GOF CTNNB1 mutations and loss-of-function (LOF) AXIN1 mutations promote canonical Wnt pathway activation in HCC (59). Genetic studies have shown that these two mutations are mutually exclusive in human HCCs (Figure 1A), further supporting that they likely function via the major common pathway during hepatocarcinogenesis. Intriguingly, considerable differences have also been revealed based on recent genomic studies (Table 2). Specifically, HCCs with GOF CTNNB1 mutations belong to the nonproliferation group, whereas HCCs with LOF AXIN1 mutations are classified into the proliferation group (40). Additional molecular analysis revealed that AXIN1-mutant HCCs show relatively low canonical Wnt pathway activation levels but higher YAP/NOTCH induction, while CTNNB1-mutant HCCs show robust canonical Wnt pathway and mTOR signaling activation (144). These data suggest that GOF CTNNB1 and LOF AXIN1 might induce overlapping but also distinct downstream molecular events during hepatocarcinogenesis. It is tempting to hypothesize that LOF AXIN1-mutant HCCs, but not GOF CTNNB1-mutant tumors, depend on the YAP cascade for growth. If so, we need to understand how YAP becomes activated downstream of LOF AXIN1, and whether targeting YAP, such as using TNKS inhibitors, will lead to regression of HCC with LOF AXIN1 mutations.
Table 2. Distinct features of HCCs with AXIN1 or CTNNB1 mutations.
What is the role of canonical Wnt/β-catenin signaling in HCCs in the absence of AXIN1 or CTNNB1 mutations?
Based on the published data and the recent genomic studies, such as TCGA analysis, it is clear that Wnt ligands and their receptors are frequently upregulated in human HCC samples. However, one can also clearly see that high expression of canonical Wnt target genes, including GLUL (encoding GS) and TBX3, tracks strongly with GOF CTNNB1 mutations in human HCC samples (Figure 1A). Therefore, upregulation of Wnt ligands/receptors obviously does not induce strong activation of the canonical Wnt/β-catenin pathway. What is the functional role of the canonical Wnt/β-catenin cascade during HCC molecular pathogenesis in the absence of AXIN1 or CTNNB1 mutations? Most studies so far have relied on HCC cell lines (60, 113, 145). However, studies have suggested that Wnt ligands are likely to be produced by cells within the microenvironment. For example, in the normal liver, Wnts are secreted from sinusoid endothelial cells (146) or Kupffer cells during liver regeneration (147). The cellular sources of Wnt ligands in HCC remain to be defined. If they are secreted by the cells within the tumor microenvironment, it would be essential to investigate this canonical Wnt/β-catenin signaling in HCC when tumor cells are in their appropriate context, such as using murine HCC models. This question is critical to determine whether targeting of Wnt ligands, such as with PORCN inhibitors, may help to treat HCC without AXIN1 or CTNNB1 mutations.
Is mTOR inhibition effective for the treatment of HCCs with GOF CTNNB1 mutations?
As we discussed above, activated β-catenin leads to mTORC1 activation, and mouse HCCs with GOF Ctnnb1 mutations are sensitive to mTOR inhibition (90). On the other hand, monotherapy of everolimus, an mTOR inhibitor, has limited efficacy against advanced HCC (148). However, no biomarker-based patient selection was conducted in this clinical trial. This issue represents a major drawback of the trial, as the mTOR pathway is modulated by multiple cascades in cancer (149), including HCC (150); in addition, HCC is a highly heterogeneous disease. One can envision that the selection of patients with GOF CTNNB1 mutations might be helpful to demonstrate the clinical efficacy of this drug. Furthermore, everolimus is a first-generation and partial mTORC1 inhibitor. The second-generation mTOR inhibitors, including mTORC1/mTORC2 inhibitors and mTOR/PI3K inhibitors, might have improved efficacy against HCCs with GOF CTNNB1 mutations (151). Additional preclinical and clinical studies are required to address this critical issue.
Can gene editing to reverse CTNNB1 mutation be useful for HCC treatment?
Recent progress with CRISPR/Cas9–based gene editing technology opens the door to genetic modification of tumor cells. GOF CTNNB1 mutations, especially point mutations, are attractive targets for such a gene editing approach for cancer treatment. One can imagine delivering the proper guide RNA into HCC cells and reversing the mutant form of the CTNNB1 allele into the wild-type sequence. However, small molecules directly targeting Wnt/β-catenin are frequently associated with significant gastrointestinal toxicity, as Wnt/β-catenin is necessary for intestinal stem cell renewal and proliferation. This toxicity substantially limits the clinical application of these small molecules. The gene editing approach has the advantage of not affecting the Wnt/β-catenin pathway in any other cells besides HCC cells that harbor the CTNNB1 mutations. However, we do not know whether conversion into the wild-type CTNNB1 sequence will lead to HCC regression, since wild-type β-catenin may be sufficient to support HCC progression. In addition, an efficient delivery method so that the guide RNAs can target all HCC cells should be developed.
Wnt inhibitors: monotherapy or combination therapy?
As we discussed above, animal studies have demonstrated that the activation of Wnt/β-catenin alone is insufficient to promote HCC development. Instead, a second oncogenic signal is required for liver tumor formation (Table 1). Therefore, it is conceivable that targeting Wnt/β-catenin alone, either directly or indirectly (such as with mTOR inhibitors), is not sufficient to induce tumor regression. In contrast, combination therapies that target multiple signaling cascades might be required for efficient therapeutics. This point is highlighted by a recent study in murine HCC models coexpressing c-Met and ΔN90–β-catenin proto-oncogenes. In these mice, combined treatment with cabozantinib, which targets c-Met, and the dual mTOR inhibitor MLN0128, which targets activated β-catenin effectors, leads to tumor regression, whereas cabozantinib or MLN0128 monotherapy does not (152). As HCC is a heterogeneous disease, it would be critical to determine the specific pathways aberrantly activated in each HCC. Then one could design effective anti–Wnt/β-catenin–based combination therapies.
In summary, in the era of precision medicine, we can readily detect HCCs harboring activated Wnt/β-catenin signaling. These HCCs have peculiar molecular and pathological features and might be treated with effective and specific targeted therapies. However, our understanding of how the Wnt/β-catenin pathway contributes to HCC molecular pathogenesis remains incomplete. Therefore, additional molecular and biochemical studies are required to investigate this vital issue to identify novel targeted therapies against HCC with aberrant Wnt/β-catenin activation.
Acknowledgments
We apologize to those investigators whose publications were not cited owing to space limitations. CX is supported by the National Science Foundation of China (grant 82073091). XC is supported by NIH grants R01CA204586, R01CA239251, and R01CA250227 as well as P30DK026743 to the UCSF Liver Center.
Version 1. 02/15/2022
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2022, Xu et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2022;132(4):e154515.https://doi.org/10.1172/JCI154515.
Contributor Information
Chuanrui Xu, Email: xcr@hust.edu.cn.
Zhong Xu, Email: xucong0567@163.com.
Yi Zhang, Email: zhangyi1@cqu.edu.cn.
Matthias Evert, Email: matthias.evert@ukr.de.
Diego F. Calvisi, Email: Diego.Calvisi@klinik.uni-regensburg.de.
Xin Chen, Email: xin.chen@ucsf.edu.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Huang DQ, et al. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valery PC, et al. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67(2):600–611. doi: 10.1002/hep.29498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raoul JL, et al. Current options and future possibilities for the systemic treatment of hepatocellular carcinoma. Hepat Oncol. 2019;6(1):HEP11. doi: 10.2217/hep-2019-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng H, et al. Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies. Am J Cancer Res. 2019;9(8):1536–1545. [PMC free article] [PubMed] [Google Scholar]
- 6.Sangro B, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 7.El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 9.Gordan JD, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 10.Perugorria MJ, et al. Wnt-β-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16(2):121–136. doi: 10.1038/s41575-018-0075-9. [DOI] [PubMed] [Google Scholar]
- 11.Pez F, et al. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59(5):1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 13.Benhamouche S, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10(6):759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Monga SP. β-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148(7):1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giles RH, et al. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu P, et al. Oncogenic mutations in armadillo repeats 5 and 6 of β-catenin reduce binding to APC, increasing signaling and transcription of target genes. Gastroenterology. 2020;158(4):1029–1043. doi: 10.1053/j.gastro.2019.11.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugter JM, et al. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21(1):5–21. doi: 10.1038/s41568-020-00307-z. [DOI] [PubMed] [Google Scholar]
- 18.Shih YL, et al. Promoter methylation of the secreted frizzled-related protein 1 gene SFRP1 is frequent in hepatocellular carcinoma. Cancer. 2006;107(3):579–590. doi: 10.1002/cncr.22023. [DOI] [PubMed] [Google Scholar]
- 19.Bengochea A, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99(1):143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rana MA, et al. Interplay of Wnt β-catenin pathway and miRNAs in HBV pathogenesis leading to HCC. Clin Res Hepatol Gastroenterol. 2019;43(4):373–386. doi: 10.1016/j.clinre.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Klingenberg M, et al. Non-coding RNA in hepatocellular carcinoma: mechanisms, biomarkers and therapeutic targets. J Hepatol. 2017;67(3):603–618. doi: 10.1016/j.jhep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Hsu HC, et al. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157(3):763–770. doi: 10.1016/S0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, et al. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155(6):1795–1801. doi: 10.1016/S0002-9440(10)65496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31(4):339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 25.Audard V, et al. Cholestasis is a marker for hepatocellular carcinomas displaying beta-catenin mutations. J Pathol. 2007;212(3):345–352. doi: 10.1002/path.2169. [DOI] [PubMed] [Google Scholar]
- 26.Cieply B, et al. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49(3):821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng SY, et al. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112(1):44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 28.An FQ, et al. Tumor heterogeneity in small hepatocellular carcinoma: analysis of tumor cell proliferation, expression and mutation of p53 AND beta-catenin. Int J Cancer. 2001;93(4):468–474. doi: 10.1002/ijc.1367. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, et al. β-Catenin mutation is correlated with a favorable prognosis in patients with hepatocellular carcinoma. Mol Clin Oncol. 2015;3(4):936–940. doi: 10.3892/mco.2015.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding X, et al. Transcriptomic characterization of hepatocellular carcinoma with CTNNB1 mutation. PLoS One. 2014;9(5):e95307. doi: 10.1371/journal.pone.0095307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu LC, et al. β-Catenin (CTNNB1) mutations are not associated with prognosis in advanced hepatocellular carcinoma. Oncology. 2014;87(3):159–166. doi: 10.1159/000362821. [DOI] [PubMed] [Google Scholar]
- 32.Wong CM, et al. β-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92(1):136–145. doi: 10.1002/1097-0142(20010701)92:1<136::AID-CNCR1301>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 33.Mao TL, et al. Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol. 2001;193(1):95–101. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH720>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Nhieu JT, et al. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155(3):703–710. doi: 10.1016/S0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calderaro J, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67(4):727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Lee JS, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12(4):410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 37.Hoshida Y, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69(18):7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169(7):1327–1341. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyault S, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45(1):42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 40.Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72(2):215–229. doi: 10.1016/j.jhep.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, et al. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol. 2002;17(9):994–1000. doi: 10.1046/j.1440-1746.2002.02774.x. [DOI] [PubMed] [Google Scholar]
- 42.Fujie H, et al. Frequent beta-catenin aberration in human hepatocellular carcinoma. Hepatol Res. 2001;20(1):39–51. doi: 10.1016/S1386-6346(00)00116-9. [DOI] [PubMed] [Google Scholar]
- 43.Torrecilla S, et al. Trunk mutational events present minimal intra- and inter-tumoral heterogeneity in hepatocellular carcinoma. J Hepatol. 2017;67(6):1222–1231. doi: 10.1016/j.jhep.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 44.de La Coste A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95(15):8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita T, et al. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67(22):10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, et al. Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. 2010;16(10):2740–2750. doi: 10.1158/1078-0432.CCR-09-2610. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, et al. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing β-catenin expression and nuclear translocation. Oncogene. 2015;34(44):5524–5535. doi: 10.1038/onc.2015.7. [DOI] [PubMed] [Google Scholar]
- 48.Liao X, et al. Oxaliplatin resistance is enhanced by saracatinib via upregulation Wnt-ABCG1 signaling in hepatocellular carcinoma. BMC Cancer. 2020;20(1):31. doi: 10.1186/s12885-019-6480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalaf AM, et al. Role of Wnt/β-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J Hepatocell Carcinoma. 2018;5:61–73. doi: 10.2147/JHC.S156701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo RC, et al. Cripto-1 contributes to stemness in hepatocellular carcinoma by stabilizing Dishevelled-3 and activating Wnt/β-catenin pathway. Cell Death Differ. 2018;25(8):1426–1441. doi: 10.1038/s41418-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung HW, et al. EPHB2 activates β-catenin to enhance cancer stem cell properties and drive sorafenib resistance in hepatocellular carcinoma. Cancer Res. 2021;81(12):3229–3240. doi: 10.1158/0008-5472.CAN-21-0184. [DOI] [PubMed] [Google Scholar]
- 52.Fan Z, et al. PTK2 promotes cancer stem cell traits in hepatocellular carcinoma by activating Wnt/β-catenin signaling. Cancer Lett. 2019;450:132–143. doi: 10.1016/j.canlet.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 53.Karabicici M, et al. Changes in Wnt and TGF-β signaling mediate the development of regorafenib resistance in hepatocellular carcinoma cell line HuH7. Front Cell Dev Biol. 2021;9:639779. doi: 10.3389/fcell.2021.639779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cadoret A, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61(8):3245–3249. [PubMed] [Google Scholar]
- 55.Harada N, et al. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64(1):48–54. doi: 10.1158/0008-5472.CAN-03-2123. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Calvisi DF. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am J Pathol. 2014;184(4):912–923. doi: 10.1016/j.ajpath.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao J, et al. Modeling a human hepatocellular carcinoma subset in mice through co-expression of met and point-mutant beta-catenin. Hepatology. 2016;64(5):1587–1605. doi: 10.1002/hep.28601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patil MA, et al. Role of cyclin D1 as a mediator of c-Met- and beta-catenin-induced hepatocarcinogenesis. Cancer Res. 2009;69(1):253–261. doi: 10.1158/0008-5472.CAN-08-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiao Y, et al. Axis inhibition protein 1 (Axin1) deletion-induced hepatocarcinogenesis requires intact β-catenin but not notch cascade in mice. Hepatology. 2019;70(6):2003–2017. doi: 10.1002/hep.30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao J, et al. Targeting β-catenin in hepatocellular cancers induced by co-expression of mutant β-catenin and K-Ras in mice. Hepatology. 2017;65(5):1581–1599. doi: 10.1002/hep.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stauffer JK, et al. Coactivation of AKT and β-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71(7):2718–2727. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charawi S, et al. LKB1 signaling is activated in CTNNB1-mutated HCC and positively regulates β-catenin-dependent CTNNB1-mutated HCC. J Pathol. 2019;247(4):435–443. doi: 10.1002/path.5202. [DOI] [PubMed] [Google Scholar]
- 63.Tao J, et al. Nuclear factor erythroid 2-related factor 2 and β-catenin coactivation in hepatocellular cancer: biological and therapeutic implications. Hepatology. 2021;74(2):741–759. doi: 10.1002/hep.31730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhan N, et al. The effect of selective c-MET inhibitor on hepatocellular carcinoma in the MET-active, β-catenin-mutated mouse model. Gene Expr. 2018;18(2):135–147. doi: 10.3727/105221618X15174108894682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pezzuto F, et al. Tumor specific mutations in TERT promoter and CTNNB1 gene in hepatitis B and hepatitis C related hepatocellular carcinoma. Oncotarget. 2016;7(34):54253–54262. doi: 10.18632/oncotarget.9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SE, et al. Frequent somatic TERT promoter mutations and CTNNB1 mutations in hepatocellular carcinoma. Oncotarget. 2016;7(43):69267–69275. doi: 10.18632/oncotarget.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dubik D, Shiu RP. Transcriptional regulation of c-myc oncogene expression by estrogen in hormone-responsive human breast cancer cells. J Biol Chem. 1988;263(25):12705–12708. doi: 10.1016/S0021-9258(18)37810-4. [DOI] [PubMed] [Google Scholar]
- 68.Kerkhoff E, et al. Regulation of c-myc expression by Ras/Raf signalling. Oncogene. 1998;16(2):211–216. doi: 10.1038/sj.onc.1201520. [DOI] [PubMed] [Google Scholar]
- 69.Ramana CV, et al. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J. 2000;19(2):263–272. doi: 10.1093/emboj/19.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 71.Rennoll S, Yochum G. Regulation of MYC gene expression by aberrant Wnt/β-catenin signaling in colorectal cancer. World J Biol Chem. 2015;6(4):290–300. doi: 10.4331/wjbc.v6.i4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shang XZ, et al. Stabilized beta-catenin promotes hepatocyte proliferation and inhibits TNFalpha-induced apoptosis. Lab Invest. 2004;84(3):332–341. doi: 10.1038/labinvest.3700043. [DOI] [PubMed] [Google Scholar]
- 73.Tong Z, et al. Steroid receptor coactivator 1 promotes human hepatocellular carcinoma progression by enhancing Wnt/β-catenin signaling. J Biol Chem. 2015;290(30):18596–18608. doi: 10.1074/jbc.M115.640490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu R, et al. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019;443:34–46. doi: 10.1016/j.canlet.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 75.Qiao Y, et al. Oncogenic potential of N-terminal deletion and S45Y mutant β-catenin in promoting hepatocellular carcinoma development in mice. BMC Cancer. 2018;18(1):1093. doi: 10.1186/s12885-018-4870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shtutman M, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 78.Delgado E, et al. β-Catenin knockdown in liver tumor cells by a cell permeable gamma guanidine-based peptide nucleic acid. Curr Cancer Drug Targets. 2013;13(8):867–878. doi: 10.2174/15680096113139990081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaur P, et al. Epigenetic silencing of sFRP1 activates the canonical Wnt pathway and contributes to increased cell growth and proliferation in hepatocellular carcinoma. Tumour Biol. 2012;33(2):325–336. doi: 10.1007/s13277-012-0331-5. [DOI] [PubMed] [Google Scholar]
- 80.Guttridge DC, et al. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19(8):5785–5799. doi: 10.1128/MCB.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein EA, Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci. 2008;121(pt 23):3853–3857. doi: 10.1242/jcs.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl) 2016;94(12):1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang B, et al. Overexpression of CTNND1 in hepatocellular carcinoma promotes carcinous characters through activation of Wnt/β-catenin signaling. J Exp Clin Cancer Res. 2016;35(1):82. doi: 10.1186/s13046-016-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cadoret A, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21(54):8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 85.Sekine S, et al. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43(4):817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 86.Lee JM, et al. β-Catenin signaling in hepatocellular cancer: Implications in inflammation, fibrosis, and proliferation. Cancer Lett. 2014;343(1):90–97. doi: 10.1016/j.canlet.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Austinat M, et al. Correlation between beta-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol Cancer. 2008;7:21. doi: 10.1186/1476-4598-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chiu M, et al. Glutamine depletion by crisantaspase hinders the growth of human hepatocellular carcinoma xenografts. Br J Cancer. 2014;111(6):1159–1167. doi: 10.1038/bjc.2014.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krall AS, et al. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7:11457. doi: 10.1038/ncomms11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adebayo Michael AO, et al. Inhibiting glutamine-dependent mTORC1 activation ameliorates liver cancers driven by beta-catenin mutations. Cell Metab. 2019;29(5):1135–1150. doi: 10.1016/j.cmet.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 92.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 93.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22(4):1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang B, et al. TBX3 functions as a tumor suppressor downstream of activated CTNNB1 mutants during hepatocarcinogenesis. J Hepatol. 2021;75(1):120–131. doi: 10.1016/j.jhep.2021.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei S, et al. KIF2C: a novel link between Wnt/β-catenin and mTORC1 signaling in the pathogenesis of hepatocellular carcinoma. Protein Cell. 2021;12(10):788–809. doi: 10.1007/s13238-020-00766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang C, et al. Silencing of KIF3B suppresses breast cancer progression by regulating EMT and Wnt/β-catenin signaling. Front Oncol. 2020;10:597464. doi: 10.3389/fonc.2020.597464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qu B, et al. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol Lett. 2014;7(4):1175–1178. doi: 10.3892/ol.2014.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin HH, et al. Inhibition of the Wnt/β-catenin signaling pathway improves the anti-tumor effects of sorafenib against hepatocellular carcinoma. Cancer Lett. 2016;381(1):58–66. doi: 10.1016/j.canlet.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 99.Kabiri Z, et al. Wnt signaling suppresses MAPK-driven proliferation of intestinal stem cells. J Clin Invest. 2018;128(9):3806–3812. doi: 10.1172/JCI99325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma B, Hottiger MO. Crosstalk between Wnt/β-catenin and NF-κB signaling pathway during inflammation. Front Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nejak-Bowen K, et al. Beta-catenin-NF-κB interactions in murine hepatocytes: a complex to die for. Hepatology. 2013;57(2):763–774. doi: 10.1002/hep.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lepourcelet M, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5(1):91–102. doi: 10.1016/S1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 103.Sukhdeo K, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104(18):7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minke KS, et al. Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur J Haematol. 2009;82(3):165–175. doi: 10.1111/j.1600-0609.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 105.Gandhirajan RK, et al. Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia. 2010;12(4):326–335. doi: 10.1593/neo.91972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wei W, et al. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int J Cancer. 2010;126(10):2426–2436. doi: 10.1002/ijc.24810. [DOI] [PubMed] [Google Scholar]
- 107.Emami KH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101(34):12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eguchi M, et al. ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med Chem. 2005;1(5):467–472. doi: 10.2174/1573406054864098. [DOI] [PubMed] [Google Scholar]
- 109.Arensman MD, et al. The CREB-binding protein inhibitor ICG-001 suppresses pancreatic cancer growth. Mol Cancer Ther. 2014;13(10):2303–2314. doi: 10.1158/1535-7163.MCT-13-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.You L, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23(36):6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 111.Gurney A, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109(29):11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dihlmann S, von Knebel Doeberitz M. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer. 2005;113(4):515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- 113.Vilchez V, et al. Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol. 2016;22(2):823–832. doi: 10.3748/wjg.v22.i2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dihlmann S, et al. Reduction of beta-catenin/T-cell transcription factor signaling by aspirin and indomethacin is caused by an increased stabilization of phosphorylated beta-catenin. Mol Cancer Ther. 2003;2(6):509–516. [PubMed] [Google Scholar]
- 115.Hawcroft G, et al. Indomethacin induces differential expression of beta-catenin, gamma-catenin and T-cell factor target genes in human colorectal cancer cells. Carcinogenesis. 2002;23(1):107–114. doi: 10.1093/carcin/23.1.107. [DOI] [PubMed] [Google Scholar]
- 116.Thorne CA, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol. 2010;6(11):829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boon EMJ, et al. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90(1):224–229. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamada Y, et al. Suppression of occurrence and advancement of beta-catenin-accumulated crypts, possible premalignant lesions of colon cancer, by selective cyclooxygenase-2 inhibitor, celecoxib. Jpn J Cancer Res. 2001;92(6):617–623. doi: 10.1111/j.1349-7006.2001.tb01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11(6):791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 120.Tian D, et al. The Wnt inhibitor LGK-974 enhances radiosensitivity of HepG2 cells by modulating Nrf2 signaling. Int J Oncol. 2017;51(2):545–554. doi: 10.3892/ijo.2017.4042. [DOI] [PubMed] [Google Scholar]
- 121.Yang XG, et al. Current advance of therapeutic agents in clinical trials potentially targeting tumor plasticity. Front Oncol. 2019;9:887. doi: 10.3389/fonc.2019.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim MK. Novel insight into the function of tankyrase. Oncol Lett. 2018;16(6):6895–6902. doi: 10.3892/ol.2018.9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 124.Lau T, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73(10):3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 125.Mizutani A, et al. RK-287107, a potent and specific tankyrase inhibitor, blocks colorectal cancer cell growth in a preclinical model. Cancer Sci. 2018;109(12):4003–4014. doi: 10.1111/cas.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waaler J, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72(11):2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- 127.Okada-Iwasaki R, et al. The discovery and characterization of K-756, a novel Wnt/β-catenin pathway inhibitor targeting tankyrase. Mol Cancer Ther. 2016;15(7):1525–1534. doi: 10.1158/1535-7163.MCT-15-0938. [DOI] [PubMed] [Google Scholar]
- 128.Busch AM, et al. Evidence for tankyrases as antineoplastic targets in lung cancer. BMC Cancer. 2013;13:211. doi: 10.1186/1471-2407-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Menon M, et al. A novel tankyrase inhibitor, MSC2504877, enhances the effects of clinical CDK4/6 inhibitors. Sci Rep. 2019;9(1):201. doi: 10.1038/s41598-018-36447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quackenbush KS, et al. The novel tankyrase inhibitor (AZ1366) enhances irinotecan activity in tumors that exhibit elevated tankyrase and irinotecan resistance. Oncotarget. 2016;7(19):28273–28285. doi: 10.18632/oncotarget.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stratford EW, et al. The tankyrase-specific inhibitor JW74 affects cell cycle progression and induces apoptosis and differentiation in osteosarcoma cell lines. Cancer Med. 2014;3(1):36–46. doi: 10.1002/cam4.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arques O, et al. Tankyrase inhibition blocks Wnt/β-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res. 2016;22(3):644–656. doi: 10.1158/1078-0432.CCR-14-3081. [DOI] [PubMed] [Google Scholar]
- 133.Ma L, et al. Tankyrase inhibitors attenuate WNT/β-catenin signaling and inhibit growth of hepatocellular carcinoma cells. Oncotarget. 2015;6(28):25390–25401. doi: 10.18632/oncotarget.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sangkhathat S, et al. In vitro RNA interference against beta-catenin inhibits the proliferation of pediatric hepatic tumors. Int J Oncol. 2006;28(3):715–722. [PubMed] [Google Scholar]
- 135.Wang XH, et al. β-Catenin siRNA regulation of apoptosis- and angiogenesis-related gene expression in hepatocellular carcinoma cells: potential uses for gene therapy. Oncol Rep. 2010;24(4):1093–1099. [PubMed] [Google Scholar]
- 136.Zeng G, et al. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007;9(11):951–959. doi: 10.1593/neo.07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Delgado E, et al. Complete response of Ctnnb1-mutated tumours to β-catenin suppression by locked nucleic acid antisense in a mouse hepatocarcinogenesis model. J Hepatol. 2015;62(2):380–387. doi: 10.1016/j.jhep.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pai SG, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101. doi: 10.1186/s13045-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chehrazi-Raffle A, et al. Wnt/β-catenin signaling and immunotherapy resistance: lessons for the treatment of urothelial carcinoma. Cancers (Basel) 2021;13(4):889. doi: 10.3390/cancers13040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harding JJ, et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25(7):2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ruiz de Galarreta M, et al. β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spranger S, et al. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 143.Pfister D, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abitbol S, et al. AXIN deficiency in human and mouse hepatocytes induces hepatocellular carcinoma in the absence of β-catenin activation. J Hepatol. 2018;68(6):1203–1213. doi: 10.1016/j.jhep.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 145.Ding Z, et al. Oncogenic dependency on β-catenin in liver cancer cell lines correlates with pathway activation. Oncotarget. 2017;8(70):114526–114539. doi: 10.18632/oncotarget.21298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng G, et al. Wnt’er in liver: expression of Wnt and frizzled genes in mouse. Hepatology. 2007;45(1):195–204. doi: 10.1002/hep.21473. [DOI] [PubMed] [Google Scholar]
- 147.Yang J, et al. β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60(3):964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu AX, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 149.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Matter MS, et al. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60(4):855–865. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu X, et al. Role of the mammalian target of rapamycin pathway in liver cancer: from molecular genetics to targeted therapies. Hepatology. 2021;73(suppl 1):49–61. doi: 10.1002/hep.31310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shang R, et al. Cabozantinib-based combination therapy for the treatment of hepatocellular carcinoma. Gut. 2021;70(9):1746–1757. doi: 10.1136/gutjnl-2020-320716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Savall M, et al. Cooperation between the NRF2 pathway and oncogenic β-catenin during HCC tumorigenesis. Hepatol Commun. 2021;5(9):1490–1506. doi: 10.1002/hep4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tao J, et al. Activation of β-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147(3):690–701. doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang S, et al. The hippo effector transcriptional coactivator with PDZ-binding motif cooperates with oncogenic β-catenin to induce hepatoblastoma development in mice and humans. Am J Pathol. 2020;190(7):1397–1413. doi: 10.1016/j.ajpath.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]