Abstract
Purpose
Human neural stem cells (NSCs) are inherently tumor-tropic, making them attractive drug delivery vehicles. Toward this goal, we retrovirally transduced an immortalized, clonal NSC line to stably express cytosine deaminase (HB1.F3.CD.C21; CD-NSCs), which converts the prodrug 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU).
Experimental Design
Recurrent high grade glioma patients underwent intracranial administration of CD-NSCs during tumor resection or biopsy. Four days later, patients began taking oral 5-FC every 6 hours for 7 days. Study treatment was given only once. A standard 3+3 dose escalation schema was used to increase doses of CD-NSCs from 10 million to 50 million and 5-FC from 75 to 150 mg/kg/day. Intracerebral microdialysis was performed to measure brain levels of 5-FC and 5-FU. Serial blood samples were obtained to assess systemic drug concentrations as well as to perform immunologic correlative studies.
Results
Fifteen patients underwent study treatment. We saw no dose-limiting toxicity (DLT) due to the CD-NSCs. There was 1 DLT (grade 3 transaminitis) possibly related to 5-FC. We did not see development of anti-CD-NSC antibodies and did not detect CD-NSCs or replication competent retrovirus in the systemic circulation. Intracerebral microdialysis revealed that CD-NSCs produced 5-FU locally in the brain in a 5-FC dose-dependent manner. Autopsy data indicate that CD-NSCs migrated to distant tumor sites and were non-tumorigenic.
Conclusions
Collectively, our results from this first-in-human study demonstrate initial safety and proof-of-concept regarding the ability of NSCs to target brain tumors and locally produce chemotherapy.
Keywords: neural stem cells, gene therapy, gliomas
INTRODUCTION
Human neural stem cells (NSCs) have emerged as promising cell-based strategies for treating central nervous system diseases and injury (1). Most current strategies aim to use NSCs for regenerative purposes—to replace damaged tissue, stimulate repair, or restore missing enzymes. In contrast, the focus of the NSC-based anti-cancer strategy described herein is to harness the intrinsic tumor-tropic properties of NSCs and use these cells as delivery vehicles to selectively target therapeutic gene products to invasive brain tumor cells. By modifying NSCs to express a prodrug-converting enzyme, potentially higher concentrations of chemotherapy can be selectively produced at tumor sites while minimizing toxicity to normal brain and other tissues in the body.
Use of NSCs can also potentially overcome a major obstacle that has limited the effectiveness of gene therapy to date—the inability of carrier cells to sufficiently distribute therapeutic agents throughout primary and distant sites of tumor. Preclinical studies have shown that human NSCs administered intratumorally, into the cerebral hemisphere opposite tumor, or even intravenously, can track to and distribute throughout the main brain tumor mass and co-localize with distant invasive micro-tumor foci in both primary and metastatic brain tumor models (2–13).
The NSC line used in this study (HB1.F3) was retrovirally transduced to express cytosine deaminase (CD) and subcloned (4, 9, 14–16). CD converts the prodrug 5-fluorocytosine (5-FC) to the cytotoxic agent 5-fluorouracil (5-FU), which then kills surrounding dividing tumor cells (Fig. 1A). This CD/5-FC prodrug strategy has a large bystander effect, predicting that one CD-NSC can kill many surrounding tumor cells (17, 18). In vitro and in vivo preclinical studies demonstrated this well characterized clonal human NSC line (HB1.F3.CD.C21; CD-NSCs) retained tumor-tropism, was non-tumorigenic, had minimal immunogenicity, and showed therapeutic efficacy (9).
Figure 1.
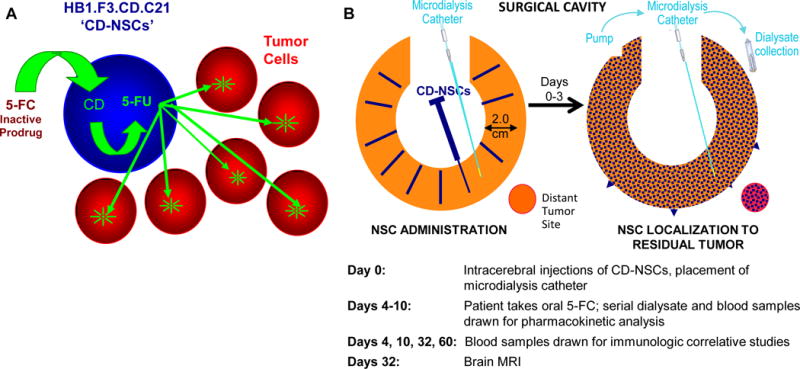
Treatment strategy for CD-NSCs in combination with 5-FC. A. Schematic of tumor-localized production of chemotherapy by CD-NSCs. Intracranially administered CD-NSCs migrate to residual tumor. The CD-NSCs stop dividing within 48 h of administration, allowing them to survive exposure to cytotoxic chemotherapy. The orally administered prodrug, 5-FC, crosses the blood-brain barrier, and the CD expressed in the NSCs converts the 5-FC into 5-FU. The 5-FU then diffuses from the NSCs and kills nearby dividing tumor cells. B. Study treatment schema. On the day of surgery (day 0), the total dose of CD-NSCs was distributed in 100–150 μl volumes and injected throughout the wall of the resection cavity (or as a single 1 ml intratumoral injection via the biopsy track [not shown]). An intracerebral microdialysis catheter was also placed in tumor or peritumoral tissue. Four days later, the patient started taking 5-FC orally every 6 h for 7 days. During this time, dialysate and blood samples were drawn to measure levels of 5-FC and 5-FU. Serial blood samples were also drawn through day 60 to assess for possible anti-NSC antibody and T cell responses and investigate if NSCs migrated into the systemic circulation. A brain MRI was performed at the end of the toxicity evaluation period, 32 days after injection of CD-NSCs.
Based on the strong preclinical data, we conducted a first-in-human study to assess the feasibility of treating recurrent high grade glioma patients with intracranially administered CD-NSCs followed by oral 5-FC. In addition to obtaining preliminary safety data, this study provides initial clinical proof-of-concept regarding NSC migration to tumor foci in the brain and the ability of NSCs to mediate localized conversion of a prodrug to chemotherapy. Results from this study serve as the foundation for designing future brain tumor clinical trials of this NSC-based anti-cancer therapy.
MATERIALS AND METHODS
Study design
The primary objective of this first-in-human study was to assess the safety of intracranially administering CD-NSCs followed by a 7 day course of oral 5-FC in patients with recurrent high grade glioma. Main secondary objectives included assessing for possible CD-NSC immunogenicity and secondary tumorigenicity, and evaluating for proof-of-concept regarding CD-NSC migration to tumor foci and localized conversion of 5-FC to 5-FU.
A standard 3 + 3 dose escalation design (19) was used to investigate 3 dose levels of CD-NSCs and 5-FC (Supplementary Table S1). Study patients underwent tumor resection or biopsy, as clinically indicated, after which an intracranial dose of CD-NSCs (10 million or 50 million) was manually administered into the wall of the resection cavity or tumor tissue. During surgery, the first 3 patients treated on each dose level also had an intracerebral microdialysis catheter temporarily placed in residual tumor or within 5–15 mm of the resection cavity to collect brain extracellular fluid (ECF) for measuring concentrations of 5-FC and 5-FU (Fig. 1B). Four days after CD-NSC administration, to allow sufficient time for the NSCs to stop dividing and distribute among tumor foci, patients began a 7-day course of oral 5-FC (75 or 150 mg/kg/day in divided doses every 6 hours). While patients were taking 5-FC, samples of brain ECF were continuously collected, and blood samples were obtained for pharmacokinetic analysis. Blood samples for immunologic correlative studies were collected through day 60. A brain MRI was performed at the end of the toxicity evaluation period (day 32), and at that point, patients could start treatment with another chemotherapy if clinically indicated, but they were still followed for possible long-term toxicity as per FDA guidelines for patients who have participated in gene therapy studies. The study was approved by the City of Hope (COH) Institutional Review Board, conducted under an Investigational New Drug Application (IND# 14041), and registered at ClinicalTrials.gov (NCT01172964). All participants gave written informed consent.
Study eligibility criteria
To be eligible to participate in this study, patients had to be at least 18 years old; have radiographic findings consistent with recurrent, supratentorial high-grade gliomas (including glioblastoma, anaplastic astrocytoma, gliosarcoma, anaplastic oligodendroglioma, or anaplastic oligoastrocytoma); and be in need of tumor resection or biopsy. Other inclusion criteria were: (a) Karnofsky performance status ≥ 60%, (b) previous treatment with brain radiation and temozolomide, (c) recovered from toxicity of prior therapy, (d) adequate bone marrow (defined as an absolute neutrophil count of ≥1500 cells/mm3 and platelet count ≥ 100,000 cells/mm3), hepatic (total bilirubin ≤ 2.0 mg/dl and AST (SGOT) ≤ 4 times the institutional upper limit of normal) and renal function (serum creatinine within normal limits), (e) a minimum of 4 weeks from previous chemotherapy (6 weeks from treatment with a nitrosourea), and (f) no anticipated physical connection between the post-resection tumor cavity and the cerebral ventricles.
Patients were excluded from study participation if they had (a) anti-HLA antibodies specific for class I or II HLA antigens expressed by the CD-NSCs, (b) a chronic or active viral central nervous system infection, (c) a coagulopathy or bleeding disorder, (e) a serious medical or psychiatric illness that could interfere with the completion of protocol treatment, or were (f) currently receiving other chemotherapy or radiation therapy, (g) or pregnant or breast-feeding.
NSCs
The primary NSCs were obtained in accordance with the Guidelines of the Anatomical Pathology Department of Vancouver General Hospital, with permission to use fetal tissue granted by the Clinical Research Screening Committee Involving Human Subjects of the University of British Columbia. A materials transfer agreement was approved for use of HB1.F3 and HB1.F3.CD-NSCs at COH. The HB1.F3 NSCs were immortalized using a retrovirus encoding the v-myc gene to retain the cells’ stem-like properties, including the ability to migrate and remain undifferentiated (14). The NSCs were then similarly transduced retrovirally to stably express Escherichia coli CD. A clonal cell population was selected (C21) and extensively characterized, demonstrating genetic and functional stability over time and passage (9). The NSCs were expanded under good manufacturing practices (GMP) conditions and release tested to establish a master cell bank (MCB) at COH.
Final CD-NSC preparation and release testing
Frozen vials of CD-NSCs from the MCB were thawed, plated, and grown for 72 h as per standard propagation protocol (9). On the day of surgery, CD-NSCs were harvested and resuspended to the target concentration in artificial cerebrospinal fluid (CSF) [Perfusion Fluid CNS; CMA Microdialysis]. The final CD-NSC product then underwent rigorous release testing prior to patient administration, including assessment for (a) bacterial or fungal contamination, (b) cell identity: >90% human nestin (NSC marker) and >70% CD (transgene expression) required, (c) cell count (+/− 20% of target dose), and (d) viability (>70 % of cells required). Please see Supplementary Methods for a description of the assays used to determine NSC identity and viability.
Intracerebral microdialysis
During surgery, a microdialysis catheter (70 Microdialysis Brain Catheter, membrane length 10 mm, membrane molecular weight cut-off 20 kDa, shaft length 100 mm; CMA Microdialysis) was placed in tumor or peritumoral tissue to collect dialysate for measuring levels of 5-FC and 5-fluorouracil (5-FU) in brain interstitium. After correct placement of the catheter was confirmed by a non-contrast brain CT scan, perfusion of the catheter with artificial CSF at 1 μl/min began by connecting the inlet tubing to a syringe pump (107 Microdialysis Pump; CMA Microdialysis). Before starting the clinical trial, we determined through in vitro recovery experiments that the fractional recoveries of 5-FC and 5-FU were 92 ± 2.1% and 91 ± 1.5%, respectively, at a flow rate of 1 μl/min. Because the in vitro recoveries of 5-FC and 5-FU were >90%, the microdialysis data in the Results section are presented as uncorrected values.
When a study patient began taking 5-FC, dialysate samples were collected continuously for analysis. The microvial at the end of the outlet tubing was replaced with a new one every 60 min during the first 24 h after the start of 5-FC, and then every 3 h until a patient finished the course of 5-FC or the microdialysis catheter stopped functioning. The catheter was removed after completing collection of the dialysate samples. Microvials containing the dialysate samples were kept on dry ice until moved to an ultralow temperature freezer (≤ −70°C), where they were stored prior to analysis.
During the dialysate collection period, blood samples were obtained to define the plasma concentration-time profiles of 5-FC and 5-FU in order to characterize the relationship between intracerebral and systemic concentrations of the two drugs. Pharmacokinetic sampling was performed before and after the 5-FC reached steady-state. On days 4 and 5, blood samples were drawn just prior to the morning dose of 5-FC and then every 30 min during the first 3 h after the morning dose was taken by the patient. Additional blood samples were collected 4 and 6 h (just prior to the next 5-FC dose) after the morning dose. On days 6, 7, and 8, blood samples were obtained just before the morning dose and then 90 min later. Within 1 h after collection, blood samples were centrifuged at 1500 × g for 10 min to separate plasma from whole blood, and then plasma was stored at ≤ −70°C until analysis. Quantitative tandem mass spectrometry analysis of 5-FC and 5-FU in plasma and dialysate was performed in the COH Analytical Pharmacology Core Facility (see Supplementary Methods).
Correlative safety studies
Assessment of possible NSC migration into the systemic circulation
Quantitative PCR (qPCR) using primer pairs specific for v-myc (a marker for the NSCs) was performed by the COH Clinical Immunobiology Correlative Studies Laboratory (CICSL) on peripheral blood mononuclear cells (PBMCs) from study participants’ blood samples obtained prior to surgery and then on days 4, 10, 32, and 60 after intracranial NSC administration to assess for the possible presence of NSCs in the blood.
Assessment of the presence of replication competent retrovirus (RCR)
RCR testing was performed by analyzing patient DNA from whole blood for RCR-specific sequences prior to surgery; at 3 months, 6 months, and 1 year after study treatment; and annually thereafter. Real time PCR with 4070A envelope-specific primers was used to screen DNA extracted from PBMCs from study patients treated with CD-NSCs.
Correlative immunologic study
Serial assessments of humoral immune responses to the NSCs were performed by CICSL. Blood was collected at enrollment, then on days 4, 10, 32, and 60 for isolation of plasma. The possible presence of antibodies against the NSCs in plasma was monitored using a qualified flow cytometric method (see Supplementary Methods).
Correlative brain tissue studies
Permission for brain autopsy was obtained from family members of two study patients. The brains were formalin fixed for 2 weeks and then serially sectioned coronally. Extensive sampling of the brain sections included tissue from prior surgical sites, ipsilateral and contralateral areas involved by tumor, grossly unremarkable gray and white matter, as well as deep nuclei, periventricular areas, and long axonal tracts. A total of 38 tissue matched samples were collected from the brain of the first patient and 26 samples from the brain of the second one. Each formalin-fixed tissue sample was divided for separate analysis of histopathology and detection of NSCs using v-myc nested PCR.
Histopathological analysis
Tissue samples were conventionally processed for paraffin embedding, and every tenth slide (10μm sections) was stained with hematoxylin and eosin and analyzed via microscopy by the study neuropathologist.
v-myc PCR analysis
Genomic DNA from tissue samples was isolated using the Puragene Kit (Qiagen). Nested PCR for v-myc was performed to detect CD-NSCs as previously described (9). The housekeeping gene used was glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the assay was repeated 3 times for each tissue sample. Detection of v-myc bands in 2 out of 3 PCR assays was considered positive for NSCs.
XY fluorescence in situ hybridization (FISH) analysis
XY FISH analysis was performed on brain tissue from the second autopsy patient who was male; NSCs were derived from female fetal tissue. Twenty-five slides of 5 μm sections were cut from each of the 3 cassettes that contained v-myc-positive tissue and from 1 cassette containing normal brain tissue that was v-myc negative (to serve as a negative control). FISH was performed with XY probes (CEPX(DXZ1) SGn #05J10-033 /Y(DYZ3)SO probe, #05J10-051; Abbott Molecular) as previously described (20). The slides were scanned and mapped using the software XY on the Bioview Duet Image analyzer (Bioview).
Proliferating cell nuclear antigen (PCNA) staining
Serial tissue sections containing female cells (NSCs) identified by XY FISH were processed for immunohistochemistry (IHC) with primary PCNA (ab2426; Abcam), followed by anti-rabbit horseradish peroxidase (K4003; Dako) conjugated and developed with 3-amino-9-ethylcarbazole (Vector Lab, SK-2000). To determine the genotype of PCNA-positive cells, IHC brightfield images of slides were scanned using Bioview Duet Image analyzer software ICHx20 TS-software task, and sequential FISH was performed before both images were superimposed to conduct a blind analysis of the slides for comparison of PCNA-positive cells to XY FISH. A minimum of 100 fields (~30-50 cells per field at 20X) were evaluated for each tissue slide.
Statistical analysis
For this first-in-human clinical trial, the United States Food and Drug Administration (FDA) specified that only one dose of CD-NSCs could be administered intracranially to study patients, and so no attempt was made to determine a maximum tolerated dose of CD-NSCs in combination with 5-FC. Instead, the goal was to define the maximum study dose (MSD), which would then be used as the starting dose for a formal phase I study in which repeated doses of study treatment would be given. The MSD was defined as the highest CD-NSC dose tested in which fewer than 33% of patients experienced a dose-limiting toxicity (DLT) attributable to the treatment regimen, when at least 6 patients were treated at that dose and were evaluable for toxicity. DLT was defined as a grade 3 or 4 non-hematologic toxicity (except grade 3 nausea/vomiting without maximal antiemetic therapy and grade 3 fatigue) or a grade 4 hematologic toxicity that was at least possibly related to the investigational agents.
All observed toxicities were summarized using descriptive statistics. Similarly, immunologic, efficacy, neuropharmacokinetic, and autopsy data were summarized using descriptive statistics and graphs. All summaries were exploratory, with the goal of developing further questions regarding the modulation of therapy and future clinical trial design.
RESULTS
Patient enrollment and characteristics
Between November 2010 and January 2013, 18 patients with recurrent high grade gliomas were screened for study enrollment. Three patients were ineligible because they had antibodies to a class I human leukocyte antigen on the NSCs. Characteristics of the 15 patients who received study treatment are provided in Supplementary Table S2. The majority of participants had recurrent glioblastoma and underwent tumor resection.
Safety results
Of the 15 patients enrolled in the study, 3 were replaced because they were not evaluable for dose escalation. Two patients died from disease progression before completing the toxicity evaluation period, and one died from a post-surgical complication, an intracerebral hemorrhage from a blood vessel at the surgical margin. CD-NSCs had not been injected in the area in which the hemorrhage occurred.
Twelve patients were fully evaluable for toxicity and dose escalation. Toxicities were graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Overall, the study treatment was well tolerated by participants. We observed no toxicities associated with intracranial administration of the CD-NSCs and no CTCAE grade 3 or 4 toxicities related to the CD-NSCs. One DLT, which was thought to be possibly due to 5-FC, occurred in a patient on dose level 3. This patient developed grade 3 elevations of alanine aminotransferase and aspartate aminotransferase, both of which returned to normal within 24 h of stopping the 5-FC. Other observed toxicities that were at least possibly related to 5-FC included grade 3 fatigue, lymphopenia, and thrombocytopenia (Table 1).
Table 1.
Toxicity summary: adverse events that were possibly, probably, or definitely related to treatment with NSCs and/or 5-FC.
| Adverse Eventa | Dose Level 1 | Dose Level 2 | Dose Level 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NCS: 10 million 5-FC: 75 mg/kg/day | NCS: 10 million 5-FC: 150 mg/kg/day | NCS: 50 million, 5-FC: 150 mg/kg/day | |||||||
| (N=4) | (N=5) | (N=6) | |||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Alanine aminotransferase increased | 1 | 0 | 0 | 2 | 0 | 0 | 3 | 1 | 1 |
| Anemia | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 |
| Anorexia | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Aspartate aminotransferase increased | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 1 |
| Constipation | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Delirium | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 1 |
| Generalized muscle weakness | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Headache | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypoalbuminemia | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| Hypokalemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hypophosphatemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hypoxia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Insomnia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphocytopenia | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Muscle weakness, left-sided | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 2 | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 0 |
| Rash, pustular | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sore throat | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary tract infection | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
Excluded from this table are adverse events whose highest reported grade was 1. There were no grade 4 or 5 toxicities related to study treatment.
Serial blood samples were collected from study patients through day 60 to assess if CD-NSCs migrated into the systemic circulation. CD-NSCs were not detected in any of the participants’ blood samples, as determined by quantitative PCR using v-myc primers. This finding is consistent with preclinical data demonstrating that NSCs injected into the brain were not detected in blood or other organs (9).
Because replication-deficient retrovirus vectors were used to transduce the NSCs ex vivo with v-myc and CD genes, we tested both the GMP cell bank and blood samples from study patients for the presence of RCR. The GMP cell bank and all of the patient blood samples, including samples obtained as much as one year after the start of study treatment, tested negative for RCR.
No evidence of humoral responses to CD-NSCs
Serum samples collected at the time of enrollment and then 32 and 60 days after CD-NSC administration were assessed for the presence of NSC-binding antibodies. Compared to results obtained with normal donor sera (negative control) and serum from a patient who was deemed ineligible because of expression of an antibody against one of the HLA class I antigens on the CD-NSCs (positive control), no anti-NSC antibodies were detected in study patients at any time (Fig. 2).
Figure 2.
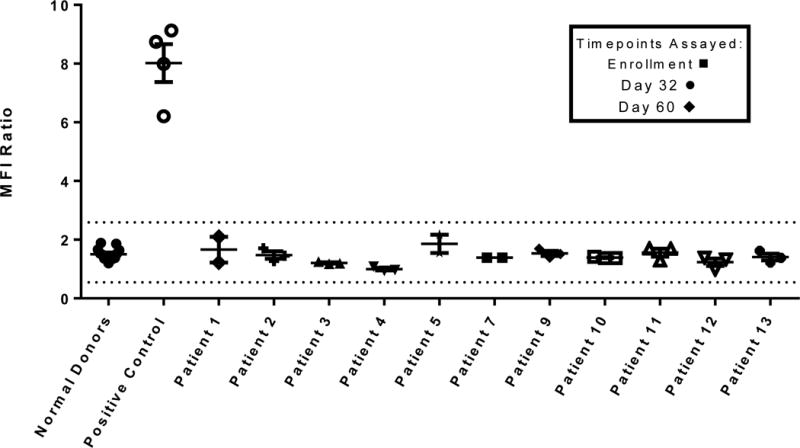
Assessment of humoral immune responses to CD-NSCs. Anti-NSC antibodies present in the serum of all patients were assessed at the time of enrollment and then on days 32 and 60 after NSC administration by flow cytometry. The dotted lines indicate the normal mean fluorescence intensity (MFI) range of 0.55 to 2.59 that represents a 95% prediction interval based on a 2.3 standard deviation in each direction. Each data point for normal sera (N=10) represents the mean of 5 independent experiments. Each data point for study patients’ sera represents the mean of triplicate assays. The “positive control” is the mean of triplicate assays on serum from a subject who was ineligible because of expression of an antibody against of the HLA I antigens (A1) expressed on the CD-NSCs. Patients 6 and 8 did not have adequate sample collections and were considered not evaluable.
Efficacy Data
For all study patients, the median progression-free survival (PFS) was 1.02 months (95% CI 0.95-1.12), and median overall survival (OS) was 8.41 months (95% CI 2.92-14.20). Regarding PFS and OS by dose of CD-NSCs, among the study patients who received 10 million CD-NSCs (dose levels 1 and 2), PFS did not exceed 2 months in any of the 9 patients treated. Of the 6 patients who received 50 million NSCs (dose level 3), 1 patient had a PFS of 5 months (the remaining 5 patients developed tumor progression in approximately 1 month). On dose levels 1-2, the median OS was 2.9 months (95% CI 1.45-11.2), while on dose level 3, the median OS was 15.4 months (95% 7.23-18.5), with p < 0.05. Two of 9 patients on dose levels 1–2 had an OS of greater than 12 months, and with the third dose level, 4 of 6 patients had an OS greater than 12 months.
Conversion of 5-FC to 5-FU by CD-NSCs
Intracerebral microdialysis was used to measure 5-FC and 5-FU concentrations in the brain, which were then compared to levels in plasma. Intracerebral microdialysis is a sampling technique that enables continuous analysis of brain ECF concentrations of biomolecules or drugs without significantly disrupting tissue function (21), and it has been applied to define the neuropharmacokinetic profiles of anti-cancer agents (22–26). Dialysate and plasma samples were collected from 9 participants while they were taking 5-FC and analyzed for drug concentrations by quantitative tandem mass spectrometry. Interstitial concentrations of both 5-FC and 5-FU increased rapidly in the brain after the first dose of 5-FC, and steady-state was achieved within the first 6–8 h (Fig.3A). Comparison of average steady-state drug concentrations in the brain across the 3 dose levels revealed a clear 5-FC dose-dependent increase in 5-FU. Increasing the dose of 5-FC from 75 to 150 mg/kg/day resulted in higher average 5-FC (53 ± 24 μM vs 183 ± 31 μM; p=0.005) and 5-FU (23 ± 9 nM vs 81 ± 29 nM; p=0.009) concentrations in the brain. Patients who were treated with 150 mg/kg/day 5-FC and either 10 million (dose level 2) or 50 million (dose level 3) CD-NSCs had average 5-FU brain interstitial concentrations of 107 ± 32 nM and 64 ± 8 nM, respectively (p>0.05), indicating there was no apparent NSC dose effect on 5-FU concentrations in the brain. Given the presumed inherent inter- and intra-patient variability in NSC biodistribution throughout the brain, and the technical variability associated with intracerebral microdialysis, it is likely that our sample size was too small to detect such an NSC-mediated dose effect.
Figure 3.
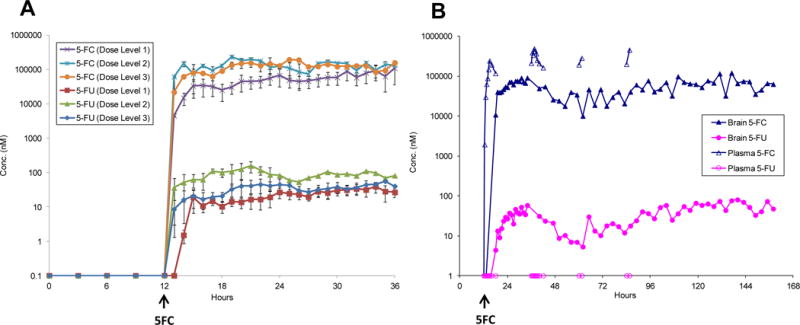
Intracerebral microdialysis data: brain interstitial 5-FC and 5-FU concentrations. A. Average brain interstitial 5-FC and 5-FU concentrations from patients on dose levels 1 (N=4), 2 (N=2) and 3 (N=3). Dose escalation was: level 1, 10 million CD-NSCs + 75 mg/kg/day 5-FC ; level 2, 10 million CD-NSCs + 150 mg/kg/day 5-FC and level 3, 50 million CD-NSCs + 150 mg/kg/day 5-FC. B. Intracerebral microdialysis data from a single patient on dose level 1 demonstrating the ability of the NSCs to convert 5-FC to 5-FU in the brain throughout the 7-day course of oral 5-FC. In this patient, no 5-FU was detected in the plasma, indicating that all of the 5-FU measured in the brain came from NSC-mediated conversion of 5-FC to 5-FU.
The average steady-state plasma 5-FC concentrations in participants taking 5-FC doses of 75 or 150 mg/kg/day were 252 ± 72 μM and 676 ± 428 μM, respectively. These 5-FC plasma concentrations are similar to previously reported values in patients taking 5-FC to treat infections (27). The mean ratio of average brain interstitial to plasma 5-FC concentrations was 25 ± 13%. In one third of participants studied, 5-FU was detected in plasma; however, the plasma 5-FU concentrations (38 ± 11 nM) were much lower than the brain interstitial 5-FU concentrations (85 ± 40 nM). This result indicates that the presence of 5-FU in brain interstitium could not be accounted for by circulating 5-FU levels in blood, providing further evidence that the CD-NSCs converted 5-FC to 5-FU locally in the brains of all participants assessed with intracerebral microdialysis.
The microdialysis catheters remained functional for the full dosing interval of 5-FC in two participants. Analysis of dialysate and plasma samples revealed that the CD-NSCs continued converting 5-FC to 5-FU throughout the entire 7 days of 5-FC administration (Fig. 3B). No 5-FU was detected in plasma samples from this participant, indicating that all of the 5-FU measured in her brain was the result of NSC-mediated conversion of 5-FC to 5-FU.
NSC migration to distant tumor foci
Brain autopsies were performed on two study participants. One was a woman who died of recurrent disease 44 days after injection of the CD-NSCs in her right frontal lobe, and the other was a man who died of progressive multifocal disease 79 days after injection of NSCs in his right parietal lobe. Both patients received a dose of 10 million NSCs. All of the sampled brain tissue was assessed for the presence of CD-NSCs by nested PCR for the v-myc gene. The detection limit of this nested PCR assay is 68 NSCs per 1 μg DNA, which is equivalent to 17 NSCs per 42,000 cells (250 ng tissue). In both brains, v-myc-positive areas were detected within tumor foci distant from the primary injection sites, including the opposite hemisphere. Additionally, v-myc was detected in the female patient’s contralateral left occipital lobe, where tumor had been resected 13 years earlier. This v-myc-positive area was devoid of obvious tumor cells but displayed moderate to marked anoxic ischemic changes (NSCs also travel to areas of anoxia-hypoxia) (28).
Figure 4A shows representative brain sections from the male patient. Nested PCR for v-myc (Fig. 4B) detected CD-NSCs in three tissue block cassettes, including the patient’s right parietal lobe, left frontal lobe/corpus callosum, and left occipital lobe. Two of these sites were located in the contralateral cerebral hemisphere, as identified in coronal brain sections (Fig. 4A). Diffuse viable tumor was confirmed by the study neuropathologist in adjacent tissue sections from all three NSC-positive PCR blocks. These NSC-positive areas were indistinguishable histologically from areas devoid of NSCs. We estimated the distance traveled by the CD-NSCs from the injection site in the right parietal lobe to the left occipital lobe (the furthest site where CD-NSCs were identified) to be approximately 11 cm.
Figure 4.
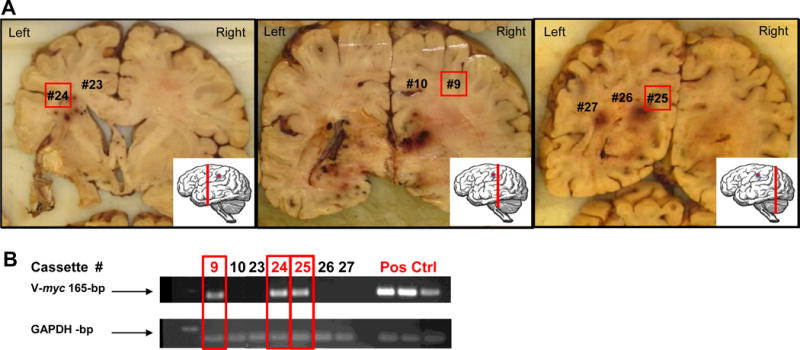
Brain autopsy data from the male patient. A. Representative coronal sections, depicted from rostral to occipital (inset shows the plane of section, red line; and the plane of NSC injection, red dot), show the classic variegated appearance of involvement by a high grade infiltrating glioma, including diffuse cerebral edema with mass effect (right side larger than left side), necrosis, and hemorrhage. Numbers on the coronal sections represent areas from which tissue samples were obtained (sampling from additional coronal sections not shown). The presence of NSCs (red boxes) was confirmed by nested PCR analysis in the right frontoparietal white matter, just occipital to the NSC injection site (cassette #9; center panel) as well as at distant sites, including the left internal capsule and corona radiata (cassette #24, left panel) and left occipital region (cassette #25, right panel). The presence of infiltrating glioblastoma was confirmed histologically at these three sampled sites. B. Representative nested PCR results. Red text and boxes indicate v-myc positive bands. GAPDH was used as a DNA integrity control and normal brain tissue spiked with NSC DNA was used as a positive control for v-myc. The assay was repeated 3 times per tissue block sample, and detection of v-myc in 2 out of 3 PCR assays was considered positive for NSCs.
Absence of CD-NSC induced tumorigenicity on short term follow up
Additionally, the male patient’s brain autopsy showed no evidence of secondary tumors derived from the CD-NSCs. To assess for the possible presence of NSC-derived secondary tumors, 5 μm tissue sections from the three v-myc positive cassettes (Fig. 4) were stained for PCNA, a cell division marker. To determine the genotype of PCNA-positive cells, sequential XY FISH staining was performed on the same slide. CD-NSCs (XX) were distinguished from the patient’s cells (XY) by superimposing PCNA and FISH images. Blind analysis by a cytogenetics technologist identified rarely present single female cells (Fig. 5), in blocks 9 (2 cells in 141 fields) and 25 (1 cell in 136 fields). All PCNA-positive cells identified were of male origin, and all female cells were PCNA-negative, indicating that the remaining NSCs in the brain were not dividing and thus not tumorigenic.
Figure 5.
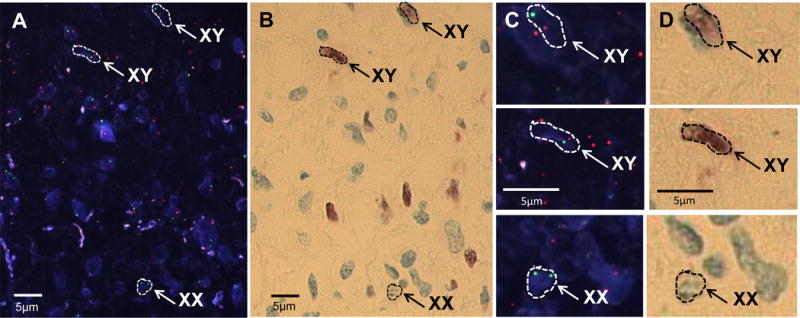
Mitotically inactive CD-NSCs within tumor parenchyma. A representative section from brain tissue in cassette #25 from the male autopsy patient, which nested PCR indicated contained NSCs (see Fig. 4), was processed for both XY FISH and PCNA, a cell division marker. A. XY FISH (X chromosomes green, Y chromosomes red) shows the presence of a rare single CD-NSC (XX) scattered among male tumor cells (XY). B. The same tissue section as in panel A, stained with PCNA and detected with the chromogenic substrate 3-amino-9-ethyl-carbazole. Patient tumor cells were PCNA-positive (brown) and CD-NSCs were PCNA-negative, indicating that the CD-NSCs were not mitotically active. FISH stained images were superimposed with PCNA brightfield images. C and D. Higher magnification fluorescent and brightfield images of the 3 cells indicated in panels A and B.
DISCUSSION
Through this first-in-human study, we have established the safety of intracranial administration of genetically modified allogeneic NSCs followed by treatment with an oral prodrug and provide initial proof-of-concept data regarding the ability of CD-NSCs to migrate to sites of tumor in the brain and locally convert a prodrug into a chemotherapy agent. Unlike many other NSC-based therapeutic approaches, where the goal is to achieve long-term engraftment and differentiation of the NSCs, this NSC-based anti-cancer strategy does not require the NSCs to permanently engraft. The cells must only remain viable in the brain long enough to produce the therapeutic agent. Our intracerebral microdialysis data showed that the CD-NSCs continued to function during the entire seven day course of 5-FC (Fig. 3B).
Based on autopsy data from two study participants, only rarely did CD-NSCs remain in the brain longer than required for prodrug conversion, which fortunately provided preliminary clinical documentation of CD-NSC migration from the injection sites to distant tumor foci, including tumor in the contralateral cerebral hemisphere. However, even a small number of CD-NSCs persisting in the brain raises a safety concern as to whether these v-myc immortalized NSCs might become tumorigenic. If residual NSCs were to continue dividing and start forming secondary tumors, the CD expressed by the NSCs could simultaneously function to kill any rapidly dividing CD-NSCs with 5-FU generated from conversion of 5-FC. Nonetheless, it is reassuring to observe that the few remaining CD-NSCs identified at autopsy did not appear to be dividing (PCNA-negative) and were present only as rare single cells in tumor, rather than as a cell mass, which might have suggested cell division had occurred. Although based on limited clinical data, these autopsy findings are consistent with preclinical data showing that CD-NSCs ceased dividing within 48 h of implantation and did not form tumors (9). Caveats to the conclusion that this NSC line is non-tumorigenic in the human brain are that each participant received only 1 intracranial dose of CD-NSCs, and the 2 autopsy patients died from progressive disease within 3 months of starting study treatment, so follow-up was limited. The potential for NSCs to form secondary tumors in the brain will continue to be assessed in future studies in which repeated doses of NSCs will be administered to study participants.
The principal advantage of NSC-based delivery of anti-cancer therapeutics is the potential to increase the tumor-specificity, and therefore, the therapeutic index of treatments. Intracerebral microdialysis data revealed that the CD-NSCs converted 5-FC to 5-FU in the brain. In a small subset of patients, low levels of 5-FU were also found in plasma, which is consistent with previous studies in patients taking 5-FC for fungal infections, and most likely results from conversion of 5-FC by intestinal microflora that express CD (29). Importantly, every patient had detectable levels of 5-FU in the brain, but not every patient had detectable levels in plasma. Furthermore, given the relatively poor CNS penetration of 5-FU when administered systemically, the fact that 5-FU levels in the brain were always higher than the levels measured in plasma, means that most, if not all, of the 5-FU detected in the brain was the result of localized production of the cytotoxic chemotherapy by the CD-NSCs.
Results from another microdialysis study (30) provide additional context for the intracerebral drug concentrations measured in the current trial. In that study, intratumoral microdialysis data were acquired from five patients who had accessible subcutaneous tumors and were treated with a 5-day continuous infusion of 1000 mg/m2/day of 5-FU. Average tumoral 5-FU concentrations were in the range of 350 to 750 nM compared to 100 nM on the current study. Although tumoral 5-FU levels in the previous study were 4-8-fold higher, in our study, using a single administration of NSCs and multiple oral doses of 5-FC, we achieved comparable 5-FU levels in the brain to those attained in peripheral tumors with continuous infusions of 5-FU. Furthermore, because we administered 5-FC for 7 days instead of only 5, the total 5-FU exposures (i.e., AUC) were even more similar.
The general threshold for 5-FU cytotoxicity is in the micromolar range; however, the cytotoxic effects of 5-FU are also time-dependent, such that longer drug exposures result in lower IC50 values. In our study, measured concentrations of 5-FU produced by the CD-NSCs were in the high nanomolar range and were maintained throughout the entire 7-day 5-FC course. It is important to point out that the concentrations of 5-FU measured by microdialysis will depend on the location of the catheter relative to the NSCs. Because the levels of 5-FU are expected to be highest directly adjacent to the cells expressing CD, the microdialysis measurements are likely underestimating the true local drug concentrations. It is also possible that with further dose escalation of the CD-NSCs and/or 5-FC, higher concentrations of 5-FU may be achievable. Alternatively, given the continuous production of 5-FU by the CD-NSCs, exposure to prolonged intracerebral concentrations of 5-FU may be sufficient to kill tumor cells as compared to bolus dosing of 5-FU. Moreover, prolonged exposure to lower concentrations of 5-FU may potentiate the cytotoxicity of other drugs and radiation therapy (31–33).
Although conversion of 5-FC to 5-FU by CD is an established prodrug/enzyme gene therapy modality, 5-FU is not a standard chemotherapy agent for gliomas. Two phase II studies of intravenously administered 5-FU in patients with recurrent primary brain tumors showed 5-FU had only minimal activity (34, 35). However, in vitro cytotoxicity studies demonstrated that glioma cells are as sensitive to 5-FU as gastrointestinal tumor cells (36). This discrepancy may be explained by the presence of the blood-brain barrier. With high grade gliomas, the blood-brain barrier is typically disrupted, but the amount of systemically administered chemotherapy that reaches these tumors is often variable and unpredictable. Moreover, the advancing edge of the tumor lies behind intact blood-brain barrier. Thus, the lack of efficacy seen in prior glioma clinical trials with 5-FU was more likely due to the limited ability of systemically administered 5-FU to cross the blood-brain barrier and achieve therapeutic levels in the brain rather than an inherent resistance of gliomas to 5-FU. Circumventing the blood-brain barrier by using NSCs to locally produce 5-FU at sites of tumor in the brain is a reasonable alternative strategy to explore.
Assessment of efficacy was not a primary objective of this pilot feasibility study, given the FDA stipulation that we first establish the safety of a single intracranial dose of CD-NSCs before treating patients with repeated doses. Indeed, no noticeable improvement in median PFS or OS was observed in this recurrent high grade glioma patient cohort as a whole. Although the finding of a statistically significant difference in median OS between study participants who received a dose of 10 million versus 50 million CD-NSCs is intriguing, there was no apparent difference in 5-FU levels measured in the brain in these two groups. Therefore, concluding that a true dose effect on OS exists after treatment with a single dose of CD-NSCs is premature and will be further assessed in a formal phase I study that is underway in patients with recurrent high grade gliomas who are being treated with escalating doses of CD-NSCs administered intracranially every 2 weeks via an indwelling brain catheter, followed each time by a 7-day course of oral 5-FC.
Regardless of whether this particular NSC-mediated enzyme/prodrug combination ultimately becomes a new treatment for gliomas, the results of this first-in-human study lay the foundation for future NSC-based clinical trials in patients with brain tumors. Viewed as a platform technology, this NSC line can be further modified for tumor-localized delivery of a variety of anti-tumor agents, including other prodrug activating enzymes (4, 10, 37, 38), oncolytic viruses (39–41), apoptotic agents (42–44), antibodies (45), or nanoparticles (46, 47), which could be given serially or in combination to maximize therapeutic benefit. Thus, this NSC-based approach potentially has widespread applicability, which could lead to improved treatment of both primary and metastatic brain tumors.
Supplementary Material
Translational Relevance.
Human neural stem cells are inherently tumor-tropic, enabling them to track to infiltrative sites of tumor in the brain. When modified to express a therapeutic transgene, neural stem cells can serve as vehicles for tumor-localized drug delivery, in effect, circumventing the blood-brain barrier to produce concentrated amounts of chemotherapy directly at sites of tumor while minimizing toxicity to normal brain tissue. In addition to obtaining preliminary safety data, this first-in-human neural stem cell study in patients with recurrent high grade gliomas provides initial clinical proof-of-concept regarding the ability of cytosine deaminase-expressing neural stem cells to migrate to tumor foci after intracranial administration and mediate localized conversion in the brain of an oral prodrug, 5-fluorocytosine, to the chemotherapy agent 5-fluorouracil. These results lay the foundation for future studies of neural stem cell-based anti-cancer strategies for the treatment of both primary and metastatic brain tumors.
Acknowledgments
We thank Rachel Magnusson, RN, for her meticulous care of the study patients and the microdialysis catheters. We also thank Keely Walker, PhD, for editing this manuscript. We are grateful to Shu Mi for qualifying and running the v-myc and RCR qPCR assays and Vivi Tran for performing the plasma antibody assay.
Financial support: This research was supported by grants from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) R21CA137639 (Co-principal investigators: J. Portnow and K.S. Aboody), Phase One Foundation (J. Portnow), Arthur and Rosalinde Gilbert Foundation (K.S. Aboody), and City of Hope. Additionally, research reported in this publication included work performed in the following City of Hope core facilities, which are supported by the NCI under award number P30CA33572: Analytical Pharmacology, Clinical Immunobiology Correlative Studies Laboratory, Analytical Cytometry, Cytogenetics, and Biostatistics. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Disclosure of potential conflicts of interest: K.S. Aboody is an officer, director, and shareholder of TheraBiologics, Inc., a clinical-stage biopharmaceutical company focused on the development of neural stem cell-mediated cancer therapies.
Authors’ contributions:
Conception and design: J.Portnow, T.W. Synold, B. Badie, K.S. Aboody.
Development of methodology: J. Portnow, T.W. Synold, S. F. Lacey, R. Tirughana, M. Metz, T. Vo, J. Najbauer, K.S. Aboody.
Acquisition of data (provided animals, acquired and managed study patients, provided facilities, etc.): J. Portnow, B. Badie, M. Chen.
Analysis and interpretation of data (eg., statistical analysis, biostatistics, computational analysis): J. Portnow, T.W. Synold, R. Tirughana, S. F. Lacey, M. D’Apuzzo, M. Metz, V. Bedell, T. Vo, M. Gutova, K.S. Aboody.
Writing, review, and or/revision of the manuscript: J. Portnow, T.W. Synold, R. Tirughana, K.S. Aboody, V. Bedell, S.F. Lacey, M. D’Apuzzo, P. Frankel, M. Metz, J. Najbauer, M. Chen, B.Badie.
Administrative, technical, or material support: (i.e., reporting or organizing of data, constructing databases): J. Portnow, P. Frankel, K.S. Aboody. J. Najbauer,
Study supervision: J. Portnow, B. Badie, P. Frankel, K.S. Aboody.
References
- 1.Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–50. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 4.Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–6. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 5.Lin D, Najbauer J, Salvaterra PM, Mamelak AN, Barish ME, Garcia E, et al. Novel method for visualizing and modeling the spatial distribution of neural stem cells within intracranial glioma. Neuroimage. 2007;37:13. doi: 10.1016/j.neuroimage.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 6.Kendall SE, Najbauer J, Johnston HF, Metz MZ, Li S, Bowers M, et al. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26:1575–86. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- 7.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–52. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 8.Seol HJ, Jin J, Seong DH, Joo KM, Kang W, Yang H, et al. Genetically engineered human neural stem cells with rabbit carboxyl esterase can target brain metastasis from breast cancer. Cancer Lett. 2011;311:152–9. doi: 10.1016/j.canlet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5:3005365. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutova M, Shackleford GM, Khankaldyyan V, Herrmann KA, Shi XH, Mittelholtz K, et al. Neural stem cell-mediated CE/CPT-11 enzyme/prodrug therapy in transgenic mouse model of intracerebellar medulloblastoma. Gene Ther. 2013;20:143–50. doi: 10.1038/gt.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SH, Lee HJ, An J, Lim I, Borlongan C, Aboody KS, et al. Human neural stem cells expressing carboxyl esterase target and inhibit tumor growth of lung cancer brain metastases. Cancer Gene Ther. 2013;20:678–82. doi: 10.1038/cgt.2013.69. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Xie R, Zhao T, Yang X, Han L, Ye F, et al. Neural stem cells preferentially migrate to glioma stem cells and reduce their stemness phenotypes. International journal of oncology. 2014;45:1989–96. doi: 10.3892/ijo.2014.2629. [DOI] [PubMed] [Google Scholar]
- 13.Teng J, Hejazi S, Badr CE, Tannous BA. Systemic anticancer neural stem cells in combination with a cardiac glycoside for glioblastoma therapy. Stem Cells. 2014;32:2021–32. doi: 10.1002/stem.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–71. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim SU. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain Dev. 2007;29:193–201. doi: 10.1016/j.braindev.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Kim SU, Lee HJ, Park IH, Chu K, Lee ST, Kim M, et al. Human nerual stem cells for brain repair. Int J Stem Cells. 2008;1:27–35. doi: 10.15283/ijsc.2008.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barresi V, Belluardo N, Sipione S, Mudo G, Cattaneo E, Condorelli DF. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther. 2003;10:396–402. doi: 10.1038/sj.cgt.7700580. [DOI] [PubMed] [Google Scholar]
- 18.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994;91:8302–6. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratain MJ, Mick R, Schilsky RL, Siegler M. Statistical and ethical issues in the design and conduct of phase I and II clinical trials of new anticancer agents. Journal of the National Cancer Institute. 1993;85:1637–43. doi: 10.1093/jnci/85.20.1637. [DOI] [PubMed] [Google Scholar]
- 20.Bedell V, Forman SJ, Gaal K, Pullarkat V, Weiss LM, Slovak ML. Successful application of a direct detection slide-based sequential phenotype/genotype assay using archived bone marrow smears and paraffin embedded tissue sections. The Journal of molecular diagnostics : JMD. 2007;9:589–97. doi: 10.2353/jmoldx.2007.070050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blakeley J, Portnow J. Microdialysis for assessing intratumoral drug disposition in brain cancers: a tool for rational drug development. Expert Opin Drug Metab Toxicol. 2010;6:1477–91. doi: 10.1517/17425255.2010.523420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergenheim AT, Capala J, Roslin M, Henriksson R. Distribution of BPA and metabolic assessment in glioblastoma patients during BNCT treatment: a microdialysis study. J Neurooncol. 2005;71:287–93. doi: 10.1007/s11060-004-1724-0. [DOI] [PubMed] [Google Scholar]
- 23.Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91:51–8. doi: 10.1007/s11060-008-9678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–8. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portnow J, Badie B, Markel S, Liu A, D’Apuzzo M, Frankel P, et al. A neuropharmacokinetic assessment of bafetinib, a second generation dual BCR-Abl/Lyn tyrosine kinase inhibitor, in patients with recurrent high-grade gliomas. Eur J Cancer. 2013;49:1634–40. doi: 10.1016/j.ejca.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portnow J, Badie B, Liu X, Frankel P, Mi S, Chen M, et al. A pilot microdialysis study in brain tumor patients to assess changes in intracerebral cytokine levels after craniotomy and in response to treatment with a targeted anti-cancer agent. J Neurooncol. 2014;118:169–77. doi: 10.1007/s11060-014-1415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwer AE, van Kan HJ, Johnson E, Rajanuwong A, Teparrukkul P, Wuthiekanun V, et al. Oral versus intravenous flucytosine in patients with human immunodeficiency virus-associated cryptococcal meningitis. Antimicrob Agents Chemother. 2007;51:1038–42. doi: 10.1128/AAC.01188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, et al. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819–29. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- 29.Harris BE, Manning BW, Federle TW, Diasio RB. Conversion of 5-fluorocytosine to 5-fluorouracil by human intestinal microflora. Antimicrob Agents Chemother. 1986;29:44–8. doi: 10.1128/aac.29.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konings IR, Sleijfer S, Mathijssen RH, de Bruijn P, Ghobadi Moghaddam-Helmantel IM, van Dam LM, et al. Increasing tumoral 5-fluorouracil concentrations during a 5-day continuous infusion: a microdialysis study. Cancer chemotherapy and pharmacology. 2011;67:1055–62. doi: 10.1007/s00280-010-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim R, Tanabe K, Inoue H, Toge T. Mechanism(s) of antitumor action in protracted infusion of low dose 5-fluorouracil and cisplatin in gastric carcinoma. International journal of oncology. 2002;20:549–55. [PubMed] [Google Scholar]
- 32.Lawrence TS, Tepper JE, Blackstock AW. Fluoropyrimidine-Radiation Interactions in Cells and Tumors. Seminars in radiation oncology. 1997;7:260–6. doi: 10.1053/SRAO00700260. [DOI] [PubMed] [Google Scholar]
- 33.Ojima E, Inoue Y, Watanabe H, Hiro J, Toiyama Y, Miki C, et al. The optimal schedule for 5-fluorouracil radiosensitization in colon cancer cell lines. Oncology reports. 2006;16:1085–91. [PubMed] [Google Scholar]
- 34.Stewart DJ, Dahrouge S, Soltys K. A phase II study of 5-fluorouracil plus folinic acid in malignant gliomas in adults. J Neurooncol. 1995;23:249–52. doi: 10.1007/BF01059957. [DOI] [PubMed] [Google Scholar]
- 35.Cascino TL, Veeder MH, Buckner JC, O’Fallon JR, Wiesenfeld M, Levitt R, et al. Phase II study of 5-fluorouracil and leucovorin in recurrent primary brain tumor. J Neurooncol. 1996;30:243–6. doi: 10.1007/BF00177275. [DOI] [PubMed] [Google Scholar]
- 36.Miller CR, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62:773–80. [PubMed] [Google Scholar]
- 37.Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS One. 2006;20 doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67:22–5. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- 39.Morshed RA, Gutova M, Juliano J, Barish ME, Hawkins-Daarud A, Oganesyan D, et al. Analysis of glioblastoma tumor coverage by oncolytic virus-loaded neural stem cells using MRI-based tracking and histological reconstruction. Cancer Gene Ther. 2015;22:55–61. doi: 10.1038/cgt.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias AL, Thaci B, Auffinger B, Rincon E, Balyasnikova IV, Kim CK, et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl Med. 2013;2:655–66. doi: 10.5966/sctm.2013-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed AU, Thaci B, Tobias AL, Auffinger B, Zhang L, Cheng Y, et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. Journal of the National Cancer Institute. 2013;105:968–77. doi: 10.1093/jnci/djt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–63. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 43.Balyasnikova IV, Ferguson SD, Han Y, Liu F, Lesniak MS. Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer Lett. 2011;310:148–59. doi: 10.1016/j.canlet.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hingtgen S, Ren X, Terwilliger E, Classon M, Weissleder R, Shah K. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and Temozolomide. Mol Cancer Ther. 2008;7:3575–85. doi: 10.1158/1535-7163.MCT-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank RT, Edmiston M, Kendall SE, Najbauer J, Cheung CW, Kassa T, et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS One. 2009;4:0008314. doi: 10.1371/journal.pone.0008314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mooney R, Roma L, Zhao D, Van Haute D, Garcia E, Kim SU, et al. Neural stem cell-mediated intratumoral delivery of gold nanorods improves photothermal therapy. ACS Nano. 2014;8:12450–60. doi: 10.1021/nn505147w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mooney R, Weng Y, Tirughana-Sambandan R, Valenzuela V, Aramburo S, Garcia E, et al. Neural stem cells improve intracranial nanoparticle retention and tumor-selective distribution. Future Oncol. 2014;10:401–15. doi: 10.2217/fon.13.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


