Abstract
Chronic kidney disease (CKD) is extremely common all over the world and is strongly linked to cardiovascular disease (CVD). The great majority of CKD patients have hypertension, which raises the risk of cardiovascular disease (CVD), end-stage kidney disease, and mortality. Controlling hypertension in patients with CKD is critical in our clinical practice since it slows the course of the disease and lowers the risk of CVD. As a result, accurate blood pressure (BP) monitoring is crucial for CKD diagnosis and therapy. Three important guidelines on BP thresholds and targets for antihypertensive medication therapy have been published in the recent decade emphasizing the way we measure BP. For both office BP and out-of-office BP measuring techniques, their clinical importance in the management of hypertension has been well defined. Although BP measurement is widely disseminated and routinely performed in most clinical settings, it remains unstandardized, and practitioners frequently fail to follow the basic recommendations to avoid measurement errors. This may lead to misdiagnosis and wrong management of hypertension, especially in CKD patients. Here, we review presently available all BP measuring techniques and their use in clinical practice and the recommendations from various guidelines and research gaps emphasizing CKD patients.
Keywords: ambulatory BP, office BP, chronic kidney disease, out-of-office BP, standardized BP
Introduction
Hypertension (HTN) affects the great majority of chronic kidney disease (CKD) patients, with prevalence ranging from 60% to 90% depending on the stage and etiology of the disease. Both HTN and CKD are pathophysiologic disorders that are intrinsically related, with chronic hypertension worsening kidney function and declining renal function worsening blood pressure regulation. Hypertension in CKD is caused by several factors including salt retention, volume overload, sympathetic overactivity, endothelial dysfunction, and changes in the renin-angiotensin-aldosterone axis.1,2 Across all age groups, it is a significant independent risk factor for cardiovascular disease, progression to end-stage kidney disease (ESKD), and mortality. Uncontrolled hypertension promotes the progression of CKD regardless of the cause.3–5 As a result, hypertension control is essential in all populations and more crucial in individuals with CKD. They often require multiple medications for blood pressure (BP) control and attempts to control hypertension in CKD to the target blood pressure remain largely ineffective.6–10 HTN epidemiology is also affected by the BP measurement techniques implemented. The prevalence of other patterns of hypertension like white coat hypertension, masked hypertension which increases the risk of cardiovascular disease (CVD) are high in CKD compared to the general population.11–14 Patients with CKD are also more likely to develop resistant HTN.13,14
The majority of hypertension clinical trials, both in the general population and in those with CKD, have used various standardized BP measurement techniques/methods for both office and out-of-office BP to define target BP goals.6–15 The recent three major guidelines for blood pressure thresholds and targets for antihypertensive drug therapy: the “2021 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the management of Blood Pressure in Chronic Kidney Disease”;14 the “2018 European Society of Cardiology and the European Society of Hypertension (ESC/ESH) Guidelines for the Management of Arterial Hypertension”;16 and the
2017 American College of Cardiology/American Heart Association 2017 (ACC/AHA) Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults17
have reemphasized for the standardization of BP measurement and its necessity for management of HTN. They have defined BP measurement techniques and conveyed proper techniques are critical for diagnosing hypertension, monitoring hypertensive patients, and evaluating therapeutic efficacy. It is also vital to know who takes your blood pressure and how well they understand the benefits and cons of different methods. The individual (doctor/nurse/clinic assistant, student) who does the BP measurement also has a considerable impact on the accuracy of the BP readings.1–4 Lack of adherence to conventional practices is not limited to nursing personnel; according to Bobrie et al18 just 20% of general practitioners, 25% of interns, and 35% of cardiologists are aware of the recommendations. Furthermore, as physicians advance in their careers, their knowledge base diminishes. The use of automated BP instruments and standardized BP methodologies are becoming more popular.19 Out-of-office BP [Home BP (HBPM) and 24-hour ambulatory BP (ABPM)] measurements are promising and tend to be the standard for diagnosis.20–24 Though all guidelines and studies recommend the need for proper BP measuring technique in office as well as out-of-office BP, still it is under practiced especially in the developing world. We summarize in this review, the various techniques of BP measurement, and the importance of standardized BP measurement along with present recommendations on BP measuring techniques by various guidelines with an emphasis on the CKD population. We also highlight the research gaps in this area, particularly in special CKD populations such as those on dialysis, kidney transplant recipients, and pediatric CKD.
Techniques of BP Measurement
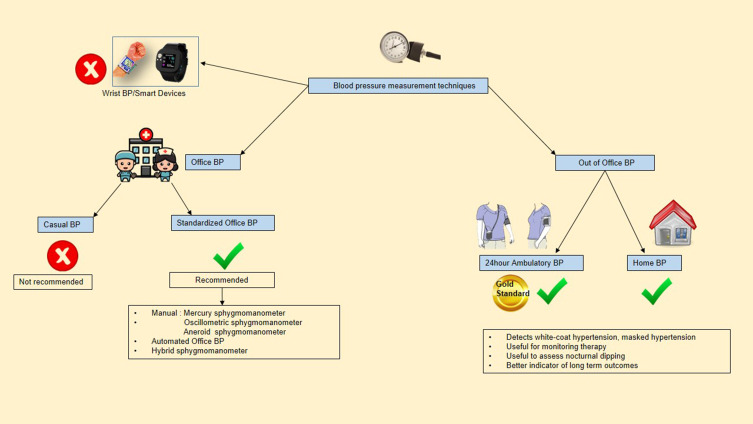
There are many BP measuring instruments available and each of these has advantages and disadvantages. The instruments available, their use in office and out-of-office setting in clinical practice are shown in Figure 1. Based on the clinical setting, BP measuring techniques can be broadly categorized into the office and out-of-office BP measurements.14,16,17
Figure 1.
BP measurement techniques.
Office BP Measurement
In clinical practice, much of our therapy and diagnosis is based on office measurements. The two non-invasive methods used are the classical auscultatory method and the oscillometric technique.25 The most widely used instrument is the oscillometric BP device in the majority of the countries for both offices and out-of-office BP.26 There are many causes for BP measurement error which may be device-related, patient-related, or process-related factors.27 The recent guidelines clearly emphasized the standardized measurement instead of routine office (casual office) BP measurement.
Standardized BP Measurement
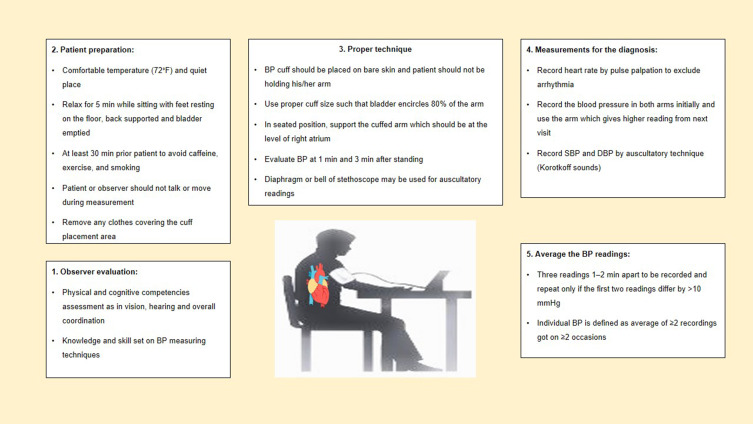
For standardized office BP measurement, oscillometric BP equipment may be preferred over a manual BP instrument. Although the automated BP (AOBP) may be more reliable than manual office BP, all guidelines emphasize the significance of measuring BP using specified protocols (Figure 2).28
Figure 2.
Standardized office BP measurement.
Casual BP Measurement
It is done without adhering to the standardized BP measurement protocol. Though discouraged by all guidelines, this is the commonly used technique in clinical practice across resource-limited centers. The major drawback is the absence of optimal cut-off-off levels, which can lead to false diagnosis.
Out-of-Office BP Measurement Techniques
Ambulatory BP Monitoring (ABPM)
As it can distinguish and identify all types of hypertension, 24-hour ABPM has been deemed the preferable measure of BP in both the general population and patients with CKD for diagnosis and therapy of hypertension. There is also a stronger link between ABPM-derived blood pressures with CVD and renal outcomes. An appropriate blood pressure cuff is worn for 24 hours in 24 hour-ABPM, with measurements taken every 15 to 20 min throughout the day and every 30 to 60 min during sleep. As a result, one benefit of ABPM is that it provides measurements during sleep that can be used to analyse nocturnal physiologic dipping (blood pressure should drop by at least 10% during sleep) and BP variability.29 “White coat hypertension” (10% −20%), “masked hypertension” (10% −30%), “nocturnal hypertension”, and “non-dippers” are highly prevalent in individuals with CKD and are associated with a higher CVD and CKD progression. In the absence of ABPM, these would be undetected. On the other hand, it is not widely available, and reimbursement for its use is limited. When accessible, 24-hour ABPM can be used to confirm increased blood pressures recorded in the clinic using an appropriate approach, particularly in cases of suspected “white coat hypertension” or “masked hypertension”. ABPM can also be used to establish that blood pressure is under control and to detect hypotension outside of the office.30
Home BP Monitoring (HBPM)
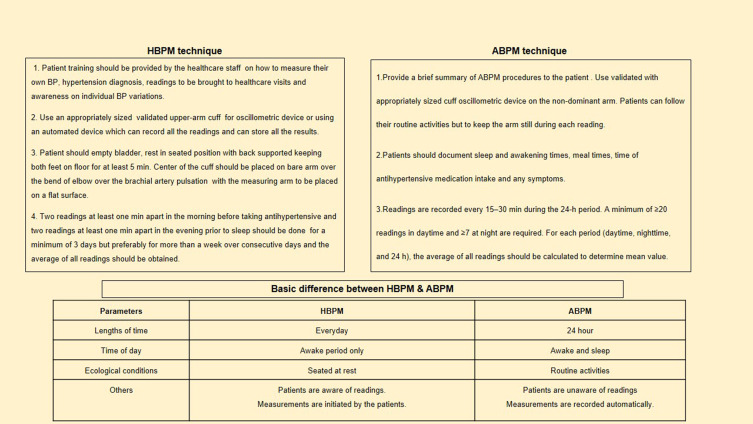
Although ABPM is the primary technique of confirmation, home BP measurements following suitable protocols are an acceptable alternative.31 When compared to routine office BP measurements, it is also observed to be more significantly linked with target organ damage and CKD progression.32–35 HBPM is more practical than ABPM and can be used to track blood pressure throughout treatment. Validated automated oscillometric instruments should be utilized, and patients should be reminded and instructed on how to measure blood pressure. Taking two readings at each session, twice a day is preferable. Although the evidence is less robust than 24-hour ABPM, home BP measurements are simple and can be a useful adjunct for improving BP control in clinical practice. The techniques of measurement and basic difference between HBPM and 24-hour ABPM are shown in Figure 3.
Figure 3.
Techniques and basic difference between Home BP (HBPM) and 24-hour ambulatory BP measurement (ABPM).
Present Evidence, Guidelines, and Their Recommendations in CKD
Office BP Measurement in CKD
Various major hypertension clinical trials in CKD have used many definitions and different techniques. They are summarized in Table 1. All the landmark trials like MDRD,36 AASK,37 REIN-2,15 ACCORD8 used standardized BP measuring protocol though the technique used varied from being the manual sphygmomanometer in the MDRD trial to the automated office BP set up in the SPRINT trial.13 The SPRINT trial has been one of the important trials forming the basis for the modified recommendations in lowering BP targets across many hypertension guidelines including the KDIGO 2021 guidelines. Nearly 30% of patients enrolled in the study had CKD. In the intensive BP control group, regardless of CKD status, there was a significant reduction in major cardiovascular events and all-cause mortality. The SPRINT trial utilized an automated BP instrument (OMRON 907XL) that could be programmed to have a 5 minute resting period followed by 3 automated BP records. Many sites had patients unattended during the BP measurement which again led to a debate on whether unattended BP would diminish the white coat effect further. An analysis of the attended versus unattended BP recordings in the SPRINT trial sites showed similar BP recordings and similar risk reduction in cardiovascular outcomes in the intensive group.38
Table 1.
Landmark Trials on Hypertension in CKD
| Study and Inclusion Criteria | BP Measurement Technique | BP Targets | Outcomes |
|---|---|---|---|
| MDRD – 199436 840 CKD patients (eGFR 13–55mL/min/1.73m2) |
3 consecutive seated BP readings of right arm measured by Hawksley random zero sphygmomanometer after 5 min rest. Mean of last 2 readings were recorded |
Usual target - MAP ≤ 107 mm Hg (for patients <60 years of age) or ≤ 113 mm Hg (for patients >61 years of age) Lower target - ≤ 92 mm Hg (for patients <60 years of age) or ≤ 98 mm Hg (for patients >61 years of age) |
Only patients with proteinuria >1 g/day benefited from lower target BP in terms of slower decline in eGFR |
| AASK – 200237 1094 hypertensive CKD (all African American) (eGFR 20–65 mL/min/1.73m2) |
3 consecutive seated BP readings were measured by Hawksley random zero sphygmomanometer after 5 min rest. Mean of last 2 were readings recorded |
Usual target – MAP 102–107 mmHg Lower target – MAP ≤ 92 mm Hg |
No benefit of lower BP target on GFR slope/GFR loss of 50%, ESRD or death |
| REIN −2 – 200515 335 patients - non-diabetic proteinuric CKD |
Mean of 3 consecutive BPs, taken 2 min apart, after 5 min rest in the sitting position, on the same arm by a standard sphygmomanometer. |
Usual target – DBP < 90mmHg Lower target – SBP <130 mmHg and DBP <80 mmHg |
No difference in the incidence of ESRD |
| ACCORD - 20108 4733 diabetic patients with creatinine <1.5mg/dl and proteinuria <1g/day |
Average of three measurements using an automated device (Omron 907) after 5 min rest with the participant seated in a chair |
Usual Target – SBP <140mmHg Lower target – SBP <120mmHg |
There is no difference in cardiovascular outcomes (nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes.) |
| SPS 3–201310 3020 patients with recent stroke |
Rest for 15 min followed by an average of 3 readings taken 2 min apart in sitting position in the same arm with an automated device (Colin 8800C) |
Usual target – SBP 130–149mmHg Lower target- SBP <130 mmHg |
No difference in outcomes for stroke or myocardial infarction or vascular death. |
| SPRINT – 201513 9361 non diabetic hypertensives (eGFR 20–60mL/min/1.72m2, proteinuria <1g/day) |
Average of 3 BP readings done in sitting position after 5 min rest, using an automated device Professional Digital Blood Pressure Monitor (Omron Healthcare, Lake Forest, model 907XL) |
Usual target – SBP <140 mmHg Lower target- SBP < 120 mmHg |
Significant reduction in rates of fatal and nonfatal major cardiovascular events and death from any cause in the lower target group |
Abbreviations: MDRD, modification of diet in renal diseases; AASK, African‑American Study of Kidney Disease and Hypertension; REIN‑2, Ramipril Efficacy in Nephropathy trial 2; ACCORD, Action to Control Cardiovascular Risk in Type 2 Diabetes; SPS3, The Secondary Prevention of Small Subcortical Strokes; SPRINT, Systolic Blood Pressure Intervention Trial; CKD, chronic kidney disease; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease.
Taking a cue from the SPRINT trial, all three guidelines mention the need for standardized BP measuring technique for office BP in the management of hypertension. The recommendations from the guidelines are summarized in Table 2. The AHA 2017 and ESH 2018 emphasize and provide clear guidelines on the methodology to be adopted for a standardized office BP measurement protocol, including having the pre-requisite checklist satisfied, compulsory rest period with a mean of at least 2–3 readings which can be practiced in the clinic setting. No specific comments have been made for the CKD population. The KDIGO 2021 guidelines have gone a step further and have suggested as a practice point that automated office BP recording may be preferred though the evidence for a recommendation is weak. In summary, office BP measurement should no longer be considered “casual“. KDIGO recommends that CKD patients with high BP be treated with a target systolic blood pressure (SBP) of <120 mm Hg, when tolerated, using standardized office BP measurement irrespective of the presence or absence of proteinuria, diabetes, or older age. KDIGO also clearly emphasizes that if BP is not measured using the standardized technique, the SBP target goal does not apply.
Table 2.
Major Hypertension Guidelines on BP Measurement Techniques and Targets
| BP Technique | ACC/AHA 201717 | ESC/ESH 2016 and 201816,18 | KDIGO 202114 |
|---|---|---|---|
| Office BP | Emphasis on standard office BP measurement protocols by validated BP measurement devices (Manual or automated) {COR- 1, LOE- EO} Standardized protocol and instructions for office BP measurement provided in the guideline |
Office BP measurement is preferred by either, auscultatory, oscillometric semi-automatic, or automatic sphygmomanometers following validated protocols Office BP to be measured in both arms. A pressure difference of 15 mmHg may indicate significant cardiovascular risk {LOE – IA} |
Recommend standardized office BP measurement compared to routine office BP measurement. No preference for manual versus automated, attended versus unattended {LOE- IB} The standard protocol to be followed as adopted from AHA 2017 guidelines Automated office BP measurement may be preferred (Practice Point) |
| 24-hour ambulatory BP | Recommended for confirming the diagnosis of HTN, titration of antihypertensive along with counselling or clinical interventions {COR- 1, LOE - A} ABPM may predict long term CVD outcomes Useful for diagnosing/titrating medications in patients with masked or white coat HTN {COR – IIa, IIb} |
Recommended as an alternative to office BP when economically or logistically feasible {LOE – IC} Recommended to detect white coat hypertension or masked hypertension, quantifying effects of treatment or side-effects {LOE- IA} A better predictor of hypertension mediated organ damage, coronary events, and stroke |
May be used to complement office BP for management of high BP. {LOE – IIB} |
| Home BP | Recommended for confirming the diagnosis of HTN, titration of antihypertensive along with counseling or clinical interventions {COR- 1, LOE - A} Useful for diagnosing/titrating medications in patients with masked or white coat HTN {COR – IIa, IIb} Instructions for Home BP monitoring have been given in the guidelines |
Recommended as an alternative to office BP when economically or logistically feasible {LOE – IC} Recommended to detect white coat hypertension or masked hypertension, quantifying effects of treatment or side-effects {LOE- IA} HBPM may better predict left ventricular hypertrophy and cardiovascular outcomes compared to office BP |
May be used to complement office BP for management of high BP. {LOE – IIB} |
| Specific recommendations/comments in CKD | CKD ± proteinuria Target BP - <130/80mmHg |
CKD ± proteinuria Target BP- <130–39/70–79 mmHg |
CKD ± proteinuria, ± Diabetes, ± old age Target BP-SBP<120 mmHg High prevalence of white coat effect and masked uncontrolled HTN in CKD which may need out-of-office BP readings. However, no major RCTs comparing clinical outcomes targeting or higher BP based on out of office BP measurements |
| Specific recommendations/comments in the HD population | Nil | Nil | Nil |
| Specific recommendations/comments in kidney transplant recipients | Use of standardized office BP to achieve target BP (< 130 /80 mm Hg) | Nil | Practice point – Use of standardized office BP to achieve target BP (< 130 /80 mm Hg) Out-of-office BP may be used as complementary modalities |
| Specific recommendations in the pediatric CKD population | Nil | A target MAP of <50th percentile for pediatric CKD with proteinuria >0.5g/g (protein creatinine ratio). For non-proteinuric pediatric CKD patients, a more liberal target of <75th percentile is recommended (ESH 2016) | 24-hour ABPM is preferred to measure for MAP targets of <50th percentile When ABPM is not available, manual auscultatory office BP is obtained in a protocol-driven standardized method for a target of SBP <90th percentile for age, sex, and height of normal children Practice point- Use of ABPM once a year and standardized office BP once in 3–6 months to achieve BP targets if standardized |
Abbreviations: COR, class of recommendation; LOE, level of evidence; ACC/AHA, 2017, American College of Cardiology/American Heart Association 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults; ESC/ESH, European Society of Cardiology and the European Society of Hypertension Guidelines for the Management of Arterial Hypertension; KDIGO, 2021 Kidney Disease: Improving Global Outcomes Clinical Practice Guideline for the management of Blood Pressure in Chronic Kidney Disease; CKD, chronic kidney disease; RCT, randomized control trial; MAP, mean arterial pressure; HBPM, home BP monitoring; ABPM, ambulatory BP monitoring.
Out-of-Office BP Measurement in CKD
BP variability in CKD: BP variability is a term that has been used to describe beat-to-beat, reading-to-reading on ABPM, circadian, within the visit, day-to-day, and between-office-visit changes. Several cross-sectional and prospective studies39–42 using ABPM in the CKD population have shown abnormalities in BP variability and circadian BP patterns, demonstrating that nocturnal non-dipping is predominant in the CKD population. Similar to ABPM, HBPM also aids in the diagnosis of various BP phenotypes such as “white-coat” hypertension, masked (reverse white-coat) hypertension, the “white-coat” effect, and masked uncontrolled which are highly prevalent in CKD patients.42,43
ABPM remains the gold standard for the diagnosis and management of hypertension in the CKD population. It has been proven to be strongly linked to unfavorable cardiovascular events and all-cause mortality in CKD patients.42,43 Proteinuria, which is a major risk factor for CKD progression, has also been correlated to ABPM.44 In a study by Minulotolo et al43 it was shown that office BP measurements did not correlate with the daytime ABPM values. ABPM was also able to predict higher cardiovascular mortality in patients with a higher night BP, including “non-dippers” and “reverse dippers”. Agarwal et al found that ABPM was much lower than office BP readings and was a stronger predictor of CKD development and ESRD than office BP readings.42 Thus, both adverse CV and renal events are well predicted by ABPM.
HBPM measurement is also a valuable tool to supplement office BP in CKD patients and has a better correlation with the 24-hour ABPM especially in patients with “nocturnal hypertension” and “masked hypertension”. Home BP, like ABPM, has been proven to be a superior predictor of left ventricular hypertrophy, fatal and nonfatal cardiovascular events, and all-cause mortality.45–47 Compared to ABPM, it may be more cost-effective and easier to implement. McManus et al35 conducted a randomized control trial on hypertensives with stroke and other high-risk groups showing patients who were randomized to self-management with home BP monitoring, self-titration of medications and telemonitoring had significantly better control of BP when compared to the usual care. Margolis et al48 showed that patients randomized to a home-based BP monitoring protocol with telemonitoring and adjustments by pharmacists showed a better office BP control when compared to the usual care. Agarwal et al49 in a similar comprehensive review and meta-analysis also emphasized the possibility of home BP measurement in conjunction with telemonitoring for better BP control. HBPM may thus enhance patient participation, promote adherence and improve BP control. Though studies show both ABPM and HBPM are preferred over office BP in terms of predicting clinical outcomes like CV events or death, the cost/benefit ratio, availability, patient acceptance are some important factors to be considered. Despite the concerns, the KDIGO 2021 guidelines14 suggest out-of-office blood pressure readings, ABPM, or home blood pressure as a supplement to office blood pressure measurements because of the possible benefits. KDIGO 202114 working group suggests that though ABPM may be the ideal method, however, home BP monitoring may be more practical especially in the scenario of the COVID-19 pandemic where visits to health-care facilities are often curtailed. Overall, as per KDIGO 2021 BP guidelines,14 the level of recommendation for out-of-office BP recording is weak (2B), because no significant RCTs have revealed differences in clinical outcomes based on office and out-of-office BP recordings in CKD. Out-of-office blood pressure readings are recommended by both the “AHA 2017 guidelines”17 and the “ESH 2018 guidelines”16 as important methods for detecting “white coat hypertension” and “masked hypertension”. The “AHA 2017 guidelines” make a strong recommendation (Class of Evidence 1 and Level of recommendation A) for using out-of-office blood pressure measures for hypertension diagnosis, medication titration, and telemedicine counseling as well as therapeutic interventions in general population. This strong recommendation stems from a comprehensive evaluation by the US Preventive Services Task Force, which found that using ABPM improved long-term CV outcome prediction.32 An ABPM (24-hour average BP) of 130/80 mm Hg (135/85 mm Hg daytime and 120/70 mm Hg overnight mean BPs) and a mean home BP of 135/85 mm Hg are expected to correspond with an office BP of 140/90 mm Hg.2 ACC/AHA 201717 guideline defines white coat hypertension when office BP is >130/80 mmHg, whereas 24-hour ABPM/HBPM is <130/80 mm Hg. The masked hypertension is vice versa (office BP <130/80 mm Hg and 24-hour ABPM/HBPM >130/80 mmHg). It also provides cut-off BP levels on ABPM and HBPM in the general population which correspond with various levels of office BP measured with auscultation or a semi-automated oscillometric device as shown in Table 3.
Table 3.
Cut off BP Levels (in mmHg) on ABPM & HBPM Based on Standardized Office BP (Adapted from ACC/AHA 2017 Guidelines)
| Standardized Office BP | Awake ABPM | Asleep ABPM | 24-Hr ABPM | HBPM |
|---|---|---|---|---|
| 120/80 | 120/80 | 100/65 | 115/75 | 120/80 |
| 130/80 | 130/80 | 110/65 | 125/75 | 130/80 |
| 140/90 | 135/85 | 120/70 | 130/80 | 135/85 |
| 160/100 | 145/90 | 140/85 | 145/90 | 145/90 |
Abbreviations: ACC/AHA-2017, American College of Cardiology/American Heart Association 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults; HBPM, Home BP monitoring; ABPM, ambulatory BP monitoring.
Hypertension Measurement Techniques in Special CKD Population
End-Stage Kidney Disease and Dialysis
Although the 2021 KDIGO guideline recommends standardized BP measurement for CKD, it does not make specific recommendations for patients on dialysis. Notably, the SPRINT trial, which heavily informs most of the recent guidelines on hypertension, excluded patients with eGFR <20mL/min/m2.
There are several issues unique to the dialysis population that prevents the extrapolation of evidence from other population groups and make the diagnosis, management, and treatment of hypertension challenging. There are various barriers50 to implementing standardized BP measurement in the peri-dialytic period which are listed below in Box 1.
Box 1.
Barriers to Standardized BP Measurement in the Peri-Dialytic Period
| (a) The presence of arteriovenous fistula prevents BP measurement in both arms (b) Measuring upper limb BP may not be possible in patients who have undergone multiple vascular access surgeries bilaterally (c) The patient may be unable to relax due to anxiety and discomfort during dialysis (d) It may be cumbersome for the dialysis nurse to take multiple readings during a hectic dialysis shift (e) Patients with severe vascular calcifications require intra-arterial measurements which is not feasible in most dialysis units. |
Moreover, even with the use of standardized techniques, there is considerable BP variability among those on dialysis depending on the timing of the measurement.51 Though assessment of BP and therapeutic decisions are most often based on measurements obtained before or after dialysis, pre-dialysis measurements tend to overestimate BP due to fluid overload and withholding of antihypertensive drugs before the dialysis session, while post-dialysis measurements underestimate BP.51,52 Studies have found that both pre- and post-dialytic BP measurements have a poor agreement with inter-dialytic BP.51 Furthermore, blood pressure measurements taken before and after dialysis are poor predictors of target organ damage, cardiovascular outcomes, and all-cause mortality.53–55 The association between peri-dialytic BP and mortality is a U-shaped curve, unlike the linear association seen in the general population.56,57 Based on these observations, the “2021 KDIGO Controversies Conference document on blood pressure and volume management in dialysis” states that pre- and post-dialytic BP measurements should not be the only means to diagnose or manage hypertension in patients on dialysis.58
ABPM – specifically, interdialytic 44-hour ABPM – has a stronger association with adverse clinical outcomes, has better reproducibility, and therefore, is considered gold-standard for assessing BP in the dialysis population.53,58–61 This method also enables the detection of circadian BP variability. It has been reported that up to 80% of patients on dialysis are non-dippers, and that reverse-dipping is five times higher in ESKD, compared to those without kidney disease.38,62 Given that these abnormal patterns are associated with increased CV risk and mortality,41,42 this is an additional compelling argument for ABPM in dialysis. However, limited availability cost constraints and poor patient acceptability limit its wide use. There is particularly poor tolerability of this technique among patients on dialysis: there is an increased risk of bruising and bleeding, and sleep disturbances (which are already a common problem in ESKD) are worsened during ABPM.63 There is also no clarity at present regarding the optimal frequency of ABPM in this population.
Considering these drawbacks of ABPM, home BP measurements taken twice a day during the interdialytic period over one to two weeks (or for four days during the mid-week interdialytic interval) have been suggested as a suitable alternative.58,61 This method addresses most of the shortcomings of peri-dialytic BP measurements and is cost-effective, practical, and feasible. Home BP measurements have also shown a stronger association with cardiovascular outcomes and mortality, compared to pre- or post-dialytic BP measurements.54 One noteworthy disadvantage of this method though is the inability to detect nocturnal hypertension and non-dippers. Out-of-dialysis BP measurement taken in-office using standardized techniques has been suggested as a third alternative.58 Studies on various BP measurements in the dialysis population are summarized in Table 4.
Table 4.
Studies on BP Measurement Techniques in a Special Population of CKD
| Study Author and Characteristics | BP Measurement Technique | Outcomes |
|---|---|---|
| End-stage kidney disease and dialysis: | ||
| 1. Pre- and post-dialysis BP Agarwal R et al, 200650 Comparison between dialysis unit BP (pre-and-post) and ambulatory BP (ABP) |
Metaanalysis The most commonly used method in most studies was an average of 12 predialysis and post-dialysis readings obtained in the month preceding the study. Space Labs 90,207, 48 hr, including the first and second dialysis sessions for ABP in most studies. |
Predialysis diastolic BP overestimated ABP and post-dialysis BP underestimated ABP |
| 2. Home BP Agarwal R et al, 200641 Using interdialytic ambulatory blood pressure recordings as the reference standard, we compared the performance of routine, standardized, and home BP monitoring in 104 predominantly black patients on chronic hemodialysis for at least 3 months. |
Dialysis unit BP recordings were averaged over 2 weeks and home BP over 1 week. Home BP monitoring: BP three times daily – on waking up, between noon and 1900 and at bedtime over 1 week using a validated self-inflating automatic oscillometric device. |
Systolic BP threshold of >150 mmHg at home averaged over 1 week was comparable to diagnosis by ambulatory BP monitoring |
| 3. ABPM Sarafidis PA et al, 201949 396 hemodialysis patients underwent 48-h ABPM monitoring during a regular hemodialysis session and the subsequent interdialytic interval |
A validated ABPM monitor was fitted in the non-fistula arm with a cuff of appropriate size and ABPM monitoring was started. The device was set to measure BP every 20 min during the daytime and every 30 min during the nighttime for a complete 48-h standard intra- and inter-dialytic period. | The prevalence of hypertension in hemodialysis patients assessed by 48-h ABPM monitoring is very high and supports the use of ABPM for HTN diagnosis and treatment in the HD population. |
| Kidney transplant recipients: | ||
| Agena et al. 201166 Comparative study between office BP, Home BP, and ABPM in 183 KTRs |
Office BP – Performed by a nurse after a rest period of 5 min, 3 readings by the automatic oscillometric device. Mean of last 2 readings taken. Home BP – BP measured twice a day, before breakfast and before dinner, three consecutive times on each occasion and for five consecutive days. An automated oscillometric device was used. ABPM – Spacelab TM ABPM device used. Day time - every 20 min and night time - every 30 min BP measured. |
Both office BP and Home BP over diagnosed patients with uncontrolled hypertension when ABPM was taken as standard. Home BP recording had more closer recordings to ABPM as compared to office BP |
| Wen KC et al. 201267 Comparative study between 24-hour ABPM and Office BP measurement in 244 prevalent KTRs |
Office BP – Single sitting BP measurement after 5 min rest by a trained nurse in a quiet room with mercury sphygmomanometer by auscultatory method ABPM – Spacelab TM oscillometric unit – day time readings every 15 min and night time every 30 min |
Office BP overestimated both daytime ABPM and 24-hour ABPM |
| Czyżewski et al. 201468 Comparative study between 24-hour ABPM and office BP in 50 KTRs |
Office BP – 10 min of rest followed by mean of 2 BP readings taken 2 min apart rest as per ECC/ESH 2013 guidelines ABPM - SpaceLabsTM 90217 device. 20 min intervals for daytime reading and 30 min for nighttime reading |
No correlation was seen between office BP and 24-hour ABPM. 18% had masked hypertension, 10% had white coat hypertension. only 16% showed nocturnal dipping in BP |
| Bhatnagar et al. 201869 Comparative study between conventional office BP versus SPRINT type automated office BP in 120 KTRs |
Conventional Office BP – rest period of 3 min, single BP recording in the presence of a doctor SPRINT type BP – Unattended BP after resting for 5 min, an average of 3 readings in 1 min interval HEM 907 XL, OMRON machine used for both recordings |
Automated office BP taken in the protocol of the SPRINT trial showed significantly lower SBP/DBP than the conventional single reading of oscillometric BP |
| Mallamaci et al. 201970 Long term comparative study between office BP and 24-hour ABPM in 260 KTRs |
Office BP – Sitting position, mean of 2–3 readings taken at 1–2 min intervals by any standard and calibrated instrument ABPM – SpacelabsTM machine. BP measurement frequency every 15 min both during the day and night |
74% of patients had nocturnal HTN. There was poor agreement between office BP and ABPM. In 37% of visits, office BP lead to wrongful anti-hypertensive adjustments |
| Pediatric CKD: | ||
| The ESCAPE Trial:73 Multicenter RCT (33 European pediatric nephrology units) 385 children Age: 3 to 18 years CKD with eGFR 15 to 80 mL per minute per 1.73 m2 of body-surface area) Fixed high dose of Ramipril for both groups (6 mg/ m2 of BSA) intensified blood-pressure control (with a target 24-hour mean arterial pressure below the 50th percentile) or conventional blood-pressure control (mean arterial pressure in the 50th to 95th percentile) |
24-hour ABPM was performed every 6 months over 5 years Spacelabs 90,207 oscillometric devices (Spacelabs Healthcare) Measurements were performed every 15 min during daytime and every 20–30 min during nighttime |
Intensified blood-pressure control, with target 24-hour blood-pressure levels in the low range of normal, confers a substantial benefit for slowing the progression of renal disease among children with progressive chronic kidney disease |
| CKiD trial:76 Twenty-Four–Hour Ambulatory Blood Pressure versus Clinic Blood Pressure Measurements and Risk of Adverse Outcomes in Children with CKD 513 participants Observational study |
Clinic BP: Performed by trained and certified personnel by auscultation method. Annually using an aneroid sphygmomanometer with an appropriately sized cuff. After at least 5 min of quiet rest, 3 consecutive seated readings were obtained at each study visit 30 seconds apart and the average of those 3 readings was considered the clinic BP for that visit ABPM: 24-hour Ambulatory BP monitoring was performed during the CKiD Study using a SpaceLabs 90,217 monitor (SpaceLabs Healthcare), with BPs taken every 20 min over 24 hours |
Clinic BPs taken in a protocol-driven setting are not consistently inferior to ambulatory BP in the discrimination of BP-related adverse outcomes in children with CKD |
Abbreviations: KTRs, Kidney transplant recipients; ESCAPE, Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CKD in Pediatric Patients; CKiD, Chronic Kidney Disease in Children Study.
While the “2021 KDIGO Controversies Conference” and the
2019 European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA)
as well as the “Hypertension in the Kidney working group of the European Society of Hypertension (ESH) consensus” documents attempts to address some of the issues regarding the optimal timing and technique of BP measurement, and BP targets in the dialysis population specifically, there are still some unanswered questions. Most of the guidance on BP assessment and monitoring in dialysis comes from studies conducted in patients on conventional thrice-weekly hemodialysis. There is a dearth of evidence in patients on peritoneal dialysis and other forms of hemodialysis, such as nocturnal daily dialysis and home dialysis. Evidence-based targets for ABPM and home-BP in the dialysis population are also awaited.
Kidney Transplant Recipients (KTRs)
The 2021 KDIGO guidelines recommend (as a starting point) monitoring blood pressure targets in KTRs using standardized office blood pressure measures, and that ABPM and home BP monitoring be used as complementary methods. KTRs belong to the high cardiovascular risk group with death related to cardiovascular events being the most common cause for mortality. Hypertension remains an important cause of adverse patient and graft outcomes.64 A combination of factors including pre-existing vascular disease, drugs like steroids and CNIs, diabetes, and renal allograft disease are considered in the initiation and potentiation of hypertension and its effects. Nocturnal hypertension and lack of nocturnal dipping are also common among KTRs.65
As with the dialysis population, there are significant knowledge gaps regarding the ideal method of assessment and monitoring of BP in KTRs. A recent systematic review found a profound difference between office BP and ABPM among KTRs in predicting clinical outcomes like renal function, markers of cardiovascular damage like left ventricular hypertrophy, carotid-intima-media thickness and endothelial dysfunction.66 A summary of a few recent studies that have explored the relationship between different BP measurement techniques with their clinical implications is summarized in Table 4.68–71 Overall, there was significant disagreement between the office BP and 24-hour ABPM measurement in KTRs in most studies. It is worth noting that, while the majority of these studies used methodologies that were comparable to “standardized” in-office BP monitoring, the protocol was not consistent. Therefore, there is a need for well-conducted studies to ascertain the effect of BP measurement techniques on clinical endpoints in this population. At present, there is insufficient data to support the incorporation of ABPM as a routine method of BP monitoring in this population. So KDIGO recommends its use at present as complementary to standardized BP like in CKD (practice point).
Hypertension in Pediatric CKD Patients
Every time a pediatric CKD patient sees a doctor, their blood pressure should be checked. Even though clinic BP readings are within the normal range, “masked hypertension” affects more than one-third of children and adolescents with CKD. “White coat hypertension” is also common in youngsters, affecting almost half of those with high office blood pressures.72
When BP recordings are consistently over the 90th percentile for the child’s age, sex, and height, or ≥130/80 mm Hg, the KDIGO 2021 clinical practice guidelines indicate that a child with CKD begins antihypertensive medicine. It recommends checking blood pressure once a year with an ABPM and every 3–6 months with a standardized auscultatory office BP. In children with CKD, KDIGO recommends lowering 24-hour mean arterial pressure by ABPM to the 50th percentile for age, sex, and height. In comparison to clinic BP results, the AHA Scientific Statement on pediatric ABPM promotes ABPM as the gold standard measure for assessing BP in children since stronger relationships between ABPM and target organ damage in children have been demonstrated.14,17,73
Even the American Academy of Pediatrics (AAP) recommends that all children with CKD get a 24-hour ABPM at least once a year to detect “white coat hypertension” and “masked hypertension”. Adults are not commonly offered ABPM, thus these recommendations are different. These guidelines are based on the Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CKD in Pediatric Patients (ESCAPE) trial, which included children with baseline CKD with eGFR 20–80 mL/min per 1.73 m2. The results of the trial lead to a redefinition of blood pressure targets in children with CKD.74,75
But in clinical practice, using 24-hour ABPM in children is still not routinely practiced. However, the logistical barrier of undertaking ambulatory BP monitoring (availability of equipment and personnel) is significant. Obtaining and returning the equipment, as well as the device’s accompanying discomfort and insurance reimbursement make ambulatory blood pressure monitoring more difficult to execute than clinic blood pressure measures. In reality, ambulatory BP monitoring to confirm maintained BP management in children is difficult in clinical practice.33,73,76,77
Manual auscultatory office BP obtained in a protocol-driven standardized setting targeting achieved SBP < 90th percentile for age, sex, and height of normal children have been advised when ABPM is not available.14 The Chronic Kidney Disease in Children Study (CKiD) also found that manual auscultatory clinic BPs taken in a protocol-driven context are not inferior to ambulatory BP in discriminating of BP-related adverse outcomes in children with CKD.76
Current KDIGO guidelines for pediatric hypertension urge routine auscultatory office BP monitoring every 3–6 months. If an electronic (oscillometric) BP monitor detects increased blood pressure, it should be confirmed with an auscultatory BP measurement. This is because the available reference values for diagnosing hypertension in children were acquired using the auscultatory method.72 The auscultatory BP evaluation, however, faces several difficulties due to the anatomic and physiological peculiarities of children. Small arm dimensions, small and elastic arteries, small waveforms, significant discrepancies in peripheral (brachial) and central (aortic) BP, substantial discrepancies in peripheral (brachial) and central (aortic) BP, as well as observer error, prejudice, and bias, as well as observer error, prejudice, bias, and terminal digit preference, are few significant ones to be considered.72,78,79
Though oscillometric devices are less expensive and require less training than auscultatory equipment as well as they prevent observer bias and errors, there is a lack of evidence for the use of automated BP in a clinical setting in pediatrics by using oscillometric devices similar to adults. In children validating BP monitoring by oscillometric method faces various obstacles due to their unique structural and functional properties, such as tiny arms with significant variation needing multiple cuff sizes and relatively low blood pressure readings. Validation techniques designed for adults do not effectively address all of the unique requirements of BP monitor validation in children. Separate validation studies in children are also required.72
Home blood pressure monitoring in children has been proven to be more effective than ambulatory monitoring in detecting “white-coat hypertension” and “masked hypertension”.80–82 It can be considered as an alternative technique for out-of-office BP, but still, there is a need for more studies.
Summary and Conclusion
The concept of standardized BP measurement has largely been restricted to clinical trials and clinical guidelines with limited penetration to the clinical practice in the past. After the SPRINT trial, the current move by recent guidelines including KDIGO 2021 has brought standardization of office BP measurement to the forefront and strongly recommends it in clinical practice to achieve BP targets and its importance in the CKD population. The benefits of obtaining reliable and accurate BP measurements by such a procedure outweigh the additional burden of time or cost. It is high time that we nephrologists implement these measures and discourage casual or unstandardized techniques. The use of out-of-office BP measurements (ABPM and HBPM) can be used complementary to office BP in our clinical practice especially in the detection of “masked hypertension”, “white coat hypertension”, “nocturnal BP” characteristics in our CKD population and seem to correlate better with clinical outcomes. Especially in the dialysis population, where BP variability is pronounced, ABPM or home BP measurements may certainly have an important role. The use of 24-hour ABPM in pediatric CKD should be encouraged among pediatricians for diagnosis and treatment as recommended by KDIGO. But at present, there are still areas of uncertainty, implementation barriers and research gaps, especially among specific subgroups like KTRs, dialysis, and pediatric CKD which preclude routine applicability of these measures. It highlights that even within the CKD population, a one-size-fits-all strategy may not be practical, and it calls for more research in these subgroups.
Funding Statement
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Horowitz B, Miskulin D, Zager P. Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis. 2015;22(2):88–95. doi: 10.1053/j.ackd.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 2.Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74(1):120–131. doi: 10.1053/j.ajkd.2018.12.044 [DOI] [PubMed] [Google Scholar]
- 3.Go A, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 4.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 5.Judd E, Calhoun DA. Management of hypertension in CKD: beyond the guidelines. Adv Chronic Kidney Dis. 2015;22(2):116–122. doi: 10.1053/j.ackd.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drexler YR, Bomback AS. Definition, identification and treatment of resistant hypertension in chronic kidney disease patients. Nephrol Dial Transplant. 2014;29(7):1327–1335. doi: 10.1093/ndt/gft346 [DOI] [PubMed] [Google Scholar]
- 7.JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertension Res. 2008;31(12):2115–2127. doi: 10.1291/hypres.31.2115 [DOI] [PubMed] [Google Scholar]
- 8.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. New Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 10.SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomized trial. Lancet. 2013;382(9891):507–515. doi: 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang AR, Lóser M, Malhotra R, Appel LJ. Blood pressure goals in patients with CKD: a review of evidence and guidelines. Clin J Am Soc Nephrol. 2019;14(1):161–169. doi: 10.2215/CJN.07440618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirkle JL, Freedman BI. Hypertension and chronic kidney disease: controversies in pathogenesis and treatment. Minerva Urol Nefrol. 2013;65(1):37. [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. SPRINT Research Group. N Engl J Med. 2015;373:2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung AK, Chang TI, Cushman WC, et al. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3):S1–87. [DOI] [PubMed] [Google Scholar]
- 15.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomized controlled trial. Lancet. 2005;365(9463):939–946. doi: 10.1016/S0140-6736(05)71082-5 [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 17.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 18.Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34(10):1887–1920. doi: 10.1097/HJH.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 19.Coomer RW, Schulman G, Breyer JA, Shyr Y. Ambulatory blood pressure monitoring in dialysis patients and estimation of mean interdialytic blood pressure. Am J Kidney Dis. 1997;29(5):678–684. doi: 10.1016/S0272-6386(97)90119-0 [DOI] [PubMed] [Google Scholar]
- 20.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–S23. [PubMed] [Google Scholar]
- 21.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines?: a framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458 [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Miller ER III, Charleston J. Improving the measurement of blood pressure: is it time for regulated standards? Ann Intern Med. 2011;154(12):838–839. doi: 10.7326/0003-4819-154-12-201106210-00014 [DOI] [PubMed] [Google Scholar]
- 23.Hagemeister J, Schneider CA, Barabas S, et al. Hypertension guidelines and their limitations – the impact of physicians’ compliance as evaluated by guideline awareness. J Hypertens. 2001;19(11):2079–2086. doi: 10.1097/00004872-200111000-00020 [DOI] [PubMed] [Google Scholar]
- 24.Verberk WJ, Kroon AA, Kessels AG, De leeuw PW. Home blood pressure measurement: a systematic review. J Am Coll Cardiol. 2005;46(5):743–751. doi: 10.1016/j.jacc.2005.05.058 [DOI] [PubMed] [Google Scholar]
- 25.Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35–e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017;35(3):421. doi: 10.1097/HJH.0000000000001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahromi SE, Haghighi G, Roozbeh J, Ebrahimi V. Comparisons between different blood pressure measurement techniques in patients with chronic kidney disease. Kidney Res Clin Pract. 2019;38(2):212. doi: 10.23876/j.krcp.18.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115(4):291–297. doi: 10.1016/S0002-9343(03)00366-8 [DOI] [PubMed] [Google Scholar]
- 29.Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. New Engl J Med. 2018;378(16):1509–1520. doi: 10.1056/NEJMoa1712231 [DOI] [PubMed] [Google Scholar]
- 30.Thomas G, Drawz PE. BP measurement techniques: what they mean for patients with kidney disease. Clin J Am Soc Nephrol. 2018;13(7):1124–1131. doi: 10.2215/CJN.12551117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siu AL. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–786. doi: 10.7326/M15-2223 [DOI] [PubMed] [Google Scholar]
- 32.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55(6):1346–1351. doi: 10.1161/HYPERTENSIONAHA.109.149336 [DOI] [PubMed] [Google Scholar]
- 34.McManus RJ, Mant J, Haque MS, et al. Targets and self-management for the control of blood pressure in stroke and at-risk groups (TASMIN-SR): a randomized controlled trial. J Hum Hypertens. 2013;27(10):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klahr S, Levey AS, Beck GJ, et al. Modification of Diet in Renal Disease Study Group: the effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/NEJM199403313301301 [DOI] [PubMed] [Google Scholar]
- 36.Fogo A, Breyer JA, Smith MC, et al.; AASK Pilot Study Investigators. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. Kidney Int. 1997;51(1):244–252. doi: 10.1038/ki.1997.29 [DOI] [PubMed] [Google Scholar]
- 37.Johnson KC, Whelton PK, Cushman WC, et al. Blood pressure measurement in SPRINT (systolic blood pressure intervention trial). Hypertension. 2018;71(5):848–857. doi: 10.1161/HYPERTENSIONAHA.117.10479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mojon A, Ayala DE, Pineiro L, et al.; Hygia Project Investigators. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30(1–2):145–158. [DOI] [PubMed] [Google Scholar]
- 39.Tanner RM, Shimbo D, Dreisbach AW, et al. Association between 24-hour blood pressure variability and chronic kidney disease: a cross-sectional analysis of African Americans participating in the Jackson heart study. BMC Nephrol. 2015;16:84. doi: 10.1186/s12882-015-0085-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMullan CJ, Hickson DA, Taylor HA, Forman JP. Prospective analysis of the association of ambulatory blood pressure characteristics with incident chronic kidney disease. J Hypertens. 2015;33(9):1939–1946. doi: 10.1097/HJH.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 41.Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(2):406–411. doi: 10.1038/sj.ki.5000081 [DOI] [PubMed] [Google Scholar]
- 42.Minutolo R, Agarwal R, Borrelli S, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171(12):1090–1098. doi: 10.1001/archinternmed.2011.230 [DOI] [PubMed] [Google Scholar]
- 43.Drawz PE, Alper AB, Anderson AH, et al. Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016;11(4):642–652. doi: 10.2215/CJN.08530815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16(7):971–975. doi: 10.1097/00004872-199816070-00010 [DOI] [PubMed] [Google Scholar]
- 45.Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B [DOI] [PubMed] [Google Scholar]
- 46.Drawz PE, Abdalla M, Rahman M. Blood pressure measurement: clinic, home, ambulatory, and beyond. Am J Kidney Dis. 2012;60(3):449–462. doi: 10.1053/j.ajkd.2012.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster-randomized clinical trial. JAMA. 2013;310(1):46–56. doi: 10.1001/jama.2013.6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57(1):29–38. doi: 10.1161/HYPERTENSIONAHA.110.160911 [DOI] [PubMed] [Google Scholar]
- 49.Sarafidis PA, Mallamaci F, Loutradis C, et al. Prevalence and control of hypertension by 48-h ambulatory blood pressure monitoring in hemodialysis patients: a study by the European Cardiovascular and Renal Medicine (EURECA-m) working group of the ERA-EDTA. Nephrol Dial Transplant. 2019;34(9):1542–1548. doi: 10.1093/ndt/gfy147 [DOI] [PubMed] [Google Scholar]
- 50.Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre-and post-dialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1(3):389–398. doi: 10.2215/CJN.01891105 [DOI] [PubMed] [Google Scholar]
- 51.Zoccali C, Tripepi R, Torino C, Tripepi G, Mallamaci F. Moderator’s view: ambulatory blood pressure monitoring and home blood pressure for the prognosis, diagnosis, and treatment of hypertension in dialysis patients. Nephrol Dial Transplant. 2015;30(9):1443–1448. doi: 10.1093/ndt/gfv241 [DOI] [PubMed] [Google Scholar]
- 52.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55(3):762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47(1):62–68. doi: 10.1161/01.HYP.0000196279.29758.f4 [DOI] [PubMed] [Google Scholar]
- 54.Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3):507–517. doi: 10.1016/S0272-6386(99)70188-5 [DOI] [PubMed] [Google Scholar]
- 55.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D. U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x [DOI] [PubMed] [Google Scholar]
- 56.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45(4):811–817. doi: 10.1161/01.HYP.0000154895.18269.67 [DOI] [PubMed] [Google Scholar]
- 57.Flythe JE, Chang TI, Gallagher MP, et al. Blood pressure and volume management in dialysis: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97(5):861–876. doi: 10.1016/j.kint.2020.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peixoto AJ, Santos SF, Mendes RB, et al. Reproducibility of ambulatory blood pressure monitoring in hemodialysis patients. Am J Kidney Dis. 2000;36(5):983–990. doi: 10.1053/ajkd.2000.19100 [DOI] [PubMed] [Google Scholar]
- 59.Parati G, Ochoa JE, Bilo G, et al. Hypertension in chronic kidney disease part 1: out-of-office blood pressure monitoring: methods, thresholds, and patterns. Hypertension. 2016;67(6):1093–1101. doi: 10.1161/HYPERTENSIONAHA.115.06895 [DOI] [PubMed] [Google Scholar]
- 60.Sarafidis PA, Persu A, Agarwal R, et al. Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transplant. 2017;32(4):620–640. [DOI] [PubMed] [Google Scholar]
- 61.Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P. An investigation of the effect of advancing uremia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997;12(11):2301–2307. doi: 10.1093/ndt/12.11.2301 [DOI] [PubMed] [Google Scholar]
- 62.Jardine AG. Con: ambulatory blood pressure measurement in patients receiving hemodialysis a sore arm and a waste of time? Nephrol Dial Transplant. 2015;30(9):1438–1441. doi: 10.1093/ndt/gfv244 [DOI] [PubMed] [Google Scholar]
- 63.Weir MR, Burgess ED, Cooper JE, et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015;26(6):1248–1260. doi: 10.1681/ASN.2014080834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasiske BL, Anjum S, Shah R, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43(6):1071–1081. doi: 10.1053/j.ajkd.2004.03.013 [DOI] [PubMed] [Google Scholar]
- 65.Pisano A, Mallamaci F, D’Arrigo G, et al. Blood pressure monitoring in kidney transplantation: a systematic review on hypertension and target organ damage. Nephrol Dial Transplant. 2021;36(7):1326–1346. doi: 10.1093/ndt/gfab076 [DOI] [PubMed] [Google Scholar]
- 66.Agena F, Prado ED, Souza PS, et al. Home blood pressure (BP) monitoring in kidney transplant recipients is more adequate to monitor BP than office BP. Nephrol Dial Transplant. 2011;26(11):3745–3749. doi: 10.1093/ndt/gfr143 [DOI] [PubMed] [Google Scholar]
- 67.Wen KC, Gourishankar S. Evaluating the utility of ambulatory blood pressure monitoring in kidney transplant recipients. Clin Transplant. 2012;26(5):E465–70. doi: 10.1111/ctr.12009. [DOI] [PubMed] [Google Scholar]
- 68.Czyżewski Ł, Wyzgał J, Kołek A. Evaluation of selected risk factors of cardiovascular diseases among patients after kidney transplantation, with particular focus on the role of 24-hour automatic blood pressure measurement in the diagnosis of hypertension: an introductory report. Ann Transpl. 2014;19:188–198. doi: 10.12659/AOT.890189 [DOI] [PubMed] [Google Scholar]
- 69.Bhatnagar A, Pein U, Markau S, et al. Influence of SPRINT study type automated office blood pressure measurements on hypertension diagnosis in kidney transplant patients. Kidney Blood Press Res. 2018;43(2):310–317. doi: 10.1159/000487900 [DOI] [PubMed] [Google Scholar]
- 70.Mallamaci F, Tripepi R, D’Arrigo G, et al. Long-term blood pressure monitoring by the office and 24-h ambulatory blood pressure in renal transplant patients: a longitudinal study. Nephrol Dial Transplant. 2019;34(9):1558–1564. doi: 10.1093/ndt/gfy355 [DOI] [PubMed] [Google Scholar]
- 71.Stergiou GS, Boubouchairopoulou N, Kollias A. Accuracy of automated blood pressure measurement in children: evidence, issues, and perspectives. Hypertension. 2017;69(6):1000–1006. doi: 10.1161/HYPERTENSIONAHA.116.08553 [DOI] [PubMed] [Google Scholar]
- 72.Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63(5):1116–1135. doi: 10.1161/HYP.0000000000000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wühl E, Trivelli A, Picca S, et al.; ESCAPE Trial Group. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. [DOI] [PubMed] [Google Scholar]
- 74.Siragy HM. ESCAPE: from hypertension to renal failure. Curr Hypertens Rep. 2010;12(4):207–209. doi: 10.1007/s11906-010-0124-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ku E, McCulloch CE, Warady BA, Furth SL, Grimes BA, Mitsnefes MM. Twenty-four–hour ambulatory blood pressure versus clinic blood pressure measurements and risk of adverse outcomes in children with CKD. Clin J Am Soc Nephrol. 2018;13(3):422–428. doi: 10.2215/CJN.09630917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nasothimiou EG, Karpettas N, Dafni MG, Stergiou GS. Patient’s preference for ambulatory versus home blood pressure monitoring. J Hum Hypertens. 2014;28(4):224–229. doi: 10.1038/jhh.2013.104 [DOI] [PubMed] [Google Scholar]
- 77.O’Brien E, Fitzgerald D. The History of Indirect Blood Pressure Measurement. Blood Pressure Measurement. Handbook of Hypertension. Amsterdam: Elsevier; 1991:1–54. [Google Scholar]
- 78.O’Sullivan J, Allen J, Murray A. A clinical study of the Korotkoff phases of blood pressure in children. J Hum Hypertens. 2001;15(3):197–201. doi: 10.1038/sj.jhh.1001140 [DOI] [PubMed] [Google Scholar]
- 79.Stergiou GS, Karpettas N, Kapoyiannis A, Stefanidis CJ, Vazeou A. Home blood pressure monitoring in children and adolescents: a systematic review. J Hypertens. 2009;27(10):1941–1947. doi: 10.1097/HJH.0b013e32832ea93e [DOI] [PubMed] [Google Scholar]
- 80.Kollias A, Dafni M, Poulidakis E, Ntineri A, Stergiou GS. Out-of-office blood pressure and target organ damage in children and adolescents: a systematic review and meta-analysis. J Hypertens. 2014;32(12):2315–2331. doi: 10.1097/HJH.0000000000000384 [DOI] [PubMed] [Google Scholar]
- 81.Karpettas N, Kollias A, Vazeou A, Stergiou GS. Office, ambulatory, and home blood pressure measurement in children and adolescents. Expert Rev Cardiovasc Ther. 2010;8(11):1567–1578. doi: 10.1586/erc.10.148 [DOI] [PubMed] [Google Scholar]
- 82.Kubrusly M, de Oliveira CM, Silva RP, Pinheiro MA, Rocha MB, Magalhães RM. Blood pressure measurement in hemodialysis: the importance of the measurement technique. Saudi J Kidney Dis Transpl. 2016;27(2):241. doi: 10.4103/1319-2442.178251 [DOI] [PubMed] [Google Scholar]