Abstract
Calcific aortic valve disease (CAVD) is a highly prevalent condition that comprises a disease continuum, ranging from microscopic changes to profound fibro-calcific leaflet remodelling, culminating in aortic stenosis, heart failure, and ultimately premature death. Traditional risk factors, such as hypercholesterolaemia and (systolic) hypertension, are shared among atherosclerotic cardiovascular disease and CAVD, yet the molecular and cellular mechanisms differ markedly. Statin-induced low-density lipoprotein cholesterol lowering, a remedy highly effective for secondary prevention of atherosclerotic cardiovascular disease, consistently failed to impact CAVD progression or to improve patient outcomes. However, recently completed phase II trials provide hope that pharmaceutical tactics directed at other targets implicated in CAVD pathogenesis offer an avenue to alter the course of the disease non-invasively. Herein, we delineate key players of CAVD pathobiology, outline mechanisms that entail compromised endothelial barrier function, and promote lipid homing, immune-cell infiltration, and deranged phospho-calcium metabolism that collectively perpetuate a pro-inflammatory/pro-osteogenic milieu in which valvular interstitial cells increasingly adopt myofibro-/osteoblast-like properties, thereby fostering fibro-calcific leaflet remodelling and eventually resulting in left ventricular outflow obstruction. We provide a glimpse into the most promising targets on the horizon, including lipoprotein(a), mineral-binding matrix Gla protein, soluble guanylate cyclase, dipeptidyl peptidase-4 as well as candidates involved in regulating phospho-calcium metabolism and valvular angiotensin II synthesis and ultimately discuss their potential for a future therapy of this insidious disease.
Keywords: Calcific aortic valve disease, Lipoprotein(a), Notch1, Ageing, Nitric oxide, Medical therapy
Graphical Abstract
Graphical Abstract.
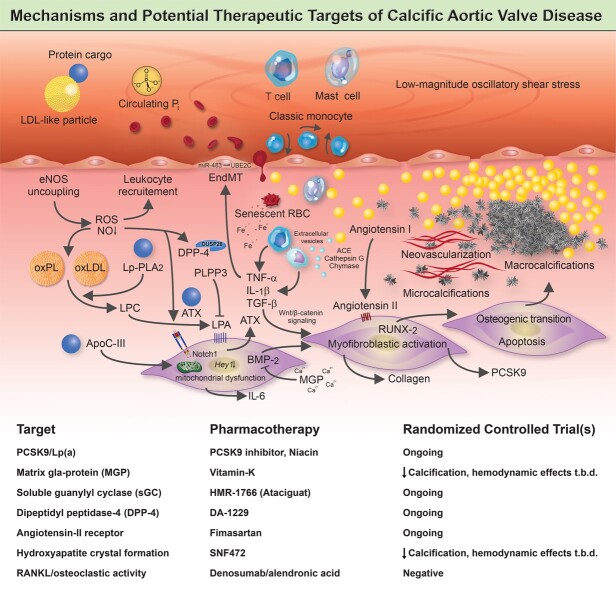
A complex network of cellular and molecular mechanisms underpins the pathobiology of calcific aortic valve disease. According to the current concept, disrupture of the endothelial layer covering the fibrosa promotes the uptake of oxidatively modified lipids (along with the protein-cargo they carry), red blood cells, and immune-cells, thereby promoting an inflammation-calcification feedback loop that results in fibro-calcific remodelling, leaflet stiffening and ultimately narrowing of the left ventricular outflow tract, with its dreadful clinical sequelae such as aortic stenosis, heart failure and premature death. Beyond LDL-C lowering by statins, other previously identified molecules, including PCSK9/Lp(a), mineral-binding matrix Gla protein, soluble guanylate cyclase, dipeptidyl peptidase-4 as well as candidates involved in regulating valvular angiotensin II synthesis and phosphocalcium metabolism, have been targeted pharmacologically in randomized controlled trials. While in some of these studies an attenuation of calcification burden could be observed, effects of target modulation on haemodynamic disease progression, a clinically much more relevant surrogate of disease burden, are uncertain and need to be rigorously assessed in future trials.
Introduction
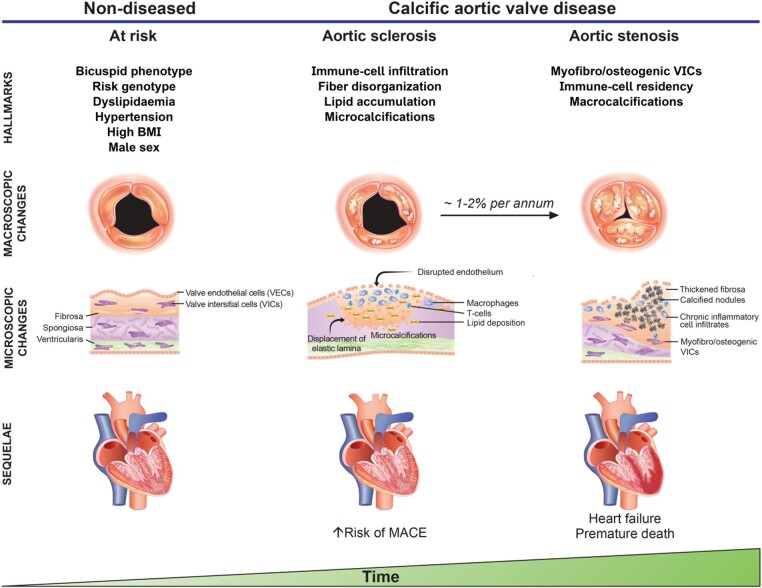
Calcific aortic valve disease (CAVD) is the most common valvular heart disease in high-income countries, encompassing a disease spectrum ranging from aortic valve (AV) sclerosis (i.e. fibro-calcific leaflet remodelling without significant impairment in leaflet motion and aortic orifice narrowing) to severe left ventricular (LV) outflow obstruction by calcific AV stenosis (AS). AV sclerosis precedes AS with roughly 9% of sclerosis cases transitioning to AS within a 5-year period,1 despite marked interindividual differences (Figure 1).2 , 3 The prevalence of CAVD sharply surges with advancing age, with >25% of people being affected >65 years, and >50% of individuals aged ≥85 years.4 , 5 In its preclinical stage, LV outflow is largely unaffected, yet already associated with high risk of adverse events, including stroke, coronary events, and premature death,5 likely mediated by the frequent co-existence of coronary atherosclerosis.6
Figure 1.
Risk factors, structural changes and sequelae of calcific aortic valve disease at different disease stages. A variety of risk factors, including a bicuspid phenotype, dyslipidaemia, hypertension, diabetes, and increased body mass index enhance the risk to develop calcific aortic valve disease. Endothelial disruption, lipid accumulation, immune-cell infiltration and collagen fibre disorganization occur early in the disease process, with fine-stippled mineralisations being a hallmark of early disease stages. Of note, patients without left ventricular outflow obstruction but sclerotic changes of the aortic valve are at increased risk for major adverse cardiovascular events, likely mediated by the frequent co-existence of coronary atherosclerosis. While the rate of transition from aortic sclerosis to symptomatic aortic valve stenosis varies considerably between patients, findings from the population-based Cardiovascular Health Study1 suggest that 1–2% of patients with aortic sclerosis progress to aortic stenosis annually, of which three-quarter develop heart failure, undergo valve replacement or die within 2 to 5 years of follow-up.
Despite a decline in mortality,7 due to an increased use of transcatheter AV implantation (TAVI) in high-risk patients,8 , 9 the global disease burden remains substantial with estimated numbers of patients requiring surgical AV replacement (SAVR) or TAVI growing at least two-fold by 2050 in both the USA and Europe.10–15 Currently, the indication for interventional therapy is driven by AS-related symptoms and a severely reduced AV area, while in asymptomatic patients at low surgical risk SAVR/TAVI can be justified only if profound LV dysfunction (LV ejection fraction <50%) is present.8 , 9 Thus, the majority of patients undergo valve replacement when myocardial remodelling and symptoms such as angina, shortness of breath, and impaired exercise performance have already developed. In fact, up to 80% of patients recruited in the prospective, multinational IMPULSE registry had symptomatic disease at baseline, of which >50% already reported severe heart failure symptoms (defined as New York Heart Association class III or IV) at a time when a diagnosis of AS was first established.16
Indeed, as CAVD progresses, the elevated LV pressure imposed by the narrowed aortic orifice induces an adaptive response to normalize LV wall stress and to temporarily compensate increased afterload at the price of marked structural changes, ranging from concentric hypertrophy and remodelling to eccentric hypertrophy, the pattern and degree of which is determined by sex, age, comorbidities and hemodynamic disease severity.17–21 Maladaptive remodelling and LV hypertrophy gradually impair coronary flow reserve (which in turn can induce angina pectoris despite angiographically lesion-free coronary arteries)22–24 through which subendocardial ischaemia, cardiomyocyte loss, and fibrosis is promoted,25 , 26 leading to reduced LV longitudinal strain.27 LV ejection fraction is generally well preserved in the majority of patients presenting with AS, and hypertrophic changes tend to regress following interventional therapy. Nevertheless, the fibrotic burden within the myocardium remains, putting patients at heightened risk for adverse outcomes, even late after valve replacement therapy.28 , 29
The growing disease burden in the elderly coupled with marked global inequity in access to interventional therapies necessitates effective pharmacological strategies to delay or even cease CAVD progression (Graphical Abstract).8 , 9 , 15 , 30 High levels of low-density lipoprotein cholesterol (LDL-C) and (systolic) hypertension are traditional risk factors that are shared among CAVD and coronary atherosclerosis (affecting 25–50% of CAVD patients),31 yet aggressive LDL-C lowering consistently failed to impact hemodynamic disease progression or clinical outcomes in well-designed randomized controlled trials (RCTs), implying differential pathogenesis (Table 1).32–35 Similarly, although experimental and observational data support a link between pathways involved in bone metabolism and CAVD pathogenesis,36 , 37 neither the receptor activator of nuclear κB ligand (RANKL) inhibitor denosumab nor the bisphosphonate alendronic acid proved effective to blunt the natural course of the disease (Table 2).38
Table 1.
Completed randomized controlled trials on the effects of statin-induced lipid-lowering on calcific aortic valve disease progression
| Trial, year published | Sample size, n | Inclusion criteria | Intervention | Follow-up period | AVA at baseline | Increase in mean and/or peak transaortic gradient during follow-up | Decrease in AVA during follow-up |
|---|---|---|---|---|---|---|---|
| ASTRONOMER,32 2010 | 269 | Asymptomatic patients aged 18–82 years, peak aortic valve velocity 2.5–4.0 m/s | Rosuvastatin (40 mg od) vs. placebo | Median, 3.5 years (IQR 2.1–4.5) | 1.49 ± 0.71 vs. 1.56 ± 0.70 cm2 | −0.19 vs. −0.16 cm2a | |
| SALTIRE,33 2005 | 155 | Patients >18 years with AS and aortic-jet velocity ≥2.5 m/s | Atorvastatin (80 mg od) vs. placebo | Median, 25 months (range, 7–36) | 1.03 ± 0.40 vs. 1.02 ± 0.41 cm2 | Peak, 6.48 ± 7.43 vs.6.56 ± 7.10 mmHg/yeara | −0.079 ± 0.107 vs. −0.083 ± 0.107 cm2/yeara |
| SEAS,34 2008 | 1873 | Asymptomatic patients aged 45–85 years with peak aortic-jet velocity of 2.5–4.0 m/s | Simvastatin (40 mg od) plus ezetimibe (10 mg od) vs. placebo | Median, 52.2 months | 1.29 ± 0.48 vs. 1.27 ± 0.46 cm2 | Mean, 2.7 ± 0.1 vs. 2.8 ± 0.1 mmHg/yearb | −0.03 ± 0.01 vs. −0.03 ± 0.01 cm2/yearb |
| TASS,35 2008 | 47 | Asymptomatic patients aged >18 years with AS, mean systolic gradients ≥15 mmHg and peak velocity ≥2.0 m/s | Atorvastatin (20 mg od) vs. placebo | Mean, 2.3 ± 1.2 years | N/A | Mean, 29.2 ± 9.1 (baseline) to 31.3 ± 12.3 (at 24 months) vs. 25.6 ± 9.3 (baseline) to 29.9 ± 14.8 (at 24 months) mmHgb | N/A |
± values are mean ± SD.
AU, denotes arbitrary units; AVA, aortic valve area; CI, confidence interval; ns, not significant; N/A, not applicable; IQR, interquartile range.
Difference in the change from baseline in the treatment vs. placebo arm with P > 0.05.
No P-value provided but reported as ns.
Table 2.
Exemplar pharmaco-therapeutic randomized controlled trials beyond low-density lipoprotein cholesterol lowering to delay calcific aortic valve disease progression
| Trial name, year registered | Key inclusion criteria | Primary outcome measure | Secondary outcome measures | Agent/pathways targeted | Status/major findings |
|---|---|---|---|---|---|
| ALFA,39 2012 |
|
Change of VO2max in cardiopulmonary exercise test |
|
|
Unknown |
| AVADEC,40 2017 |
|
Change in AV calcification |
|
|
Ongoing |
| BASIK-2,41 2016 |
|
Change in AV calcium metabolism (18F-NaF PET/CT) |
|
|
Ongoing |
| CAVS,42 2015 |
|
Changes in aortic valve calcium levels (CT) |
|
|
Completed |
| CaLIPSO,43 2016 |
|
Change in log CAC volume score from baseline to Week 52 for the combined dose groups vs. placebo |
|
|
|
| DECAV-K2,45 2017 |
|
Hemodynamic disease progression on echocardiography (change in pressure gradients, AVA, peak aortic jet velocity) |
|
|
Recruiting |
| DIP-CAVD,46 2019 |
|
Change in AV calcium volume (96 weeks) |
|
|
Recruiting |
| EAVaLL,47 2014 |
|
Calcium score progression by cardiac CT |
|
|
Unknown |
| PCSK9 Inhibitors in the Progression of Aortic Stenosis,48 2017 |
|
Progression of calcium score (CT and NaF-PET) |
|
|
Unknown |
| SALTIRE-II,49 2014 |
|
Change in AV calcium score |
|
|
|
| SLOW,50 2020 |
|
|
N/A |
|
Recruiting |
| Vitamin K Supplement for Inhibition of the Progress in Aortic Valve Calcification,51 2008 |
|
|
|
|
|
AS, denotes aortic stenosis; AU, arbitrary units; AV, aortic valve; AVA, aortic valve area; AVR, aortic valve replacement; BAV, bicuspid aortic valve; CAC, coronary artery calcium; CT, computed tomography; EF, ejection fraction; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; Lp(a), lipoprotein (a); LDL-C, low-density lipoprotein cholesterol; LV, left ventricular; LVEF, left ventricular ejection fraction; MGP, matrix Gla protein; MSCT, multislice computed tomography; N/A, not applicable; NYHA, New York Heart Association; PCSK9, proprotein convertase subtilisin/kexin type 9; PET, positron emission tomography; RANKL, receptor activator of NF-κB ligand; VKA, vitamin K antagonist; VO2max, maximum oxygen consumption.
Multifaceted mechanisms are intricately linked to calcific aortic valve disease pathogenesis
The native AV is an avascular tissue, characterized by a trileaflet architecture, whereby each leaflet comprises three layers, the fibrosa (facing the aorta), the ventricularis (facing the LV outflow tract), and the glycosamino- and proteoglycan-rich spongiosa (residing in-between the former two). The ventricularis is abundant in radial elastin, and the fibrosa is rich in circumferentially aligned type-I collagen fibres, which provides leaflet structural integrity. In non-diseased AVs, all three layers are populated by valvular interstitial cells (VICs),53 a heterogeneous cell pool comprising at least five phenotypes,54 with the majority representing quiescent fibroblast-like cells.55 VICs residing within the AV largely originate from cells of the endocardial cushion that undergo endocardial-to-mesenchymal transition during valvulogenesis, a version of which is reactivated as CAVD evolves (reviewed by Ma et al.56). During disease initiation and progression, VICs undergo myofibroblastic and osteogenic differentiation, thereby evoking extracellular matrix (ECM) remodelling, collagen deposition, nucleation loci formation (via apoptotic bodies or extracellular vesicles) and eventually osteoblastic VIC-mediated bone formation.57 The osseous metaplasia is rare and found only in 10–13% of surgically removed AVs.58 By harnessing single-cell RNA sequencing on normal vs. CAVD tissues, Xu et al.59 identified 14 different cell subtypes populating AV tissues, with resident VICs comprising at least 3 subpopulations. The intricacy of VIC differentiation and possible crosstalks of myofibro- and osteogenesis are further highlighted by the study of Hjortnaes et al.60, showing that the osteoblastic differentiation of VICs loaded into three-dimensional (3D) hydrogel constructs is preceded by and, at least in part, depends on their myofibroblastic activation. This is in line with a recent -omics-based study, showing that the fibrotic stage represents an intermediate gene expression profile between non-diseased and calcific tissues.61
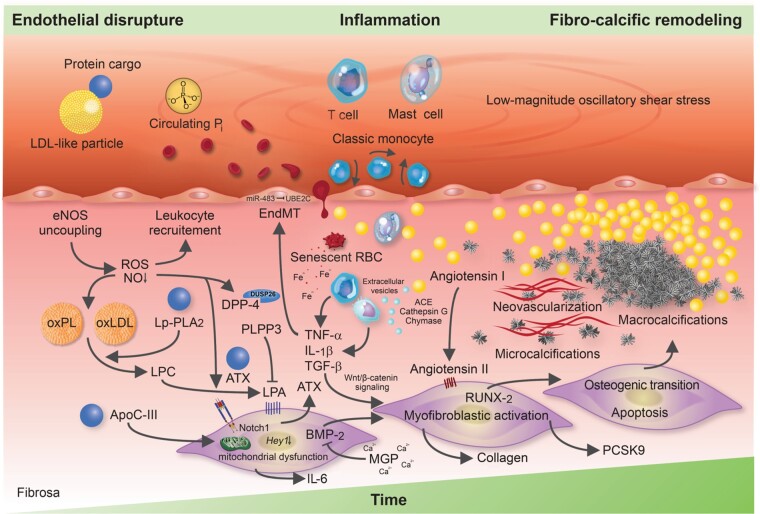
Circumferentially aligned valvular endothelial cells (VECs) sit on the surface of the AV, where they form a physical barrier, sense environmental changes, and through their paracrine actions, maintain tissue homeostasis, which involves nitric oxide (NO) signalling, among others.62 Although our understanding of their involvement in disease initiation is ambiguous, it is a well-accepted concept that injurious insults on the endothelial layer abet the uptake of lipids along with the protein cargo they carry, immune cells and red blood cells (RBCs), which coincides with the nucleation of calcium and phosphorus within the AV, collectively perpetuating a pro-inflammatory milieu during which VICs become progressively activated, culminating in fibro-calcific leaflet remodelling and eventually LV outflow obstruction (Figure 2).63–66 While early phases of CAVD are characterized by lipid and collagen depositions (typically accompanied by stippled microcalcifications that originate within the base of the fibrosa), macrocalcifications predominate more advanced disease stages, with prominent sex-specific differences in the burden of AV calcifications, reflecting in lower Agatston unit thresholds in women compared to men for a diagnosis of severe AS.8 , 9 , 17 , 67–70 Indeed, artificial intelligence based models applied on the transcriptomic data of AV tissues obtained from propensity score matched AS patients of both sexes revealed a marked enrichment of fibrotic pathways in calcified vs. non-diseased AV tissues in females, indicating that the transcriptomic signature of CAVD is strongly determined by sex, which may underpin differential disease phenotypes.71 While multifaceted aspects certainly contribute to sex-specific dissimilarities in fibro-calcific leaflet remodelling, including differences across pro-inflammatory, pro-apoptotic, pro-angiogenic and pro-fibrotic pathways,71–73 it is thought-provoking that female AV leaflets exhibit accentuated baseline expression of the ECM-embedded endogenous calcification inhibitor mineral-binding matrix Gla protein (MGP).73 Presently, mechanistic insight into and potential therapeutic implications of sex-specific aspects of human CAVD is scant, yet the hitherto available studies unequivocally suggest that differences among both pro-fibrotic and anti-calcific mechanisms account for the observed dissimilarities in CAVD pathobiology (reviewed in detail by Summerhill et al.74).
Figure 2.
Molecular and cellular mechanisms involved in calcific aortic valve disease pathogenesis. The injured endothelium covering the fibrosa fosters the uptake of immune-cells, red blood cells as well as low-density lipoprotein-like particles and their protein cargo, such as autotaxin and lipoprotein-associated phospholipase A2. Reactive oxygen species formation, enhanced by nitric oxide synthase uncoupling, aggravates the oxidative modification of lipids, promotes endothelial immune-cell trafficking and induces valvular interstitial cell apoptosis—yielding apoptotic bodies which may form additional nidi for the deposition of calcium and phosphorus crystals. While lipoprotein-associated phospholipase A2 hydrolyses the ester bond of oxidized phospholipids, autotaxin—which is secreted by valvular interstitial cells—catalyzes lysophosphatidic acid synthesis by choline group removal. Importantly, apoC-III colocalizes with calcific regions, promotes mitochondrial stress and increases interleukin-6 and bone morphogenetic protein-2 expression in human valvular interstitial cells. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinases imbalances disrupt extracellular matrix homeostasis and promote leaflet stiffening, while bone morphogenetic protein-2 drives osteogenic transition of valvular interstitial cells through increased expression of pro-osteogenic transcription factors, such as RUNX2. Infiltrated mast cells release chymase which facilitates angiotensin II synthesis, thereby promoting valvular interstitial cell-mediated collagen production and thus stiffening of aortic valve leaflets—a potent promoter of osteogenic valvular interstitial cell differentiation. Neovascularization, fuelled by vascular endothelial growth factor secretion, exacerbates immune-cell recruitment and cytokine secretion, which in turn boosts the fibro-calcific response.
Alterations in paracrine nitric oxide signalling occur early in the disease process
Endothelium-dependent vasomotion is profoundly impaired in early-stage CAVD,75 and altered NO signalling is implicated in accelerated disease progression.2 In non-diseased AVs, endothelial nitric oxide synthase (eNOS) protein abundance is almost five times lower on the aortic compared with the ventricular side,76 possibly owing to shear stress that corresponds to ≈20–30% of the magnitude encountered by the ventricular surface,77 coinciding with increased propensity of VICs to undergo myofibro-/osteogenesis.62 , 78–81 Endothelial NO deficiency is further aggravated by eNOS uncoupling (i.e. switch from its classical NO synthesis function to superoxide production due to tetrahydrobiopterin depletion),82 which contributes to enhanced valvular reactive oxygen species (ROS) formation (reviewed in detail by Greenberg et al.83), as it is typically observed in pericalcific regions in human CAVD tissues.84 , 85 Consequently, VEC-derived NO has emerged as an important mediator to maintain valvular homeostasis by modulating the behaviour of VICs in a paracrine fashion, as previously reported.62 , 76 , 81 , 86
For instance, Gould et al.81 showed that the myofibroblastic potential of porcine VICs seeded on polyethylene glycol (PEG) hydrogels with varying degrees of elasticity was diminished if co-cultured with VECs, and that these effects were reversible upon L-NAME exposure. Notably, blockage of soluble guanylyl cyclase activity, the main downstream effector of NO promoting GTP transformation to cyclic GMP (cGMP), or pharmacological ROCK stimulation abolished the protective effects conferred by VECs, implying ROCK-dependent mechanisms. Mice null for eNOS [≈25–45% of which present bicuspid aortic valve (BAV) pathology]62 , 78 elicit profound AV fibrosis, irrespective of valvular phenotype, with accelerated AV calcification being confined to BAVs, suggesting that disturbed flow represents an important trigger for valvular calcifications.79 Bosse et al.62 were the first to establish a link between NO and NOTCH1 signalling, a transcriptional regulator utterly essential for proper AV development, with NOTCH1 mutations enhancing the susceptibility for CAVD in humans.87 Indeed, endothelial-derived NO inhibits RUNX2 dependent calcification,76 at least in part by NOTCH1 activation and subsequent Hey1 down-regulation.88 Recently, Majumdar et al.86 deepened mechanistic insight by showing that VEC-derived NO inhibits VIC-driven calcification through S-nitrosylation of USP9X, stabilization of MIB1, activation of NOTCH1, and in turn diminished activation of RUNX2. Importantly, AV tissues obtained from patients undergoing SAVR displayed blunted S-nitrosylation of USP9X, diminished MIB1 levels, and increased nuclear localization of the NOTCH1 intracellular domain (NICD), while the amount of S-nitrosylated USP9X inversely correlated with CAVD severity, providing insights into new pathways during human CAVD pathogenesis.86
Beyond the downstream mechanism outlined above, Choi et al.89 recently reported that endothelial dysfunction evoked by NO depletion enhances dipeptidyl peptidase-4 [DPP-4; a multifunctional protein whose stability is regulated by dual-specificity phosphatase 26 (DUSP26)]90 expression in VICs, in turn limiting autocrine insulin-like growth factor-1 (IGF-1) signalling, and thus accelerating CAVD progression. These findings were recapitulated in vivo using eNOS-deficient mice and a rabbit model, in which a CAVD-like phenotype was established by high cholesterol diet coupled with daily vitamin D2 supplementation. Taken together, these studies indicate that depletion of VEC-derived NO, as it occurs already early in the disease process, fuels several pro-fibrotic and pro-calcific processes involving, at least in part, ROCK-, NOTCH1-, and IGF-1-dependent mechanisms. Considering that NO homeostasis is perturbed already early in the disease process, coupled with the evolving availability of pharmaceutical approaches to specifically interfere with downstream acteurs of the NO signalling pathway (e.g. HMR1766; Table 2), an increased understanding of its role in CAVD pathogenesis is crucial for the appropriate design of future RCTs (see section ‘Pharmacotherapies: moving from past to contemporary clinical trials’).
Mechanical stress disrupts endothelial structure and function
Seminal histological studies on early-diseased AV tissues revealed that leaflet thickening and the formation of microcalcifications preferentially affect the aortic side, with the endothelium covering the lesion being disrupted, and the underlying elastic lamina displaced.91 Subendothelial lipid deposits superimposed by immune cells tend to align parallel to the valvular endothelium,91 , 92 and subjacent microcalcifications predominantly evolve in regions where disturbed flow occurs,93–95 collectively suggesting endothelial injury as a prime driver of CAVD. For instance, individuals with a BAV, a congenital condition with incomplete cusp separation during embryogenesis,96 are at accentuated risk to develop CAVD prematurely, and despite a low prevalence of 0.5–1.5%, account for up to 50% of patients undergoing SAVR.97 Although mechanisms beyond hemodynamics likely contribute to the high prevalence and exacerbated disease progression, it is interesting to note that shear stress abnormalities are most pronounced near the base of the fused cusps,94 where calcifications most frequently occur.98
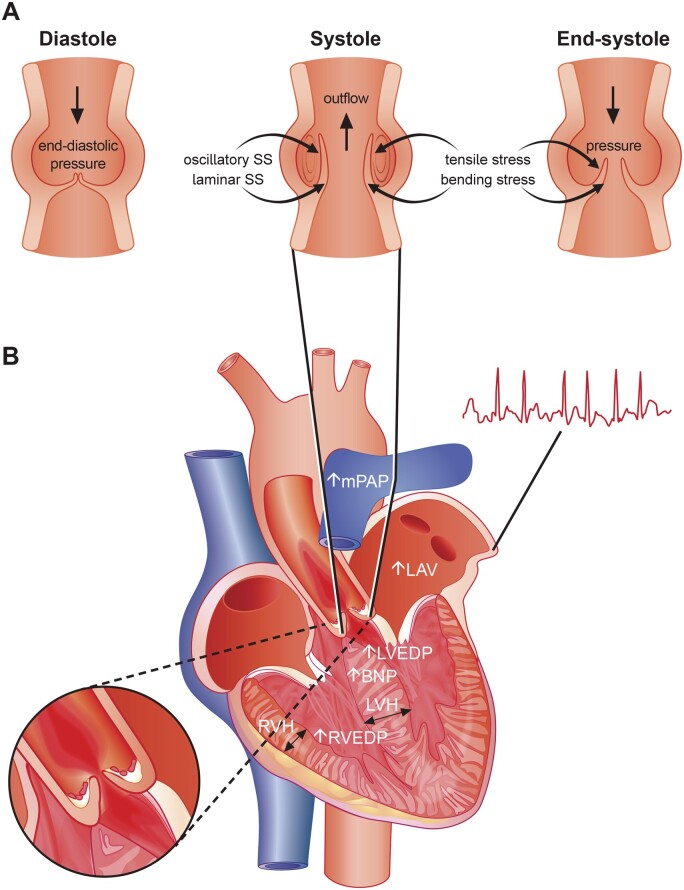
The laminar shear stress encountered by the surface of the ventricular side reaches up to 64–91 dyne/cm2,77 whilst shear stress on the aortic side shows both anterograde and retrograde components (i.e. oscillatory) and peaks already at 19 dyne/cm2 (Figure 3),93 known to induce endothelial dysfunction, hamper barrier function, and to shape the expression of key mediators governing the transition of VICs into a myofibro- and osteoblastic phenotype, respectively.99–101 Indeed, by employing a physiologically relevant bioreactor system, Mahler et al.102 provided compelling evidence that decreasing shear stress magnitudes upregulates ICAM-1 and nuclear factor κB (NFκB) expression in porcine VECs, with low-magnitude oscillatory shear stress promoting their invasion and transdifferentiation into myofibroblastic VICs, a process termed endothelial-to-mesenchymal transition (EndMT). The genetic lineage tracing study of Gee et al.103 proposed that induction of EndMT mainly relies on NFκB activation and might be activated in the postnatal stage solely during diseased conditions, despite former studies ascribing EndMT a physiological function.104 , 105 The findings of Fernandez Esmerats et al.99 further implicated miR-483 and in turn ubiquitin E2 ligase C (UBE2C) in this process, and highlighted the importance of endothelial inflammation, as disturbed flow regulated EndMT via enhanced UBE2C-mediated activation of the pro-inflammatory hypoxia-inducible factor 1α (HIF1α) pathway. Other inflammatory mediators, such as tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), have also been shown to accelerate EndMT, likely acting through the Akt/NFκB pathway.104 Increased rates of EndMT, triggered by the mechanisms outlined above, can perturb endothelial structure and thus hamper its barrier function, thereby allowing blood-derived cargo to invade the valvular interstitium.106
Figure 3.
Hemodynamic flow across the aortic valve and myocardial alterations occurring with advanced calcific aortic valve disease. (A) The hemodynamic forces aortic leaflets are exposed to are shown. Note that disturbed hemodynamic flow can perturb tissue homeostasis by acting on pro-inflammatory and pro-fibrotic signalling, thereby promoting calcific aortic valve disease progression and eventually the development of aortic stenosis. (B) As calcific aortic valve disease progresses and impediments in left ventricular outflow occur, left ventricular hypertrophy and myocardial fibrosis evolves leading to reduced left ventricular longitudinal function, although left ventricular ejection fraction typically remains unchanged in the majority of patients. If left untreated, the left atrium enlarges, enhancing the susceptibility to atrial fibrillation. Due to left ventricular hypertrophy and the reduced diastolic pressure gradient, coronary flow reserve can substantially decrease leading to cardiomyocyte loss further perpetuating processes underlying myocardial fibrosis. At late disease stages, secondary pulmonary hypertension and right-ventricular dysfunction evolves.
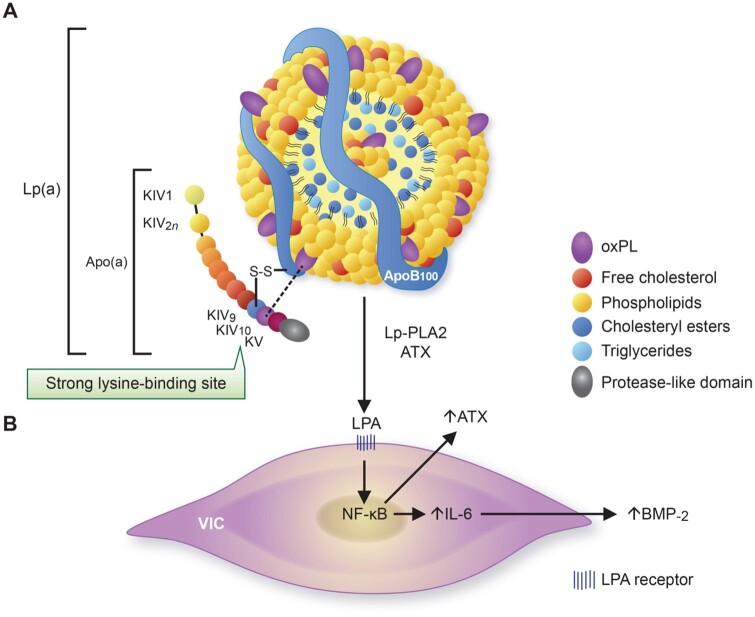
In contrast to the ventricular side, the endothelium covering the disease-susceptible fibrosa shows areas of denudation,63 , 107 which fosters the intraleaflet accumulation of RBCs during early phases of CAVD, while neovessel formation may act as an additional source of intraleaflet RBCs at more advanced disease stages.63 Impairments in endothelial barrier function coupled with the enhanced expression of endothelial scavenger receptors (SR) also promotes the uptake of lipoproteins. For instance, the SR-A1 and the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) are abundantly expressed in CAVD tissues, with LOX-1 showing high affinity to oxidatively modified LDL-like particles.108 , 109 Both LDL-C and lipoprotein(a) [Lp(a)] emerged as important risk factors for CAVD, with the latter also spurring disease progression.1 , 110–113 Structurally, Lp(a) consists of an LDL-like moiety covalently bound to apolipoprotein(a), with strong fibrin and lysine binding sites, likely facilitating its valvular interaction upon endothelial injury (Figure 4).114–117 These apolipoprotein B100-containing lipoproteins bind oxidized phospholipids (oxPLs), autotaxin (ectonucleotide pyrophosphatase/phosphodiesterase 2), angiotensin-converting enzyme (ACE), apoC-III, and lipoprotein-associated phospholipase A2 (Lp-PLA2), all of which are mechanistically implicated in CAVD pathogenesis.66 , 110 , 118–122 The importance of mechanical stress in CAVD pathogenesis is further underscored by the independent association of elevated systolic blood pressure (SBP) and accelerated AV calcification,123 and by a recent population-based Mendelian randomization (MR) study showing an up to three-fold increased risk for incident AS per 20-mmHg increase in SBP.124 While the mechanisms linking heightened SBP and CAVD warrant further study, it is tempting to speculate that amplified tensile stress experienced by the fibrosa coupled with changes in quality and magnitude of shear stress may contribute to this phenomenon.
Figure 4.
Structure of lipoprotein(a) and its pro-osteogenic effects on valvular interstitial cells. (A) Lipoprotein(a) is characterized by a low-density lipoprotein-like particle (note the single apoB100 molecule) that is covalently linked to the unique apolipoprotein(a) glycoprotein which is encoded by the LPA gene. While its lipid core consists mainly of cholesteryl esters and (some) triglycerides, its outer shell is mainly composed of phospholipids and free cholesterol. Although the majority of oxidized phospholipid is bound to apolipoprotein(a), lipids can also be covalently linked to apoB100 or even found freely in the lipid-shell. Twelve domains form apolipoprotein(a), with 10 (i.e. KIV1–KIV10) being homologous to plasminogen kringle-IV and one representing a kringle-V-like domain (i.e. KV) which is followed by an inactive protease-like domain. Different functions have been ascribed to each, with KIV10 being characterized by a strong lysine-binding site crucial for oxidized phospholipid binding. (B) Lipoprotein-associated phospholipase A2 and autotaxin can transform oxidized phospholipid to lysophosphatidylcholine and lysophosphatidic acid, respectively, thereby promoting endogenous interleukin-6 and autotaxin production through NF-κB activation. Interleukin-6 can induce increased bone morphogenetic protein-2 expression in a paracrine manner resulting in osteogenic transition of adjacent valvular interstitial cells and eventually aortic valve calcification.
Inflammation drives fibro-calcific leaflet remodelling
Impaired endothelial structure and function evoked by the mechanisms outlined above promotes the uptake of numerous blood-derived components (including lipoproteins, the protein cargo they carry and RBCs), which, coupled with alterations in paracrine signalling, perpetuates an inflammation-calcification feedback loop that culminates in LV outflow obstruction by fibro-calcific leaflet remodelling. Chronic inflammation causes valvular calcifications in hyperlipidaemic mice,125 , 126 a phenomenon also well established in humans by fluorodeoxyglucose (FDG) imaging and histology.65 , 127 Indeed, years before symptoms manifest, the human AV already harbours a variety of immune cells in the subendothelium of the fibrosa, including macrophages, mast cells, and CD8+ T cells, with microcalcifications being largely confined to the lesion’s base, suggesting their transendothelial recruitement.91 , 128 , 129
Infiltrated macrophages, predominantly from the M1 subtype,130 secrete pro-inflammatory cytokines, including TNF-α and IL-6, which contribute to ECM remodelling, ignite EndMT, and drive the evolution of micro- and eventually macrocalcifications.131 Indeed, IL-6 expression correlates with CAVD severity in humans, and exposures of human VICs to recombinant IL-6 induces their osteoblastic activation, while its knockdown alleviates VIC-driven calcification.36 , 132 Recently, Schlotter et al.66 explored the CAVD-specific apolipoproteome and found that apoC-III, an apolipoprotein known to interact with Lp(a),120 is abundantly expressed in the disease-prone fibrosa and accelerates VIC-driven calcification in vitro, likely also via increased IL-6 production. Of note, a recent genome-wide association study using data of four European cohorts identified IL6 as a novel risk loci for incident AS.133 Activity of Lp-PLA2 is enhanced during such pro-inflammatory states,134 and its expression is increased in CAVD tissues,122 implying accentuated enzymatic activity. Mechanistically, Lp-PLA2 transforms oxPL, a highly atherogenic molecule bound to Lp(a), into lysophosphatidylcholine, upon which it undergoes autotaxin-mediated conversion to lysophosphatidic acid. Lysophosphatidic acid is a strong promotor of osteoblastic transition of VICs and, thus, AV calcification, with its degrading enzymes (i.e. phospholipid phosphatases) being increasingly acknowledged as pivotal drivers of its activity.110 , 118 , 135 , 136 Furthermore, activated macrophages release extracellular vesicles (providing a scaffold for nucleation of calcium-phosphate crystals)137–139 and express matrix metalloproteinase (MMP)-1, -2, -3, -9, and -10,140–142 able to modulate ECM elasticity, which in turn determines VIC activation in response to biochemical cues.143 Activated VICs (along with invaded mast cells) are an important source of pro-angiogenic factors such as VEGF-A,144 , 145 thereby stimulating neovessel formation and thus further accelerating the uptake of blood-derived components, such as lipoproteins and immune cells. In support of this notion, deprivation of the antiangiogenic chondromodulin-I elicits high Vegf-A expression, lipid deposition, immune-cell invasion, and AV calcification in aged mice.146
The infiltration of both macrophages and mast cells, along with LDL- and VIC-derived ACE,147 , 148 also accelerates local angiotensin II synthesis. In fact, ACE−, chymase, and cathepsin G are highly expressed in calcific vs. normal AV tissues, thereby promoting enhanced angiotensin II production.121 , 149 Notably, exposure of rat VICs to angiotensin II increases type I collagen synthesis, likely via binding to the angiotensin II type 1 receptor (AT-1R).147 Accordingly, high-dose angiotensin II administration to Apoe − / − mice induces myofibroblastic activation of VICs and subsequent AV thickening, effects that can be suppressed by concomitant olmesartan but not by hydralazine administration, suggesting that angiotensin II exerts pro-fibrotic effects via AT-1R independent of blood-pressure lowering,150 findings corroborated in hypercholesterolaemic rabbits.151 Côté et al. found that preoperative plasma levels of angiotensin II correlate strongly with the tissue expression of TNF-α and IL-6 in excised CAVD tissues,152 and showed in a follow-up study that the use of angiotensin receptor blockers (ARBs) associates with lower IL-6 expression and fibrotic remodelling.36 Although the valvular renin–angiotensin system (RAS) and its implications in CAVD pathogenesis warrants further study, it is interesting to note that a very recent report highlighted a link between the activity of the angiotensin II-degrading enzyme ACE2 and the degree of AV calcification but failed to establish an association with hemodynamic disease severity,153 opening an exciting avenue for future research on the role of the valvular RAS in CAVD pathogenesis beyond its effects on blood pressure regulation. Given the so-far contradictory results obtained across different clinical studies (see section ‘Pharmacotherapies: moving from past to contemporary clinical trials’),154–158 we must deepen our mechanistic understanding of the valvular RAS in human CAVD. This may provide the basis for the right timing and proper selection of both the study population as well as type of intervention in future RCTs to eventually convincingly assess the impact of RAS modulation on CAVD progression.
Dysregulated calcium-phosphate metabolism promotes aortic valve mineralization
Besides pathogenic processes directed by the mechanisms outlined above, dysregulation in systemic phospho-calcium metabolism/homeostasis, as it occurs during chronic kidney disease (CKD) or osteoporosis,159 is also implicated in CAVD. This pathway acts through distinct mechanisms (reviewed in detail by Bäck and Michel160) but exhibits multiple points of crosstalk that may operate simultaneously within the same AV.53 CAVD is a common comorbidity of CKD, hallmarked by premature manifestation and accelerated disease progression, with alterations in systemic calcium-phosphate metabolism being implicated in its pathogenesis. Similarly, osteoporosis and enhanced bone resorption activity have been consistently linked to CAVD,37 , 161 suggesting that deranged calcium-phosphate homeostasis is involved in its pathogenesis.
Phosphates represent essential structural (nucleic acids, phospholipids) and functional (purinergic system and pyrophosphate metabolism) building blocks for proper cell function, involving its inorganic (Pi) and biologically active (organic) form. As metabolic alterations occur, such as CKD or osteoporosis,161–163 phosphates are shifted from their organic to inorganic form, loose their intracellular predominance, and can initiate the mineralization process within the valvular ECM.160 A variety of sources contribute to their extracellular abundance, including plasma Pi, phospholipids (derived from lipoproteins, cell- or exosome-derived membranes), and nucleotides,160 with the former two playing a predominant role in CKD-associated CAVD.159 , 164 Calcium typically precipitates on exposed phosphates when its product (Ca × Pi) approximates its saturation point. Therefore, hyperphosphataemia, for instance due to CKD, may enhance the propensity for hydroxyapatite deposition within valvular and vascular tissues, yet endogenous inhibitors that act on systemic (fetuin-A, Klotho) or local levels (MGP, osteopontin) may oppose calcium-phosphate precipitation.
For instance, liver-derived fetuin-A limits calcium-phosphate precipitation by forming colloidal calciprotein particles, and interferes with Wnt/β-catenin signalling.165 Since fetuin-A deprivation elicits more profound calcifications in hyperphosphataemic mice,166–168 it might well be that fetuin-A exerts protective effects only when calcium-phosphate metabolism is disturbed,169 which may explain the conflicting results obtained across different observational studies.170 , 171 In contrast, Klotho—a fibroblast growth factor-23 (FGF-23) co-receptor—regulates Pi by diminishing renal phosphate reabsorption, and its loss evokes high valvular RUNX2 expression in vivo, indicating the osteoblastic activation of VICs.172 At the valvular level, VICs synthesize MGP that, following vitamin K-dependent post-translational γ-carboxylation of its glutamic acids, is incorporated in the valvular ECM.173 Carboxylated MGP limits calcium-phosphate precipitation mainly by calcium binding174 but may also suppress bone morphogenetic protein (BMP)-2 and BMP-4 expression.175 , 176 Loss of murine MGP leads to severe arterial calcifications,177 and its expression is upregulated in human calcified AVs—concomitantly with osteocalcin and Gla-rich protein, suggesting a pivotal role in CAVD.178
Beyond these mechanisms, CKD patients accumulate endogenous toxins, such as indoxyl sulphate, and have heightened Lp(a) as well as oxLDL levels,179 highlighting important crosstalks to phosphate-independent mechanisms. In addition, other aspects that contribute to the almost tripled prevalence of CAVD among CKD patients may include frequently observed (systolic) hypertension, chronic volume overload, and accentuated mechanical stress across the AV due to the presence of an arteriovenous fistula/graft and regular hemodialysis.164 , 180
Pharmacotherapies: moving from past to contemporary clinical trials
Low-density lipoprotein cholesterol
Despite the wealth of data supporting a causal role for LDL-C in CAVD,181–183 aggressive LDL-C lowering has consistently failed to blunt disease progression in well-designed RCTs (Table 1).32–35 Indeed, in the SALTIRE study enrolling 155 patients with AS (defined as AV calcification on echocardiography and aortic-jet velocity ≥2.5 m/s) atorvastatin reduced LDL-C by 53% over a median follow-up of 25 months, but failed to impact disease progression.33 Similarly, in the large-scale SEAS study, in which 1873 patients with mild-to-moderate AS were randomized to receive simvastatin (40 mg od) plus ezetimibe (10 mg od) or placebo, no effect on the primary outcome was observed during a median follow-up of 52.2 months, despite a mean reduction in LDL-C of 53.8%.34 Also, in the smaller ASTRONOMER trial, rosuvastatin-mediated LDL-C lowering of 54.5% in relatively young patients with similar AS severity had no effect on disease progression over a median follow-up of 3.5 years.32 Likely, the insufficient macrophage-driven lipid removal mechanism in CAVD, a well-documented pathophysiologic process in atherogenesis, contributes to these findings.66 , 184 Also, off-target effects of statins, ranging from perturbed glucose homeostasis185 , 186 and increased Lp(a) levels187 to pro-osteogenic properties,188 , 189 could counterbalance their LDL-C lowering effects. Finally, and in stark contrast to Lp(a), LDL-C does not associate with hemodynamic disease progression in observational studies,1 , 110 , 190 , 191 questioning the effectiveness of pharmaceutical strategies directed at this target when AS has already evolved.
Lipoprotein(a)
Landmark MR studies imply a causal role for Lp(a) in CAVD,111 , 192 , 193 with preclinical studies providing mechanistic insights into its role as a carrier of culprits involved in VIC-driven calcification, including autotaxin and oxPLs.119 , 135 , 194 A post hoc analysis of the FOURIER trial195 suggests that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors may exert protective effects, likely because they not only lower LDL-C but also reduce Lp(a) by up to 25–30% and interfere with pathways directly involved in valvular remodelling.196–201 Yet, based on estimates to lower the risk and improve outcomes of coronary artery disease, a greater Lp(a) reduction might be required to achieve benefit.202 , 203 In this context, novel antisense oligonucleotides targeting hepatic LPA mRNA might represent promising tools for aggressive Lp(a) lowering,204 as interventions currently applied in ongoing RCTs (Table 2), such as niacin, only modestly lower Lp(a) by up to 30%. On the other hand, overreliance on Lp(a)-directed pharmacotherapies should be avoided, as current evidence highlights that high Lp(a) (exceeding 175 nmol/L) only accounts for up to 7% of AS cases,205 thus likely benefitting only a minority of patients afflicted by CAVD.
Mineral-binding matrix gla protein
Considering the largely non-vascularized architecture of the native AV, boosting the activity of ECM-embedded calcification inhibitors, such as MGP (which is intriguingly highly expressed in fibrotic areas of CAVD tissues61), could represent a promising avenue for future drug development. In support of this theory, loss of MGP evokes profound valvular calcification in mice,177 whereas liver-derived fetuin(a) deficiency necessitates a hyperphosphataemic mileu.168 In line with previous reports,206–208 a retrospective substudy of the population-based DANCAVAS trial209 with 15 048 participants revealed that vitamin-K antagonist (VKA) use confers heightened propensity for AV calcification, with an increase in computed tomography (CT)-detected AV calcium by 6%/year on VKA. However, the retrospective character and unavailability of surrogates of MGP carboxylation status (e.g. plasma-derived as used in previous studies)210 are strong limitations and thus warrant further study. A small prospective proof-of-concept study found that daily vitamin K supplementation slightly blunted the progression of AV calcification during 12 months of follow-up (10.0% in patients undergoing treatment vs. 22.0% in the placebo arm)52 but unfortunately lacked power to assess effects on more clinically relevant parameters of disease progression. The DECAV-K2 trial45, designed to assess the effects of vitamin K supplementation on hemodynamic disease progression with a sample size 1.5 times larger than the afore-mentioned trial, started recruiting patients in 2017, with results likely available by 2022/2023.
Renin–angiotensin system
Angiotensin II is abundantly expressed in CAVD tissues and induces the myofibroblastic activation and collagen deposition of VICs in vitro,147 while contributing to valvular ECM remodelling and eventually leaflet thickening in vivo.150 Independent of its effects on blood pressure, AT-1R blockage attenuated myofibroblastic VIC activation and AV thickening in two independent preclinical models,150 , 151 suggesting that AT-1R inhibition exerts anti-fibrotic effects. In their retrospective study, Côté et al. reported that preoperative ARB use associated with diminished AV remodelling,154 while in another study, ARBs slowed hemodynamic disease progression, whereas ACE inhibitors have failed.155 This is in line with the initial report of Rosenhek et al.156 showing neutral effects of ACE inhibition on hemodynamic disease progression. Yet, O’Brian et al.157 reported that the use of ACE inhibitors is associated with diminished increase in CT-detected AV calcium load,157 while in the prospective RIAS trial158 only a trend toward slower hemodynamic disease progression could be established. In aggregate, these studies suggest that ARBs may diminish valvular remodelling, while ACE inhibition has thus far yielded conflicting results across different reports. Likely, the abundance of mast cell- or macrophage-derived chymase/cathepsin G necessitates targeted downstream inhibition to provide benefit. Prospectively designed studies, such as the currently running ALFA trial39, are urgently warranted to convincingly assess the efficacy of RAS-modulating agents to impact CAVD progression.
Targets involved in nitric oxide and IGF-1 signalling
Minute amounts of endothelial-derived NO activate soluble guanylate cyclase (sGC) via its prosthetic haem moiety (whose reduced form binds NO), thereby inducing the conversion of GTP to cGMP, a key player of VIC quiescence and maintenance of valvular homeostasis.81 Non-sGC sources of AV cGMP, e.g. via the particulate guanylate cyclase Npr2, also contribute to valvular homeostasis, embryonic development, and inhibition of fibrosis/calcification in mice—pointing towards a broader importance of valvular cGMP levels in maintaining AV health.211 Early-stage CAVD is associated with systemic endothelial dysfunction,75 and derangements in paracrine NO signalling drives pro-fibrotic and pro-calcific processes that underpin CAVD pathogenesis.62 , 81 , 86 HMR-1766, an sGC activator that acts independently of NO and preferably interacts with oxidized sGC,212 was shown to exert anti-fibrotic effects in a rat model of myocardial infarction,213 while a preliminary report also implies a role in valvular BMP-2 signalling.214 The CAVS42 trial, in which patients with AS were randomized to receiving 200 mg HMR-1766 daily or matching placebo, is undergoing analysis currently. VEC-derived NO depletion, as it occurs during CAVD progression, enhances DPP-4 expression, and induces the osteoblastic activation of VICs via accelerated IGF-1 degradation.89 The DIP-CAVD trial46 will test whether selective DPP-4 inhibition by orally administered evogliptin (DA-1229) once daily can alter the progression of AV calcification over 96 weeks, with first study results likely being available by 2024. These RCTs open an exciting avenue for future research to study drugs to reinstate paracrine VEC/VIC homeostasis, a process likely deranged early in the disease process.
Phosphate/calcium-metabolism-associated targets
Epidemiological and preclinical evidence linking CAVD with dysregulated phosphocalcium metabolism has stimulated RCTs to assess the effectiveness of pharmaceutical strategies directed at targets interfering with hydroxyapatite crystal formation (SNF472), the RANKL/RANK/osteoprotegerin axis (denosumab), or osteoclastic activity (bisphosphonates). As noted earlier, the almost tripled prevalence of CAVD in CKD patients is secondary to a combination of factors, with a predominant role of deranged mineral metabolism.160 The landmark CaLIPSO trial44 showed that 52-week treatment with SNF472, a myo-inositol hexaphosphate that selectively inhibits hydroxyapatite formation,215 significantly attenuated AV calcium volume score progression in CKD patients on long-term haemodialysis and adjunct therapies (57% of which had AV calcifications at baseline), equalling a progression of 98% with placebo vs. 14% with active treatment. Although further studies are needed to study the effects on hard cardiovascular endpoints, including hemodynamic disease progression, the clinical implications could be huge, particularly in patients with high propensity for CAVD, yet at high risk for adverse outcomes following interventional valve replacement therapies.164
The RANKL/RANK/osteoprotegerin axis regulates bone turnover and is mechanistically implicated in osteoporosis pathogenesis, a condition linked to high CAVD prevalence. RANKL is upregulated in calcific lesions of the AV,216 and promotes matrix calcification and osteoblastic activation of VICs,217 while its inhibition by osteoprotegerin attenuates CAVD in Ldlr-/- ApoB100/100 mice.218 Observationally, bisphosphonate use is associated with reduced hemodynamic AS progression and lower prevalence of AV calcifications,37 , 219 while bone density correlates inversely with incident AS.161 In the recently published SALTIRE II trial,38 150 AS patients with a mean peak aortic jet velocity of 3.36 m/s were randomized to receiving denosumab, placebo injection, alendronic acid, or placebo capsule and were subjected to serial echocardiography, CT AV calcium scoring, and 18F-NaF positron emission tomography (PET)/CT during 24-month follow-up. A decline in serum C-terminal telopeptide by >50% confirmed efficacy of both active drugs, yet neither a change in AV calcium load/activity nor peak aortic jet velocity could be established, highlighting the need for the identification of novel therapeutic targets beyond the RANKL/RANK/osteoprotegerin axis.
Conclusions
Several mediators, including Lp(a) (mainly via bound oxPLs), NO, RAS, DPP-4/IGF-1, MGP, autotaxin (via enhanced lysophosphatidic acid), and IL-6, have emerged as pivotal drivers of CAVD, some of which are already being therapeutically targeted in ongoing trials (Table 2). Despite the progress made, our understanding of the mechanisms operative in valvular tissues in response to hemodynamic and biochemical cues is still incomplete, with well-designed randomized controlled trials targeting LDL-C or key players of bone metabolism showing disappointing results.32–35 However, phase II studies harnessing interventions of MGP carboxylation (by oral vitamin K supplementation) or hydroxyapatite crystal formation inhibition (by intravenous myo-inositol hexaphosphate administration) have yielded promising results in specific patient populations. Whether these interventions impact hemodynamic disease progression and, in turn, the necessity for interventional therapy, in both male and female patients, needs to be shown in larger RCTs, some of which are currently ongoing (e.g. AVADEC,40 BASIK2,41 and DECAV-K245). Lastly, there is a pressing need to design tailored RCTs investigating the effects of aggressive Lp(a) lowering on CAVD progression, as such an approach might represent a promising remedy for patients with elevated Lp(a).220
In parallel, preclinical efforts aimed at characterizing the pathobiology of different CAVD stages should be continued incessantly. For the discovery of novel mediators and final common pathways of CAVD initiation and progression, the application of spatiotemporally resolved omics studies coupled with the rigorous validation of promising therapeutic targets in ex vivo/in vivo models merit consideration.221–224 Finally, the identification of patients best suited for medical therapy intertwined with the development of more sensitive screening modalities for the detection of early fibro-calcific changes, which markedly differ between sexes, will be key if interventional trials are to be efficiently conducted and novel drugs shown to be effective are to be broadly applied. As indicated, the disease-causing mechanisms may change as CAVD evolves, with women typically showing a more fibrotic phenotype compared to men.17 , 67 , 68 Therefore, novel composite endpoints—depending on the study population recruited and disease stage targeted—need to be established, as emerging surrogates of inflammation and fibro-calcific remodelling (e.g. assessed by PET/CT with 18F-NaF/18F-FDG) can provide incremental information on top of echocardiography, and likely represent more accurate measures of disease activity.
Funding
Research of SK and TFL was supported by the Swiss Heart Foundation (FF20094) and the Foundation of Cardiovascular Research—Zurich Heart House (Donation of H.H. Sheikh Khalifa bin Hamad Al-Thani). E.A.’s laboratory is supported by National Institutes of Health grants R01HL136431, R01HL141917, and R01HL147095.
Conflict of interest: There are no conflicts of interests related to this article. Outside this field, T.F.L. has received educational and research grants by Abbot, Amgen, Boehringer Ingelheim, Daichi-Sankyo, Novartis, Sanofi, Servier, and Vifor and honoraria from Amgen, Daichi-Sankyo, Novartis, and Pfizer. G.G.C. is a coinventor on the International Patent WO/2020/226993 filed in April 2020. The patent relates to the use of antibodies which specifically bind IL (interleukin)-1α to reduce various sequelae of ischaemia–reperfusion injury to the central nervous system. G.G.C. is a consultant to Sovida solutions limited. The other authors report no conflicts.
Contributor Information
Simon Kraler, Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; University Heart Center, Department of Cardiology, University Hospital, Rämistrasse 100, 8091 Zurich, Switzerland.
Mark C Blaser, Center for Interdisciplinary Cardiovascular Sciences, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 3 Blackfan Street, Boston, MA 02115, USA.
Elena Aikawa, Center for Interdisciplinary Cardiovascular Sciences, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 3 Blackfan Street, Boston, MA 02115, USA; Center for Excellence in Vascular Biology, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 77 Ave Louis Pasteur, NRB7, Boston, MA 02115, USA.
Giovanni G Camici, Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; University Heart Center, Department of Cardiology, University Hospital, Rämistrasse 100, 8091 Zurich, Switzerland; Department of Research and Education, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Thomas F Lüscher, Center for Molecular Cardiology, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; Heart Division, Royal Brompton & Harefield Hospitals, Sydney Street, London SW3 6NP, UK; National Heart and Lung Institute, Imperial College, Guy Scadding Building, Dovehouse Street, London SW3 6LY, UK.
References
- 1. Novaro GM, Katz R, Aviles RJ et al. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease. The Cardiovascular Health Study. J Am Coll Cardiol 2007;50:1992–1998. [DOI] [PubMed] [Google Scholar]
- 2. Sverdlov AL, Ngo DTM, Chan WPA et al. Determinants of aortic sclerosis progression: implications regarding impairment of nitric oxide signalling and potential therapeutics. Eur Heart J 2012;33:2419–2425. [DOI] [PubMed] [Google Scholar]
- 3. Faggiano P, Antonini-Canterin F, Erlicher A et al. Progression of aortic valve sclerosis to aortic stenosis. Am J Cardiol 2003;91:99–101. [DOI] [PubMed] [Google Scholar]
- 4. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142–147. [DOI] [PubMed] [Google Scholar]
- 5. Coffey S, Cox B, Williams MJA. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol 2014;63:2852–2861. [DOI] [PubMed] [Google Scholar]
- 6. Williams MC, Massera D, Moss AJ et al. Prevalence and clinical implications of valvular calcification on coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2021;22:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bevan GH, Zidar DA, Josephson RA, Al-Kindi SG. Mortality due to aortic stenosis in the United States, 2008-2017. JAMA 2019;321:2236–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vahanian A, Beyersdorf F, Praz F et al. ; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. Published online ahead of print 28 August 2021. [DOI] [PubMed] [Google Scholar]
- 9. Otto CM, Nishimura RA, Bonow RO et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/. American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 10. Osnabrugge RLJ, Mylotte D, Head SJ et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002–1012. [DOI] [PubMed] [Google Scholar]
- 11. Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart 2016;102:75–85. [DOI] [PubMed] [Google Scholar]
- 12. Yadgir S, Johnson CO, Aboyans V et al. ; Global Burden of Disease Study 2017 Nonrheumatic Valve Disease Collaborators. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation 2020;141:1670–1680. [DOI] [PubMed] [Google Scholar]
- 13. Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø Study. Heart 2013;99:396–400. [DOI] [PubMed] [Google Scholar]
- 14. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 15. Owens DS, Bartz TM, Buzkova P et al. Cumulative burden of clinically significant aortic stenosis in community-dwelling older adults. Heart 2021;107:1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lutz M, Messika-Zeitoun D, Rudolph TK et al. Differences in the presentation and management of patients with severe aortic stenosis in different European centres. Open Heart 2020;7:e001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simard L, Côté N, Dagenais F et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis. Circ Res 2017;120:681–691. [DOI] [PubMed] [Google Scholar]
- 18. Villari B, Vassalli G, Schneider J, Chiariello M, Hess OM. Age dependency of left ventricular diastolic function in pressure overload hypertrophy. J Am Coll Cardiol 1997;29:181–186. [DOI] [PubMed] [Google Scholar]
- 19. Lindman BR, Arnold SV, Madrazo JA et al. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail 2011;4:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pagé A, Dumesnil JG, Clavel MA et al. ; ASTRONOMER Investigators. Metabolic syndrome is associated with more pronounced impairment of left ventricle geometry and function in patients with calcific aortic stenosis. A substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin). J Am Coll Cardiol 2010;55:1867–1874. [DOI] [PubMed] [Google Scholar]
- 21. Buttrick P, Scheuer J. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation 1992;86:1336–1338. [DOI] [PubMed] [Google Scholar]
- 22. Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation 2003;107:3170–3175. [DOI] [PubMed] [Google Scholar]
- 23. Rajappan K, Rimoldi OE, Dutka DP et al. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002;105:470–476. [DOI] [PubMed] [Google Scholar]
- 24. Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation 1997;95:892–898. [DOI] [PubMed] [Google Scholar]
- 25. Hein S, Arnon E, Kostin S et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human: heart structural deterioration and compensatory mechanisms. Circulation 2003;107:984–991. [DOI] [PubMed] [Google Scholar]
- 26. Treibel TA, López B, González A et al. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rassi AN, Pibarot P, Elmariah S. Left ventricular remodelling in aortic stenosis. Can J Cardiol 2014;30:1004–1011. [DOI] [PubMed] [Google Scholar]
- 28. Azevedo CF, Nigri M, Higuchi ML et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278–287. [DOI] [PubMed] [Google Scholar]
- 29. Dweck MR, Joshi S, Murigu T et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271–1279. [DOI] [PubMed] [Google Scholar]
- 30. McGuire C, Yip AM, MacLeod JB et al. Regional differences in aortic valve replacements: atlantic Canadian experience. Can J Surg 2018;61:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rapp AH, Hillis LD, Lange RA, Cigarroa JE. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am J Cardiol 2001;87:1216–1217. [DOI] [PubMed] [Google Scholar]
- 32. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J; ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (Astronomer) trial. Circulation 2010;121:306–314. [DOI] [PubMed] [Google Scholar]
- 33. Cowell SJ, Newby DE, Prescott RJ et al. ; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352:2389–2397. [DOI] [PubMed] [Google Scholar]
- 34. Rossebø AB, Pedersen TR, Boman K et al. ; SEAS Investigators. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N Engl J Med 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 35. Dichtl W, Alber HF, Feuchtner GM et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am J Cardiol 2008;102:743–748. [DOI] [PubMed] [Google Scholar]
- 36. Côté N, Mahmut A, Fournier D et al. Angiotensin receptor blockers are associated with reduced fibrosis and interleukin-6 expression in calcific aortic valve disease. Pathobiology 2014;81:15–24. [DOI] [PubMed] [Google Scholar]
- 37. Skolnick AH, Osranek M, Formica P, Kronzon I. Osteoporosis treatment and progression of aortic stenosis. Am J Cardiol 2009;104:122–124. [DOI] [PubMed] [Google Scholar]
- 38. Pawade TA, Doris MK, Bing R et al. Effect of denosumab or alendronic acid on the progression of aortic stenosis: a double-blind randomized controlled trial. Circulation 2021;143:2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. A Randomized Trial of Angiotensin Receptor Blocker Fimasartan in Aortic Stenosis (ALFA Trial). https://clinicaltrials.gov/ct2/show/NCT01589380 (28 May 2021).
- 40. The Aortic Valve DECalcification (AVADEC) Trial (AVADEC). https://clinicaltrials.gov/ct2/show/NCT03243890 (28 May 2021)
- 41. Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcium Metabolism on 18F-NaF PET/MRI (BASIK-II). https://clinicaltrials.gov/ct2/show/NCT02917525 (28 May 2021).
- 42. A Study Evaluating the Effects of Ataciguat (HMR1766) on Aortic Valve Calcification (CAVS). https://clinicaltrials.gov/ct2/show/NCT02481258 (28 May 2021).
- 43. Effect of SNF472 on Progression of Cardiovascular Calcification in End-Stage-Renal-Disease (ESRD) Patients on Hemodialysis (HD). https://clinicaltrials.gov/ct2/show/NCT02966028 (28 May 2021).
- 44. Raggi P, Bellasi A, Bushinsky D et al. Slowing progression of cardiovascular calcification with snf472 in patients on hemodialysis: results of a randomized phase 2b study. Circulation 2020;141:728–739. [DOI] [PubMed] [Google Scholar]
- 45. Decalcification of the Aortic Valve by Vitamin K2 (Menaquinone-7) (DECAV-K2). https://clinicaltrials.gov/ct2/show/NCT03305536 (28 May 2021).
- 46. Clinical Study to Evaluate Efficacy and Safety of DA-1229 in Patients With CAVD (DIP-CAVD). https://clinicaltrials.gov/ct2/show/NCT04055883 (28 May 2021).
- 47. Early Aortic Valve Lipoprotein(a) Lowering Trial (EAVaLL). https://clinicaltrials.gov/ct2/show/NCT02109614 (28 May 2021).
- 48. PCSK9 Inhibitors in the Progression of Aortic Stenosis. https://clinicaltrials.gov/ct2/show/NCT03051360 (28 May 2021).
- 49. Study Investigating the Effect of Drugs Used to Treat Osteoporosis on the Progression of Calcific Aortic Stenosis. (SALTIRE II). https://clinicaltrials.gov/ct2/show/NCT02132026 (28 May 2021).
- 50. SLOW-Slower Progress of caLcificatiOn With Vitamin K2. https://clinicaltrials.gov/ct2/show/NCT04429035 (28 May 2021).
- 51. Vitamin K Supplement for Inhibition of the Progress in Aortic Valve Calcification (08-002). https://clinicaltrials.gov/ct2/show/NCT00785109 (28 May 2021).
- 52. Brandenburg VM, Reinartz S, Kaesler N et al. Slower Progress of Aortic Valve Calcification With Vitamin K Supplementation. Circulation 2017;135:2081–2083. [DOI] [PubMed] [Google Scholar]
- 53. Aikawa E, Libby P. A rock and a hard place chiseling away at the multiple mechanisms of aortic stenosis. Circulation 2017;135:1951–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 2007;171:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis 2004;13:841–847. [PubMed] [Google Scholar]
- 56. Ma X, Zhao D, Yuan P et al. Endothelial-to-mesenchymal transition in calcific aortic valve disease. Acta Cardiol Sin 2020;36:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rutkovskiy A, Malashicheva A, Sullivan G et al. Valve interstitial cells: the key to understanding the pathophysiology of heart valve calcification. J Am Heart Assoc 2017;6:e006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torre M, Hwang DH, Padera RF, Mitchell RN, VanderLaan PA. Osseous and chondromatous metaplasia in calcific aortic valve stenosis. Cardiovasc Pathol 2016;25:18–24. [DOI] [PubMed] [Google Scholar]
- 59. Xu K, Xie S, Huang Y et al. Cell-type transcriptome atlas of human aortic valves reveal cell heterogeneity and endothelial to mesenchymal transition involved in calcific aortic valve disease. Arterioscler Thromb Vasc Biol 2020;40:2910–2921. [DOI] [PubMed] [Google Scholar]
- 60. Hjortnaes J, Goettsch C, Hutcheson JD et al. Simulation of early calcific aortic valve disease in a 3D platform: a role for myofibroblast differentiation. J Mol Cell Cardiol 2016;94:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schlotter F, Halu A, Goto S et al. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation 2018;138:377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bosse K, Hans CP, Zhao N et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J Mol Cell Cardiol 2013;60:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morvan M, Arangalage D, Franck G et al. Relationship of Iron Deposition to Calcium Deposition in Human Aortic Valve Leaflets. J Am Coll Cardiol 2019;73:1043–1054. [DOI] [PubMed] [Google Scholar]
- 64. Gomel MA, Lee R, Grande-Allen KJ. Comparing the role of mechanical forces in vascular and valvular calcification progression. Front Cardiovasc Med 2018;5:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abdelbaky A, Corsini E, Figueroa AL et al. Early aortic valve inflammation precedes calcification: a longitudinal FDG-PET/CT study. Atherosclerosis 2015;238:165–172. [DOI] [PubMed] [Google Scholar]
- 66. Schlotter F, de Freitas RCC, Rogers MA et al. ApoC-III is a novel inducer of calcification in human aortic valves. J Biol Chem 2021;296:100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thomassen HK, Cioffi G, Gerdts E et al. Echocardiographic aortic valve calcification and outcomes in women and men with aortic stenosis. Heart 2017;103:1619–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Völzke H, Haring R, Lorbeer R et al. Heart valve sclerosis predicts all-cause and cardiovascular mortality. Atherosclerosis 2010;209:606–610. [DOI] [PubMed] [Google Scholar]
- 69. Veulemans V, Piayda K, Maier O et al. Aortic valve calcification is subject to aortic stenosis severity and the underlying flow pattern. Heart Vessels 2021;36:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Linde L, Carter-Storch R, Christensen NL et al. Sex differences in aortic valve calcification in severe aortic valve stenosis: association between computer tomography assessed calcification and valvular calcium concentrations. Eur Heart J Cardiovasc Imaging 2021;22:581–588. [DOI] [PubMed] [Google Scholar]
- 71. Sarajlic P, Plunde O, Franco-Cereceda A, Bäck M. Artificial Intelligence Models Reveal Sex-Specific Gene Expression in Aortic Valve Calcification. JACC Basic Transl Sci 2021;6:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Parra-Izquierdo I, Castaños-Mollor I, López J et al. Lipopolysaccharide and interferon-γ team up to activate HIF-1α via STAT1 in normoxia and exhibit sex differences in human aortic valve interstitial cells. Biochim Biophys Acta Mol Basis Dis 2019;1865:2168–2179. [DOI] [PubMed] [Google Scholar]
- 73. Parra-Izquierdo I, Castaños-Mollor I, López J et al. Calcification induced by type I interferon in human aortic valve interstitial cells is larger in males and blunted by a Janus Kinase inhibitor. Arterioscler Thromb Vasc Biol 2018;38:2148–2159. [DOI] [PubMed] [Google Scholar]
- 74. Summerhill VI, Moschetta D, Orekhov AN, Poggio P, Myasoedova VA. Sex-specific features of calcific aortic valve disease. Int J Mol Sci 2020;21:5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Poggianti E, Venneri L, Chubuchny V, Jambrik Z, Baroncini LA, Picano E. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J Am Coll Cardiol 2003;41:136–141. [DOI] [PubMed] [Google Scholar]
- 76. Richards J, El-Hamamsy I, Chen S et al. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol 2013;182:1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yap CH, Saikrishnan N, Yoganathan AP. Experimental measurement of dynamic fluid shear stress on the ventricular surface of the aortic valve leaflet. Biomech Model Mechanobiol 2012;11:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000;101:2345–2348. [DOI] [PubMed] [Google Scholar]
- 79. El Accaoui RN, Gould ST, Hajj GP et al. Aortic valve sclerosis in mice deficient in endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 2014;306:1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kennedy JA, Hua X, Mishra K, Murphy GA, Rosenkranz AC, Horowitz JD. Inhibition of calcifying nodule formation in cultured porcine aortic valve cells by nitric oxide donors. Eur J Pharmacol 2009;602:28–35. [DOI] [PubMed] [Google Scholar]
- 81. Gould ST, Matherly EE, Smith JN, Heistad DD, Anseth KS. The role of valvular endothelial cell paracrine signaling and matrix elasticity on valvular interstitial cell activation. Biomaterials 2014;35:3596–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gebhart V, Reiß K, Kollau A, Mayer B, Gorren ACF. Nitric Oxide Site and mechanism of uncoupling of nitric-oxide synthase : Uncoupling by monomerization and other misconceptions. Nitric Oxide 2019;89:14–21. [DOI] [PubMed] [Google Scholar]
- 83. Greenberg HZE, Zhao G, Shah AM, Zhang M. Role of oxidative stress in calcific aortic valve disease and its therapeutic implications. Cardiovasc Res 2021; doi: 10.1093/cvr/cvab142. Published online ahead of print 21 April 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 2008;52:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Farrar EJ, Huntley GD, Butcher J. Endothelial-derived oxidative stress drives myofibroblastic activation and calcification of the aortic valve. PLoS One 2015;10:e0123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Majumdar U, Manivannan S, Basu M et al. Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Sci Adv 2021;7:eabe3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Garg V, Muth AN, Ransom JF et al. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270–274. [DOI] [PubMed] [Google Scholar]
- 88. Nigam V, Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol 2009;47:828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Choi B, Lee S, Kim SM et al. Dipeptidyl peptidase-4 induces aortic valve calcifcation by inhibiting insulin-like growth factor-1 signaling in valvular interstitial cells. Circulation 2017;135:1935–1950. [DOI] [PubMed] [Google Scholar]
- 90. Wang Y, Han D, Zhou T et al. DUSP26 induces aortic valve calcification by antagonizing MDM2-mediated ubiquitination of DPP4 in human valvular interstitial cells. Eur Heart J 2021;42:2935–2951. [DOI] [PubMed] [Google Scholar]
- 91. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis: histological and immunohistochemical studies. Circulation 1994;90:844–853. [DOI] [PubMed] [Google Scholar]
- 92. Deck JD. Endothelial cell orientation on aortic valve leaflets. Cardiovasc Res 1986;20:760–767. [DOI] [PubMed] [Google Scholar]
- 93. Yap CH, Saikrishnan N, Tamilselvan G, Yoganathan AP. Experimental measurement of dynamic fluid shear stress on the aortic surface of the aortic valve leaflet. Biomech Model Mechanobiol 2012;11:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chandra S, Rajamannan NM, Sucosky P. Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech Model Mechanobiol 2012;11:1085–1096. [DOI] [PubMed] [Google Scholar]
- 95. Cao K, Sucosky P. Computational comparison of regional stress and deformation characteristics in tricuspid and bicuspid aortic valve leaflets. Int J Numer Method Biomed Eng 2017;33:1–21. [DOI] [PubMed] [Google Scholar]
- 96. Michelena HI, Prakash SK, Della Corte A et al. ; BAVCon Investigators. Bicuspid aortic valve identifying knowledge gaps and rising to the challenge from the international bicuspid aortic valve consortium (BAVCON). Circulation 2014;129:2691–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005;111:920–925. [DOI] [PubMed] [Google Scholar]
- 98. Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999;74:14–26. [DOI] [PubMed] [Google Scholar]
- 99. Fernandez Esmerats J, Villa-Roel N, Kumar S et al. Disturbed flow increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, inducing aortic valve calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1α (Hypoxia-Inducible Factor-1α) pathway in endothelial cells. Arterioscler Thromb Vasc Biol 2019;39:467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Souilhol C, Serbanovic-Canic J, Fragiadaki M et al. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat Rev Cardiol 2020;17:52–63. [DOI] [PubMed] [Google Scholar]
- 101. Thijssen DHJ, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 2009;53:986–992. [DOI] [PubMed] [Google Scholar]
- 102. Mahler GJ, Frendl CM, Cao Q, Butcher JT. Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol Bioeng 2014;111:2326–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gee T, Farrar E, Wang Y et al. NFκB (nuclear factor κ-light-chain enhancer of activated B cells) activity regulates cell-type-specific and context-specific susceptibility to calcification in the aortic valve. Arterioscler Thromb Vasc Biol 2020;40:638–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mahler GJ, Farrar EJ, Butcher JT. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol 2013;33:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Paranya G, Vineberg S, Dvorin S et al. Aortic valve endothelial cells undergo transforming growth factor-β-mediated and non-transforming growth factor-β-mediated transdifferentiation in Vitro. Am J Pathol 2001;159:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ma W, Zhang W, Wang C. Microfissure on the aortic valve endothelium: the root of all evil? J Am Coll Cardiol 2019;74:163. [DOI] [PubMed] [Google Scholar]
- 107. Harasaki H, Hanano H, Tanaka J, Tokunaga K, Torisu M. Surface structure of the human cardiac valve. A comparative study of normal and diseased valves. J Cardiovasc Surg (Torino) 1978;19:281–290. [PubMed] [Google Scholar]
- 108. Akhmedov A, Sawamura T, Chen CH, Kraler S, Vdovenko D, Lüscher TF. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): a crucial driver of atherosclerotic cardiovascular disease. Eur Heart J 2021;42:1797–1807. [DOI] [PubMed] [Google Scholar]
- 109. Syväranta S, Alanne-Kinnunen M, Öörni K et al. Potential pathological roles for oxidized low-density lipoprotein and scavenger receptors SR-AI, CD36, and LOX-1 in aortic valve stenosis. Atherosclerosis 2014;235:398–407. [DOI] [PubMed] [Google Scholar]
- 110. Zheng KH, Tsimikas S, Pawade T et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients With Aortic Stenosis. J Am Coll Cardiol 2019;73:2150–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Thanassoulis G, Campbell CY, Owens DS et al. ; CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Perrot N, Thériault S, Dina C et al. Genetic variation in LPA, calcific aortic valve stenosis in patients undergoing cardiac surgery, and familial risk of aortic valve microcalcification. JAMA Cardiol 2019;4:620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Larsson SC, Gill D, Mason AM et al. Lipoprotein(a) in Alzheimer, atherosclerotic, cerebrovascular, thrombotic, and valvular disease. Circulation 2020;141:1826–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hajjar KA, Gavish D, Breslow JL, Nachman RL. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature 1989;339:303–305. [DOI] [PubMed] [Google Scholar]
- 115. Loscalzo J, Weinfeld M, Fless GM, Scanu AM. Lipoprotein(a), fibrin binding, and plasminogen activation. Arteriosclerosis 1990;10:240–245. [DOI] [PubMed] [Google Scholar]
- 116. Hughes SD, Lou XJ, Ighani S et al. Lipoprotein(a) vascular accumulation in mice. In vivo analysis of the role of lysine binding sites using recombinant adenovirus. J Clin Invest 1997;100:1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lou XJ, Boonmark NW, Horrigan FT, Degen JL, Lawn RM. Fibrinogen deficiency reduces vascular accumulation of apolipoprotein(a) and development of atherosclerosis in apolipoprotein(a) transgenic mice. Proc Natl Acad Sci USA 1998;95:12591–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bouchareb R, Mahmut A, Nsaibia MJ et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation 2015;132:677–690. [DOI] [PubMed] [Google Scholar]
- 119. Rogers MA, Aikawa E. An (Auto)Taxing effort to mechanistically link obesity and calcific aortic valve disease. JACC Basic Transl Sci 2020;5:898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Capoulade R, Torzewski M, Mayr M et al. ApoCIII-Lp(a) complexes in conjunction with Lp(a)-OxPL predict rapid progression of aortic stenosis. Heart 2020;106:738–745. [DOI] [PubMed] [Google Scholar]
- 121. Helske S, Lindstedt KA, Laine M et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol 2004;44:1859–1866. [DOI] [PubMed] [Google Scholar]
- 122. Mahmut A, Boulanger MC, El Husseini D et al. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: implications for valve mineralization. J Am Coll Cardiol 2014;63:460–469. [DOI] [PubMed] [Google Scholar]
- 123. Tastet L, Capoulade R, Clavel MA et al. Systolic hypertension and progression of aortic valve calcification in patients with aortic stenosis: results from the PROGRESSA study. Eur Heart J Cardiovasc Imaging 2017;18:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K et al. Systolic Blood Pressure and Risk of Valvular Heart Disease: a Mendelian Randomization Study. JAMA Cardiol 2019;4:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Honda S, Miyamoto T, Watanabe T et al. A novel mouse model of aortic valve stenosis induced by direct wire injury. Arterioscler Thromb Vasc Biol 2014;34:270–278. [DOI] [PubMed] [Google Scholar]
- 126. Artiach G, Carracedo M, Plunde O et al. Omega-3 Polyunsaturated Fatty Acids Decrease Aortic Valve Disease through the Resolvin E1 and ChemR23 Axis. Circulation 2020;142:776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dweck MR, Jones C, Joshi NV et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76–86. [DOI] [PubMed] [Google Scholar]
- 128. Després AA, Perrot N, Poulin A et al. Lipoprotein(a), oxidized phospholipids, and aortic valve microcalcification assessed by 18F-sodium fluoride positron emission tomography and computed tomography. CJC Open 2019;1:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bartoli-Leonard F, Zimmer J, Aikawa E. Innate and adaptative immunity: the understudied driving force of heart valve disease. Cardiovasc Res 2021. Aug 25; doi: 10.1093/cvr/cvab273. Published online ahead of print 25 August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Li G, Qiao W, Zhang W, Li F, Shi J, Dong N. The shift of macrophages toward M1 phenotype promotes aortic valvular calcification. J Thorac Cardiovasc Surg 2017;153:1318–1327. e1. [DOI] [PubMed] [Google Scholar]
- 131. Passos LSA, Lupieri A, Becker-Greene D, Aikawa E. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis 2020;306:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. El Husseini D, Boulanger MC, Mahmut A et al. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol 2014;72:146–156. [DOI] [PubMed] [Google Scholar]
- 133. Thériault S, Dina C, Messika-Zeitoun D et al. Genetic association analyses highlight IL6, ALPL, and NAV1 As 3 new susceptibility genes underlying calcific aortic valve stenosis. Circ Genom Precis Med 2019;12:431–441. [DOI] [PubMed] [Google Scholar]
- 134. Gonçalves I, Edsfeldt A, Ko NY et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol 2012;32:1505–1512. [DOI] [PubMed] [Google Scholar]
- 135. Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol 2019;16:305–318. [DOI] [PubMed] [Google Scholar]
- 136. Mkannez G, Gagné-Ouellet V, Jalloul Nsaibia M et al. DNA methylation of a PLPP3 MIR transposon-based enhancer promotes an osteogenic programme in calcific aortic valve disease. Cardiovasc Res 2018;114:1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nadra I, Mason JC, Philippidis P et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res 2005;96:1248–1256. [DOI] [PubMed] [Google Scholar]
- 138. Hutcheson JD, Goettsch C, Bertazzo S et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater 2016;15:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rogers MA, Buffolo F, Schlotter F et al. Annexin A1-dependent tethering promotes extracellular vesicle aggregation revealed with single-extracellular vesicle analysis. Sci Adv 2020;6:eabb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol 2000;9:281–286. [DOI] [PubMed] [Google Scholar]
- 141. New SEP, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res 2011;108:1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Matilla L, Roncal C, Ibarrola J, Arrieta V et al. A role for MMP-10 (matrix metalloproteinase-10) in calcific aortic valve stenosis. Arterioscler Thromb Vasc Biol 2020;40:1370–1382. [DOI] [PubMed] [Google Scholar]
- 143. Yip CYY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol 2009;29:936–942. [DOI] [PubMed] [Google Scholar]
- 144. SyväRanta S, Helske S, Laine M et al. Vascular endothelial growth factor-secreting mast cells and myofibroblasts: a novel self-perpetuating angiogenic pathway in aortic valve stenosis. Arterioscler Thromb Vasc Biol 2010;30:1220–1227. [DOI] [PubMed] [Google Scholar]
- 145. Gendron N, Rosa M, Blandinieres A et al. Human Aortic Valve Interstitial Cells Display Proangiogenic Properties During Calcific Aortic Valve Disease. 2021;41:415–429. [DOI] [PubMed] [Google Scholar]
- 146. Yoshioka M, Yuasa S, Matsumura K et al. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med 2006;12:1151–1159. [DOI] [PubMed] [Google Scholar]
- 147. Katwa LC, Ratajska A, Cleutjens JP et al. Angiotensin converting enzyme and kininase-II-like activities in cultured valvular interstitial cells of the rat heart. Cardiovasc Res 1995;29:57–64. [PubMed] [Google Scholar]
- 148. O'Brien KD, Shavelle DM, Caulfield MT et al. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 2002;106:2224–2230. [DOI] [PubMed] [Google Scholar]
- 149. Helske S, Syväranta S, Kupari M et al. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J 2006;27:1495–1504. [DOI] [PubMed] [Google Scholar]
- 150. Fujisaka T, Hoshiga M, Hotchi J et al. Angiotensin II promotes aortic valve thickening independent of elevated blood pressure in apolipoprotein-E deficient mice. Atherosclerosis 2013;226:82–87. [DOI] [PubMed] [Google Scholar]
- 151. Arishiro K, Hoshiga M, Negoro N et al. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J Am Coll Cardiol 2007;49:1482–1489. [DOI] [PubMed] [Google Scholar]
- 152. Côté N, Pibarot P, Pépin A et al. Oxidized low-density lipoprotein, angiotensin II and increased waist cirumference are associated with valve inflammation in prehypertensive patients with aortic stenosis. Int J Cardiol 2010;145:444–449. [DOI] [PubMed] [Google Scholar]
- 153. Ramchand J, Patel SK, Kearney LG et al. Plasma ACE2 activity predicts mortality in aortic stenosis and is associated with severe myocardial fibrosis. JACC Cardiovasc Imaging 2020;13:655–664. [DOI] [PubMed] [Google Scholar]
- 154. Côté N, Couture C, Pibarot P, Després JP, Mathieu P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur J Clin Invest 2011;41:1172–1179. [DOI] [PubMed] [Google Scholar]
- 155. Capoulade R, Clavel MA, Mathieu P et al. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur J Clin Invest 2013;43:1262–1272. [DOI] [PubMed] [Google Scholar]
- 156. Rosenhek R, Rader F, Loho N et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004;110:1291–1295. [DOI] [PubMed] [Google Scholar]
- 157. O'Brien KD, Probstfield JL, Caulfield MT et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005;165:858–862. [DOI] [PubMed] [Google Scholar]
- 158. Bull S, Loudon M, Francis JM et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril in Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging 2015;16:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rattazzi M, Bertacco E, Del Vecchio A, Puato M, Faggin E, Pauletto P. Aortic valve calcification in chronic kidney disease. Nephrol Dial Transplant 2013;28:2968–2976. [DOI] [PubMed] [Google Scholar]
- 160. Bäck M, Michel J. From organic and inorganic phosphates to valvular and vascular calcifications. Cardiovasc Res 2021;117:2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw K-T. Inverse association between bone mineral density and risk of aortic stenosis in men and women in EPIC-Norfolk prospective study. Int J Cardiol 2015;178:29–30. [DOI] [PubMed] [Google Scholar]
- 162. Samad Z, Sivak JA, Phelan M, Schulte PJ, Patel U, Velazquez EJ. Prevalence and outcomes of left-sided valvular heart disease associated with chronic kidney disease. J Am Heart Assoc 2017;6:e006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Vavilis G, Bäck M, Occhino G et al. Kidney dysfunction and the risk of developing aortic stenosis. J Am Coll Cardiol 2019;73:305–314. [DOI] [PubMed] [Google Scholar]
- 164. Shroff GR, Bangalore S, Bhave NM et al. ; American Heart Association Council on the Kidney in Cardiovascular Disease and Stroke Council. Evaluation and management of aortic stenosis in chronic kidney disease: a scientific statement from the American Heart Association. Circulation 2021;143:e1088–e1114. [DOI] [PubMed] [Google Scholar]
- 165. Khan K, Yu B, Kiwan C et al. The role of Wnt/β-catenin pathway mediators in aortic valve stenosis. Front Cell Dev Biol 2020;8:862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Jahnen-Dechent W, Schinke T, Trindl A et al. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem 1997;272:31496–31503. [DOI] [PubMed] [Google Scholar]
- 167. Schafer C, Heiss A, Schwarz A et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 2003;112:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Westenfeld R, Schäfer C, Krüger T et al. Jahnen-Dechent W. Fetuin-A protects against atherosclerotic calcification in CKD. J Am Soc Nephrol 2009;20:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Carracedo M, Bäck M. Fetuin A in aortic stenosis and valve calcification: not crystal clear. Int J Cardiol 2018;265:77–78. [DOI] [PubMed] [Google Scholar]
- 170. Kubota N, Testuz A, Boutten A et al. Impact of Fetuin-A on progression of calcific aortic valve stenosis - The COFRASA - GENERAC study. Int J Cardiol 2018;265:52–57. [DOI] [PubMed] [Google Scholar]
- 171. Koos R, Brandenburg V, Mahnken AH et al. Association of fetuin-A levels with the progression of aortic valve calcification in non-dialyzed patients. Eur Heart J 2009;30:2054–2061. [DOI] [PubMed] [Google Scholar]
- 172. Chen J, Lin Y, Sun Z. Deficiency in the anti-aging gene Klotho promotes aortic valve fibrosis through AMPKα-mediated activation of RUNX2. Aging Cell 2016;15:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Dowd P, Hershline R, Ham SW, Naganathan S. Vitamin K and energy transduction: a base strength amplification mechanism. Science 1995;269:1684–1691. [DOI] [PubMed] [Google Scholar]
- 174. Jaminon AMG, Dai L, Qureshi AR et al. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci Rep 2020;10:6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chiyoya M, Seya K, Yu Z et al. Matrix Gla protein negatively regulates calcification of human aortic valve interstitial cells isolated from calcified aortic valves. J Pharmacol Sci 2018;136:257–265. [DOI] [PubMed] [Google Scholar]
- 176. Sweatt A, Sane DC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J Thromb Haemost 2003;1:178–185. [DOI] [PubMed] [Google Scholar]
- 177. Luo G, Ducy P, McKee MD et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997;386:78–81. [DOI] [PubMed] [Google Scholar]
- 178. Viegas CSB, Rafael MS, Enriquez JL et al. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol 2015;35:399–408. [DOI] [PubMed] [Google Scholar]
- 179. Ferro CJ, Mark PB, Kanbay M, Sarafidis P et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 2018;14:727–749. [DOI] [PubMed] [Google Scholar]
- 180. Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension 2005;46:514–520. [DOI] [PubMed] [Google Scholar]
- 181. Mundal LJ, Hovland A, Igland J et al. Association of low-density lipoprotein cholesterol with risk of aortic valve stenosis in familial hypercholesterolemia. JAMA Cardiol 2019;4:1156–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Stewart BF, Siscovick D, Lind BK et al. Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 183. Allara E, Morani G, Carter P et al. Genetic determinants of lipids and cardiovascular disease outcomes: a wide-angled Mendelian randomization investigation. Circ Genom Precis Med 2019;12:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013;13:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010;55:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Rees-Milton KJ, Norman P, Babiolakis C et al. Statin use is associated with insulin resistance in participants of the canadian multicentre osteoporosis study. J Endocr Soc 2020;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J 2020;41:2275–2284. [DOI] [PubMed] [Google Scholar]
- 188. Shah SR, Werlang CA, Kasper FK, Mikos AG. Novel applications of statins for bone regeneration. Natl Sci Rev 2015;2:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Okuyama H, Langsjoen PH, Hamazaki T et al. Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Rev Clin Pharmacol 2015;8:189–199. [DOI] [PubMed] [Google Scholar]
- 190. Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol 2002;40:1723–1730. [DOI] [PubMed] [Google Scholar]
- 191. Capoulade R, Yeang C, Chan KL, Pibarot P, Tsimikas S. Association of mild to moderate aortic valve stenosis progression with higher lipoprotein(a) and oxidized phospholipid levels: secondary analysis of a randomized clinical trial. JAMA Cardiol 2018;3:1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Arsenault BJ, Boekholdt SM, Dubé MP et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis a prospective mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet 2014;7:304–310. [DOI] [PubMed] [Google Scholar]
- 193. Chen HY, Cairns BJ, Small AM et al. Association of FADS1/2 locus variants and polyunsaturated fatty acids with aortic stenosis. JAMA Cardiol 2020;5:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Nsaibia MJ, Mahmut A, Boulanger M-C et al. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J Intern Med 2016;280:509–517. [DOI] [PubMed] [Google Scholar]
- 195. Bergmark BA, O'Donoghue ML, Murphy SA et al. An exploratory analysis of proprotein convertase subtilisin/kexin type 9 inhibition and aortic stenosis in the FOURIER trial. JAMA Cardiol 2020;5:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Perrot N, Valerio V, Moschetta D et al. Genetic and in vitro inhibition of PCSK9 and calcific aortic valve stenosis. JACC Basic Transl Sci 2020;5:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Poggio P, Songia P, Cavallotti L et al. PCSK9 involvement in Aortic Valve Calcification. J Am Coll Cardiol 2018;72:3225–3227. [DOI] [PubMed] [Google Scholar]
- 198. Desai NR, Kohli P, Giugliano RP et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C assessment with proprotein convertase subtilisi. Circulation 2013;128:962–969. [DOI] [PubMed] [Google Scholar]
- 199. Raal FJ, Giugliano RP, Sabatine MS et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol 2014;63:1278–1288. [DOI] [PubMed] [Google Scholar]
- 200. Gaudet D, Watts GF, Robinson JG et al. Effect of alirocumab on lipoprotein(a) Over ≥1.5 Years (from the Phase 3 ODYSSEY Program). Am J Cardiol 2017;119:40–46. [DOI] [PubMed] [Google Scholar]
- 201. O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk insights from the FOURIER trial. Circulation 2019;139:1483–1492. [DOI] [PubMed] [Google Scholar]
- 202. Burgess S, Ference BA, Staley JR et al. ; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD) Consortium. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: A Mendelian randomization analysis. JAMA Cardiol 2018;3:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lamina C, Kronenberg F; Lp(a)-GWAS-Consortium. Estimation of the required lipoprotein(a)-lowering therapeutic effect size for reduction in coronary heart disease outcomes: a Mendelian randomization analysis. JAMA Cardiol 2019;4:575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I et al. ; AKCEA-APO(a)-LRx Study Investigators. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
- 205. Welsh P, Welsh C, Celis-Morales CA et al. Lipoprotein(a) and cardiovascular disease: prediction, attributable risk fraction, and estimating benefits from novel interventions. Eur J Prev Cardiol 2020; doi: 10.1093/eurjpc/zwaa063. Published online ahead of print 7 October 2020. [DOI] [PubMed] [Google Scholar]
- 206. Yamamoto K, Koretsune Y, Akasaka T et al. ; Japanese Aortic Stenosis Study-2 (JASS-2) Investigators. Effects of vitamin K antagonist on aortic valve degeneration in non-valvular atrial fibrillation patients: prospective 4-year observational study. Thromb Res 2017;160:69–75. [DOI] [PubMed] [Google Scholar]
- 207. Fusaro M, Tripepi G, Noale M et al. Vertebral Fractures And Vascular Calcifications Study Group. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr Vasc Pharmacol 2015;13:248–258. [DOI] [PubMed] [Google Scholar]
- 208. Koos R, Mahnken AH, Mühlenbruch G et al. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol 2005;96:747–749. [DOI] [PubMed] [Google Scholar]
- 209. Sønderskov PS, Lindholt JS, Hallas J et al. Association of aortic valve calcification and vitamin K antagonist treatment. Eur Heart J Cardiovasc Imaging 2020;21:718–724. [DOI] [PubMed] [Google Scholar]
- 210. Nigwekar SU, Bloch DB, Nazarian RM et al. Vitamin K-Dependent Carboxylation of Matrix Gla Protein Influences the Risk of Calciphylaxis. J Am Soc Nephrol 2017;28:1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Blaser MC, Wei K, Adams RLE et al. Deficiency of natriuretic peptide receptor 2 promotes bicuspid aortic valves, aortic valve disease, left ventricular dysfunction, and ascending aortic dilatations in mice. Circ Res 2018;122:405–416. [DOI] [PubMed] [Google Scholar]
- 212. Zhou Z, Pyriochou A, Kotanidou A et al. Soluble guanylyl cyclase activation by HMR-1766 (ataciguat) in cells exposed to oxidative stress. Am J Physiol Heart Circ Physiol 2008;295:H1763–71. [DOI] [PubMed] [Google Scholar]
- 213. Fraccarollo D, Galuppo P, Motschenbacher S, Ruetten H, Schäfer A, Bauersachs J. Soluble guanylyl cyclase activation improves progressive cardiac remodeling and failure after myocardial infarction. Cardioprotection over ACE inhibition. Basic Res Cardiol 2014;109:421. [DOI] [PubMed] [Google Scholar]
- 214. Zhang B, Roos C, Hagler M et al. Abstract 123: Activation of Oxidized Soluble Guanylate Cyclase Slows Progression of Aortic Valve Calcification. Arterioscler Thromb Vasc Biol 2019;39:A123–A123. [Google Scholar]
- 215. Perelló J, Joubert PH, Ferrer MD, Canals AZ, Sinha S, Salcedo C. First-time-in-human randomized clinical trial in healthy volunteers and haemodialysis patients with SNF472, a novel inhibitor of vascular calcification. Br J Clin Pharmacol 2018;84:2867–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Nagy E, Lei Y, Martínez-Martínez E et al. Interferon-γ Released by Activated CD8+ T Lymphocytes Impairs the Calcium Resorption Potential of Osteoclasts in Calcified Human Aortic Valves. Am J Pathol 2017;187:1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kaden JJ, Bickelhaupt S, Grobholz R et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol 2004;36:57–66. [DOI] [PubMed] [Google Scholar]
- 218. Weiss RM, Lund DD, Chu Y et al. Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice. PLoS One 2013;8:e65201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Elmariah S, Delaney JAC, O'Brien KD et al. Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (The Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010;56:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Tzolos E, Dweck MR. Threshold effect for lipoprotein(a) in aortic stenosis. Heart 2021;107:1367–1368. [DOI] [PubMed] [Google Scholar]
- 221. Sider KL, Blaser MC, Simmons CA. Animal models of calcific aortic valve disease. Int J Inflam 2011;2011:364310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Blaser MC, Kraler S, Lüscher TF, Aikawa E. Multi-omics approaches to define calcific aortic valve disease pathogenesis. Circ Res 2021;128:1371–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. D van der V, C van der V, Blaser M et al. Engineering a 3D-bioprinted model of human heart valve disease using nanoindentation-based biomechanics. Nanomaterials 2018;8:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Rogers MA, Aikawa E. Cardiovascular calcification: artificial intelligence and big data accelerate mechanistic discovery. Nat Rev Cardiol 2019;16:261–274. [DOI] [PubMed] [Google Scholar]