The unifying theme of myeloproliferative neoplasm (MPN) driver mutations, including JAK2V617F(1–4), CALR(5, 6), MPL(7), and SH2B3(8) is activation of thrombopoietin receptor signaling, highlighting the critical importance of this pathway in the pathogenesis of MPN. JAK2V617F associates with a number of homodimeric type I cytokine receptors, including erythropoietin receptor (EPOR), thrombopoietin receptor (MPL), and granulocyte-colony stimulating factor receptor (G-CSFR) leading to constitutive activation(9), whereas mutant CALR has been found to only associate with MPL and leads to its constitutive activation(10, 11). This selectivity for activation of MPL explains why CALR mutations are seen in essential thromobocythemia (ET) and myelofibrosis (MF) but not polycythemia vera (PV).
Ba/F3 cells are a useful tool to dissect cytokine receptor signaling pathways and to test the oncogenicity of mutations. Parental Ba/F3 require IL-3 to provide a survival signal utilizing endogenous IL-3R(12). Ectopic expression of activated tyrosine kinases or constitutively active cytokine receptors provide Ba/F3 cells with a survival signal which obviates the need for supplemental IL-3(9, 13). We find that in vivo passage of Ba/F3 cells expressing either CALR type 1 (52 base pair deletion, CALRDEL) or type 2 (5 base pair insertion, CALRINS) mutations selects for the outgrowth of cells which have upregulated endogenous MPL allowing these cells to expand in mice. These findings solidify the exclusivity of MPL as the requisite cytokine receptor binding partner for CALR mediated transformation and highlight the central role for activation of thrombopoieitin receptor (MPL) signaling in the pathogenesis of MPN.
We created Ba/F3 cells stably expressing hCALRDEL, hCALRINS, hCALRWT, or empty vector using an MSCV-IRES-GFP (MIG) based retrovirus. Ba/F3 cells expressing mMPL using an MSCV-IRES-hCD4 vector were also produced. BCR-ABL p210 MIG Ba/F3 cells were used as a positive control for cytokine independent growth. Consistent with other reports(10, 11), expression of CALRDEL or CALRINS was not sufficient to render Ba/F3 cells cytokine independent in vitro (Supplemental Figure 1). To further evaluate the oncogenic potential of Ba/F3 cells expressing mutant calreticulin we injected CALRDEL, CALRINS, and CALRWT Ba/F3 cells into BALB/c mice (2.5×106 cells each) and monitored for expansion of GFP+ cells in the peripheral blood. GFP+ cells emerged in the peripheral blood in two out of ten mice each injected with CALRDEL or CALRINS cells, but not in mice injected with CALRWT cells. Expansion of GFP+ cells caused mice to become moribund and require sacrifice (Figure 1A, Supplemental Table 1).
Figure 1. Ba/F3 cells expressing CALRDEL and CALRINS mutations expand in a fraction of mice and gain cytokine independence.
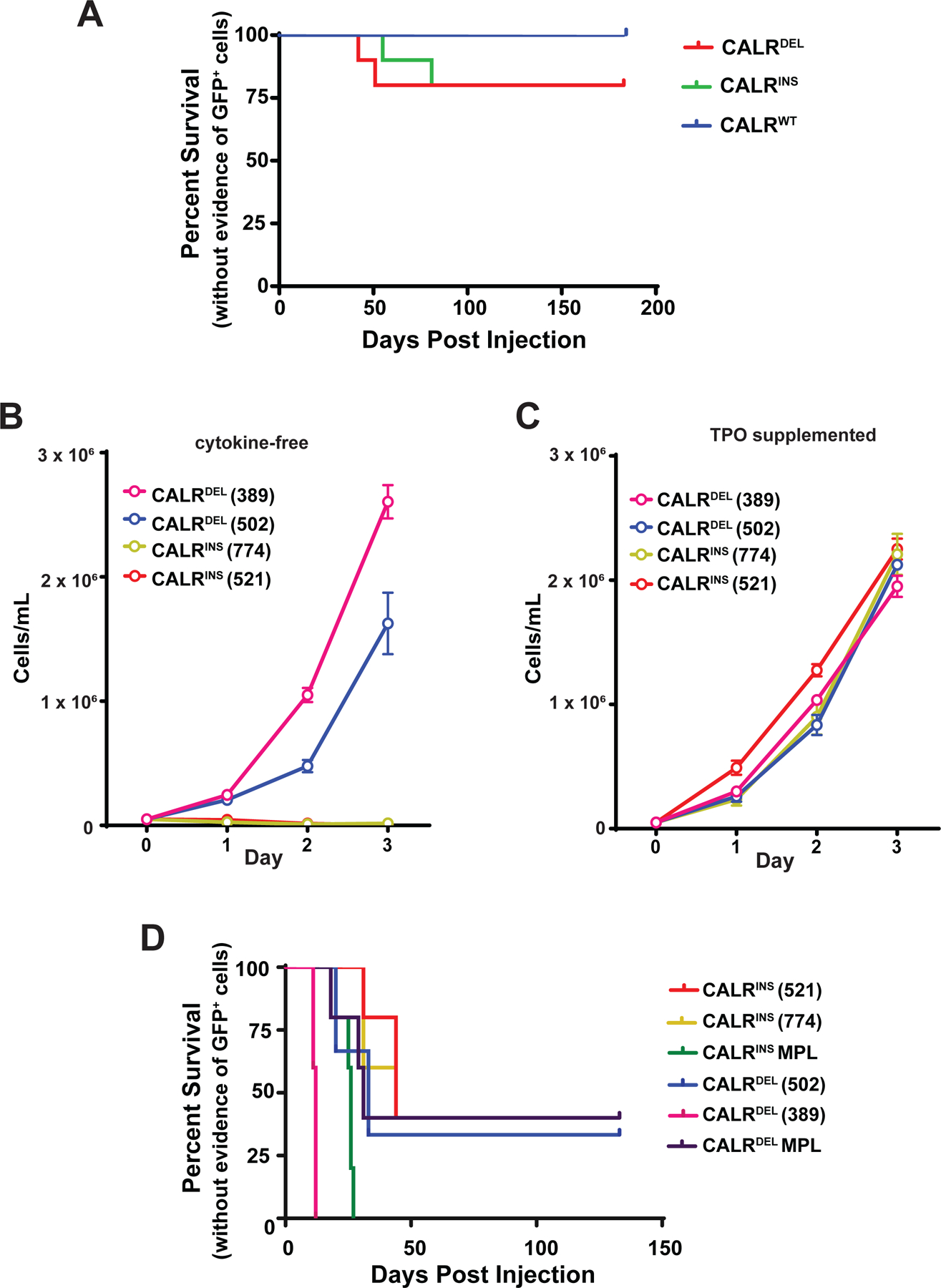
(A) Survival of mice injected with 2.5 × 106 CALRWT, CALRDEL, or CALRINS Ba/F3 cells (n=10 mice each). All mice remaining mice were sacrificed at day 183 post-transplant, no GFP+ cells were detected in the peripheral blood, bone marrow, or spleen of any of these mice. (B) Growth of post-mouse cell lines in cytokine free media. (C) Growth of post-mouse cells lines in media supplemented with 10ng/mL TPO. (D) Survival of mice injected with 2.5 × 106 post-mouse cell lines (389, 502, 521, 774), Ba/F3 CALRDEL MPL, or Ba/F3 CALRINS MPL (n=5 each).
We retrieved GFP+ cells by flow cytometry from the bone marrow of moribund mice and tested their ability to grow in vitro in the absence of cytokines. GFP+ cells retrieved from mice (referred to hereafter as post-mouse) injected with CALRDEL Ba/F3 cells were able to grow in the absence of cytokines, however GFP+ cells retrieved from mice injected with CALRINS Ba/F3 cells did not grow in the absence of cytokines (Figure 1B) but grew well with supplemental IL-3 (Supplemental Table 2). We tested the ability of multiple cytokines, including thrombopoietin (TPO), erythropoietin (EPO), granulocyte-macrophage colony-stimulating factor (G-CSF), interleukin-11 (IL-11), interleukin-7 (IL-7), interleukin-11 (IL-11), Stem Cell Factor (SCF), and Interferon-gamma (IFNγ) to support growth of post-mouse Ba/F3 CALRINS, only TPO was able to support growth of post-mouse Ba/F3 CALRINS cells (Figure 1D, Supplemental Table 2).
This suggested that the Ba/F3 cells which expanded in vivo expressed endogenous MPL. Indeed, we found that all post-mouse cell lines expressed MPL (Figure 2A) on Western Blot. Next, we stimulated the cell lines with TPO and measured activation of ERK and STAT5 (Figure 2B). All post-mouse cells activated ERK and STAT5 upon TPO stimulation, providing further evidence for presence of MPL on the cell surface. had Activation of ERK and STAT5 was present without stimulation in CALRDEL post-mouse cells as well as CALRDEL cells with ectopic MPL, consistent with constitutive activation of MPL signaling. CALRINS post-mouse cells also had activation of STAT5 without TPO suggesting constitutive activation of MPL.
Figure 2. Post-mouse cell lines have upregulated endogenous MPL.
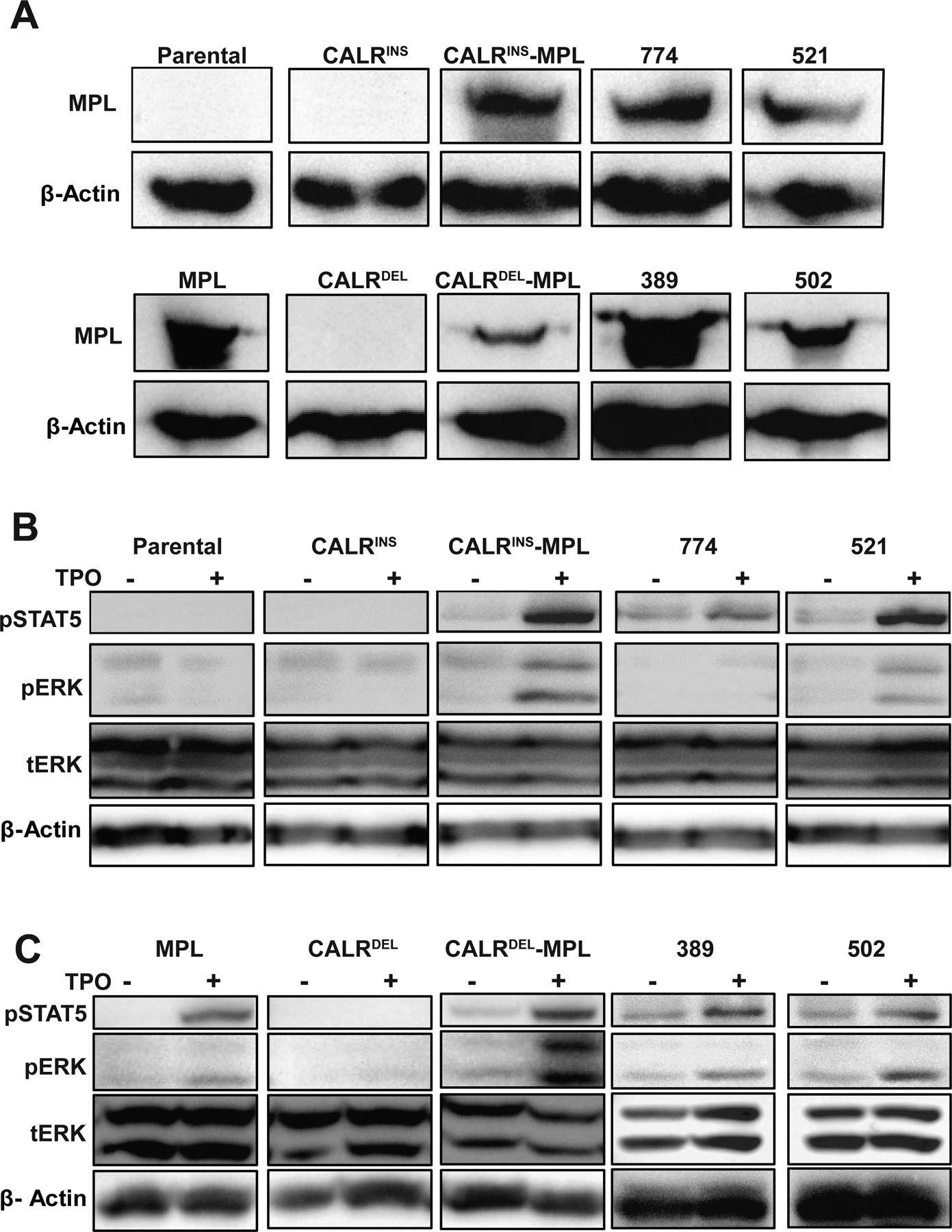
(A) Western blot demonstrating MPL expression in post-mouse cell lines. (B) Western blot of cells stimulated with TPO (50ng/mL) or PBS and harvested 15 minutes later to assess activation of downstream signaling pathways.
After expansion in vitro post-mouse cell lines were injected back into BALB/c mice to compare the ability of pre- versus post-mouse cells to expand in vivo. Ba/F3 CALRDEL and CALRINS cells with ectopic expression of MPL (mMPL-MSCV-hCD4 vector) were also used as a comparator. We found that all post-mouse cell lines expanded and caused death with similar kinetics as Ba/F3 CALRDEL and CALRINS with ectopic expression of MPL (Figure 1D).
Here we demonstrate that in vivo passage of Ba/F3 cells expressing mutant CALR leads to the selection of cells which have upregulated endogenous MPL thus allowing for their subsequent expansion in mice. This work highlights the exclusivity of MPL as the requisite scaffold cytokine receptor for mutant calreticulin and solidifies the central importance of activated MPL signaling in the pathogenesis of MPN.
MATERIALS AND METHODS:
Cell lines:
Ba/F3 cells were transduced with GFP- or human CD4-tagged MSCV retrovirus expressing murine MPL or human CALR wild-type (CALRWT), 52bp deletion (CALRDEL), or 5bp insertion (CALRINS). Cells were sorted for GFP or hCD4 positivity (FACSAria Fusion, BD Biosciences). For double-transduced cell lines, Ba/F3 cells expressing MPL were clone-sorted and expanded prior to transduction with CALR retroviruses. Cells were maintained in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin, streptomycin, and L-glutamine (PSL), and 5% WEHI-3 conditioned medium as a source of murine IL-3.
Mice:
BALB/c mice were retro-orbitally injected with Ba/F3 cells expressing CALRWT, CALRDEL (n=10), CALRINS (n=10), post-mouse cell lines (389, 502, 521, 774) (n=5 each), CALRDEL MPL (n=5) or CALRINS MPL (n=5). Mice were monitored for presence of Ba/F3 cells (GFP+ cells) in the peripheral blood every 1–2 weeks post-transplant. Mice with expanding GFP+ cells in the peripheral blood (>10%) were monitored twice daily and sacrificed once they appeared moribund at which time GFP+ cells were sorted from the bone marrow or spleen (FACSAria Fusion, BD Biosciences).
Cytokine independence assay:
Ba/F3 cells were washed four times with RPMI-1640 and plated at a concentration of 50,000 cells/ml in duplicate in either cytokine-free R10 medium (RPMI-1640 + 10% FBS + Pen/Strep/L-glutamine) or R10 supplemented with 5ng/ml murine TPO (BioLegend). Cells were counted daily by flow cytometry (Accuri C6, BD Biosiences) and dead cells were excluded by gating via FSC vs. SSC.
Western blotting:
Ba/F3 cells were plated in RPMI-1640 medium without FBS for 4 hours prior to stimulation with murine thrombopoietin (TPO) for 15 minutes. Cells were washed with PBS then lysed in RIPA buffer containing phosphatase and protease inhibitors (Sigma-Aldrich). Protein concentration was measured via BCA assay (Pierce). 30μg protein was run on 12% polyacrylamide gels then transferred onto nitrocellulose membranes. Membranes were blocked with 5% BSA and probed with antibodies detecting phospho-STAT5 (BD Biosciences), phospho-ERK1/2, total ERK1/2 (Cell Signaling Technologies), MPL, β-actin, and calreticulin (Abcam). Proteins were detected using HRP-conjugated secondary antibodies (Abcam) and chemiluminescence (Pierce) and visualized by a CCD imager (G Box, Syngene).
Supplementary Material
Funding:
This work was funded by a V Foundation Scholar Award (AGF), and NIH T32 Training Grant in Cancer Biology 5T32CA009054-39 (SAB)
Footnotes
Competing Interests: No authors have any competing financial interest
REFERENCES:
- 1.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. [DOI] [PubMed] [Google Scholar]
- 2.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005;352(17):1779–90. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer cell. 2005;7(4):387–97. [DOI] [PubMed] [Google Scholar]
- 4.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. [DOI] [PubMed] [Google Scholar]
- 5.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. The New England journal of medicine. 2013;369(25):2391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. The New England journal of medicine. 2013;369(25):2379–90. [DOI] [PubMed] [Google Scholar]
- 7.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS medicine. 2006;3(7):e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs KD Jr., et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116(6):988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(52):18962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balligand T, Achouri Y, Pecquet C, Chachoua I, Nivarthi H, Marty C, et al. Pathologic activation of thrombopoietin receptor and JAK2-STAT5 pathway by frameshift mutants of mouse calreticulin. Leukemia. 2016;30(8):1775–8. [DOI] [PubMed] [Google Scholar]
- 11.Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu RI, Marty C, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127(10):1325–35. [DOI] [PubMed] [Google Scholar]
- 12.Palacios R, Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41(3):727–34. [DOI] [PubMed] [Google Scholar]
- 13.Daley GQ, Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(23):9312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


