Abstract
Ferroptosis, a new form of non-apoptotic, regulated cell death characterized by iron dependency and lipid peroxidation, is involved in many pathological conditions such as neurodegenerative diseases, heart ischemia/reperfusion injury, acute renal failure, and cancer. While metabolic dysfunctions can lead to excessive lipid peroxidation culminating in ferroptotic cell death, glutathione peroxidase 4 (GPX4) resides in the center of a network that functions to prevent lipid hydroperoxides from accumulation, thereby suppressing ferroptosis. Indeed, RSL3 and other small-molecule GPX4 inhibitors can induce ferroptosis in not only cultured cancer cells but also tumor xenografts implanted in mice. Similarly, erastin and other system Xc− inhibitors can deplete intracellular glutathione required for GPX4 function, leading to lipid peroxidation and ferroptosis. As therapy-resistant cancer cells are sensitive to GPX4-targeted therapeutic regimens, the agents capable of inducing ferroptosis hold great promises to improve current cancer therapy. This review will outline the molecular basis of ferroptosis, but focus on the strategies and the agents developed in recent years for therapeutic induction of ferroptosis. The potentials of these ferroptosis-inducing agents, which include system Xc− inhibitors, GPX4 inhibitors, and iron-based nanoparticles, in cancer therapy will be subsequently discussed.
Keywords: Cancer therapy, Erastin, Ferroptosis, GPX4, Lipid peroxidation, Nanomedicine, RSL3, System Xc-
Introduction
Despite great advances in the molecular understanding of cancer, cancer remains as a leading cause of human death worldwide. A major challenge faced with the current cancer therapy is cancer recurrence owing to re-growth of therapy-resistant malignant cells. While many cancer therapeutics intend to kill cancer cells by inducing apoptotic cell death, resistant or persister cells often find ways to evade apoptosis.1 It is thus thought that therapeutic agents that can induce cancer cells to undergo non-apoptotic forms of death would hold promises to improve the clinical outcomes of current cancer therapy.2 Indeed, recent studies have demonstrated that therapy-resistant cancer cells are more sensitive to agents that can induce ferroptosis, an iron-dependent, non-apoptotic form of cell death.3,4
Ferroptosis has not been characterized until 2012 when erastin - a small molecule thought to selectively inhibit the growth of H-Ras-transformed BJ fibroblasts5 - was found to induce cancer cells to undergo a new type of iron-dependent, programmed death characterized by shrinking mitochondria with decreased crista and condensed/ruptured membranes.6 Ferroptosis lacks the hallmarks of apoptosis (e.g., chromatin condensation and caspase activation), and is also distinct from necroptosis as knockdown of necroptosis mediators RIPK1 and RIPK3 does not impair ferroptosis.6 Rather, ferroptosis is the final fate of cells with excessive lipid peroxidation, and can be regulated by multiple metabolic pathways that intersect to maintain cellular redox hemostasis.7, 8, 9, 10 Although ferroptosis was first characterized in transformed cells, this non-apoptotic cell death is also involved in other pathological conditions, such as neurodegenerative diseases, heart and hepatic ischemia/reperfusion injury, acute renal failure, and drug-induced hepatotoxicity.9 Intriguingly, while ferroptosis induced by loss of the major ferroptosis-regulatory gene Slc7a11 leads to suppression of growth of pancreatic ductal adenocarcinoma in mice,11 conventional cancer therapeutics, such as cisplatin,12 irradiation,13, 14, 15 and checkpoint blockage,13,16 can also induce ferroptosis in cancer cells. Moreover, a number of ferroptosis inducers (FINs) have been developed and shown to effectively kill cancer cells in various preclinical animal models.10,17 Therefore, ferroptosis-inducing agents have potentials to become a new class of therapeutic agents for treating cancers, particularly advanced, therapy-resistant cancers. This article will outline the strategies for inducing ferroptosis, and then focus on the diverse array of ferroptosis-inducing agents and their potentials in cancer therapy.
The molecular basis of ferroptosis
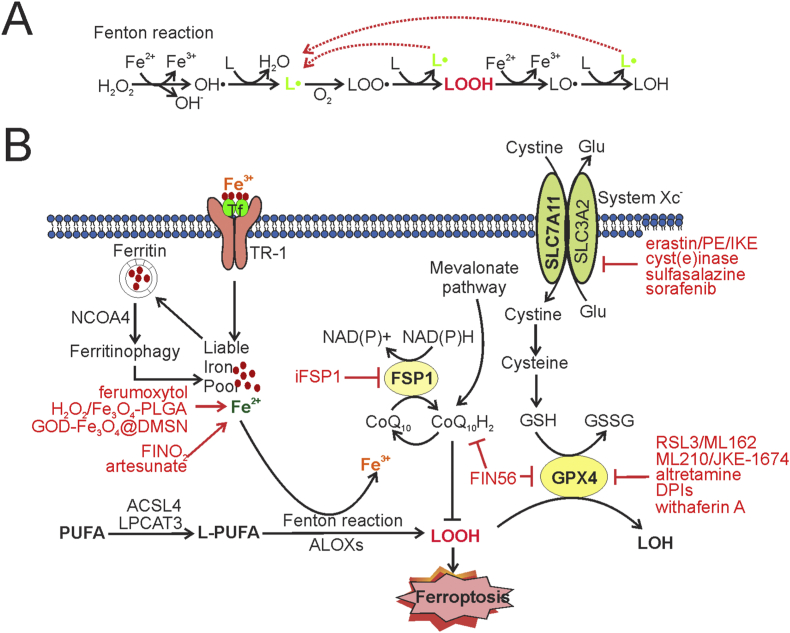
Ferroptosis is defined as a form of programmed cell death that is dependent on iron and caused by excessive peroxidation of membrane phospholipids.6 Lipid peroxidation occurs when free radicals, such as the hydroxyl radical (OH·) produced by H2O2-catalyzed oxidation of soluble ferrous irons (Fe2+) (i.e., the Fenton reaction), react with polyunsaturated fatty acids (PUFAs) inserted in the membrane phospholipids (L in Fig. 1A), yielding lipid radicals (L·) (Fig. 1A). Lipid radicals then react with oxygen (O2) to yield lipid peroxyl radicals (LOO·), which in turn react with an adjacent PUFA-phospholipid to produce a lipid hydroperoxide (LOOH) as well as a new lipid radical (Fig. 1A). While the new lipid radical can initiate a new lipid radical reaction, LOOH can also be converted into an alkoxyl radical (LO·) in the presence of Fe2+ and subsequently oxidize an adjacent PUFA-phospholipid to yield a lipid radical for another lipid radical reaction (Fig. 1A). This auto-amplifying chain reaction thus causes accumulation of lipid peroxides, leading to loss of membrane fluidity/integrity, damage of cellular proteins, and eventually ferroptotic cell death.18 As Fe2+ can provide free radicals to promote lipid peroxidation, overloading cells with iron by treating cells with ammonium sulfate ((NH4)2Fe(SO4)2 or ion chloride (FeCl2) can induce ferroptosis.19,20 Likewise, hemin and hemoglobin can be metabolized to Fe2+ through a reaction catalyzed by heme oxygenase 1 (HMOX1), thereby inducing cell death via ferroptosis.19,20 In addition to the Fe2+-dependent reactions, lipogenesis, e.g., arachidonate 5-lipogenease (ALOX5) can catalyze dioxygenation of PUFAs, yielding lipid hydroperoxides to promote ferroptosis21(Fig. 1B).
Figure 1.
The molecular basis of ferroptosis and the strategies for therapeutic induction of ferroptosis. (A) The lipid radical chain reactions triggered by ferrous irons (Fe2+) yield and amplify lipid radicals (L·) for the production of lipid hydroperoxides (LOOH). Free radicals produced by Fenton reactions oxidize phospholipids to yield L·, which in turn reacts with O2 to generate lipid peroxyl radicals (LOO·) for subsequent production of LOOH. These reactions can re-generate lipid radicals leading to excessive lipid peroxidation if remaining unblocked. (B) While the increase of the liable iron pool caused by ferric import through transferrin (Tf) and its receptor (TR-1), or ferritin degradation via ferritinophagy, can promote lipid peroxidation and subsequent ferroptosis, two major mechanisms respectively mediated by GPX4 and FSP1 serve to prevent lipid hydroperoxides from accumulation thereby suppressing ferroptosis. GPX4 is the only peroxidase that can reduce lipid peroxides, and its activity is regulated by the intracellular GSH level. System Xc− imports cystine for the production of cysteine and GSH, and thus plays a crucial role in the regulation of GPX4 activity. FSP1, on the other hand, may reduce CoQ10 for the suppression of lipid peroxidation. Multiple metabolic pathways, such as the mevalonate pathway and those for lipid synthesis or iron hemostasis, intersect to regulate ferroptosis. Accordingly, system Xc– inhibitors (e.g., erastin), GPX4 inhibitors (e.g., RSL3), and iron-based nanoparticles (e.g., ferumoxytol) represent the 3 major classes of ferroptosis-inducing agents that hold great promises for cancer therapy.
While intracellular lipophilic anti-oxidants (e.g., vitamin E) can neutralize lipid radicals by donating electrons and break the lipid radical chain reactions, a major mechanism utilized by cells to prevent lipid peroxidation and subsequent ferroptosis is mediated by glutathione peroxidase 4 (GPX4).22 As a selenocysteine-containing proteins, GPX4 is the only enzyme that can reduce membrane lipid hydroperoxides (LOOH) into non-toxic lipid alcohols (LOH) at the expense of reduced glutathione (GSH)22(Fig. 1B). It is thus not surprising that genetic depletion of GPX4 or inhibition of GPX4 activity by small molecules (e.g., (1S,3R)-RSL3, or RSL3 in short) results in excessive lipid peroxidation and subsequent ferroptotic cell death.22 RSL3 and other GPX4-binding compounds (e.g., ML210) belong to a major class of small molecules that can induce ferroptosis by inactivating the lipid peroxidase17,23 (Fig. 1B).
Because GPX4 mainly utilizes anti-oxidant GSH to reduce lipid hydroperoxides, the GPX4 activity is largely dependent on the intracellular GSH level. GSH is synthesized in the cytosol through two-step reactions that require cysteine. While cysteine can be obtained through reduction from cystine in the cytosol, uptake of cystine from the extracellular environment through system Xc− serves as the rate-limiting step in providing cysteine for GSH synthesis in many cancer cells.24 As a cystine/glutamate antiporter, system Xc− is composed of a light-chain subunit (xCT) encoded by SLC7A11 and a heavy-chain subunit (4F2hc) encoded by SLC3A2, and mediates the exchange of extracellular cystine and intracellular glutamate across the plasma membrane24,25 (Fig. 1B). In support of the important role of system Xc−-mediated cystine uptake in the prevention of ferroptosis, cystine deprivation,26 or treatments with erastin, an inhibitor of system Xc−,27 induces accumulation of lipid peroxides and subsequent ferroptosis. Therefore, by regulating cysteine availability and GSH synthesis, system Xc− acts together with GPX4 to drive a pathway that serves to reduce excessive lipid peroxidation and suppress ferroptosis (Fig. 1B). The agents that can inhibit system Xc− or limit cystine availability (e.g., erastin) are thus classified as a class of ferroptosis inducers that can indirectly inactivate GPX4 by depleting intracellular GSH10,17(Fig. 1B).
Coenzyme Q10 (CoQ10), also known as ubiquinone, is a potent intracellular lipophilic anti-oxidant that can be produced from isopentenyl-5-pyrophosphate, the end product of the mevalonate metabolic pathway. Ubiquinol (CoQ10H2), the reduced form of CoQ10, can trap lipid peroxyl radicals capable of catalyzing lipid peroxidation, thereby suppressing ferroptosis by preventing accumulation of lipid peroxides.28 Ferroptosis suppressor protein 1(FSP1), formerly known as apoptosis-inducing factor mitochondria-associated 2 (AIFM2), can reduce CoQ10 and catalyze regeneration of CoQ10H2 using NAD(P)H, thereby promoting CoQ10H2–mediated suppression of lipid peroxidation.29,30 Indeed, genetic screens identified FSP1 as one of the top hits that can prevent ferroptosis induced by GPX4 inactivation.29,30 Although a recent study argues against the role of CoQ10 in FSP1-mediated ferroptosis suppression,31 FSP1 likely mediates an alternative pathway that suppresses ferroptosis independent of GPX4 (Fig. 1B).
Strategies for therapeutic induction of ferroptosis
Given that it is an iron-dependent process associated with excessive lipid peroxidation, ferroptosis can be induced by targeting the major mediators of the ferroptosis-suppressing pathways (e.g., GPX4 and system Xc−), or increasing the liable iron pool (Fig. 1B). Indeed, a number of ferroptosis inducers (FINs) have been identified or developed that can either directly inhibit the GPX4 activity by binding to (e.g., RSL3, ML210) or depleting (e.g., FIN56) GPX4, or indirectly do so by inhibiting system Xc− (e.g., erastin, sulfasalazine, and sorafenib) or depleting extracellular cystine/cysteine (i.e., cyst(e)inase)10,17 (Fig. 1B). In addition, agents that can cause iron overload (e.g., FeCl2 and heme) or regulate ion oxidation (FINO2) can also induce ferroptosis in cancer cells. As iron plays an important role in the regulation of redox hemostasis in normal cells, systemic administration of the agents regulatory for iron metabolism likely causes severe side effects. Although a number of iron-based nanoparticles/nanomaterials have been developed that can deliver and release irons selectively at tumor sites by virtue of their dimensions and the defective tumor neovascularity – an effect often referred to as enhanced permeability and retention (EPR) effect - in animal models (Fig. 1B), the EPR effect is often compromised in human cancers, particularly in advanced cancers, due to their high heterogeneity and/or complexity.32 Therefore, the GPX4-mediated pathway remains as the ideal target for therapeutic induction of ferroptosis in human cancers. In support of this notion, overexpression of SLC7A11, the gene encoding the unique subunit (xCT) of system Xc−,24,25 often occurs in human cancers and is associated with reduced patient survival.11
As genetic knockout of Slc7a11 in mice does not result in lethality or obvious defects,33,34 system Xc− inhibitors are expected to be well tolerable. Intriguingly, although T cell proliferation in culture is dependent on SLC7A11 expression, system Xc− deficiency does not appear to abolish anti-cancer immunity in vivo.35 However, while the main function of system Xc− is to import cystine and supply cysteine, cysteine can not only be synthesized through the transsulfuration pathway,36 but also be imported through system ASC transporters such as ASCT1 and ASCT2.37 Moreover, GPX4 can use protein thiols as donors of electrons to reduce lipid hydroperoxides in the absence of GSH.38 Therefore, only cancer cells whose growth is largely dependent on cystine uptake, such as pancreatic cancer cell lines (MIA PaCa-2, PANC-1 and BxPC-3) and a subset of triple-negative breast cancer lines, are sensitive to system Xc− inhibition. On the other hand, Gpx 4-knockout mice are embryonic lethal,39 suggesting the importance of defining therapeutic windows for safely administrating GPX4 inhibitors in patients. Of note, although ferroptosis can be suppressed by FSP1, depletion or inhibition of FSP1 alone is not sufficient to induce ferroptosis, but rather sensitize cancer cells to undergo ferroptosis induced by GPX4 inactivation.29,30 In the following sections, we will summarize the pharmacological properties and the therapeutic potentials of major classes of ferroptosis-inducing agents (Figs. 1B and 2).
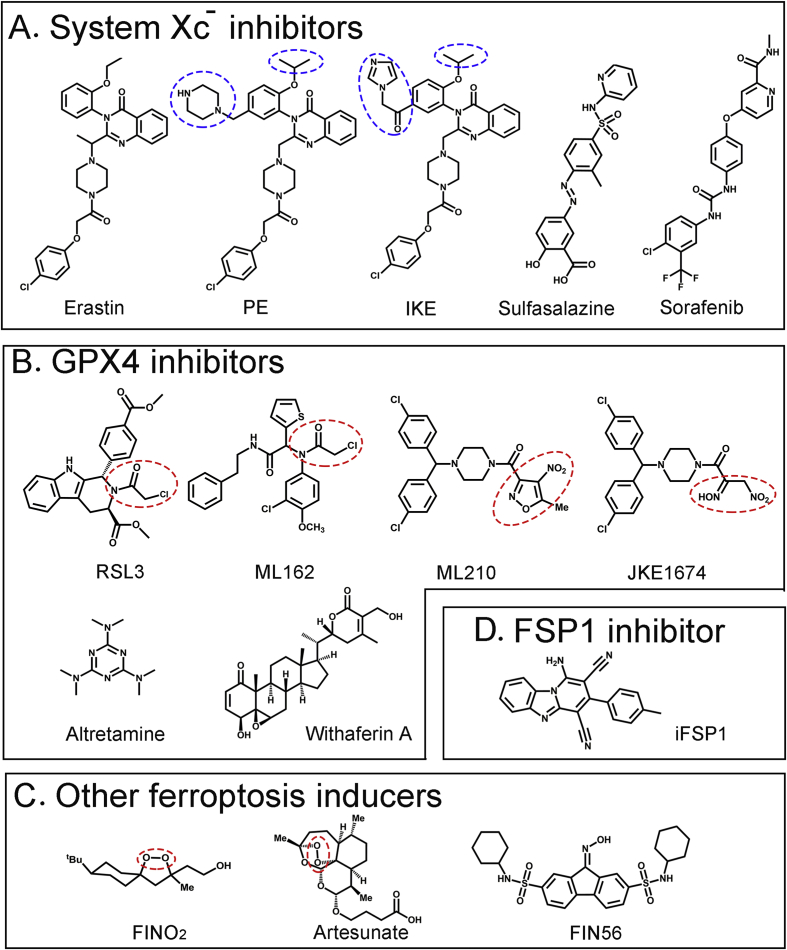
Figure 2.
Chemical structures of representative ferroptosis-inducing small molecules. (A) System Xc− inhibitors. PE, piperazine erastin; IKE, Imidazole ketone erastin. The groups distinct from erastin are indicated by blue dashed circles (B) GPX4 inhibitors. The moieties indicated by red dashed circles are proposed warheads, i.e., chloroacetamide for RSL3 and ML162, and nitroketoxime for ML210 and JKE1674 (C) Other ferroptosis inducers. The endoperoxide moiety is indicated by red circles (D) FSP1 inhibitor.
Agents depleting GSH (system xc− inhibitors and cyst(e)inase)
Erastin and its derivatives
Erastin (2-[1-[4-[2-(4-Chlorophenoxy)acetyl]-1-piperazinyl]ethyl]-3-(2-ethoxyphenyl)-4(3H)-Quinazolinone) (Fig. 2) was first identified as a small molecule that selectively causes death of transformed cells expressing oncogenic HRAS, KRAS, or BRAF in a high-throughput drug screening.5 While subsequent studies failed to confirm such selectivity, erastin was shown to bind the outer mitochondrial membrane proteins - voltage-dependent anion channel 2 and 3 (VDAC2/3) - to induce oxidative stress-dependent, non-apoptotic cell death,40 and was later identified as the first FIN that can inhibit system Xc− and decrease the intracellular GSH level at low micromolar concentrations.6 Cancer cell lines originated from diffuse large B cell lymphoma and renal cell carcinoma cells are often sensitive to erastin-induced ferroptosis.22 Erastin can also induce ferroptosis in head & neck, gastric, melanoma, lung cancer cells,41, 42, 43, 44 and muscle-derived embryonal and alveolar rhabdomyosarcoma cells.45
As the prototype of FINs, erastin has also been tested for its anti-cancer effects in combination with common therapeutic agents. For example, it was shown that erastin synergizes with cisplatin to induce death of HCT116 and A549 cells.46 Likewise, low-cytotoxic doses of erastin significantly enhance the activities of cytarabine and doxorubicin, two first-line anticancer drugs for acute myeloid leukemia, in HL60 cells.47 Erastin also enhances the radiosensitivity of non-small cell lung cancer (NSCLC) cells (e.g., A549-R and H460-R), partly because irradiation can induce SLC7A11 expression and increase the system Xc− activity.48
However, erastin has intermediate water solubility (0.086 mM) and is metabolically liable, precluding its in vivo applications for cancer therapy. Piperazine erastin (PE) is an analog of erastin, which adds a piperazine moiety on the meta position of the aniline ring to improve water solubility (1.4 mM) while substituting the ethoxyl moiety with isopropoxy to improve metabolic stability (Fig. 2). Initial subcutaneous injection of 40 mg/ml of PE followed by intravenous PE injection (30 mg/mL) starting one day after implantation of HT1080 cells into athymic nude mice significantly inhibits the xenograft growth without overt toxicity in this prevention model.22 However, PE has a limited effect on the growth of established tumors, perhaps due to its intermediate potency.49 Imidazole ketone erastin (IKE) is another erastin analog that replaces the piperazine moiety of PE with a carbonyl (Fig. 2), which can form a reversible covalent interaction with a lysine side chain in the binding site of a protein, resulting in more than 100-fold increase in potency.49 IKE also adds an imidazole moiety to increase water solubility (1 mM at pH 3; 0.25 mM at pH 7) and the stability of the ketone (Fig. 2). Intraperitoneal injections of IKE (23 and 40 mg/kg) can significantly slow the tumor growth in a xenograft mouse model of diffuse large B cell lymphoma (DLBCL).50 Tumor samples from IKE-treated mice express higher levels of ferroptosis markers (e.g., COX-2, MDA), demonstrating that IKE induces ferroptosis in vivo.50 As IKE is more soluble under acidic conditions than neutral conditions, nanoparticles (NP) based on biocompatible and biodegradable PEG-PLGA di-block copolymers have also been tested for their utility as an IKE carrier system.50 The PEG block was used to prevent clearance and prolong circulation lifetime while the PLGA block was used to provide a hydrophobic core to incorporate and sustainably release IKE. Although it does not improve the anti-tumor efficacy, the IKE NP formulation appears to exert less toxicity.50 These results have demonstrated the potentials of erastin analogs in the treatment of cancer.
Sulfasalazine
Sulfasalazine (SAS, 2-Hydroxy-5-((4-((2-pyridinylamino)sulfonyl)phenyl)azo)benzoic acid) (Fig. 2) is a FDA-approved drug commonly used for the treatment of rheumatoid arthritis and inflammatory bowel disease.51 Taken by mouth, SAS is metabolized by intestinal bacteria into two active metabolites, sulfapyridine and 5-aminosalicylic acid. In a 2001 study, SAS, but not its colonic metabolites, was shown to inhibit cystine uptake at 0.3 mM, and consequently identified as a potent system Xc− inhibitor that can markedly inhibit the growth of lymphoma without major toxicity to the hosts when administrated intraperitoneally at a dose of 200 or 250 mg/kg in a rat xenograft model.52 SAS was later shown to induce ferroptosis, but this activity is much lower than that of erastin.6,27 SAS-induced cell death can be reversed by β-mercaptoethanol, a compound which can reduce extracellular cystine to cysteine and thus bypass the function of system Xc−,27 confirming that SAS acts as a system Xc− inhibitor to induce ferroptosis. SAS has been shown to inhibit the growth of several cancer cells, including prostate, small cell lung, liver, head & neck, and triple-negative breast cancer cells.41,53, 54, 55, 56 Similar to erastin, SAS can sensitize cancer cells to first-line therapies. For example, as human glioblastomas (GBM) express SLC7A11, intraperitoneal injection of SAS (30–40 mg/kg) synergizes with gamma knife radiosurgery to prolong survival of nude rats carrying GBM xenografts.57 Likewise, while SLC7A11 expresses in one-third of triple negative breast tumors, SAS increases the efficacy of carboplatin against these tumors.56 Moreover, SAS can potentiate the cytotoxicity of cisplatin to colorectal, and head & neck cancer.41,58 However, clinical trials that test SAS efficacy in treatments of gliomas and lymphomas were unsuccessful,59 likely due to its low potency and metabolic instability. SAS is rapidly metabolized through cleavage of the diazo bond. Analogs with an alkyne group replacing the diazo group were synthesized, but their potencies in inhibiting system Xc− need improvement.60
Sorafenib
Sorafenib (4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide, BAY43-9006) (Fig. 2) is a broad-spectrum protein kinase inhibitor that can inhibit kinases including VEGFR, PDGFR, and Raf kinases, and is approved by FDA for the treatment of advanced renal cell carcinoma, advanced hepatocellular carcinoma, and thyroid carcinoma. While it remains uncertain whether the anti-cancer activity of sorafenib is due to its inhibition of kinases,61 sorafenib was found to induce a form of caspase-independent, but reactive oxygen species-dependent death in cancer cells.62,63 It was soon found that sorafenib is a potent system Xc− inhibitor, and it acts alone to induce ferroptosis in a range of cancer cell lines, including lung, kidney, pancreatic, colon, and liver cancer cells.27,64,65 Unlike erastin, however, sorafenib causes non-ferroptotic death at higher concentrations and thus triggers ferroptosis only within a narrow concentration window. While it is the only known protein kinase inhibitor that can induce ferroptosis, sorafenib may inhibit an unknown kinase whose activity is necessary for system Xc− activity, or bind to a non-kinase target regulatory for system Xc− activity.27
Cyst(e)inase
System Xc− inhibitors induce ferroptosis by blocking cystine uptake and consequently limiting the supply of cysteine required for GSH synthesis. While it has been demonstrated that culturing cancer cells in cystine-free medium sufficiently induces ferroptosis,26 an alternative strategy for decreasing the intracellular cysteine level in cancer cells is pharmacological depletion of extracellular cystine and cysteine by cyst(e)inase, an engineered cystathionine-γ-lyase (CGL) conjugated to methoxy PEG succinimidyl carboxyl ester.66 The wildtype CGL can degrade both cysteine and cystine, but the kinetic rates are too slow to be relevant for clinical applications. The engineered enzyme has two substitutions (E59T and E339V) that largely increases the binding affinity to cysteine and cystine.66 While the half-life of cyst(e)inase can reach to 25 h in mice, a single dose (8 mg/kg, i.p.) of cyst(e)inase results in a marked reduction of the blood cysteine/cystine level with no apparent toxicity in cynomolgus monkeys, indicating its potential application in cancer therapy.66 Indeed, while cyst(e)inase potently decreases the viability of mouse (HMVP2) and human (PC3 and DU145) prostate and breast (MDA-MB-361) cancer cells, treating mice harboring xenografts from these cells with cyst(e)inase (50 and 100 mg/kg) every 4 days for 4 weeks dramatically inhibits the growth of tumors with no overt adverse effects.66 Cyst(e)inase also prolongs the survival of genetically-engineered mice mimicking human chronic lymphocytic leukemia.66 Based on the observation that the growth of pancreatic ductal adenocarcinoma (PDAC) is critically dependent on system Xc− to import cystine and suppress ferroptosis, a recent study treated mice harboring established PDACs with cyst(e)inase, and showed that this treatment induces PDACs to undergo ferroptosis, resulting in tumor regression or stabilization.11 Of note, this study also reveals that PDAC cells can use cysteine to synthesize coenzyme A and its derivative CoQ10, two metabolites which can suppress ferroptosis in PDAC cells.11 Thus, system Xc− inhibitors may also induce ferroptosis through a mechanism independent of GSH depletion.
Agents inhibiting GPX4 activity (GPX4 inhibitors)
RSL3 and ML162
RSL3 ((1S,3R)-Methyl 2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1-[4-(methoxycarbonyl)phenyl]-1H-pyrido [3,4-b]indole-3-carboxylate) (Fig. 2) was first identified in a high-throughput screening as a compound that can selectively induce non-apoptotic, iron-dependent, oxidative cell death in transformed cells harboring activated H-Ras.67 However, unlike erastin, RSL3-induced cell death is independent of VDAC2/VDAC3 and system Xc−. Affinity purification experiments identifies GPX4 as a direct target of RSL3.22 RSL3 via its electrophilic chloroacetamide moiety covalently binds to the selenocysteine residue of GPX4, and thus directly inhibits the catalytic activity of the peroxidase in an irreversible manner.21 A number of cancer cell lines in the NCI60 panel (e.g., HOP-92, H522, CAKI-1, DU145 and PC3) are sensitive to RSL3, and nanomolar concentrations of RSL3 are often sufficient to decrease cell viability.67 RSL3 fails to induce lipid peroxidation and ferroptosis in GPX4-knockout cells, indicating that RSL3-induced ferroptosis is due to its inhibition of GPX4.22 Interestingly, recent studies show that drug-tolerant persistent cells and therapy-resistant cancer cells with mesenchymal characteristics are sensitive to RSL3 and other GPX4 inhibitors.3,4 As drug-tolerant persister cells represent surviving cells from which fully drug-resistant cells may eventually emerge to cause tumor relapse, these results support the potentials of these GPX4 inhibitors in the treatment of recurrent cancer. Moreover, the cells from clear-cell carcinomas (CCCs), which are highly aggressive malignancies commonly originating in the kidney and ovary, exhibit substantially higher sensitivity to GPX4 inhibition than normal cells, indicating the presence of a therapeutic window for inducing ferroptosis to treat CCC.68 Although it has been shown that intratumoral injection of RSL3 (100 mg/kg) inhibits the growth of xenografted BJeLR cells in mice,22 the poor solubility and unfavorable pharmacokinetic property of RSL3 preclude its systematic use in vivo.
ML162 (DPI7, α-[(2-chloroacetyl) (3-chloro-4-methoxyphenyl)amino]-N-(2-phenylethyl)-2-thiopheneacetamide) (Fig. 2) is another chloroacetamide-containing GPX4 inhibitor identified in a high-throughput screening for selective H-Ras-targeting compounds.69 Co-crystallization experiments demonstrate that ML162 binds the selenocysteine site of GPX4 via its reactive α-chloroacetamide group.23 ML162 exhibits a similar ferroptosis-inducing activity as RSL3.
ML210 and JKE-1674
The GPX4 inhibitors RSL3 and ML162 bind GPX4 via a chloroacetamide moiety. In addition to poor pharmacokinetic property, a major limitation of this group of GPX inhibitors lies in the fact that the highly reactive chloroacetamide moiety mediates promiscuous binding to cellular proteins, resulting in substantial off-target effects.23 Indeed, ferrostatin-1, a lipophilic radical-trapping antioxidant which specifically rescues cellular effects caused by the loss of GPX4 activity, only partly rescues cell death caused by RSL3 or ML162 23. ML210 ([4-[bis(4-chlorophenyl)methyl]-1-piperazinyl](5-methyl-4-nitro-3-isoxazolyl)-methanone) (Fig. 2), identified along with ML162, represents another class of GPX4 inhibitors that do not harbor a chloroacetamide group. ML210 exhibits a pattern of cell killing across 821 cancer cell lines that is similar to that of RSL3 and ML162. Although ML210 via a nitroisoxazole group can bind the selenocysteine reside of GPX4 in cells, it does not bind GPX4 in vitro, suggesting that ML210 is rather a prodrug. Indeed, cellular activation of ML210 generates the α-nitroketoxime JKE-1674, which retains the same GPX4-binding and–inhibitory activity.23 While ML210 and JKE-1674 are metabolically stable and bind a significantly-reduced number of cellular proteins, cell death caused by these GPX4 inhibitors can be fully rescued by ferrostatin-1, indicating their high selectivity toward GPX4 inhibition. Importantly, the α-nitroketoxime group of JKE-1674 significantly improves the water solubility. As a result, JKE-1674 can be detected in the serum of mice when orally administrated in a PEG400/ethanol solution.23 Therefore, JKE-1674 and its optimized derivatives have great potentials in cancer therapy.
Other GPX4 inhibitors
Altretamine (2,4,6-Tris (dimethylamino)-1,3,5-triazine) (Fig. 2) is an oral drug approved by FDA for palliative treatment of patients with persistent or recurrent ovarian cancer. Altretamine is structurally similar to the alkylating agent triethylenemelamine. While the precise mechanism underlying its anti-cancer effects remains unknown, N-demethylation of altretamine may produce reactive intermediates that covalently bind to DNA, causing DNA damage. In a network perturbation analysis, altretamine and SAS were identified as an anti-cancer compound pair that shares a same mechanism of action.70 However, unlike SAS, altretamine (0.5 mM) does not decrease the intracellular GSH level, but rather inhibits the GPX4 activity, resulting in lipid peroxidation.70 It remains elusive whether altretamine can induce ferroptosis in vivo and to what extent the ferroptosis-inducing activity contributes to the anti-cancer effects of altretamine in patients.
High throughput screening also identifies several other GPX4 inhibitors with diverse structures.22 Among them, DPI3, DPI4, DPI6, DPI8, DPI9, DPI12, DPI13, DPI15, and DPI19 contain a chloroacetamide group like RSL3, while DPI10 is highly similar to ML162 and contains a nitroisoxazole warhead. DPI17 and DPI18 have a triazine ring, which is similar to altretamine. DPI12, DPI13, and DPI19 has been confirmed to inhibit GPX4 activity. These compounds are not well characterized so far. In addition, a natural product withaferin A (WA) was also shown to inhibit GPX4 activity and induce ferroptosis in neuroblastoma IMR-32 and SK-N-SH cells.20 WA can bind GPX4 at a high concentration (10 μM), causing a decrease in the GPX4 protein level. WA at a low concentration (1 μM), however, binds Keap 1 and induces ferroptosis through targeting the Keap-1-Nrf 2-HMOX1 pathway to increase the liable iron pool.20 WA can also induce apoptosis in cancer cells,71 which may confound the interpretation of its anti-cancer activity in vivo.
Iron-based nanomaterials
Nanoparticles encapsulating iron oxide
Nanomaterials/nanoparticles are designed to specifically accumulate at tumor sites due to the EPR effect, and thus are ideal carriers of iron ions whose release in acidic lysosomes accelerates Fenton reactions, leading to ferroptotic death of cancer cells (Fig. 1B). Although ferumoxytol injection (Feraheme), the iron oxide nanoparticles approved by FDA for the treatment of anemia in patients of chronic kidney disease, can promote leukemia cells to produce hydroxides/hydroperoxides and induce them to die,72 this agent rather indirectly kill solid tumor cells by inducing macrophage polarization in the tumor microenvironment.73 Indeed, the intracellular H2O2 level in cancer cells is often too low for iron oxide nanoparticles to catalyze Fenton reactions to produce sufficient amounts of hydroperoxides for the induction of ferroptosis.74 A solution to this problem is to treat cancer cells with iron-based nanocatalysts encapsulated with H2O2. For example, a H2O2/Fe3O4-PLGA polymersome encapsulates H2O2 in the hydrophilic core of a Fe3O4- poly (lactic-co-glucolic acid) (PLGA) nanocarrier. Disruption of this polymersome by ultrasound can readily release both H2O2 and Fe3O4 in cancer cells, thereby triggering Fenton reactions to produce excessive reactive oxygen species to kill cancer cells.75 However, premature leakage of the strong oxide from the carriers may cause oxidative damage to normal tissues. An alternative strategy is to use a sequential catalytic nanosystem to produce H2O2 specifically within cancer cells.74 The nanocatalyst, referred to as GOD-Fe3O4@DMSNs, integrates glucose oxidase (GOD) and ultrasmall Fe3O4 nanoparticles into large pore-sized and biodegradable dendritic silica nanoparticles (DMSNs). GOD generates H2O2 by oxidizing glucose in cancer cells, thereby providing H2O2 for production of hydroperoxides in presence of iron. Intravenous injection of 10 mg/kg of GOD-Fe3O4@DMSNs inhibits the growth of 4T1 and U87 xenografts by 65% without obvious toxicities.74 Single-atom nanocatalysts have improved catalytic activities, and are often nontoxic. Recently, PEGylated single-atom iron-containing nanocatalysts (PSAF NCs) were developed that isolate the Fe atom in nitrogen-doped carbon nanomaterials.76 Although the addition of H2O2 is required for PSAF NCs to catalyze the production of hydroperoxides and induce ferroptosis in vitro, a single dose (20 mg/kg) of intravenous injection of the single-atom nanocatalysts to mice suppresses the growth of 4T1 xenografts by 63.5%.76 A mild photothermal process, i.e., exposing the xenografts to an 808 nm laser for 5 min, can accelerate the Fenton reactions catalyzed by PSAF NCs, leading to complete tumor regression.76 However, it is worth noting that the iron-based nanomaterials often induce both apoptosis and ferroptosis in cancer cells.
Complex nanoparticles containing other therapeutics
Iron-based metal–organic networks (MON) can also induce ferroptosis in cancer cells. One such agent, MON-p53, carrying not only ferric irons and tannic acid (TA) but also a plasmid expressing p53, is designed to maximally induce lipid peroxidation and ferroptosis by virtue of MON-induced Fenton reactions and p53-mediated downregulation of SLC7A11.77 MON-p53 was shown to induce ferroptosis of HT1080, 4T1 and SCC-7 cancer cells, and suppress the growth of HT1080 xenografts with negligible systemic toxicity.77 A similar agent SRF@FeIIITA (SFT) consists of a network-like corona self-deposited by Fe3+ and TA onto sorafenib nanocores.78 The entrapped sorafenib can inhibit system Xc− when released along with the corona dissociation in lysosomes of cancer cells, and thus may cooperate with iron-catalyzed Fenton reactions to induce ferroptosis.78 These studies also support the feasibility of a strategy for the development of complex nanomaterials that not only induce ferroptosis, but also encapsulate other therapeutic agents for best therapeutic benefits. For example, the magnetic nanoparticle FeGd-HN@Pt@LF/RGD2 loads Fe3O4/Gd2O3 hybrid nanoparticles with the common anti-cancer drug cisplatin (Pt), lactoferrin (LF) for ready transportation of the nanoparticles across the blood–brain barrier, and a RGD2 peptide whose binding to integrin αvβ3 on the surfaces of cancer cells can facilitate endocytosis of the nanoparticles by cancer cells.79 This iron-based agent indeed exhibits a profound anti-cancer activity that not only kills U-87MG cancer cells in vitro, but also significantly suppresses the growth of orthotopically-implanted glioblastoma cells in animals.79
Other ferroptosis-inducing agents
FIN56 (N2,N7-dicyclohexyl-9-(hydroxyimino)-9H-fluorene-2,7-disulfonamide) (Fig. 2) is an analog of a small-molecule ferroptosis-inducer CIL56 identified through a screening for compounds inducing caspase-independent death of HT1080 cells. FIN56 induces ferroptosis likely through depleting GPX4 while binding and inhibiting squalene synthase (SQS) required for CoQ10 synthesis.28 Unlike FIN56, FINO2 ((5α,8α)-8-(1,1-dimethylethyl)-3-methyl-1,2-dioxaspiro [4,5]decane-3-ethanol) (Fig. 2), an analog of platinic acids, rather indirectly inhibits the GPX4 activity and oxidize ferrous irons via its endoperoxide moiety, leading to widespread lipid peroxidation and ferroptosis.80 Artesunate (ART, 1-[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano [4,3-j]-1,2-benzodioxepin-10-yl] ester) (Fig. 2), the first-line medication used to treat malaria, also contains an endoperoxide moiety. While it has long been known that ART can induce cell death in a manner dependent on ferrous irons and ROS generation, this well-tolerate, FDA-approved drug was recently shown to induce ferroptosis in pancreatic, head & neck cancer cells, and Burkitt's lymphoma.81, 82, 83, 84 However, the in vivo ferroptosis-inducing and anti-cancer activities of these small molecules remain unknown.
Several other nanoparticles that can induce ferroptosis in cancer cells have also been developed. For example, low-density lipoprotein docosahexaenoic acid nanoparticles (LDL-DHA) reconstituted with the natural ω-3 fatty acid, docosahexaenoic acid were shown to deplete GSH and inactivate GPX4, leading to accumulation of lipid peroxidation and subsequent ferroptosis in hepatocellular carcinoma cells.85 Intratumoral injection of LDL-DHA can also severely suppress the tumor growth in a cohort of mice bearing human HepG2 xenografts.85 αMSH-PEG-C′ dots, ultrasmall near-infrared fluorescent silica nanoparticles coated by PEG with diameters down to 10 nm and surface-functionalized with alpha-melanocyte stimulating hormone (αMSH), the melanoma-targeting peptide, can also induce ferroptosis in multiple cancer cells in nutrient-deprived conditions while inhibiting the growth of HT1080 and 786-O xenografts when intravenously injected into mice.86 It was proposed that the αMSH peptide mediates the internalization and cancer-cell targeting while the silica particles can absorb and incorporate iron from the extracellular environment to promote Fenton reactions.86
Regulators of cancer cell sensitivity to ferroptosis
As ferroptosis-inducing agents are often effective in only a subset of cancer cells, great efforts have been put forth to understand the molecular basis of cell sensitivity to ferroptosis. As a result, a large number of genes have been identified that can promote or suppress ferroptosis by regulating the major ferroptosis mediators (i.e., system Xc− and GPX4), or the metabolic pathways crucial for triggering ferroptosis (Fig. 3A). Many of these genes are potential drug targets for sensitizing cancer cells to ferroptosis-based therapies.
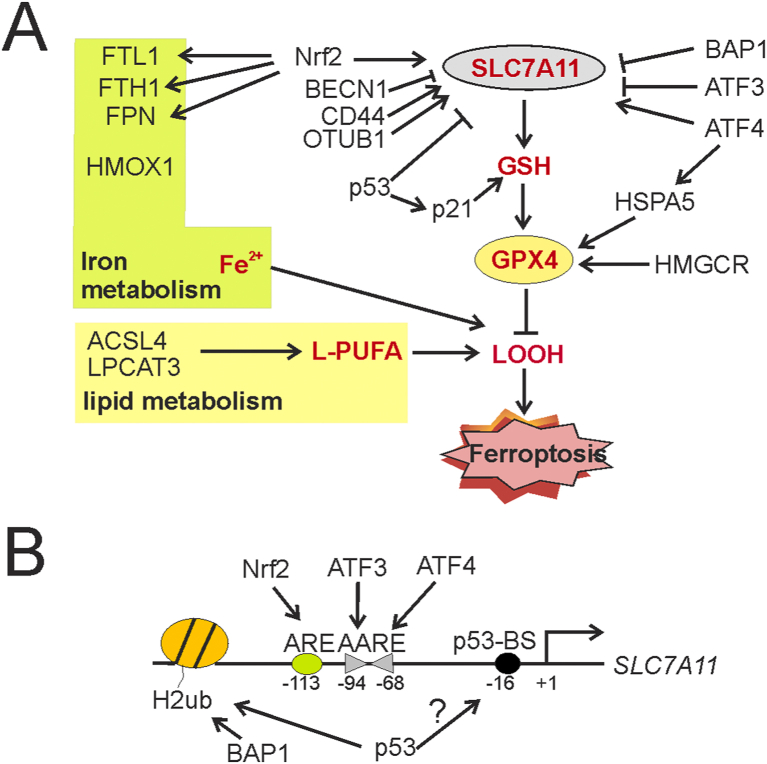
Figure 3.
Genetic factors regulate cancer cell sensitivity to ferroptosis-inducing agents. (A) Proteins that can regulate GSH synthesis, iron hemostasis, or lipid metabolism alter the sensitivity of cancer cells to ferroptosis induction. These proteins include transcription factors (e.g., p53, ATF3, ATF4, and Nrf 2) and epigenetic factors (e.g., BAP1 and OTUB1) that regulate GPX4 translation/stability or expression of SLC7A11 and iron-regulatory genes (e.g., FPN), and enzymes (e.g., ACSL4) that regulate the synthesis of phospholipids (B) Multiple transcription factors and epigenetic factors bind to the human SLC7A11 promoter and regulate SLC7A11 transcription. Note that the p53-binding element (p53-BS) appears to be dispensable for the regulation of the activity of the human SLC7A11 promoter. ARE, antioxidant response element; AARE, amino acid response element; H2ub, ubiquitinated histone H2.
SLC7A11 regulators
A major group of such regulators are transcription factors (TFs) that can regulate transcription of SLC7A11 – the gene encoding the light chain unique to system Xc− (Fig. 3B). Downregulation of SLC7A11 expression would impair cystine uptake by system Xc−, thereby depleting GSH leading to an increase in cancer cell sensitivity to ferroptosis-inducing agents. The tumor suppressor p53 was the first-identified TF that promotes erastin- or oxidative stress-induced ferroptosis by repressing SLC7A11 transcription.87 Although p53 was shown to directly bind to a p53-consensus site (p53-BS) in the mouse SLC7A11 promoter,87 deletion of the corresponding fragment in the human SLC7A11 promoter (Fig. 3B) does not prevent p53 from repressing the human gene,88 suggesting that the binding of p53 to this putative site is unlikely required for p53-mediated SLC7A11 repression.89 P53 can also promote ferroptosis by inducing expression of glutaminase 2 (GLS2) and spermidine/spermine N1-acetyltransferase 1 (SAT1).90,91 Paradoxically, p53 was also shown to decrease the sensitivity of cancer cells to ferroptosis by inducing p21 expression to promote GSH synthesis,92 or blocking the activity of DPP4 in a transcription-independent manner,93 in other cell contexts. Activating transcription factor 3 (ATF3) is another SLC7A11 transcriptional repressor that can suppress system Xc− and predispose cells to a state sensitive to ferroptosis induced by various FINs.88 ATF3 binds the SLC7A11 promoter at two adjacent sites that are in opposite orientation but coincide with the amino acid response elements (AAREs) (Fig. 3B) presumably bound by transcription activators C/EBP or other ATF/CREB members, thereby likely repressing SLC7A11 expression by competing with these transcription activators for binding to the promoter.88 Indeed, ATF4, a family member of ATF3, was shown to bind these AAREs (Fig. 3B), but the binding rather leads to SLC7A11 transactivation and suppression of ferroptosis in human glioma cells.94 ATF4 can also transactivate HSPA5, which encodes a heat shock protein capable of blocking the degradation of GPX495(Fig. 3A). Accordingly, knockdown of ATF4 was shown to indirectly regulate the GPX4 protein level and promote human pancreatic ductal adenocarcinoma (PDAC) cells to undergo ferroptosis.95 However, as Atf4-knockout mice are embryonic lethal, drugging ATF4 could result in severe side effects. In addition to TFs, SLC7A11 transcription is also repressed by BRCA1-associated protein 1 (BAP1) – a major component of a deubiquitinase (DUB) complex which catalyzes deubiquitination of histone 2 A (H2A) associated with the SLC7A11 promoter.96 Not surprisingly, BAP1 promotes ferroptosis in a DUB-dependent manner.96 Other SLC7A11/system Xc− regulators include CD44 and OTUB1, which suppress ferroptosis by directly binding the SLC7A11 protein and increasing the stability of the latter.97,98 Beclin-1 (BECN1), a key autophagy regulator, on the other hand, inhibits system Xc− by binding SLC7A11, and consequently promotes ferroptosis induced by system Xc− inhibitors.99
GPX4 regulators
In contrast to SLC7A11/system Xc−, the GPX4 activity is mainly regulated at the posttranscriptional level. As mentioned earlier, HSPA5, also known as GRP78 or BIP, can bind GPX4 and stabilize the peroxidase, and thus negatively regulate ferroptosis induced by erastin.95 In line with this activity, knockdown of HSPA5 or pharmacologic inhibition of HSPA5 by epigallocatechin gallate (EGCG) promotes GPX4 protein degradation, thereby promoting erastin-induced ferroptosis in pancreatic cancer cells.95 GPX4 is a selenoprotein containing the unusual amino acid selenocysteine (Sec) at its active site. The translation/synthesis of GPX4 requires selenocysteine-tRNA[sec]ser whose functionality depends on isopentenylation - a biochemical reaction catalyzed by tRNA isopentenyl transferase using isopentenyl pyrophosphate (IPP) as the isopentenyl donor.38,100 As IPP is an intermediate of the mevalonate pathway, statins (e.g., fluvastatin, lovastatin, and simvastatin), the inhibitors of the rate-limiting enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) in this pathway, can decrease the GPX4 protein level, and sensitize cancer cells to ferroptosis induced by FIN56 or RSL3.3,28 Statins can also promote ferroptosis by depleting CoQ10 produced by the mevalonate pathway, or inhibiting tRNA isopentenylation via TRIT13,28,.101
Nuclear factor erythroid 2-related factor 2 (Nrf 2)
Nrf 2 is a master anti-oxidant TF whose level is mainly regulated by proteasomal degradation mediated by kelch-like ECH-associated protein 1 (Keap 1).102 Nrf2 along with small Maf proteins bind to antioxidative response elements (ARE) to transactivate a number of genes involved in redox hemostasis, iron metabolism, and lipid metabolism.103 SLC7A11 and GPX4 are among the genes induced by Nrf2103,104 (Fig. 3B). This master antioxidant TF can also transactivate genes encoding both the light chain (FTL1) and heavy chain (FTH1) of ferritin, the major iron storage protein, as well as the only known iron-transporter ferroportin (FPN), and thus suppress ferroptosis by reducing the level of liable iron pool103(Fig. 3A). Indeed, Nrf2 overexpression, or inhibition of its negative regulator Keap1, can confer ferroptosis resistance in glioblastoma and head & neck cancer cells, while knockdown of Nrf2 promotes cancer cells to undergo ferroptotic cell death.83,105 Accordingly, trigonelline, a coffee alkaloid which can inhibit Nrf2 nuclear translocation and transactivation activity, enhances erastin/sorafenib-induced ferroptosis in hepatocellular carcinoma cells, or artesunate-induced ferroptosis in head & neck cancer cells.83,106 These studies thus support a notion that Nrf2-sepcific inhibitors could be of value in use to promote ferroptosis in cancer cells. However, it is important to note that trigonelline is not a specific Nrf2 inhibitor.
Ferroptosis suppressor protein 1 (FSP1)
FSP1 mediates a ferroptosis-suppressing pathway that can compensate for GPX4 inactivation in cancer cells (Fig. 1B). Indeed, FSP1 expression directly correlates with resistance to GPX4 inhibitors (including RSL3, ML162, and ML210) in a panel of 860 cancer cell lines.29,30 3 μM of iFSP1 (1-amino-3-(4-methylphenyl)-pyrido [1,2-a]benzimidazole-2,4-dicarbonitrile) (Fig. 2), a FSP1 inhibitor identified from a screen of 10,000 drug-like compounds, was shown to sensitize cancer cell lines from different origins to RSL3-induced ferroptosis.29 However, further chemical optimization appears to be necessary before applying this agent for in vivo studies.29
Other ferroptosis regulatory proteins
Many other proteins can also regulate the sensitivity to ferroptosis (Fig. 3B). For example, knockdown of acyl-CoA synthase 4 (ACSL4), which can convert long-chain fatty acids into fatty acyl-CoA esters, or lysophosphatidylcholine acyltransferase 3 (LPCAT3), which catalyzes the insertion of acylated fatty acids into phospholipids, prevents ferroptosis induced by erastin or RSL3.107, 108, 109, 110 Knockdown of cysteinyl-tRNA synthetase (CARS) or several other tRNA synthetases results in upregulation of the transsulfuration pathway and cysteine biosynthesis, thereby suppressing ferroptosis induced by system Xc− inhibitors.111 In addition, many proteins regulating iron metabolism, such as iron response element-binding protein 2 (IREB2), heat shock factor binding protein 1 (HSPB1), and CDGSH iron sulfur domain 1 (CISD1) can also regulate cancer cell sensitivity to ferroptosis.6,112,113
Concluding remarks
While therapy-resistant cancer cells are vulnerable to GPX4 inhibition, conventional cancer therapeutics (e.g., cisplatin, radiation, and immunotherapy) can also kill cancer cells by inducing ferroptosis. It has thus become clear that therapeutic induction of ferroptosis holds great promises for improving the outcome of current cancer therapy.10 Although a number of ferroptosis-inducing small molecules have been developed and demonstrated to induce death of cultured cancer cells, many of them have poor bioavailability, precluding their usages in cancer therapy. While medicinal chemistry optimization can improve pharmacological properties of these small-molecule agents, it is worth noting that the fact that human cancers are more heterogeneous and complex than mouse tumors used in animal models32 may limit the success of ferroptosis-inducing nanoparticles in clinical settings. On the other hand, as only a subset of cancer cells are sensitive to ferroptosis-inducing agents, the success of ferroptosis-based cancer therapies depends on the identification of reliable biomarkers for predicting the responsiveness of a cancer. However, the fact that ferroptosis results from dysregulation of intersected metabolic pathways9 hinders the efforts toward this direction. Indeed, while the intracellular level of NADPH, an essential reductant for lipid radicals, was found to determine cancer cell sensitivity to GPX4 inhibitors,28 it remains unclear how broad this biomarker can be used to predict cancer responsiveness to GPX4 inhibitors. In a similar vein, although FSP1 emerges as a novel ferroptosis suppressor, the FSP1 inhibitor (i.e., iFSP1) appears to only sensitize a subset of cancer cell lines to GPX4 inhibition.29 Notwithstanding these limitations, while we have been making great strides toward a better understanding of ferroptosis and its regulatory network, we have many reasons to be optimistic that therapeutic induction of ferroptosis will eventually become a new therapeutic option for cancer patients. Indeed, the recent identification of transferrin receptor 1 as a ferroptosis marker114 would allow for characterization of ferroptosis in vivo, and thus represents a significant step toward the success of ferroptosis-based cancer therapy.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Institutes of Health grant (No. R01CA240966) and the US Department of Defense award (No. W81XWH1910587 to CY).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Vallette F., Olivier C., Lézot F., et al. Dormant, quiescent, tolerant and persister cells: four synonyms for the same target in cancer. Biochem Pharmacol. 2019;162:169–176. doi: 10.1016/j.bcp.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Su Z., Yang Z., Xie L., DeWitt J., Y C. Cancer therapy in the necroptosis era. Cell Death Differ. 2016;23:747–756. doi: 10.1038/cdd.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan V., Ryan M., Dhruv H., et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547(7664):453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hangauer M., Viswanathan V., Ryan M., et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolma S., Lessnick S.L., Hahn W.C., Stockwell B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 6.Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y., Hou W., Song X., et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26(3):165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockwell B., Friedmann Angeli J., Bayir H., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassannia B., Vandenabeele P., Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Badgley M., Kremer D., Maurer H., et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J., Xu B., Han Q., et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat. official journal of Korean Cancer Association. 2018;50(2):445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang X., Green M., Wang W., et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye L., Chaudhary K., Zandkarimi F., et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15(2):469–484. doi: 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei G., Zhang Y., Koppula P., et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., Green M., Choi J., et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockwell B., Jiang X. The chemistry and biology of ferroptosis. Cell Chem Biol. 2020;27(4):365–375. doi: 10.1016/j.chembiol.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassannia B., Vandenabeele P., Vandem Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Li Q., Han X., Lan X., et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2(7):e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassannia B., Wiernicki B., Ingold I., et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest. 2018;128(8):3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W., Kim K., Gaschler M., Patel M., Shchepinov M., Stockwell B. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Nat Acad Sci USA. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W., SriRamaratnam R., Welsch M., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton J., Furst L., Ruberto R., et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol. 2020;16(5):497–506. doi: 10.1038/s41589-020-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewerenz J., Hewett S., Huang Y., et al. The cystine/glutamate antiporter system Xc- in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bridges R., Natale N., Patel S. System Xc- cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon S., Patel D., Welsch M., et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada K., Skouta R., Kaplan A., et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doll S., Freitas F., Shah R., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 30.Bersuker K., Hendricks J., Li Z., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai E., Zhang W., Cong D., Kang R., Wang J., Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun. 2020;523(4):966–971. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 32.Shi J., Kantoff P., Wooster R., Farokhzad O. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato H., Shiiya A., Kimata M., et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280(45):37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 34.McCullagh E., Featherstone D. Behavioral characterization of system xc- mutant mice. Behav Brain Res. 2014;265:1–11. doi: 10.1016/j.bbr.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Arensman M., Yang X., Leahy D., et al. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc Natl Acad Sci USA. 2019;116(19):9533–9542. doi: 10.1073/pnas.1814932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J., Berisa M., Schwörer S., Qin W., Cross J., Thompson C. Transsulfuration activity can support cell growth upon extracellular cysteine limitation. Cell Metab. 2019;30(5):865–876. doi: 10.1016/j.cmet.2019.09.009. e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandasamy P., Gyimesi G., Kanai Y., Hediger M. Amino acid transporters revisited: new views in health and disease. Trend Biochem Sci. 2018;43(10):752–789. doi: 10.1016/j.tibs.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Labunskyy V., Hatfield D., Gladyshev V. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo S., Chen L., Na R., et al. Gpx 4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic Biol Med. 2012;52(9):1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yagoda N., von Rechenberg M., Zaganjor E., et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh J., Kim E., Jang H., Park J., Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381(1):96–103. doi: 10.1016/j.canlet.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 42.Hao S., Yu J., He W., et al. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia. 2017;19(12):1022–1032. doi: 10.1016/j.neo.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang K., Wu L., Zhang P., et al. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol Carcinog. 2018;57(11):1566–1576. doi: 10.1002/mc.22878. [DOI] [PubMed] [Google Scholar]
- 44.Huang C., Yang M., Deng J., Li P., Su W., Jiang R. Upregulation and activation of p53 by erastin-induced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncol Rep. 2018;40(4):2363–2370. doi: 10.3892/or.2018.6585. [DOI] [PubMed] [Google Scholar]
- 45.Codenotti S., Poli M., Asperti M., Zizioli D., Marampon F., Fanzani A. Cell growth potential drives ferroptosis susceptibility in rhabdomyosarcoma and myoblast cell lines. J Cancer Res Clin Oncol. 2018;144(9):1717–1730. doi: 10.1007/s00432-018-2699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato M., Kusumi R., Hamashima S., et al. The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin's cytotoxicity in cancer cells. Sci Rep. 2018;8(1):968. doi: 10.1038/s41598-018-19213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y., Xie Y., Cao L., et al. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol. 2015;2(4):e1054549. doi: 10.1080/23723556.2015.1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan X., Lin Z., Jiang D., et al. Erastin decreases radioresistance of NSCLC cells partially by inducing GPX4-mediated ferroptosis. Oncology letters. 2019;17(3):3001–3008. doi: 10.3892/ol.2019.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larraufie M., Yang W., Jiang E., Thomas A.G., Slusher B., Stockwell B. Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg Med Chem Lett. 2015;25(21):4787–4792. doi: 10.1016/j.bmcl.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Tan H., Daniels J.D., et al. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol. 2019;26(5):623–633. doi: 10.1016/j.chembiol.2019.01.008. e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plosker G., Croom K. Sulfasalazine: a review of its use in the management of rheumatoid arthritis. Drugs. 2005;65(13):1825–1849. doi: 10.2165/00003495-200565130-00008. [DOI] [PubMed] [Google Scholar]
- 52.Gout P., Buckley A., Simms C., Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001;15(10):1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 53.Doxsee D., Gout P., Kurita T., et al. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate. 2007;67(2):162–171. doi: 10.1002/pros.20508. [DOI] [PubMed] [Google Scholar]
- 54.Guan J., Lo M., Dockery P., et al. The xc- cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother Pharmacol. 2009;64(3):463–472. doi: 10.1007/s00280-008-0894-4. [DOI] [PubMed] [Google Scholar]
- 55.Guo W., Zhao Y., Zhang Z., et al. Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer Lett. 2011;312(1):55–61. doi: 10.1016/j.canlet.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 56.Timmerman L., Holton T., Yuneva M., et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24(4):450–465. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sleire L., Skeie B., Netland I., et al. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion. Oncogene. 2015;34(49):5951–5959. doi: 10.1038/onc.2015.60. [DOI] [PubMed] [Google Scholar]
- 58.Ma M., Chen G., Wang P., et al. Xc- inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH-dependent mechanism. Cancer Lett. 2015;368(1):88–96. doi: 10.1016/j.canlet.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 59.Robe P., Martin D., Nguyen-Khac M., et al. Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer. 2009;9:372. doi: 10.1186/1471-2407-9-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shukla K., Thomas A., Ferraris D., et al. Inhibition of xc⁻ transporter-mediated cystine uptake by sulfasalazine analogs. Bioorg Med Chem Lett. 2011;21(20):6184–6187. doi: 10.1016/j.bmcl.2011.07.081. [DOI] [PubMed] [Google Scholar]
- 61.Wilhelm S., Carter C., Lynch M., et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 62.Katz S., Zhou L., Chao G., et al. Sorafenib inhibits ERK1/2 and MCL-1(L) phosphorylation levels resulting in caspase-independent cell death in malignant pleural mesothelioma. Canc Biol Ther. 2009;8(24):2406–2416. doi: 10.4161/cbt.8.24.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coriat R., Nicco C., Chéreau C., et al. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol Cancer Therapeut. 2012;11(10):2284–2293. doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- 64.Louandre C., Ezzoukhry Z., Godin C., et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133(7):1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 65.Lachaier E., Louandre C., Godin C., et al. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 2014;34(11):6417–6422. [PubMed] [Google Scholar]
- 66.Cramer S., Saha A., Liu J., et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23(1):120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang W., Stockwell B. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou Y., Palte M., Deik A., et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10(1):1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weïwer M., Bittker J., Lewis T., et al. Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg Med Chem Lett. 2012;22(4):1822–1826. doi: 10.1016/j.bmcl.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woo J., Shimoni Y., Yang W., et al. Elucidating compound mechanism of action by network perturbation analysis. Cell. 2015;162(2):441–451. doi: 10.1016/j.cell.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chirumamilla C., Perez-Novo C., Van Ostade X., Vanden Berghe W. Molecular insights into cancer therapeutic effects of the dietary medicinal phytochemical withaferin A. Proc Nutr Soc. 2018;76(2):96–105. doi: 10.1017/S0029665116002937. [DOI] [PubMed] [Google Scholar]
- 72.Trujillo-Alonso V., Pratt E., Zong H., et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol. 2019;14(6):616–622. doi: 10.1038/s41565-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zanganeh S., Hutter G., Spitler R., et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huo M., Wang L., Chen Y., Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8(1):357. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li W., Su C., Chang Y.C., Lin Y.J., Yeh C. Ultrasound-induced reactive oxygen species mediated therapy and imaging using a Fenton reaction activable polymersome. ACS Nano. 2016;10(2):2017–2027. doi: 10.1021/acsnano.5b06175. [DOI] [PubMed] [Google Scholar]
- 76.Huo M., Wang L., Wang Y., Chen Y., Shi J. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano. 2019;13(2):2643–2653. doi: 10.1021/acsnano.9b00457. [DOI] [PubMed] [Google Scholar]
- 77.Zheng D., Lei Q., Zhu J., et al. Switching apoptosis to ferroptosis: metal-organic network for high-efficiency anticancer therapy. Nano Lett. 2017;17(1):284–291. doi: 10.1021/acs.nanolett.6b04060. [DOI] [PubMed] [Google Scholar]
- 78.Liu T., Liu W., Zhang M., et al. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano. 2018;12(12):12181–12192. doi: 10.1021/acsnano.8b05860. [DOI] [PubMed] [Google Scholar]
- 79.Shen Z., Liu T., Li Y., et al. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano. 2018;12(11):11355–11365. doi: 10.1021/acsnano.8b06201. [DOI] [PubMed] [Google Scholar]
- 80.Gaschler M., Andia A., Liu H., et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14(5):507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ooko E., Saeed M., Kadioglu O., et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22(11):1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Eling N., Reuter L., Hazin J., Hamacher-Brady A., Brady N. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2(5):517–532. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roh J., Kim E., Jang H., Shin D. Nrf 2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang N., Zeng G., Yin J., Bian Z. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt's Lymphoma. Biochem Biophys Res Commun. 2019;519(3):533–539. doi: 10.1016/j.bbrc.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 85.Ou W., Mulik R., Anwar A., McDonald J., He X., Corbin I. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med. 2017;112:597–607. doi: 10.1016/j.freeradbiomed.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim S.E., Zhang L., Ma K., et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11(11):977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang L., Kon N., Li T., et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L., Liu Y., Du T., et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc- Cell Death Differ. 2020;27(2):662–675. doi: 10.1038/s41418-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gridasova A., Henry R. The p53 tumor suppressor protein represses human snRNA gene transcription by RNA polymerases II and III independently of sequence-specific DNA binding. Mol Cell Biol. 2005;25(8):3247–3260. doi: 10.1128/MCB.25.8.3247-3260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jennis M., Kung C., Basu S., et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30(8):918–930. doi: 10.1101/gad.275891.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ou Y., Wang S., Li D., Chu B., Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci USA. 2016;113(44):E6806–E6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tarangelo A., Magtanong L., Bieging-Rolett K., et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 2018;22(3):569–575. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie Y., Zhu S., Song X., et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20(7):1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 94.Chen D., Fan Z., Rauh M., Buchfelder M., Eyupoglu I., Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36(40):5593–5608. doi: 10.1038/onc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu S., Zhang Q., Sun X., et al. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res. 2017;77(8):2064–2077. doi: 10.1158/0008-5472.CAN-16-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y., Shi J., Liu X., et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasegawa M., Takahashi H., Rajabi H., et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7(11):11756–11769. doi: 10.18632/oncotarget.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu T., Jiang L., Tavana O., Gu W. The deubiquitiylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79(8):1913–1924. doi: 10.1158/0008-5472.CAN-18-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song X., Zhu S., Chen P., et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system xc(-) activity. Curr Biol. 2018;28(15):2388–2399. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moosmann B., Behl C. Selenoproteins, cholesterol-lowering drugs, and the consequences: revisiting of the mevalonate pathway. Trends Cardiovasc Med. 2004;14(7):273–281. doi: 10.1016/j.tcm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Fradejas N., Carlson B., Rijntjes E., Becker N., Tobe R., Schweizer U. Mammalian Trit1 is a tRNA([Ser]Sec)-isopentenyl transferase required for full selenoprotein expression. Biochem J. 2013;450(2):427–432. doi: 10.1042/BJ20121713. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi A., Kang M., Okawa H., et al. Oxidative stress sensor Keap 1 functions as an adaptor for cul3-based E3 ligase to regulate proteasomal degradation of Nrf 2. Mol Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dodson M., Castro-Portuguez R., Zhang D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ye P., Mimura J., Okada T., et al. Nrf 2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol Cell Biol. 2014;34:3421–3434. doi: 10.1128/MCB.00221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fan Z., Wirth A., Chen D., et al. Nrf 2-Keap 1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6(8):e371. doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun X., Ou Z., Chen R., et al. Activation of the p62-Keap 1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dixon S., Winter G., Musavi L., et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10(7):1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan H., Li X., Zhang X., Kang R., Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478(3):1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 109.Kagan V., Mao G., Qu F., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doll S., Proneth B., Tyurina Y., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayano M., Yang W., Corn C., Pagano N., Stockwell B. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23(2):270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun X., Ou Z., Xie M., et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34(45):5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuan H., Li X., Zhang X., Kang R., Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478(2):838–844. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 114.Feng H., Schorpp K., Jin J., et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30(10):3411–3423. doi: 10.1016/j.celrep.2020.02.049. e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]