Abstract
The frequency of human suffering from cancer is increasing annually across the globe. This has fueled numerous investigations aimed at the prevention and cure of various cancers. Long non-coding RNA (lncRNA) are known to play a crucial role in cancer. For instance, cancer susceptibility candidate 11 (CASC11), as one of the long non-coding RNAs, has been reported to be overexpressed in various tumors. This review elucidates the mechanism by which lncRNA CASC11 regulates tumors' biological processes and affirms its value as a therapeutic target for tumors. Through systematic analysis and review of relevant articles in PubMed, we revealed the pathophysiological mechanism of CASC11 on the tumor by regulating the biological processes of tumor such as proliferation, autophagy, apoptosis, thereby promoting tumor metastasis. We also revealed the regulatory pathways of CASC11 in different tumors, for instance by acting on a variety of microRNAs, oncogenic proteins, carcinogens, and transcription factors. Consequently, CASC11 regulates cancer proliferation, apoptosis, and invasion by altering the WNT/β-catenin signaling pathway and epithelial–mesenchymal transition (EMT). Furthermore, CASC11 expression has a high pertinence with clinical prognosis, suggesting that it is a potential marker for malignant tumors or a clinical adjuvant therapy in the future.
Keywords: CASC11, Long non-coding RNA, Malignant tumors, microRNA, Therapeutic targets
Introduction
As a nucleic acid that does not encode proteins, long non-coding RNA (LncRNA) has become a research hotspot attracting wide attention. LncRNAs mainly couples and interact with upstream and downstream signaling molecules regulating gene expression. They are primarily transcribed by Pol II and considered as transcriptional noise.1 In recent years, the association of lncRNA with tumors has been confirmed by an increasing number of scholars. For instance, lncRNA PTAR, in combination with miR-101-3p, positively affects the expression of ZEB1, thereby enhancing the aggressiveness of ovarian cancer cells.2 FEZF1antisenseRNA1 (FEZF1-AS1), as one of the most highly expressed lncRNA in colorectal cancer, also promotes the malignant progression of CRC by activating STAT3 signal in combination with PKM2.3 The expression of lncRNA MIR22HG mediates the STAT3/c-Mcy/FAK axis to promote tumorigenesis of Esophageal adenocarcinoma.4
CASC11 (cancer susceptibility candidate 11), located in human chromosome 8q24.21, is a member of lncRNAs and is significantly overexpressed in various tumors.5 Variations in the human 8q24 region are associated with many cancers. This region is not only associated with CASC11 but also with other lncRNAs. For example, lncRNA PVT1 located in the 8q24 region mediates angiogenesis in gastric cancer, which is attributed to the activation of the STAT3/VEGFA axis.6 High expression of lncRNA CCAT2, localized in the 8q24 region, can promote osteosarcoma progression by regulating the miR-200b/VEGF axis.7 Moreover, CASC11 acts on various microRNAs, oncogenic proteins, carcinogens, transcription factors, altering the EMT (epithelial–mesenchymal transition) pathway, WNT/beta-catenin pathway, and other pathways. Consequently, proliferation and apoptosis of cancer cells are regulated, leading to malignant tumor events.
This review explores the mechanism of CASC11-mediated tumorigenesis and affirms its potential value as a marker for malignancy.
Molecular mechanisms of lncRNA regulation of cancer
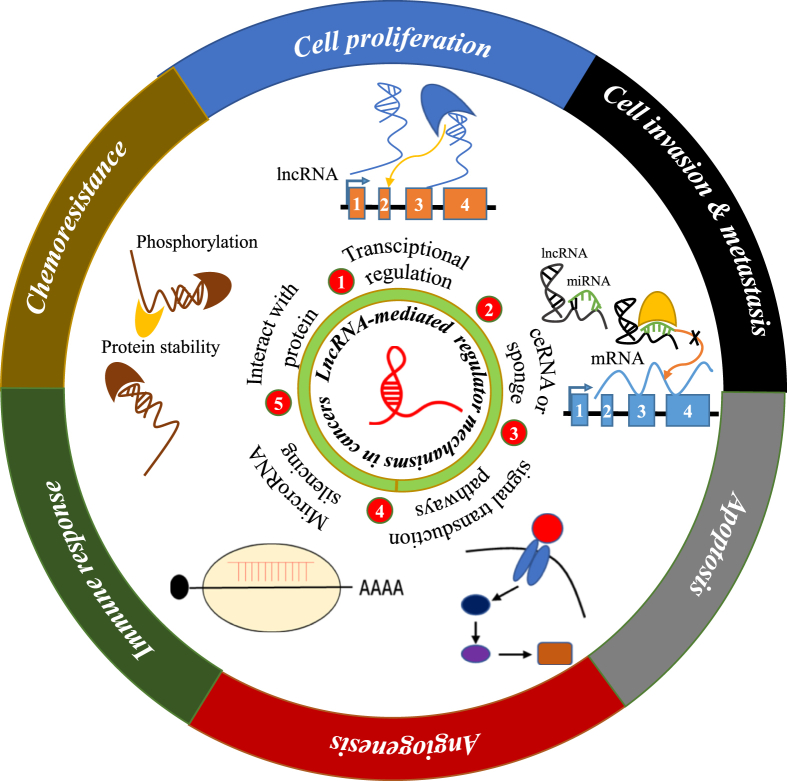
Various changes in gene expression contribute to the development of cancer. Such changes can lead to tumor development and metastasis by altering cell proliferation, invasion, and angiogenesis. Increasing evidence suggests that lncRNAs play a crucial role in cancer development by inducing the proliferation of tumor cells.
LncRNAs interact with chromatin remodeling complexes to induce heterochromatin formation at specific genomic loci, resulting in reduced expression of target genes. Thus, lncRNAs are key molecules in tumors as they regulate processes such as chromatin remodeling, transcription, and post-transcriptional processing.1,8,9
Concurrently, a wealth of studies in recent years have found that lncRNAs potentially function as endogenous sponges regulating miRNA expression and function. miRNAs bind to lncRNAs and regulate their stability. Competitive endogenous RNA (ceRNA) regulatory networks have also revealed that lncRNAs work as sponges by competitively binding to microRNAs and inhibiting their function.9 At the same time, the EMT process is regulated by lncRNAs. Of note, EMT confers the ability to metastasis and invasion on tumor cells, whereas lncRNAs act as ceRNAs to influence the occurrence of EMT, this subsequently influences the invasion and metastasis of malignant tumors.10 In recent years, more and more people have realized the target of RNA-Binding protein (RBPs), and believed that protein-RNA interaction is a key link in many cellular processes.11 In addition, the effect of LncRNA on multiple signaling pathways in cancer is also extremely important.
Several lncRNAs aberrantly expressed in different tumors promote malignant biological behaviors of tumor cells, such as proliferation, invasion, and metastasis. In addition, lncRNAs exhibit high tissue-specific stability and have diagnostic and prognostic potential as potential therapeutic targets and biomarkers.12 (Fig. 1).
Figure 1.
LncRNA-mediated molecular mechanisms in cancer.
Dysregulation of lncRNA CASC11 in different types of cancer
Overexpression of CASC11 in ovarian cancer, neuroblastoma, bladder cancer and esophageal adenocarcinoma
Ovarian cancer (OC) is a gynecological tumor characterized by high mortality and poses a serious threat to women's physical and mental health.13 Using quantitative polymerase chain reaction (qPCR) assay, studies have shown that CASC11 is up-regulated in OC. In vitro experiments have also demonstrated that overexpression of CASC11 can promote OC growth by inhibiting cancer cell apoptosis and promoting cancer cell proliferation.14 Besides, overexpression of CASC11 in ovarian squamous cell carcinoma mediates the resistance of cancer cells to chemotherapeutic drugs.15 Neuroblastoma (NB) is a common tumor in children, which develops from immature nerve cells.16 Real-time fluorescence quantitative PCR (qRT-PCR) analyses have revealed that CASC11 is overexpressed in NB. Notably, the progression of NB is inhibited via CASC11 silencing.17 Bladder cancer (BC) results from the malignant proliferation of bladder epithelium. Notably, BC affects the function of the urinary system and is prone to recurrence and metastasis.18 Compared to normal plasma, the expression of CASC11 in plasma of BC patients is significantly higher. Furthermore, the elevation of CASC11 significantly promotes the progression of BC, suggesting that CASC11 may serve as a new prognostic marker for BC.18,19 Esophageal adenocarcinoma (ECA) and esophageal squamous cell carcinoma (ESCC), collectively referred to as esophageal carcinoma (ECa), show varying degrees of dysphagia.20 The expression level of lncRNA in ECa tissues and adjacent normal tissues is detectable via QRTPCR, whereby the expression of CASC11 in ECa is significantly increased. Thus, it is suggested that high expression of CASC11 is associated with the pathological stage and poor prognosis of ECa.21
Overexpression of CASC11 in gastric cancer, glioma, colorectal cancer and lung cancer
Early gastric cancer GC is easily confused with some benign diseases of the stomach, so early and accurate diagnosis is highly important.22 QPCR and Transwell assay show that the expression of CASC11 in GC tissues is higher than that in paracancerous tissues, whereas overexpression of CASC11 potentially promotes the invasion and migration of GC cells.23,24 Glioma is a kind of tumor originating from abnormal proliferation of glial cells. Different cell types of glioma can be classified as astrocytoma, oligodendroglioma, and astrocytoma.25 Ectopic overexpression of CASC11 in glioma specimens and cells suggests poor prognosis in glioma patients.26 As a high-risk gastrointestinal tumor, the incidence of colorectal cancer (CRC) is on the rise.27 Studies have demonstrated that up-regulated CASC11 levels in CRC tissues are associated with tumor enlargement, serosal invasion, lymph node metastasis, and tumor lymph node metastasis (TNM) stage. Moreover, functional experiments show that CASC11 can promote the proliferation and metastasis of CRC cells in vivo and in vitro.28 Lung cancer (LC) is a kind of tumor with an incidence of more than 96%. It originates from the bronchial area of the lung and occurs either as a central type or peripheral type according to different parts of the bronchi.29 CASC11 is highly expressed in LC cell lines. In a study, after inhibition of CASC11 expression, the low proliferative capacity of LC cells was reported.30 Of note, in SCLC, overexpression of CASC11 elevates the stemness of SCLC cancer cells, an indication that the level of CASC11 expression may predict patient survival.31
Overexpression of CASC11 in hepatocellular carcinoma, cervical cancer and osteosarcoma
Hepatocellular carcinoma (HCC) is a heterogeneous tumor with multifactorial effects. Chronic hepatitis B virus (HBV) infection and cirrhosis are the two leading causes of HCC death in 45% of liver cancer-related deaths worldwide.32 Studies have demonstrated that the expression of CASC11 is up-regulated in tumor tissues of HCC patients. According to the Tumor Genome Atlas (TCGA) database and follow-up studies, high levels of CASC11 are significantly associated with poor survival. Further analysis has revealed that the loss of CASC11 function can effectively inhibit HCC cell migration, invasion, and epithelial–mesenchymal transition (EMT). It is suggested that CASC11 expression is a potential independent prognostic indicator in HCC patients.33,34 In addition, CASC11 down-regulation inhibits the viability of HCC cells after carboplatin treatment. This suggests that up-regulated CASC11 expression is associated with the formation of carboplatin resistance.35 HPV virus infection, sexual dysfunction, and precocious puberty are the leading causes of cervical cancer (CC). Multiple functions mediated by LncRNA have been widely reported in CC.36 Besides, the up-regulated expression of CASC11 is positively correlated with tumor size, FIGO stage, whereas it is negatively associated with patient survival in CC tissues and cell lines. Upon CASC11 silencing, the proliferation, migration, and invasion ability of CC cells are inhibited, whereas overexpression of CASC11 inhibits the apoptosis of CC cells.5 Osteosarcoma (OS) is a more prevalent malignant bone tumor in adolescents or children aged below 20 years, accounting for about 5% of childhood tumors.37 Clinical association analysis has demonstrated that the expression of CASC11 in OS tissues is significantly higher than that in normal tissues. In addition, clinical association analysis and gain-of-function and loss-of-function analysis show that high expression of CASC11 is not only closely associated with the clinical stage, distant metastasis, and poor prognosis of OS patients, but also promotes the migration, invasion, epithelial–mesenchymal transition (EMT), and metastasis of OS cells.38
LncRNA CASC11-mediated tumorigenesis
Upstream transcription factors regulating CASC11
CASC11 is overexpressed in multiple human cancers. To elucidate the different expression patterns of CASC11 in various cancers, we explored the upstream regulators of CASC11. Recent studies have shown that transcriptional activation is vital in up-regulation of lncRNA, the transcription factor SP1, and STAT3, which are key transcription factors regulating the expression of CASC11. Both can mediate the activation and overexpression of CASC11 in tumors. Transcription factors exert different functions in tumors, which leads to differential expression of CASC11. Based on the information retrieved from the online database software, SP1, a transcription factor, is abnormally upregulated in gliomas, which can bind to the CASC11 promoter and activate its transcription. Chip experiment further confirms that overexpression of SP1 activates CASC11 expression. What's more, analysis in StarBase has shown that miR-498 is a potential CASC11 target, whereas FOXK1 is the downstream miR-498 target. The absence of CASC11 inhibits FOXK1 expression and exerts an anti-tumor effect. As a result, SP1 mediates the activation of CASC11 and promotes glioma migration via miR-498 down-regulation and FOXK1 activation.26
Additionally, in hepatocellular carcinoma (HCC), Han et al revealed that STAT3, a signal transduction and transcription activator related to the interaction of tumor proliferation and differentiation cells, can activate CASC11. The activation of CASC11, in combination with EZH2, silence PTEN and promote the PI3K/AKT pathway, inducing tumorigenesis of HCC.34
In colorectal cancer, Zhang et al pointed out that CASC11 potentially activates WNT/β-catenin signaling in CRC by binding to hnRNP-K. The WNT/β-catenin pathway has previously been shown to be a classic pathway in cancer, an indication that CASC11 may be involved in CRC pathogenesis as a WNT signal regulator. Moreover, the promoter region of CASC11 binds directly to the oncogene c-Myc39 and enhances CASC11 expression by elevating histone acetylation in this region. Collectively, LncRNA CASC11, as an important diagnostic factor of CRC, has future prospects as an important strategy for clinical treatment of CRC.28
Downstream microRNA targets of CASC11 in diverse tumors
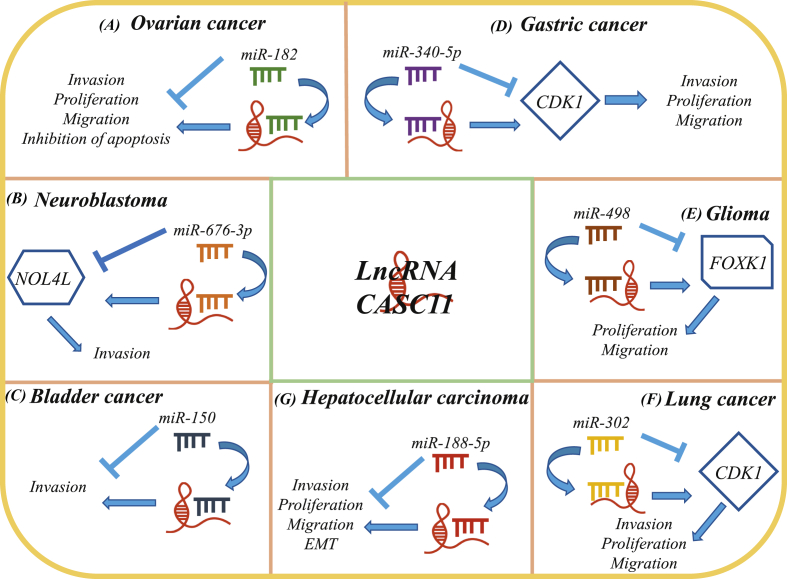
The mechanism by which CASC11 regulates the procession of tumors varies in how they associate with diverse microRNAs. Multiple microRNAs targeted by CASC11 have manifested regulatory roles in tumorigenesis, including miR-182, miR-637-3p, miR-150, miR-340-5p, miR-498, miR-302, and miR-188-5p.
Particularly, in ovarian cancer, miR-182, a well-known carcinogen in various tumors, is also significantly upregulated in OC tissues. Studies have revealed an interaction between CASC11 and miR-182 in OC, which has not been found in normal tissues. Further assessments demonstrate that CASC11 overexpression induces the expression of miR-182 and promotes the proliferation of OC cells. This suggests that CASC11 potentially interacts with miR-182 mediated by other factors. Notably, CASC11 has been as an adverse factor for OC, though the specific mechanism requires further elucidation.14
In NB, miR-676-3p is negatively regulated by CASC11, which is a highly potential target. Moreover, NOL4L, over-expressed in tumor tissue, is directly targeted by miR-676-3p. The deletion of CASC11 impairs the aggressiveness of NB, but this effect can be reversed by overexpression of NOL4L. Thus, CASC11 promotes the malignant transformation of NB mediated by NOL4L.40
MiR-150, a tumor suppressor and the most potent molecule, is significantly underexpressed in the plasma of bladder cancer (BC) patients. In BC patients, miR-150 is negatively regulated by CASC11. Interestingly, in healthy people, there is no significant correlation between miR-150 and CASC11 expression. Besides, overexpression of CASC11 enhances the invasiveness of BC cells mediated by miR-150 down-regulation. However, overexpressed levels of miR-150 impair the cancer-promoting effect induced via CASC11 overexpression. Thus, CASC11 may be a potential marker for early bladder cancer detection.19
Besides, cell cycle pathway analysis has revealed that in gastric cancer (GC), the cell cycle pathway involving CDK1 is activated. CDK1, a cyclin-dependent kinase, has been reported to mediate the proliferation of tumors induced by cell cycle regulation. Online tools such as TargetScan also show that miR-340-5p targets CASC11, whereas miR-340-5p can directly target CDK1. In GC, CDK1 is significantly upregulated, while miR-340-5p is inhibited substantially. Besides, overexpression of CDK1 demonstrates its ability to promote GC invasion; however, miR-340-5p overexpression weakens this cancer-promoting effect. In a nutshell, CASC11 induces the expression of CDK1 mediated by the inactivation of miR-340-5p, which promote the proliferation of GC.24
In Glioma, Jin J et al has revealed that miR-498 is a potential target of CASC11. The luciferase experiment shows that FOXK1 is the downstream target of miR-498 and is negatively regulated by miR-498. The absence of CASC11 inhibits the expression of FOXK1 and shows anti-tumor effect. Therefore, CASC11 promotes glioma migration mediated by the down-regulated miR-498 as well as the activation of FOXK1.26
In lung cancer (LC), Tong et al verified the down-expression of miRNA-302 as well as the over-expression of CDK1. They reported the expression and activation of CDK1in various cancers, and a higher expression of CDK1 implied a lower tumor survival rate. Dual-luciferase reporter assay demonstrated that miRNA-302 is the downstream factor of CASC11, whereas CDK1 is targeted directly by miRNA-302. microRNA-302 knockout reverses the inhibitory effect of CASC11 on CDK1 expression; thus CASC11 promotes tumorigenesis of LC by upregulating CDK1 expression mediated by miRNA-302.41
In hepatocellular carcinoma (HCC), miR-188-5p, which is significantly down-regulated, is targeted by CASC11. The miR-188-5p expression is negatively regulated by CASC11. Furthermore, the activation of miR-188-5p abolish the promotion of HCC cell proliferation by overexpressed CASC11. A study suggested that LncRNA CASC11 may promote tumorigenesis of HCC by inhibiting miR-188-5p.33 (Fig. 2, Table 1).
Figure 2.
LncRNA CASC11 facilitated tumor progression via sponging miRNAs in cancers. (A) Ovarian cancer: CASC11 overexpression induces the expression of miR-182 and promotes the proliferation of OC cells. (B) Neuroblastoma: CASC11 promotes the malignant transformation of NB mediated by NOL4L. (C) Bladder cancer: overexpression of CASC11 enhances the invasiveness of BC cells mediated by miR-150 down-regulation. (D) Gastric cancer: CASC11 induces the expression of CDK1 mediated by the inactivation of miR-340-5p. (E) Glioma: CASC11 promotes glioma migration mediated by the down-regulated miR-498 as well as the activation of FOXK1. (F) Lung cancer: CASC11 promotes tumorigenesis of LC by upregulating CDK1 expression mediated by miRNA-302. (G) Hepatocellular carcinoma: CASC11 may promote tumorigenesis of HCC by inhibiting miR-188-5p. Abbreviations: NOL4L: Nucleolar protein 4 like; CDK1: Cyclin-dependent kinase 1; FOXK1: Forkhead Box K1.
Table 1.
Methodologies for exploring the expression, distribution, and function of lncRNA CASC11.
| Research purpose | Assays |
|---|---|
| Genomic data | Tumor Genome Atlas (TCGA) database |
| Expression | Real-time fluorescence quantitative PCR (qRT-PCR) analyses quantitative polymerase chain reaction (qPCR) assay |
| Target | The luciferase experiment, Dual-luciferase reporter assay |
| Proliferation | Kaplan–Meier experiment |
| Apoptosis | |
| Migration and Invasion | Transwell assay |
| EMT | qRT-PCR |
Tumor regulation by CASC11 through other pathways
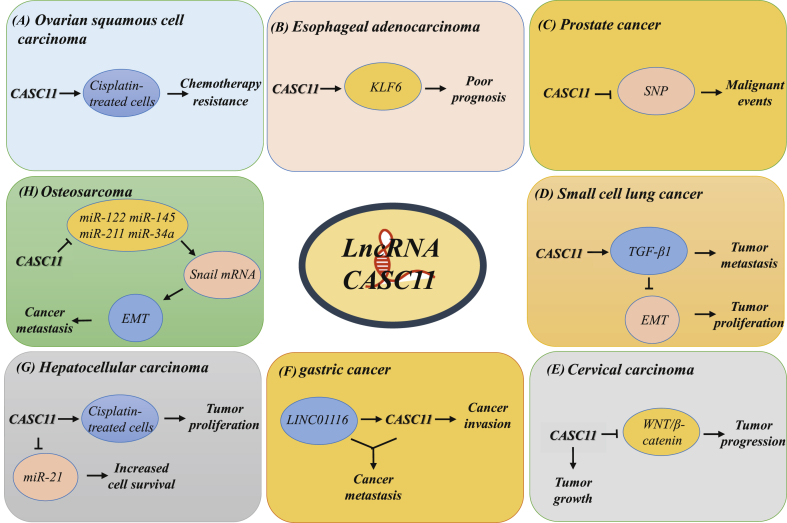
In terms of tumor regulation, CASC11 not only is dependent on the interaction with upstream and downstream microRNAs but can also mediate tumor development via numerous mechanisms. Such mechanisms include promoting chemical resistance, inhibiting the expression of transcription factor KLF6, interacting with single nucleotide polymorphism, activating signal pathways, enhancing the expression of the transforming factor, among others.
Oxaliplatin, tetraplatin, cisplatin, and carboplatin, which are clinically used to treat ovarian squamous cell carcinoma (OSCC), is quite effective in the early stage. But due to the severe drug resistance in the late stages, the treatment faces a considerable bottleneck. The expression of CASC11 is significantly higher after treatment with the above-drugs than pre-treatment, indicating that CASC11 may be involved in the chemical resistance of ovarian cancer. To verify the hypothesis that CASC11 led to the resistance of OSCC cells to cisplatin, OSCC cells were cultured at different concentrations of cisplatin. Compared to the normal group, the proliferation and invasion of OSCC cells with high concentrations of CASC11 were higher but could be is reversed by further CASC11 knockdown. Thus, CASC11 is a potential diagnostic indicator. However, the mechanism by which CASC11 contributes to OSCC resistance needs extensive exploration.15
In esophageal adenocarcinoma, KLF6, a zinc finger protein transcription factor that regulates cell growth and apoptosis, is significantly inhibited in ECa. Besides, CASC11 knockdown promotes the apoptosis of ECa cells by activating the expression of KLF6. Consequently, CASC11 may lead to the poor prognosis of ECa by regulating KLF6.42
In prostate cancer, Lin et al hold a perspective that two SNPs of CASC11 are associated with the susceptibility to PC. These two SNPs interact with other SNPs, causing malignant events in PC.40
Additionally, Fu et al revealed that lncRNA CASC11 and TGF-β 1 were positively correlated with the percentage of stem cells in SCLC cell lines, suggesting that the changes of CASC11 and TGF-β 1 could predict the survival rate of SCLC patients. Further experiments found that high expression of CASC11 mediated the upregulation of TGF-β 1 in SCLC cells, and this effect could be attenuated by TGF-β inhibitors but not by exogenous TGF-β 1. These findings show that TGF-β mediates tumor metastasis by inducing epithelial–mesenchymal transition, this contributes to the regulation of cancer cell stem cells. Additionally, the plasma TGF-β 1 mRNA in SCLC patients was significantly higher than that in healthy controls, indicating that the upregulation of TGF-β 1 is associated with the increase in SCLC stem cells. Notably CASC11 may mediate the prognosis of SCLC by up-regulating TGF-β1 to increase the number of cancer stem cells; thus, it is considered a valuable strategy for detecting the occurrence of small cell lung cancer.31
In cervical carcinoma, Hsu et al found that LncRNA CASC11 is not only abnormally highly expressed in CC tissues but also inhibits the growth of cancer cells after CASC11 knockout by treatment with siRNA technology. Additionally, the Kaplan–Meier experiment confirmed that the expression of CASC11 is closely related to the rate of tumor proliferation and the patient's survival rate. WNT signaling plays a vital role in various physiological processes in animals, but if the essential protein in this signaling pathway is mutated, causing abnormal activation of the signal, it may induce cancer. The study further confirmed that the overexpression of CASC11 could increase the activity of WNT/β-catenin. This suggested that the activity change of the WNT/β-catenin signaling pathway is correlated to the mechanism by which CASC11 promotes cervical cancer progression.5
In gastric cancer, LINC01116 and CASC11 coordinatively promote GC metastasis. The over-expression of LINC01116 and CASC11 has been revealed in GC tissues. Besides, LINC01116 activation induces the expression of CASC11, whereas its overexpression enhances the invasiveness of GC. Further, CASC11 knockdown exerts cytotoxic effects against GC cells.23
Elsewhere, Liu et al found that CASC11 could promote the proliferation of carboplatin-treated HCC cells. Through in vitro culture, overexpression of miR-21 and CASC11 both elevated the survival rate of carboplatin-treated HCC cells.35
In osteosarcoma (OS), Song et al demonstrated that CASC11 is associated with EMT inducer Snail mRNA and could enhance its stability. The binding of CASC11 to Snail mRNA blocks the Snail inhibition via miR-122, miR-145, miR-211, and miR-34a. Snail, a classical epithelial–mesenchymal transition (EMT) inducing molecule, has been widely reported to be abnormally highly expressed in various malignant tumor tissues. Among them, Snail-expressing tumor cells undergo EMT to acquire the invasive properties of mesenchymal-like cells and some characteristics of stem cells. Collectively, CASC11 is a potential new therapeutic target for OS as it binds to Snail mRNA, inhibiting its degradation, promoting OS metastasis and EMT transformation.43 (Fig. 3, Table 2).
Figure 3.
The molecular mechanisms of CASC11 in different human cancers. (A) Ovarian squamous cell carcinoma: CASC11 may mediate cisplatin resistance in OSCC cells. (B) Esophageal adenocarcinoma: CASC11 may lead to the poor prognosis of ECa by regulating KLF6. (C) Prostate cancer: SNPs of CASC11 are associated with the susceptibility to PC. (D) Small cell lung cancer: Activation of TGF-β1 is mediated by CASC11 and induces the EMT process, which in turn promotes tumor metastasis and proliferation. (E) Cervical carcinoma: the activity change of the WNT/β-catenin signaling pathway is correlated to the mechanism by which CASC11 promotes CC progression. (F) gastric cancer: LINC01116 and CASC11 coordinatively promote GC metastasis and invasion. (G) Hepatocellular carcinoma: CASC11 not only promotes the proliferation of HCC cells after carboplatin treatment, but also interacts with miR-21 to improve the survival rate of HCC cells. (H) Osteosarcoma: CASC11 binds to Snail mRNA, inhibiting its degradation, promoting OS metastasis and EMT transformation. KLF6: Zinc finger protein transcription; SNPs: Single nucleotide polymorphisms; TGF-β1: Transforming growth factor-β.
Table 2.
Tumor associated lncRNA CASC11 reported biological functions and the affected pathways.
| Cancer types | Biological significance | Genes/proteins/pathways | Property | Expression | References |
|---|---|---|---|---|---|
| Ovarian cancers | Invasion Proliferation migration Inhibition of apoptosis | miR-182 drug resistance | Oncogene | Up-Regulation | 13,14 |
| Neuroblastoma | Invasion | CASC11/miR-637-3p/NOL4L axis | Oncogene | Up-Regulation | 16,39 |
| Bladder cancer | Invasion | miR-150 | Oncogene | Up-Regulation | 17,18 |
| Esophageal adenocarcinoma | Invasion Inhibition of apoptosis | KLF6 | Oncogene | Up-Regulation | 20,41 |
| Gastric cancer | Invasion Proliferation Migration | LINC01116 CASC11/miR-340-5p/CDK1 axis | Oncogene | Up-Regulation | 22,23 |
| Prostate cancer | Invasion Migration | SNP | Oncogene | Up-Regulation | 39 |
| Glioma | Proliferation Migration | CASC11/miR-498/FOXK1 axis | Oncogene | Up-Regulation | 24,25 |
| Colorectal cancer | Invasion Proliferation Migration | hnRNP-K WNT/β-catenin c-Myc | Oncogene | Up-Regulation | 27,38 |
| Lung cancer | Invasion Proliferation Migration | CASC11/miR-302/CDK1 TGF-β1 | Oncogene | Up-Regulation | 29,30 |
| Hepatocellular carcinoma | Invasion Proliferation Migration EMT | CASC11/miR-188-5p STAT3/CASC11/PTEN/PI3K/AKT miR-21 | Oncogene | Up-Regulation | 32, 33, 34 |
| Cervical cancer | Invasion Proliferation Migration Inhibition of apoptosis | WNT/β-catenin | Oncogene | Up-Regulation | 36 |
| Osteosarcoma | Invasion Proliferation Migration Inhibition of apoptosis | CASC11/Snail mRNA | Oncogene | Up-Regulation | 37,42 |
Expression of lncRNA CASC11 in different tissues
LncRNA CASC11 not only mediates the regulation of many tumors but also has corresponding molecular mechanisms in the regulation of some non-tumor diseases. CASC11 can regulate the apoptosis and proliferation of vascular smooth muscle cells by downregulating the expression of cytokine IL-9, thereby improves atherosclerosis.44 When CASC11 is upregulated in osteoporosis, the occurrence of osteoporosis is enhanced via TNF-alpha upregulation.45 Of note, the downregulation of LncRNA CASC11 in coronary heart disease promotes the expression of TGF-β1. Overexpression of TGF-β1 promotes the development of CAD, regulated by downstream genes, such as sphingosine kinase 1 and tissue inhibitor of metalloproteinase-1, which consequently regulates cardiomyocyte behavior, such as apoptosis.46 The above mechanisms suggest that CASC11 shows great potential in the treatment of human diseases.
Conclusions and outlook
In summary, lncRNA CASC11, a characteristic cancer-associated lncRNA, regulating proliferation, autophagy, apoptosis, and other biological tumor processes that promotes metastasis. The transcription network of CASC11 in tumors evolution is mainly elucidated from upstream transcription factors and downstream microRNAs, among other mechanisms. CASC11 can promote the progression of various cancers, including ovarian cancer, neuroblast cancer, bladder cancer, esophageal adenocarcinoma, gastric cancer, prostate cancer, glioma, colorectal cancer, lung cancer, hepatocellular carcinoma, cervical cancer, osteosarcoma, etc. CASC11 mainly alters the WNT/β-catenin signaling pathway and epithelial-stromal transformation to promote tumor invasion. More importantly, there is a strong correlation between the expression of CASC11 and clinical prognosis, suggesting that CASC11 may be a therapeutic target for cancer management. Thus, CASC11 is a highly potential target in future studies on the impact of non-neoplastic diseases.
Conflict of interests
The authors declare that they have no conflict of interest.
Funding
The work was supported by the grants from National Natural Science Foundation of China (No. 81773959 to C.F. Yuan and No. 81974528 to C.F. Yuan), Open Foundation for Tumor Microenvironment and Immunotherapy Key Laboratory of Hubei province in China (No. 2019KZL09 to C.F. Yuan), Health commission of Hubei Province scientific research project in China (No. WJ2019H527 to C.F. Yuan), and the central government guides the special funds for the development of local science and technology (No. 2020ZYYD016 to C.F. Yuan).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2020.11.016.
List of abbreviations and acronyms
- LncRNA
Long non-coding RNA
- CASC11
Cancer susceptibility candidate 9
- OC
Ovarian cancer
- CRC
Colorectal cancer
- OSCC
Ovarian squamous cell carcinoma
- NB
Neuroblastoma
- NOL4L
Nucleolar protein 4 like
- FOXK1
Forkhead Box K1
- BC
Bladder cancer
- ESCC
Esophageal squamous cell carcinoma
- ECA
Esophageal adenocarcinoma
- EC
Esophageal carcinoma
- GC
Gastric cancer
- CDKs
Cyclin-dependent kinases
- CDK1
Cyclin-dependent kinase 1
- KLF6
Zinc finger protein transcription
- PC
Prostate cancer
- SNPs
Single nucleotide polymorphisms
- TMN
Tumor-node-metastasis
- SP1
Specific protein 1
- CHIP
Chromatin immunoprecipitation
- hnRNP-K
heterogeneous ribonucleoprotein K
- LC
Lung cancer
- TGF-β1
Transforming growth factor-β 1
- HCC
Hepatocellular carcinoma
- TCGA
The Cancer Genome Atlas
- EZH2
Enhancer of Zeste 2
- PTEN
Phosphatase and tensin homolog delet2ed on chromosome ten
- CC
Cervical carcinoma
- OS
Osteosarcoma
- EMT
Epithelial-mesenchymal transition
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bergmann J., Spector D. Long non-coding RNAs: modulators of nuclear structure and function. Curr Opin Cell Biol. 2014;26:10–18. doi: 10.1016/j.ceb.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang H., Yu T., Han Y., et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer. 2018;17(1):119. doi: 10.1186/s12943-018-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bian Z., Zhang J., Li M., et al. LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin Cancer Res. 2018;24(19):4808–4819. doi: 10.1158/1078-0432.CCR-17-2967. [DOI] [PubMed] [Google Scholar]
- 4.Su W., Guo C., Wang L., et al. LncRNA MIR22HG abrogation inhibits proliferation and induces apoptosis in esophageal adenocarcinoma cells via activation of the STAT3/c-Myc/FAK signaling. Aging. 2019;11(13):4587–4596. doi: 10.18632/aging.102071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu W., Liu L., Chen X., Zhang Y., Zhu W. LncRNA CASC11 promotes the cervical cancer progression by activating Wnt/beta-catenin signaling pathway. Biol Res. 2019;52(1):33–44. doi: 10.1186/s40659-019-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J., Du P., Cui P., et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37(30):4094–4109. doi: 10.1038/s41388-018-0250-z. [DOI] [PubMed] [Google Scholar]
- 7.Liu J., Kong D., Sun D., Li J. Long non-coding RNA CCAT2 acts as an oncogene in osteosarcoma through regulation of miR-200b/VEGF. Art Cell Nanomed Biotechnol. 2019;47(1):2994–3003. doi: 10.1080/21691401.2019.1640229. [DOI] [PubMed] [Google Scholar]
- 8.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhade V., Pal D., Kanduri C. Long noncoding RNA: Genome organization and mechanism of action. Adv Exp Med Biol. 2017;1008:47–74. doi: 10.1007/978-981-10-5203-3_2. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Li Z., Zheng W., et al. LncRNA-ATB: an indispensable cancer-related long noncoding RNA. Cell Prolif. 2017;50(6):12381. doi: 10.1111/cpr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrè F., Colantoni A., Helmer-Citterich M. Revealing protein-lncRNA interaction. Briefings Bioinf. 2016;17(1):106–116. doi: 10.1093/bib/bbv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tano K., Akimitsu N. Long non-coding RNAs in cancer progression. Front Genet. 2012;3:219–224. doi: 10.3389/fgene.2012.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guleria S., Jensen A., Toender A., Kjaer S.K. Risk of epithelial ovarian cancer among women with benign ovarian tumors: a follow-up study. Cancer Causes Control. 2020;31(1):25–31. doi: 10.1007/s10552-019-01245-4. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y., Shen G., Zhou D., Wu F. CASC11 overexpression predicts poor prognosis and regulates cell proliferation and apoptosis in ovarian carcinoma. Cancer Manag Res. 2020;12:523–529. doi: 10.2147/CMAR.S226801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen F., Feng L., Zhou J., et al. Overexpression of CASC11 in ovarian squamous cell carcinoma mediates the development of cancer cell resistance to chemotherapy. Gene. 2019;710:363–366. doi: 10.1016/j.gene.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Ward E., DeSantis C., Robbins A., Kohler B., Jemal A. Childhood and adolescent cancer statistics, 2014. CA Canc J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 17.Yu Z., Zhang J., Han J. Silencing CASC11 curbs neonatal neuroblastoma progression through modulating microRNA-676-3p/nucleolar protein 4 like (NOL4L) axis. Pediatr Res. 2019;87(4):662–668. doi: 10.1038/s41390-019-0625-z. [DOI] [PubMed] [Google Scholar]
- 18.Amir H., Khan M.A., Feroz S., et al. CARLo-7-A plausible biomarker for bladder cancer. Int J Exp Pathol. 2019;100(1):25–31. doi: 10.1111/iep.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo H., Xu C., Le W., Ge B., Wang T. LncRNA CASC11 promotes cancer cell proliferation in bladder cancer through miRNA-150. J Cell Biochem. 2019;120(8):13487–13493. doi: 10.1002/jcb.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vja K. Reports SRJCg. Barrett’s esophagus and esophageal carcinoma: can biomarkers guide clinical practice? Curr Gastroenterol Rep. 2019;21(4):14–22. doi: 10.1007/s11894-019-0685-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen S.G., Wang C.H., He R.Q., Xu R.Y., Ji C.B. LncRNA CASC11 promotes the development of esophageal carcinoma by regulating KLF6. Eur Rev Med Pharmacol Sci. 2019;23(20):8878–8887. doi: 10.26355/eurrev_201910_19283. [DOI] [PubMed] [Google Scholar]
- 22.Lai Y., Xu P., Liu J., et al. Decreased expression of the long non-coding RNA MLLT4 antisense RNA 1 is a potential biomarker and an indicator of a poor prognosis for gastric cancer. Oncol Lett. 2017;14(3):2629–2634. doi: 10.3892/ol.2017.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su X., Zhang J., Luo X., et al. LncRNA LINC01116 promotes cancer cell proliferation, migration and invasion in gastric cancer by positively interacting with lncRNA CASC11. OncoTargets Ther. 2019;12:8117–8123. doi: 10.2147/OTT.S208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Kang W., Lu X., Ma S., Dong L., Zou B. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle. 2018;17(15):1886–1900. doi: 10.1080/15384101.2018.1502574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Ma Q., Long W., Xing C., et al. Cancer stem cells and immunosuppressive microenvironment in glioma. Front Immunol. 2018;9:2924–2936. doi: 10.3389/fimmu.2018.02924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin J., Zhang S., Hu Y., Zhang Y., Guo C., Feng F. SP1 induced lncRNA CASC11 accelerates the glioma tumorigenesis through targeting FOXK1 via sponging miR-498. Biomed Pharmacother. 2019;116:118968–118975. doi: 10.1016/j.biopha.2019.108968. [DOI] [PubMed] [Google Scholar]
- 27.Rao X., Wang J., Song H.M., Deng B., Li J.G. KRT15 overexpression predicts poor prognosis in colorectal cancer. Neoplasma. 2020;67(2):410–414. doi: 10.4149/neo_2019_190531N475. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z., Zhou C., Chang Y., et al. Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/beta-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett. 2016;376(1):62–73. doi: 10.1016/j.canlet.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 29.W N., J Q., Md X., et al. Prognostic and predictive value of blood tumor mutational burden in patients with lung cancer treated with docetaxel. J Natl Compr Cancer Netw. 2020;18(5):582–589. doi: 10.6004/jnccn.2019.7383. [DOI] [PubMed] [Google Scholar]
- 30.Tong W., Han T.C., Wang W., Zhao J. LncRNA CASC11 promotes the development of lung cancer through targeting microRNA-302/CDK1 axis. Eur Rev Med Pharmacol Sci. 2019;23(15):6539–6547. doi: 10.26355/eurrev_201908_18539. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y., Zhang P., Nan H., et al. LncRNA CASC11 promotes TGF-β1, increases cancer cell stemness and predicts postoperative survival in small cell lung cancer. Gene. 2019;704:91–96. doi: 10.1016/j.gene.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Pinter M., Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;48(6):598–609. doi: 10.1111/apt.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng N., Wu J., Yin M., et al. LncRNA CASC11 promotes cancer cell proliferation in hepatocellular carcinoma by inhibiting miRNA-188-5p. Biosci Rep. 2019;39(4):1–12. doi: 10.1042/BSR20190251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Y., Chen M., Wang A., Fan X. STAT3-induced upregulation of lncRNA CASC11 promotes the cell migration, invasion and epithelial-mesenchymal transition in hepatocellular carcinoma by epigenetically silencing PTEN and activating PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2019;508(2):472–479. doi: 10.1016/j.bbrc.2018.11.092. [DOI] [PubMed] [Google Scholar]
- 35.Liu H., Liu T., Zhou Y., Song X., Wei R. Overexpression of long non-coding RNA cancer susceptibility 11 is involved in the development of chemoresistance to carboplatin in hepatocellular carcinoma. Oncol Lett. 2020;19(3):1993–1998. doi: 10.3892/ol.2020.11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P., Zhang W., Chen Y., Zheng X., Yang D. Comprehensive analysis of aberrantly expressed long non-coding RNAs, microRNAs, and mRNAs associated with the competitive endogenous RNA network in cervical cancer. Mol Med Rep. 2020;22(1):405–415. doi: 10.3892/mmr.2020.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meazza C., Bastoni S., Scanagatta P. What is the best clinical approach to recurrent/refractory osteosarcoma? Expet Rev Anticancer Ther. 2020;20(5):415–428. doi: 10.1080/14737140.2020.1760848. [DOI] [PubMed] [Google Scholar]
- 38.Song K., Yuan X., Li G., Ma M., Sun J. Long noncoding RNA CASC11 promotes osteosarcoma metastasis by suppressing degradation of snail mRNA. Am J Canc Res. 2019;9(2):300–311. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Kajino T., Shimamura T., Gong S., et al. Divergent lncRNA MYMLR regulates MYC by eliciting DNA looping and promoter-enhancer interaction. EMBO J. 2019;38(17):e98441. doi: 10.15252/embj.201798441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin H.Y., Callan C.Y., Fang Z., Tung H.Y., Park J.Y. Interactions of PVT1 and CASC11 on prostate cancer risk in African Americans. Cancer Epidemiol Biomark Prev. 2019;28(6):1067–1075. doi: 10.1158/1055-9965.EPI-18-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong W., Han T.C., Wang W., Zhao J. Medical ZJJErf, sciences p. LncRNA CASC11 promotes the development of lung cancer through targeting microRNA-302/CDK1 axis. Eur Rev Med Pharmacol Sci. 2019;23(15):6539–6547. doi: 10.26355/eurrev_201908_18539. [DOI] [PubMed] [Google Scholar]
- 42.Chen S.G., Wang C.H., He R.Q., Xu R.Y., Ji C.B. Medical JCJErf, sciences p. LncRNA CASC11 promotes the development of esophageal carcinoma by regulating KLF6. Eur Rev Med Pharmacol Sci. 2019;23(20):8878–8887. doi: 10.26355/eurrev_201910_19283. [DOI] [PubMed] [Google Scholar]
- 43.K S., X Y., G L., M M. Research SJJAjoc. Long noncoding RNA CASC11 promotes osteosarcoma metastasis by suppressing degradation of snail mRNA. Am J Cancer Res. 2019;9(2):300–311. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Tao K., Hu Z., Zhang Y., Jiang D., Cheng H. LncRNA CASC11 improves atherosclerosis by downregulating IL-9 and regulating vascular smooth muscle cell apoptosis and proliferation. Biosci Biotechnol Biochem. 2019;83(7):1284–1288. doi: 10.1080/09168451.2019.1597621. [DOI] [PubMed] [Google Scholar]
- 45.Yu H., Zhou W., Yan W., Xu Z., Xie Y., Zhang P. LncRNA CASC11 is upregulated in postmenopausal osteoporosis and is correlated with TNF-alpha. Clin Interv Aging. 2019;14:1663–1669. doi: 10.2147/CIA.S205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J., Dang J. LncRNA CASC11 was downregulated in coronary artery disease and inhibits transforming growth factor-beta 1. J Int Med Res. 2020;48(3):1–9. doi: 10.1177/0300060519889187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.