Abstract
Endochondral bone formation is an important route for bone repair. Although emerging evidence has revealed the functions of long non-coding RNAs (lncRNAs) in bone and cartilage development, the effect of lncRNAs in endochondral bone repair is still largely unknown. Here, we identified a lncRNA, named Hypertrophic Chondrocyte Angiogenesis-related lncRNA (HCAR), and proved it to promote the endochondral bone repair by upregulating the expression of matrix metallopeptidase 13 (Mmp13) and vascular endothelial growth factor α (Vegfa) in hypertrophic chondrocytes. Lnc-HCAR knockdown in hypertrophic chondrocytes restrained the cartilage matrix remodeling and decrease the CD31hiEmcnhi vessels number in a bone repair model. Mechanistically, we proved that lnc-HCAR was mainly enriched in the cytoplasm using fluorescence in situ hybridization (FISH) assay, and it acted as a molecular sponge for miR-15b-5p. Further, in hypertrophic chondrocytes, lnc-HCAR competitively bound to miR-15b-5p to increase Vegfa and Mmp13 expression. Our results proved that lncRNA is deeply involved in endochondral bone repair, which will provide a new theoretical basis for future strategies for promoting fracture healing.
Keywords: Bone marrow mesenchymal stem cells, Bone repair, Chondrocyte, Enchondral bone repair, Long non coding RNA
Abbreviations: lncRNA, long no-coding RNA; HCAR, hypertrophic chondrocyte angiogenesis-related lncRNA; Mmp13, matrix metallopeptidase 13; Vegfa, vascular endothelial growth factor α; OA, osteoarthritis; Runx2, runt-related transcription factor 2; Hoxa2, homeobox A2; Mef2c, myocyte enhancer factor 2C; Osx, osterix; BMSCs, bone marrow mesenchymal stem cells
Introduction
Bone healing is a remarkable process that can deliver fully functional and integrated new tissue, without scar formation. However, 10% of all fractures do not completely heal, resulting in failed bridging of the bone defect, called a non-union. The bone defect and non-union always show impaired endochondral ossification, which is the primary bone formation route in adult bone repair. Endochondral bone repair is characterized by bone replacing cartilage, which is mediated by hypertrophic chondrocytes.1,2 However, the gene regulation of hypertrophic chondrocytes in endochondral bone repair is still largely unknown. In recent years, long non-coding RNAs (lncRNAs) have become a hotspot for gene regulation. Although emerging evidence reveals the functions of long non-coding RNAs (lncRNAs) in bone and cartilage development,3 the effect of lncRNAs in endochondral bone repair have not been demonstrated.
Endochondral bone repair relies on the cartilage to bone transition, characterized by matrix remodeling and endochondral angiogenesis. Cartilage matrix remodeling mediated by matrix metallopeptidase 13 (MMP13) expressed by hypertrophic chondrocytes is a prerequisite for the recruits of vascular endothelial cells, precursor osteoclasts, and osteogenic progenitor cells.4 The cartilage matrix degraded by MMP13 forms canals and cavities. Until then, the blood vessels invade the cartilaginous matrix and bring precursor osteogenic progenitor cells for bone formation. In the meantime, the differentiation of hypertrophic chondrocytes is accompanied by the expression of angiogenic growth factors, especially vascular endothelial growth factor (VEGF), allowing the blood vessels to invade the cartilage.5
Research has evidenced that VEGF secreted by hypertrophic chondrocytes was precisely coupled to hypertrophic cartilage remodeling, ossification and angiogenesis.6 In metaphysis, recent studies revealed that CD31hiEmcnhi vessels couple angiogenesis and osteogenesis in during bone formation.7 The CD31hiEmcnhi capillaries located distal to the skeletal artery network may represent a central building block of the metabolically specific tissue environment with the privilege of obtaining oxygen and nutrients, which may affect the growth potential and metabolism of other cell types during endochondral ossification.8
Based on the above, hypertrophic chondrocytes are the bridge of cartilage to bone transition. More systematic studies on the controls of angiogenesis-related genes in hypertrophic chondrocytes may modulate endochondral ossification. LncRNAs are transcripts longer than 200 nucleotides that are not translated into proteins and are involved in biological regulation at multiple levels.9,10 Evidence has revealed that the lncRNAs participate in the bone and cartilage development. For example, ROCR, a lncRNA is specifically expressed in the cartilage, regulates SOX9 expression. SOX9 expression is significantly reduced in the absence of ROCR, and overexpression of SOX9 rescues MSC differentiation to chondrocytes.11 LncRNAs are demonstrated to regulate the degradation of the extracellular matrix of osteoarthritis (OA) chondrocytes. After knockdown of lncRNA-CIP, the formation of collagen and aggrecan in cartilage ECM increase, and the expression of related genes such as MMP13 and ADAMTS5 in matrix degradation decrease.12 However, little is known about the function of lncRNAs on gene regulation of hypertrophic chondrocytes and its role in endochondral bone repair.
In this study, we identified a lncRNA highly expressed in hypertrophic chondrocytes and named it “Hypertrophic Chondrocyte Angiogenesis-related lncRNA (HCAR)” for consideration of its effect on angiogenesis during endochondral bone repair in mouse bone repair model. The function and mechanism of lnc-HCAR on endochondral bone repair have been demonstrated in the present study, which provides a theoretical basis for exploring new therapeutic strategies for bone defects and non-union.
Materials and methods
Cells and reagents
Four-week-old Balb/c mice (male and female) were from the Laboratory Animals Center of Army Medical University (Third Military Medical University, Chongqing, China). All experiments in this research were carried out according to the Third Military Medical University Sciences Guide for Laboratory Animals. Mouse bone marrow-derived mesenchymal stem cells (BMSCs) were isolated from tibia and femur as described.13 After euthanization of Balb/c mouse, we collected bone marrow cells from femur and tibia with aseptic operation, then cultured them in minimum essential medium (DMEM; Gbico, Waltham, MA, USA) supplemented with 100U/ml penicillin (Sigma–Aldrich, St. Louis, MO, USA), 100 g/ml streptomycin sulfate (Sigma–Aldrich) and 20% fetal bovine serum (FBS; Gbico) at 37 °C in a 5% CO2 humidified incubator. After 72 h, we removed non-adherent cells and cultured adherent cells for an additional 7d with a single media change.
Chondrogenic and hypertrophic differentiation in vitro
For chondrogenic and hypertrophic differentiation,14 1 × 106 BMSCs were placed in polypropylene tubes, centrifuge at a speed of 500 g for 20 min. Then remove the medium and add 500 μL of low-glucose DMEM supplemented with 10−7 M dexamethasone (Sigma–Aldrich), 1%(v/v) ITS (Sigma–Aldrich), 50 μM ascorbate-2-phosphate (Sigma–Aldrich), 1 mM sodium pyruvate, 50 μg/ml proline (Sigma–Aldrich) and 20 ng/ml TGFβ3. Change the medium three times a week and culture for 14d. Then, the serum-free chondrogenic medium was replaced with hypertrophic medium supplemented with 50 nM thyroxine (Sigma–Aldrich), 10−8 M dexamethasone, 250 μM ascorbate-2-phosphate, 1 mM sodium pyruvate and 50 μg/ml proline. Change the medium three times a week and culture for another 14d.
Plasmids and lentivirus production
To construct a lentiviral vector for lnc-HCAR expression, the full length of HCAR were chemically synthesized and inserted into the pLVX-puro plasmid (MiaolingBio, Wuhan, China).
To construct a lentiviral vector for the lnc-HCAR knockdown, the lnc-HCAR targeting sequence for shRNA (5′- ATCACTTTATGTCGTGTATGAA -3′) were designed according to the previous study.15 Then, DNA oligonucleotides were chemically synthesized and annealed to form double-stranded DNA fragments that were inserted into the pLKO.1 lentivirus vector (Addgene).
To construct Cola10a1-driven shRNA vector, the mouse Col10a1 promoter, including 1000 bp upstream from transcription initiation site, first exon and the first intron (25), were cloned from the genomic DNA of mouse C3H10T1/2 cells by PCR and inserted into pLVX-puro to replace the CMV promoter. Then, the framework of the GFP tandem hairpin structure was chemically synthesized and added behind the Col10a1 promoter.
For lentivirus production, the psAX2, pMD2.G, and pLKO.1-shHCAR or pLVX-HCAR-puro or Col10a1-shHCAR were co-transfected into the 293FT cells (Invitrogen, Waltham, MA, USA).
Femoral fracture repair model and histological staining
The study was conducted according to the Third Military Medical University Sciences Guide for Laboratory Animals. Twelve adults male Wistar rats weighing 250–280 g were used. Rats were randomly divided into two groups (n = 6/group): 1 experimental group and one control group. One week before the start of the experiment, the rats were housed in cages with three rats. For the experimental procedure, in brief, the rats were anesthetized with an intraperitoneal injection of 0.5% sodium pentobarbital, and then the knee to the greater trochanter side incision was made through the shaved skin and the right fascia. The plane between the blood vessel and the hamstring was opened by blunt dissection to expose the femur, and the right femur of each animal was fractured using a 3-point bending device and stabilized using a syringe needle. The fascia and skin are closed with absorbable sutures. Three days after surgery, 100 μL of shHCAR lentivirus with a titer of 106 was injected into the fracture area of the experimental group. The control group was injected with the same volume of shNC lentivirus. After 2w, the fracture site was taken, and the cross section was sectioned. The samples were then fixed in 4% formalin for 24 h and decalcified in 10% EDTA solution for four weeks. A total of 3 sections of each animal were randomly selected for paraffin embedding and used for and used for Safranin O-fast green and Masson staining.
Real-time quantitative PCR
Total RNA extraction was used RNAiso Plus reagent (Takara, Kusatsu, Shiga, Japan). cDNA was prepared from 1 μg of total RNA using PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara) according to the manufacturer's instructions. The PCR amplifications were carried out using specific primers for each gene as follows:
Hoxa2(F) 5′-CTGAGTGCCTGACATCTTTTCC -3′, (R) 5′-GTGTGAGCGTCGAGGTCTT -3′; Mef2c (F) 5′-ATCCCGATGCAGACGATTCAG -3′, (R) 5′-AACAGCACACAATCTTTGCCTA -3′; Runx2 (F) 5′-ATGCTTCATTCGCCTCACAAA -3′, (R) 5′-GCACTCACTGACTCGGTTGG -3′; Col10a1(F) 5′-TTCTGCTGCTAATGTTCTTGACC -3′, (R) 5′-GGGATGAAGTATTGTGTCTTG GG -3′;Mmp13 (F) 5′-CTTCTTCTTGTTGAGCTGGACTC -3′, (R) 5′-CTGTGGAGGTCACTGTAGACT -3′; Osx (F) 5′-ATGGCGTC CTCTCTGCTTG -3′, (R) 5′-TGAGGTCAGCGTATGGCTT -3′; Vegfa (F): 5′-CTGCCGTCCGATTGAGACC -3′, (R): 5′-GCTGC CTCTTCCTTCTAC -3′.
Western blots
Cells were lysed in cell lysis buffer (Beyotime Biotechnology, Shanghai, China). Twenty microgram of protein samples were subjected to SDS-PAGE for electrophoresis. Next, the proteins were transferred to PVDF membranes (Merck Millipore, Germany). After blocking in 5% skim milk for 2 h, membranes were incubated with rabbit antibodies against Runx2 (1:1000; Abcam, Cambridge, MA, USA), Hoxa2 (1:1000; CST, Danvers, MA, USA), Collagen X (1:1000, Abcam), Mmp13(1:1000, Abcam), Vegf (1:1000, CST), Osx (1:1000, CST), Mef2c (1:1000, CST), and β-actin (1:1000, Bioss, Wuhan, Hubei, China) overnight at 4 °C. Then, the membranes were incubated for 2 h with secondary antibody (1:1000, Bioss) at room temperature. After washing in TBST, chemiluminescent signals were detected using Bio-Rad Molecular Imager ChemiDocTM XRS+ system (Bio-Rad, Hercules, CA, USA). β-actin was used as loading control.
Fluorescence in situ hybridization (FISH)
The FISH probe sequence is labeled with biotin at the 5′ end. The hybrid kit was purchased from RiboBio Co., Ltd. (Guangzhou, China). All procedures are performed according to the kit instructions. Hybridization of lnc-HCAR in hypertrophic chondrocytes seeded on confocal dishes using the HCAR FISH probe. Before observed under confocal microscopy, the nuclei were stained with Hoechst 33342 Solution (20 mM; Thermo Scientific Pierce, Waltham, MA, USA).
Dual-luciferase reporter assay
The miR-15b-5p target binding sequence in wild-type lnc-HCAR (HCAR) and a mutant (HCAR-mut, with mutation of individual bases in the binding site) were synthesized and cloned downstream of the dual luciferase reporter system in psiCheck 2.0 luciferase plasmid (Promega, Madison, WI, USA). To determine whether miR-15b-5p directly targets Vegfa 3′-UTR and the Mmp13 3′-UTR, the dual luciferase reporter plasmid psiCheck 2.0 containing the upstream and downstream 200bp length of Mmp13 or Vegfa 3′UTR with miR-15b-5p wide type binding site or the mutation site were constructed. The above plasmids and miRNA mimics (NC and miR15b) were co-transfected into cells. Firefly and Renilla luciferase activities were measured consecutively by Dual Luciferase Assay kit (Promega) at 48 h after transfection. All experiments were repeated five times.
Statistical analysis
The unpaired, two-tailed Student's t-tests were used for comparisons between two groups and one-way analysis of variance (ANOVA) with Bonferroni post hoc test for multiple comparisons. For all experiments, P < 0.05 were considered to be significant and indicated by ‘∗’; P < 0.01 were indicated by ‘∗∗’.
Results
The expression of lnc-HCAR is associated with chondrocyte hypertrophy
In previous studies, we found that a large number of differentially expressed lncRNAs are involved in the differentiation of MSCs into chondrocyte and hypertrophic chondrocytes. We focused on NONMMUT038035 located on mouse chromosome 2:75,808,120–675,809,467 (+strand, mm10) because it displayed markedly higher expression in the hypertrophic differentiation in this study (Fig. 1A, B). To verify the microarray results, we used qPCR to detect lnc-HCAR expression during chondrocytes hypertrophy. The level of lnc-HCAR showed 4.3-fold increase in hypertrophic chondrocytes, which was consistent with the microarray assay (Fig. 1C). To investigate the correlation between NONMMUT038035 and hypertrophic cartilage-related gene expression, we performed an expression correlation analysis using the ChIPBase v2 website (http://rna.sysu.edu.cn/chipbase/). We found that gm13669 has a positive expression related to hypertrophic cartilage-related genes, including Vegfa, Mmp13, and Runx2 (Fig. 1D).
Figure 1.
The lnc-HCAR is associated with chondrocyte hypertrophy. (A) The heat map that clusters the results of the microarray. The lnc-HCAR markedly increased in hypertrophic differentiation. (B) Schematic representation of HCAR on the genome. (C) Quantitative PCR analysis of lnc-HCAR during hypertrophic differentiation. (D) Correlation analysis between lnc-HCAR levels and hypertrophic chondrocytes-related genes using ChIPBase v2 database.Data are shown as mean ± s.d. ∗P < 0.05, ∗∗P < 0.01. “NONMMUT038035” and “ENSMUST00000122366.1” are the same transcript (lnc-HCAR) of the “Gm13669” gene.
The lnc-HCAR up-regulates Vegfa and Mmp13 expression in hypertrophic chondrocyte
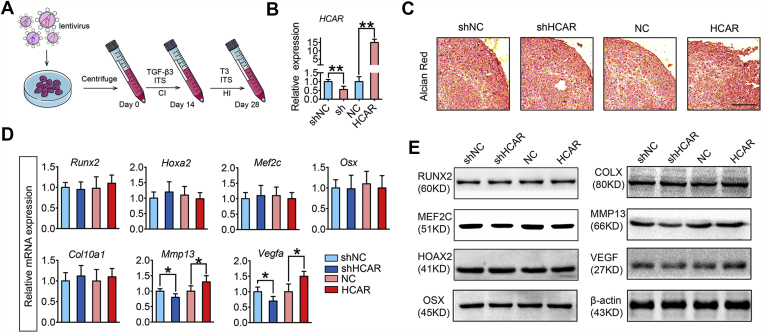
To investigate whether lnc-HCAR regulates chondrocyte hypertrophy during endochondral ossification, we knocked down or overexpressed lnc-HCAR in bone marrow mesenchymal stem cells (BMSCs) with lentiviruses. The cell pellet culture method is used to induce hypertrophic differentiation in vitro, which is an ideal model for the in vitro recapitulation of chondrocyte hypertrophic differentiation during endochondral ossification. Marker genes of hypertrophic chondrocyte were detected using qPCR and Western blot analysis. The qPCR results showed that there were no significant differences in chondrocyte hypertrophic differentiation-related genes, such as runt-related transcription factor 2 (Runx2), homeobox A2 (Hoxa2), myocyte enhancer factor 2C (Mef2c) and osterix (Osx), after lnc-HCAR overexpression or knockdown. The Western blot results of the above marker genes were consistent with qPCR, and no significant difference was observed after overexpression or knockdown of lnc-HCAR. However, Vegfa and MMP13, two essential genes involved in the remodeling and angiogenesis of cartilage matrix in endochondral ossification, both changed in mRNA and protein levels after the lnc-HCAR knockdown. Then lnc-HCAR was overexpressed, the expression levels of Vegfa and MMP13, both in mRNA and protein, were significantly increased compared with the control group. In contrast, knockdown of lnc-HCAR resulted in a decrease in both Vegfa and MMP13 gene expression compared with the control group. Further, to investigate the effect of lnc-HCAR in matrix mineralization in vitro, we performed alizarin red staining on slides of cell pellets. Alizarin red staining results showed that overexpression or knockdown of lnc-HCAR did not make a significant difference in the intensity of alizarin red staining (Fig. 2C). Based on the above observations, we concluded that lnc-HCAR has no effect on chondrocyte hypertrophic differentiation and matrix mineralization in vitro, but promotes Vegfa and Mmp13 expression in chondrocyte hypertrophic differentiation during endochondral ossification. We inferred that lnc-HCAR might promote the remodeling of cartilage-derived angiogenesis and collagen X-riched hypertrophic cartilage matrix by promoting the expression of Vegfa and Mmp13.
Figure 2.
lnc-HCAR modulates Vegfa and Mmp13 in hypertrophic chondrocytes during the chondrocytes hypertrophy without regulate matrix mineralization in vitro.(A) Schematic diagram of the model for hypertrophic differentiation in vitro. (B) Quantitative analysis of the expression level of lnc-HCAR by qPCR after lentiviral knockdown or overexpression of HCAR. (C) Representative images of Alizarin red staining of BMSCs pellet culture during hypertrophic differentiation. Scale bar, 200 μm. (D) Quantitative PCR analysis of hypertrophic marker genes after hypertrophic induction. (E) Representative Western blot images of hypertrophic marker genes after hypertrophic induction. Data are shown as mean ± s.d. ∗ P < 0.05, ∗∗P < 0.01.
Knockdown of lnc-HCAR restrains the endochondral bone repair
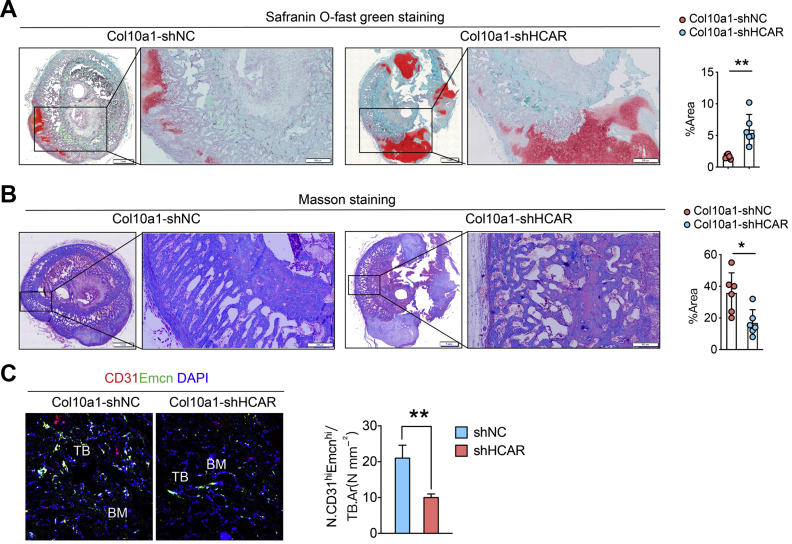
To investigate the role of lnc-HCAR in endochondral bone repair in vivo, we used Col10a1-specific promoter lentivirus to knock down lnc-HCAR expression in hypertrophic chondrocytes in a fracture repair model. The degradation of the cartilage matrix was detected by Safranin O-fast green staining. The results showed that Safranin O staining intensities were significantly increased after knockdown the lnc-HCAR expression in hypertrophic chondrocytes (Fig. 3A). Consistently, the results of Masson staining showed a significant decrease in the new bone formation after silencing lnc-HCAR (Fig. 3B). The above results indicated that lnc-HCAR is required for the endochondral ossification. The CD31hiEmcnhi endothelial cells were also detected using immunofluorescence to obverse the effect of lnc-HCAR on endochondral angiogenesis. The results showed that after knockdown the lnc-HCAR in hypertrophic chondrocytes, the number of CD31hiEmcnhi endothelial cells decreased significantly (Fig. 3C).
Figure 3.
Specific knockdown of lnc-HCAR in hypertrophic chondrocytes delayed remodeling of cartilage matrix and attenuates angiogenesis of CD31hiEmcnhi vessels in endochondral ossification during fracture repair. Knockdown lentivirus containing the Col10a1-specific promoter was used to specifically knock down the lnc-HCAR in hypertrophic chondrocytes in hypertrophic chondrocytes in fracture repair model. (A) Representative images and quantitative analysis of Safranin O-fast green staining in the cross section of fracture area in each group. Scale bar, 500 μm. (B) Representative images and quantitative analysis of Masson staining in the cross section of fracture area in each group. Scale bar, 200 μm. (C) Representative images of CD31 (red) and Emcn (green) immunostaining with quantification of CD31hiEmcnhi (yellow) cells. Scale bar, 50 mm. Data are shown as mean ± s.d. ∗P < 0.05, ∗∗P < 0.01.
The lnc-HCAR acts as a sponge for miR-15b-5p
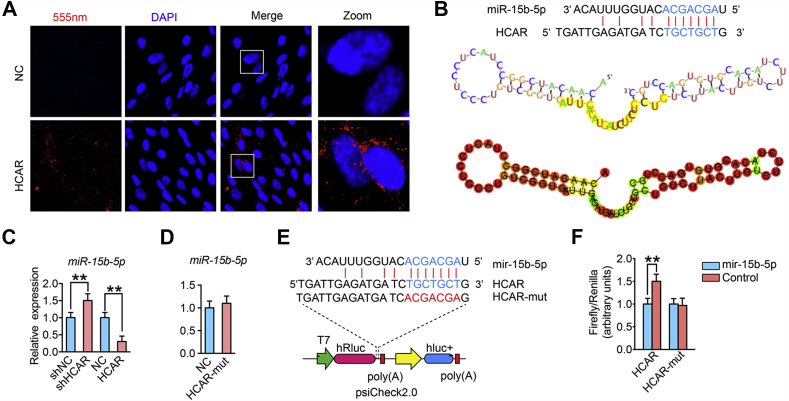
Since lncRNA functionality depends on intracellular localization, the cellular sublocalization of lnc-HCAR was determined using specific fluorescence in situ hybridization (FISH) probe. According to the results of the FISH assay, lnc-HCAR was mainly distributed in the cytoplasm, suggesting that lnc-HCAR may act as a miRNA sponge to reduce their inhibition effect on target mRNAs (Fig. 4A). We predicted miRNAs that may bind to lnc-HCAR by using the RegRNA2.0 (regrna2.mbc.nctu.edu.tw/detection.html) website with setting limits for a score and free energy to >150 and <15, respectively. In all results, we focused on the possible binding of miR-15b-5p to lnc-HCAR with a score of 152 and free energy of 12. Due to the more relaxed secondary structure of lnc-HCAR at the miR-15b-5p binding site, miR-15b-5p has more potential for lnc-HCAR binding than other microRNAs (Fig. 4B). The secondary structure near the miR-15b-5p binding site in lnc-HCAR is shown in Fig. 4B.
Figure 4.
lnc-HCAR acts as a sponge of miR-15b-5p. (A) Confocal representative image of lnc-HCAR fluorescence in situ hybridization (FISH). Scale bar, 500 μm n = 3 per group. (B) Sequence of miR-15b-5p binding sites on lnc-HCAR and the secondary structure of lnc-HCAR near the miR-15b-5p binding site. (C) Quantitative PCR analysis of miR-15b-5p after overexpression or knockdown of lnc-HCAR in hypertrophic chondrocytes. (D) Quantitative PCR analysis of miR-15b-5p levels after overexpression of lnc-HCAR mutated at the miR-15b-5p binding site. (E) Schematic diagram of the dual luciferase reporter plasmid psiCheck 2.0 containing the full length lnc-HCAR with miR-15b-5p wide type binding site or the mutation site. (F) Quantitative statistics of the ratio of Firefly to Renilla in 293FT cells when adding miR-15b-5p or Control to a dual luciferase reporter gene system containing wild-type or mutant lnc-HCAR. Data are shown as mean ± s.d. ∗P < 0.05, ∗∗P < 0.01.
To confirm that lnc-HCAR regulates miR-15b-5p in hypertrophic chondrocytes, miR-15b-5p expression was detected in lnc-HCAR overexpressed and silenced hypertrophic chondrocytes, respectively. The qPCR results showed that miR-15b-5p was significantly increased in lnc-HCAR knockdown hypertrophic chondrocytes. Conversely, miR-15b-5p was significantly reduced in hypertrophic chondrocytes overexpressing lnc-HCAR (Fig. 4C). Furthermore, the miR-15b-5p expression was not substantially different from the negative control (NC) group when transfected with lnc-HCAR-miR15b-mut vector in hypertrophic chondrocytes (Fig. 4D). To further demonstrate that miR-15b-5p binds directly to lnc-HCAR, a dual luciferase reporter assay was performed. The dual luciferase reporter vector (psiCheck2.0) and the sequence of lnc-HCAR wild-type and miR-15b-binding site mutant-type are shown in Fig. 4E. The lnc-HCAR wild-type or lnc-HCAR-miR-15b-mut mutant was cloned into psiCheck2.0 vector. We found that the fluorescence intensity ratio of Firefly/Renilla in lnc-HCAR wild-type group was significantly decreased when miR-15b-5p mimics was introduced. In contrast, there was no significant change in the Firefly/Renilla ratio in the lnc-HCAR-mut group (Fig. 4F). Taken together, the above results demonstrate that miR-15b-5p binds directly to lnc-HCAR and lnc-HCAR acts as a miR-15b-5p sponge in hypertrophic chondrocytes.
Lnc-HCAR increased VEGF and MMP13 levels through sponging miR-15b-5p
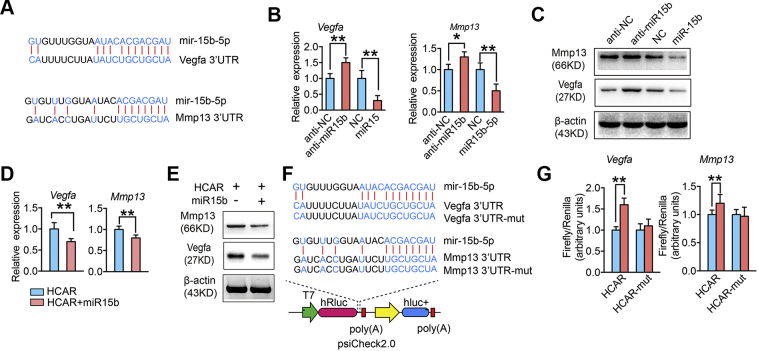
The miR-15b-5p was predicted to bind to Vegfa and Mmp13 3′UTR by Targetscan (http://www.targetscan.org/mmu_71/) (Fig. 5A). The above results showed that the mechanisms by which lnc-HCAR modulates Vegfa and Mmp13 may be competitive binding to miR-15b-5p. To investigate whether miR-15b-5p regulates Vegfa and Mmp13 expression, anti-miR15b and miR15b mimics were transfected to hypertrophic chondrocytes. We found that the mRNA levels of Vegfa and Mmp13 in hypertrophic chondrocytes transfected with anti-miR15b were significantly higher than those in miR15b mimics transfected cells (Fig. 5B). Also, the results of Western blot analysis showed that miR-15b-5p inhibited the expression of MMP13 and VEGF protein level in hypertrophic chondrocytes, which were consistent with the qPCR results (Fig. 5C). The above results demonstrated that miR-15b-5p negatively regulates VEGF and MMP13 levels. To demonstrate whether lnc-HCAR regulates Vegfa and Mmp13 expression through miR-15b-5p, we transferred anti-miR15b into hypertrophic chondrocytes overexpressing lnc-HCAR. The qPCR results showed that the Vegfa and Mmp13 mRNA levels were significantly decreased compared with the control group when miR-15b-5p mimics were introduced (Fig. 5D). Similarly, the results of Western blot analysis were consistent with qPCR (Fig. 5E). Dual luciferase reporter assay was used to investigate whether miR-15b-5p binds directly to Vegfa and Mmp13 3′UTR. The wild-type and mutant Vegfa and Mmp13 3′UTR were cloned into the dual-luciferase reporter vector, respectively. Dual luciferase reporter constructs are shown in Fig. 5F. When miR-15b-5p was introduced, Firefly/Renilla increased significantly in the wild-type Vegfa group, and the same results were not found in the Vegfa group. Similarly, miR-15b-5p significantly increased Firefly/Renilla in the wild-type Vegfa group but not in the Mmp13-mut group (Fig. 5G). These results demonstrated that miR-15b-5p directly bind to Vegfa and Mmp13 3′UTR.
Figure 5.
lnc-HCAR increases MMP13 and VEGF levels by competitive binding to mir-15b-5p. (A) Schematic diagram of the sequence complementation relationship of miR-15b-5p in Mmp13 and Vegfa 3′UTR. (B) Quantitative PCR analysis of Mmp13 or Vegfa after overexpression or knockdown of lnc-HCAR, overexpression of miR-15b-5p using miR-15b-5p mimics or knockdown of miR-15b-5p using anti-miR-15b in hypertrophic chondrocytes. n = 3 per group. (C) Representative images of Western blot of MMP13 or VEGF after overexpression of miR-15b-5p using miR-15b-5p mimics or knockdown of miR-15b-5p using anti-miR-15b in hypertrophic chondrocytes. (D) Quantitative PCR analysis of Mmp13 or Vegfa after knockdown of miR-15b-5p or not in lnc-HCAR overexpressed hypertrophic chondrocytes. (E) Representative images of Western blot of MMP13 or VEGF after knockdown of miR-15b-5p or not in lnc-HCAR overexpressed hypertrophic chondrocytes. (F) Schematic diagram of the dual luciferase reporter plasmid psiCheck 2.0 containing the upstream and downstream 200bp length of Mmp13 or Vegfa 3′UTR with miR-15b-5p wide type binding site or the mutation site. (G) Quantitative statistics of the ratio of Firefly to Renilla in 293FT cells when adding miR-15b-5p or Control to a dual luciferase reporter gene system containing wild-type or mutant Mmp13 or Vegfa 3′UTR. Data are shown as mean ± s.d. ∗P < 0.05, ∗∗P < 0.01.
Discussion
In the present study, we identified a highly expressed lncRNA during chondrocyte hypertrophic differentiation, and it was observed mainly distributed in the cytoplasm by FISH. Dual luciferase reporter demonstrated that lncRNA competitively binds miR-15b-5p and increases the expression of Vegfa and Mmp13. Our findings provide a new direction for investigating the regulation mechanism of endochondral bone repair, that is, the regulation of lncRNA on the endochondral angiogenesis-related genes in hypertrophic chondrocytes.
Our results are consistent with previous results, further confirming that hypertrophic chondrocytes play a bridge role in endochondral ossification during bone repair. Our in vitro studies have demonstrated that HCAR modulates the expression of MMP13 and VEGF in hypertrophic chondrocytes. As previous studies have shown that MMP13 and VEGF play an important role in the “cartilage to bone transition” in endochondral ossification, so we draw a hypothesis that when HCAR is knocked down in hypertrophic chondrocytes, it may affect “cartilage to bone transition” process.16,17 Due to technical limitations, it is very difficult to specifically overexpress a gene in hypertrophic chondrocytes, but it is easy to achieve specific knockdown of a gene. Therefore, in this study, we used the local application of lentivirus to knock down the HACR in hypertrophic chondrocytes to indirectly explain the necessary role of HCAR on the process of “cartilage to bone transition”. The results of safranine O-fast green staining showed that when HCAR was knocked down, the relative volume of cartilage tissue increased. In contrast, there was more trabecular bone formation after HCAR was knocked down compared to the negative control group using Masson staining. The above results suggest that the cartilage to bone transition is damaged after HCAR knocked down. Combined with the evidence of previous studies, our results indicate that HCAR is involved in the process of cartilage to bone transition during endochondral ossification.
The HCAR may also modulate a new specific subtype bone capillary, the CD31hiEmcnhi blood vessel. It is a specific subtype bone capillary that couples angiogenesis and osteogenesis in bone development and bone repair. These special capillaries subtypes were primarily distributed within the metaphysis and endosteum area, where cells maintain their growth and proliferation abilities and sustain bone development.7,18, 19, 20 A recent study showed that the defective CD31hiEmcnhi vessels formation was closely related to the delayed osteogenesis under osteoporotic conditions.21 When the HCAR is knocked down, the number of CD31hiEmcnhi vessels decreased. However, the mechanisms by which HCAR regulates the CD31hiEmcnhi vessels formation remains to be studied.
Recently, several studies have characterized the regulation of ncRNA on chondrocytes.22,23 Among these ncRNAs, microRNAs (miRNAs) and lncRNAs have attracted a large number of researchers and become the main focus. More and more evidence reveal that miRNAs are essential for post-transcriptional regulation of chondrogenic and hypertrophic differentiation and thus regulate the biological behavior of cartilage differentiation, chondrocyte maturation, apoptosis and degradation of cartilage matrix. Our previous study demonstrated that miR-145 could target Sox9 and thus inhibit chondrogenic differentiation of MSCs.24 To further investigate the role of lncRNA in endochondral bone development, we used lncRNA microarray to find a large number of differentially expressed lncRNAs during the process of hypertrophic differentiation of chondrocytes. When some of the highly expressed lncRNAs were knocked down, the marker gene expression of hypertrophic differentiation decreased, suggesting that lncRNA may regulate the hypertrophic differentiation of chondrocytes. In addition, some differentially expressed lncRNAs have a significant regulatory effect on the development of OA, indicating their potential role in the loss of chondrocytes phenotype and degradation of cartilage extracellular matrix (ECM). In the current study, we identified a lncRNA, HCAR, which was high expressed in hypertrophic chondrocytes and mainly distributed in the cytoplasm. The lnc-HCAR promoted the expression of Vegfa and MMP13 in hypertrophic chondrocytes by acting as miRNA-15b-5p sponge.
LncRNAs can serve as scaffolds or guides to regulate the interaction between proteins and genes, and they can act as bait to combine proteins or miRNAs.25, 26, 27 In addition, they may also function as enhancers to regulate the transcription of their target after transcription from the enhancer region or its neighboring loci. Interestingly, recent studies had reported a novel lncRNA regulatory circuit in which lncRNAs can act as competitive endogenous RNAs (ceRNAs) and cross-react with mRNA by competitively binding their common miRNAs. Recent evidence has shown that lncRNA could regulate the differentiation of osteoblasts and chondrocytes through the mechanism of miRNA sponge.28,29 For example, lncRNA MALAT1 regulated the expression of Smad4 through sponging miR-204, acting as a positive regulator of osteogenic differentiation of VICs; besides, lncRNA H19 promoted OB differentiation and bone formation of hMSCs, and miR-675 partially mediated H19-induced pro-osteogenic activity.
In addition, studies have demonstrated that miR-15 microRNA precursor family regulates angiogenesis in multiple myeloma (MM). miR-15a/16 directly targets VEGFA expression and thus inhibits angiogenesis of multiple myeloma.30 Similar to this study, we demonstrated by dual-luciferase reporter assay that miR-15b-5p, a member of the miR-15 microRNA precursor family, directly targeted the 3′UTR of Vegfa mRNA and thereby inhibited Vegfa expression in hypertrophic chondrocytes.
However, the limitation of this study is that only the effects of lnc-HCAR on Vegfa and Mmp13 expression in hypertrophic chondrocytes in the bone fracture model were demonstrated, and the effect of endochondral ossification on bone development still needs to be further confirmed in HCAR-deficient transgenic animals.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgments
This work was supported by Key Program of Natural Science Foundation of China (No. 81930067), General Program of Nature Science Foundation of China (No. 31870962), the Key Project of Logistics Research Plan of the PLA (No. AWS17J004-02-06), the Medical Science and Technology Youth Cultivation Project of PLA (No. 20QNPY022), and Medical innovation capability upgrading Plan of Southwest Hospital (No. SWH2018LJ-03).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Berendsen A.D., Olsen B.R. Bone development. Bone. 2015;80:14–18. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega N., Behonick D.J., Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14(2):86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q., Jia L., Li X., et al. Long noncoding RNAs: new players in the osteogenic differentiation of bone marrow- and adipose-derived mesenchymal stem cells. Stem Cell Rev. 2018;14(3):297–308. doi: 10.1007/s12015-018-9801-5. [DOI] [PubMed] [Google Scholar]
- 4.Kosaki N., Takaishi H., Kamekura S., et al. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun. 2007;354(4):846–851. doi: 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- 5.Yamagiwa H., Tokunaga K., Hayami T., et al. Expression of metalloproteinase-13 (Collagenase-3) is induced during fracture healing in mice. Bone. 1999;25(2):197–203. doi: 10.1016/s8756-3282(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 6.Bonyadi Rad E., Musumeci G., Pichler K., et al. Runx2 mediated induction of novel targets ST2 and Runx3 leads to cooperative regulation of hypertrophic differentiation in ATDC5 chondrocytes. Sci Rep. 2017;7(1):17947. doi: 10.1038/s41598-017-18044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J.M., Gao Y., Cheng P.Z., et al. CD31hiEmcnhi vessels support new trabecular bone formation at the frontier growth area in the bone defect repair process. Sci Rep. 2017;7(1):4990. doi: 10.1038/s41598-017-04150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y., Zhang M., Wang N., et al. Epigenetic silencing of tumor suppressor gene CDKN1A by oncogenic long non-coding RNA SNHG1 in cholangiocarcinoma. Cell Death Dis. 2018;9(7):746. doi: 10.1038/s41419-018-0768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Sun Y., Cai R., Wang G., Shu X., Pang W. Long noncoding RNA: multiple players in gene expression. BMB reports. 2018;51(6):280–289. doi: 10.5483/BMBRep.2018.51.6.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barter M.J., Gomez R., Hyatt S., et al. The long non-coding RNA ROCR contributes to SOX9 expression and chondrogenic differentiation of human mesenchymal stem cells. Development. 2017;144(24):4510–4521. doi: 10.1242/dev.152504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q., Zhang X., Dai L., et al. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 2014;66(4):969–978. doi: 10.1002/art.38309. [DOI] [PubMed] [Google Scholar]
- 13.Gao S., Mao F., Zhang B., et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-kappaB and signal transducer and activator of transcription 3 pathways. Exp Biol Med. 2014;239(3):366–375. doi: 10.1177/1535370213518169. [DOI] [PubMed] [Google Scholar]
- 14.Bai Y., Liu C., Fu L., et al. Mangiferin enhances endochondral ossification-based bone repair in massive bone defect by inducing autophagy through activating AMP-activated protein kinase signaling pathway. FASEB J. 2018;32(8):4573–4584. doi: 10.1096/fj.201701411R. [DOI] [PubMed] [Google Scholar]
- 15.Sandy P., Ventura A., Jacks T. Mammalian RNAi: a practical guide. Biotechniques. 2005;39(2):215–224. doi: 10.2144/05392RV01. [DOI] [PubMed] [Google Scholar]
- 16.Beamer B., Hettrich C., Lane J. Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS J. 2010;6(1):85–94. doi: 10.1007/s11420-009-9129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelzer E., Mamluk R., Ferrara N., Johnson R.S., Schipani E., Olsen B.R. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131(9):2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Zhou F., Zhang P., et al. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 2017;8(5):e2760. doi: 10.1038/cddis.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M., Li C.J., Sun X., et al. MiR-497 approximately 195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1alpha activity. Nat Commun. 2017;8:16003. doi: 10.1038/ncomms16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie H., Cui Z., Wang L., et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20(11):1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Gao Y., Cheng P., et al. CD31hiEmcnhi vessels support new trabecular bone formation at the frontier growth area in the bone defect repair process. Sci Rep. 2017;7(1):4990. doi: 10.1038/s41598-017-04150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F., Sun J.G., Huang S.L., Su G., Pi G.F. LncRNA GAS5 overexpression reverses LPS-induced inflammatory injury and apoptosis through up-regulating KLF2 expression in ATDC5 chondrocytes. Cell Physiol Biochem. 2018;45(3):1241–1251. doi: 10.1159/000487455. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y.H., Tan H.L., Dai P.Y. Kruppel-like factor 2 regulates degradation of type II collagen by suppressing the expression of matrix metalloproteinase (MMP)-13. Cell Physiol Biochem. 2017;42(6):2159–2168. doi: 10.1159/000479991. [DOI] [PubMed] [Google Scholar]
- 24.Yang B., Guo H., Zhang Y., Chen L., Ying D., Dong S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One. 2011;6(7):e21679. doi: 10.1371/journal.pone.0021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei H., Hu W., Guo Z., et al. Cancer Research; 2018. Long Non-coding RNA CRYBG3 Blocks Cytokinesis by Directly Binding G-Actin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chai W., Li J., Shangguan Q., et al. Lnc-ISG20 inhibits influenza A virus replication by enhancing ISG20 expression. J Virol. 2018;92(16) doi: 10.1128/JVI.00539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang M., Zhang S., Yang Z., et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell. 2018;173(4):906–919. doi: 10.1016/j.cell.2018.03.064. [DOI] [PubMed] [Google Scholar]
- 28.Xiao X.X., Zhou T.W., Guo S.C., et al. LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int J Cardiol. 2017;243:404–412. doi: 10.1016/j.ijcard.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y.P., Zheng Y.F., Jia L.F., Li W.R. Long noncoding RNA H19 promotes osteoblast differentiation via TGF-beta 1/Smad3/HDAC signaling pathway by deriving miR-675. Stem Cell. 2015;33(12):3481–3492. doi: 10.1002/stem.2225. [DOI] [PubMed] [Google Scholar]
- 30.Sun C.Y., She X.M., Qin Y., et al. miR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis. 2013;34(2):426–435. doi: 10.1093/carcin/bgs333. [DOI] [PubMed] [Google Scholar]