Abstract
Background
Sarcopenia, the age-related loss of skeletal muscle mass/function, has been identified as a marker of frailty. We examined the association between sarcopenia and adverse events following transcatheter aortic valve implantation (TAVI).
Methods
A retrospective cohort study was conducted at Toronto General Hospital. All patients who underwent TAVI in the time period 2007-2017 with preoperative computed tomography were included. Skeletal muscle index (SMI) was calculated radiographically using psoas muscle area at the L3 vertebral level, divided by height. Various measures of sarcopenia, including mean SMI, SMI below the sex-specific median, and SMI in the lowest sex-specific quartile were calculated. The primary outcome was postoperative adverse events, defined as a composite of in-hospital mortality and morbidity including cardiovascular, pulmonary, neurologic, access-related, and gastrointestinal complications. Univariate and multivariate logistic regression were performed to determine the association between sarcopenia and adverse events.
Results
A total of 468 patients (mean age: 80.7 years) were included. Baseline comorbidity burden was high, particularly congestive heart failure (93.4%). Postoperative adverse events occurred in 62 patients (13.2%). Univariate logistic regression demonstrated that postoperative adverse events were correlated with mean SMI (odds ratio [OR] 0.81, 95% confidence interal [CI] 0.66-0.97), events were less than the SMI (OR 2.16, 95% CI 1.24-3.84), and SMI in the sex-specific lowest quartile (OR 2.34, 95% CI 1.33-4.07). On multivariate analysis, SMI in the sex-specific lowest quartile was an independent predictor of adverse events (OR 2.53, 95% CI 1.41-4.50).
Conclusions
Sarcopenia defined by radiologic psoas muscle measurements was independently associated with in-hospital mortality and morbidity following TAVI.
Résumé
Contexte
La sarcopénie, soit la perte de masse et de fonction des muscles squelettiques liée à l’âge, a été identifiée comme un marqueur de fragilité. Nous avons examiné l’association entre la sarcopénie et les événements indésirables suivant l’implantation valvulaire aortique par cathéter (IVAC).
Méthodologie
Une étude de cohorte rétrospective a été menée au Toronto General Hospital. Tous les patients ayant subi une IVAC avec tomodensitométrie préopératoire au cours de la période 2007-2017 ont été inclus. L’indice de masse musculaire squelettique (IMMS) a été calculé par radiographie en utilisant la surface du psoas au niveau de la vertèbre L3, divisée par la taille. Diverses mesures de la sarcopénie, y compris l’IMMS moyen, l’IMMS sous la médiane selon le sexe et l’IMMS dans le quartile inférieur selon le sexe, ont été calculées. Le critère d’évaluation principal était les événements indésirables postopératoires, définis comme un critère composite comprenant la mortalité et la morbidité à l’hôpital, notamment les complications cardiovasculaires, pulmonaires, neurologiques, gastro-intestinales et liées à l’accès vasculaire. Des régressions logistiques univariée et multivariée ont été effectuées pour déterminer l’association entre la sarcopénie et les événements indésirables.
Résultats
Un total de 468 patients (âge moyen : 80,7 ans) ont été inclus. Le fardeau de comorbidité au départ était élevé, en particulier pour ce qui est de l’insuffisance cardiaque congestive (93,4 %). Des événements indésirables postopératoires sont survenus chez 62 patients (13,2 %). La régression logistique univariée a montré que les événements indésirables postopératoires étaient en corrélation avec un IMMS moyen (rapport des cotes [RC] : 0,81, intervalle de confiance [IC] à 95 % : 0,66 à 0,97), un IMMS sous la médiane selon le sexe (RC : 2,16; IC à 95 % : 1,24 à 3,84) et un IMMS dans le quartile inférieur selon le sexe (RC : 2,34; IC à 95 % : 1,33 à 4,07). Lors de l’analyse multivariée, un IMMS situé dans le quartile inférieur selon le sexe était un prédicteur indépendant d’événements indésirables (RC : 2,53; IC à 95 % : 1,41 à 4,50).
Conclusions
La sarcopénie définie par les mesures radiologiques du psoas était indépendamment associée à la mortalité et à la morbidité à l’hôpital à la suite d’une IVAC.
Transcatheter aortic valve implantation (TAVI) was initially offered to patients who were at prohibitive or high risk for conventional surgery.1 Despite the minimally invasive nature of TAVI, older patients with a substantial burden of comorbidity remain at substantial risk of incurring adverse events postoperatively.2,3 Identifying risk factors for poor outcomes can inform clinical decision-making and management strategies.
Sarcopenia, the age-related loss of skeletal muscle mass and function, has been investigated as a potential marker of frailty and adverse events among hospitalized patients.4 A validated method for quantifying sarcopenia is the measurement of psoas muscle area using cross-sectional computed tomography (CT) imaging.5 Using the psoas area as a surrogate measure, previous studies have demonstrated an association between sarcopenia and morbidity/mortality in patients with abdominal aortic aneurysm,6 colorectal cancer,7 and pulmonary embolism.8 However, the literature on sarcopenia in patients undergoing TAVI is heterogenous in terms of the measurement of sarcopenia, with a prevalence ranging from 21% to 70%, depending on the definitions used.9 This range is associated with variability in outcome prediction measurements, with mortality effect sizes ranging from odds ratios and hazard ratios of 1.12-11.30.9
Given the heterogeneity of sarcopenia measurements and results in the literature, we quantified psoas area radiographically and defined sarcopenia using various skeletal muscle index thresholds to determine its association with postoperative death and complications in patients undergoing TAVI.
Methods
Design and study population
We conducted a retrospective cohort study at Toronto General Hospital between January 18, 2007 and October 1, 2017. The research ethics board of the University Health Network, Toronto, Canada, approved this study and waived the requirement for informed consent. All patients who underwent TAVI and had preoperative CT imaging were included. For patients who had multiple pre-procedure CT scans, the examination closest to the date of the TAVI procedure was analyzed. Preoperative CT is routinely done at our institution to assess the size of the aortic annulus, the degree of valve calcification, and the status of peripheral access vessels. Use of this approach is corroborated by other groups.10,11 At our institution, TAVI candidates undergo comprehensive assessment by a team of cardiac surgeons, cardiologists, and vascular surgeons, with consideration of clinical and anatomic factors relevant to TAVI, including severity of disease based on symptoms and echocardiogram findings, suitability of access vessels, and comorbidities. This approach is the one recommended in the literature.12,13
Data collection
Our study used data collected in the CorHealth Ontario TAVI Registry. This database contains demographic, comorbidity, and procedural variables from hospitals across the province of Ontario, Canada that perform TAVI. The database has been validated through chart audits and core laboratory analysis.14
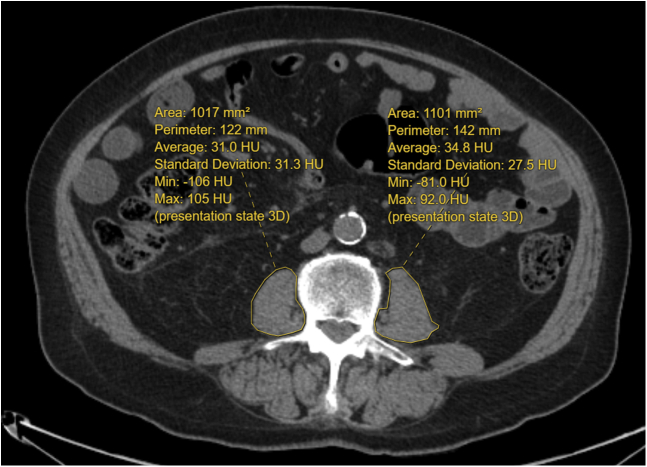
Psoas muscle area and radiodensity were measured from CT scans within 6 months of the TAVI procedure. A trained researcher (S.G.) quantified the cross-sectional area of the bilateral psoas muscles, in centimeters squared, at the third lumbar vertebra (L3), with verification by
a board-certified radiologist (A.D.B.). The L3 level was landmarked by counting the vertebral levels on the CT scan, and measurement was made when the spinous processes became visible at the middle of L3. These muscles in the region of interest were outlined freehand, , with the use of an in-house PACS workstation (Coral Workstation 3.6, University Health Network, Toronto; Fig. 1). Single-slice abdominal cross-sectional areas at the L3 vertebra are strongly correlated with whole-body volumes of muscle and have been used in prior studies.15 The skeletal muscle index (SMI) was defined as muscle area at L3, in cm2, divided by height in m2. Given the various ways sarcopenia can be defined,16,17 we chose to use 2 cutoff values for dividing populations into sarcopenic and non-sarcopenic groups. With the first method, patients with SMIs in the lowest quartile were considered sarcopenic, whereas in the second method, patients with SMIs below the median were considered sarcopenic—in both instances, sex-specific cutoffs were used. Skeletal muscle radiodensity represents muscle quality and was measured using the average radiation attenuation of tissue in Hounsfield units.
Figure 1.
Bilateral psoas muscle cross-sectional area measurement at the third lumbar vertebral level (L3), using freehand circumscribed region of interest. HU, Hounsfield units; Max, maximum; Min, minimum; 3D, 3-dimensional.
Outcomes
The CorHealth registry tracks mortality and 20 serious postoperative complications that may manifest during the period from post-TAVI procedure to time of discharge. Our primary endpoint was postoperative adverse events, defined as a composite of in-hospital death and complications, including low cardiac output syndrome (defined as a decrease in cardiac index to < 2.0 L/min per m2 and a systolic blood pressure < 90 mm Hg with signs of tissue hypoperfusion18) and gastrointestinal complications (defined based on clinical, biochemical, and/or endoscopic examinations and included bleeding, pancreatitis, liver failure, and mesenteric ischemia19). The remaining complications are captured by the Valve Academic Research Consortium-2 (VARC-2)20 clinical endpoints following TAVI, including myocardial infarction, unplanned vascular surgery, low cardiac output syndrome, cardiac arrest, major arrhythmia, new-onset atrial fibrillation/flutter, cardiac tamponade, mitral valve apparatus damage or dysfunction, cerebrovascular event, pulmonary embolism, pneumonia, vascular access site and access-related complications, vascular access hemorrhage, access-site hemorrhage, access-site infection, initiation of antibiotic treatment, multisystem organ failure, new initiation of dialysis, gastrointestinal complications, and prolonged ventilation after 24 hours.
Statistical analysis
Baseline characteristics were reported for patients with vs without postoperative adverse events as mean ± standard deviation (SD), or number and proportion. Kruskal-Wallis or χ2 tests were used to compare categorical variables between groups. The independent t-test was used to compare continuous variables between groups. Odds ratios (ORs) and 95% confidence intervals (CIs) estimating the risk of postoperative adverse events were calculated using univariate logistic regression for imaging-based characteristics representative of sarcopenia. Backward stepwise multivariable logistic regression of significant predictors on univariate analysis was performed to identify independent predictors of adverse events. Statistical analysis was performed in R (version 4.0.3) (https://www.R-project.org/) using the dplyr 1.0.3 and tidyverse 1.3.0 packages. Significance was set at P ≤ 0.05.
Results
Patient characteristics
A total of 468 patients underwent TAVI during the study period. Postoperative adverse events occurred in 62 patients (13.2%). The mean ± SD age of the study population was 80.7 ± 9.6 years, and 56% were male. Mean body mass index (BMI) was 29.2 kg/m2, with no significant difference between groups. The baseline comorbidity burden in these patients was high, including a history of congestive heart failure (93.4%). About half of the patients had previously undergone a cardiac procedure, 19.0% had a prior myocardial infarction, and 19.4% had previously undergone coronary bypass grafting. Most patients were hypertensive (73.9%) and had advanced New York Heart Association functional class (III or IV for 52.3%). Baseline characteristics were similar between patients with vs without postoperative adverse events (Table 1).
Table 1.
Baseline patient characteristics
| All patients (n = 468) | Adverse event (n = 62) | No adverse event (n = 406) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean (SD) | 80.7 (9.6) | 82.3 (8.0) | 80.5 (9.8) | 0.18 |
| Female | 206 (44.0) | 29 (46.8) | 177 (43.6) | 0.74 |
| BMI, kg/m2, mean (SD) | 29.2 (13.8) | 28.0 (8.2) | 29.4 (14.5) | 0.48 |
| Clinical characteristics | ||||
| NYHA functional class | ||||
| Asymptomatic | 4 (0.9) | 0 (0.0) | 4 (1.0) | 0.92 |
| I | 12 (2.6) | 2 (3.2) | 10 (2.5) | |
| II | 207 (44.2) | 27 (43.5) | 180 (44.3) | |
| III | 220 (47.0) | 30 (48.4) | 190 (46.8) | |
| IV | 25 (5.3) | 3 (4.8) | 22 (5.4) | |
| LVEF, % | ||||
| > 50 | 355 (75.9) | 49 (79.0) | 306 (75.4) | 0.47 |
| 31–50 | 77 (16.5) | 10 (16.1) | 67 (16.5) | |
| 21–30 | 29 (6.2) | 3 (4.8) | 26 (6.4) | |
| < 21 | 7 (1.5) | 0 (0.0) | 7 (1.7) | |
| Aortic valve mean gradient, mm Hg, mean (SD) | 43.4 (16.4) | 40.7 (15.5) | 43.8 (16.5) | 0.17 |
| Cardiac comorbidities | ||||
| Congestive heart failure | 437 (93.4) | 56 (90.3) | 381 (93.8) | 0.45 |
| Prior myocardial infarction | 89 (19.0) | 9 (14.5) | 80 (19.7) | 0.42 |
| Previous cardiac procedure∗ | 232 (49.6) | 38 (61.3) | 194 (47.8) | 0.07 |
| Prior coronary artery bypass graft | 91 (19.4) | 15 (24.2) | 76 (18.7) | 0.40 |
| Prior valve surgery | 41 (8.8) | 5 (8.1) | 36 (8.9) | 1.0 |
| Atrial fibrillation | 129 (27.6) | 20 (32.3) | 109 (26.8) | 0.46 |
| Permanent pacemaker | 53 (11.3) | 4 (6.5) | 49 (12.1) | 0.28 |
| Noncardiac comorbidities | ||||
| Hypertension | 346 (73.9) | 47 (75.8) | 299 (73.6) | 0.84 |
| Diabetes | 123 (26.3) | 17 (27.4) | 106 (26.1) | 0.95 |
| Chronic lung disease | 114 (24.4) | 14 (22.6) | 100 (24.6) | 0.85 |
| Peripheral vascular disease | 179 (38.2) | 26 (41.9) | 153 (37.7) | 0.62 |
| Cerebrovascular disease | 79 (16.9) | 7 (11.3) | 72 (17.7) | 0.28 |
| Dialysis | 9 (1.9) | 1 (1.6) | 8 (2.0) | 1.0 |
Values are n (%), unless otherwise indicated.
BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
Documented history of cardiac procedure not requiring opening of sternum, including cardiac device implantation and interventional cardiology procedures (eg, transcatheter aortic valve implantation, percutaneous coronary intervention).
Postoperative adverse events
Overall, 62 patients suffered adverse events, including 2 deaths and 75 complications. The 2 deaths were due to left ventricular rupture and complete heart block. Complication types included 44 cardiovascular (32 major arrhythmia, 10 new-onset atrial fibrillation/flutter, 1 cardiac arrest, 1 cardiac tamponade), 3 pulmonary (2 pneumonia, 1 prolonged ventilation), 7 cerebrovascular, 20 vascular access–related (5 major, 15 minor) and 1 gastrointestinal bleed (Table 2).
Table 2.
In-hospital adverse events
| Event | n (%) |
|---|---|
| Deaths | 2 (3.2) |
| Cardiovascular complications | |
| Major arrhythmia | 32 (51.6) |
| New-onset atrial fibrillation / flutter | 10 (16.1) |
| Cardiac arrest | 1 (1.6) |
| Cardiac tamponade | 1 (1.6) |
| Pulmonary complications | |
| Pneumonia | 2 (3.2) |
| Prolonged ventilation after 24 h | 1 (1.6) |
| Neurologic complications | |
| Cerebrovascular event | 7 (11.3) |
| Access-related complications | |
| Major vascular∗ | 5 (8.1) |
| Minor vascular† | 15 (24.2) |
| Gastrointestinal complications | |
| Bleed | 1 (1.6) |
Values are of n = 62 patients.
Major vascular complications: (i) any aortic dissection, aortic rupture, annulus rupture, left ventricle perforation, or new apical aneurysm/pseudo-aneurysm; OR (ii) access-site or access-related vascular injury (dissection, stenosis, perforation, rupture, arterio-venous fistula, pseudoaneurysm, hematoma, irreversible nerve injury, compartment syndrome, percutaneous closure device failure) leading to death, life-threatening or major bleeding, visceral ischemia, or neurologic impairment; OR (iii) distal embolization (non-cerebral) from a vascular source requiring surgery or resulting in amputation or irreversible end-organ damage OR the use of unplanned endovascular or surgical intervention associated with death, major bleeding, visceral ischemia or neurologic impairment; OR (iv) any new ipsilateral lower-extremity ischemia documented by patient symptoms, physical exam, and/or decreased or absent blood flow on lower-extremity angiogram; OR (v) surgery for access site–related nerve injury OR (vi) permanent access site–related nerve injury.
Minor vascular complications: (i) access-site or access-related vascular injury (dissection, stenosis, perforation, rupture, arterio-venous fistula, pseudoaneurysm, hematoma, percutaneous closure device failure) not leading to death, life-threatening, or major bleeding, visceral ischemia, or neurologic impairment; OR (ii) distal embolization treated with embolectomy and/or thrombectomy and not resulting in amputation or irreversible end-organ damage; OR (iii) any unplanned endovascular stenting or unplanned surgical intervention not meeting the criteria for a major vascular complication; OR (iv) vascular repair or the need for vascular repair (via surgery, ultrasound-guided compression, transcatheter embolization, or stent-graft); OR (v) percutaneous closure device failure; OR (vi) failure of a closure device to achieve hemostasis at the arteriotomy site leading to alternative treatment (other than manual compression or adjunctive endovascular ballooning).
Association between sarcopenia and adverse events
Sarcopenia was quantified using mean SMI, SMI below the sex-specific median, and SMI in the lowest sex-specific quartile. Patients with postoperative adverse events had a lower mean SMI (45.3 [SD 15.1] vs 49.9 [SD 15.1] cm2/m2; OR 0.81, 95% CI 0.66-0.97, P = 0.03). Furthermore, patients with postoperative adverse events were more likely to have an SMI below the sex-specific median (male < 52.6 cm2/m2; female < 40.9 cm2/m2; 66.1% vs 47.5%; OR 2.16, 95% CI 1.24-3.84, P = 0.007) and SMI in the lowest sex-specific quartile (male < 42.9 cm2/m2; female < 33.6 cm2/m2; 40.3% vs 22.4%; OR 2.34, 95% CI 1.33-4.07, P = 0.003). There was no difference in skeletal muscle density measured in Hounsfield units between patients with vs without adverse events. On stepwise multivariate analysis of significant predictors on univariate regression (mean SMI, SMI below sex-specific median, and SMI in the lowest sex-specific quartile), SMI in the sex-specific lowest quartile was the only independent predictor of postoperative adverse events (OR 2.53, 95% CI 1.41-4.50, P = 0.002; Table 3). Patients with SMIs in the sex-specific lowest quartile had a significantly higher adverse event rate than those with SMIs above the sex-specific lowest quartile (21.6% [n = 25 of 116] vs 10.5% [n = 37 of 352], P = 0.009).
Table 3.
Psoas measurements and associations with postoperative adverse events
| Psoas measurements | All patients (n = 468) | Adverse event (n = 62) | No adverse event (n = 406) | Univariate logistic regression, OR (95% CI) | Multivariate logistic regression, OR (95% CI) |
|---|---|---|---|---|---|
| SMD, HU, mean (SD) | 49.7 (10.8) | 48.9 (9.01) | 49.8 (11.0) | 0.99 (0.97–1.02) P = 0.53 | NS |
| SMI, cm2/m2, mean (SD) | 49.3 (1.53) | 45.3 (1.51) | 49.9 (1.51) | 0.81 (0.66–0.97) P = 0.03 | NS |
| SMI below sex-specific median,∗ n (%) | 234 (50.0) | 41 (66.1) | 193 (47.5) | 2.16 (1.24–3.84) P = 0.007 | NS |
| SMI in lowest sex-specific quartile,† n (%) | 116 (24.8) | 25 (40.3) | 91 (22.4) | 2.34 (1.33–4.07) P = 0.003 | 2.53 (1.41–4.50) P = 0.002 |
Multivariate logistic regression was performed for significant univariate predictors (mean skeletal muscle density [SMI], SMI below sex-specific median, and SMI in lowest sex-specific quartile). Boldface indicates statistical significance.
CI, confidence interval; HU, Hounsfield unit; NS, not significant; OR, odds ratio; SD, standard deviation; SMD, skeletal muscle density.
SMI below sex-specific median in our study population was < 52.6 cm2/m2 for male patients and < 40.9 cm2/m2 for female patients.
SMI in the lowest sex-specific quartile in our study population was < 42.9 cm2/m2 for male patients and < 33.6 cm2/m2 for female patients.
Discussion
Main findings
In this retrospective cohort study of 468 patients who underwent TAVI at a single institution, sarcopenia was an independent predictor of in-hospital mortality and morbidity. Multiple measures of sarcopenia, including mean SMI, SMI below the sex-specific median, and SMI in the lowest sex-specific quartile were associated with adverse events. On multivariate analysis, SMI in the lowest sex-specific quartile was an independent predictor, with thresholds of < 42.9 cm2/m2 for men and < 33.6 cm2/m2 for women. Baseline characteristics, including age, gender, BMI, and comorbidities were not associated with adverse events.
Comparison to existing literature
The quantification of sarcopenia, using radiographic psoas measurements, and its correlation to outcomes in cardiac procedures have been reported previously. Hawkins et al. demonstrated that psoas muscle index predicted adverse outcomes in patients undergoing open surgical aortic valve replacement.21 We have observed a similar association in patients who received TAVI, a less-invasive approach. An association between sarcopenia and poor outcomes was previously observed in 152 patients undergoing TAVI.22 However, the investigators employed an infrequently used definition of sarcopenia (psoas muscle area indexed to body surface area) and did not report whether the sarcopenia thresholds used were sex-specific.22 We demonstrated similar findings using sex-specific sarcopenia thresholds based on the validated measurement of SMI. Our findings corroborate those reported in a recent systematic review of 18 observational studies demonstrating a correlation between radiographic measurements of sarcopenia and poor outcomes following TAVI.9 Given the heterogeneity of CT-based quantification of sarcopenia in the literature, an important strength of our analysis is the consideration of multiple measurement thresholds for sarcopenia to identify the most significant predictor of adverse outcomes.
There remains no standardized cutoff value for dividing populations into sarcopenic and non-sarcopenic groups. Damluji et al.23 used absolute sex-specific cutoffs (women: SMI < 39 cm2/m2; men: SMI < 55 cm2/m2), and Yoon et al.24 analyzed sarcopenic patients based on SMI tertiles. This heterogeneity led us to explore different methods for quantifying sarcopenia from psoas area. We demonstrated that multiple measures of sarcopenia were associated with adverse events; however, only a single method of quantification (SMI in the lowest sex-specific quartile) was an independent predictor. The reason only 1 of the 3 SMI measurements was an independent predictor is likely the collinearity between the measurements and the fact that SMI in the lowest sex-specific quartile had the strongest association with adverse events. This finding may inform the development of a standardized definition for sarcopenia using SMI quartiles.
Explanation for findings
The mechanism by which sarcopenia contributes to adverse outcomes has not been fully elucidated.25 It is considered a marker of older age and more comorbidities26; however, we observed that sarcopenia was an independent predictor of adverse events, whereas age and comorbidity burden were not. Therefore, declining muscle mass may increase the propensity for poor outcomes independent of age and comorbidities. We suspect that sarcopenia is an indicator of frailty,27 broadly defined as age-associated decline in physiological reserve.28 There are a number of measurement scales for frailty, including the Frailty Phenotype29 and Frailty Index.30, 31 Our study suggests that psoas muscle measurements may provide a timely and objective indicator of frailty, which improves reliability and practicality in the clinical setting, particularly for patients who routinely undergo preoperative CT imaging. Future studies are required to validate the use of psoas measurements as an indicator of frailty.
The assessment of frailty is complex, given the multiple contributing factors, including demographic, clinical, and psychosocial considerations.32 Phenotypic criteria include weakness, slowness, low level of physical activity, self-reported exhaustion, and unintentional weight loss.29 In our study, the mean BMI was 29, which was higher than those in other cohorts, which range from 20 to 27.33, 34, 35, 36 This variability suggests that BMI alone is insufficient to characterize frailty, given that weight-loss phenotypes often neglect sarcopenic obesity.37 Furthermore, the assessment of frailty in TAVI candidates can be challenging due to the high volumes of patients and limited time/expertise of cardiovascular specialists to perform complete frailty evaluations.38 Given that TAVI patients undergo routine preoperative CT, and interventionalists have expertise in assessing imaging studies, we hypothesize that our modality will be practical in the clinical setting.39 Patients suspected to have frailty can then be referred for comprehensive geriatrics assessment and undergo targeted, multidimensional, prehabilitation interventions to improve TAVI outcomes.40
Limitations
This study has several limitations. First, it involves the experience of a single institution using retrospectively collected data. Second, our registry currently captures in-hospital outcomes, and future studies analyzing longer-term results are needed. Third, only 2 deaths occurred in the cohort, and therefore, mortality was not analyzed as an individual outcome, owing to the low event rate. Fourth, urgency of TAVI was not recorded. Fifth, debate remains regarding the level at which the psoas area should be measured, with some authors using L4 as the reference level.41 We measured at L3, given that skeletal muscle area has been reported to peak at this level16 and L3 measurements are more commonly reported in the literature.8,42 Sixth, freehand planimetry has the disadvantage of including intramuscular fat as part of the measured muscle area. Future studies can consider Hounsfield-based segmentation of muscle pixels for more accurate measurement of muscle mass with radiologic validation of cutoff Hounsfield thresholds for the psoas muscle. Seventh, muscle strength cannot be gleaned from CT imaging data. The European Working Group on Sarcopenia in Older People (EWGSOP) 2018 revised guidelines use muscle strength as the primary parameter defining sarcopenia, given its strong correlation with adverse outcomes in patients with sarcopenia.43 Therefore, future studies on this topic should consider the importance of muscle strength in the diagnosis of sarcopenia. Eighth, patient-relevant outcomes, including length of stay, discharge to institutions, and quality of life post-TAVI were not captured in our study.
Conclusions
Accurate assessment of the postoperative risk of death and complications remains critically important, even in minimally invasive procedures such as TAVI. We demonstrated that sarcopenia defined as SMI in the lowest sex-specific quartile (< 42.9 cm2/m2 for men and < 33.6 cm2/m2 for women in our study population) was an independent predictor of adverse events following TAVI. As indications for TAVI expand to include younger patients with lower rates of comorbidity, routine assessment of sarcopenia may identify those at highest risk of poor outcomes.
Acknowledgments
Funding Sources
M.O. is partially supported by the Antonio and Helga DeGasperis Chair in Clinical Trials and Outcomes Research at University Health Network. The other authors have no sources of funding to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research ethics board of the University Health Network, Toronto, Canada, approved this study and waived the requirement for informed consent.
See page 178 for disclosure information.
References
- 1.Alsara O., Alsarah A., Laird-Fick H. Advanced age and the clinical outcomes of transcatheter aortic valve implantation. J Geriatr Cardiol. 2014;11:163–170. doi: 10.3969/j.issn.1671-5411.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lansky A.J., Brown D., Pena C., et al. Neurologic complications of unprotected transcatheter aortic valve implantation (from the Neuro-TAVI Trial) Am J Cardiol. 2016;118:1519–1526. doi: 10.1016/j.amjcard.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Czerwińska-Jelonkiewicz K., Michałowska I., Witkowski A., et al. Vascular complications after transcatheter aortic valve implantation (TAVI): risk and long-term results. J Thromb Thrombolysis. 2014;37:490–498. doi: 10.1007/s11239-013-0996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su H., Ruan J., Chen T., Lin E., Shi L. CT-assessed sarcopenia is a predictive factor for both long-term and short-term outcomes in gastrointestinal oncology patients: a systematic review and meta-analysis. Cancer Imaging. 2019;19:1–15. doi: 10.1186/s40644-019-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J.S.J., He K., Harbaugh C.M., et al. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53:912–917. doi: 10.1016/j.jvs.2010.10.111. [DOI] [PubMed] [Google Scholar]
- 7.Lieffers J.R., Bathe O.F., Fassbender K., Winget M., Baracos V.E. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akkoc I., Toptas M., Yalcin M., Demir E., Toptas Y. Psoas muscle area measured with computed tomography at admission to intensive care unit: prediction of in-hospital mortality in patients with pulmonary embolism. Biomed Res Int. 2020;2020:1586707. doi: 10.1155/2020/1586707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertschi D., Kiss C.M., Schoenenberger A.W., Stuck A.E., Kressig R.W. Sarcopenia in patients undergoing transcatheter aortic valve implantation (TAVI): a systematic review of the literature. J Nutr Heal Aging. 2021;25:64–70. doi: 10.1007/s12603-020-1448-7. [DOI] [PubMed] [Google Scholar]
- 10.Lehmkuhl L., Foldyna B., Haensig M., et al. Role of preprocedural computed tomography in transcatheter aortic valve implantation. Rofo. 2013;185:941–949. [PubMed] [Google Scholar]
- 11.Matsumoto S., Yamada Y., Hashimoto M., et al. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur Radiol. 2017;27:1963–1970. doi: 10.1007/s00330-016-4547-4. [DOI] [PubMed] [Google Scholar]
- 12.Cerrato E., Nombela-Franco L., Nazif T., et al. Evaluation of current practices in transcatheter aortic valve implantation: The WRITTEN (WoRldwIde TAVI ExperieNce) survey. Int J Cardiol. 2017;228:640–647. doi: 10.1016/j.ijcard.2016.11.104. [DOI] [PubMed] [Google Scholar]
- 13.Shirakawa K., Murata M. Significance of echocardiographic evaluation for transcatheter aortic valve implantation. Cardiovasc Interv Ther. 2020;35:85–95. doi: 10.1007/s12928-019-00617-6. [DOI] [PubMed] [Google Scholar]
- 14.Tu J.V., Ko D.T., Guo H., et al. Determinants of variations in coronary revascularization practices. CMAJ. 2012;184:179–186. doi: 10.1503/cmaj.111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen W., Punyanitya M., Wang Z.M., et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 16.Derstine B.A., Holcombe S.A., Ross B.E., et al. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8:11369. doi: 10.1038/s41598-018-29825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishigori T., Tsunoda S., Obama K., et al. Optimal cutoff values of skeletal muscle index to define sarcopenia for prediction of survival in patients with advanced gastric cancer. Ann Surg Oncol. 2018;25:3596–3603. doi: 10.1245/s10434-018-6728-7. [DOI] [PubMed] [Google Scholar]
- 18.Lomivorotov V.V., Efremov S.M., Kirov M.Y., Fominskiy E.V., Karaskov A.M. Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth. 2017;31:291–308. doi: 10.1053/j.jvca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz A.T., Arslan M., Demirkiliç U., et al. Gastrointestinal complications after cardiac surgery. Eur J Cardio-Thorac Surg. 1996;10:763–767. doi: 10.1016/s1010-7940(96)80337-x. [DOI] [PubMed] [Google Scholar]
- 20.Kappetein A.P., Head S.J., Généreux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document (varc-2) Eur J Cardio-thoracic Surg. 2012;42:545–560. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins R.B., Mehaffey J.H., Charles E.J., et al. Psoas muscle size predicts risk-adjusted outcomes after surgical aortic valve replacement. Ann Thorac Surg. 2018;106:39–45. doi: 10.1016/j.athoracsur.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg L., Agrawal S., Pew T., et al. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol. 2017;119:457–460. doi: 10.1016/j.amjcard.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Damluji A.A., Rodriguez G., Noel T., et al. Sarcopenia and health-related quality of life in older adults after transcatheter aortic valve replacement. Am Heart J. 2020;224:171–181. doi: 10.1016/j.ahj.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon Y.H., Ko Y., Kim K.W., et al. Prognostic value of baseline sarcopenia on 1-year mortality in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2021;139:79–86. doi: 10.1016/j.amjcard.2020.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Rong S., Wang L., Peng Z., et al. The mechanisms and treatments for sarcopenia: Could exosomes be a perspective research strategy in the future? J Cachexia Sarcopenia Muscle. 2020;11:348–365. doi: 10.1002/jcsm.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walston J.D. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–627. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gingrich A., Volkert D., Kiesswetter E., et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019;19:120. doi: 10.1186/s12877-019-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima G., Liljas A.E.M., Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23–30. doi: 10.2147/RMHP.S168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 30.Burn R., Hubbard R.E., Scrase R.J., et al. A frailty index derived from a standardized comprehensive geriatric assessment predicts mortality and aged residential care admission. BMC Geriatr. 2018;18:319. doi: 10.1186/s12877-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dent E., Kowal P., Hoogendijk E.O. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Chen X., Mao G., Leng S. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433. doi: 10.2147/CIA.S45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afilalo J., Lauck S., Kim D.H., et al. Frailty in older adults undergoing aortic valve replacement: The FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Muneretto C., Bisleri G., Moggi A., et al. Treating the patients in the “grey-zone” with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg. 2015;20:90–95. doi: 10.1093/icvts/ivu340. [DOI] [PubMed] [Google Scholar]
- 35.Kapadia S.R., Tuzcu E.M., Makkar R.R., et al. Long-term outcomes of inoperable patients with aortic stenosis randomly assigned to transcatheter aortic valve replacement or standard therapy. Circulation. 2014;130:1483–1492. doi: 10.1161/CIRCULATIONAHA.114.009834. [DOI] [PubMed] [Google Scholar]
- 36.Maleszka A., Kleikamp G., Zittermann A., Serrano M.R.G., Koerfer R. Simultaneous aortic and mitral valve replacement in octogenarians: a viable option? Ann Thorac Surg. 2008;86:1804–1808. doi: 10.1016/j.athoracsur.2008.07.116. [DOI] [PubMed] [Google Scholar]
- 37.Barazzoni R., Bischoff S.C., Boirie Y., et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. 2018;37:1787–1793. doi: 10.1016/j.clnu.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Hanna K., Ditillo M., Joseph B. The role of frailty and prehabilitation in surgery. Curr Opin Crit Care. 2019;25:717–722. doi: 10.1097/MCC.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 39.Zwart A.T., van der Hoorn A., van Ooijen P.M.A., et al. CT-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J Cachexia Sarcopenia Muscle. 2019;10:1060–1069. doi: 10.1002/jcsm.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norris C.M., Close J.C.T. Prehabilitation for the frailty syndrome: improving outcomes for our most vulnerable patients. Anesth Analg. 2020;130:1524–1533. doi: 10.1213/ANE.0000000000004785. [DOI] [PubMed] [Google Scholar]
- 41.Saji M., Lim D.S., Ragosta M., et al. Usefulness of psoas muscle area to predict mortality in patients undergoing transcatheter aortic valve replacement. Am J Cardiol. 2016;118:251–257. doi: 10.1016/j.amjcard.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 42.Gu D.H., Kim M.Y., Seo Y.S., et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319–330. doi: 10.3350/cmh.2017.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]