Abstract
Canada is a wealthy nation with a geographically diverse population, seeking health innovations to better serve patients in accordance with the Canada Health Act. In this country, population and geography converge with social determinants, policy, procurement regulations, and technological advances with the goal to achieve equity in the management and distribution of health care. Rural and remote patients are a vulnerable population; when managing chronic conditions like cardiovascular disease, there is currently inequity to accessing specialist physicians at the recommended frequency—increasing the likelihood of poor health outcomes. Ensuring equitable care for this population is an unrealized priority of several provincial and federal government mandates. Virtual care technology might provide practical, economical, and innovative solutions to remedy this discrepancy. We conducted a scoping review of the literature pertaining to the use of virtual care technologies to monitor patients living in rural areas of Canada with cardiovascular disease. A search strategy was developed to identify the literature specific to this context across 3 bibliographic databases. Two hundred thirty-two unique citations were ultimately assessed for eligibility, of which 37 met the inclusion criteria. In our assessment of these articles, we provide a summary of the interventions studied, their reported effectiveness in reducing adverse events and mortality, the challenges to implementation, and the receptivity of these technologies among patients, providers, and policy-makers. Furthermore, we glean insight into the barriers and opportunities to ensure equitable care for rural patients and conclude that there is an ongoing need for clinical trials on virtual care technologies in this context.
Résumé
Le Canada, pays riche dont la population est répartie dans des régions géographiquement diversifiées, reste à l’affût des innovations en matière de santé pour mieux servir les patients conformément à la Loi canadienne sur la santé. Dans ce pays, la population et la géographie ainsi que les déterminants sociaux, les politiques, la réglementation des marchés publics et les progrès technologiques convergent vers un objectif d’équité dans la gestion et la distribution des soins de santé. Les patients des régions rurales et éloignées constituent une population vulnérable; la prise en charge de maladies chroniques comme les maladies cardiovasculaires est marquée par des inégalités en ce qui concerne l’accès aux médecins spécialistes à la fréquence recommandée – ce qui augmente le risque de problèmes de santé. La garantie d’un accès équitable aux soins de santé pour cette population constitue une priorité non concrétisée pour plusieurs gouvernements provinciaux et fédéraux. Les technologies des soins virtuels pourraient offrir des solutions pratiques, économiques et novatrices afin de remédier à la disparité qui persiste. Nous avons effectué une revue exploratoire de la littérature relative à l’utilisation des technologies des soins virtuels pour le suivi des patients atteints de maladies cardiovasculaires vivant dans les régions rurales du Canada. Une stratégie de recherche a été élaborée pour recenser les articles visant spécifiquement ce contexte dans trois bases de données bibliographiques. Au terme de la recherche, 232 références uniques ont été évaluées en fonction des critères d’admissibilité; 37 y répondaient. Dans notre évaluation des articles, nous résumons les interventions étudiées, leur efficacité rapportée quant à la réduction des événements indésirables et de la mortalité, les difficultés de mise en œuvre et la réceptivité des patients, des fournisseurs de soins et des décideurs politiques aux technologies utilisées. En outre, nous offrons un aperçu des obstacles à surmonter et des occasions à saisir pour garantir un accès équitable aux soins de santé dans les régions rurales et nous concluons que des études cliniques sur les technologies des soins virtuels demeurent nécessaires dans ce contexte.
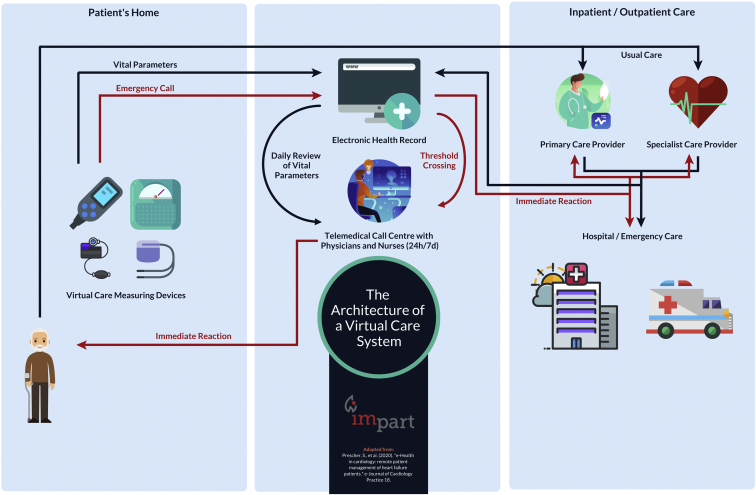
On the basis of its geographic size and distributed population density, Canada is well positioned to become a leader of innovation for remote medical care—whether the nation is optimally capitalizing on this is unclear. Rural populations are essential to the well-being of a nation, because they are naturalized environmental stewards; farmers and harvesters provide most food and other natural resources that enable urban living and a trade economy. In contrast to urban populations, rural patients are a vulnerable group in need of special attention to ensure equitable health care delivery.1, 2, 3 The inequities affecting rural populations are complex, but a major concern is timely access to specialist physicians in accordance with clinical guideline recommendations.4, 5, 6 Disparities in health care negatively affect all clinical outcomes, and thus provisions to secure equitable health care and comparable health outcomes for vulnerable groups are necessary. The scientific and clinical efforts toward addressing this are increasing and multiple approaches to improve care for this population are being studied.7 One approach is the opportunity for health care workers, researchers, and policy makers to adopt innovative virtual care and remote monitoring platforms to improve clinical outcomes, reduce hospital wait times, or increase the quality of life for patients and their families.8,9 An example of how such a system could work is depicted in Figure 1, which has been adapted from Prescher et al.10 In the 1970s, Canada was a leader in virtual care, yet now lags behind other countries. In a recent interview, the president of the Canadian Medical Association, Dr Gigi Osler, expressed that: “The reasons for Canada’s sluggishness to adapt vary from province to province. Some lack the policy and legislation that would enable physicians to reach out to patients outside their offices; some pay only for in-person visits. Health providers are often restricted from dealing with patients outside their provinces’ borders,” and that all of this “adds up to an inefficient system that makes it difficult for some people to get health care when they need it, especially in remote communities.”11 There is now increasing demand by Canadian physicians for more virtual care technology, training, and payment models to support and integrate/hybridize with standard/historical clinical practices. In an organized initiative, the Canadian Medical Association Virtual Care Task Force recently created a national strategy of practice recommendations.12
Figure 1.
The architecture of a virtual care system. Data from Prescher et al.10
There is no widely accepted definition of virtual care, telehealth, telemedicine, or e-medicine, and these terms are often used interchangeably, along with many others. The World Health Organization defines e-health as “the cost-effective and secure use of information and communication technologies in support of health and health-related fields, including health care services, health surveillance, health literature, and health education, knowledge, and research.”13 The use of virtual care technologies continues to grow and includes electronic virtual visits, referrals, consults, prescriptions, medical records, vital sign monitoring, digital therapeutics, care flow-ordered checklists, telepresence, and robotic surgery. For the purpose of this review, virtual care is defined as health care services provided directly to patients using information telecommunications technology (that is, medical care provided without traditional dependence on physical contact, paper, or archaic technologies such as fax machines). We refer to all further instances of these terms simply as virtual care. Health care providers can use virtual care to remotely diagnose, treat, and manage patients, in many but not all instances. Thus, virtual care ensures continuity and integration with practice-level operations, not displacement or replacement. Patients can be seen virtually by primary care providers in their homes, places of work, or anywhere else they have access to the Internet. The ability to connect providers and patients without regard for their respective locations is a compelling benefit of virtual care services for patients and providers.14 It is also considered to be essential infrastructure to occupational health, safety, and productivity by employers by reducing down-time and sick days, and an optimized work culture. Virtual care simply provided by video and audio alone has diverse applications, but numerous disease-specific platforms and devices have also been developed to remotely provide the consulting physician with ever more information to improve decision-making. These devices include stethoscopes, glucometers, oxygen saturation monitors, weigh scales, temperature, and blood pressure devices, along with more invasive technologies such as otoscopes, continuous positive airway pressure sleep devices, or even implantable cardiac defibrillators.15,16
As was alluded to previously, virtual care technologies are not intended to replace traditional health care delivery methods, but rather to augment and integrate with services to ensure more productive, efficient, and equitable health care for all citizens. Regular health monitoring of patients at-risk of—or diagnosed with—cardiovascular disease (CVD), has the potential to reduce the need for significant treatment interventions, including invasive cardiac surgery or readmissions for past interventions.17, 18, 19 Virtual care mitigates the need for rurally located patients to defer care or make burdensome commutes to visit with specialist clinicians for follow-up appointments. This can improve postoperative health outcomes. Because these technologies enable more productive, efficient, sustainable, supported, and convenient care management, they have important economic implications to health care payers, whether they are government or private allied insurers. CVD ranks among the largest chronic comorbidity and cause of morbidity and mortality in Canada.20 Therefore, our research question was directed at understanding: what efforts in the realm of rural CVD management using virtual care technologies have recently been conducted in Canada? The purpose of this review is to summarize the relevant studies, the effectiveness of virtual care systems to reduce adverse events, improve adherence to medication and lifestyle modifications to affect all-cause mortality—recognizing barriers to implementation or acceptance of these technologies among payers, patients, and providers.
Methods
Eligibility criteria
Using a priori-developed selection criteria, we included studies that reported on the use of virtual care technologies in the context of patients living with CVD in rural areas of Canada. We used the definition of CVD outlined by the World Health Organization’s International Classification of Diseases Tenth Edition (ICD-10), which encompasses all diseases of the circulatory system, primarily: (1) coronary artery disease, or ischemic heart disease, caused by low myocardial perfusion, and thus precipitating angina, myocardial infarction, and/or heart failure (HF); (2) cerebrovascular disease, including disorders that affect the blood supply to the brain, including stroke and transient ischemic attack; and (3) peripheral artery disease, involving the limbs or claudication in peripheral vasculature. Additionally, hypertension, valvular pathologies, aortic aneurysms, and cardiac arrhythmias were all considered to be cardiovascular pathologies that fit this definition.21
This is a scoping review (as defined by Paré et al.22) and therefore includes all study designs, including quantitative pilot and retrospective comparison studies, and qualitative analyses of the various aspects involved with the adoption of virtual care technologies by health care providers, patients, and their families. Therefore, the population of interest included patients diagnosed with CVD or individuals caring for patients diagnosed with CVD at the time of study. We only included studies that explicitly mentioned rurally located patients living in Canada, because the economic and political climate of a particular health care network has proven to be a critical factor regulating how these virtual care technologies are being disseminated and studied. Despite this, in some subsections, we reflect on Canadian studies that did not explicitly focus on rural patients to contextualize the state of the technology for a given disease. The articles also had to have been published in English within the past 10 years, because the technology is rapidly evolving, and older technologies are becoming irrelevant.
Literature search strategy
Literature search strategies were developed with the assistance of an experienced information services librarian (Jackie Phinney, W.K. Kellogg Health Sciences Library, Dalhousie University), who reviewed and refined the search criteria through iterative discussion. We adopted relevant elements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).23 An initial search of the literature was performed by the primary author (R.B.) in May of 2020 using PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL). All articles that listed in the title or abstract the terms, “telehealth,” “omnicare,” “e-medicine,” “electronic medicine,” “remote consultation,” or “telemedicine” were linked through the Boolean operator “and” to rural-patient–identifying terms (“rural,” “remote,” “distance,” “rural population,” and “rural health services”), as well as the terms “Canada,” and terms relating to the diseases of interest (“hypertension” or “cardiovascular disease”). Medical Subject Headings (MeSH) terms were used where appropriate and when possible. Using the features built into each of these respective databases, the results were limited to studies published in English within the past 10 years before being imported into Covidence (https://community.cochrane.org/help/tools-and-software/covidence). The search was then updated in May of 2021.
Study selection
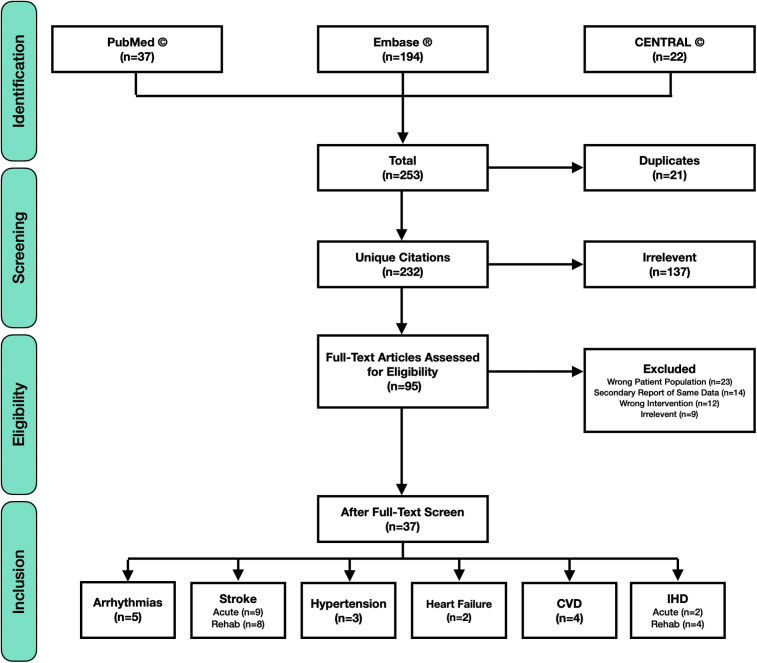
After being imported into the review software, any duplicate articles were automatically removed. The remaining titles and abstracts were screened for relevance to the research question and were removed if they did not meet the a priori-defined criteria. The full text of each of the remaining articles were then individually obtained and reviewed in depth to determine their applicability. At this stage, each article was either removed, or included and subsequently categorized on the basis of the subtype of CVD in the study. A miscellaneous category was created for studies that included multiple CVD subtypes. The primary author (R.B.) led the literature search and the study selection process with constructive and direct contributions and guidance from the coauthors. A total of 232 unique citations were identified and assessed for eligibility, yet only 37 studies met the inclusion criteria and are discussed in this review (Figure 2).
Figure 2.
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram depicting literature search and study extraction processes, where n represents the number of studies in each group. CVD, cardiovascular disease; IHD, ischemic heart disease; Rehab, rehabilitation.
Results
We summarized our findings in Table 1 and categorized them on the basis of which specific disease subtype the reported interventions were targeting, with a miscellaneous category when only the generic term, CVD, was used, or when the study concerned multiple subtypes. Despite that the collective studies lacked the desired scientific rigour, or were of limited metadata, the qualitative insights provide valuable recommendations for researchers and clinicians planning to deploy virtual care technologies in the future. These have been summarized through a thematic compilation process on the basis of the readings in their entirety and are depicted in Figures 3 and 4.
Table 1.
Summary of virtual care studies
| Disease | Sample size | Community setting | Virtual care components | Author-reported outcomes | Reference |
|---|---|---|---|---|---|
| CVD | 48 | Rural and urban (BC) | Assessment of stakeholder perceptions | Strong stakeholder support; concerns regarding data accuracy and security | Jarvis-Selinger et al.24 |
| 129 | Rural (ON) | Remote chronic disease self-management program | Noninferior improvements in self-efficacy, health behaviours, and health status | Jaglal et al.25 | |
| 213 | Rural (ON) | Remote chronic disease self-management program | Geographic barriers for rural patients were demonstrably overcome | Cameron et al.26 | |
| N/R (> 100,000 events) | Rural (ON) | All modalities approved for billing | > 10-fold increase in utilization of services after onset of the Covid-19 pandemic | Chu et al.27 | |
| Heart failure | 69 | Rural and urban (BC) | Home vital data monitoring (BP, HR, O2, weight); self-management | Stakeholder satisfaction; decreased emergency department visits and length of stay | Lauscher et al.28,∗ |
| 240 | Rural and urban (ON) | Home vital data monitoring (BP, HR, ECG, weight); with telephone nursing visits | Demonstrated technologic feasibility among rural patients; concerns regarding data accuracy and security; improved access to specialist care | Jaana and Sherrand29 | |
| Arrhythmias | N/R | Rural (AB) | Remote consultations and home vital data monitoring | Noninferior patient management; reduced travel costs; stakeholder satisfaction | Amelio and Manchak30,∗ |
| 5 | Rural (ON) | Home data monitoring (device setup) | Technologic challenges, influenced by gender, age, and experience with technology; technologic feasibility | Sparkes et al.31 | |
| 350 | Rural and urban (BC) | Remote consultations | Patient satisfaction; challenges to integrate into current practices; improved access to specialist care | Forman et al.32,∗ | |
| 116 | Rural (BC) | Assessment of stakeholder perceptions | Strong stakeholder support, particularly to expedite access to specialist care | Rush et al.33 | |
| 14 | Rural (BC) | Assessment of stakeholder perceptions | Variability in stakeholder receptiveness, on the basis of past experiences and perceptions | Rush et al.34 | |
| IHD; acute | 208 | Rural (QC) | Remote ECG monitoring | Early diagnosis and treatment; improved patient outcomes (rerouting of ambulances toward PCI centres) | Tanguay et al.35 |
| 728 | Rural (QC) | Remote ECG monitoring | Early diagnosis and treatment; improved patient outcomes (rerouting of ambulances toward PCI centres) | Tanguay et al.36 | |
| IHD; rehabilitation | 86 | Rural (BC) | Virtual cardiac rehabilitation and vital data monitoring (BP, HR, weight) | Noninferior patient outcomes (exercise capacity, blood indices, blood pressure); improved program adherence | Lear et al.37 |
| N/R | Rural (BC) | Virtual cardiac rehabilitation with health education component | Robust patient interest, uptake, satisfaction, and improved self-efficacy | Pistawka et al.38,∗ | |
| 38 | Rural (BC) | Virtual cardiac rehabilitation and SMS nursing follow-up | Improved patient follow-up when access to hospital-based CRPs is limited; technologic challenges | Mendell et al.39 | |
| Hypertension | 243 | Indigenous rural (ON, QC, NB) | SMS-based health education regarding lifestyle modification | Noninferior blood pressure control; feasibility of remote blood pressure monitoring by nonmedical staff | Tobe et al.40 |
| CeVD; acute | N/R | Rural (ON) | Synthesized delivery of training for health care providers | Harmonized standards of practice for physicians in network | Zimmer et al.41,∗ |
| 211 | Rural (AB) | Virtual consultations and diagnostic imaging interpretation | Geographic barriers for rural patients were demonstrably overcome; decreased need for patient transfers | Khan et al.42,∗ | |
| 18 | Rural (AB) | Remote consultations and portable diagnostic imaging equipment | Portable CT scanners and virtual consultations were successfully used to evaluate remote stroke patients | Shuaib et al.43 | |
| 68 | Rural (ON) | Comparison of stroke outcomes (virtual vs in-person care) | Noninferior patient outcomes (time to definitive treatment, rate of complications, length of stay, and mortality) | Khan et al.44,∗ | |
| 498 | Rural (AB) | Comparison of stroke outcomes (virtual vs in-person care) | Noninferior patient outcomes (time to definitive treatment and rate of hemorrhagic complications) | Jeerakathil et al.45,∗ | |
| N/R | Rural (AB) | Telestroke simulations using virtual consultations | Health care providers reported confidence to competently manage patients | Taralson et al.46,∗ | |
| 119 | Rural (AB) | Remote consultations and portable diagnostic imaging equipment | Mobile stroke units served as a triage mechanism before transport to a tertiary care centre | Shuaib and Jeerakathil47 | |
| CeVD; rehabilitation | 7 | Indigenous rural (ON) | Remote home safety assessments | Improved access to occupational therapy services; clinical utility; technologic challenges | Linkewich et al.48,∗ |
| 10 | Rural (ON) | Virtual stroke rehabilitation (video/audio) with interdisciplinary team | Stakeholder satisfaction; concerns/difficulties with the technology | French et al.49 | |
| N/R | Indigenous rural (ON) | Virtual stroke rehabilitation (video/audio) | Successful development of culturally appropriate services and supports in partnership with communities | Bodnar50,∗ | |
| 19 | Rural (ON) | Virtual, group-based stroke rehabilitation program (video/audio) | Stakeholder satisfaction; decreased travel requirements; loss of personal connection when communicating | Taylor et al.51 | |
| 184 | Rural (SK) | Remote consultations | Stakeholder satisfaction; decreased travel requirements (cost savings) | Whelan et al.52,∗ | |
| 75 | Rural (ON) | Remote consultations | Stakeholder satisfaction; decreased wait times and visit duration; decreased travel requirements (cost savings) | Appireddy et al.53 |
AB, Alberta; BC, British Columbia; BP, blood pressure; CRP, cardiac rehabilitation program; CeVD, cerebrovascular disease; CT, computed tomography; CVD, cardiovascular disease; ECG, electrocardiogram; HR, heart rate; IHD, ischemic heart disease; NB, New Brunswick; N/R, not reported; O2, oxygen saturation; ON, Ontario; PCI, percutaneous coronary intervention; QC, Quebec; SK, Saskatchewan; SMS, short message service.
Abstract.
Figure 3.

A thematic summary of virtual care recommendations.
Figure 4.
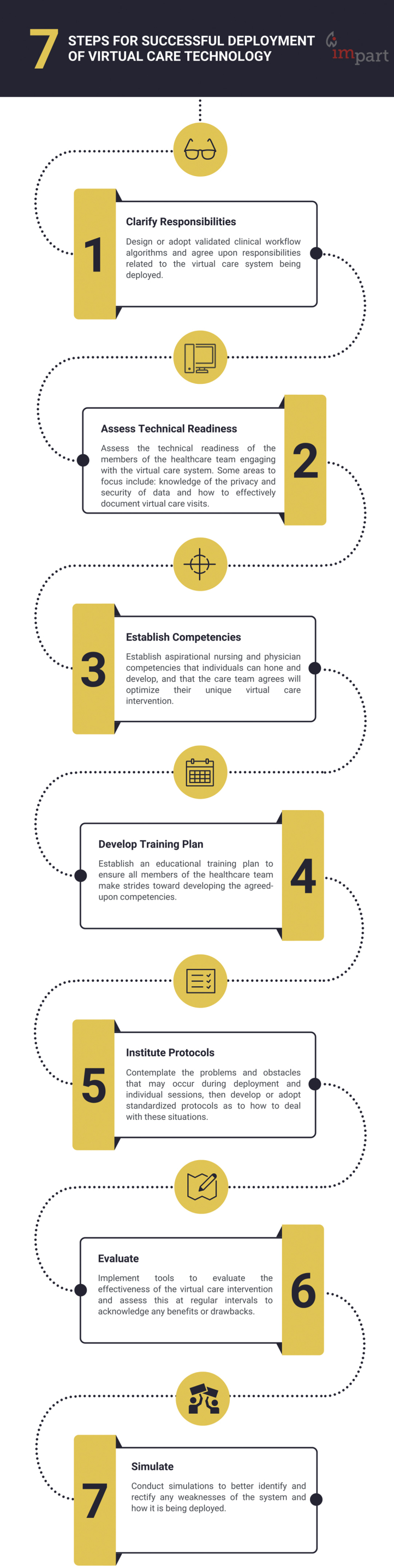
Thematic summary of practical objectives for future virtual care deployments.
Study characteristics
The studies that met the inclusion criteria of this review reported on virtual care programs from 5 provinces: Ontario (12), British Columbia (8), Alberta (6), Quebec (2), and Saskatchewan (1). One study specifically included Indigenous Nations in 3 provinces (Ontario, Quebec, and New Brunswick), which strengthens the validity of inclusiveness in a rural Canadian context for that study.40 The remaining 7 articles were commentary pieces or reviews that did not enroll patients, but still provided insights relevant to our objectives.54, 55, 56, 57, 58, 59, 60 The articles included 23 full-length articles and 14 abstracts. The articles were published from 2010 to 2021, with nearly half (n = 16) published in the past 5 years.
General CVD interventions
Our findings suggest that health care providers and patients supported the use of virtual care in the management of CVD, with the greatest benefits derived from the ability to share patient data and support patient self-management.24, 25, 26 Two concerns commonly expressed were the accuracy of patient self-reported data and security, therefore support for implementing such systems needs to be tempered by a clear understanding of how these concerns will be mitigated.24 Some reassurance might be derived from the fact that many of these systems include automatic vital sign reporting to the provider (as well as an option for manual input by the patient), and most devices adhere to federal regulatory standards with respect to privacy and security. Taken together, these studies show that virtual care initiatives can be a viable means of overcoming geographic barriers for rural patients, and that patients and providers in rural areas might be uniquely receptive to these innovations.26 Outcomes produced from these programs, including those related to self-efficacy, and mental and physical health status, can be comparable to in-person programs, thereby enhancing the overall reach and equity of health care services.25,26 Recent work done by Chu et al. shows, not surprisingly, that the COVID-19 pandemic has rapidly accelerated this adoption process among rural Ontarians; this is likely a glimpse into a national trend, though evidence for this was not sourced.27
HF interventions
Andrikopoulou and colleagues described a conceptual model of HF disease management in which they state that virtual care is effective because it allows for early detection of worsening HF symptoms. Further, device data might prompt providers to optimize medication regimens and might allow patients to better understand their unique causes of decompensation.61 The real-world literature in this context confirmed these benefits, because the deployment of virtual care technologies generally resulted in a reduced rate and duration of hospital admissions for HF decompensation.28,62 A common point of note was that the success of these programs were largely attributable to the cardiovascular nurses’ ability to assess and manage complex patients and are therefore a key factor in the effectiveness of this model of care.28,62,63 Reassuringly, Jaana and Sherrard concluded that rural HF patients might not be perceived as extensive users of resources, nor patients who represent challenges in terms of feasibility of virtual care use.29 This is encouraging, because it suggests that rural patients, with the potential to benefit more from virtual care services than their urban counterparts, should not believe that they are being perceived as “depriving health care dollars” from urban taxpayers when advocating for its increased promotion and use within their own communities.
Cardiac arrhythmia interventions
The Canadian Cardiovascular Society and Canadian Heart Rhythm Society position statement on virtual care technology for cardiovascular implantable electronic device follow-up suggested that integrating virtual care technologies into clinical practice could accelerate the identification of clinical events and device problems and recommended that virtual care be available at all cardiovascular implantable electronic device follow-up clinics as standard of care.64 The collective studies we identified that met our criteria in this category reported near-universal satisfaction and support for the use of virtual care to augment traditional clinical visits, provided they are tailored to be inclusive of patients living in a rural demographic area, and thereby preserve the vital role of primary care physicians. Virtual care use tended to decrease patient anxiety levels, improved communication of device information, and facilitated patients living in rural communities’ access to specialized care.30,32, 33, 34 A particular point of note was the significant cost and time savings that these technologies allowed. Several challenges were also mentioned, such as changes to clinic workflow, compliance and usability by patients, and the need to develop effective education strategies when initiating monitoring with new patients.30, 31, 32, 33, 34 Taken together, these studies show the importance of involving patients as stakeholders when designing virtual care technology and dissemination strategies. The evidence also suggests that, if designed correctly, patients might not need to attend a preliminary face-to-face visit with their care providers before initiating a virtual care-based health care relationship.31 Established self-learning and orientation using video or walk-through modules is one way to empower patients in this regard.
Ischemic heart disease interventions
Accurate and efficient interpretation of prehospital 12-lead electrocardiograms has been shown to produce favourable patient outcomes because it reduces the delay to reperfusion through fibrinolysis or percutaneous coronary intervention (PCI) therapies.35,65 Our search revealed 2 virtual care-based ST-elevation myocardial infarction detection programs that allow for the transmission of an electrocardiogram every 2 minutes for remote interpretation of abnormalities by a physician and potential diversion to primary PCI facilities.35,36 Both studies illustrate that virtual care can help give timely access to PCI for rural populations that would not otherwise have access to this treatment. Integrating virtual care with paramedic and emergency departments can further augment this capacity to deliver on-time care to rural patients. Despite this, however, one could argue that truly remote patients will never be able to reach a PCI centre within the recommended time window, and thus these results might not be applicable. After an acute event of cardiac ischemia, cardiac rehabilitation programs, consisting of a combination of lifestyle and risk factor management, can improve psychosocial outcomes and reduce premature mortality and future cardiac events. Because these programs are traditionally offered in large urban centres, geography is a common reason for decreased attendance and inaccessibility. The review by Lear summarizes key work done in Canada that leverages virtual care technologies for use in remote cardiac rehabilitation programs.54 The specific studies we found that investigated this model of care showed that they are cost efficient and readily sustainable because of the robust patient interest, uptake, and improved self-efficacy.37, 38, 39 However, Stone presented an alternative opinion in their recent editorial, in which it was claimed that, despite a recent finding that there are > 700 mobile applications available that are dedicated to CVD care, most have failed to find a mainstream space within the everyday delivery of cardiovascular care. Specifically, they argue that most have “failed to find the right interface on the right device in the right practice environment with the right metrics for the right patient and the right payer at the right time and in the right place.”66 This suggests that simply making downloadable applications available passively without provider engagement is not conducive to virtual care uptake or utility and should be avoided as a stand-alone strategy.
Hypertension interventions
The prevalence of hypertension in Canada continues to increase and coordinated efforts to improve the treatment and control of hypertension are needed.67 Despite the high prevalence rate, we only identified 1 primary research article that specifically investigated the use of virtual care for hypertensive patients living in rural areas in Canada. Tobe et al. conducted the Diabetes Risk Evaluation and Management (DREAM-GLOBAL) study to improve hypertension awareness, treatment, and management in Canadian Indigenous populations living in rural areas using virtual care.40,55 Their quantitative results suggest that virtual care can be effective for lowering blood pressure for Indigenous community members under the direction of community leadership.40 Contrary to some studies we discuss herein, their qualitative results revealed that 76% of patients enrolled did not have concerns about the privacy of their data in this context.55 This study also gives credence to the idea that community leaders can be trained to serve as intermediaries between patients in a given community and health care providers at a distance, thereby enhancing communication and participant engagement. This would align with empowered community care, equity, and inclusiveness with Indigenous reconciliation. In an editorial commentary about this study, Padwal suggested that passive virtual care interventions like text messages are unlikely to be effective and that more dynamic interventions such as tele-transmitting blood pressure devices are a better strategy.56 Further cultural engagement with Indigenous communities is needed to have 2-eyed seeing initiated with Indigenous ways of knowing to evaluate the approaches and goals for CVD management.68
Cerebrovascular disease interventions
The phrase “time-is-tissue” is used by cardiologists and neurologists to emphasize that tissue is lost quickly as an infarction progresses without reperfusion, therefore emergent evaluation and rapid therapy are required.69 For this reason, virtual care is paramount to individuals affected by—and involved in the care of—patients with these conditions when geographically distant from health care centres specializing in stroke treatment. The Canadian Stroke Best Practice Guidelines state that virtual care services are a cost-effective tool to support health systems in closing the urban/rural and tertiary/primary care gap.70 In their 2012 article, Dr Hakim57 suggests that the delivery of thrombolysis with the help of virtual care is safe and achieves clinical outcomes similar to the same treatment delivered at stroke centres. He further suggests that a virtual care approach could be used to provide training, teaching, and therapy in the recovery phase for stroke patients living in remote regions.57
Several articles reported on the training necessary to establish a stroke-focused virtual care system, the components of which include: clinical workflow algorithms, nursing/physician competencies, an education/training flow chart, protocols, technical readiness, evaluation tools, and resource contact information.41,46,60 Comprehensive training systems seem to have facilitated efficiencies and standardization of practice to ensure optimal acute stroke management.41 An enthusiastic local leadership team, with strong physician support, and an equally supportive hub site stroke team were found to be necessary for successful implementation, following which regular participation in mock code drills helped to maintain staff confidence and competency.45,46
Access to computed tomography (CT) scanners is paramount to differentiating an acute stroke from an intracranial bleed, which call for entirely antagonistic treatments. Several studies show that remote interpretation of these scans could be carried out by a single hub-site physician responding to emergencies over a large geographic area, allowing patients to be treated effectively without the need for transfer.42,44,45 Stroke quality indicators have been compared between patients treated remotely using these programs and those who received care from an on-site stroke specialist, and they were indeed comparable.44,45 The rationale for not yet nationalizing such a virtual care program remains unclear. Although they are becoming increasingly more affordable, CT scanners are an expensive piece of equipment and are not yet widely available in rural areas. We identified studies that show portable CT scanners can be brought into rural sites and can be operated after minimal training.43,47 These examples of advanced paramedic technology suggest the potential for decentralizing care in remote areas to bring the “hospital to the patient.” Although initial success has been documented, the question of cost effectiveness, particularly in the rural setting, requires further study and should be an important part of ongoing research studies.58
The disparities in CVD rates among Canadian Indigenous populations contribute to increased rates of stroke, and therefore some researchers have partnered with Indigenous organizations to work toward developing culturally appropriate services and support programs. Bodnar reported on projects undertaken within the Northwestern Ontario Regional Stroke Network centred around the development of educational materials and strategies, and virtual care-based initiatives designed to meet the unique needs of Indigenous people.50
After stroke, extensive rehabilitation is often needed, involving physiotherapists, occupational and speech therapists, and social workers, many of whom are not accessible to remote populations. There were a number of studies identified that investigated the use of virtual care to bypass the need for members of these communities to attend in-person sessions.48,49,51, 52, 53 There was strong stakeholder support for most interventions, largely because of significant time and cost savings, derived from the lack of travel, shorter wait times to obtain an appointment, and shorter visit durations, all while maintaining a high degree of patient satisfaction. Although some quantitative data describing these benefits were available, the evidence was sparse and variable, therefore incorporating health economic analyses into virtual care pilots, implementation, and monitoring should be emphasized in future studies.52,53 These health value demonstration initiatives are ideally well powered and longitudinal partnerships between academic institutes and health authorities.
As is a common theme in this review, health care, community, and virtual care partnerships were critical to the success of this program.59,60 Health care providers generally reported confidence in making practice recommendations using these programs, however, a comment about needing to “sense” the client in a different way was repeatedly made, suggesting that reduced nonverbal body language could be a disadvantage of such tools.49,51 Technology-based experiential quality is a surmountable issue and can be overcome through cell tower and emerging satellite high-speed connectivity. These findings also emphasize that the goal of virtual care should not be to eliminate in-person systems, but rather to integrate and augment the standard of care to enhance productivity, efficiency, automation, and patient outcome or experience benefit.
Discussion
To our knowledge, this is the first review of recent publications that examine the use of virtual care technology seeking to benefit patients with CVD who are living in rural Canadian locations. We have attempted to understand the effectiveness of the various interventions, which have generally had positive effects among the patient population of interest. The specific subjective benefits of virtual care include reduced hospitalization and readmission rates, improved mortality rates, increased cost effectiveness for patients and hospitals, improved quality of life for patients, and an increase in self-management of CVD. Further benefit is derived through better disease prevention and increased patient investment in their health span. Virtual care is not a panacea for all rural medicine needs. However, it is clear that value is being extracted, predominantly by 3 Canadian provinces (British Columbia, Ontario, and Quebec), which is concerning because these provinces have the greatest urban concentrations of people.71 These provinces also account for most research pertaining to rural medicine in general, which indicates potential inequities in medical research contrary to the objectives of the Canada Health Act. Remarkably, the Western Prairies, Territories, and the Atlantic provinces (with seemingly the most need and the most to gain from virtual care innovations) are not well represented in this subsection of the literature. In the Canadian context, this puts all provinces at a disadvantage by being over-reliant on federal health transfers to deliver the minimum expectations of care without overpayments for special projects that might be highly relevant to their unique needs (ie, Indigenous and rural communities, to name a few). As an example, Toronto might expect to receive extra payments to conduct urban efficiency studies whereas Manitoba might be ideally suited to a distributed health care optimization program because of their centralized government and unique geography, respectively. There are never sufficient resources to provide all things to all people, however, so the solution might be interprovincial collaboration expectations with regard to health innovation rollouts (ie, Ontario and Manitoba, Quebec and New Brunswick, or Alberta and North West Territories). As such, we should present the best opportunities for Canadians, unbound by provincial political boundaries. Establishing an online, virtual care institute that could intentionally reduce geographic or political barriers might be possible, yet most supercluster and centres of excellence are concentrated in dense urban zones without nodes for change managers in regional areas of priority. Moving forward, exploring this potential inequity from a legal, social, and humanities perspective should be of interest to researchers.
Although still largely unadopted on a national level, the data indicate that systems in place for acute pathologies like stroke and ST-elevation myocardial infarction are the most well established and far-reaching, whereas systems focused on more chronic subtypes of CVD like HF, hypertension, and arrhythmias are lagging and largely remain in the pilot phase. This is an important point to highlight, because the management of chronic disease is likely to have the greatest effect. Studies performed within government by health departments or industry in the form of white papers were inaccessible for review. As a result, very few randomized controlled trials were identified and much of the work in this area is only being shared in conference proceedings. In the wake of the COVID-19 pandemic, this dynamic is likely to rapidly evolve because of the expedited policy changes surrounding the use of virtual care technologies.72 Innovative solutions like the ones discussed herein are now of critical importance because of their ability to reduce contact between health care providers and vulnerable patient populations. However, the lack of coordinated planning to optimize and automate systems of virtual care could also negatively affect perceptions of utility, particularly if telephone-alone use was adopted as a means of avoided in-person visits.
Overall, there is an interest to support virtual care among the stakeholders investigated in this study, despite some perceived barriers compared with in-person approaches to health care. Despite this enthusiasm, there is still insufficient evidence to objectively support the outcomes, health economics, physician/patient satisfaction, and privacy concerns because of a lack of trials and peer-reviewed evidence at this time. Because of the low number of study participants and the overall diversity among the articles, extrapolating definitive, objective conclusions from this data set is prone to bias and therefore should be considered weak evidence to form consensus in any proposed interpretation. CVD risk factor management using virtual care needs peer-reviewed study in the Canadian context to build an evidence base that informs policy development. Such studies should emphasize the rationale, define the intended benefit, and the comparator arm, and address cost effectiveness or any barriers to implementation. Until that time, it remains presumed but uncertain whether virtual care in Canada can assist in the prevention of CVD, compared with more specified conditions requiring interventional support.
Despite that the collective studies lacked the desired scientific rigour, or are of limited metadata, the qualitative insights provide valuable recommendations for researchers planning to deploy virtual care technologies in the future. As mentioned, these have been thematically summarized in Figures 3 and 4. Briefly, a common point of note was the need to involve patients as stakeholders during the planning of virtual care distribution strategies. Kim and colleagues provide insightful commentary as to how best to involve patients.73 Another was the need to clarify the roles and responsibilities of all members of the health care team related to the virtual care system, specifically stressing who will be responsible for the training of patients and informal care providers. This might represent an obstacle best overcome by online education videos or other patient-assistive products and services. Role clarification is needed between the payer and the provider for services and should be explored in future studies. Canada remains predominantly a single, public-paying system and is slow to integrate innovations predominantly developed by the private sector. Establishing a priori criteria to be met by the private sector to deliver virtual care would be welcomed, because the preferences of payers are a critical policy consideration for health authorities. One concern in particular that would require private sector attention is that, in Canada, provincial regulatory standards supersede federal standards with regard to patient confidentiality mandates. Therefore, the onus will be on the provincial authorities to individually vet the security protocols of each system they consider adopting. Rather, such authority should be set as a pass/fail system by Health Canada to avoid interprovincial disparity and inequity. To fulfil their obligations, private companies will need to work with governments to ensure they meet their standards or else risk losing the contract to a competitor. Establishing victory conditions (that is, clear target end points) would avoid any further languishing of incorporating health care technology innovation that is greatly needed, to be prepared for crisis rather than react to it in the example of implementing virtual care during a pandemic.
The current disparities in health metrics suggest that patients in rural areas, particularly Indigenous nations, desire greater diversity in health care delivery, desire to contribute culturally, and require specific accommodations. In addition to virtual care services, advanced care paramedics, mobile care units, increased scope of practice for rural pharmacists, augmented extramural nursing and home care options, and physician assistants could all contribute to rural health care teams. As mentioned, the goal of virtual care should not be to eliminate in-person systems, but rather to focus them and integrate them with existing care processes to enhance productivity, automation, efficiency, and benefit patient outcome or experience. Many of these auxiliary topics, in addition to the challenges of policy and privacy, were not well addressed in these studies and should be the subjects of future research and policy development. Similarly, little in the way of economic analyses were reported, nor is there a consensus for adopting cost-saving or cost-deferring innovations or establishment of time-dependent value thresholds. Studies that did report on the cost savings of the intervention averaged approximately CAD$475 per appointment for patients living in rural communities. Using a reported rural Canadian population of 19%, data showing approximately 500,000 HF patients, and an estimated 10 HF-related health care appointments per year per patient, equating to $451 million in lost opportunity, illustrates that virtual care tools could significantly reduce out-of-pocket expenses for patients.74,75 To further illustrate, we will assume that 60% of all in-person visits could be performed using virtual care tools and systems, as has been the low estimate during the COVID-19 pandemic. The Commonwealth Fund reports that the average annual physician visits per capita in Canada in 2017 was 6.8.76 For a current population of 37.59 million, and an opportunity cost ranging between $31 and $500 per visit, this extrapolates to potential cost savings of approximately $4.8-$77 billion. This is to say nothing with respect to the cost savings for the health care system itself, which would likely be enough to recoup the costs of purchasing and implementing a virtual care system within a short period of time. Industry-partnered health value demonstration initiatives dedicated to regions with a high need can accelerate access to this opportunity by engaging with academic institutions and health authorities in tri-lateral objectives to patient-centred care.
Study limitations and strengths
This systematic review has several limitations. We evaluated published articles written in English from 3 relevant databases, therefore, virtual care interventions described in other languages or those not peer-reviewed, but communicated internally within government health departments were likely missed. Additional search results might have been found using other databases and sources (eg, grey literature, books, etc) and were therefore not included in this review. We also did not evaluate articles that were published before 2010, because we decided that 10 years was a sufficient amount of time to gather material germane to our objectives in a country with a paucity of standard virtual care. Because of the highly specific inclusion criteria, this review risks implying that these are the only interventions that serve rural populations, which is not the case. There are likely to be many virtual care services based in an urban/semirural setting (because that is where specialty services tend to reside) that also serve a wide range of Canadians, including those in rural and remote locales. When these interventions are evaluated, they do not always include an explicit subgroup analysis on the basis of rurality. Furthermore, only a single reviewer screened the included studies, therefore unconscious selection bias might be at play, however, studies show that this approach still represents an appropriate methodological approach.77 Strengths of this review include: a diverse writing group with multiple relevant disciplines represented, the assistance of an experienced librarian for the development of the search strategy, and adherence to the PRISMA guidelines.
Future directions
Many important areas relating to virtual care were not well addressed and require further study. These include: (1) the role of the public and private sectors in evaluating, disseminating, and overseeing the use of virtual care technology; (2) appropriate remuneration rates for using virtual care technology; (3) incentivizing a priori criteria for using and adopting virtual care technology; and (4) preparing future health care providers to incorporate virtual care technology in cardiovascular practices, such as providing training in undergraduate medical education programs and providing continuing medical education credits.
For a successful virtual care program to be developed, it must first meet or ideally improve on desired health outcomes. This must be quantitatively determined through solid surrogate or direct metrics that are clinically meaningful, like rehospitalization, mortality, blood pressure control, etc. Crossover studies will then be required to compare these outcomes between virtual and traditional care practices. Ideally, superiority will be shown, however, noninferiority with virtual care is sufficient if there is a lower cost. The system should be shown, over the long-term, to improve health economics and lower costs (in that the initial investment in technology should yield tangible returns over time through automation, prevention, accessibility, and/or efficiency). When these components have been satisfied, patient and provider satisfaction will need to be shown to be acceptable, scalable, equitable, and adherent to the principles of the Canada Health Act.78
Conclusion
In summary, the systems for acute pathologies seem to be the most well established, whereas those focused on chronic cardiac conditions remain in the pilot phase. Many research groups focused on soft end points like satisfaction without first confirming quantitative noninferiority in health outcome metrics, which will need to be more thoroughly addressed to effect practice change. The evidence presented herein indicates a potential for long-term health care savings with virtual care solutions but are as yet unproven because it has been understudied or indirectly assessed. Using the themes identified in this review—virtual care technologies are likely efficacious, well accepted, and economical, we believe that these systems should be adopted and actively integrated into clinical cardiology practices to care for patients diagnosed with chronic CVD who are living in rural areas of Canada. To this end, the current trajectory of virtual care adoption is running contrary to the principles of the Canada Health Act; specifically, the ideas of universality and accessibility are being violated in that some provinces such as British Columbia and Ontario have great virtual care services, with innovative access and integration, whereas other provinces are years behind or have none at all in practical application. It is our belief that this has reached a point requiring federal intervention, and so we call upon organizations like the Canadian Cardiovascular Society, the Heart and Stroke Foundation of Canada, Hypertension Canada, and the Canadian Institutes of Health Research Institute for Circulatory and Respiratory Health to assist with these advancement efforts and work to establish policies for innovation testing and adoption in collaboration across provincial lines with health authorities, clinicians, and academics. There needs to be virtual care legislation mandated for equitable delivery of these opportunities to the more dispersed and less populated provinces that, in fact, could likely benefit from them the most.
Acknowledgements
The authors acknowledge the help of Jackie Phinney (Librarian, W.K. Kellogg Health Sciences Library, Dalhousie University) who assisted in the development of the literature search strategy.
Funding Sources
This work was generously supported by AGE-WELL NCE, Inc, a member of the Networks of Centres of Excellence program, the New Brunswick Health Research Foundation and the New Brunswick Innovation Foundation. Neither of these organizations played a role in the design, data collection, analysis, or interpretation of this literature review, or in the preparation of the report.
Disclosures
K.R.B. has a direct financial interest in medical technology innovation companies as a director or shareholder of NBBM, Inc, and Routinify, Inc; has received partnership grant/contract funding or financial in-kind support from or serves as a medical science advisor to Ausculsciences, Inc, BaioTeq, Inc, Cloud DX Inc, eVisitNB (Maple Inc), Medtronic Canada Inc, Servier Canada, Inc, IBM Canada, and the Government of New Brunswick. H.C. has a direct financial interest in medical technology innovation companies as a director or shareholder of eVisitNB (Maple, Inc). J.-F.L. and S.L. have received medical technology innovation partnership grant/contract funding or financial in-kind support from Medtronic, Inc, and Servier Canada Inc. The remaining authors have no conflicts of interest to disclose. None of these enterprises listed in this section played a role in the design, data collection, analysis, or interpretation of this literature review, or in the preparation of the report.
Footnotes
Ethics Statement: This scoping review of the literature did not require research ethics board approval at our institution.
See page 145 for disclosure information.
References
- 1.Canadian Institute for Health Information; 2012. Disparities in Primary Health Care Experience Among Canadians with Ambulatory Care Sensitive Conditions.https://secure.cihi.ca/free_products/PHC_Experiences_AiB2012_E.pdf Available at: [Google Scholar]
- 2.Garcia M.C., Faul M., Massetti G., et al. Reducing potentially excess deaths from the five leading causes of death in the rural United States. MMWR Surveill Summ. 2017;66:1–7. doi: 10.15585/mmwr.ss6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subedi R., Greenberg T.L., Roshanafshar S. Does geography matter in mortality? An analysis of potentially avoidable mortality by remoteness index in Canada. Health Rep. 2019;30:3–15. doi: 10.25318/82-003-x201900500001-eng. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S., Shahid R.K. Disparity in cancer care: a Canadian perspective. Curr Oncol. 2012;19:e376–e382. doi: 10.3747/co.19.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luu J., Kirychuk S., Karunanayake C., et al. Access to routine medical care and cardiovascular disease outcomes in a rural Canadian Population (abstract) Can J Cardiol. 2017;33 [Google Scholar]
- 6.Benchimol E.I., Kuenzig M.E., Bernstein C.N., Nguyen G.C., Guttmann A., Jones J.L., et al. Rural and urban disparities in the care of Canadian patients with inflammatory bowel disease: a population-based study. Clin Epidemiol. 2018;10:1613–1626. doi: 10.2147/CLEP.S178056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian Medical Association Ensuring Equitable Access to Care: Strategies For Governments, Health System Planners, and the Medical Profession. https://policybase.cma.ca/en/permalink/policy11062 Available at:
- 8.Canadian Institute for Health Information . 2012. Health Care in Canada: A Focus on Wait Times. [Google Scholar]
- 9.Owens B. Telemedicine on the rise but lagging in Canada. CMAJ. 2018;190:E1149–E1150. doi: 10.1503/cmaj.109-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prescher S., Koehler J., Koehler F. e-Health in cardiology: remote patient management of heart failure patients. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-18/e-health-in-cardiology-remote-patient-management-of-heart-failure-patients Available at:
- 11.Osman L. Canada losing head start on ‘virtual’ health care: task force. https://www.ctvnews.ca/health/canada-losing-head-start-on-virtual-health-care-task-force-1.4806401?cache = yes%3Fot%3DAjaxLayout%3FclipId%3D373266%3FautoPlay%3Dtrue Available at:
- 12.Canadian Medical Association Virtual Care: Recommendations for Scaling up Virtual Medical Services. https://www.cma.ca/sites/default/files/pdf/virtual-care/ReportoftheVirtualCareTaskForce.pdf Available at:
- 13.World Health Organization Fifty-Eighth World Health Assembly. WHA58.28. e-Health. https://www.who.int/healthacademy/media/WHA58-28-en.pdf Available at:
- 14.Castro D., Miller B., Nager A. Unlocking the Potential of Physician-to-Patient Telehealth Services. https://itif.org/publications/2014/05/12/unlocking-potential-physician-patient-telehealth-services Available at:
- 15.Nesbitt TS. The evolution of telehealth: where have we been and where are we going? In: The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. Available at: https://www.ncbi.nlm.nih.gov/books/NBK207141. Accessed September 29, 2020.
- 16.Cannon C. Telehealth, mobile applications, and wearable devices are expanding cancer care beyond walls. Semin Oncol Nurs. 2018;34:118–125. doi: 10.1016/j.soncn.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James P.A., Oparil S., Carter B.L., Cushman W.C., Dennison-Himmelfarb C., Handler J., et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 19.Padwal R.S., So H., Wood P.W., Mcalister F.A., Siddiqui M., Norris C.M., et al. Cost-effectiveness of home blood pressure telemonitoring and case management in the secondary prevention of cerebrovascular disease in Canada. J Clin Hypertens (Greenwich) 2019;21:159–168. doi: 10.1111/jch.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang J.J., Alam S., Cahill L.E., Drucker A.M., Gotay C., Kayibanda J.F., et al. Global Burden of Disease Study trends for Canada from 1990 to 2016. CMAJ. 2018;190:E1296–E1304. doi: 10.1503/cmaj.180698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 22.Paré G., Trudel M.-C., Jaanac M., Kitsioud S. Synthesizing information systems knowledge: A typology of literature reviews. Information & Management. 2015;52:183–199. [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis-Selinger S., Bates J., Araki Y., Lear S.A. Internet-based support for cardiovascular disease management. Int J Telemed Appl. 2011;2011:342582. doi: 10.1155/2011/342582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaglal S.B., Haroun V.A., Salbach N.M., Hawker G., Voth J., Lou W., Kontos P., et al. Increasing access to chronic disease self-management programs in rural and remote communities using telehealth. Telemed J E Health. 2013;19:467–473. doi: 10.1089/tmj.2012.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron J.E., Voth J., Jaglal S.B., Guilcher S.J.T., Hawker G., Salbach N.M. “In this together”: social identification predicts health outcomes (via self-efficacy) in a chronic disease self-management program. Soc Sci Med. 2018;208:172–179. doi: 10.1016/j.socscimed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Chu C., Cram P., Pang A., Stamenova V., Tadrous M., Bhatia R.S. Rural telemedicine use before and during the COVID-19 pandemic: repeated cross-sectional study. J Med Internet Res. 2021;23 doi: 10.2196/26960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak Lauscher H., Ho K., Cordeiro J., Bhullar A., Abu Laban R., Christenson J., et al. TEC4Home heart failure: using home telemonitoring to decrease ED readmissions and clinical flow (abstract) CJEM. 2018;20:S56–S57. [Google Scholar]
- 29.Jaana M., Sherrard H. Rural-urban comparison of telehome monitoring for patients with chronic heart failure. Telemed J E Health. 2019;25:101–108. doi: 10.1089/tmj.2017.0303. [DOI] [PubMed] [Google Scholar]
- 30.Amelio J., Manchak A. Telehealth arrhythmia clinic: initial experience report (abstract) Can J Cardiol. 2010;26:133D. [Google Scholar]
- 31.Sparkes J., Valaitis R., McKibbon A. A usability study of patients setting up a cardiac event loop recorder and BlackBerry gateway for remote monitoring at home. Telemed J E Health. 2012;18:484–490. doi: 10.1089/tmj.2011.0230. [DOI] [PubMed] [Google Scholar]
- 32.Forman J., Flavelle S., Van Breemen O., et al. Evaluating the integration of remote monitoring for cardiovascular implantable electronic device follow-up (abstract) Can J Cardiol. 2014;30:S357–S358. [Google Scholar]
- 33.Rush K.L., Burton L., Van Der Merwe F., Hatt L., Galloway C. Atrial fibrillation care in rural communities: a mixed methods study of physician and patient perspectives. BMC Fam Pract. 2019;20:144. doi: 10.1186/s12875-019-1029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rush K.L., Hatt L., Gorman N., Janicki L., Polasek P., Shay M. Planning telehealth for older adults with atrial fibrillation in rural communities: understanding stakeholder perspectives. Clin Nurs Res. 2019;28:130–149. doi: 10.1177/1054773818758170. [DOI] [PubMed] [Google Scholar]
- 35.Tanguay A., Lebon J., Lau L., Hébert D., Bégin F. Detection of STEMI using prehospital serial 12-lead electrocardiograms. Prehosp Emerg Care. 2018;22:419–426. doi: 10.1080/10903127.2017.1399185. [DOI] [PubMed] [Google Scholar]
- 36.Tanguay A., Dallaire R., Hébert D., Bégin F., Fleet R. Rural patient access to primary percutaneous coronary intervention centers is improved by a novel integrated telemedicine prehospital system. J Emerg Med. 2015;49:657–664. doi: 10.1016/j.jemermed.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Lear S.A., Singer J., Banner-Lukaris D., Horvat D., Park J.E., Bates J., et al. Randomized trial of a “virtual” cardiac rehabilitation program delivered at a distance via the internet. Circ Cardiovasc Qual Outcomes. 2014;7:952–959. doi: 10.1161/CIRCOUTCOMES.114.001230. [DOI] [PubMed] [Google Scholar]
- 38.Pistawka A., Gabelhouse J., Butts D. Telehealth delivery of cardiac education to rural communities (abstract) Can J Cardiol. 2017;33:S224–S225. [Google Scholar]
- 39.Mendell J., Bates J., Banner-Lukaris D., Horvat D., Kang B., Singer J., et al. What do patients talk about? A qualitative analysis of online chat sessions with health care specialists during a “virtual” cardiac rehabilitation program. Telemed J E Health. 2019;25:71–78. doi: 10.1089/tmj.2017.0206. [DOI] [PubMed] [Google Scholar]
- 40.Tobe S.W., Yeates K., Campbell N.R.C., Maar M.A., Perkins N., Liu P.P., et al. Diagnosing hypertension in Indigenous Canadians (DREAM-GLOBAL): a randomized controlled trial to compare the effectiveness of short message service messaging for management of hypertension: main results. J Clin Hypertens (Greenwich) 2019;21:29–36. doi: 10.1111/jch.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmer L., Kelloway L. A clinical toolkit to support acute stroke management using Telestroke in Ontario (abstract) Can J Neurol Sci. 2010;37:S96. [Google Scholar]
- 42.Khan K., Shuaib A., Whittaker T., et al. Telestroke in northern Alberta, Canada: a two-year experience with small remote hospitals. Stroke. 2010;41:e262–e263. doi: 10.1017/s0317167100051489. [DOI] [PubMed] [Google Scholar]
- 43.Shuaib A., Khan K., Whittaker T., Amlani S., Crumley P. Introduction of portable computed tomography scanners, in the treatment of acute stroke patients via telemedicine in remote communities. Int J Stroke. 2010;5:62–66. doi: 10.1111/j.1747-4949.2010.00408.x. [DOI] [PubMed] [Google Scholar]
- 44.Khan F.B., Hall R.E., Stamplecoski M., et al. Acute stroke care: telestroke vs. on-site stroke specialist care. Results from the Ontario telestroke experience (abstract) Stroke. 2011;42:e340. [Google Scholar]
- 45.Jeerakathil T., Shuaib A., Fang S., Butcher K., Saqqur M., Burridge D., et al. Thrombolysis using telehealth has comparable results to non-telehealth thrombolysis across northern Alberta: the Alberta provincial stroke strategy (APSS) (abstract) Stroke. 2012;43(suppl 1):A3107. [Google Scholar]
- 46.Taralson C., Gaucher S. Telestroke in a remote site: one Northern Alberta hospital’s experience establishing acute stroke treatment by telehealth (abstract) Stroke. 2014;45:e285. [Google Scholar]
- 47.Shuaib A., Jeerakathil T. The mobile stroke unit and management of acute stroke in rural settings. CMAJ. 2018;190:E855–E858. doi: 10.1503/cmaj.170999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linkewich E., Harrington K., Oikonen A. “Virtual” home safety assessment after stroke in remote aboriginal communities (abstract) Can J Neurol Sci. 2010;37:S100. [Google Scholar]
- 49.French E.H., Reinikka K.J., Huijbregts M.P. Tele-rehab: the feasibility of using telemedicine to deliver interprofessional stroke rehabilitation consultations in northern, rural and remote communities. Stroke. 2010;41:e506. [Google Scholar]
- 50.Bodnar P. Developing an aboriginal stroke strategy in Northwestern Ontario (abstract) Stroke. 2011;42:e622–e623. [Google Scholar]
- 51.Taylor D.M., Stone S.D., Huijbregts M.P. Remote participants’ experiences with a group-based stroke self-management program using videoconference technology. Rural Remote Health. 2012;12:1947. [PubMed] [Google Scholar]
- 52.Whelan K., Gardner A., Kelly M. The Saskatchewan cerebrovascular center: Telehealth pilot project (abstract) Stroke. 2014;45:e278–e279. [Google Scholar]
- 53.Appireddy R., Khan S., Leaver C., Martin C., Jin A., Durafourt B.A., et al. Home virtual visits for outpatient follow-up stroke care: cross-sectional study. J Med Internet Res. 2019;21 doi: 10.2196/13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lear S.A. The delivery of cardiac rehabilitation using communications technologies: the “Virtual” Cardiac Rehabilitation Program. Can J Cardiol. 2018;34(10 suppl 2):S278–S283. doi: 10.1016/j.cjca.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Barsky J., Hunter R., McAllister C., Yeates K., Campbell N., Liu P., et al. Analysis of the implementation, user perspectives, and feedback from a mobile health intervention for individuals living with hypertension (DREAM-GLOBAL): mixed methods study. JMIR MHealth UHealth. 2019;7 doi: 10.2196/12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padwal R.S. Designing interventions for blood pressure control in challenging settings: active not passive intervention is needed. J Clin Hypertens (Greenwich) 2019;21:37–38. doi: 10.1111/jch.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hakim A.M. The future of stroke thrombolysis. Ann N Y Acad Sci. 2012;1268:8–13. doi: 10.1111/j.1749-6632.2012.06706.x. [DOI] [PubMed] [Google Scholar]
- 58.Butt A., Jeerakathil T. Equitable access to stroke care in Canada - the geographic conundrum. Can J Neurol Sci. 2020;47:285–286. doi: 10.1017/cjn.2020.34. [DOI] [PubMed] [Google Scholar]
- 59.Taralson C., Kelloway L., Green T., Lindsay P. The expansion of telestroke across the continuum of care. Stroke. 2014;45:e265–e266. [Google Scholar]
- 60.Glasser E., Lindsay P., Alcock S., Taralson C. Nursing competencies for telestroke. Int J Stroke. 2015;10:48. [Google Scholar]
- 61.Andrikopoulou E., Abbate K., Whellan D.J. Conceptual model for heart failure disease management. Can J Cardiol. 2014;30:304–311. doi: 10.1016/j.cjca.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 62.TEC4Home Healthcare Innovation Community Supporting heart failure patient transitions from acute to community care with home telemonitoring technology: a protocol for a provincial randomized controlled trial (TEC4Home) JMIR Res Protoc. 2016;5:e198. doi: 10.2196/resprot.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toback M. The effectiveness of telehealth nursing in heart failure self-management. Can J Cardiol. 2017;33:S213–S214. [Google Scholar]
- 64.Yee R., Verma A., Beardsall M., Fraser J., Philippon F., Exner D.V. Canadian Cardiovascular Society/Canadian Heart Rhythm Society joint position statement on the use of remote monitoring for cardiovascular implantable electronic device follow-up. Can J Cardiol. 2013;29:644–651. doi: 10.1016/j.cjca.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 65.O’Gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Jr., Chung M.K., de Lemos J.A., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 66.Stone J.A. Mobile medicine: digital dynamo or virtual vaporware. Can J Cardiol. 2017;33:216–218. doi: 10.1016/j.cjca.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 67.Campbell N., Young E.R., Drouin D., Legowski B., Adams M.A., Farrell J., et al. A framework for discussion on how to improve prevention, management, and control of hypertension in Canada. Can J Cardiol. 2012;28:262–269. doi: 10.1016/j.cjca.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Peltier C. An application of two-eyed seeing: indigenous research methods with participatory action research. Int J Qual Methods. 2018;17:1–12. [Google Scholar]
- 69.Saver J.L. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 70.Blacquiere D., Lindsay M.P., Foley N., Taralson C., Alcock S., Balg C., et al. Canadian stroke best practice recommendations: Telestroke best practice guidelines update 2017. Int J Stroke. 2017;12:886–895. doi: 10.1177/1747493017706239. [DOI] [PubMed] [Google Scholar]
- 71.Statistics Canada Canada goes urban. https://www150.statcan.gc.ca/n1/pub/11-630-x/11-630-x2015004-eng.htm Available at:
- 72.Arnold R.H., Tideman P.A., Devlin G.P., Carroll G.E., Elder A., Lowe H., et al. Rural and remote cardiology during the COVID-19 pandemic: Cardiac Society of Australia and New Zealand (CSANZ) Consensus Statement. Heart Lung Circ. 2020;29:e88–93. doi: 10.1016/j.hlc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim K.K., Khodyakov D., Marie K., Taras H., Meeker D., Campos H.O., et al. A novel stakeholder engagement approach for patient-centered outcomes research. Med Care. 2018;56(suppl 10):S41–S47. doi: 10.1097/MLR.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross H., Howlett J., Arnold J.M., Liu P., O'Neill B.J., Brophy J.M., et al. Treating the right patient at the right time: access to heart failure care. Can J Cardiol. 2006;22:749–754. doi: 10.1016/s0828-282x(06)70290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Statistics Canada Canada’s rural population since 1851. https://www12.statcan.gc.ca/census-recensement/2011/as-sa/98-310-x/98-310-x2011003_2-eng.cfm Available at:
- 76.The Commonwealth Fund Average annual number of physician visits per capita. 2017. https://www.commonwealthfund.org/international-health-policy-center/system-stats/annual-physician-visits Available at:
- 77.Waffenschmidt S., Knelangen M., Sieben W., Bühn S., Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol. 2019;19:132. doi: 10.1186/s12874-019-0782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Government of Canada Canada Health Act (R.S.C., 1985, c. C-6) https://laws-lois.justice.gc.ca/eng/acts/c-6/ Available at: