Abstract
The treatment of cancer mainly involves surgical excision supplemented by radiotherapy and chemotherapy. Chemotherapy drugs act by interfering with tumor growth and inducing the death of cancer cells. Anti-tumor drugs were developed to induce apoptosis, but some patient’s show apoptosis escape and chemotherapy resistance. Therefore, other forms of cell death that can overcome the resistance of tumor cells are important in the context of cancer treatment. Ferroptosis is a newly discovered iron-dependent, non-apoptotic type of cell death that is highly negatively correlated with cancer development. Ferroptosis is mainly caused by the abnormal increase in iron-dependent lipid reactive oxygen species and the imbalance of redox homeostasis. This review summarizes the progression and regulatory mechanism of ferroptosis in cancer and discusses its possible clinical applications in cancer diagnosis and treatment.
Keywords: Cancer, Cancer therapy, Clinical application, Ferroptosis, Lipid peroxidation, Pathogenesis
Introduction
Cell death is the irreversible termination of cell life processes and plays a significant role in organism survival and development.1,2 There are many common forms of cell death, including necrosis and apoptosis.3, 4, 5 Initially, only apoptosis was thought to be the only programmed form of cell death, but as research progressed, it was found that there are more types, including cysteine protease-1 (Caspase-1)-dependent apoptosis and receptor-interacting protein kinase 1 (RIPKl)-dependent necroptosis.6, 7, 8, 9
Ferroptosis is a mode of iron-dependent non-apoptotic cell death, discovered in 2012, that is characterized by the excessive accumulation of lipid peroxides and reactive oxygen species (ROS).10 Ferroptosis differs from apoptosis, necrosis, and other types of cell death by the morphology, genetics, metabolism, and molecular biology of the dying cell.11,12 Studies have found that ferroptosis can inhibit the proliferation of tumor cells in liver, pancreatic, kidney, prostate, and breast cancer.13, 14, 15, 16, 17 Therefore, inducing ferroptosis is expected to provide a new method for anti-tumor therapy and bring new hope to cancer patients. On the other hand, activation of ferroptosis can accelerate the progression of acute kidney injury and neurodegenerative diseases such as Alzheimer's disease.18,19 Other studies have similarly suggested the therapeutic benefits of inhibiting ferroptosis in patients who have an ischemia/reperfusion injury (IRI). Induction of ferroptosis could therefore concerningly lead to further neuronal damage, cardiomyopathy, and renal tubular cell death in brain, heart, and kidney IRI's respectively.20
Cell death escape is one of the most important characteristics of cancer cells, regardless of the cell death type.21, 22, 23 Therefore, a detailed study on the molecular mechanisms and biochemical characteristics of a cell death pathway can provide a set of possible targets for cancer treatment.24 Ferroptosis, in particular, has attracted a lot of attention in the research community because of its important role in the development, progression, and multidrug resistance of cancers.25, 26, 27 This study reviews the definition and characteristics of ferroptosis and summarizes its regulation mechanism in cancers as well as its possible clinical application in anti-tumor therapy.
Forms of regulated cell death
There are two distinct cell death types, regulated cell death (RCD) and accidental cell death (ACD).28,29 RCD relies on specialized molecular mechanisms and can be modulated (delayed or accelerated). It is also not unique to multicellular life forms.28 RCD occurs during the development or regeneration of single-cell eukaryotes (yeast and Dictyostelium discoideum) as well as certain prokaryotes (Escherichia coli). In these cases, RCD is also known as programmed cell death (PCD), which refers to completely physiologic forms of RCD.12,28 ACD is the instantaneous and disorganized death of cells under physical (high pressure, temperature, or osmotic force), chemical (extreme pH changes), or mechanical (shear force) conditions.29
Historically, cell death has been divided into three different categories according to the observed changes in cell morphology (Table 1). This includes type I cell death (apoptosis) where the morphology of cells is characterized by cytoplasmic contraction, chromatin consolidation, nuclear fragmentation, plasma membrane bubbling, and finally formation of undisrupted regular vesicles (commonly known as apoptotic bodies). Type II cell death (autophagy) involves extensive cytoplasmic vacuolization, which is followed by autophagosome-lysosome fusion and cell content degradation. Finally, type III cell death (necrosis) occurs without the involvement of autophagosomes and lysosomes, and its endpoint instead is phagocytic clearance.30,31
Table 1.
Characteristics of the classic forms of cell death.
| Apoptosis | Autophagy | Necrosis | |
|---|---|---|---|
| Inducing factors | Altered expression of genes under physiological conditions | Nutrient deficiency or hormones | Extreme physicochemical conditions or strong pathological stimuli |
| Cell morphology at the initial stages of cell death | Cells become smaller in size | Cells produce vacuoles | Cells become swollen and deformed |
| Cell membrane | Preserves continuity, forms apoptotic bodies | Intact structure | Damaged structure |
| Organelles | Intact structure | Contained in autophagosomes, digested in lysosomes and vacuoles | Deformed or swollen |
| Chromatin | Condensed or fragmented | No typical features | Collapsed |
| DNA | Broken into fragments of varying size | Randomly degraded | Randomly degraded |
| Specific enzymes | Caspase | Lysosomal enzymes | Lysosomal enzymes |
| Characteristic proteins | Caspase 3, Bcl-2, Cytochrome c, DAP | PI3K, mTOR, LC3, DAP | DAP |
| Inflammatory response | Not induced | Can be induced | Can be induced |
DNA, deoxyribo nucleic acid; BCL-2, B cell lymphoma-2; DAP, death-associated protein; PI3K, phosphatidyl inositol 3-kinase; mTOR, mammalian target of rapamyoin; LC3, light chain 3.
The discovery of ferroptosis
In 2003, Dolma et al investigated the mechanism of action of the anti-tumor drug erastin in RAS-mutated cancer cells and found a distinct pattern of cell death that differs from apoptosis. However, the new mechanism was not yet named at that time.32 In 2008, Yang and Stockwell identified two compounds, Ras-selective lethal 3 (RSL3) and Ras-selective lethal 5 (RSL5), which cause cell death in a manner very similar to that of erastin. The resultant cell death process is inhibited by iron chelators and antioxidants, which suggests that it is influenced by iron and ROS.33 Moreover, when Dixon et al treated a human fibrosarcoma HT-1080 cell line harboring the N-RAS mutation with erastin in 2012, analysis by flow cytometry with fluorescent probe labeling showed an increase in the level of cellular lipid ROS 2 h after treatment. After 6 h, the significant increase in the level of ROS was accompanied by an increase in cell death. However, after co-incubation with deferoxamine (DFO), cell death was significantly reduced. Therefore, it can be assumed that the newly discovered cell death mode is directly related to the iron concentration. This was also confirmed by the fact that the addition of ferric ammonium citrate, ferric citrate, ferric chloride, or exogenous iron ions to cell culture as a substitute for other metal ions (Cu2+, Mn2+, Ni2+, Co2+) could increase the level of intracellular ROS as well as the cell death rate.10,34 Because Dixon et al found that the increased cell death rate occurred over the period of ROS accumulation and the antioxidants could inhibit this process, they concluded that the examined cell death mode was different from apoptosis, necrosis, and autophagy, and named it ferroptosis.10
The process and characteristics of ferroptosis
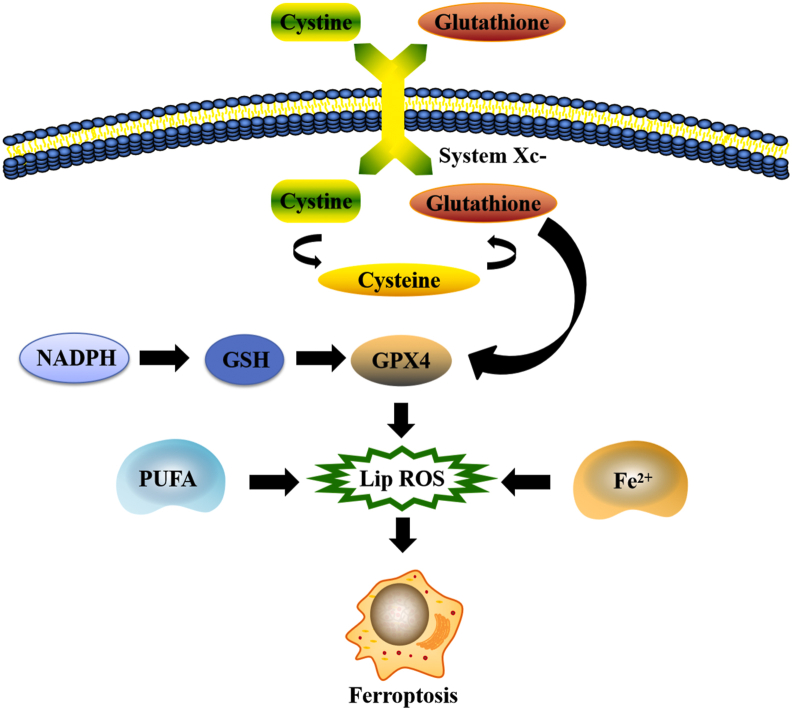
Cystine/glutamate transporter (System ) is composed of solute carrier family 7 member 11 (SLC7A 11) and solute carrier family 3 member 2 (SLC3A 2) and is an important intracellular antioxidant molecule.35,36 System is the upstream transporter molecule in the process of ferroptosis, and its main function is to maintain the balance between cystine (Cys–Cys) intake and glutamate (Glu) excretion. The molecule binds Cys–Cys and reduces it to cysteine (Cys), which is involved in the glutathione (GSH) synthesis pathway.37 Glu reduces ROS and reactive nitrogen species (RNS), which are catalyzed by glutathione peroxidase 4 (GPX 4). Therefore, both Glu depletion and GPX 4 degradation contribute to cellular ferroptosis.10
Ferroptosis is caused mainly by an abnormal increase in iron-dependent lipid reactive oxygen species and an imbalance of redox homeostasis in cells. Induction of ferroptosis in cancer cells can inhibit its growth, which bring new possibilities for the development of anti-tumor treatments and solves the problem of chemotherapy resistance.38, 39, 40 There are many differences between ferroptosis and classical cell death modes (apoptosis, autophagy, necrosis, necroptosis, and pyroptosis) in morphology, biochemistry, genetics, and inducers, which are summarized in Table 2.
Table 2.
Differences between ferroptosis and classical forms of cell death.
| Forms of cell death | Biochemical characteristics | Characteristics observed under the electron microscope | Regulatory mechanism |
|---|---|---|---|
| Ferroptosis | Participation of iron ions, accumulation of iron-dependent ROS | Increased density of cell outer membrane, decreased volume of mitochondria, absence of mitochondrial cristae, ruptured outer mitochondrial membrane | Iron ion metabolism, iron-dependent ROS metabolic pathways, Ras/Raf/MEK/ERK metabolic pathway |
| Apoptosis | Activation of the Caspase pathway, cytoplasmic Ca2+ involvement, formation of Bcl-2-bound oligonucleotide nucleosomes and DNA fragmentation, degradation of mitochondrial transmembrane potential protein | Cells shrunken and round, contracted cell membrane with vesicular projections, condensed and fragmented nucleus, chromatin edge collection, degraded DNA, invaginated cell membrane, apoptotic bodies | FasL/FasR, TNF-α/TNFR1, and other exogenous pathways, endogenous pathways such as Bcl-2 and Caspase pathway |
| Autophagy | Atg, LC3, or p62 as LC3 ligand | Autophagosomes containing cytoplasm and organelles, intact cytoskeleton, degraded organelles such as Golgi apparatus, ribosomes, and endoplasmic reticulum | Atg12-Atg5 and Atg-PE pathways |
| Necrosis | Consumption of ATP, RIP1, RIP3, and MLKL, release of DAMPs, hyperactivation of PARP1 | Swollen cells and organelles, degraded lysosomes, ruptured cell membrane, degraded nuclear chromosome, damaged mitochondria, cell lysis | RIP1 and RIP3, PRR pathways |
| Necroptosis | Fatal influx of Ca2+, formation of RIPK1-RIPK3-MLKL complex | Ruptured cell membrane, swollen organelles and dysfunctional mitochondria | TNF-mediated pathway |
| Pyroptosis | NLRP1b, NLRP3, NLRC4 and AIM2 inflammasome | Cytoplasmic swelling, shrunken cell nucleus, fragmented chromatin, cell membranes with multiple vesicular projections resulting in uneven shear stress and cell membrane rupture | Caspase 1-dependent, accompanied by the release of a large number of proinflammatory factors |
ROS, reactive oxygen species; Ras, rat sarcoma virus; Raf, rapidly accelerated fibrosarcoma; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; BCL-2, B cell lymphoma-2; FasL, fas receptors ligands; FasR, fas receptors; TNF-α, tumor necrosis factor-α; TNFR1, tumor necrosis factor receptor subtype-1; Atg, autophagy-related genes; LC3, light chain 3; ATP, adenosine triphosphate; RIP1, receptor-interacting protein 1; RIP3, receptor-interacting protein 3; MLKL, mixed lineage kinase domain-like; DAMPs, damage associated molecular patterns; PARP1, poly(ADP-ribose) polymerase 1; PRR, pattern recognition receptor; NLRP1b, nucleotide-binding oligomerization domain-like receptor 1b; NLRP3, nucleotide-binding oligomerization domain-like receptor 3; NLRC4, NLR family CARD domain-containing protein 4; AIM2, absent in melanoma 2.
Metabolic regulatory mechanisms of ferroptosis
Iron, lipids, and amino acids are important regulators of ferroptosis, therefore the metabolism of ferroptosis; therefore, the metabolism of these substances plays a significant role in the process of cell death (Fig. 1).
Figure 1.
Regulation of iron metabolism, lipid metabolism and amino acid metabolism in ferroptosis.
Iron metabolism
Normal iron metabolism is crucial for the survival of organisms. It is mainly involved in oxygen transport, DNA biosynthesis, the tricarboxylic acid (TCA) cycle, and the electron transport chain as a coenzyme. It also affects the synthesis of ATP.41 However, excessive levels of iron ions in cells can catalyze the formation of ROS that exhibit metabolic toxicity, leading to cell damage or death.42 Iron is also one of the elements necessary for lipid peroxide accumulation and ferroptosis. Therefore, both flux and storage of iron can affect the occurrence of ferroptosis.43, 44, 45 Studies have shown that Fe3+ binds to transferrin receptor 1 (TFR1) anchored in the cell membrane and is then transferred to the endosome. In endosomes, Fe3+ is reduced to Fe2+, and divalent metal transporter 1 (DMT1) releases it to replenish unstable iron pools in the cytoplasm. Excess iron is coupled to the ferritin light chain (FTL) and the ferritin heavy chain 1 (FTH1), where it is stored in the protein-bound form.46,47 Dolma et al found increased levels of TFR1 and decreased levels of ferritin (FTL and FTH1) in RAS-mutated cells compared with non-mutated controls, which correlated with the occurrence of ferroptosis.10 This outcome suggests that cells with RAS mutations have increased iron intake and reduced iron storage capacity, and the resultant iron overload can induce ferroptosis.48,49 Studies have shown that the use of iron chelating agents (deferramine) inhibit erastin-induced ferroptosis, and supplementation with exogenous iron increases erastin-induced ferroptosis.50, 51, 52 Therefore, regulation of iron ion levels can significantly affect the occurrence of cellular ferroptosis. TFR1 transports iron from the extracellular environment into the cell, which is a necessary step in ferroptosis. This process is regulated by iron-responsive element binding protein 2 (IREB2), as silencing of the IREB2 gene is known to reduce the occurrence of ferroptosis.10 Iron ion levels are also regulated by phosphorylase kinase catalytic subunit gamma 2 (PHKG2), and silencing of this gene is similarly found to reduce the occurrence of ferroptosis.53,54 Autophagy of ferritin also regulates ferroptosis by affecting the level of protein-bound iron in the cell.55,56 Several other cellular proteins that affect iron metabolism, such as heat shock protein beta 1 (HSPB1) and CDGSH iron sulfur domain 1 (CISD1), also regulate ferroptosis.57,58 HSPB1 can inhibit the circulatory metabolism of transferrin receptor 1 (TFR1), which in turn reduces the content of iron in cells, and therefore reduces the sensitivity to ferroptosis.57 On the other hand, CISD1 can increase iron uptake and lipid oxidation in mitochondria, thus enhancing the sensitivity to ferroptosis.58 Therefore, the control over iron metabolism and autophagy of ferritin can potentially be used as a way to regulate the occurrence of ferroptosis.
Lipid metabolism
Dyslipidemia is a signal of cell death by ferroptosis, and lipid metabolism is also closely related to cell ferroptosis.59 Polyunsaturated fatty acids containing diallyl hydrogen atoms are sensitive to lipid peroxidation, and it is one of the elements necessary for ferroptosis.33 Membrane phospholipids containing the polyunsaturated fatty acid (PUFA) are enzymatically oxidized to form lipid peroxides which induce cellular ferroptosis.60 Moreover, under the further action of excess iron, lipid peroxides continue to generate toxic lipid radicals that act on adjacent PUFA, triggering a new round of lipid peroxidation that further accelerates the process of cellular ferroptosis.61 The synthesis of PUFA-containing phospholipids requires Acyl-CoA synthetase long-chain family member 4 (ACSL4) and recombinant lysophosphatidylcholine acyltransferase 3 (LPCAT3) as catalysts.62 The lack of genes encoding these enzymes prevent PUFA from being inserted into membrane phospholipids, thus preventing the formation of PUFA-containing phospholipids. This inhibits lipid peroxidation and reduces the sensitivity of cells to ferroptosis.63 Lipoxygenase (LOX) is an active enzyme that catalyzes the formation of lipid peroxides from PUFA-containing membrane phospholipids. Wenzel et al found that phosphatidylethanolamine binding protein 1 (PEBP1) can bind to LOX and change its structure in a way that enhances lipid peroxidation.64 On the other hand, it was found that elevated glutathione peroxidase 4 (GPX4) activities in cells could inhibit lipid peroxide formation and avoid cellular ferroptosis. The content and location of polyunsaturated fatty acids determine the degree of lipid peroxidation in cells and thus the degree of ferroptosis.65 Lipidomic studies have shown that phosphatidylethanolamine containing arachidonic acid or its derivative, epinephrine, is a key phospholipid that is oxidized and causes ferroptosis.66
Amino acid metabolism
Amino acid metabolism is closely related to the regulation of ferroptosis. The key positive regulators in this process are glutamate and glutamine.67 Glutamate is exchanged with cystine in a 1: 1 ratio by reverse transporter , when concentrations of glutamate are high, this antiporter is inhibited, thereby inducing cellular ferroptosis.10 Similarly, the degradation product of glutamine can provide substrates for the tricarboxylic acid cycle and lipid biosynthesis. Therefore, the absence of glutamine or the lack of glutamine decomposition inhibits cellular ferroptosis by preventing lipids from being synthesized and thus oxidized.55 In vivo animal studies have demonstrated that inhibiting glutamine breakdown can reduce ischemia-reperfusion-induced heart and kidney injury as well as cerebral hemorrhage.67,68 The first step in the decomposition of glutamine is the conversion of glutamine to glutamate, which is catalyzed by glutaminases GLS1 and GLS2. Although GLS1 and GLS2 are highly similar in structure, only GLS2 has been found to be involved in the regulation of ferroptosis. GLS2 is a downstream target gene of p53, and upregulation of GLS2 can promote p53-dependent ferroptosis.69 Since ferroptosis is closely related to the mechanism of cell death in damaged tissues, treatment targeting the glutamine degradation pathway may be a promising method for post-injury organ repair.
Inducers and inhibitors of ferroptosis
A variety of substances can induce ferroptosis, including erastin, RSL3 and RSL5, Buthioninesulfoximine (BSO), acetaminophen, lanperisone, salazosulfopyridine, sorafenib, and artesunate.70 There are also substances that are known to inhibit ferroptosis, such as vitamin E, GSH, ferrostatin-1, liproxstatin −1, and iron chelating agents, as shown in Table 3.
Table 3.
Inducers and inhibitors of ferroptosis.
| Classification | Molecules | Mechanism of action |
|---|---|---|
| Inducers | Erastin, sorafenib, p53 | Inhibit GPX4 function indirectly by inhibiting the glutathione/cystine reverse transport system and glutathione synthesis |
| RSL3, DPI7, DPI10, DPI12, DPI13, DPI17, DPI18, DPI19 | Inhibit GPX4 function directly | |
| FIN56, FINO2, NOX-1, artemisinin | Promote intracellular ROS accumulation and lead to lipid peroxidation | |
| PHKG2 | Activates ferroptosis by increasing iron availability | |
| Inhibitors | Antioxidants (Vitamin E, Trolox, U0126) | Reduce the intracellular ROS accumulation |
| Iron inhibitor and its derivatives, iron chelating agents | Reduce the intracellular content of iron ions | |
| HSPB1 | Reduces the intracellular content of iron ions and lipid ROS | |
| NRF2, MT1 | Block GSH depletion | |
| Desferrioxamine mesylate | Chelates lysosomal or unstable iron | |
| Cyclopyridyl ethanolamine | Prevents lipid peroxidation in the cytoplasm |
GPX4, glutathione peroxidase 4; RSL3, Ras-selective lethal 3; DPI7, diphenylene iodonium 7; DPI10, diphenylene iodonium 10; DPI12, diphenylene iodonium 12; DPI13, diphenylene iodonium 13; DPI17, diphenylene iodonium 17; DPI18, diphenylene iodonium 18; DPI19, diphenylene iodonium 19; FIN56, ferroptosis-inducing 56; FINO2, 1,2-dioxolane; NOX-1, NADPH oxidase 1; ROS, reactive oxygen species; PHKG2, phosphorylase kinase G2; HSPB1, heat shock protein family B member 1; NRF2, nuclear factor erythroid 2-related factor 2; MT1, membrane type 1; GSH, glutathione.
Inducers of ferroptosis
The currently known inducers of ferroptosis include molecules regulating the oxidative stress response and iron ion metabolism. erastin, the first discovered ferroptosis inducer, can mobilize voltage-dependent anion channels (VDACs) to trigger mitochondrial dysfunction.71 Erastin-induced ferroptosis can happen by the production of ROS that damage mitochondria or by direct inhibition of System . This process is additionally enhanced by heme oxygenase-1 (HO-1), which increases the availability of intracellular iron ions and induces ROS production.72, 73, 74, 75 Piperazine erastin, a derivative of erastin, is more soluble and stable in cells than erastin and inhibits the proliferation of human fibroblast HT-1080 cells by inducing ferroptosis.76 Imidazole ketone erastin (IKE), an analog of the original drug known for its greater water solubility and metabolic capacity, also has been shown to more effective at inducing ferroptosis.77 Similar to erastin, lanperisone also induces the production of ROS, causing ferroptosis.78 Another molecule, sorafenib, has been widely used in the treatment of advanced liver cancer. It can enhance the toxicity of System to liver cancer cells, inhibit their proliferation, and promote the sustained cancer decline.79, 80, 81 Studies have found that nuclear factor E2-related factor 2 (NRF2) can regulate the production of GSH and activate the p62-KEAP1-NRF2 pathway to achieve antioxidant activity and prevent cellular ferroptosis.82 After NRF2 and its target genes were knocked out, the activity of sorafenib was enhanced, which led to the decreased proliferation of liver cancer cells. After p62 knockout, HO-1, NAD(P)H quinone oxidoreductase 1 (NQO1), and FTH1 significantly enhanced the activity of ferroptosis inducers such as sorafenib, erastin, and sulfasalazine.82 Small molecule compounds RSL3, DPI2/7/10, and Fin56 can directly inhibit GPX4, and thus increase the production of lipid reactive oxygen species increases, leading to ferroptosis.83, 84, 85 Inducer RSL5 directly interacts with VDAC2/3 to produce ROS and lead to ferroptosis. Buthionine sulfoximine (BSO) irreversibly inhibits γ-glutamyl cysteine synthase (γ-GCS) activity, thereby reducing GSH synthesis and inhibiting its activity. Similarly, an overdose of acetaminophen causes an excessive amount of the reactive metabolite N-acetyl-p-benzoquinone imine to form, thereby reducing GSH stores and inducing ferroptosis.86 As mentioned before, excessive extracellular glutamate also inhibits System activity, which results in ferroptosis induction.87 During the treatment of breast cancer, siramesine can modify the iron content in cells by regulating the expression of transferrin, which can lead to ferroptosis upon iron accumulation. Siramesine also acts as a ferroptosis inducer in conjunction with lapatinib.88
Inhibitors of ferroptosis
Regulation of intracellular iron metabolism, increase in GSH levels, increase in GPX4 activity, and direct inhibition of ROS production can all inhibit cellular ferroptosis. Inhibitors of oxidative stress, such as ferrostatin-1 (first-generation ferroptosis inhibitor) rely on aromatic amines to inhibit the accumulation of specific lipid ROS. They have been proven to inhibit ferroptosis induced by erastin and RSL3.89 However, second-generation (SRS11-92) and third-generation ferroptosis inhibitors (SRS16-86) showed more stable metabolism and significantly improved resistance to tissue damage than first-generation ferroptosis inhibitors.90 As mentioned earlier, ferroptosis can be inhibited by up-regulating NRF2 expression. It can also be prevented by using zileuton to inhibit 5-LOX activity and reduce ROS production.91 Recently, it was discovered that ferroptosis suppressor protein 1 (FSP1) also acts as a ferroptosis inhibitor. FSP1 (formerly known as apoptosis-inducing factor mitochondrial 2), is recruited to the plasma membrane through the process of myristoylation, where it then acts as an oxidoreductase that reduces coenzyme Q10 (CoQ). This process creates a lipophilic radical-trapping antioxidant (RTA) that stops the formation of lipid peroxides, thereby inhibiting ferroptosis.92 In addition, trolox, ebselen, tocopherol, vitamin E, lostoxin A, pepstatin methyl ester, butyl hydroxytoluene, and ammonium chloride all inhibit ferroptosis by interfering with lipid peroxidation pathway.93, 94, 95
Inhibitors regulating iron metabolism effectively inhibit erastin-induced ferroptosis by optimally reducing excessive iron uptake. For example, heat shock protein beta 1 (HSPB1) is involved in the uptake of iron into cells and can affect the expression of its own gene HSPB1 by inhibiting the expression of heat shock transcription factor-1 (HSF-1), thus inhibiting erastin-induced ferroptosis.57 The reduction of TFR1 expression by shRNA also inhibits ferroptosis induced by erastin. Iron chelators, such as DFO and deferoxamine mesylate, inhibit inhibit ferroptosis by reducing the amount of iron available as the electron donor in the process of lipid peroxidation, thus preventing the production of ROS and negatively regulating erastin-induced ferroptosis in human cervical cancer cells, osteosarcoma cells, and prostate cancer cells.70,89,96, 97, 98
Ferroptosis plays an important role in inhibiting tumorigenesis, and cancer cells reduce cell death by inhibiting ferroptosis. solute carrier family 7 member 11 (SLC7A11) is highly expressed in human cancers, and its overexpression inhibits the ferroptosis of cancer cells. When SLC7A11 has been inhibited, ferroptosis can be induced.99,100 Studies have shown that GPX4 plays an important role in the fight against cellular ferroptosis by reducing phospholipid hydrogen peroxide and inhibiting lipoxygenase-mediated lipid peroxidation.101,102 GPX4 uses GSH as a cofactor to catalyze the reduction of lipid peroxides, protect cells and cell membranes from peroxides, and inhibit the occurrence of cellular ferroptosis.103 Studies have shown that mitoNEET (CISD1) protein, an iron-containing mitochondrial outer membrane protein, is used to regulate mitochondrial iron uptake and respiratory function, and can negatively regulate the ferroptosis of cancer cells.58,104
Clinical implications
Biomarker
ACSL4 is a member of the long-chain acyl coenzyme A (acyl-CoA) synthase (ACSL) family. It catalyzes the synthesis of arachidonic acid CoA and adrenal acid CoA and is involved in the synthesis of phosphatidylethanolamine, phosphatidylinositol, and other negatively charged membrane phospholipids.58,62 As an important gene of the lipid metabolism pathway, knockout of the ACSL4 gene in mouse and human cells can effectively reduce the cell mortality induced by erastin and RSL3.105,106 ACSL4 expression was significantly downregulated of ferroptosis-resistant cells (such as LNCaP and K562) compared with that in ferroptosis-sensitive cells (such as HepG2 and HL60). Using CRISPR/Cas9 to perform genome-wide screening and genomic microarray analysis, ACSL4 was found to be an executive factor of ferroptosis, making it a promising biomarker of the process.62
Reduced nicotinamide adenine dinucleotide phosphate (NADPH) is a coenzyme of GSH reductase and therefore plays an important role in maintaining intracellular GSH levels.107 Classical inducers of ferroptosis (erastin, RSL3, and Fin56) were studied in 12 different cell lines, including fibrosarcoma, osteosarcoma, and striated cancer cells.108,109 In said cell lines, intracellular NAD(H) and NADP(H) levels were significantly reduced, and the formation of lipid ROS was identified.110 The consumption of NAD(P) (H) represents the degree of lipid peroxidation, and its level may be a useful biomarker for monitoring the susceptibility of cells to ferroptosis inducers.111,112
The gene encoding cyclooxygenase-2 (COX-2) is a widely-used biomarker of ferroptosis in cancer cells induced by erastin or RSL3.66 In vitro experiments have shown that ferroptosis can be detected by various methods involving the measurement of cell activity, iron content, and ROS levels. However, it is still difficult to prove the existence of ferroptosis in vivo.72
Clinical treatment
Suitable ferroptosis inducers or inhibitors can play a useful therapeutic role in different types of cancers. As a newly discovered type of programmed cell death, ferroptosis kills cancer cells utilizing a unique mechanism and signaling pathway, especially in cancer cells that are not sensitive to death by chemoradiotherapy and apoptosis.113 For example, pancreatic cancer cells are resistant to chemotherapy drug-induced apoptosis but are sensitive to artemisinin-induced ferroptosis.114 In addition, dihydroartemisinin has been proven to induce ferroptosis in head and neck squamous cell carcinoma.115 Lapatinib can induce ferroptosis in breast cancer cells, and acetaminophen can induce ferroptosis in liver injury and hepatotoxicity.88,116,117 Low-level laser irradiation followed by gallic acid treatment was found to treat melanoma cancer cells by inactivating GPX4 activity and thereby inducing ferroptosis.118 The occurrence of ferroptosis in gastric cancer cells was limited by restoring intracellular GSH level, activating GPX4 expression, inhibiting lipid peroxidation, and little ROS accumulation.119,120 Moreover, several molecules, such as RSL3, RSL5, artesunate (ART), docosahexaenoic acid (DHA), and a series of small-molecule ferroptosis-inducing agents (FINs), have been shown to promote ferroptosis in cancer cells.121, 122, 123 Among 60 examined cancer cell lines from eight different sources from the National Cancer Institute (NCI), kidney cancer cells and leukemia cells were more sensitive to the ferroptosis inducer erastin than the cells derived from the other six cancer lines (lung, colon, central nervous system, melanocytes, ovaries, and breast).109 In addition, erastin has been proven to enhance the therapeutic effects of temozolomide, cisplatin, cytarabine, and doxorubicin in specific cancers.124 Traditional Chinese medicine (TCM) has played a significant role in the clinical treatment of cancers, but its mechanism is still unclear.125,126 Recently, it has been found that baicalein can be used as a new ferroptosis inhibitor, and its effect is significantly better than that of typical ferroptosis inhibitors (such as Fer-1 and ferriamine mesylate).127 This provides a basis for further studies comparing the effects of TCM on ferroptosis with the effects of related drugs. Finally, as mentioned earlier, ACSL4 is a promising biomarker for ferroptosis induction. Doll et al recently discovered that this enzyme is significantly expressed in a subset of triple-negative breast cancer cell lines. With a new possibility of inducing ferroptosis into breast cancer cells with this biomarker, this finding provides a promising outlook for potential treatments of this deadly disease.62
Conclusion and future directions
Ferroptosis is a newly discovered form of cell death. At present, the research on ferroptosis is still in the primary stage, and there are many problems to be solved. Further research on the mechanism of cellular ferroptosis could be helpful for the clinical treatment of cancers, and therefore has great developmental potential. Research on the molecular mechanism of ferroptosis as well as potential inducers and inhibitors of the process are likely to be the main focus of future studies. In addition, drugs that are currently widely used in the clinic, such as sorafenib, may find new applications in the context of ferroptosis, which will have an important value in basic research and clinical applications.
Conflict of interests
The authors declare that they have no competing interests.
Acknowledgements
This study was supported by The National Natural Science Foundation of China (No. 81904231,82072978,82072979), the China Postdoctoral Science Foundation (No. 2020M672369), the Natural Science Foundation of Hubei Province (No. 2020CFB861) and the Postdoctoral Innovation Practice Post in Hubei Province (No. 34).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Jianxiang Liu, Email: liujianxiangljx@163.com.
Zengwu Shao, Email: 1985XH0536@hust.edu.cn.
References
- 1.Su Z., Yang Z., Xu Y., et al. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. doi: 10.1186/s12943-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tower J. Programmed cell death in aging. Ageing Res Rev. 2015;23(Pt A):90–100. doi: 10.1016/j.arr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green D.R., Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7(12):a006080. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Arcy M.S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss R.S., Strasser A., McDunn J.E., et al. Cell death. N Engl J Med. 2009;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sollberger G., Strittmatter G.E., Grossi S., et al. Caspase-1 activity is required for UVB-induced apoptosis of human keratinocytes. J Invest Dermatol. 2015;135(5):1395–1404. doi: 10.1038/jid.2014.551. [DOI] [PubMed] [Google Scholar]
- 7.Liao J., Yang F., Tang Z., et al. Inhibition of Caspase-1-dependent pyroptosis attenuates copper-induced apoptosis in chicken hepatocytes. Ecotoxicol Environ Saf. 2019;174:110–119. doi: 10.1016/j.ecoenv.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 8.Yu X., Zhou Y., Cao J., et al. Caspase-1 participates in apoptosis of salivary glands in rhipicephalus haemaphysaloides. Parasites Vectors. 2017;10(1):225. doi: 10.1186/s13071-017-2161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalini S., Dorstyn L., Dawar S., et al. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22(4):526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., Geng Y., Lu X., et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci U S A. 2019;116(8):2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L., Vitale I., Aaronson S.A., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Du L., Qiao Y., et al. Ferroptosis is governed by differential regulation of transcription in liver cancer. Redox Biol. 2019;24:101211. doi: 10.1016/j.redox.2019.101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao D., Yang G., Yang Y., et al. Identification of pannexin 2 as a novel marker correlating with ferroptosis and malignant phenotypes of prostate cancer cells. OncoTargets Ther. 2020;13:4411–4421. doi: 10.2147/OTT.S249752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badgley M.A., Kremer D.M., Maurer H.C., et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M., Gai C., Li Z., et al. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110(10):3173–3182. doi: 10.1111/cas.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y., Palte M.J., Deik A.A., et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10(1):1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan N., Zhang J. Iron metabolism, ferroptosis, and the links with alzheimer's disease. Front Neurosci. 2019;13:1443. doi: 10.3389/fnins.2019.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z., Zhang H., Yang S.K., et al. Emerging role of ferroptosis in acute kidney injury. Oxid Med Cell Longev. 2019:8010614. doi: 10.1155/2019/8010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan H.F., Tuo Q.Z., Yin Q.Z., et al. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool Res. 2020;41(3):220–230. doi: 10.24272/j.issn.2095-8137.2020.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fricker M., Tolkovsky A.M., Borutaite V., et al. Neuronal cell death. Physiol Rev. 2018;98(2):813–880. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg A.D., Dudek-Peric A.M., Romano E., et al. Immunogenic cell death. Int J Dev Biol. 2015;59(1–3):131–140. doi: 10.1387/ijdb.150061pa. [DOI] [PubMed] [Google Scholar]
- 23.Garg A.D., Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280(1):126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 24.Haschka M., Karbon G., Fava L.L., et al. Perturbing mitosis for anti-cancer therapy: is cell death the only answer? EMBO Rep. 2018;19(3):e45440. doi: 10.15252/embr.201745440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang C., Zhang X., Yang M., et al. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31(51):e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 26.Wang W., Green M., Choi J.E., et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedmann Angeli J.P., Krysko D.V., Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19(7):405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 28.Tang D., Kang R., Berghe T.V., et al. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vossenkamper A., Warnes G. Flow cytometry reveals the nature of oncotic cells. Int J Mol Sci. 2019;20(18):4379. doi: 10.3390/ijms20184379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y., Overholtzer M. After-death functions of cell death. Yale J Biol Med. 2019;92(4):687–694. [PMC free article] [PubMed] [Google Scholar]
- 31.Dhuriya Y.K., Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15(1):199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolma S., Lessnick S.L., Hahn W.C., et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 33.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta G., Gliga A., Hedberg J., et al. Cobalt nanoparticles trigger ferroptosis-like cell death (oxytosis) in neuronal cells: potential implications for neurodegenerative disease. FASEB J. 2020;34(4):5262–5281. doi: 10.1096/fj.201902191RR. [DOI] [PubMed] [Google Scholar]
- 35.Singer E., Judkins J., Salomonis N., et al. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. doi: 10.1038/cddis.2014.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutter J., Huang Y., Marovca B., et al. Image-based RNA interference screening reveals an individual dependence of acute lymphoblastic leukemia on stromal cysteine support. Oncotarget. 2014;5(22):11501–11512. doi: 10.18632/oncotarget.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C., Ding Q., Shen B., et al. Insights into the authentic active ingredients and action sites of oral exogenous glutathione in the treatment of ischemic brain injury based on pharmacokinetic-pharmacodynamic studies. Drug Metab Dispos. 2020;48(1):52–62. doi: 10.1124/dmd.119.089458. [DOI] [PubMed] [Google Scholar]
- 38.Doll S., Conrad M. Iron and ferroptosis: a still ill-defined liaison. IUBMB Life. 2017;69(6):423–434. doi: 10.1002/iub.1616. [DOI] [PubMed] [Google Scholar]
- 39.Lachaier E., Louandre C., Godin C., et al. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 2014;34(11):6417–6422. [PubMed] [Google Scholar]
- 40.Xu T., Ding W., Ji X., et al. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med. 2019;23(8):4900–4912. doi: 10.1111/jcmm.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauline M., Verghese S.T., Srinivasu B.Y., et al. Effect of ascorbic acid rich, micro-nutrient fortified supplement on the iron bioavailability of ferric pyrophosphate from a milk based beverage in Indian school children. Asia Pac J Clin Nutr. 2018;27(4):792–796. doi: 10.6133/apjcn.092017.07. [DOI] [PubMed] [Google Scholar]
- 42.Xu D., He Y.B., Chu X., et al. Synthesis of lithium iron phosphate/carbon microspheres by using polyacrylic acid coated iron phosphate nanoparticles derived from iron(III) acrylate. ChemSusChem. 2015;8(6):1009–1016. doi: 10.1002/cssc.201403060. [DOI] [PubMed] [Google Scholar]
- 43.Ong D.C., de Luna M.D.G., Pingul-Ong S.M.B., et al. Manganese and iron recovery from groundwater treatment sludge by reductive acid leaching and hydroxide precipitation. J Environ Manag. 2018;223:723–730. doi: 10.1016/j.jenvman.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 44.Dam T.T.N., Hoang N.T., Nguyen A.T.N., et al. Colloidal dynamics of freshly formed iron oxides under the influence of silicic acid. J Environ Qual. 2019;48(3):670–676. doi: 10.2134/jeq2018.10.0365. [DOI] [PubMed] [Google Scholar]
- 45.Jeong D., Kim K., Min D.W., et al. Freezing-enhanced dissolution of iron oxides: effects of inorganic acid anions. Environ Sci Technol. 2015;49(21):12816–12822. doi: 10.1021/acs.est.5b04211. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y., Li X., Zhao B., et al. Iron metabolism gene expression and prognostic features of hepatocellular carcinoma. J Cell Biochem. 2018;119(11):9178–9204. doi: 10.1002/jcb.27184. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Li X., Mu Y., et al. The iron chelator desferrioxamine synergizes with chemotherapy for cancer treatment. J Trace Elem Med Biol. 2019;56:131–138. doi: 10.1016/j.jtemb.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Manz D.H., Blanchette N.L., Paul B.T., et al. Iron and cancer: recent insights. Ann N Y Acad Sci. 2016;1368(1):149–161. doi: 10.1111/nyas.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao M., Monian P., Jiang X. Metabolism and iron signaling in ferroptotic cell death. Oncotarget. 2015;6(34):35145–35146. doi: 10.18632/oncotarget.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Yu L., Ding J., et al. Iron metabolism in cancer. Int J Mol Sci. 2018;20(1):95. doi: 10.3390/ijms20010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H., An P., Xie E., et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66(2):449–465. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao M., Monian P., Pan Q., et al. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu B., Chen X.B., Ying M.D., et al. The role of ferroptosis in cancer development and treatment response. Front Pharmacol. 2017;8:992. doi: 10.3389/fphar.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mou Y., Wang J., Wu J., et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao M., Monian P., Quadri N., et al. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou W., Xie Y., Song X., et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun X., Ou Z., Xie M., et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34(45):5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan H., Li X., Zhang X., et al. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478(2):838–844. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 59.Kagan V.E., Mao G., Qu F., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang W.S., Kim K.J., Gaschler M.M., et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez M.A., Magtanong L., Dixon S.J., et al. Dietary lipids induce ferroptosis in caenorhabditiselegans and human cancer cells. Dev Cell. 2020;54(4):447–454. doi: 10.1016/j.devcel.2020.06.019. e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doll S., Proneth B., Tyurina Y.Y., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dixon S.J., Winter G.E., Musavi L.S., et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10(7):1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wenzel S.E., Tyurina Y.Y., Zhao J., et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171(3):628–641. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedmann Angeli J.P., Schneider M., Proneth B., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stoyanovsky D.A., Tyurina Y.Y., Shrivastava I., et al. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2019;133:153–161. doi: 10.1016/j.freeradbiomed.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Q., Han X., Lan X., et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2(7) doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 69.Kang R., Kroemer G., Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–168. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie Y., Hou W., Song X., et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skonieczna M., Cieslar-Pobuda A., Saenko Y., et al. The Impact of DIDS-induced inhibition of voltage-dependent anion channels (VDAC) on cellular response of lymphoblastoid cells to ionizing radiation. Med Chem. 2017;13(5):477–483. doi: 10.2174/1573406413666170421102353. [DOI] [PubMed] [Google Scholar]
- 72.Park E., Chung S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10(11):822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geng N., Shi B.J., Li S.L., et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci. 2018;22(12):3826–3836. doi: 10.26355/eurrev_201806_15267. [DOI] [PubMed] [Google Scholar]
- 74.Liu N., Lin X., Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br J Cancer. 2020;122(2):279–292. doi: 10.1038/s41416-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y.Q., Chang S.Y., Wu Q., et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front Aging Neurosci. 2016;8:308. doi: 10.3389/fnagi.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang W.S., SriRamaratnam R., Welsch M.E., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larraufie M.H., Yang W.S., Jiang E., et al. Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg Med Chem Lett. 2015;25(21):4787–4792. doi: 10.1016/j.bmcl.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaw A.T., Winslow M.M., Magendantz M., et al. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci U S A. 2011;108(21):8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao L., Zhao T., Song Y., et al. The emerging role of ferroptosis in non-cancer liver diseases: hype or increasing hope? Cell Death Dis. 2020;11(7):518. doi: 10.1038/s41419-020-2732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang H., Chen D., Li C., et al. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int J Pharm. 2019;572:118782. doi: 10.1016/j.ijpharm.2019.118782. [DOI] [PubMed] [Google Scholar]
- 81.Tang H., Li C., Zhang Y., et al. Targeted manganese doped silica nano GSH-cleaner for treatment of liver cancer by destroying the intracellular redox homeostasis. Theranostics. 2020;10(21):9865–9887. doi: 10.7150/thno.46771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun X., Ou Z., Chen R., et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sui X., Zhang R., Liu S., et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imai H., Matsuoka M., Kumagai T., et al. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–170. doi: 10.1007/82_2016_508. [DOI] [PubMed] [Google Scholar]
- 85.Shin D., Kim E.H., Lee J., et al. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 86.Yamada N., Karasawa T., Kimura H., et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020;11(2):144. doi: 10.1038/s41419-020-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bridges R.J., Natale N.R., Patel S.A. System xc(-) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165(1):20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma S., Henson E.S., Chen Y., et al. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y., Sun C., Zhao C., et al. Ferroptosis inhibitor SRS 16-86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Res. 2019;1706:48–57. doi: 10.1016/j.brainres.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 91.Kang Y., Tiziani S., Park G., et al. Cellular protection using Flt3 and PI3Kalpha inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nat Commun. 2014;5:3672. doi: 10.1038/ncomms4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bersuker K., Hendricks J.M., Li Z., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yotsumoto S., Muroi Y., Chiba T., et al. Hyperoxidation of ether-linked phospholipids accelerates neutrophil extracellular trap formation. Sci Rep. 2017;7(1):16026. doi: 10.1038/s41598-017-15668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinman A., Holst C.R., Latham J.C., et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PloS One. 2018;13(8):e0201369. doi: 10.1371/journal.pone.0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zilka O., Shah R., Li B., et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. 2017;3(3):232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11–12):2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu H., Guo P., Xie X., et al. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21(4):648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J., Cao F., Yin H.L., et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang L., Kon N., Li T., et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lang X., Green M.D., Wang W., et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forcina G.C., Dixon S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19(18) doi: 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- 102.Ingold I., Berndt C., Schmitt S., et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172(3):409–422. doi: 10.1016/j.cell.2017.11.048. e21. [DOI] [PubMed] [Google Scholar]
- 103.Ursini F., Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–185. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 104.Kang R., Tang D. Autophagy and ferroptosis - what's the connection? Curr Pathobiol Rep. 2017;5(2):153–159. doi: 10.1007/s40139-017-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li W., Li W., Leng Y., et al. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39(2):210–225. doi: 10.1089/dna.2019.5097. [DOI] [PubMed] [Google Scholar]
- 106.Muller T., Dewitz C., Schmitz J., et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74(19):3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Winkler B.S., DeSantis N., Solomon F. Multiple NADPH-producing pathways control glutathione (GSH) content in retina. Exp Eye Res. 1986;43(5):829–847. doi: 10.1016/s0014-4835(86)80013-6. [DOI] [PubMed] [Google Scholar]
- 108.Fan Z., Wirth A.K., Chen D., et al. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6(8):e371. doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu T., Shi L., Yu C., et al. Ferroptosis promotes photodynamic therapy: supramolecular photosensitizer-inducer nanodrug for enhanced cancer treatment. Theranostics. 2019;9(11):3293–3307. doi: 10.7150/thno.32867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimada K., Hayano M., Pagano N.C., et al. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chem Biol. 2016;23(2):225–235. doi: 10.1016/j.chembiol.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lv H., Zhen C., Liu J., et al. Beta-phenethyl isothiocyanate induces cell death in human osteosarcoma through altering iron metabolism, disturbing the redox balance, and activating the MAPK signaling pathway. Oxid Med Cell Longev. 2020;2020:5021983. doi: 10.1155/2020/5021983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shimada K., Skouta R., Kaplan A., et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu Y., Qin Z., Ma J., et al. Recent progress in nanotechnology based ferroptotic therapies for clinical applications. Eur J Pharmacol. 2020;880:173198. doi: 10.1016/j.ejphar.2020.173198. [DOI] [PubMed] [Google Scholar]
- 114.Yamaguchi Y., Kasukabe T., Kumakura S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol. 2018;52(3):1011–1022. doi: 10.3892/ijo.2018.4259. [DOI] [PubMed] [Google Scholar]
- 115.Lin R., Zhang Z., Chen L., et al. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016;381(1):165–175. doi: 10.1016/j.canlet.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 116.Villalpando-Rodriguez G.E., Blankstein A.R., Konzelman C., et al. Lysosomal destabilizing drug siramesine and the dual tyrosine kinase inhibitor lapatinib induce a synergistic ferroptosis through reduced heme oxygenase-1 (HO-1) levels. Oxid Med Cell Longev. 2019;2019:9561281. doi: 10.1155/2019/9561281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma S., Dielschneider R.F., Henson E.S., et al. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PloS One. 2017;12(8):e0182921. doi: 10.1371/journal.pone.0182921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khorsandi K., Kianmehr Z., Hosseinmardi Z., et al. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020;20:18. doi: 10.1186/s12935-020-1100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hao S., Yu J., He W., et al. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia. 2017;19(12):1022–1032. doi: 10.1016/j.neo.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao Z., Deng G., Li Y., et al. Actinidia chinensis planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed Pharmacother. 2020;126:110092. doi: 10.1016/j.biopha.2020.110092. [DOI] [PubMed] [Google Scholar]
- 121.Ou W., Mulik R.S., Anwar A., et al. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med. 2017;112:597–607. doi: 10.1016/j.freeradbiomed.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lei G., Zhang Y., Koppula P., et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30(2):146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Y., Chen H., Xuan N., et al. Induction of ferroptosis-like cell death of eosinophils exerts synergistic effects with glucocorticoids in allergic airway inflammation. Thorax. 2020;75(11):918–927. doi: 10.1136/thoraxjnl-2020-214764. [DOI] [PubMed] [Google Scholar]
- 124.Basu S., Barnoud T., Kung C.P., et al. The African-specific S47 polymorphism of p53 alters chemosensitivity. Cell Cycle. 2016;15(19):2557–2560. doi: 10.1080/15384101.2016.1215390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu J., Mao J.J., Wang X.S., et al. Evaluation of traditional Chinese medicine herbs in oncology clinical trials. Cancer J. 2019;25(5):367–371. doi: 10.1097/PPO.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 126.Wang S., Long S., Wu W. Application of traditional Chinese medicines as personalized therapy in human cancers. Am J Chin Med. 2018;46(5):953–970. doi: 10.1142/S0192415X18500507. [DOI] [PubMed] [Google Scholar]
- 127.Xie Y., Song X., Sun X., et al. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem Biophys Res Commun. 2016;473(4):775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]