Abstract
Cancer stem cells (CSCs) are a distinct population of cells within tumors with capabilities of self-renewal and tumorigenicity. CSCs play a privotal role in cancer progression, metastasis, and relapse and tumor resistance to cytotoxic therapy. Emerging scientific evidence indicates that CSCs adopt several mechanisms, driven by cellular plasticity, senescence and quiescence, to maintain their self-renewal capability and to resist tumor microenvironmental stress and treatments. This poses major hindrances for CSC-targeting anti-cancer therapies: cell plasticity maintains stemness in CSCs and renders tumor cells to acquire stem-like phenotypes, contributing to tumor heterogeneity and CSC generation; cellular senescence induces genetic reprogramming and stemness activation, leading to CSC-mediated tumor progression and metastasis; cell quienscence facilitates CSC to overcome their intrinsic vulnerabilities and therapeutic stress, inducing tumor relapse and therapy resistance. These mechanisms are subjected to spatiotemporal regulation by hypoxia, CSC niche, and extracellular matrix in the tumor microenvironment. Here we integrate the recent advances and current knowledge to elucidate the mechanisms involved in the regulation of plasticity, senescence and quiescence of CSCs and the potential therapeutic implications for the future.
Keywords: Cancer stem cells, Plasticity, Senescence, Quiescence, Tumor microenvironment, Therapy resistance
Introduction
Stem cells are cells with both self-renewing and differentiating abilities, that are capable of developing into many different cell types. Most cancers comprise a heterogenous population of cells with different capacities of tumor initiating ability. Only distinct populations of less differentiated cells found within tumors have a high self–renewal ability and tumorigenic potential. These cells are referred as cancer stem cells (CSCs), tumor-propagating cells, or tumor initiating cells, which are pluripotent and have the ability to repopulate tumors (M Al-Hajj & Clarke, 2004), (Reya, Morrison, Clarke, & Weissman, 2001). CSCs were first identified in leukemia using the experimental approach of xenotransplantation (Bonnet & Dick, 1997). By now, most blood cancers and solid tumors, such as breast, brain, and colon cancers and melanoma, have been shown to possess unique populations of CSCs (Muhammad Al-Hajj, Wicha, Benito-Hernandez, Morrison, & Clarke, 2003), (Singh et al., 2004), (Galli et al., 2004), (O’Brien, Pollett, Gallinger, & Dick, 2007), (Schatton et al., 2008). CSCs are refractory to cytotoxic treatment and significantly contribute to tumor resistance to radio/chemotherapy; CSCs also initiate metastasis and are responsible for cancer relapse through their ability to self-renew and proliferate into the bulk of the tumor (Shiozawa, Nie, Pienta, Morgan, & Taichman, 2013), (Reya et al., 2001). Development of new therapies that are effective at eradication of CSCs, therefore, is urgently needed.
Although the concept of CSCs has been established for several decades, our understanding of their biology and therapeutic implication is still evolving (Nguyen, Vanner, Dirks, & Eaves, 2012), (Kreso & Dick, 2014), (Capp, 2019). Notably, evolving concepts of plasticity, senescence, and quiescence have indicated some caveats associated with the classical concepts of CSCs. For instance, accumulating evidence suggests the possibility that CSC-like cells thrive in a dynamic and plastic state, where a non-stem like cell can acquire stem-like characteristics, a phenomenon that is contradictory to the unidirectional and binary view of CSCs as a rare population of cells that can repopulate bulk tumor cells (Batlle & Clevers, 2017), (Gupta et al., 2011). Plasticity has complicated the identification and targeting of CSCs. Another insight from recent studies highlights the possibility of reprogramming of chemotherapy-induced senescent cells into a stem-like state (Milanovic, Fan, et al., 2018), highlighting the possibility of senescence being a reversible process. In addition to transition between diverse differentiation states, CSCs can also switch into a dormant or quiescent state, a property that has been associated with incurring therapeutic resistance (W. Chen, Dong, Haiech, Kilhoffer, & Zeniou, 2016). While such emerging concepts have reshaped our understanding of CSCs, we need to revisit some of the clinical implications of CSC plasticity, senescence, and quiescence from the recent studies involving single-cell approaches and other advanced technologies. In this review we will discuss the recent advances in our understanding of the mechanisms and pathways involved in the maintenance of plasticity, senescence and quiescence of CSCs and their potential therapeutic implications for the future.
CSCs and tumor heterogeneity
Most cancers contain a heterogenous population of cells. For example, bulk transcriptomic analyses of glioblastoma (GBM) tumors identified the existence of several tumor subtypes including proneural, classical, and mesenchymal, all of which exist in the same tumors with prominent heterogeneity (Verhaak et al., 2010). Recent single-cell RNA sequencing analysis highlighted intratumoral heterogeneity and revealed interconvertible transcriptional states in GBM (Neftel et al., 2019). Similarly, a single-cell RNAseq study showed intratumoral heterogeneity in triple negative breast cancer (S. Zhou et al., 2021). Not all cells within the tumor have equal potential to proliferate and give rise to the bulk of the tumor. There are two postulated models on tumor heterogeneity. The first one, called the stochastic model or the cancer stem cell model, states that tumor cells are organized in a hierarchical manner, with the cells at the apex possessing stem-like properties. These specialized cells referred as CSCs, are capable of self-renewal and repopulate the tumor (Tang, 2012). An alternative view, known as the clonal evolution model, posits that heterogeneity in tumors arise from sub clonal differences. Contrary to the stochastic model, this model states that all tumor cells may contribute to tumor maintenance but in varying degree. According to this model, tumor cells are rapidly evolving due to genetic and epigenetic changes, giving rise to sub clones with varying capacity of tumorigenesis (Campbell & Polyak, 2007), (Greaves & Maley, 2012). In other words, while the CSC model attributes to functional heterogeneity with no regards to their genetic/epigenetic identity, the clonal evolution model attributes to genetic and epigenetic heterogeneity ignoring the intrinsic, functional, and phenotypic differences within individual sub clones of the tumors. Over time, other reviews have pointed out that these two models are not mutually exclusive (Shackleton, Quintana, Fearon, & Morrison, 2009), (Marusyk, Almendro, & Polyak, 2012), (Sreekumar, Roarty, & Rosen, 2015). In both blood and some solid tumors, studies have revealed that there is ample genetic diversity within stem cell populations, indicating both these models exert their respective influences on tumorigenesis (Anderson et al., 2011), (Shipitsin et al., 2007). It is also to be noted that there is evidence of multiple distinct stem cell populations in breast cancer, with variable therapeutic susceptibilities, which again deviates from the classical view of existence of a single stem cell population in cancer. In summary, although the concepts of tumor heterogeneity are continuously evolving, there is an emerging consensus of tumors consisting of stem-like cells that are defined by a distinct ‘cellular-state’ rather than phenotype.
Characteristics of CSCs
Both normal stem cells and CSCs are known to possess self-renewing and differentiating ability, also referred as asymmetric cell division. This maintains a steady stem cell population in a tissue, and therefore they share common stem cell markers. Classically CSCs were identified based on certain marker expressions. In acute myeloid leukemia, CD34+CD38− cells are considered as the stem cell population (Bonnet & Dick, 1997). Similarly, CD133 expression is associated with CSCs in brain tumors including medulloblatoma (MB) and glioblastoma (GBM), and CD44+CD24− cells were identified as breast cancer CSCs (Singh et al., 2004), (Muhammad Al-Hajj et al., 2003). Although CSC surface markers have proven to be useful for enrichment of CSCs, they have limitations owing to heterogeneity in expression and regulation by the microenvironment (Visvader & Lindeman, 2012). This embarks upon the importance of functional assays to identify CSC-like characteristics. CSCs can be defined by functional analysis: sphere formation and limiting dilution assays are used to verify the self-renewing ability and determine the stem cell frequency in CSCs, while serial tumor transplantation is considered a gold standard to detect tumorigenic potential of CSCs. Finally, CSCs can be characterized by activation of a number of pathways and transcription factors that specifically regulate stemness, including Wnt/β-catenin, Notch, and hedgehog signaling pathways. OCT4, NANOG, SOX2 are some well-established transcription factors that regulate stemness in GBM and are often used as CSC markers (Prager, Bhargava, Mahadev, Hubert, & Rich, 2020). Wnt/β-catenin pathway is another prominent regulator of stemness in multiple cancers (Atlasi, Looijenga, & Fodde, 2014), (Reya & Clevers, 2005). All these pathways play major roles in acquiring and maintaining stem-like features such as self-renewal ability, plasticity, quiescence, about which we shall discuss in more detail in the following sections.
Plasticity, senescence, and quiescence – major hindrances in targeting CSCs
Growing evidence has established that stem-like cells are more resistant to conventional therapies including chemotherapy, radiation, and molecularly targeted therapy, compared to non-stem-like cells in the tumors. To develop new effective therapies targeting the stem-like population of tumor cells, we need to have a thorough understanding of the characteristics of CSCs and their mechanisms of resistance induction and acquisition of stem-cell like characteristics, which seems to be highly related to their plasticity, senescence, and quiescence.
While it is postulated that CSCs give rise to the bulk tumor cells by asymmetric division, this hierarchy is not unidirectional, and tumor cells can de-differentiate and acquire stem-like properties, a process referred as plasticity (Figure 1). Plasticity may occur through genetic and epigenetic changes, again highlighting the non-mutual exclusivity of the two models of heterogeneity. In GBM, tumor cells expressing CSC markers do not represent a functionally distinct clonal entity but rather a plastic state that most cells can attain in response to microenvironmental cues (Dirkse et al., 2019). Recent single-cell RNA sequencing analyses of human GBM tumors highlight intratumoral heterogeneity (Patel et al., 2014), (Lopes & Vinga, 2020), and suggest that tri-lineage cancer hierarchy and cell plasticity co-exist in the tumors (Couturier et al., 2020), (Neftel et al., 2019). In basal-like subtype of breast cancer, non-CSC tumor cells have the ability to switch into a CSC-like state through ZEB1 (Chaffer et al., 2013). Plasticity not only refers to de-differentiation processes but can also refer to transitions between different cellular states comprising heterogeneities within tumors, as indicated by the coexistence of four cellular states within individual tumors in GBM, namely neural progenitor-, oligodendrocyte-, astrocyte-, and mesenchymal-like states, which can interconvert into each other (Neftel et al., 2019). Because of such rapid changes in cell states, cell plasticity may pose a major hindrance to treatments targeting a particular cell-state.
Figure 1. Plasticity in cancer stem cells –
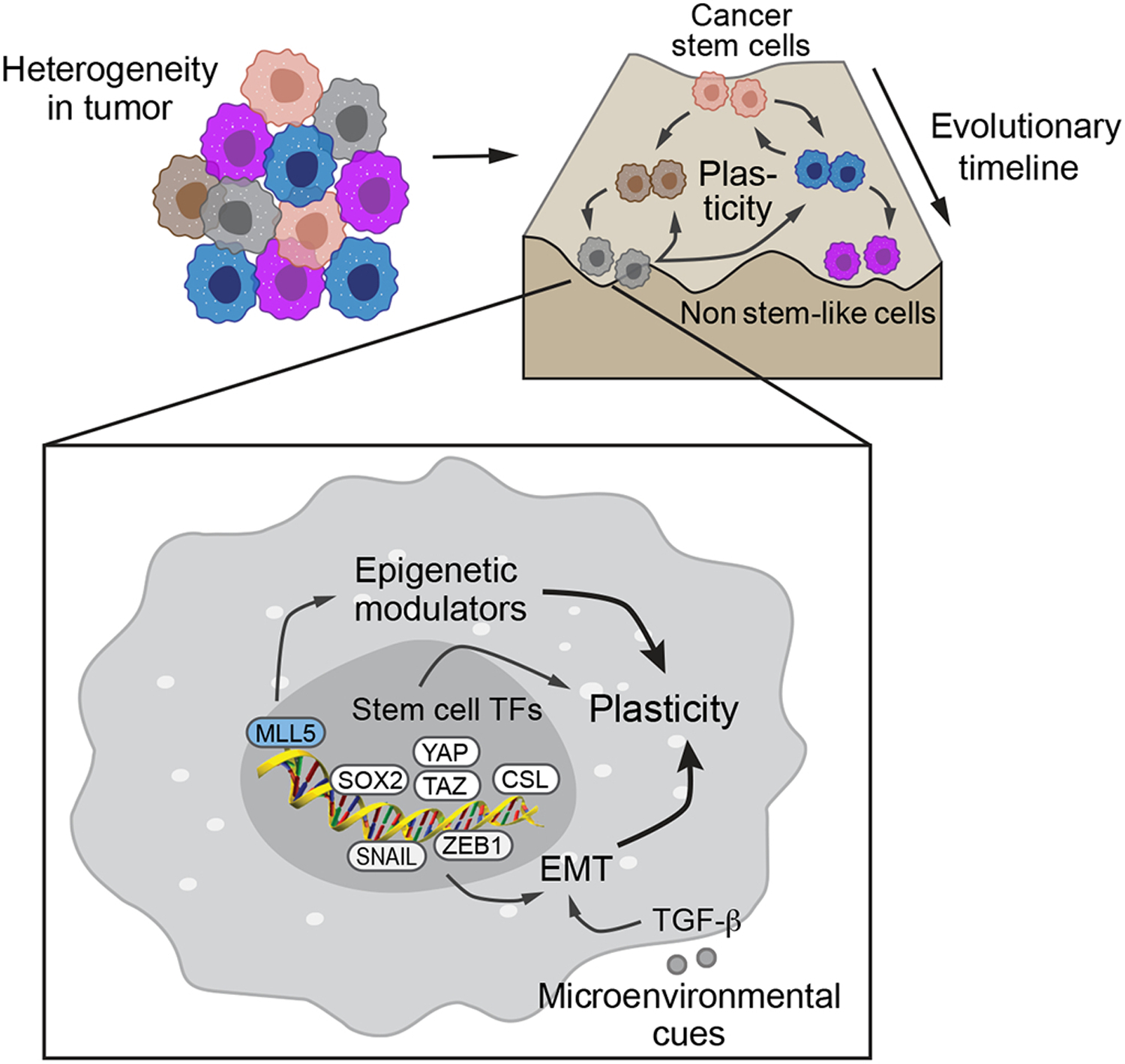
Tumors are heterogenous population of cells, composed of cells belonging to different hierarchies in the epigenetic landscape (adapted from Corad Waddington’s diagram). The cells at the top of the hierarchy are the cancer stem cells (CSCs) with the potential to give rise to all other cells in the tumor. The cells at the bottom of the landscape are the non-stem cells which usually have a limited tumorigenic potential, and stay within their respective state. Upregulation of stem cell pathways in these cells, can make them transition into a cell state higher up in the hierarchy, which is reffered as plasticity. The pathways inducing the push toward stemness are mediated through eptithelial-mesenchymal transition (EMT) transcriptiom factors such as SNAIL and ZEB1, stem cell transcription factors such as SOX2, Hippo/YAP/TAZ, NOTCH/CSL, and epigenetic modulator MLL5.
Cellular senescence is a terminal cellular state characterized by growth arrest and cessation of cell division (Figure 2) (Campisi & D’Adda Di Fagagna, 2007), (Di Micco, Krizhanovsky, Baker, & d’Adda di Fagagna, 2021). Consistent with its well-established tumor-inhibitory role, cellular senescence can limit the tumorigenic potentials of neoplastic cells and also contribute to the outcome of cytotoxic therapy (Serrano, Lin, McCurrach, Beach, & Lowe, 1997), (Braig et al., 2005), (Michaloglou et al., 2005), (Schmitt et al., 2002), (Dörr et al., 2013). However, senescence and stemness functions are co-regulated by overlapping signaling pathways including p16, p21, and p53, suggesting that senescence may induce genetic reprogramming and stemness activation and contribute to CSC-mediated tumor progression, metastasis, and therapy resistance (Zon, 2008), (Milanovic, Fan, et al., 2018). This is further supported by recent work showing that cells with therapy-induced senescence displayed high tumor initiating ability and upregulated canonical Wnt signaling (Milanovic, Yu, & Schmitt, 2018), indicating that senescent cell cycle arrest may not be terminal and possibly reversible, despite the traditional view of cellular senescence being a final and irreversible phase of a cell cycle. This suggests that dynamic senescence in tumors may promote CSC phenotypes. Recent studies also provide molecular insights into the dysfunctional senescence: in response to genotoxic insults or oncogenic stress, tumor cells upregulate a large-scale chromatin remodeling, involving repressive methylation marks that stably repress S-phase promoting genes, but also upregulates the secretion of pro-tumorigenic factors and stimulates stem cell transcriptional factors such as WNT/LEF1 (Lecot, Alimirah, Desprez, Campisi, & Wiley, 2016) (Milanovic, Fan, et al., 2018). Elucidation of the pathways involved in such reprogramming mechanisms during cellular senescence will help us navigate through the difficulties posed by the current therapies to successfully targeting the CSCs in future.
Figure 2. Senescence and quiescence in cancer stem cells –
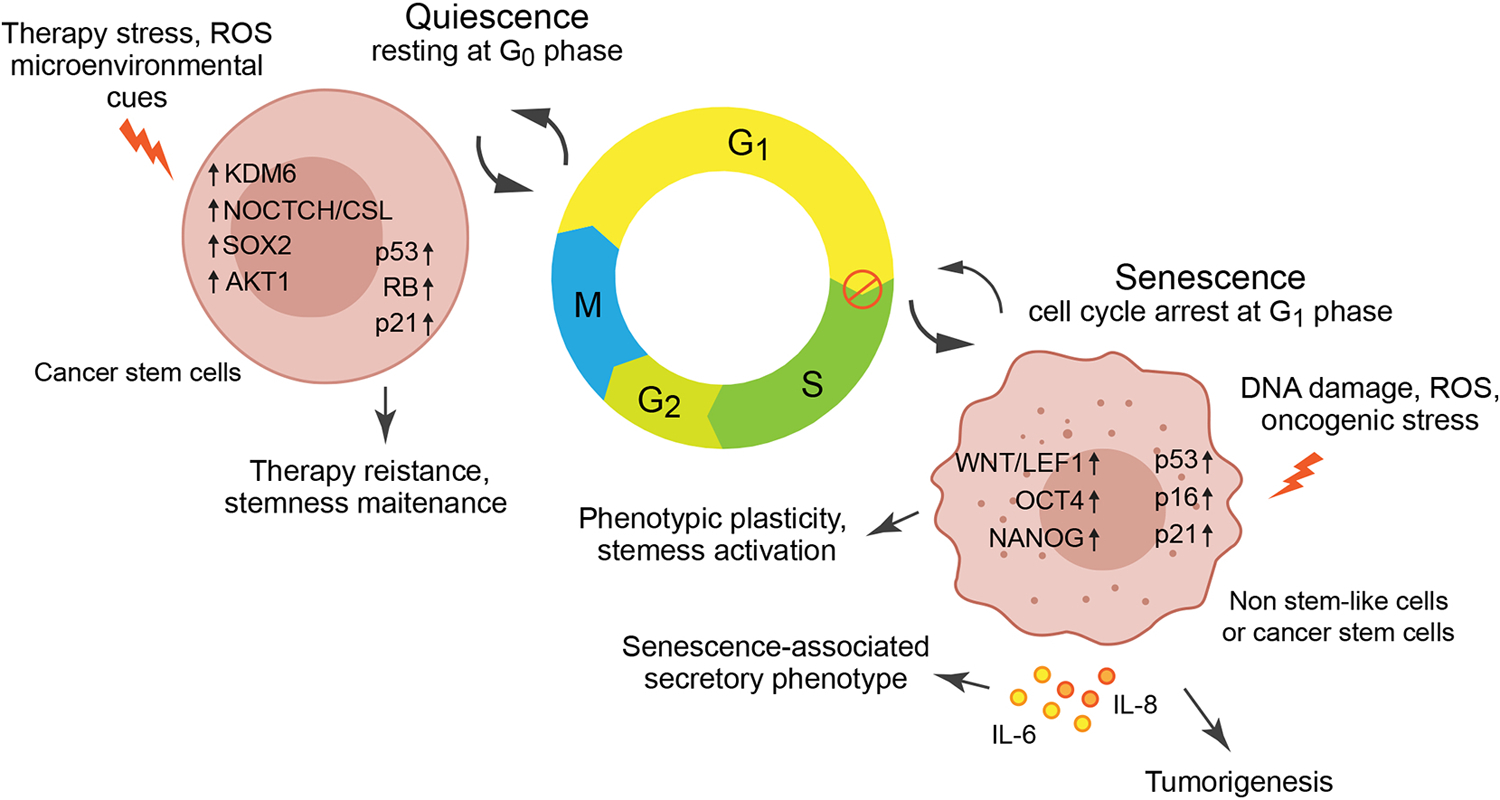
Senescence is a halt mainly in cell cycle G1, induced by persistent DNA damage, reactive oxygen species (ROS), and oncogenic stress. Senescence-associated secretory phenotype, a feature of senescence, induces secretion of cytokines such as IL-6 and IL-8 to promote tumorigenesis via paracrine effects. Senescent cells also undergo reprogramming and activation of stem cell transcriptional factors including WNT/LEF1, OCT4 and NANOG to induce plasticity and stemness activation. The paracrine effect and the genetic reprograming may lead to reversal of cell senescence. Quiescence is a cell state of resting a G0 phase to overcome vulnerabilities and maintain stemness, which is induced by therapeutic stress, ROS, or microenvironmental cues. Quiescence is a reversible state where cells switch into a diving state upon favorable conditions through KDM6, NOTCH/CSL, SOX, AKT1.
Another characteristic of CSCs that exhibit a hindrance to standard therapy is their ability to switch into a dormant or a quiescent state (Figure 2). CSCs can enter reversible G0 phase of cell cycle and remain in a dormant state, particularly upon treatment (W. Chen et al., 2016). Such a state transition is often triggered by micro-environmental cues such as hypoxia, nutrient deprivation, oxidative stress, or a selection pressure such as that posed by chemotherapeutics. Since most standard care therapy for cancer targets proliferating cells, quiescent CSCs can escape the fate of such therapies and often switch back into proliferative state with the emergence of favorable conditions (Sosa, Bragado, & Aguirre-Ghiso, 2014), (De Angelis, Francescangeli, La Torre, & Zeuner, 2019). Slow cycling CSCs in GBM, identified through lineage tracing, resist temozolomide treatment, and interestingly, ablation of this population re-renders susceptibility to temozolomide (J. Chen et al., 2012). Identification of such CSC-derived quiescence or dormant cell population and the pathways involved in their maintenance is key to successful therapy of cancers. To be noted, while senescence is an active cell cycle arrest mostly in G1-S phase of cell cycle, quiescence is a temporary halt of cell cycle as cell transition into Go phase (Terzi, Izmirli, & Gogebakan, 2016) (Figure 2). Also quiescence may be triggered by lack of some nutrients and growth factors which are often a result of therapy or the microenvironmental cues. On the other hand senescence involves greater extent of metabolic stress and DNA damage. Hence it is imperative that multiple CSC populations can exist in a tumor. While some can be fast cycling and plastic, some can be senescent and some can be slow cycling and quiescent.
Pathways playing major roles in plasticity of CSCs
Epigenetic changes triggered by microenvironmental cues may govern plasticity in cancers. In the last decade, the field has evolved from marker-based approaches to lineage-tracing, and more recently, to single cell transcriptomic approaches to identify CSCs and the regulated pathways that control CSC self-renewal capability. This has advanced our understanding of the various intrinsic and microenvironmental factors that govern plasticity and has also elucidated their therapeutic vulnerabilities. Epithelial-Mesenchymal transition (EMT) has long been proposed to be closely related to the gain of CSC-like properties (Mani et al., 2008). Currently, EMT is considered to be one of the major mechanisms that regulate CSC plasticity (Wahl & Spike, 2017). In basal-like subtype of breast cancer, a less-tumorigenic population of tumor cells characterized by CD44low phenotype generates tumors with a spontaneous conversion to CD44high cells which are highly tumorigenic (Chaffer et al., 2011), (Chaffer et al., 2013). ZEB1, a key EMT transcriptional factor/repressor behind this plasticity and stemness activation, is maintained in a bivalent chromatin configuration, which switches between a repressive and transcriptionally active state in response to TGF-β (Scheel & Weinberg, 2012), (Zhang, Sun, & Ma, 2015). This highlights that microenvironmental cues like TGF-β can reshape the epigenetic makeup of the cells to bring plasticity. More recently, EMT conversion is viewed as a fluidic process with the intermediate or hybrid EMT states differing in metastatic potential, invasiveness and plasticity as compared to either the epithelial or the mesenchymal extremities of the spectrum (Pastushenko et al., 2018). While ZEB1 is required for transiting out of the extreme epithelial state, the more tumorigenic intermediate EMT state is driven by Snail, another key EMT transcription factor (Bierie et al., 2017), (Kröger et al., 2019).
SOX2 is a key transcription factor that regulates stem cell-like functions in CSCs. SOX2 is required for de-differentiation of serum-differentiated glioblastoma cells and their acquisition of stem-cell phenotype (Berezovsky et al., 2014). SOX2 along with POU3F2, SALL2, and OLIG2, a key set of neuro-developmental transcription factors, activate and reprogram differentiated glioblastoma cells to stem-like tumor-propagating cells (Suvà et al., 2014). Notably, recent single-cell analysis-based studies provide deeper insights into plasticity pathways that gives rise to tumor heterogeneity and regulate CSC stemness. YAP/TAZ has been identified as a master regulator of glioma CSC function through single-cell RNA sequencing (Castellan et al., 2021): serum-induced differentiation leads to dissociation of YAP/TAZ and its translocation into the cytoplasm; while this process is reversible upon retrieval of serum, after inhibition of YAP/TAZ, the de-differentiation of the tumor cells to GSC-like state is completely blocked upon retrieval of serum, suggesting that YAP/TAZ-mediated cell plasticity is critical for CSC stemness. Several studies have pointed out various epigenetic mechanisms such as DNA methylation, changes in chromatin structure, histone modifications in mediating CSC plasticity (Chaffer et al., 2013), (Poli, Fagnocchi, & Zippo, 2018), (Natsume et al., 2013). Forced expression of MLL5, an epigenetic repressor in non-stem cells, sufficiently induced plasticity by repressing pro-neural differentiation in GBM (Gallo et al., 2015). Cancer cell plasticity can also be induced as a result of therapy. Ionizing radiation reprogrammed breast cancer stem cells that lacked stem cell properties into stem-like cells through high Notch activity (Lagadec, Vlashi, Della Donna, Dekmezian, & Pajonk, 2012). Another recent study shows that circulating tumor cells, isolated from Her2− patients who underwent multiple courses of treatment, acquire a Her2+ subpopulations with high Notch activity which readily interconverted between Her2− positive and negative state (Jordan et al., 2016). These studies illustrate that plasticity poses a major threat to current CSC-targeting therapies and suggest that modulation of these potential pathways that regulate CSC plasticity may tackle this problem (Figure 1).
Mechanisms regulating senescence of CSCs
Cellular senescence is a state of cell-cycle arrest that is triggered in response to stress (Lecot et al., 2016). It is generally characterized by irreversible growth arrest, high lysosomal activity, persistent DNA damage and distinct morphological changes such as enlarged cell volume (B. Wang, Kohli, & Demaria, 2020). Under normal circumstances cells have limited proliferative potential and hence undergo senescence due shortening of telomere length; however in cancer, responses to certain oncogenic signals like RAS and cytotoxic chemotherapeutic agents can trigger senescence (Serrano et al., 1997), (Roninson, 2003). The cell cycle arrest is mediated by elevated expression and accumulation of p53 and cell cycle inhibitors - p16, p21 (Hernandez-Segura, Nehme, & Demaria, 2018). These molecular determinants are also part of the apoptotic pathway. Whether or not a cell will enter apoptosis or senescence depends on the levels and activity of p53 and p21 and the degree of DNA damage (Childs, Baker, Kirkland, Campisi, & Deursen, 2014). For example, long exposure to low dose of doxorubicin results in high levels of reactive oxygen species (ROS) and induces senescence, while short term and high dose of doxorubicin results in low levels of ROS and apoptosis in breast cancer cells (Song, Lee, & Hwang, 2005). Chemotherapeutic agents that induce both senescence and apoptosis have proved to be successful in targeting CSCs (Chiao, Cheng, Yang, Shen, & Ko, 2013). However, the accumulation of senescent cells over time can ironically promote tumorigenesis (Coppé et al., 2008). This could be mediated by an increased expression and secretion of pro-inflammatory chemokines and cytokines, a common phenotype during senescence referred as the senescence-associated secretory phenotype (SASP). SASP results in the release of inflammatory cytokines, such as IL-6, IL-8, and IL-1a, which are known to promote tumorigenesis and CSC phenotypes in a paracrine manner (Figure 2) (Kuilman et al., 2008), (Laberge et al., 2015).
While the secretory cytokines can promote growth of non-senescent adjacent cells, questions remain if senescence directly provokes any cell-intrinsic tumor promoting abilities. Senescence and stem cell functions are co-regulated by overlapping signaling pathways including p16, p21, and p53, suggesting that senescence induced by activation of these pathways may lead to genetic reprogramming and stemness activation (Zon, 2008), (Milanovic, Fan, et al., 2018).
Some studies provide evidence of restoration of a dividing cell population from radiation induces senescent cell population post treatment of transformed cells (Chitikova et al., 2014), (Roberson, Kussick, Vallieres, Chen, & Wu, 2005), (Sabisz & Skladanowski, 2009). The surviving cells post radiation have high expression of some stem cell markers, NANOG and OCT4, providing the possibility of senescence as a reversible state which could switch tumor cells to a stem-like state in a context-dependent manner (Chitikova et al., 2014). For CSCs in prostate cancer, BMP7 induces senescence by activating p38 MAP kinase and increases expression of p21, which is reversible upon withdrawal of BMP7 (Kobayashi et al., 2011). Recently, in a genetically switchable model system of senescence in leukemia, cells released from senescence re-enter cell cycle with enhanced Wnt signaling, suggesting a crosstalk between senescence and stemness activation (Milanovic, Fan, et al., 2018). This study also shows that cellular senescence could reprogram non-stem bulk leukemia cells into leukemia-initiating cells, i.e., CSCs, with a high self-renewing ability, and highlights the detrimental potential of senescence induction to CSC-mediated cancer relapse (Milanovic, Fan, et al., 2018). In future more studies in different types of cancers are required to elucidate whether reversal of senescence is dependent on the type of cancer and to identify robust and specific markers of reversible senescence. Also, some open questions remain regarding how long senescence serves as beneficial or therapeutically desirable, in regards to CSC generation, and whether forcing cells to apoptosis rather than senescence, eradicating CSCs, is the way forward for a long-lasting clinical success,.
Mechanisms regulating quiescence of CSCs
Cell quiescence is a growth stagnant state, i.e., cells enter and exist in a resting G0 phase, and the progression of normal cell cycle is halted. The temporary and reversible state of cell quiescence is distinct from senescence, whereby cells are irreversibly arrested in G0, eventually leading to degeneration and cell death (Figure 2) (Cho et al., 2019). CSCs can switch between quiescent and actively cycling states and therefore evade therapies that target actively dividing cells (Sau Har Lee, Reed-Newman, Anant, & Ramasamy, 2020), (W. Chen et al., 2016). Quiescence can be initiated due to altered signaling in response to new microenvironmental cues or absence of favorable contextual cues such as posed by drug treatments (Recasens & Munoz, 2019). p53, RB, and CDK inhibitors p21 and p27 are some of the molecular determinants implicated for imparting quiescence (Cheung & Rando, 2013). These sets of tumor suppressors and cell cycle inhibitors are usually activated by different upstream stimuli and cell signaling pathways. Like in squamous cell carcinoma, TGF-β regulates quiescence by driving the expression of p21. p21 not only bestows slow cycling properties to the squamous cell carcinoma progenitors, but also protects them from high levels of ROS created after treatment with cisplatin by stabilizing NRF2, a key transcription factor regulating the expression of genes required for glutathione metabolism (Oshimori, Oristian, & Fuchs, 2015). Glioma CSCs can switch into a slow cycling state in response to inhibitors of receptor tyrosine kinase (RTK), in which slow cell cycling was dependent on the histone demethylase KDM6 and high Notch activity (Liau et al., 2017). Consistent with these findings, Notch induces radio-resistance in glioma CSCs by activating AKT (J. Wang et al., 2010), and promotes recurrence from dormant tumor cells following HER2 targeted therapy in breast cancer (Abravanel et al., 2015).
CSCs play a crucial role in cancer metastasis (Shiozawa et al., 2013). During metastasis, tumor cells leave the primary site and enter circulation which are often referred as disseminated cancer cells or circulating tumor cells (CTCs). Glioma CTCs exhibit Wnt-mediated, CSC-like properties (T. Liu et al., 2018). These cells that seed for metastasis are thought to be dormant (Risson, Nobre, Maguer-Satta, & Aguirre-Ghiso, 2020). Latent CTCs has been shown to display high SOX transcriptional activity which maintains a slow cycling state and evasion from immune surveillance (Malladi et al., 2016). In breast cancer, reduction in AKT pathway activity has been linked to metastatic dormancy (Schewe & Aguirre-Ghiso, 2008). Increasing evidence suggests that reduction in AKT activity can lead to activation of autophagy, which may serve as the mechanism of survival for the dormant metastatic cells (Vera-Ramirez, Vodnala, Nini, Hunter, & Green, 2018), (Aqbi et al., 2018).
Role of the microenvironment in maintenance of CSCs
Hypoxia
The stem cell niche or the microenvironment defines an entity surrounding the stem cells that provides signals in the form of secreted signaling molecules that determine proliferation, fate of the daughter cells, protect the stem cells from exhaustion, and more importantly retain the stem-cell characteristics of CSCs (Figure 3) (Jones & Wagers, 2008), (Plaks, Kong, & Werb, 2015), (Oskarsson, Batlle, & Massagué, 2014), (Sainz, Carron, Vallespinós, & Machado, 2016), (Lau, Ho, & Lee, 2017), (Huang et al., 2020). Tumor microenvironment characterized by hypoxia has long been indicated to be a key regulator of CSCs (Plaks et al., 2015), (Finger & Giaccia, 2010), (Semenza, 2000), (Keith & Simon, 2007). Hypoxia is considered as a hallmark of cancer and CSC phenotypes in GBM and other tumors (Colwell et al., 2017), (Bar, 2011). In GBM, HIF1-α, a master transcription factor that controls adaptive cell responses to hypoxia, sustains treatment resistance through expression of pro-survival factors in glioma CSCs that are a major source for tumor radioresistance (Marampon et al., 2014), (Soeda et al., 2009), (Bao, Wu, McLendon, et al., 2006), (Das, Srikanth, & Kessler, 2008). Interestingly, HIF2-α is selectively responsible for cell survival and stemness activation in glioma CSCs but not in normal stem cells (Li et al., 2009), (Seidel et al., 2010). HIF2-α also incurs plasticity by promoting stem-like phenotype in non-stem tumor cell population by upregulating stemness-associated transcriptional factors including NANOG, OCT4 and c-MYC (Heddleston, Li, McLendon, Hjelmeland, & Rich, 2009). Recent studies show that hypoxia stimulates EMT during tumor progression and metastasis, suggesting an emerging role of hypoxia in regulation of cell plasticity in CSCs (Cannito et al., 2008), (Ye et al., 2016). Furthermore, hypoxia induces quiescence in tumor cells and CSCs via HIF-1α, Akt, and CREBBP/Creb Binding Protein (CBP) (Kida & Kahn, 2014), (Endo, Okuyama, Ohue, & Inoue, 2014). In addition, hypoxia inhibits senescence and maintains stem cell phenotypes via HIF-1α-mediated downregulation of p21 in CSCs and other progenitor and stem cells (Tsai et al., 2011), (Sang Hun Lee et al., 2013), (Welford & Giaccia, 2011).
Figure 3. Cancer stem cell niche regulating plasticity, senescence and quiescence –
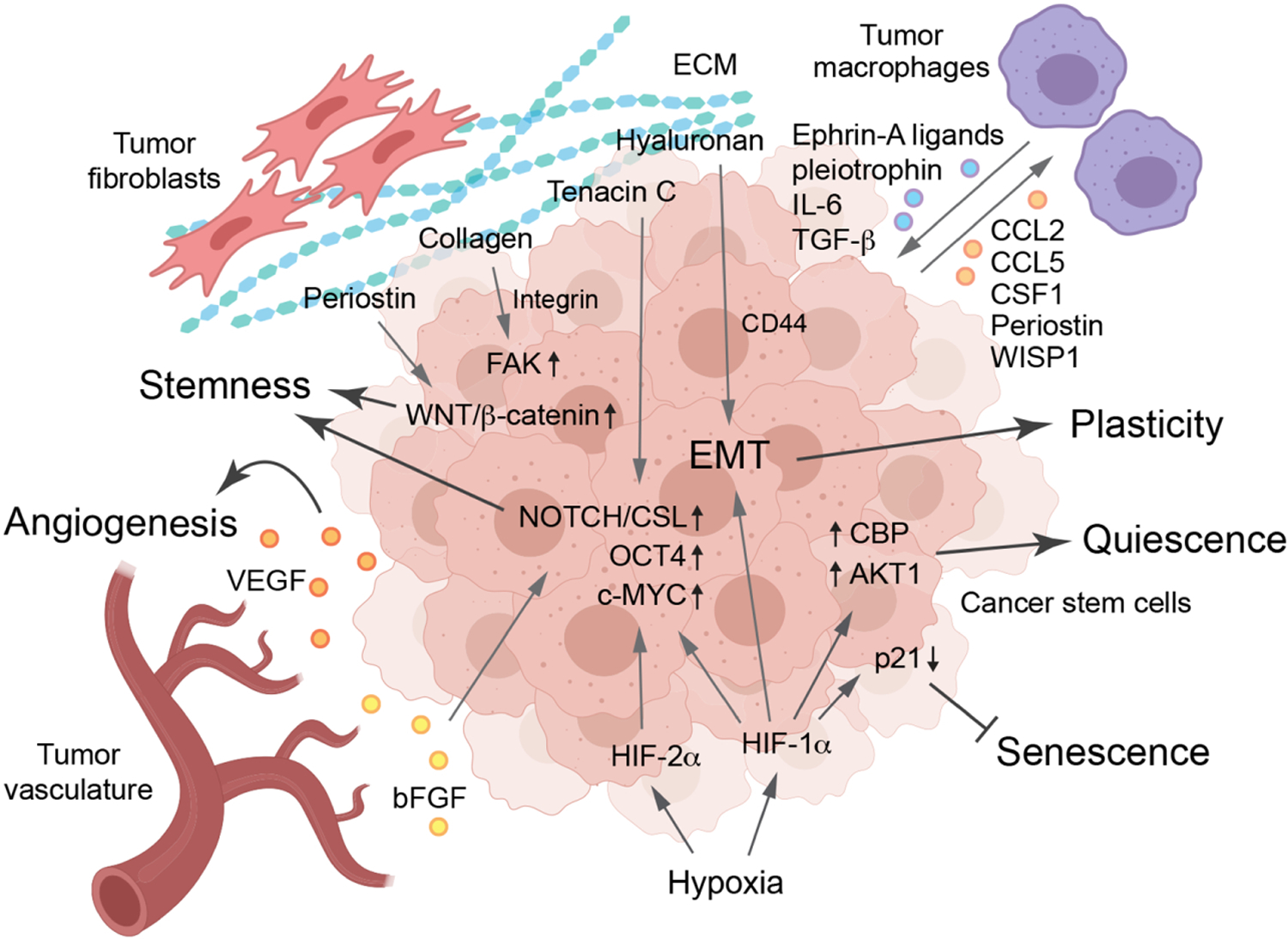
The niche of CSCs is defined by the support provided by their peripheral environment, namely, the vasculature, fibroblasts, and extracellular matrix (ECM), which is characterized by hypoxia. Hypoxia downregulates p21 to inhibit senescence, modulates Akt and CBP activity to induce cell quiescence, and activaes EMT and stem-cell pathways Notch/CSL, OCT4, and c-MYC to regulate plasticity and stemness. The CSCs and vasculature support each other by secreting various growth factors including VEGF and bFGF. ECM proteins, including periostin, collagen, hyaluronan, and tenacin-C, mainly derived from fibroblasts, activate stemness-activating pathways mediated through WNT/β-catenin, FAK, EMT, and NOTCH/CSL. Tumor-associated macrophages foster CSC phenotypes through ephrin-A ligands, pleiotrophin, IL-6, and TGF-β, and CSCs reciprocally induce macrophage recruitment and polarization through CCL2, CCL5, CSF1, periostin, and WISP1.
Tumor endothelial cells
CSCs reside within specialized tumor niches that maintain their stemness and malignant properties (Plaks et al., 2015), (Oskarsson et al., 2014), (Sainz et al., 2016), (Melzer, von der Ohe, Lehnert, Ungefroren, & Hass, 2017), (M. Heddleston et al., 2011). The CSC niche plays a major role in maintaining phenotypic plasticity and dormancy, which predominantly comprises of vascular endothelial cells (ECs) and extracellular matrix (Figure 3). Vascular ECs not only play an important role in tumor angiogenesis, but also form a vascular niche by secreting soluble factors and interacts with CSCs to support CSC survival, plasticity, and quiescence (Gilbertson & Rich, 2007), (Brooks & Parrinello, 2017). The perivascular niche is most well characterized in GBM (Calabrese et al., 2007). The interaction between the glioma CSCs and vascular niche is bidirectional where both the CSC and the ECs influence and support each other. CSCs secrete vascular endothelial growth factor (VEGF) that induces local angiogenesis and ECs produce nitric oxide that activates Notch signaling in glioma CSCs (Gilbertson & Rich, 2007), (Charles et al., 2010), (Bao, Wu, Sathornsumetee, et al., 2006). ECs can also secrete basic fibroblast growth factor (bFGF) to enhance expression of stem-cell markers and sphere forming ability of differentiated glioblastoma cells (Fessler, Borovski, & Medema, 2015). We suggest that vascular niche-dependent mechanisms converge on CSC stemness capacity, either inherited (hierarchical theory, via maintaining CSC stemness) or acquired through transformation which leads to a stem cell-like permissive epigenome (stochastic theory, gain of stem cell properties via enhanced plasticity in tumor cells). In addition, the vascular niche also plays a critical role in inducing quiescence in CTCs and promoting survival of CSCs and CTCs during metastasis and contribute to its dormancy (Favaro, Amadori, & Indraccolo, 2008), (Rak, Milsom, & Yu, 2008), (Ingangi, Minopoli, Ragone, Motti, & Carriero, 2019), (Phan & Croucher, 2020). A recent study shows that vascular niche-derived thrombospondin-1 suppresses CTC growth and sustained dormancy in breast cancer (Ghajar et al., 2013), suggesting disruption of vascular niche as a strategy to overcome tumor resistance to therapy.
Extracellular matrix
The extracellular matrix (ECM) is another important component of the tumor microenvironment and CSC niche (Birnie et al., 2008), (Reinhard, Brösicke, Theocharidis, & Faissner, 2016), (Nallanthighal, Heiserman, & Cheon, 2019), (Brown, Hua, & Tanwar, 2019). Recent studies show that ECM receptors including laminin, vitronectin, and collagen receptors and integrins are required for stemness phenotypes in normal stem cells and CSCs, suggesting ECM is an requisite aspect of the CSC niche (Villa-Diaz, Kim, Laperle, Palecek, & Krebsbach, 2016), (Goel et al., 2014), (Pontier & Muller, 2009), (Lathia et al., 2010), (Seguin, Desgrosellier, Weis, & Cheresh, 2015). Niche ECM is a complex network of macromolecules mainly secreted by cancer-associate fibroblasts (CAFs), macrophages, and ECs governing tissue architecture and intracellular signaling (Frantz, Stewart, & Weaver, 2010), (Winkler, Abisoye-Ogunniyan, Metcalf, & Werb, 2020), (Poltavets, Kochetkova, Pitson, & Samuel, 2018). ECM remodeling in CSC niches enables two-way abnormal interactions between CSCs and the ECM, which is critical for regulation of phenotypic plasticity, senescence and quiescence in CSCs. ECM components can initiate cellular responses in several ways, such as by binding to cell surface receptors like integrins, by sequestering and presenting growth factors and chemokines, and by providing tensile strength cues. For example, periostin, an ECM protein secreted by CAFs, facilitates the recruitment of Wnt ligands and thus augments Wnt signaling in CSCs, thereby promoting their stemness and growth (Malanchi et al., 2012). Moreover, hedgehog ligand reprograms CAFs to provide a supportive niche for the acquisition of plasticity and chemoresistant CSC phenotype through FGF5 expression and production of fibrillar collagen in breast cancer (Cazet et al., 2018). Expression of another ECM protein, tenascin C is associated with aggressiveness of lung metastasis (Oskarsson et al., 2011). It’s interaction with CSCs at the invasive front enhances signaling by the Notch and Wnt pathways, resulting in downregulation of differentiation signals, promoting self-renewability and viability of CSCs and the re-initiation of metastatic outgrowth (Oskarsson et al., 2011), which hightlights the possible role of ECM in switching of quiescent CTCs into a proliferative state required for metastatic colonization. Hyaluronan, a major component of ECM, is frequently overexpressed in aggressive cancer, and its receptor CD44 is a commonly used CSC marker (Caon et al., 2020), (M. Liu, Tolg, & Turley, 2019). ECM can also induce EMT and thereby promote cell plasticity. A recent study shows that hyaluronan, produced by tumor cells, induces expression of EMT transcription factors, such as SNAIL and TWIST, through activation of TGF-β signaling pathway, leading to expansion of CSC population (Chanmee et al., 2014). In addition, integrin – FAK pathway, a well characterized ECM signaling pathway, has been linked with CSC functions (Luo et al., 2013), (Kolev et al., 2017), (Hirata et al., 2015). While the components of ECM regulate plasticity and stemness of CSCs in these studies, the CSCs can also remodel the ECM and is critical for CSC-mediated cancer invasion and metastasis (Winkler et al., 2020). Senescent cells undergo ECM remodelling and often express matrix metalloproteases (MMPs) as SASP factors, creating a permissive environment for invasion and metastasis (Gonzalez-Meljem, Apps, Fraser, & Martinez-Barbera, 2018).
Immune cells
The tumor microenvironment is generally considered to be immunosuppressive. There is growing appreciation of the reciprocal communication between CSCs and immunosuppressive tumor-infiltrating immune cells, which facilitates immune evasion, tumour progression, and therapeutic resistance (Hinshaw & Shevde, 2019), (Ferguson, Diaz, & Reya, 2021) (Bayik & Lathia, 2021). Myeloid cells, primarily including macrophages and myeloid-derived suppressor cells (MDSCs) that exhibit pro-tumorigenic and immunosuppressive phenotypes, are the most well studied component that interacts with CSCs in the tumor immune microenvironment (Bayik & Lathia, 2021). Tumor-associated macrophages foster CSC phenotypes through producing soluble factors including ephrin-A ligands, pleiotrophin, IL-6, and TGF-β (H. Lu et al., 2014), (Shi et al., 2017), (Jinushi et al., 2011), (Bellomo, Caja, & Moustakas, 2016). Reciprocally, CSCs are imperative to macrophage recruitment and immunosuppressive polarization through secreting CCL2, CCL5, CSF1, periostin, and WISP1 (Bayik & Lathia, 2021), (Guo et al., 2017) (W. Zhou et al., 2015), (Tao et al., 2020). However, there is limited knowledge on the precise mechanisms by which the immune microenvironment regulates plasticity, senescence and quiescence of CSCs. A recent report shows that interactions between macrophages and tumor cells drive glioblastoma into a mesenchymal state (Hara et al., 2021), suggesting a potential role of macrophages in dictating plasticity. In summary, these studies collectively suggest that the stem cell niche comprised of the vasculature, ECM, fibroblasts, and immune cells may regulate plasticity, senescence and quiescence in CSCs and is essential for CSC maintenance and therapy resistance.
Therapeutic implications and concluding remarks
Over the past decades major efforts have been made to identify the vulnerabilities of CSCs for molecularly targeted therapy. Cell plasticity, quiescence and senescence currently pose major threats to selectively and efficiently targeting the CSCs. Rationally designed combination treatment strategies to curb plasticity and quiescence without causing toxicities would promise a better efficacy. Several common pathways have been implicated in mediating plasticity, senescence, and quiescence in CSCs, and targeting Wnt, Notch, Hedgehog pathways, acting as major nodes in CSCs have proved to be beneficial (Yang et al., 2020). Additional therapeutic targets include the key regulatory molecules that control metabolic, genetic, epigenetic alternations in CSCs. Nanoparticle based delivery systems holds promise for targeting CSCs in a specific manner (B. Lu, Huang, Mo, & Zhao, 2016). While designing future therapeutic prospective, the role of the tumor microenvironment in dictating various CSC mechanisms should be kept in mind. Single-cell studies of the tumor microenvironment in the future would help us explore such mechanisms, understand the heterogenicity of CSC niches, and translate the findings into CSC therapies. As such, reprogramming tumor microenvironment represents a promising strategy to overcome treatment resistance in CSCs. Furthermore, targetted immunotherapy has great potential to eliminate CSCs. Since CSCs express a number of unique markers and neoantigens, immunotherapy by adoptive cells transfer with chimeric antigen receptor (CAR)- and T cell receptor (TCR)-modified T cells that specifically recognized CSC-associated tumor antigen holds great promise. A recent report shows that CD133-specific CAR-T cells induced cytotoxicity in GBM CSCs in vitro and in an orthotopic tumor model in vivo (Zhu et al., 2015). Finally, deeper investigation exploring the limitations posed by current therapeutic approaches targeting the pathways essential for plasticity, senescence and quiescence of CSCs will be required to successfully eradicate CSCs. Future interventions and novel therapeutic strategies that involve not only targeting a particular cellular state but a landscape of cellular potential, along with its supporting niche would advance cancer treatment for the future.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grants R01NS094533 (to Y.F.), R01NS106108 (to Y.F.), and R01CA241501 (to J.D., and Y.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of abbreviations
- CSC
Cancer stem cell
- GBM
Glioblastoma
- EMT
Epithelial-Mesenchymal transition
- ROS
Reactive oxygen species
- SASP
Senescence-associated secretory phenotype
- RTK
Receptor tyrosine kinase
- CTC
Circulating tumor cell
- EC
Endothelial cell
- ECM
Extracellular matrix
- CAF
Cancer-associated fibroblast
- CAR
Chimeric antigen receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
All authors declare no competing interests.
Reference
- Abravanel DL, Belka GK, Pan TC, Pant DK, Collins MA, Sterner CJ, & Chodosh LA (2015). Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. Journal of Clinical Investigation. 10.1172/JCI74883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, & Clarke MF (2004). Self-renewal and solid tumor stem cells. Oncogene, 23, 7274–7282. 10.1038/sj.onc.1207947 [DOI] [PubMed] [Google Scholar]
- Al-Hajj Muhammad, Wicha MS, Benito-Hernandez A, Morrison SJ, & Clarke MF (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K, Lutz C, Van Delft FW, Bateman CM, Guo Y, Colman SM, … Greaves M (2011). Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 10.1038/nature09650 [DOI] [PubMed] [Google Scholar]
- Aqbi HF, Tyutyunyk-Massey L, Keim RC, Butler SE, Thekkudan T, Joshi S, … Manjili MH (2018). Autophagy-deficient breast cancer shows early tumor recurrence and escape from dormancy. Oncotarget 10.18632/oncotarget.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlasi Y, Looijenga L, & Fodde R (2014). Cancer Stem Cells, Pluripotency, and Cellular Heterogeneity. A WNTer Perspective. In Current Topics in Developmental Biology. 10.1016/B978-0-12-416022-4.00013-5 [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, … Rich JN (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, … Rich JN (2006). Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Research 10.1158/0008-5472.CAN-06-1010 [DOI] [PubMed] [Google Scholar]
- Bar EE (2011). Glioblastoma, cancer stem cells and hypoxia. Brain Pathology 10.1111/j.1750-3639.2010.00460.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, & Clevers H (2017). Cancer stem cells revisited. Nature Medicine 10.1038/nm.4409 [DOI] [PubMed] [Google Scholar]
- Bayik D, & Lathia JD (2021). Cancer stem cell–immune cell crosstalk in tumour progression. Nature Reviews Cancer 10.1038/s41568-021-00366-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo C, Caja L, & Moustakas A (2016). Transforming growth factor β as regulator of cancer stemness and metastasis. British Journal of Cancer. 10.1038/bjc.2016.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovsky AD, Poisson LM, Cherba D, Webb CP, Transou AD, Lemke NW, … de Carvalho AC (2014). Sox2 promotes malignancy in glioblastoma by regulating plasticity and astrocytic differentiation. Neoplasia (United States). 10.1016/j.neo.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, … Weinberg RA (2017). Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1618298114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie R, Bryce SD, Roome C, Dussupt V, Droop A, Lang SH, … Collins AT (2008). Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biology 10.1186/gb-2008-9-5-r83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, & Dick JE (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 10.1038/nm0797-730 [DOI] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AHFM, Schlegelberger B, … Schmitt CA (2005). Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 10.1038/nature03841 [DOI] [PubMed] [Google Scholar]
- Brooks LJ, & Parrinello S (2017). Vascular regulation of glioma stem-like cells: a balancing act. Current Opinion in Neurobiology. 10.1016/j.conb.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Brown Y, Hua S, & Tanwar PS (2019). Extracellular matrix-mediated regulation of cancer stem cells and chemoresistance. International Journal of Biochemistry and Cell Biology. 10.1016/j.biocel.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, … Gilbertson RJ (2007). A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 10.1016/j.ccr.2006.11.020 [DOI] [PubMed] [Google Scholar]
- Campbell LL, & Polyak K (2007). Breast tumor heterogeneity: Cancer stem cells or clonal evolution? Cell Cycle. 10.4161/cc.6.19.4914 [DOI] [PubMed] [Google Scholar]
- Campisi J, & D’Adda Di Fagagna F (2007). Cellular senescence: When bad things happen to good cells. Nature Reviews Molecular Cell Biology. 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- Cannito S, Novo E, Compagnone A, di Bonzo LV, Busletta C, Zamara E, … Parola M (2008). Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis 10.1093/carcin/bgn216 [DOI] [PubMed] [Google Scholar]
- Caon I, Bartolini B, Parnigoni A, Caravà E, Moretto P, Viola M, … Passi A (2020). Revisiting the hallmarks of cancer: The role of hyaluronan. Seminars in Cancer Biology. 10.1016/j.semcancer.2019.07.007 [DOI] [PubMed] [Google Scholar]
- Capp JP (2019). Cancer stem cells: From historical roots to a new perspective. Journal of Oncology 10.1155/2019/5189232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan M, Guarnieri A, Fujimura A, Zanconato F, Battilana G, Panciera T, … Piccolo S (2021). Single-cell analyses reveal YAP/TAZ as regulators of stemness and cell plasticity in glioblastoma. Nature Cancer. 10.1038/s43018-020-00150-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazet AS, Hui MN, Elsworth BL, Wu SZ, Roden D, Chan CL, … Swarbrick A (2018). Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nature Communications 10.1038/s41467-018-05220-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, … Weinberg RA (2011). Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1102454108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, … Weinberg RA (2013). XPoised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 10.1016/j.cell.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanmee T, Ontong P, Mochizuki N, Kongtawelert P, Konno K, & Itano N (2014). Excessive hyaluronan production promotes acquisition of cancer stem cell signatures through the coordinated regulation of twist and the transforming growth factor β (TGF-β)-snail signaling axis. Journal of Biological Chemistry, 289(38), 26038–26056. 10.1074/jbc.M114.564120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, & Holland EC (2010). Perivascular Nitric Oxide Activates Notch Signaling and Promotes Stem-like Character in PDGF-Induced Glioma Cells. Cell Stem Cell 10.1016/j.stem.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, & Parada LF (2012). A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 10.1038/nature11287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Dong J, Haiech J, Kilhoffer MC, & Zeniou M (2016). Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem Cells International 10.1155/2016/1740936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, & Rando TA (2013). Molecular regulation of stem cell quiescence. Nature Reviews Molecular Cell Biology. 10.1038/nrm3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao MT, Cheng WY, Yang YC, Shen CC, & Ko JL (2013). Suberoylanilide hydroxamic acid (SAHA) causes tumor growth slowdown and triggers autophagy in glioblastoma stem cells. Autophagy 10.4161/auto.25664 [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, & Deursen JM (2014). Senescence and apoptosis: dueling or complementary cell fates? EMBO Reports 10.15252/embr.201439245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitikova ZV, Gordeev SA, Bykova TV, Zubova SG, Pospelov VA, & Pospelova TV (2014). Sustained activation of DNA damage response in irradiated apoptosis-resistant cells induces reversible senescence associated with mTOR downregulation and expression of stem cell markers. Cell Cycle 10.4161/cc.28402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IJ, Lui PPW, Obajdin J, Riccio F, Stroukov W, Willis TL, … Watt FM (2019). Mechanisms, Hallmarks, and Implications of Stem Cell Quiescence. Stem Cell Reports 10.1016/j.stemcr.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell N, Larion M, Giles AJ, Seldomridge AN, Sizdahkhani S, Gilbert MR, & Park DM (2017). Hypoxia in the glioblastoma microenvironment: Shaping the phenotype of cancer stem-like cells. Neuro-Oncology 10.1093/neuonc/now258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, … Campisi J (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier CP, Ayyadhury S, Le PU, Nadaf J, Monlong J, Riva G, … Petrecca K (2020). Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nature Communications 10.1038/s41467-020-17186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Srikanth M, & Kessler JA (2008). Cancer stem cells and glioma. Nature Clinical Practice Neurology 10.1038/ncpneuro0862 [DOI] [PubMed] [Google Scholar]
- De Angelis ML, Francescangeli F, La Torre F, & Zeuner A (2019). Stem cell plasticity and dormancy in the development of cancer therapy resistance. Frontiers in Oncology 10.3389/fonc.2019.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, Krizhanovsky V, Baker D, & d’Adda di Fagagna F (2021). Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology. 10.1038/s41580-020-00314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkse A, Golebiewska A, Buder T, Nazarov PV, Muller A, Poovathingal S, … Niclou SP (2019). Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nature Communications 10.1038/s41467-019-09853-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Däbritz JHM, … Schmitt CA (2013). Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 10.1038/nature12437 [DOI] [PubMed] [Google Scholar]
- Endo H, Okuyama H, Ohue M, & Inoue M (2014). Dormancy of cancer cells with suppression of AKT activity contributes to survival in chronic hypoxia. PLoS ONE 10.1371/journal.pone.0098858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro E, Amadori A, & Indraccolo S (2008). Cellular interactions in the vascular niche: Implications in the regulation of tumor dormancy. APMIS 10.1111/j.1600-0463.2008.01025.x [DOI] [PubMed] [Google Scholar]
- Ferguson LP, Diaz E, & Reya T (2021). The Role of the Microenvironment and Immune System in Regulating Stem Cell Fate in Cancer. Trends in Cancer 10.1016/j.trecan.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler E, Borovski T, & Medema JP (2015). Endothelial cells induce cancer stem cell features in differentiated glioblastoma cells via bFGF. Molecular Cancer 10.1186/s12943-015-0420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, & Giaccia AJ (2010). Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer and Metastasis Reviews. 10.1007/s10555-010-9224-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, & Weaver VM (2010). The extracellular matrix at a glance. Journal of Cell Science, 123, 4195–4200. 10.1242/jcs.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, … Vescovi A (2004). Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Research 10.1158/0008-5472.CAN-04-1364 [DOI] [PubMed] [Google Scholar]
- Gallo M, Coutinho FJ, Vanner RJ, Gayden T, Mack SC, Murison A, … Dirks PB (2015). MLL5 Orchestrates a Cancer Self-Renewal State by Repressing the Histone Variant H3.3 and Globally Reorganizing Chromatin. Cancer Cell 10.1016/j.ccell.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, … Bissell MJ (2013). The perivascular niche regulates breast tumour dormancy. Nature Cell Biology. 10.1038/ncb2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, & Rich JN (2007). Making a tumour’s bed: Glioblastoma stem cells and the vascular niche. Nature Reviews Cancer 10.1038/nrc2246 [DOI] [PubMed] [Google Scholar]
- Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, … Mercurio AM (2014). Regulated Splicing of the α6 Integrin Cytoplasmic Domain Determines the Fate of Breast Cancer Stem Cells. Cell Reports 10.1016/j.celrep.2014.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meljem JM, Apps JR, Fraser HC, & Martinez-Barbera JP (2018). Paracrine roles of cellular senescence in promoting tumourigenesis. British Journal of Cancer. 10.1038/s41416-018-0066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M, & Maley CC (2012). Clonal evolution in cancer. Nature. 10.1038/nature10762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai X, … Zhao B (2017). Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes and Development. 10.1101/gad.294348.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, & Lander ES (2011). Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 10.1016/j.cell.2011.07.026 [DOI] [PubMed] [Google Scholar]
- Hara T, Chanoch-Myers R, Mathewson ND, Myskiw C, Atta L, Bussema L, … Tirosh I (2021). Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell 10.1016/j.ccell.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, & Rich JN (2009). The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 10.4161/cc.8.20.9701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Segura A, Nehme J, & Demaria M (2018). Hallmarks of Cellular Senescence. Trends in Cell Biology. 10.1016/j.tcb.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Hinshaw DC, & Shevde LA (2019). The tumor microenvironment innately modulates cancer progression. Cancer Research 10.1158/0008-5472.CAN-18-3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, … Sahai E (2015). Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK Signaling. Cancer Cell 10.1016/j.ccell.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, … Cheng SY (2020). Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 10.7150/thno.41648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingangi V, Minopoli M, Ragone C, Motti ML, & Carriero MV (2019). Role of microenvironment on the fate of disseminating cancer stem cells. Frontiers in Oncology 10.3389/fonc.2019.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, … Tahara H (2011). Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1106645108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, & Wagers AJ (2008). No place like home: anatomy and function of the stem cell niche. Nature Reviews. Molecular Cell Biology, 9(january), 11–21. 10.1038/nrm2319 [DOI] [PubMed] [Google Scholar]
- Jordan NV, Bardia A, Wittner BS, Benes C, Ligorio M, Zheng Y, … Haber DA (2016). HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 10.1038/nature19328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, & Simon MC (2007). Hypoxia-Inducible Factors, Stem Cells, and Cancer. Cell. 10.1016/j.cell.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida A, & Kahn M (2014). Hypoxia Selects for a Quiescent, CML Stem/Leukemia Initiating-Like Population Dependent on CBP/Catenin Transcription. Current Molecular Pharmacology 10.2174/1874467207666140219121219 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, … Watabe K (2011). Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. Journal of Experimental Medicine. 10.1084/jem.20110840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev VN, Tam WF, Wright QG, McDermott SP, Vidal CM, Shapiro IM, … Weaver DT (2017). Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget 10.18632/oncotarget.18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreso A, & Dick JE (2014). Evolution of the cancer stem cell model. Cell Stem Cell 10.1016/j.stem.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Kröger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, … Weinberg RA (2019). Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1812876116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, … Peeper DS (2008). Oncogene-Induced Senescence Relayed by an Interleukin-Dependent Inflammatory Network. Cell 10.1016/j.cell.2008.03.039 [DOI] [PubMed] [Google Scholar]
- Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, … Campisi J (2015). MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nature Cell Biology. 10.1038/ncb3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadec C, Vlashi E, Della Donna L, Dekmezian C, & Pajonk F (2012). Radiation-induced reprogramming of breast cancer cells. Stem Cells 10.1002/stem.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, MacSwords J, … Rich JN (2010). Integrin Alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 10.1016/j.stem.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EYT, Ho NPY, & Lee TKW (2017). Cancer stem cells and their microenvironment: Biology and therapeutic implications. Stem Cells International 10.1155/2017/3714190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecot P, Alimirah F, Desprez PY, Campisi J, & Wiley C (2016). Context-dependent effects of cellular senescence in cancer development. British Journal of Cancer. 10.1038/bjc.2016.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Sang Hun, Lee JH, Yoo SY, Hur J, Kim HS, & Kwon SM (2013). Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1α-TWIST-p21 Axis. Arteriosclerosis, Thrombosis, and Vascular Biology. 10.1161/ATVBAHA.113.301931 [DOI] [PubMed] [Google Scholar]
- Lee Sau Har, Reed-Newman T, Anant S, & Ramasamy TS (2020). Regulatory Role of Quiescence in the Biological Function of Cancer Stem Cells. Stem Cell Reviews and Reports 10.1007/s12015-020-10031-8 [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, … Rich JN (2009). Hypoxia-Inducible Factors Regulate Tumorigenic Capacity of Glioma Stem Cells. Cancer Cell 10.1016/j.ccr.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau BB, Sievers C, Donohue LK, Gillespie SM, Flavahan WA, Miller TE, … Bernstein BE (2017). Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell 10.1016/j.stem.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Tolg C, & Turley E (2019). Dissecting the dual nature of hyaluronan in the tumor microenvironment. Frontiers in Immunology 10.3389/fimmu.2019.00947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xu H, Huang M, Ma W, Saxena D, Lustig RA, … Fan Y (2018). Circulating glioma cells exhibit stem cell-like properties. Cancer Research 10.1158/0008-5472.CAN-18-0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MB, & Vinga S (2020). Tracking intratumoral heterogeneity in glioblastoma via regularized classification of single-cell RNA-Seq data. BMC Bioinformatics 10.1186/s12859-020-3390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Huang X, Mo J, & Zhao W (2016). Drug delivery using nanoparticles for cancer stem-like cell targeting. Frontiers in Pharmacology 10.3389/fphar.2016.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, … Weinberg RA (2014). A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nature Cell Biology. 10.1038/ncb3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Zhao X, Chen S, Liu S, Wicha MS, & Guan JL (2013). Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Research, 73(734), 5591–5602. 10.1158/0008-5472.CAN-13-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddleston MJ, Hitomi M, Venere M, Flavahan AW, Yang K, Kim Y, … Hjelmeland BA, (2011). Glioma Stem Cell Maintenance: The Role of the Microenvironment. Current Pharmaceutical Design 10.2174/138161211797249260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez a., Susanto E, Peng H, Lehr H. -a., Delaloye J-F, & Huelsken J (2012). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature, 481, 85–89. 10.1158/1538-7445.AM2012-SY28-02 [DOI] [PubMed] [Google Scholar]
- Malladi S, MacAlinao DG, Jin X, He L, Basnet H, Zou Y, … Massagué J (2016). Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell 10.1016/j.cell.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, … Weinberg RA (2008). The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marampon F, Gravina GL, Zani BM, Popov VM, Fratticci A, Cerasani M, … Festuccia C (2014). Hypoxia sustains glioblastoma radioresistance through ERKs/DNA-PKcs/HIF- 1α functional interplay. International Journal of Oncology. 10.3892/ijo.2014.2358 [DOI] [PubMed] [Google Scholar]
- Marusyk A, Almendro V, & Polyak K (2012). Intra-tumour heterogeneity: A looking glass for cancer? Nature Reviews Cancer. 10.1038/nrc3261 [DOI] [PubMed] [Google Scholar]
- Melzer C, von der Ohe J, Lehnert H, Ungefroren H, & Hass R (2017). Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Molecular Cancer. 10.1186/s12943-017-0595-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LCW, Soengas MS, Denoyelle C, Kuilman T, Van Der Horst CMAM, … Peeper DS (2005). BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 10.1038/nature03890 [DOI] [PubMed] [Google Scholar]
- Milanovic M, Fan DNY, Belenki D, Däbritz JHM, Zhao Z, Yu Y, … Schmitt CA (2018). Senescence-associated reprogramming promotes cancer stemness. Nature 10.1038/nature25167 [DOI] [PubMed] [Google Scholar]
- Milanovic M, Yu Y, & Schmitt CA (2018). The Senescence–Stemness Alliance – A Cancer-Hijacked Regeneration Principle. Trends in Cell Biology. 10.1016/j.tcb.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Nallanthighal S, Heiserman JP, & Cheon D-J (2019). The Role of the Extracellular Matrix in Cancer Stemness. Frontiers in Cell and Developmental Biology. 10.3389/fcell.2019.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume A, Ito M, Katsushima K, Ohka F, Hatanaka A, Shinjo K, … Kondo Y (2013). Chromatin regulator PRC2 is a key regulator of epigenetic plasticity in glioblastoma. Cancer Research 10.1158/0008-5472.CAN-13-0109 [DOI] [PubMed] [Google Scholar]
- Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, … Suvà ML (2019). An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 10.1016/j.cell.2019.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, & Eaves CJ (2012). Cancer stem cells: An evolving concept. Nature Reviews Cancer. 10.1038/nrc3184 [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, & Dick JE (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 10.1038/nature05372 [DOI] [PubMed] [Google Scholar]
- Oshimori N, Oristian D, & Fuchs E (2015). TGF-β Promotes Heterogeneity and Drug Resistance in Squamous Cell Carcinoma. Cell. 10.1016/j.cell.2015.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH-F, Vanharanta S, Tavazoie SF, Morris PG, … Massagué J (2011). Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nature Medicine, 17(7), 867–874. 10.1038/nm.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Batlle E, & Massagué J (2014). Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell. 10.1016/j.stem.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, … Blanpain C (2018). Identification of the tumour transition states occurring during EMT. Nature 10.1038/s41586-018-0040-3 [DOI] [PubMed] [Google Scholar]
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, … Bernstein BE (2014). Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 10.1126/science.1254257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, & Croucher PI (2020). The dormant cancer cell life cycle. Nature Reviews Cancer 10.1038/s41568-020-0263-0 [DOI] [PubMed] [Google Scholar]
- Plaks V, Kong N, & Werb Z (2015). The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 10.1016/j.stem.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V, Fagnocchi L, & Zippo A (2018). Tumorigenic cell reprogramming and cancer plasticity: Interplay between signaling, microenvironment, and epigenetics. Stem Cells International 10.1155/2018/4598195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavets V, Kochetkova M, Pitson SM, & Samuel MS (2018). The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Frontiers in Oncology 10.3389/fonc.2018.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier SM, & Muller WJ (2009). Integrins in mammary-stem-cell biology and breast-cancer progression--a role in cancer stem cells? Journal of Cell Science, 122, 207–214. 10.1242/jcs.040394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager BC, Bhargava S, Mahadev V, Hubert CG, & Rich JN (2020). Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends in Cancer. 10.1016/j.trecan.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak J, Milsom C, & Yu J (2008). Vascular determinants of cancer stem cell dormancy - Do age and coagulation system play a role? APMIS. 10.1111/j.1600-0463.2008.01058.x [DOI] [PubMed] [Google Scholar]
- Recasens A, & Munoz L (2019). Targeting Cancer Cell Dormancy. Trends in Pharmacological Sciences. 10.1016/j.tips.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Reinhard J, Brösicke N, Theocharidis U, & Faissner A (2016). The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. The International Journal of Biochemistry & Cell Biology, 1–10. 10.1016/j.biocel.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Reya T, & Clevers H (2005). Wnt signalling in stem cells and cancer. Nature 10.1038/nature03319 [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, & Weissman IL (2001). Stem cells, cancer, and cancer stem cells. Nature. 10.1038/35102167 [DOI] [PubMed] [Google Scholar]
- Risson E, Nobre AR, Maguer-Satta V, & Aguirre-Ghiso JA (2020). The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nature Cancer 10.1038/s43018-020-0088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson RS, Kussick SJ, Vallieres E, Chen SYJ, & Wu DY (2005). Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Research 10.1158/0008-5472.CAN-04-1270 [DOI] [PubMed] [Google Scholar]
- Roninson IB (2003). Tumor cell senescence in cancer treatment. Cancer Research. [PubMed] [Google Scholar]
- Sabisz M, & Skladanowski A (2009). Cancer stem cells and escape from drug-induced premature senescence in human lung tumor cells: Implications for drug resistance and in vitro drug screening models. Cell Cycle 10.4161/cc.8.19.9758 [DOI] [PubMed] [Google Scholar]
- Sainz B, Carron E, Vallespinós M, & Machado HL (2016). Cancer Stem Cells and Macrophages: Implications in Tumor Biology and Therapeutic Strategies. Mediators of Inflammation. 10.1155/2016/9012369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, … Frank MH (2008). Identification of cells initiating human melanomas. Nature 10.1038/nature06489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, & Weinberg RA (2012). Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Seminars in Cancer Biology. 10.1016/j.semcancer.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe DM, & Aguirre-Ghiso JA (2008). ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.0800939105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, & Lowe SW (2002). A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 10.1016/S0092-8674(02)00734-1 [DOI] [PubMed] [Google Scholar]
- Seguin L, Desgrosellier JS, Weis SM, & Cheresh D. a. (2015). Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends in Cell Biology, 25(4), 234–240. 10.1016/j.tcb.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel S, Garvalov BK, Wirta V, Von Stechow L, Schänzer A, Meletis K, … Acker T (2010). A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2α. Brain. 10.1093/brain/awq042 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2000). Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Critical Reviews in Biochemistry and Molecular Biology. 10.1080/10409230091169186 [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, & Lowe SW (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16(INK4a). Cell. 10.1016/S0092-8674(00)81902-9 [DOI] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER, & Morrison SJ (2009). Heterogeneity in Cancer: Cancer Stem Cells versus Clonal Evolution. Cell. 10.1016/j.cell.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Shi Y, Ping YF, Zhou W, He ZC, Chen C, Bian BSJ, … Bian XW (2017). Tumour-associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth. Nature Communications 10.1038/ncomms15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Nie B, Pienta KJ, Morgan TM, & Taichman RS (2013). Cancer stem cells and their role in metastasis. Pharmacology and Therapeutics. 10.1016/j.pharmthera.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, … Polyak K (2007). Molecular Definition of Breast Tumor Heterogeneity. Cancer Cell 10.1016/j.ccr.2007.01.013 [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, … Dirks PB (2004). Identification of human brain tumour initiating cells. Nature 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, … Park DM (2009). Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 10.1038/onc.2009.252 [DOI] [PubMed] [Google Scholar]
- Song YS, Lee BY, & Hwang ES (2005). Dinstinct ROS and biochemical profiles in cells undergoing DNA damage-induced senescence and apoptosis. Mechanisms of Ageing and Development. 10.1016/j.mad.2004.11.008 [DOI] [PubMed] [Google Scholar]
- Sosa MS, Bragado P, & Aguirre-Ghiso JA (2014). Mechanisms of disseminated cancer cell dormancy: An awakening field. Nature Reviews Cancer. 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar A, Roarty K, & Rosen JM (2015). The mammary stem cell hierarchy: A looking glass into heterogeneous breast cancer landscapes. Endocrine-Related Cancer. 10.1530/ERC-15-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvà ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, … Bernstein BE (2014). Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 10.1016/j.cell.2014.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG (2012). Understanding cancer stem cell heterogeneity and plasticity. Cell Research 10.1038/cr.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Chu C, Zhou W, Huang Z, Zhai K, Fang X, … Bao S (2020). Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nature Communications 10.1038/s41467-020-16827-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi MY, Izmirli M, & Gogebakan B (2016). The cell fate: senescence or quiescence. Molecular Biology Reports. 10.1007/s11033-016-4065-0 [DOI] [PubMed] [Google Scholar]
- Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, & Hung SC (2011). Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 10.1182/blood-2010-05-287508 [DOI] [PubMed] [Google Scholar]
- Vera-Ramirez L, Vodnala SK, Nini R, Hunter KW, & Green JE (2018). Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nature Communications 10.1038/s41467-018-04070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, … Hayes DN (2010). Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Diaz LG, Kim JK, Laperle A, Palecek SP, & Krebsbach PH (2016). Inhibition of Focal Adhesion Kinase Signaling by Integrin α6β1 Supports Human Pluripotent Stem Cell Self-Renewal. Stem Cells 10.1002/stem.2349 [DOI] [PubMed] [Google Scholar]
- Visvader JE, & Lindeman GJ (2012). Cancer stem cells: Current status and evolving complexities. Cell Stem Cell. 10.1016/j.stem.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Wahl GM, & Spike BT (2017). Cell state plasticity, stem cells, EMT, and the generation of intra-tumoral heterogeneity. Npj Breast Cancer. 10.1038/s41523-017-0012-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Kohli J, & Demaria M (2020). Senescent Cells in Cancer Therapy: Friends or Foes? Trends in Cancer. 10.1016/j.trecan.2020.05.004 [DOI] [PubMed] [Google Scholar]
- Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, … Sullenger BA (2010). Notch promotes radioresistance of glioma stem cells. Stem Cells 10.1002/stem.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford SM, & Giaccia AJ (2011). Hypoxia and senescence: The impact of oxygenation on tumor suppression. Molecular Cancer Research. 10.1158/1541-7786.MCR-11-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, & Werb Z (2020). Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nature Communications 10.1038/s41467-020-18794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, … Cui H (2020). Targeting cancer stem cell pathways for cancer therapy. Signal Transduction and Targeted Therapy 10.1038/s41392-020-0110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, … Liang TB (2016). Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Research 10.1158/0008-5472.CAN-15-0977 [DOI] [PubMed] [Google Scholar]
- Zhang P, Sun Y, & Ma L (2015). ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 10.1080/15384101.2015.1006048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Huang Y. e., Liu H, Zhou X, Yuan M, Hou F, … Jiang W (2021). Single-cell RNA-seq dissects the intratumoral heterogeneity of triple-negative breast cancer based on gene regulatory networks. Molecular Therapy - Nucleic Acids. 10.1016/j.omtn.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, … Bao S (2015). Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nature Cell Biology. 10.1038/ncb3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, & Niedermann G (2015). Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget. 10.18632/oncotarget.2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI (2008). Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 10.1038/nature07038 [DOI] [PubMed] [Google Scholar]


