Abstract
The noncanonical inflammasome, comprising inflammatory caspases 4, 5, or 11, monitors the cytosol for bacterial lipopolysaccharide (LPS). Intracellular LPS-elicited autoproteolysis of these inflammatory caspases leads to the cleavage of the pore-forming protein gasdermin D (GSDMD). GSDMD pore formation induces a lytic form of cell death known as pyroptosis and the release of inflammatory cytokines and DAMPs, thereby promoting inflammation. The noncanonical inflammasome-dependent innate sensing of cytosolic LPS plays important roles in bacterial infections and sepsis pathogenesis. Exciting studies in the recent past have significantly furthered our understanding of the biochemical and structural basis of the caspase-4/11 activation of GSDMD, caspase-4/11’s substrate specificity, and the biological consequences of noncanonical inflammasome activation of GSDMD. This review will discuss these recent advances and highlight the remaining gaps in our understanding of the noncanonical inflammasome and pyroptosis.
Introduction
The innate immune system is composed of an evolutionarily conserved set of germ-line encoded pattern recognition receptors (PRR) and effectors that serve critical roles in host surveillance and defense against pathogens [1,2]. PRRs are strategically positioned to recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) in the intracellular and extracellular compartments. Once engaged, PRRs activate key transcription factors, including nuclear factor kappa B (NF-κB) or interferon regulatory factors (IRF)-3/7, to trigger the transcription of proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF) or type I interferons, respectively [3].
Inflammasomes, a special class of PRRs, are oligomeric complexes that assemble in response to various PAMPs and DAMPs [4,5]. Canonical inflammasomes consist of, in most cases, a sensor, an adaptor molecule, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD) (ASC), and the effector protein caspase-1 [6]. Cellular perturbations including ion fluxes, bacterial secretion system proteins, and DNA activate the canonical inflammasomes such as NLRP3, NAIP/NLRC4, and AIM2, respectively, to induce the autoproteolysis of caspase-1 [5,7]. Once activated, caspase-1 cleaves the latent forms of proinflammatory cytokines IL-1β and IL-18 into their active forms. At the same time, caspase-1 cleaves gasdermin D (GSDMD), releasing the inhibitory C-terminal domain from the pore-forming N-terminal domain [8–11]. The N-terminal domain of GSDMD migrates to the plasma membrane and forms pores, leading to lytic death known as pyroptosis. During pyroptosis, GSDMD pores and plasma membrane rupture allow the release of cytokines, DAMPs, and alarmins that amplify local and systemic inflammation.
A noncanonical form of inflammasome devoid of dedicated receptor and adapter proteins has been uncovered almost a decade ago [12–15]. In this abridged version of the inflammasome, the effector caspases—caspases 4 and 5 in humans and caspase-11 in rodents that are closely related to the prototypical inflammatory caspase-1—function as the receptor also [12]. These inflammatory caspases (4/5/11) directly sense lipopolysaccharide (LPS), an abundant gram-negative bacterial cell wall component. The CARD domain of caspase-11/4/5 binds to the lipid A of LPS leading to caspase oligomerization and activation [12]. While the hexa-acylated lipid A of LPS is the optimal ligand for caspases 4 and 11 [12,14], caspase-4, not caspase-11, can sense tetraacylated lipid A [16]. This is one of the few functional differences between human and murine noncanonical inflammasomes. As pathogenic human bacteria such as Yersinia and Francisella underacylate their lipid A to evade TLR4 recognition [17,18], caspase-4’s capacity to detect such underacylated lipid A can be considered a host strategy to counter pathogen evasion mechanisms. The cytosolic localization of LPS occurs during both cytosolic and noncytosolic bacterial infections. In the latter scenario, LPS-rich outer membrane vesicles (OMV) secreted by bacteria play a critical role in the cytosolic localization of LPS [19].
Furthermore, host factors such as HMGB1, secretoglobin-3A2 syndecan-1, and heparin-sensitive glycocalyx degradation have been shown to play a role in the intracellular access of LPS [20–22]. Since the lipid A moiety of LPS is buried in the outer membrane of bacteria and OMVs, caspase-4’s access to its ligand, lipid A, is limited. An ordered assembly of interferon-inducible guanylate binding proteins (GBPs) on bacterial membranes serves as a platform to recruit caspase-4 and facilitate its access to lipid A [23–31]. Once activated, caspase-4/5/11 directly cleave GSDMD to induce pyroptosis. Concomitantly, active GSDMD is considered to indirectly trigger NLRP3 inflammasome assembly leading to the processing of IL-1β and IL-18 by caspase-1 [32–35].
Unique pyroptotic substrate recognition by inflammatory caspases
Upon their activation by intracellular LPS, inflammatory caspases 4 and 11 cleave the pore-forming protein gasdermin D (GSDMD). In addition, caspase-4 has been shown to cleave IL-18 and the executioner caspase, caspase-7 [23,26,36]. Interestingly, IL-18 is a substrate for caspase-4 but not caspase-11 implying potential differences in the outcomes of noncanonical inflammasome activation in humans versus rodents especially in cells lacking a functional caspase-1. Moreover, TAR DNA binding protein of 43 kDa (TDP-43) has also been shown to be a substrate of caspase-4 but not caspase-11 [37]. As TDP-43 plays a role in amyotrophic lateral sclerosis (ALS) [38], caspase-4 cleavage of and subsequent cytosolic accumulation of TDP-43 may be involved in neurodegeneration in ALS. Furthermore, a recent proteomic study employing a degenerate monoclonal antibody library determined that the caspase-4 cleavage motif is found in over 300 proteins including several spliceosome components, indicating that there are additional physiological protein substrates for the noncanonical inflammasome [36].
Nonetheless, GSDMD remains the most predominant and well-characterized noncanonical inflammasome substrate identified thus far. GSDMD belongs to a family of gasdermins, which includes GSDMA, GSDMB, GSDME, and GSDMF [39]. Recent studies showed that IRF1 and IRF2 control the constitutive expression of GSDMD [40,41]. GSDMD is composed of two domains connected by a linker region containing a protease cleavage site [8,9]. Cleavage by the inflammatory caspases 1/4/5/11 at Asp276 (mouse) or Asp275 (human) releases the auto-inhibitory C terminal domain from the cytotoxic N terminal domain. It has been recently appreciated that there is a complex interaction between GSDMD and caspase-11 that is unique among caspases [42]. In general, the prevailing dogma is that caspases recognize a tetrapeptide motif (XXXD) within their substrate. For example, the preferred recognition motifs of apoptotic caspase 3, 8, and 9 are DEVD, LETD, and (W/L)EHD, respectively [43]. However, it has been demonstrated that upon LPS sensing, caspase-11/4 autocleaves at Asp289/285 in the p20-p10 interdomain linker Structural and biochemical studies of caspase-1/4/11-GSDMD complexes revealed that the autoproteolysis of caspase-11/4 at the p20-p10 interdomain linker [42,44] generates two anti-parallel β-strands in the p10 domain that bind tightly to the C-terminal domain of GSDMD at a hydrophobic interface [42]. This p10 exosite-driven interaction between caspase-1/4/11 and the C-terminal domain of GSDMD stabilizes dimerization-mediated caspase activation leading to the cleavage of GSDMD in the linker region in a tetrapeptide-independent manner [42]. The two distinguishing features of substrate binding by caspase-11/4, namely the exosite involvement and the tetrapeptide-independency, may explain their relatively narrow substrate range.
Consequences of GSDMD processing by inflammatory caspases
Membrane perforation by GSDMD
The liberated N-terminal fragments of GSDMD oligomerize into a pre-pore complex that preferentially binds to acidic phospholipids. GSDMD most favorably binds phosphatidylinositol phosphates and phosphatidylserine on the inner leaflet of the plasma membrane, thereby forming plasma membrane pores [8–10,45]. The Ragulator-Rag complex-mediated production of reactive oxygen species (ROS) promotes the gasdermin D oligomerization and pore formation [46]. The inner diameter of GSDMD pores ranges from 10–20 nm, depending on the lipid composition at the insertion site. The pore is large enough to allow the passage of processed IL-1β and IL-18, ions, and smaller DAMPs such as galectins [47–50], but not the passage of high molecular weight intracellular proteins such as LDH and HMGB1, which require plasma membrane rupture for release [39]. It was recently appreciated that there is a degree of specificity in releasing molecules through GSDMD pores. Pore electrostatics specifically allow the robust release of mature IL-1β and IL-18 over the pro-forms of the cytokines, although the pore is physically large enough for the unprocessed cytokines to pass [47]. Furthermore, the N-terminal fragment of GSDMD (GSDMD-N) has an affinity for additional lipid species such as cardiolipin associated with mitochondrial and bacterial membranes [10]. Consistent with its affinity for a mitochondrial lipid, GSDMD-N targets and permeabilizes mitochondria leading to the release of cytochrome C and generation of mitochondrial ROS that activates the apoptosome and the canonical NLRP3 inflammasome, respectively [34,35]. Thus, plasma membrane and organelle membrane perturbations by GSDMD-N links the noncanonical inflammasome, canonical inflammasome, and apoptotic pathways. This crosstalk amplifies the cytotoxic effects of GSDMD and indirectly triggers additional cellular responses such as the activation of IL-1β and IL-18 downstream of the noncanonical inflammasome. GSDMD-N binding of cardiolipin has also been implicated in its targeting and direct killing of bacteria [10]. It has been shown that GSDMD pores are subject to a cellular repair mechanism; calcium ion influx through GSDMD pores activates the endosomal sorting complexes required for transport (ESCRT) proteins I and III. Upon assembly, ESCRT proteins encapsulate GSDMD pores at the membrane into vesicles and patch up the plasma membrane, halting the release of inflammatory proteins and progression to cell lysis [51]. These regulatory steps are necessary to allow for membrane repair in situations where the inflammasome activation is sub-optimal.
Cell death and plasma membrane rupture
Upon optimal inflammasome activation, the cell proceeds to die with several events occurring downstream of GSDMD pore formation [52]. First, extensive ionic flux through GSDMD pores disrupts cellular osmolality, leading to swelling of the cells [8]. During this process, the disruption of mitochondrial membrane potential and organelle structures coincides with cells irreversibly committing to death [52,53]. Eventually, the plasma membrane breaks apart, spilling the intracellular contents. The plasma membrane rupture (PMR) was assumed to be a passive event, however, a recent forward genetic screen identified the cell-surface protein ninjurin-1 (NINJ1) as the critical molecule mediating PMR [54]. NINJ1 is widely expressed in myeloid cells and has been associated with inflammation and tumor suppression [55,56]. NINJ1 is a small 15-kDa protein inserted into the plasma membrane such that both N- and C- terminal ends are in the extracellular space. BMDMs deficient in NINJ1 display attenuated release of larger intracellular proteins such as LDH and HMGB1 upon the activation of both canonical and noncanonical inflammasomes. However, NINJ1 deficiency did not affect the release of smaller molecules, including mature IL-1β and IL-18, which positions NINJ1 downstream of GSDMD pore formation. Interestingly, the secondary PMR that can occur following apoptosis is also dependent on NINJ1, but the PMR happening during MLKL-driven necroptosis is only partially dependent on NINJ1, which imply key differences in the plasma membrane perturbations by GSDMD and MLKL.
In vivo studies revealed that NINJ1−/− mice are not protected against endotoxin shock, suggesting that pyroptotic death itself and the release of smaller DAMPs are sufficient to drive lethality. On the other hand, NINJ1 plays a role in host survival during Citrobacter rodentium infection [54]. Mechanistically, the N-terminal extracellular region is predicted to adopt an amphipathic helix, which is proposed to play a role in the PMR [54]. NINJ1 exists in unstimulated cells as a dimer or trimer and undergoes higher-order oligomerization upon pyroptotic signaling [54]. The exact signal for NINJ1’s transition to the oligomeric state remains to be identified.
Release of DAMPs and alarmins upon noncanonical inflammasome activation
Apoptosis is generally considered an immunologically silent form of cell death, whereas programmed necrosis is inflammatory partly due to DAMP/alarmin release [57,58]. In fact, DAMP/alarmin release is considered a hallmark of inflammatory cell death pathways, such as necroptosis and pyroptosis [58–60]. Unlike the conventional cytokines with an N-terminal signaling sequence that directs their release through the ER-Golgi secretory pathway, DAMPs and alarmins lack the signal sequence for the ER-Golgi secretion [61]. These intracellular proteins are released when plasma membrane integrity is compromised and function as DAMPs/alarmins in the extracellular space [58,60–62]. Emerging proteomic studies are revealing the global secretome of pyroptosis and the differences between the secretome of pyroptosis and necroptosis [50,63,64]. During pyroptosis, GSDMD pores, and microvesicles, and cell lysis contribute to the release of DAMPs/alarmins to varying degrees [48,65–67]. Secretory lysosomes also contribute to unconventional cytokine release [68]. Comparatively, most proteins are secreted in soluble form rather than in EVs during pyroptosis [50].
DAMPs can act on a wide range of cell types and regulate both innate and adaptive immune responses. Several biological functions ranging from inflammation to immunoregulation have been attributed to DAMPs [58–60]. As the circulating levels of DAMPs/alarmins are elevated during inflammatory conditions, they are also considered as biomarkers. HMGB1 is one such inflammatory DAMP released by cells undergoing pyroptosis [69]. Extracellular HMGB1 plays a detrimental role in several inflammatory conditions such as sepsis and ischemia-reperfusion injury. Increased levels of HMGB1 have been found in patients with severe sepsis and septic shock and were associated with morbidity and mortality [70–72]). HMGB1 exerts its inflammatory effects via TLR4 and receptor for advanced glycation end products (RAGE) [58].
While extracellular LPS signaling through TLR4 can trigger the conventional secretion of cytokines like TNF, cytosolic LPS sensing leads to the unconventional release of cytokines, like IL-1β and IL-18 as well as DAMPs/alarmins [13,73]. Whereas leaderless proteins released during canonical inflammasome-driven pyroptosis are known, those released upon noncanonical inflammasome activation remain poorly defined [73]. A liquid phase fractionation-based proteomic approach identified that the noncanonical inflammasome activation leads to the release of galectin-1 [48]. Galectin-1 belongs to a family of β-galactoside-binding proteins that lack signal peptides for the classical secretion [74]. As galectin-1 is a 14.5 kDa protein, its release is mediated via GSDMD pores without requiring terminal cell lysis. Moreover, galectin-1 release occurs upon canonical inflammasome activation and necroptosis, indicating that its release is a universal outcome of inflammatory cell death [48]. Human sepsis patients have increased levels of galectin-1 in the serum [48]. Importantly, galectin-1 binds the glycan ligands generated by Mgat5 and C2gnt1 and limits the anti-inflammatory activity of CD45, enhancing inflammatory responses during endotoxemia. In agreement with this, functional studies in vivo revealed a detrimental role for galectin-1 in sepsis. [69]
SQSTM1 is another DAMP released during caspase-11-GSDMD mediated pyroptosis [75]. In addition, SQSTM1’s release can also be triggered by TLR4 signaling; TBK1 activated by TLR4 can phosphorylate SQSTM1 at serine 403, leading to SQSTM1’s release. Furthermore, secreted SQSTM1 signals through the insulin receptor to activate NF-kB and mediates the pathophysiology of septic shock. Moreover, circulating SQSTM1 levels correlated positively with sepsis severity—as indicated by sequential organ failure assessment (SOFA) and disseminated intravascular coagulopathy (DIC) scores—and mortality in humans [75].
Functions of the noncanonical inflammasome in health and disease
Cytosolic LPS sensing by caspase-11 plays a protective role in several bacterial infections [73,76]. For example, caspase-11 deficient mice are highly susceptible to infection with cytosol-invasive Burkholderia thailandensis and B. pseudomallei [77,78]. Although T3SS of Burkholderia can activate GSDMD via the NLRC4 inflammasome-caspase-1 pathway to induce pyroptosis, the NLRC4 pathway is insufficient to protect against B. thailandensis infection, and GSDMD-activated by caspase-11 is crucial for bacterial clearance. Similarly, caspase-4 mediates intracellular restriction of B. pseudomallei in human alveolar epithelial cells [79]. Furthermore, caspase-11−/− mice have defective neutrophilic responses and bacterial clearance during Klebsiella pneumoniae or Acinetobacter baumannii infections [80,81]. Caspase-11 activation has also been shown to trigger neutrophil extracellular traps and cell death and contribute to bacterial control during Salmonella infection [82].
While cytosolic LPS sensing has beneficial functions in certain bacterial infections, the uncontrolled activation has detrimental consequences for the host and can be lethal [13,69,78]. Several regulatory mechanisms have been shown to limit noncanonical inflammasome activation and its deleterious effects [83–86]. An interferon inducible immunity-related GTPase, Irgm2, has been found to negatively regulate caspase-11 activation and sepsis in cooperation with the noncanonical autophagy protein Gate-16 [85,87]. Furthermore, the oxidative stress sensor GPx8 binds covalently to caspase-4/11 and inhibits their oligomerization, and mice deficient for GPx8 have enhanced caspase-11 activation and mortality in endotoxemia [87]. SERPINB1 and the E3 ubiquitin ligase Nedd4 suppress noncanonical inflammasome responses by targeting caspase-11 oligomerization and protein level, respectively [88,89]. The second messenger, cAMP, has also been shown to inhibit caspase-11 activation and attenuate endotoxin shock [90].
Dysregulated responses to cytosolic LPS is a significant contributor to lethality during sepsis as caspase-11- or GSDMD-deficient mice are highly protected in murine models of sepsis [13,48,91]. The mechanisms underlying caspase-11 mediated lethality include pyroptosis and the simultaneous release of DAMPs such as HMGB1, galectin-1, and SQSTM1 [13,21,48,69,71,75]. DAMPs secreted through GSDMD pores and cell lysis fuel inflammation, and their pharmacological or genetic inhibition reduces caspase-11-driven lethality [21,48,69,71,75]. Disseminated intravascular coagulation (DIC) also contributes to noncanonical inflammasome-induced death [92,93]. Calcium influx induced by GSDMD pore formation triggers the exposure of phosphatidylserine on the plasma membrane via the activation of transmembrane protein 16F. Phosphatidylserine enhances the coagulant activity of tissue factor-3, which leads to DIC [92,93]. The noncanonical inflammasome has also been shown to be involved in multiple non-infectious diseases such as colitis, colitis-associated cancer, colonic dysmotility, allergic airway inflammation, multiple sclerosis, atherosclerosis, and nonalcoholic steatohepatitis [76,94] [95,96] [97] [98][93] [99] [100][101,102]. In summary, cytosolic LPS sensing is important for the defense against certain bacterial infections; however, its hyperactivation has detrimental effects.
Cell type-specific physiological and pathological roles of the noncanonical inflammasome
A broad range of hematopoietic and nonhematopoietic cells—such as macrophages, dendritic cells (DC), neutrophils, intestinal epithelial cells (IEC), endothelial cells, airway epithelial cells, fibroblasts, and neurons [8,9,12,82,91,103–105]—express caspase-4/11 and GSDMD and are responsive to intracellular LPS. Growing evidence suggests cell-type-specific contributions of the noncanonical inflammasome to antibacterial host responses and pathophysiological manifestations of sepsis. Caspase-11 activation in endothelial cells (EC) has been shown to contribute to acute lung injury [91]. Conditional deletion of endothelial caspase-11 conferred protection against endotoxin shock and polymicrobial sepsis. Hepatocyte-specific expression of caspase-11 also contributes to the release of DAMPs such as HMGB1 and IL-1α and lethality during endotoxin shock [21].
Furthermore, we recently identified differential contributions of monocyte/macrophage-, DC, neutrophil-, and IEC-specific caspase-11 to a spectrum of host responses to cytosolic LPS [106]. Mice lacking caspase-11 in monocytes/macrophages were more resistant than wild-type mice to LPS challenge. Surprisingly, mice lacking caspase-11 in DCs and IECs were also protected, albeit to a small extent, against lethal LPS shock. On the other hand, neutrophil-specific deletion of caspase-11 did not protect mice against LPS-induced lethality. Moreover, splenic and hepatic GSDMD cleavage mainly occurs in monocytes, macrophages, and DCs during endotoxemia [106]. Additionally, cytosolic LPS sensing in monocytes/macrophages is a primary contributor to DAMP release and organ damage.
On the other hand, caspase-11 in neutrophils and monocytes/macrophages was crucial for bacterial clearance and host survival during infection with B. thailandensis [106,107]. DC- and IEC-intrinsic caspase-11 expression is dispensable for the host protection against B. thailandensis infection [106]. Overall, cytosolic LPS sensing in monocyte/macrophages and neutrophils provides antibacterial protection. In contrast, cytosolic LPS sensing in monocytes/macrophages, DCs, endothelial cells, hepatocytes, and IECs contribute to the pathophysiology of sepsis to varying degrees. Furthermore, enteric-associated neuron-intrinsic caspase-11 mediates rapid and persistent neuronal loss and long-term gastrointestinal abnormalities during enteric infections [105]. All these studies show that the noncanonical inflammasome functions in several cellular compartments to control inflammatory and antimicrobial responses.
Suppression of the noncanonical inflammasome by bacterial pathogens
It is becoming increasingly apparent from recent studies that bacterial pathogens have evolved to inhibit the noncanonical inflammasome. OspC3, an effector protein of Shigella flexneri, directly targets caspase-4 to prevent its dimerization and activation [108]. A S. flexneri mutant lacking OspC3 triggers enhanced cell death and is more sensitive to bacterial clearance in a guinea pig model. Coxiella burnetii, the causative agent of Q fever, has been shown to target caspase-11-LPS interaction and suppress noncanonical inflammasome responses via its T4SS effector IcaA [109]. A type III secretion system effector, NleF, produced by Enteropathogenic E. coli (EPEC) inhibits the catalytic activity of caspase-4 by binding to its catalytic domain. Similarly, NleF of C. rodentium targets caspase-11 to suppress IL-18 activation and early neutrophil influx [110,111]. Furthermore, bacteriophage-encoded Shiga toxin (Stx) of enterohemorrhagic E. coli (EHEC) has recently been shown to suppress caspase-11 activation [112]. An EHEC mutant strain lacking Stx induces stronger noncanonical inflammasome responses than wild-type EHEC, and purified Stx2 is sufficient to suppress noncanonical inflammasome responses in macrophages and mice. Importantly, a genetically engineered C. rodentium strain that expresses Stx, a recently established murine model for EHEC infection, inhibits noncanonical inflammasome responses in vivo [112]. The molecular basis by which Stx inhibits the caspase-11-GSDMD interaction is yet to be fully elucidated, and it appears that Stx targets GSDMD downstream of caspase-11 activation. Given the evolutionary advantage of subverting the innate immune sensing, it is likely that additional bacterial proteins also interfere with the noncanonical inflammasome pathway.
Conclusions & outstanding questions
Rapid progress has been made in mapping out the signaling and functions of the noncanonical inflammasome pathway within a decade of its discovery. The interaction between the noncanonical inflammasome and the effector GSDMD is critical to the innate inflammatory response and host defense against bacterial insults. While this interaction’s structural and mechanistic basis is emerging, there is still much to learn regarding certain aspects of the noncanonical inflammasome. The cell biological mechanisms involved in the cytosolic translocation of LPS are still unclear, and whether endosomal transporters actively mediate this process is an area of interest. Caspases 4 and 11 have been presumed to have a limited set of substrates. However, recent work suggests additional substrates for the noncanonical inflammasome. How the processing of these potential new substrates shapes the responses to intracellular LPS during bacterial infections warrants further investigation. Additional studies are also needed to further understand the roles DAMPs and alarmins play in mediating noncanonical inflammasome functions. The discovery of NINJ1 as a mediator of PMR—challenging the long-held notion that the terminal lysis of cells undergoing pyroptotic death is a passive event—prompts many exciting questions including whether additional proteins participate in this programmed event. Though both human caspase-4 and caspase-5 are similar to caspase-11, the role of caspase-5 in innate immunity remains less clear. Finally, additional work on the role of noncanonical inflammasome in human diseases including sepsis could facilitate the development of new therapeutics.
Figure 1. Noncanonical inflammasome sensing of cytosolic LPS and activation of GSDMD.
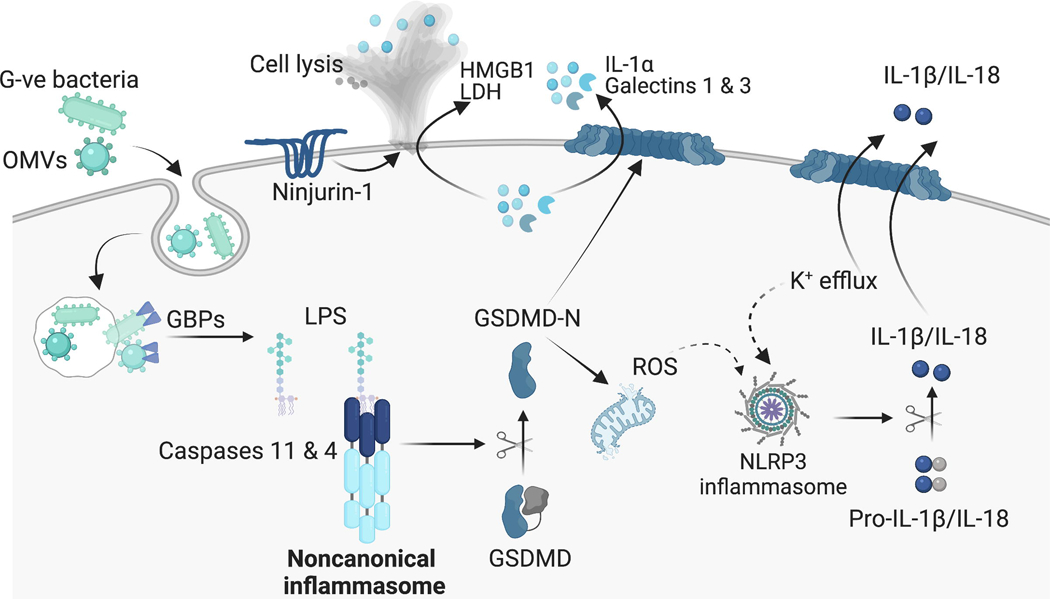
LPS associated with intracellular Gram-negative bacteria or outer membrane vesicles (OMVs) gains access to the cytosol. GBPs act on the bacterial membranes to liberate LPS for recognition by the noncanonical inflammasome. The lipid A moiety of cytosolic LPS interacts with the CARD of caspase-11/4, resulting in caspase-11/4 oligomerization and activation. Active caspase-11/4 interact with GSDMD in an exosite-dependent but tetrapeptide-independent fashion and cleave it. The freed N-terminal domain of GSDMD forms of pores on the plasma membrane leading to the release of DAMPs/alarmins and the processing of IL-1β and IL-18 by the NLRP3 inflammasome. The pyroptotic cascade activates ninjurin-1, which mediates the plasma membrane rupture and terminal cell lysis (Illustration created with BioRender.com.)
Highlights.
Inflammatory caspases of the noncanonical inflammasome function as intracellular LPS sensors.
Active caspase-11/4 interact with GSDMD in an exosite-dependent but tetrapeptide-independent fashion and cleave it.
N-terminal domain of GSDMD forms of pores on the plasma membrane leading to the release of DAMPs/alarmins and activation of the NLRP3 inflammasome.
The pyroptotic cascade activates ninjurin-1, which mediates the plasma membrane rupture and terminal cell lysis.
Noncanonical inflammasome-mediated sensing of cytosolic LPS plays a critical role in numerous bacterial infections and sepsis pathogenesis.
Acknowledgements
The authors apologize to those investigators whose original papers could not be cited because of the space limitation. The Rathinam laboratory is supported by the US National Institutes of Health (R01AI119015 and R01AI148491).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
All authors contributed to the writing of the review.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Mushegian A, Medzhitov R, Evolutionary perspective on innate immune recognition, J Cell Biology 155 (2001) 705–710. 10.1083/jcb.200107040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kawai T, Akira S, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors, Nat Immunol 11 (2010) 373 384. 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- [3].Brubaker SW, Bonham KS, Zanoni I, Kagan JC, Innate Immune Pattern Recognition: A Cell Biological Perspective, Annu Rev Immunol 33 (2015) 1–34. 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rathinam VAK, Fitzgerald KA, Inflammasome Complexes: Emerging Mechanisms and Effector Functions., Cell 165 (2016) 792 800. 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Broz P, Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling., Nat Rev Immunol 16 (2016) 407 420. 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- [6].von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE, Recognition of Bacteria by Inflammasomes, Annu Rev Immunol 31 (2013) 73–106. 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- [7].Rathinam VAK, Vanaja SK, Fitzgerald KA, Regulation of inflammasome signaling, Nat Immunol 13 (2012) 333 332. 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F, Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death, Nature 526 (2015) 660–665. 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- [9].Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM, Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling, Nature 526 (2015) 666–671. 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- [10].Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J, Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores, Nature 535 (2016) 153–158. 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].He W, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang Z-H, Zhong C-Q, Han J, Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion, Cell Res 25 (2015) 1285–1298. 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F, Inflammatory caspases are innate immune receptors for intracellular LPS, Nature 514 (2014) 187. 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- [13].Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM, Non-canonical inflammasome activation targets caspase-11, Nature 479 (2011) 117. 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- [14].Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM, Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4, Science 341 (2013) 1246–1249. 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- [15].Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA, Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock, Science 341 (2013) 1250–1253. 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lagrange B, Benaoudia S, Wallet P, Magnotti F, Provost A, Michal F, Martin A, Lorenzo FD, Py BF, Molinaro A, Henry T, Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11., Nat Commun 9 (2018) 242. 10.1038/s41467-017-02682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E, Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response, Nat Immunol 7 (2006) 1066–1073. 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- [18].Okan NA, Kasper DL, The atypical lipopolysaccharide ofFrancisella, Carbohyd Res 378 (2013) 79 83. 10.1016/j.carres.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VAK, Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation, Cell 165 (2016) 1106–1119. 10.1016/j.cell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang Y, Wang X, Li Z, He Z, Yang X, Cheng X, Peng Y, Xue Q, Bai Y, Zhang R, Zhao K, Liang F, Xiao X, Andersson U, Wang H, Billiar TR, Lu B, Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties, Immunity (2021). 10.1016/j.immuni.2021.01.007. [DOI] [PubMed] [Google Scholar]
- [21].Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z, Huang Y, Peng H, Xiao L, Tang D, Scott MJ, Wang Q, Liu J, Xiao X, Watkins S, Li J, Yang H, Wang H, Chen F, Tracey KJ, Billiar TR, Lu B, The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis., Immunity 49 (2018) 740 753.e7. 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yokoyama S, Cai Y, Murata M, Tomita T, Yoneda M, Xu L, Pilon AL, Cachau RE, Kimura S, A novel pathway of LPS uptake through syndecan-1 leading to pyroptotic cell death, Elife 7 (2018) e37854. 10.7554/elife.37854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wandel MP, Kim B-H, Park E-S, Boyle KB, Nayak K, Lagrange B, Herod A, Henry T, Zilbauer M, Rohde J, MacMicking JD, Randow F, Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms, Nat Immunol 21 (2020) 880–891. 10.1038/s41590-020-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Meunier E, Dick MS, Dreier RF, Schürmann N, Broz DK, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, Yamamoto M, Broz P, Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases., Nature 509 (2014) 366 370. 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- [25].Santos JC, Dick MS, Lagrange B, Degrandi D, Pfeffer K, Yamamoto M, Meunier E, Pelczar P, Henry T, Broz P, LPS targets host guanylate‐binding proteins to the bacterial outer membrane for non‐canonical inflammasome activation, Embo J 37 (2018) e98089. 10.15252/embj.201798089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Santos JC, Boucher D, Schneider LK, Demarco B, Dilucca M, Shkarina K, Heilig R, Chen KW, Lim RYH, Broz P, Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria, Nat Commun 11 (2020) 3276. 10.1038/s41467-020-16889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kutsch M, Sistemich L, Lesser CF, Goldberg MB, Herrmann C, Coers J, Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions, Embo J 39 (2020) e104926. 10.15252/embj.2020104926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J, Guanylate binding proteins promote caspase-11–dependent pyroptosis in response to cytoplasmic LPS, Proc National Acad Sci 111 (2014) 6046–6051. 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fisch D, Bando H, Clough B, Hornung V, Yamamoto M, Shenoy AR, Frickel E, Human GBP1 is a microbe‐specific gatekeeper of macrophage apoptosis and pyroptosis, Embo J 38 (2019) e100926. 10.15252/embj.2018100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fisch D, Clough B, Domart M-C, Encheva V, Bando H, Snijders AP, Collinson LM, Yamamoto M, Shenoy AR, Frickel E-M, Human GBP1 Differentially Targets Salmonella and Toxoplasma to License Recognition of Microbial Ligands and Caspase-Mediated Death, Cell Reports 32 (2020) 108008. 10.1016/j.celrep.2020.108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Park E-S, Kim B-H, Kumar P, Tretina K, Maminska A, Philbrick WM, Flavell RA, MacMicking JD, A hierarchical GBP network promotes cytosolic LPS recognition and sepsis, Biorxiv (2021) 2021.08.25.457662. 10.1101/2021.08.25.457662. [DOI] [Google Scholar]
- [32].Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V, Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells., Eur J Immunol 45 (2015) 2911 2917. 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- [33].Rühl S, Broz P, Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux., Eur J Immunol 45 (2015) 2927 2936. 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- [34].Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES, Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation, Nat Commun 10 (2019) 1689. 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Platnich JM, Chung H, Lau A, Sandall CF, Bondzi-Simpson A, Chen H-M, Komada T, Trotman-Grant AC, Brandelli JR, Chun J, Beck PL, Philpott DJ, Girardin SE, Ho M, Johnson RP, MacDonald JA, Armstrong GD, Muruve DA, Shiga Toxin/Lipopolysaccharide Activates Caspase-4 and Gasdermin D to Trigger Mitochondrial Reactive Oxygen Species Upstream of the NLRP3 Inflammasome, Cell Reports 25 (2018) 1525–1536.e7. 10.1016/j.celrep.2018.09.071. [DOI] [PubMed] [Google Scholar]
- [36].Davies CW, Stowe I, Phung QT, Ho H, Bakalarski CE, Gupta A, Zhang Y, Lill JR, Payandeh J, Kayagaki N, Koerber JT, Discovery of a caspase cleavage motif antibody reveals insights into noncanonical inflammasome function, Proc National Acad Sci 118 (2021) e2018024118. 10.1073/pnas.2018024118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yin P, Guo X, Yang W, Yan S, Yang S, Zhao T, Sun Q, Liu Y, Li S, Li X-J, Caspase-4 mediates cytoplasmic accumulation of TDP-43 in the primate brains, Acta Neuropathol 137 (2019) 919–937. 10.1007/s00401-019-01979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen-Plotkin AS, Lee VM-Y, Trojanowski JQ, TAR DNA-binding protein 43 in neurodegenerative disease, Nat Rev Neurol 6 (2010) 211–220. 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Broz P, Pelegrín P, Shao F, The gasdermins, a protein family executing cell death and inflammation, Nat Rev Immunol (2019) 1–15. 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- [40].Benaoudia S, Martin A, Gamez MP, Gay G, Lagrange B, Cornut M, Krasnykov K, Claude J, Bourgeois CF, Hughes S, Gillet B, Allatif O, Corbin A, Ricci R, Henry T, A genome‐wide screen identifies IRF2 as a key regulator of caspase‐4 in human cells, Embo Rep 20 (2019) e48235. 10.15252/embr.201948235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kayagaki N, Lee BL, Stowe IB, Kornfeld OS, O’Rourke K, Mirrashidi KM, Haley B, Watanabe C, Roose-Girma M, Modrusan Z, Kummerfeld S, Reja R, Zhang Y, Cho V, Andrews TD, Morris LX, Goodnow CC, Bertram EM, Dixit VM, IRF2 transcriptionally induces GSDMD expression for pyroptosis, Sci Signal 12 (2019) eaax4917. 10.1126/scisignal.aax4917. [DOI] [PubMed] [Google Scholar]
- [42].Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, Li Z, Wang Y, Zhao Q, Shao F, Ding J, Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis, Cell 180 (2020) 941–955.e20. 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- [43].Julien O, Wells JA, Caspases and their substrates, Cell Death Differ 24 (2017) 1380–1389. 10.1038/cdd.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ross C, Chan AH, Pein JV, Boucher D, Schroder K, Dimerization and auto-processing induce caspase-11 protease activation within the non-canonical inflammasome, Life Sci Alliance 1 (2018) e201800237. 10.26508/lsa.201800237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S, GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death, Embo J 35 (2016) 1766–1778. 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, Doench JG, LaFleur MW, Sharpe AH, Thiagarajah JR, Kagan JC, Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway, Cell (2021). 10.1016/j.cell.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, Wang L, Fu T-M, Jacobson MP, Greka A, Lieberman J, Ruan J, Wu H, Gasdermin D pore structure reveals preferential release of mature interleukin-1, Nature 593 (2021) 607–611. 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Russo AJ, Vasudevan SO, Méndez-Huergo SP, Kumari P, Menoret A, Duduskar S, Wang C, Sáez JMP, Fettis MM, Li C, Liu R, Wanchoo A, Chandiran K, Ruan J, Vanaja SK, Bauer M, Sponholz C, Hudalla GA, Vella AT, Zhou B, Deshmukh SD, Rabinovich GA, Rathinam VA, Intracellular immune sensing promotes inflammation via gasdermin D–driven release of a lectin alarmin, Nat Immunol 22 (2021) 154–165. 10.1038/s41590-020-00844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen Y, Wang H, Shen J, Deng R, Yao X, Guo Q, Lu A, Sun B, Zhang Y, Meng G, Gasdermin D Drives the Nonexosomal Secretion of Galectin-3, an Insulin Signal Antagonist., J Immunol 203 (2019) 2712–2723. 10.4049/jimmunol.1900212. [DOI] [PubMed] [Google Scholar]
- [50].Phulphagar K, Kühn LI, Ebner S, Frauenstein A, Swietlik JJ, Rieckmann J, Meissner F, Proteomics reveals distinct mechanisms regulating the release of cytokines and alarmins during pyroptosis, Cell Reports 34 (2021) 108826. 10.1016/j.celrep.2021.108826. [DOI] [PubMed] [Google Scholar]
- [51].Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P, ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation, Science 362 (2018) 956–960. 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- [52].de Vasconcelos NM, Opdenbosch NV, Gorp HV, Parthoens E, Lamkanfi M, Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture., Cell Death Differ 26 (2018) R568. 10.1038/s41418-018-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].DiPeso L, Ji DX, Vance RE, Price JV, Cell death and cell lysis are separable events during pyroptosis, Cell Death Discov 3 (2017) 17070. 10.1038/cddiscovery.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, Zhang J, Lee WP, McKenzie BS, Ulas G, Payandeh J, Roose-Girma M, Modrusan Z, Reja R, Sagolla M, Webster JD, Cho V, Andrews TD, Morris LX, Miosge LA, Goodnow CC, Bertram EM, Dixit VM, NINJ1 mediates plasma membrane rupture during lytic cell death, Nature 591 (2021) 131–136. 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- [55].Yang HJ, Zhang J, Yan W, Cho S-J, Lucchesi C, Chen M, Huang EC, Scoumanne A, Zhang W, Chen X, Ninjurin 1 has two opposing functions in tumorigenesis in a p53-dependent manner, Proc National Acad Sci 114 (2017) 11500–11505. 10.1073/pnas.1711814114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee H-J, Ahn BJ, Shin MW, Jeong J-W, Kim JH, Kim K-W, Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development, Cell Death Differ 16 (2009) 1395–1407. 10.1038/cdd.2009.78. [DOI] [PubMed] [Google Scholar]
- [57].Elliott MR, Ravichandran KS, Clearance of apoptotic cells: implications in health and disease, J Cell Biology 189 (2010) 1059–1070. 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gong T, Liu L, Jiang W, Zhou R, DAMP-sensing receptors in sterile inflammation and inflammatory diseases, Nat Rev Immunol 20 (2020) 95–112. 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- [59].Zindel J, Kubes P, DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation, Annu Rev Pathology Mech Dis 15 (2019) 1–26. 10.1146/annurev-pathmechdis-012419-032847. [DOI] [PubMed] [Google Scholar]
- [60].Emerging inflammasome effector mechanisms., Nat Rev Immunol 11 (2011) 213 220. 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- [61].Nickel W, Rabouille C, Mechanisms of regulated unconventional protein secretion., Nat Rev Mol Cell Biology 10 (2009) 148–55. 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- [62].Dimou E, Nickel W, Unconventional mechanisms of eukaryotic protein secretion, Curr Biol 28 (2018) R406 R410. 10.1016/j.cub.2017.11.074. [DOI] [PubMed] [Google Scholar]
- [63].Tanzer MC, Frauenstein A, Stafford CA, Phulphagar K, Mann M, Meissner F, Quantitative and Dynamic Catalogs of Proteins Released during Apoptotic and Necroptotic Cell Death, Cell Reports 30 (2020) 1260–1270.e5. 10.1016/j.celrep.2019.12.079. [DOI] [PubMed] [Google Scholar]
- [64].Keller M, Rüegg A, Werner S, Beer H-D, Active Caspase-1 Is a Regulator of Unconventional Protein Secretion, Cell 132 (2008) 818 831. 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- [65].Carta S, Lavieri R, Rubartelli A, Different Members of the IL-1 Family Come Out in Different Ways: DAMPs vs. Cytokines?, Front Immunol 4 (2013) 123. 10.3389/fimmu.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC, The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages, Immunity 48 (2018). 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Piccioli P, Rubartelli A, The secretion of IL-1β and options for release, Semin Immunol 25 (2013) 425–429. 10.1016/j.smim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- [68].Monteleone M, Stow JL, Schroder K, Mechanisms of unconventional secretion of IL-1 family cytokines., Cytokine 74 (2015) 213 218. 10.1016/j.cyto.2015.03.022. [DOI] [PubMed] [Google Scholar]
- [69].Lamkanfi M, Sarkar A, Walle LV, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti T-D, Dixit VM, Inflammasome-Dependent Release of the Alarmin HMGB1 in Endotoxemia, J Immunol 185 (2010) 4385–4392. 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kim JY, Park JS, Strassheim D, Douglas I, del Valle FD, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E, HMGB1 contributes to the development of acute lung injury after hemorrhage, Am J Physiol-Lung C 288 (2005) L958–L965. 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- [71].Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ, HMG-1 as a Late Mediator of Endotoxin Lethality in Mice, Science 285 (1999) 248–251. 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 72.[] Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ, Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock*, Crit Care Med 33 (2005) 564–573. 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- [73].Rathinam VAK, Zhao Y, Shao F, Innate immunity to intracellular LPS, Nat Immunol 20 (2019) 527–533. 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sundblad V, Morosi LG, Geffner JR, Rabinovich GA, Galectin-1: A Jack-of-All-Trades in the Resolution of Acute and Chronic Inflammation., J Immunol Baltim Md 1950 199 (2017) 3721–3730. 10.4049/jimmunol.1701172. [DOI] [PubMed] [Google Scholar]
- [75].Zhou B, Liu J, Zeng L, Zhu S, Wang H, Billiar TR, Kroemer G, Klionsky DJ, Zeh HJ, Jiang J, Tang D, Kang R, Extracellular SQSTM1 mediates bacterial septic death in mice through insulin receptor signalling, Nat Microbiol 5 (2020) 1576–1587. 10.1038/s41564-020-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Russo AJ, Behl B, Banerjee I, Rathinam VAK, Emerging Insights into Noncanonical Inflammasome Recognition of Microbes, J Mol Biol 430 (2018) 207–216. 10.1016/j.jmb.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA, Caspase-11 Protects Against Bacteria That Escape the Vacuole, Science 339 (2013) 975–978. 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Aachoui Y, Kajiwara Y, Leaf IA, Mao D, Ting JP-Y, Coers J, Aderem A, Buxbaum JD, Miao EA, Canonical Inflammasomes Drive IFN-γ to Prime Caspase-11 in Defense against a Cytosol-Invasive Bacterium, Cell Host Microbe 18 (2015) 320–332. 10.1016/j.chom.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Srisaowakarn C, Pudla M, Ponpuak M, Utaisincharoen P, Caspase-4 Mediates Restriction of Burkholderia pseudomallei in Human Alveolar Epithelial Cells, Infect Immun 88 (2020). 10.1128/iai.00868-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wang J, Shao Y, Wang W, Li S, Xin N, Xie F, Zhao C, Caspase-11 deficiency impairs neutrophil recruitment and bacterial clearance in the early stage of pulmonary Klebsiella pneumoniae infection., Int J Med Microbiol 307 (2017) 490 496. 10.1016/j.ijmm.2017.09.012. [DOI] [PubMed] [Google Scholar]
- [81].Wang W, Shao Y, Li S, Xin N, Ma T, Zhao C, Song M, Caspase-11 Plays a Protective Role in Pulmonary Acinetobacter baumannii Infection, Infect Immun 85 (2017) e00350–17. 10.1128/iai.00350-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K, Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps., Sci Immunol 3 (2018) eaar6676. 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- [83].Finethy R, Dockterman J, Kutsch M, Orench‐Rivera N, Wallace GD, Piro AS, Luoma S, Haldar AK, Hwang S, Martinez J, Kuehn MJ, Taylor GA, Coers J, Dynamin‐related Irgm proteins modulate LPS‐induced caspase‐11 activation and septic shock, Embo Rep 21 (2020) e50830. 10.15252/embr.202050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Eren E, Planès R, Bagayoko S, Bordignon P, Chaoui K, Hessel A, Santoni K, Pinilla M, Lagrange B, Burlet‐Schiltz O, Howard JC, Henry T, Yamamoto M, Meunier E, Irgm2 and Gate‐16 cooperatively dampen Gram‐negative bacteria‐induced caspase‐11 response, Embo Rep 21 (2020) e50829. 10.15252/embr.202050829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Linder A, Hornung V, Irgm2 and Gate‐16 put a break on caspase‐11 activation, Embo Rep 21 (2020) e51787. 10.15252/embr.202051787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sakaguchi N, Sasai M, Bando H, Lee Y, Pradipta A, Ma JS, Yamamoto M, Role of Gate-16 and Gabarap in Prevention of Caspase-11-Dependent Excess Inflammation and Lethal Endotoxic Shock, Front Immunol 11 (2020) 561948. 10.3389/fimmu.2020.561948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hsu J, Chou J, Chen T, Hsu J, Su F, Lan J, Wu P, Hu C, Lee EY, Lee W, Glutathione peroxidase 8 negatively regulates caspase‐4/11 to protect against colitis, Embo Mol Med 12 (2020) e9386. 10.15252/emmm.201809386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Choi YJ, Kim S, Choi Y, Nielsen TB, Yan J, Lu A, Ruan J, Lee H-R, Wu H, Spellberg B, Jung JU, SERPINB1-mediated checkpoint of inflammatory caspase activation, Nat Immunol 20 (2019) 276–287. 10.1038/s41590-018-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liu Q, Zhang S, Sun Z, Guo X, Zhou H, E3 ubiquitin ligase Nedd4 is a key negative regulator for non-canonical inflammasome activation, Cell Death Differ 26 (2019) 2386–2399. 10.1038/s41418-019-0308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chen R, Zeng L, Zhu S, Liu J, Zeh HJ, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D, Kang R, cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis, Sci Adv 5 (2019) eaav5562. 10.1126/sciadv.aav5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, Gao X, An S, Mittal M, Vogel SM, Miao EA, Rehman J, Malik AB, Caspase-11–mediated endothelial pyroptosis underlies endotoxemia-induced lung injury, J Clin Invest 127 (2017) 4124–4135. 10.1172/jci94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H, Wu J, Wang Z, Liu Y, Chen F, Xiao X, Mackman N, Billiar TR, Han J, Lu B, Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure, Immunity 51 (2019) 983–996.e6. 10.1016/j.immuni.2019.11.005. [DOI] [PubMed] [Google Scholar]
- [93].Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, Grover SP, Zhang X, Li L, Xiang B, Shi J, Li X-A, Daugherty A, Smyth SS, Kirchhofer D, Shiroishi T, Shao F, Mackman N, Wei Y, Li Z, Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis, Immunity 50 (2019) 1401–1411.e4. 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Agnew A, Nulty C, Creagh EM, Regulation, Activation and Function of Caspase-11 during Health and Disease, Int J Mol Sci 22 (2021) 1506. 10.3390/ijms22041506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Oficjalska K, Raverdeau M, Aviello G, Wade SC, Hickey A, Sheehan KM, Corr SC, Kay EW, O’Neill LA, Mills KHG, Creagh EM, Protective Role for Caspase-11 during Acute Experimental Murine Colitis, J Immunol 194 (2015) 1252–1260. 10.4049/jimmunol.1400501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Demon D, Kuchmiy A, Fossoul A, Zhu Q, Kanneganti TD, Lamkanfi M, Caspase-11 is expressed in the colonic mucosa and protects against dextran sodium sulfate-induced colitis., Mucosal Immunol 7 (2014). 10.1038/mi.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Flood B, Manils J, Nulty C, Flis E, Kenealy S, Barber G, Fay J, Mills KHG, Kay EW, Creagh EM, Caspase-11 regulates the tumour suppressor function of STAT1 in a murine model of colitis-associated carcinogenesis, Oncogene 38 (2019) 2658–2674. 10.1038/s41388-018-0613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zasłona Z, Flis E, Wilk MM, Carroll RG, Palsson-McDermott EM, Hughes MM, Diskin C, Banahan K, Ryan DG, Hooftman A, Misiak A, Kearney J, Lochnit G, Bertrams W, Greulich T, Schmeck B, McElvaney OJ, Mills KHG, Lavelle EC, Wygrecka M, Creagh EM, O’Neill LAJ, Caspase-11 promotes allergic airway inflammation, Nat Commun 11 (2020) 1055. 10.1038/s41467-020-14945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yi Y-S, Caspase-11 Noncanonical Inflammasome: A Novel Key Player in Murine Models of Neuroinflammation and Multiple Sclerosis, Neuroimmunomodulat (2021) 1–9. 10.1159/000516064. [DOI] [PubMed] [Google Scholar]
- [100].Ye L, Li G, Goebel A, Raju AV, Kong F, Lv Y, Li K, Zhu Y, Raja S, He P, Li F, Mwangi SM, Hu W, Srinivasan S, Caspase-11–mediated enteric neuronal pyroptosis underlies Western diet–induced colonic dysmotility, J Clin Invest 130 (2020) 3621–3636. 10.1172/jci130176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jiang M, Sun X, Liu S, Tang Y, Shi Y, Bai Y, Wang Y, Yang Q, Yang Q, Jiang W, Yuan H, Jiang Q, Cai J, Caspase-11-Gasdermin D-Mediated Pyroptosis Is Involved in the Pathogenesis of Atherosclerosis, Front Pharmacol 12 (2021) 657486. 10.3389/fphar.2021.657486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhu Y, Zhao H, Lu J, Lin K, Ni J, Wu G, Tang H, Caspase-11–Mediated Hepatocytic Pyroptosis Promotes the Progression of Nonalcoholic Steatohepatitis, Cell Mol Gastroenterology Hepatology 12 (2021) 653–664. 10.1016/j.jcmgh.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA, Noncanonical Inflammasome Activation of Caspase-4/Caspase-11 Mediates Epithelial Defenses against Enteric Bacterial Pathogens, Cell Host Microbe 16 (2014) 249–256. 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang J, Sahoo M, Lantier L, Warawa J, Cordero H, Deobald K, Re F, Caspase-11-dependent pyroptosis of lung epithelial cells protects from melioidosis while caspase-1 mediates macrophage pyroptosis and production of IL-18, Plos Pathog 14 (2018) e1007105. 10.1371/journal.ppat.1007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Matheis F, Muller PA, Graves CL, Gabanyi I, Kerner ZJ, Costa-Borges D, Ahrends T, Rosenstiel P, Mucida D, Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss, Cell 180 (2020) 64–78.e16. 10.1016/j.cell.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kumari P, Russo AJ, Wright SS, Muthupalani S, Rathinam VA, Hierarchical cell-type-specific functions of caspase-11 in LPS shock and antibacterial host defense, Cell Reports 35 (2021) 109012. 10.1016/j.celrep.2021.109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kovacs SB, Oh C, Maltez VI, McGlaughon BD, Verma A, Miao EA, Aachoui Y, Neutrophil Caspase-11 Is Essential to Defend against a Cytosol-Invasive Bacterium, Cell Reports 32 (2020) 107967. 10.1016/j.celrep.2020.107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kobayashi T, Ogawa M, Sanada T, Mimuro H, Kim M, Ashida H, Akakura R, Yoshida M, Kawalec M, Reichhart J-M, Mizushima T, Sasakawa C, The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection., Cell Host Microbe 13 (2013) 570–83. 10.1016/j.chom.2013.04.012. [DOI] [PubMed] [Google Scholar]
- [109].Cunha LD, Ribeiro JM, Fernandes TD, Massis LM, Khoo CA, Moffatt JH, Newton HJ, Roy CR, Zamboni DS, Inhibition of inflammasome activation by Coxiella burnetii type IV secretion system effector IcaA, Nat Commun 6 (2015) 10205. 10.1038/ncomms10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Song T, Li K, Zhou W, Zhou J, Jin Y, Dai H, Xu T, Hu M, Ren H, Yue J, Liang L, A Type III Effector NleF from EHEC Inhibits Epithelial Inflammatory Cell Death by Targeting Caspase-4, Biomed Res Int 2017 (2017) 1–11. 10.1155/2017/4101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pallett MA, Crepin VF, Serafini N, Habibzay M, Kotik O, Sanchez-Garrido J, Santo JPD, Shenoy AR, Berger CN, Frankel G, Bacterial virulence factor inhibits caspase-4/11 activation in intestinal epithelial cells, Mucosal Immunol 10 (2017) 602–612. 10.1038/mi.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Havira MS, Ta A, Kumari P, Wang C, Russo AJ, Ruan J, Rathinam VA, Vanaja SK, Shiga toxin suppresses noncanonical inflammasome responses to cytosolic LPS, Sci Immunol 5 (2020) eabc0217. 10.1126/sciimmunol.abc0217. [DOI] [PMC free article] [PubMed] [Google Scholar]


