Abstract
Background:
We aimed to investigate the associations of glycemic markers (hemoglobin A1C [HbA1C], fasting plasma glucose [FPG] and glycemic status [normoglycemia, prediabetes and diabetes]) with incident heart failure (HF) and its subtypes, among Blacks.
Methods:
We included 2,290 community-dwelling Blacks(64% women, mean age 58 years) without prevalent HF from the Jackson Heart Study who attended the second exam (2005–2008). The associations between glycemic markers and incident HF (and subtypes including HF with preserved ejection fraction [HFpEF] and reduced ejection fraction [HFrEF]) were evaluated using Cox proportional hazards regression models, adjusting for risk factors and coronary heart disease.
Results:
There were 119 incident HF events (48 HFpEF, 58 HFrEF, and 13 unclassified HF events) over a median follow-up of 10.5 years. Higher levels of HbA1C (HR per SD increment, 1.30; 95% CI 1.12, 1.51) and FPG (HR per SD increment FPG: 1.32; 95% CI: 1.17, 1.48) were associated with a higher risk of incident HF. Compared to normal glycemia, diabetes status was associated with a higher risk of incident HF (HR: 1.24; 95%CI: 1.02, 2.05). HbA1C was significantly associated with higher risks of HFpEF (HR per SD increment: 1.41, 95% CI: 1.18, 1.69) and HFrEF (HR per SD increment: 1.32; 95% CI: 1.12, 1.56). FPG was significantly associated with higher risk of HFpEF (HR per SD increment: 1.35, 95% CI: 1.14, 1.62) but not HFrEF (HR per SD increment: 1.12; 95% CI: 0.53, 2.35).
Conclusions:
Among community-dwelling Blacks, higher levels of glycemic markers were associated with higher risk of HF subtypes.
Clinical Trials. gov Identifier:
NCT00005485) of the Jackson Heart Study.
Keywords: Diabetes, Fasting glucose, Glycosylated hemoglobin, Heart Failure subtypes
INTRODUCTION
Heart failure (HF) and type 2 diabetes are highly prevalent and commonly co-occurring conditions (1,2). Individuals with diabetes are at increased risk of HF compared to those without diabetes (3). One in three HF patients has diabetes (4), and HF is a common complication of diabetes (5). In the US, Blacks are disproportionately affected by both HF and diabetes (1,2). An exploration of the relation of various hyperglycemic markers and HF can be useful to improve our understanding of HF pathogenesis, as well as its control and prevention strategies among Blacks. However, a limited number of studies have comprehensively assessed the relation between glycemic markers and incident HF among Blacks (6,7). These studies have included a limited number of Blacks, and have not examined the whole spectrum of measures of glucose homeostasis, which would capture different aspects of the pathophysiology of diabetes-related cardiac dysfunction. Indeed, several biological mechanisms have been proposed to explain the independent link of hyperglycemia to cardiac dysfunction (8). The variety of mechanisms suggest that the different markers of hyperglycemia (fasting blood glucose [FPG] and glycosylated hemoglobin [HbA1C]) could capture different aspects of the hyperglycemia and HF relation (8). This may be particularly important, given the glucose independent differences in HbA1C observed across ethnicities; with higher levels of HbA1C at similar levels of glycemia in Blacks compared to other ethnic groups (9).
Using the community-based Jackson Heart Study (JHS) cohort, we evaluated the associations of several glycemic markers (FPG, HbA1C, and overall glycemic status) with incident HF hospitalizations, among Blacks.
METHODS
Study sample
The JHS recruited Blacks aged 21 to 94 years, from the Jackson, Mississippi, metropolitan area (10). The JHS study design and methods have been described elsewhere (10). After completing a baseline clinic visit (examination 1 [2000–2004]), participants returned for two additional clinic visits, examination 2 (2005–2008) and examination 3 (2009–2013).
The present study included participants who underwent the examination 2 (n=4,205) because the ascertainment of HF only started at examination 2 (Figure 1). We excluded participants with missing data on HbA1C and FPG (n=1370), prevalent HF (n=50), missing outcome (n=420), and missing data on the covariates to be used in the analyses (n=125).
Figure 1:
Process of selecting eligible participants for the analyses
The study protocol was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University and Tugaloo College, all located in Jackson, Mississippi, USA. All the participants provided informed consent.
Markers of Glucose Metabolism
FPG and HbA1C were assessed at examination 2. HbA1C was measured using high-performance liquid chromatography (Tosoh G7, Tosoh Corporation, Tokyo, Japan). The coefficient of variation for HbA1C assay ranged from 1.4% to 1.9%. A National Glycohemoglobin Standardization Program (NGSP)-certified assay was used to measure HbA1C. Fasting plasma glucose was measured using the glucose oxidase method. Glucose assays were run in duplicate; the intra-assay coefficient of variation was <3%.
Diabetes was defined according to the American Diabetes Association (ADA) criteria as a FPG ≥ (126 mg/dL) or HbA1C ≥ 6.5% (11), self-reported of physician-diagnosed diabetes or confirmed use diabetes medications. Prediabetes was defined as a FPG of 100 to 125 mg/dL or HbA1C of 5.7 to 6.4% (11).
Incident Heart Failure Assessment
The primary outcome of interest was incident HF hospitalization. The process of adjudication of HF events in the JHS has been described previously (12). Briefly, HF hospitalizations were identified through annual follow-up telephone interviews and confirmed with HF hospitalization records. The hospital discharge lists with relevant International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes were obtained from Jackson, Mississippi tri-county area hospitals and out of catchment area hospitals. The formal physician-adjudicated diagnosis of HF hospitalization begun in January 2005 and included probable or definite HF hospitalization. The medical records that include ICD-9-CM codes for primary diagnoses of HF were reviewed, and an adjudication through examination of clinical documentation including presenting signs and symptoms, laboratory tests and imaging (echocardiography and magnetic resonance imaging). The adjudication of HFpEF versus HFrEF was conducted in the subset of subset of individuals with available hospitalization data on cardiac imaging (echocardiogram or other cardiac imaging such as cardiac magnetic resonance imaging). HFpEF was defined as a HF event with an ejection fraction ≥50% (12).. The evaluation of adjudicated incident HF events was performed through December 31, 2015.
Covariates
The covariates, including demographic and behavioral characteristics, as well as medical history and medication use, were assessed at examination 2. The methods of risk factor ascertainment in JHS have been reported elsewhere (10).
Information on personal medical and family history, medication use, income, physical activity, alcohol use (within the past 12 months), and current smoking was obtained using standardized questionnaires.
Height, and weight were measured and body mass index (BMI) was calculated (kg/m2). Blood pressure (BP) was measured twice in the left arm of the seated subject with a mercury column sphygmomanometer. The average of the two readings was used as the examination BP, and hypertension was defined as systolic BP ≥130 mm Hg or diastolic BP ≥80 mm Hg, or self-reported antihypertensive medication use. Serum creatinine was measured using the rate Jaffe reaction, and the kidney function was assessed using the estimated glomerular filtration rate (eGFR) calculated by the CKD-EPI study equation (13). Plasma total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides concentrations were measured using standard enzymatic methods, on a Vitros 950 or 250, Ortho-Clinical Diagnostics analyzer (Raritan, NJ) in accordance with the College of American Pathologists Proficiency Testing Program.(14) Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation (which was not used for individuals with triglycerides levels ≥400 mg/dL) (15).
Statistical Analysis
We classified participants into clinically relevant categories of HbA1C (<5.7%; 5.7 % to 6.4% and ≥ 6.5%) and compared differences in baseline characteristics of participants’ variables across HbA1C groups, using ANOVA (for continuous variables) or the Chi square test (for categorical variables). We also assessed the baseline characteristics of participants by glycemic categories (defined by FPG alone, or FPG and HbA1C concomitantly).
Crude HF incidence rates and 95% confidence intervals were calculated by exposure levels (clinically relevant categories of FPG and HbA1Cand diabetes status). The person-time of follow up from baseline (examination 2) until the first occurrence of a) HF outcomes b) death or c) censoring (date of the last available follow-up). Heart failure-free survivor curves were plotted using the Kaplan–Meier method, and differences between event-free survivor probabilities between the different categories of HbA1C were compared using the log-rank test. For the multivariable analyses, we fitted Cox proportional hazards regression models to relate each glycemic marker to incident HF, after verification of the assumption of proportionality of hazards tested using Schoenfeld residuals and the plots of these residuals over time. We modelled each glycemic marker both as a continuous and categorical variable using the relevant categories.
For each glycemic marker, identical covariates in models. These variables included age, sex, smoking, alcohol use, physical activity, body mass index, ratio of total to HDL-cholesterol, systolic blood pressure, use of antihypertensive medications, use of statins, eGFR, and CHD (time-varying covariate).
For the HFpEF and HFrEF separate analyses, we used cause-specific Cox proportional hazards models, treating the other type of HF as a censoring event.
Two-sided P values of <0.05 were considered statistically significant, including for interaction terms. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Source of funding
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The funding agencies had no role in the formulation, editing, or decision to submit this manuscript. Justin Echouffo Tcheugui was supported by NIH/NHLBI grant K23 HL153774. Robert J. Mentz was supported by the National Institutes of Health (U01HL125511–01A1 and R01AG045551–01A1). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
Study Sample
The final study population included 2,290 participants (63.7 % women, mean age 58 years). Table 1 summarizes the baseline characteristics of participants by categories of HbA1C. Compared to the lowest HbA1C category, individuals with higher HbA1C were older, had higher FPG levels, systolic blood pressure, and body mass index, but had worse renal function, and were more likely to have an atherogenic lipid profile and less likely to be active. In additional analysis, the distributions of the characteristics of participants were examined by categories of FPG alone, FPG and HbA1C (Supplementary Tables 2, 3, 4 &5). The distributions of the characteristics of participants were roughly similar across levels of worsening glycemia using samples defined by categories of FPG alone, FPG and HbA1C.
Table 1:
Characteristics of Jackson Heart Study participants by categories of glycated hemoglobin at Examination2
| Glycated hemoglobin (HbA1C) categories | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | Total (N=2,290) | <5.7 % (N=1050) | 5.7 – 6.4 % (N = 834) | ≥6.5 % (N = 406) | P-value |
| Age, years | 57 (12) | 54.7 + 0.36 | 58.7 + 0.40 | 60.6 + 0.58 | <.0001 |
| Male, n (%) | 830 (36.2) | 395 (37.6) | 288 (34.5) | 147 (36.2) | 0.3835 |
| Low income status, n (%) | 250 (11.0) | 108 (10.3) | 92 (11.0) | 45 (11.1) | |
| Current Smokers, n (%) | 225 (9.8) | 91 (8.7) | 100 (12.0) | 34 (8.4) | 0.0307 |
| Physical Activity categories: | |||||
| Low | 1021 (44.5) | 420 (40.0) | 390 (46.8) | 211 (52.0) | <0.0001 |
| Moderate | 771 (33.7) | 362 (34.5) | 279 (33.5)) | 130 (32.0) | |
| Ideal | 498 (21.8) | 268 (25.5) | 165 (19.8) | 65 (16.0) | |
| Body mass index, kg/m2 | 31.9 (6.9) | 30.5 + 0.21 | 32.2 + 0.23 | 34.7 + 0.33 | <.0001 |
| Obese (BMI>30 kg/m2), n (%) | 1252 (54.7) | 474 (45.1) | 484 (58.0) | 294 (72.4) | <.0001 |
| Fasting plasma glucose (FPG), mg/dL | 93.3 + 0.78 | 99.9 + 0.88 | 144.3 + 1.26 | <.0001 | |
| Hemoglobin A1C, % | 5.4 + 0.02 | 6.0 + 0.02 | 7.6 + 0.03 | <.0001 | |
| FPG <100 mg/dL | 1325 (57.9) | 842 (80.2) | 445 (53.4) | 155 (9.4) | <.0001 |
| FPG: 100 – 125 mg/dL | 718 (31.4) | 200 (19.0) | 363 (43.5) | 155 (38.2) | |
| FPG: >126 mg/dL | 247(10.8) | 8 (0.8) | 26 (3.1) | 213 (52.5) | |
| Diabetes, n (%) | 568 (24.8) | 51 (4.9) | 111 (13.3) | 406 (100.0) | <.0001 |
| Systolic blood pressure, mmHg | 127 (19) | 125.4 + 0.57 | 126.9 + 0.64 | 131.5 + 0.92 | <.0001 |
| Diastolic blood pressure, mmHg | 75 (10) | 75.1 + 0.31 | 74.0 + 0.35 | 74.3 + 0.50 | <.0.06 |
| Hypertension, n (%) | 1502 (65.6) | 578 (55.0) | 588 (70.2) | 336 (82.8) | <.0001 |
| Antihypertensive medications, n (%) | 127 (19) | 528 (50.3) | 544 (65.2) | 336 (82.8) | <.0001 |
| Total cholesterol, mg/dL | 197 (41) | 196.5 + 1.25 | 200.2 + 1.41 | 191.2 + 2.02 | 0.005 |
| HDL-cholesterol, mg/dL | 54.2 (15.1) | 55.5 + 0.46 | 53.9 + 0.52 | 51.2 + 0.75 | <.0001 |
| Total to HDL-cholesterol ratio | 123.0 (36.8) | 3.75 + 0.04 | 3.94 + 0.04 | 3.96 + 0.06 | 0.0004 |
| LDL -cholesterol, mg/dL | 3.86 (1.21) | 123.2 + 1.13 | 126 + 1.27 | 116.9 + 1.85 | 0.0003 |
| eGFR, mL/min/1.73 m2 | 97.2 (19.7) | 99.6 + 0.61 | 95.3 + 0.68 | 95.2 + 0.97 | 0.011 |
| Prevalent CHD, n (%) | 88 (3.8) | 23 (2.2) | 40 (4.8) | 25 (6.2) | 0.0004 |
Values are reported as mean ± SD for continuous traits and n (%) for dichotomous traits. BP: blood pressure, CHD: coronary heart disease, eGFR: estimated glomerular filtration rate, HDL: high-density lipoprotein, LDL: low-density lipoprotein
The comparison of the characteristics of the included and excluded participants is detailed in Supplementary Table 1. Given the difference between the included and excluded participants, we performed sensitivity analyses to test for potential bias. We estimated propensity scores using logistic regression and the distribution are summarized in Supplementary Figure 1. In addition, to account for missingness and thus minimize bias, we converted the scores into inverse probability weighting and included it in the Cox proportional hazard regression models, using previously described approaches. (16) We observed that the including of weights to account for missingness had no effect on our effect estimates, suggesting that our inclusion-exclusion criteria did not introduce bias.
Associations of Glycemic Markers and Incident Heart Failure
Over a median follow-up of 10.5 years, there were 119 incident HF events observed (48 HFpEF events, 58 HFrEF events, and 13 unclassified HF events). The overall incidence rate of HF was 5.17 (4.32, 6.19) per 1,000 person-years.
The incidence rate of HF increased across levels of HbA1C, FPG, glycemic categories (as defined by both FPG and HbA1C) (Table 2). Indeed, the incidence rate for HF among individuals with diabetes was at least a 3-fold higher than that of individuals without diabetes (Table 2).
Table 2:
Event Rates of Incident of Heart Failure Rates among Jackson Heart Study Participants
| Cases/No. at Risk | 1000-Person Years | Event Rates (per 1000 Person-Years) | |
|---|---|---|---|
| Glycated hemoglobin (HbA 1C ) | |||
| ≤5.7 % | 40/1050 | 10591 | 3.8 (2.77, 5.15) |
| 5.7 – 6.4 % | 36/834 | 8447 | 4.26 (3.07, 5.91) |
| ≥ 6.4 % | 43/406 | 3972 | 10.8 (8.03, 14.6) |
| Pooled | 119/2290 | 23012 | 5.17 (4.32, 6.19) |
| Fasting Plasma Glucose (mg/dL) | |||
| <100 mg/dL | 54/1325 | 13334 | 4.05 (3.1, 5.29) |
| 100–125 mg/dL | 35/718 | 7273 | 4.81 (3.46, 6.70) |
| ≥ 125 mg/dL | 30/247 | 2404 | 12.5 (8.72, 17.8) |
| Pooled | 119/2290 | 19945 | 5.17 (4.32, 6.19) |
| Hyperglycemia status | |||
| Normal | 27/687 | 6903 | 3.91 (2.68, 5.70) |
| Pre-diabetes | 39/1035 | 10538 | 3.70 (2.70, 5.06) |
| Diabetes | 53/568 | 5569 | 9.52 (7.27, 12.50) |
| Pooled | 119/2290 | 21587 | 5.17 (4.32, 6.19)) |
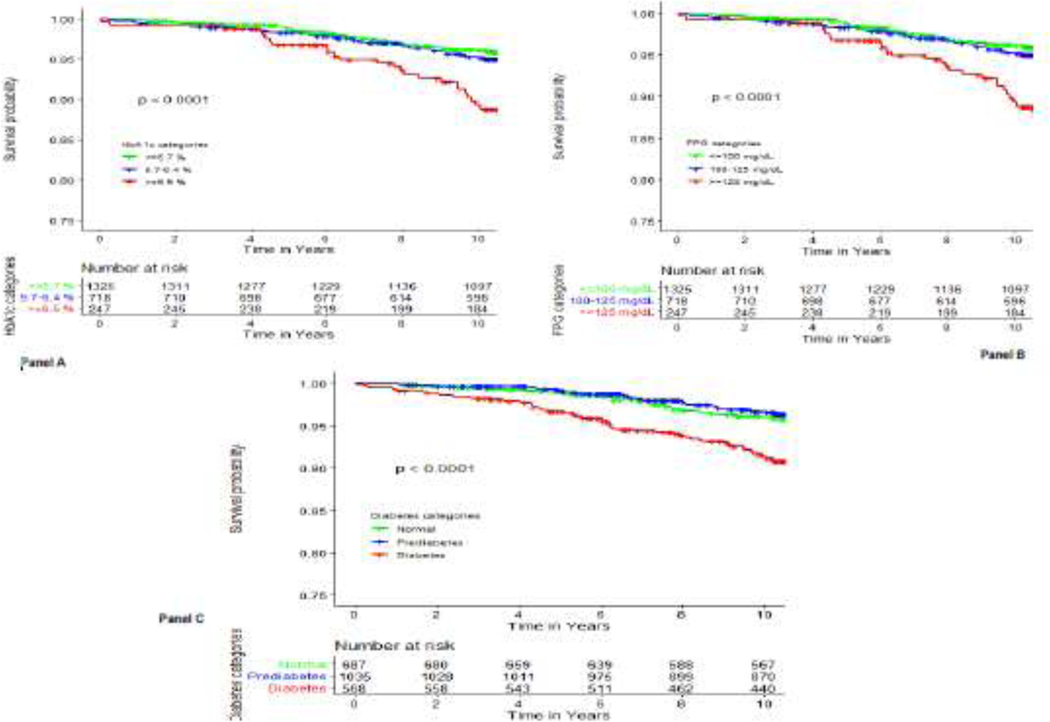
Compared to the lowest clinically relevant category, individuals in the highest levels of HbA1C or FPG exhibited at least a three-time higher incidence rates of HF events (Table 2&Figure 2, P-log rank <0.001 for both HbA1C and FPG).
Figure 2:
Survival plot comparing the risk of heart failure hospitalization across levels of glycemic markers.
Panel A: by levels of glycosylated hemoglobin (HbA1C), Panel B: by levels of by levels of fasting plasma glucose (FPG), Panel C: by glycemic status categories
In multivariable adjusted Cox proportional hazards (Table 3), compared to the referent HbA1C category (<5.7%), higher levels of HbA1c (≥ 6.5%) were associated with incident HF (hazard ratio [HR] 1.65; 95% confidence interval [CI]: 1.03, 2.62). When modeled as a continuous predictor, HbA1C was also associated with greater risk of heart failure (HR per SD increment in HbA1C: 1.30; 95% CI 1.12, 1.51) (Table 3).
Table 3:
Estimates of the Multivariable Adjusted Association of glycemic markers and incident heart failure
| Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | P-value | |
|---|---|---|---|
| Glycated hemoglobin (HbA 1C ) | |||
| ≤5.7 % | 1 (Reference) | 1 (Reference) | |
| 5.7 – 6.4 % | 1.12 (0.72, 1.77) | 0.84 (0.52, 1.33) | 0.45 |
| ≥ 6.5 % | 2.90 (1.88, 4.45) | 1.65 (1.03, 2.62) | 0.0355 |
| 1 SD increment of HbA 1C | 1.38 (1.24, 1.52) | 1.30 (1.12, 1.51) | 0.0007 |
| Fasting Plasma Glucose (mg/dL) | |||
| <100 mg/dL | 1 (Reference) | 1 (Reference) | |
| 100–125 mg/dL | 1.19 (0.78, 1.81) | 0.81 (0.51, 1.26) | 0.34 |
| ≥ 125 mg/dL | 3.13 (2.00, 4.89) | 1.92 (1.20, 3.08) | 0.0070 |
| 1-SD increment of fasting Glucose | 1.29 (1.17, 1.42) | 1.32 (1.17, 1.48) | <0.0001 |
| Hyperglycemia status (defined by both fasting plasma glucose and HbA 1C ) | |||
| Normal | 1 (Reference) | 1 (Reference) | |
| Pre-diabetes | 0.96 (0.58, 1.57) | 0.68 (0.41, 1.13) | 0.14 |
| Diabetes | 2.36 (2.03, 3.79) | 1.24 (1.02, 2.05) | 0.03 |
Values are hazard ratios (95% confidence interval [CI]) and P values. The hazard ratios are adjusted for age, sex, smoking, income, alcohol use, physical activity, body mass index, ratio of total to HDL-cholesterol, systolic blood pressure, use of antihypertensive medications, use of statins, eGFR, and CHD (time-varying covariate).
Higher levels of FPG (>125 mg/dL) as compared to lower levels (<100 mg/dL), were associated with an increased risk of incident HF (HR, 1.92; 95% CI: 1.20, 3.08). When fitted as a continuous variable, FPG was associated with a higher risk of incident HF (HR per SD increment in FPG: 1.32; 95% CI: 1.17, 1.48, Table 3).
Having diabetes (as defined by both HbA1C and FPG) was associated with incident HF (HR: 1.24; 95% CI: 1.02, 2.05), compared to no-diabetes (Table 3). Prediabetes as not associated with incident HF.
Additionally accounting for the use of medications such angiotensin converting enzyme inhibitors, angiotensin receptor blockers and beta-blockers did not affect our results in terms of overall HF (Supplementary Table 6).
The incidence rates of HFpEF and HFrEF were higher with increasing glycemia (Supplementary Tables 7 & 9). Higher levels of HbA1C were associated with higher relative risks of HFpEF (HR for each SD change in HbA1C: 1.41, 95%CI: 1.18, 1.69) and HFrEF (HR for each SD change in HbA1C: 1.32, 95% CI: 1.12, 1.56]) (Supplementary Tables 8 & 10). FPG was significantly associated with higher risk of HFpEF (HR per SD increment: 1.35, 95% CI: 1.14, 1.62) but not significantly HFrEF (HR per SD increment: 1.12; 95% CI: 0.53, 2.35). Diabetes status was not significantly associated with higher relative risks of HFpEF and HFrEF (Supplementary Tables 8 & 10).
DISCUSSION
In a community-based sample of Blacks, we found that increasing levels of HbA1C and FPG, as well as the diabetes status (as defined by a combination of HbA1C and FPG) were independently associated with a higher risk incident HF hospitalization. HbA1C was significantly associated with higher risks of both HFpEF and HFrEF, and to a similar extent. FPG was associated with a higher risk of both HFpEF.
Our observations provide additional insights into the relation of dysglycemia and HF. Several studies have reported on the association of glycemic markers or hyperglycemia status and incident HF, but have these included a limited number of participants of African descent both (3). These studies have reported relative risk for the diabetes and HF association of similar magnitude to that reported in our investigation (3).The prior studies have generally focused on HbA1C (6,7), other on FPG and others on both (3), showing positive association of these markers with incident HF (7,17,18). Other studies of HbA1C have focused on individuals with diabetes only (6,19). There is a paucity of studies on the relation of glycemia with the subtypes of HF (20). The extant studies of HF subtypes have shown that diabetes status is associated with both HFpEF and HFrEF, in similar fashion (20), but have not estimated the individual associations with FPG HbA1C or, and have not accounted for HbA1C in their definition of diabetes. Our observations extend the literature on this topic, by providing additional evidence on the association between glycemic markers and the future risk of incident HF, as well as it subtypes. Our study attempts to capture the whole spectrum of dysglycemia (including prediabetes and diabetes), as well as the various pathways representing the metabolic milieu associated with dysglycemia that contribute to cardiac dysfunction, as possibly captured by various glycemic markers. These pathways include glucotoxicity, lipotoxicity, and hyperinsulinemia (captured by FPG but also by HbA1C), or increased tissue glycation leading to fibrosis (captured by HbA1C) (8). Our finding of a lack of a significant association between the diabetes status and HF subtypes (HFpEF or HFrEF) may be due to a lack of power. Futhermore, the observed significant association of HbA1C with HF suggests that the diabetes status is not the only determinant of HF, and confirms that the extent of blood glucose control matters (19).
Our study suggests that although HbA1C may differ by racial/ethnic groups, its has a important prognostic value among Blacks in terms of HF. Our study highlight diabetes as a high-risk condition that should be the focus of HF preventive efforts among Blacks, who are disproportionally affected by both diabetes and HF compared to other racial/ethnic groups in the US (1,2). Such a preventative approach can combine lifestyle changes and the use of of novel diabetes therapies wih a cardiovascular benefit such as sodium-glucose co-transporter-2 (SGLT-2) inhibitors showing improved HF outcomes among individuals with diabetes in clinical trials including those with HFrEF (21,22) and those with HFpEF. (23,24). This importance is recognized in contemporary guidelines that recommend the use of specific therapies in the context of HF and diabetes, to optimize management (25,26).
In a hypothesis-generating study, we studied the association of HF with several glycemic biomarkers, namely FPG and HbA1C, which may all represent distinct biological pathways that would contribute to HF occurrence. Data from labaratory-based studies suggest the potential pathways through which hyperglycemia leads to HF include among others : i) increased concentration of advanced glycation end products (AGEs) may promote myocardial collagen deposition and fibrosis; ii) hyperglycemia-related oxidative stress may induce myocardial injury and fibrosis; iii) mitochondrial dysfunction and autonomic perturbations related to diabetes may also contribute to myocardial dysfunction and iv) hyperinsulinemia related to insulin resistance (8). While laboratory-based studies suggest distinct pathways by which diabetes leads to HFpEF (27), we did not find significant differences in the risk of HFpEF and HFrEF related to hyperglycemia.
Some limitations of our study should be acknowledged. First, the participants were Blacks from Jackson in Mississippi; thus, our results may not be generalizable to Blacks elsewhere in the US and to other racial/ethnic groups. Second, we did not have data on 2 hour-post load glucose levels, and thus we may have underestimated the effect of diabetes on HF risk. Third, the ascertainment of the incidence of HF relied solely on hospitalizations, and did not include outpatient HF cases, which may have led to an underestimation of cases of HF in the community. Fourth, we may have been underpowered to examine the association with the HF subtypes, as evidenced by the low event rates for each subtype. Lastly, because of the observational nature of our analysis, the study findings may be predisposed to residual confounding.
The strengths of this study include a well-characterized community-based sample of Blacks, the availability of several glycemic markers, the examination of various HF subtypes, a rigorous adjustment that included accounting for CHD as time-varying covariates in our analyses.
CONCLUSION
In conclusion, in a community-based sample of Blacks adults, high levels of several glycemic markers were associated with an increased risk of incident HF. These results suggest that the link to abnormal glucose metabolism could represent the combined influence of different biological pathways leading to heart failure. Overall, our findings are of contemporary significance given the rising burden of HF among patients with diabetes in the United States, especially among Blacks.
Supplementary Material
Acknowledgements:
The authors wish to thank the staff and participants of the Jackson Heart Study.
Funding: The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The funding agencies had no role in the formulation, editing, or decision to submit this manuscript. Justin Echouffo Tcheugui was supported by NIH/NHLBI grant K23 HL153774. Robert J. Mentz was supported by the National Institutes of Health (U01HL125511-01A1 and R01AG045551-01A1)
Footnotes
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Competing interests: Robert J. Mentz receives research support from Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Regent, Medtronic, Merck, Novartis and Sanofi; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, Roche, Sanofi and Vifor; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim. All the other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation. 2021;143(8):e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9. [DOI] [PubMed] [Google Scholar]
- 3.Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019; 62(9):1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echouffo-Tcheugui JB, Xu H, DeVore AD, Schulte PJPJ, Butler J, Yancy CW, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: Findings from Get With The Guidelines–Heart Failure registry. Am Heart J. 2016;182: 9–20. [DOI] [PubMed] [Google Scholar]
- 5.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazin-Filho A, Kottgen A, Bertoni AG, Russell SD, Selvin E, Rosamond WD, et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2008;51(12):2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, et al. The association of hemoglobin A1c with incident heart failure among people without diabetes: The atherosclerosis risk in communities study. Diabetes. 2010;59(8):2020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circulation Research. 2018;122(4):624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman WH, Cohen RM. Racial and Ethnic Differences in the Relationship between HbA1c and Blood Glucose: Implications for the Diagnosis of Diabetes. J Clin Endocrinol Metab. 2012;97(4):1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor HA. The Jackson Heart Study: an overview. Ethn Dis. 2005;15(4 Suppl 6):S6–1–3. [PubMed] [Google Scholar]
- 11.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–S33. [DOI] [PubMed] [Google Scholar]
- 12.Keku E, Rosamond W, Taylor HA, Garrison R, Wyatt SB, Richard M, et al. Cardiovascular disease event classification in the Jackson Heart Study: Methods and procedures. Ethn Dis. 2005;15(4 Suppl 6):S6–62-70. [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–44. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: The example of smoking and cognitive decline. Epidemiology. 2012;23(1):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pazin-Filho A, Kottgen A, Bertoni AG, Russell SD, Selvin E, Rosamond WD, et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2008. 51(12):2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalogeropoulos A, Georgiopoulou V, Harris TB, Kritchevsky SB, Bauer DC, Smith AL, et al. Glycemic Status and Incident Heart Failure in Elderly Without History of Diabetes Mellitus: The Health, Aging, and Body Composition Study. J Card Fail. 2009;15(7):593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erqou S, Lee C-TC, Suffoletto M, Echouffo-Tcheugui JB, de Boer R a, van Melle JP, et al. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. Eur J Heart Fail. 2013;15(2):185–93. [DOI] [PubMed] [Google Scholar]
- 20.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018;6(8):701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393(10166):31–39. [DOI] [PubMed] [Google Scholar]
- 22.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2020;6(2): 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385(16):1451–1461. [DOI] [PubMed] [Google Scholar]
- 24.Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. 2021;144(16):1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America. Circulation. 2019;140(7):e294–e324. [DOI] [PubMed] [Google Scholar]
- 26.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J;2020;41(2):255–323. [DOI] [PubMed] [Google Scholar]
- 27.Packer M. Differential Pathophysiological Mechanisms in Heart Failure With a Reduced or Preserved Ejection Fraction in Diabetes. JACC Heart Fail. 2021;9(8):535–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.