Summary
Common executive functioning (cEF) is a domain-general factor that captures shared variance in performance across diverse executive function tasks. To investigate the neural mechanisms of individual differences in cEF (e.g., goal maintenance, biasing), we conducted the largest fMRI study of multiple executive tasks to date (N=546). Group average activation during response inhibition (antisaccade task), working memory updating (keep track task), and mental set shifting (number–letter switch task) overlapped in classic cognitive control regions. However, there were no areas across tasks that were consistently correlated with individual differences in cEF ability. Although similar brain areas are recruited when completing different executive function tasks, activation levels of those areas are not consistently associated with better performance. This pattern is inconsistent with a simple model in which higher cEF is associated with greater or less activation of a set of control regions across different task contexts; however, it is potentially consistent with a model in which individual differences in cEF primarily depend on activation of domain-specific targets of executive function. Brain features that explain commonalities in executive function performance across tasks remain to be discovered.
Introduction
Executive functions (EFs) are a family of cognitive processes that regulate goal-related behavior. Individual differences in EF abilities are “important to just about every aspect of life” (Diamond, 2013, p. 137). The variance shared across diverse EF tasks, a Common Executive Functioning (cEF) factor (Friedman & Miyake, 2017), appears to be a particularly important dimension of individual differences, showing stronger relationships to outcomes compared to individual EF tasks. In particular, cEF is associated with important life outcomes including academic achievement (Cantin et al., 2016), self-regulation (Gustavson et al., 2015), subjective well-being (Toh et al., 2020), psychopathology (Friedman et al., 2020; Harden et al., 2020; McTeague, Goodkind, & Etkin, 2016; Snyder et al., 2015), and substance use (Gustavson et al., 2017; Jones et al., 2020). Despite this importance, we still know very little about the neural basis of individual differences in cEF. Although the areas that are typically more active during EF-demanding compared to baseline conditions across tasks are well documented (e.g., Niendam et al., 2012), it is not known whether activation differences in these areas or other areas across tasks are associated with better cEF ability. To date, only a handful of studies with modest N (for individual differences questions) have examined individual differences, and those tend to focus on individual tasks or EF abilities, such as a response inhibition or task set shifting (Jamadar et al., 2010; Wager, Jonides, Smith, & Nichols, 2005; Wager et al., 2005). Here, we investigate the neural correlates of a cEF factor score in the largest multi-task fMRI study (N = 546) of EFs to date. We evaluate whether individual differences in a highly stable cEF factor that captures shared variance in across three separable EF components (response inhibition, working memory updating, and mental set shifting) are associated with similar patterns of brain activation or functional connectivity across tasks tapping each of these EF components.
Most neuroimaging studies have focused on the frontoparietal and cingulo-opercular areas that tend to activate across individuals during EF tasks (Collette et al., 2005; Duncan, 2010; Fedorenko, Duncan, & Kanwisher, 2013; Nee et al., 2013; Dosenbach et al., 2006; Niendam et al., 2012), rather than areas that distinguish between individuals as a function of their level of EF performance. Yet the brain regions that activate consistently across individuals for any given task may not necessarily be sensitive to individual differences (Yarkoni & Braver, 2010).
Of those studies that do investigate the neural basis of individual differences in EFs, there are two notable issues. First, they have focused on single EF tasks. One obstacle to understanding individual differences in EFs is that EFs are by definition domain general processes that control lower-level processes in diverse contexts. Any one EF task offers only a glimpse of this unobservable construct, in which the EF of interest cannot be separated from the particular context in which it is observed (e.g., a categorization task that also requires stimulus processing, response mappings, etc.). Thus, to get a full picture of an EF, one has to measure performance in multiple contexts or tasks and look at what is common across these contexts (Miyake et al., 2000). This multi-task approach has become popular in the behavioral literature, but is rarely adopted within the neuroimaging literature. Most studies focus on a single EF task (e.g., Ravizza & Carter, 2008, Jamadar et al., 2010; Burgess et al., 2011; Wager, Jonides, Smith, & Nichols, 2005, Purkayastha, Wager, & Nichols, 2008).
Second, most fMRI studies of EF individual differences to date are modest in sample size (e.g., N = 18, Jamadar et al., 2010; N = 43, Wager et al., 2005). Small sample sizes are particularly limiting for fMRI studies of individual differences, which require larger sample sizes to detect reliable and reproducible associations), compared to studies that focus on group mean effects (Yarkoni, 2009). For example, nearly 200 participants are required for 80% power to detect a small effect, r = 0.20, as is commonly observed in the imaging literature, at an alpha = 0.05 (not accounting for multiple comparisons). Consortium-level and biobank-scale projects (such as the UK Biobank; Bycroft et al., 2018) are attempting to overcome the limits of small sample sizes. However, by design, they usually fall victim to the first concern by not measuring EFs with high resolution in an effort to maximize the sample size. To date, no study has simultaneously addressed both of these concerns by deeply phenotyping EFs in a sample that is appropriately sized for individual differences analyses.
Here, we present the first large study (N = 546) to investigate associations of task-related fMRI activations/connectivity in multiple tasks with individual differences in a highly reliable cEF factor (Friedman & Miyake, 2017). We measured cEF with a battery of six tasks tapping response inhibition, working memory updating, and mental set shifting abilities. Three of these tasks (one per EF component) were administered in an fMRI context, and three were administered outside the scanner. This design allowed us to evaluate neural correlates of individual differences in cEF across multiple tasks that tap separable EF constructs.
Specifically, we use activation and connectivity analyses to evaluate hypotheses related to the two main mechanisms proposed to drive cEF individuals differences: actively maintaining goals and using those goals to bias ongoing processing (Friedman & Miyake, 2017). From a neural perspective, goal maintenance refers to sustained activation or attractor dynamics (Braver & Cohen, 2000) in frontal areas that allow information to be held on-line so it is accessible in the focus of attention. The neural implementation of goals involves linking desired actions/states with the multimodal information relevant to those states, which is particularly important in difficult EF tasks when the desired link between goals and sensory information rapidly changes based on tasks demands, or is poorly established by default (Miller & Cohen, 2001). Goal maintenance is hypothesized to be supported by lateral prefrontal cortex, whereas processing of lower-level information relevant to goals is distributed across relevant portions of association cortex and thus should spatially vary based on the specifics of the task at hand.
Hence, our first set of analyses leverages multi-task conjunctions to ask Question 1: Is variability in cEF associated with an overlapping or task-specific spatial pattern of activation across three EF tasks? We are primarily interested in the spatial pattern of individual differences results within frontoparietal regions that are commonly implicated in EF tasks. We propose four possible outcomes: 1a) If individual differences in cEF reflect high-level goal maintenance, we should observe that cEF differences are related to activation of dorsolateral prefrontal cortex during all three tasks. 1b) If individual differences reflect variation in top-down attentional control, in a domain-general manner, to lower-level goal-related information (such as increasing activation to goal-relevant stimuli dimensions; Jessen et al., 1999), we might observe common activation in regions that are more proximal to the target/s of cognitive control, such as the lateral parietal cortex. 1c) If instead cEF differences reflect variation in processing of task-specific lower-level associative information, we would expect to see that activation differences related to cEF are spatially inconsistent across tasks and include lower-level sensory areas. 1d) Finally, individual differences in EF may be may be related to other brain regions not typically activated during goal-directed behavior. For example, some work suggests the default network may have functions complementary to those of the frontoparietal network such as formation of conceptual maps (Constantinescu, O’Reilly, & Behrens, 2016) or integration of prior knowledge to inform new situations (Schlichting & Preston, 2015). That said, the internal mentation functions commonly associated with the default network (Andrews-Hanna, 2011) could also be seen as distracting in the context of demanding externally-directed tasks, so it is not yet clear whether more or less default activation will be associated with individual differences in cEF.
Goal maintenance and other mechanisms that could manifest in fMRI activations may not be the only mechanisms relevant to individual differences in EF. One can maintain the goal and yet fail to implement it when appropriate, as illustrated by “goal neglect” (Duncan et al., 1996). This observation is consistent with Friedman and Miyake’s (2017) suggestion that goal maintenance and the use of those goals to bias ongoing processing may be separable mechanisms, with the latter related to connectivity between brain regions. Specifically, in computational models of EF, individual differences in biasing are implemented by manipulating connectivity strength between frontal maintenance areas and posterior areas that process lower-level information necessary for the task at hand (i.e., targets of control; Herd et al., 2014). An important area involved in such biasing is middle frontal gyrus (MFG), which can adaptively connect to other cortical areas based on task demands (Cole et al., 2013; Depue et al., 2015).
Thus, our second set of analyses used task-based functional connectivity analysis to evaluate Question 2: Is variability in cEF associated with connectivity of lateral prefrontal cortex across the three neuroimaging tasks? If lateral prefrontal cortex is responsible for biasing the activity of other brain areas, task-based functional connectivity analyses should reveal that cEF is associated with change in connectivity of those areas involved in biasing (lateral prefrontal cortex) when tasks become more demanding. We propose three possible outcomes for the task-based functional connectivity analyses: 2a) Lateral prefrontal cortex biases processing in a posterior area common to all EF contexts. 2b) Lateral prefrontal cortex biases processing in task-specific posterior areas. 2c) Lateral prefrontal cortex is not involved in biasing as measured by task-based functional connectivity.
Methods
Resource Availability
Data and Code Availability.
All unthresholded statistical maps will be made available in Neurovault (neurovault.org) upon publication. Further information and requests for data should be directed to the lead contact, Andrew Reineberg (andrew.reineberg@colorado.edu).
Participant Details
Analyses used data from a total of 546 individuals who had data for at least one task (237 male/309 female; Mage = 28.67 years, SDage = 0.63 years, range = 28 - 32 years): n = 443 for the antisaccade task, n = 488 for the keep track task, and n = 480 for the number–letter task; 358 participants had usable data for all three scanner tasks. These individuals were a subset of the initial sample scanned (587 individuals), after data were removed due to incidental anatomical findings or excessive movement during the scanning session based on the criteria of greater than 3 mm translation (motion in x, y, or z plane) or 3° rotation (roll, pitch, or yaw motion). Participants were part of the Longitudinal Twin Study (LTS), a long-term longitudinal study of twins in Colorado recruited from the Colorado Twin Registry based on birth records (see Corley et al., 2019; Rhea et al., 2006, 2013, for additional information). Of the 546 individuals, there were 119 pairs of monozygotic (MZ) twins, 109 pairs of dizygotic (DZ) twins, 41 MZ twin singletons, and 49 DZ twin singletons. Singletons are members of twin pairs whose cotwins either did not participate or were excluded from analysis. Based on self-report, the entire LTS sample is 92.6% White, 5.0% more than one race, <1% American Indian/Alaskan Native, <1% Pacific Islander, and 1.2% unknown/not reported. Hispanic individuals composed 9.1% of the sample. Participants were paid $150 for participation in the study or $25 per half an hour for those who did not finish the entire 3-hour session.
Ethics Statement.
All study procedures were approved by the Institutional Review Board of the University of Colorado Boulder.
Procedure
The study was run in a single 3-hour session. Following informed consent, participants were familiarized with the imaging procedures including practice versions of the tasks to ensure comprehension later in the scanner. They first completed a 1.5-h scanning session. The following scanning sequence order was used for all participants: scout localizer scan, 6-minute resting-state scan (not analyzed in the current study), structural scan, antisaccade task, keep track task, number–letter task, and a diffusion tensor imaging sequence (not analyzed in the current study). After the scans, participants returned to a behavioral testing room to complete three additional EF tasks – Stroop, category-switch, and letter memory, in that order. If both twins of a pair participated on the same day, the twins completed the protocol consecutively (twin order randomized) with the same ordering of behavioral testing and imaging acquisition.
Participants were scanned in a Siemens Tim Trio 3T (n = 259) or Prisma 3T (n = 287) scanner (the Trio scanner was upgraded approximately halfway through the study). Scanner type was included as a nuisance regressor in all analyses. Neuroanatomical data were acquired with T1-weighted magnetization prepared using rapid gradient echo magnetization sequence (acquisition parameters: repetition time (TR) = 2400 ms, echo time (TE) = 2.07, matrix size = 320 × 320 × 224, voxel size = 0.80 × 0.80 × 0.80mm, flip angle (FA) =8.00°, slice thickness = 0.80mm). Functional data were acquired with T2*-weighted echo-planar functional scans. Acquisition parameters were: number of volumes = 966 for each run of the antisaccade task (2 runs total), 784 for each run of the keep track task (3 runs total), 1588 for each run of the number–letter task (2 runs total); TR = 460 ms; TE = 27.2 ms; multi-band acceleration factor = 8; matrix size = 82 × 82 × 56; voxel size = 3.02 × 3.02 × 3.00 mm; FA = 44.0°; slice thickness = 3.00 mm; field of view (FOV) = 248 mm.
Behavioral and Imaging Tasks
The battery of six tasks is an abbreviated version of the nine-task battery used in the LTS study in prior waves of data collection (see Friedman et al., 2016). It contained two tasks from each of three EF components: response inhibition, working memory updating, and mental set shifting. One task from each component was administered during fMRI (antisaccade, keep track, and number–letter) and one task from each category was administered outside the scanner (Stroop, letter memory, and category-switch). These tasks were chosen to align the current study with the rich longitudinal historical data available for LTS participants and to allow for future longitudinal analyses. We have found these tasks effective at eliciting genetic and environmental individual differences in prior waves of data collection (Friedman et al., 2016) and useful in prior individual differences analyses exploring EF relationships with psychopathology (du Pont, Rhee, Corley, Hewitt, & Friedman, 2019; Friedman et al., 2020), substance use (Gustavson et al., 2017) , and stressful life events (Morrison et al., 2020), among many other associations. To maintain continuity with our prior work with this sample and ensure that we did not change the constructs of interest, we maintained key aspects of the tasks (such as the short trial times used in the antisaccade), even when those would not be considered typical for a scanner task (as most scanner tasks are not focused on eliciting individual differences in performance).
The design of the three non-scanner tasks was identical to that used in the age 23 battery administered to this sample (Friedman et al., 2016). All tasks included additional practice trials and “warm-up” trials at the beginning of each block that were not analyzed.
The Stroop task (adapted from Stroop, 1935) captures the ability to stop a prepotent word reading response and instead name the color in which the words were printed. Participants voiced the color (red, blue, or green) of text presented on a black screen as quickly as possible. Reaction times (RTs) were measured with a ms-accurate voice key. There were three trial types: a block of 42 neutral trials in which 3-5 asterisks were presented in one of three colors (red, blue, and green); a block of 42 congruent trials in which color words were presented in matched font color (e.g., the word “RED” displayed in red font); and two blocks of 42 trials each of incongruent trials in which color words were presented in non-matched font color (e.g., the word “RED” displayed in blue ink). Stimuli disappeared as soon as the voice key detected the response. Trials were separated by a 250 ms white fixation cross. The dependent measure was the mean RT difference between correct incongruent and neutral trials.
The letter memory task (adapted from Morris & Jones, 1990) captures the ability to maintain and update items in working memory. In each of 12 trials, participants viewed a series of 9, 11, or 13 consonants, with each letter appearing for 3 s. As each letter appeared, they had to say aloud the last four letters they viewed, including the current letter. The dependent measure was the proportion of 132 sets in which they reported the set of letters in the correct order.
The category-switch task (adapted from Mayr & Kliegl, 2000) captures the ability to shift between mental sets. In each trial, participants categorized a word according to animacy (i.e., living vs. non-living) or size (i.e., smaller or larger than a soccer ball), depending on a cue (heart or crossed arrows, respectively). The cue preceded the word by 350 ms and remained above the word until the participant responded with one of two buttons on a ms-accurate button box. The stimuli disappeared from the screen when the participant responded. There was a 350 ms delay between responses and the next trial. A 200-ms buzz sounded for errors. The task began with two single-task blocks of 32 trials each, in which participants categorized words only by animacy then only by size. Then participants completed two mixed blocks of 64 trials each, in which half the trials required switching the categorization criterion. The dependent measure was the local switch cost — the difference between average response times on correct switch and no-switch trials within mixed blocks. RTs for trials following errors were also excluded from analysis, as the switch vs. repeat classification would be incorrect if participants were using the incorrect task set on those trials.
Three tasks were adapted for fMRI from the versions used by Friedman et al. (2016) with this sample. The antisaccade task (Roberts, Hager, & Heron, 1994) requires inhibiting reflexive eye movements to a cue stimulus, instead saccading to the opposite side of the screen in time to see a briefly appearing target stimulus. Participants completed 20 s blocks of prosaccade, anti-saccade, and fixation trials (12 blocks of each across two runs; 5 trials per block for the prosaccade and antisaccade blocks). Each block was preceded by a jittered instruction (TOWARD, AWAY, or FIXATION for 2, 4, or 6 s) indicating the direction to which they should direct their attention relative to the cue. After a jittered fixation lasting 1–3 s, a small visual cue flashed on one side of the computer screen. The cue lasted for 234 ms, however, the duration of this cue was changed to 284 ms after the first 276 participants as we noticed low average performance in an interim analysis for another project. After the cue, a target (a digit from 0 to 9) appeared for 150 ms before being masked. The mask lasted 1650 ms, during which time the participant was instructed to vocalize the target. The cue and target appeared on the same side of the screen during prosaccade trials and opposite sides during anti-saccade trials. Hence, in order to identify the number on the antisaccade trials, participants had to avoid the tendency to saccade to the cue and instead immediately look in the opposite direction. The behavioral dependent measure was the proportion of correctly identified targets on the 60 antisaccade trials. The main fMRI contrast of interest was antisaccade trials versus prosaccade trials. The antisaccade task was broken into 2 runs.
The keep-track task (Yntema, 1963) captures the ability to maintain and update information in working memory. Each trial was preceded by a 1,500 ms instruction (REMEMBER, READ, or FIXATION) indicating the trial type. On each remember trial, 500 ms after the instruction disappeared, a fixation cross appeared in the center of the screen, and below it appeared 3 or 4 target categories (animals, colors, countries, distances, metals, or relatives). The categories remained on the screen throughout the trial. After a duration of 2, 4, or 6 s, a series of 16 words (2s per word) appeared where the fixation was; each word belonged to one of the six categories. Participants had been shown the full list of words during the practice trials, so were familiar with them and the categories to which they belonged. After presentation of the words, a prompt (“???”) appeared for 10 s and participants were instructed to orally recall the last exemplar of each target category. Because each list of 16 words contained 1-3 exemplars of each category, they had to update which words to remember and ignore words from irrelevant categories. In addition to these “Remember” trials, the scanner version of the task included baseline conditions of “Read” trials, in which participants just silently read the words without trying to remember them (followed by a 4 s “---” prompt during which they remained silent), and 20 s rest (fixation) trials. The behavioral dependent measure was the proportion of the 45 words correctly recalled out of all trials where they were asked to remember words. The main fMRI contrast of interest was viewing the words in remember trials versus read trials. The keep track task was broken into 3 runs, each with 3 recall trials (two with 4 words to recall and one with 3), 3 read trials (also two with 4 categories and one with 3 categories present with the words), and 3 fixation trials.
The number-letter task (Rogers & Monsell, 1995) captures the ability to shift between mental sets. In each trial, participants viewed a box sectioned into four quadrants. The borders of one quadrant were darkened as a cue for 350 ms before a number–letter or letter–number pair (e.g., 3F, G7) appeared inside. Participants were instructed to categorize the number as odd/even if the cued quadrant was one of the upper 2 quadrants, or the letter as consonant/vowel if the cued quadrant was one of the lower 2 quadrants, using two buttons on a ms-accurate button box. The stimuli disappeared from the screen when categorized. There was 350 ms delay between response and the next trial. The trials were arranged in blocks, and rest blocks (20 s) were intermixed with the task blocks. Each block was preceded by a jittered instruction (TOP, BOTTOM, MIXED, or FIXATION for 2, 4, or 6 s) that indicated where the stimuli would appear for that block. In mixed blocks, half the trials were repeat trials in which the task stayed the same as the previous trial; the other trials required a switch in categorization task. Each block consisted of 13 trials. The first trial in each block was not counted because it was neither switch nor repeat. The behavioral dependent measure was the difference between average RTs on correct switch trials (i.e., trials in which a switch of mental set was made) versus correct trials in which no switch was made. As in the category-switch task, RTs for trials following errors were also excluded from analysis, as the switch vs. repeat classification would be incorrect if participants were using the incorrect task set on those trials. To equate all tasks based on the difficulty of their respective baseline conditions, the main contrasts of interest in the number-letter task analysis is switch vs. repeat trials in single-task blocks because repeat trials in the mixed context are more cognitively demanding than the baseline condition in the other imaging tasks. We also report results from the switch versus repeat trials in mixed blocks contrast (i.e., reflecting local switch cost) in the supplemental results for comparison to prior work that utilized this contrast. The task was broken into 2 runs, each containing eight mixed blocks, four single-task blocks (two number and two letter blocks), and four 20 s fixation blocks.
Statistical Analyses
Behavioral data were processed with the same pipeline we used in a previous manuscript (Reineberg et al., 2018). Reaction times were trimmed within-subject to obtain the best measures of central tendency within conditions (Wilcox & Keselman, 2003). Extreme high and low scores at the between-subjects level (greater than 3 SDs from the group mean) were Windsorized by replacing them with the cutoff value of 3 SDs above or below the mean to improve normality and reduce the impact of extreme scores while maintaining these scores in the distribution. Behavioral data from the antisaccade task were z-scored within each version (234 and 284 ms cue versions) prior to between-subject trimming to remove mean differences due to cue duration.
After the trimming procedures, behavioral data from all six tasks were input to a confirmatory factor analysis in Mplus. The model for these six tasks was similar to the one used in prior waves of this longitudinal study with nine tasks (Friedman et al., 2016): There were three orthogonal factors: a cEF factor on which all 6 tasks loaded, an orthogonal Updating-specific factor on which the keep track and letter memory tasks loaded, and an orthogonal Shifting-specific factor on which the number–letter and category-switch tasks loaded. To identify the orthogonal 2-indicator specific factors, the loadings for each specific factor were constrained to be equal after first scaling the tasks variances to be similar that the standardized loadings would be equal. The resulting model fit was reasonable according to recommended thresholds for confirmatory fit index (CFI>.95) and standardized root mean residual (SRMR<.08), although the chi-square statistic was significant and the root-mean squared error of approximation exceeded the recommended value (RMSEA<.06): χ2(7) = 33.74, p < .001, CFI = .954, RMSEA = .081, SRMR = .040. As a similar model fit well and was used in the two prior waves of this longitudinal study, we proceeded with this model as specified and extracted cEF, Updating-specific, and Shifting-specific factor scores using the “SAVE=FSCORES” option in Mplus.
Image processing and data analysis were implemented using FSL version 5.0.9 (FMRIB, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl/). A standard pre-processing was applied: motion correction, brain extraction, high pass filter (0.01 Hz), 8mm FWHM spatial smoothing, and registration and spatial normalization to the Montreal Neurological Institute (MNI) 152-T1 2-mm template. Additionally, we applied an ICA-based single-subject denoising procedure (implemented in FSL’s AROMA tool) to each participant’s functional scan to remove artifact signal associated with breathing, heartbeat, movement, and other noise sources.
Data were analyzed using FSL’s general linear model tool. Lower-level model regressors were task-specific, with each task having regressors of interest (e.g., antisaccade trials, prosaccade trials) and confound regressors (e.g., inter-trial intervals, error trials) as well as 6 linear head movement parameters (X, Y, Z, roll, pitch, yaw) and their squared values. Each task had several 20-second fixation blocks, which were left as the un-modeled baseline per standard FSL procedure. The main contrast of interest for each of the three EF tasks was between demanding EF events (antisaccade trials for antisaccade, remember trials for keep-track, and switch trials for number–letter) and less demanding events (prosaccade trials, read trials, and repeat trials within the single trial blocks) or fixation blocks controlling for nuisance events (e.g., pre-stimulus fixation cross events).
For each lower-level contrast, an intermediate model was used to combine multiple runs, and one-sample t-tests were performed at the highest level to obtain the group average activation. The main focus of the current study was an additional higher-level model with a covariate for cEF to obtain an estimate of which brain region’s activity during the demanding versus less demanding condition covaried with cEF factor scores. These covariate (individual differences) models ask about activation differences for those who are different in their cEF ability and should not be confused with group average models. Task-specific covariates were used in secondary analyses (i.e., antisaccade performance covariate for antisaccade task analysis, etc.). In addition to a gender covariate, all models included a scanner covariate to account for the fact that approximately 50% of the sample’s data was obtained using a Siemens Trio scanner before upgrading to a Siemens Prisma scanner. In post-hoc analyses suggested during review, we checked for significant motion-BOLD relationships in all areas with significant cEF-BOLD relationships. A single region from the antisaccade task cEF covariate results had a significant motion-activation relationship, however the effect of cEF persisted after statistically controlling for individual differences in motion.
FSL’s PALM permutation testing tool was used to account for non-independence associated with twin pairs. This tool allowed targeted permutation between and within twin pairs. We used FDR-corrected output from PALM unless otherwise noted. For conjunction analysis of the group average and covariate results, we binarized FDR-corrected stat maps for each task at a pfdr < 0.05 threshold. Binary maps were overlapped and any location in the brain with a sum of 3 was plotted as a three-way conjunction.
In addition to the standard task activation analyses outlined above, we performed functional connectivity analyses via the psychophysiological interaction (PPI) framework (Friston et al., 1997). We were interested in whether individual differences in cEF were associated with the change in MFG connectivity from the less demanding to the more demanding EF condition of each task. In particular, we were interested in whether cEF was related to MFG connectivity with the regions implicated by the individual differences models from the task activation analyses. To perform the PPI analyses, we first extracted the time course for a classic cognitive control region (MFG; mask taken from the Harvard-Oxford atlas) for each participant and run. A contrast-coded regressor was created for each run of each task for the demanding condition (1) versus the less demanding condition (−1). Additionally, a dummy-coded regressor for the combined demanding and less demanding (both coded as 1) conditions was created. Using FEAT, we prepared a new lower-level model for each run that included the new contrast and dummy-coded regressors, the MFG time course, a regressor of nuisance components, and the interaction of the MFG time course and the contrast-coded regressor. The interaction effect is the main component of interest in PPI analyses, as it represents the location in the brain where connectivity to MFG changes as a function of task demands. We subjected the lower-level PPI results to the same intermediate models (to combine runs) and higher-level group models (with cEF covariate and nuisance regressors) as described above. To ascertain whether the regions from the task activation analyses emerged in the task-based connectivity cEF covariate maps, we masked the results of the PPI models in each task by the respective individual differences maps from the task activations analyses. For this analysis, we did not utilize a correction for multiple comparisons because we considered the masked areas from the task activation covariate maps a priori areas of interest.
Results
Behavioral Results
Descriptive statistics for all six behavioral tasks and three factor scores are provided in Table 1. The 234- and 284-ms cue versions of the antisaccade task differed in mean accuracy but not reliability. cEF, Shifting-specific, and Updating-specific latent variables are orthogonal. However, their factor scores are moderately correlated because they are imperfect approximations of latent variables due to factor score indeterminacy. Factor score determinacy estimates for the complete data pattern were 0.815, 0.652, and 0.733 for cEF, Updating-specific, and Shifting-specific factors respectively. cEF factor scores positively correlated with Updating-specific (r = 0.360, p < 0.001) and Shifting-specific (r = 0.253, p < 0.001) scores, whereas Updating-specific and Shifting-specific factor scores were negatively correlated (r = −0.317, p < 0.001). Regarding correlations of the behavioral scores from the three scanner tasks, antisaccade performance was positively correlated with keep track performance (r = 0.34, p < 0.001). After reverse scoring the category–switch task so that higher scores would indicate better performance (lower shift costs), category-switch performance was positively correlated with Antisaccade performance (r = 0.34 p < 0.001) and keep track performance (r = 0.21, p < 0.001). Descriptive statistics, reliability, and the pattern of relationship among cEF, Updating-specific and Shifting-specific factor scores closely replicate the results of an identical analysis of EF behavior in the first 250 participants of the current wave of data collection in the LTS sample (Reineberg et al., 2018).
Table 1.
Descriptive statistics for behavioral variables.
| n | mean | sd | min | max | skew | kurtosis | Reliability | 6-year stability† | |
|---|---|---|---|---|---|---|---|---|---|
| Antisaccade (234 ms cue) | 276 | 44.42 | 21.03 | 6.67 | 96.67 | 0.35 | −0.70 | 0.94* | 0.68° |
| Antisaccade (284 ms cue) | 289 | 58.95 | 21.25 | 8.33 | 96.67 | −0.36 | −0.77 | 0.93* | |
| Stroop | 580 | 145.93 | 75.09 | −163.32 | 373.03 | 0.55 | 0.84 | 0.96* | 0.48 |
| Keep track | 579 | 0.77 | 0.13 | 0.36 | 1.00 | −0.69 | 0.14 | 0.74^ | 0.51 |
| Letter memory | 585 | 73.28 | 13.97 | 35.61 | 100.00 | −0.05 | −0.97 | 0.93^ | 0.84 |
| Number-letter | 568 | 182.22 | 120.73 | −60.54 | 565.48 | 0.93 | 0.94 | 0.93* | 0.55 |
| Category switch | 583 | 180.32 | 149.90 | −103.05 | 664.08 | 1.22 | 1.55 | 0.93* | 0.66 |
| Common EF | 587 | 0.00 | 0.81 | −2.42 | 2.02 | −0.13 | −0.45 | 0.80 | |
| Shifting-specific EF | 586 | 0.00 | 0.65 | −2.11 | 1.70 | −0.32 | −0.26 | 0.62 | |
| Updating-specific EF | 584 | 0.00 | 0.73 | −2.52 | 1.73 | −0.81 | 0.65 | 0.68 |
EF = Executive Functioning.
Split-half reliability (odd/even for Stroop task and category-switch task or run1/run2 for antisaccade task and number–letter task), adjusted with the Spearman-Brown prophecy formula.
Cronbach’s alpha across 3 runs for keep-track and 4 sets of trials for letter memory.
Pearson’s r between behavioral performance at current time and prior wave of data collection.
6-year stability for antisaccade task calculated across 234 and 284 ms cue versions.
Because the ability to measure robust intercorrelations among cognitive tasks depends, in part, on the reliability of the individual tasks (Draheim et al., 2021, Rouder et al., Hedge, Powell, & Sumner, 2018), we assessed the internal reliability (Table 1) and 6-year test-retest reliability of our tasks. Internal reliability was high for all tasks (.74 to .96). Consistent with our goal of measuring the same individual differences constructs we had previously measured in this sample, performance in the three scanner tasks correlated well with the behavioral versions of the same tasks administered 6 years earlier (Friedman et al., 2016), rs = 0.51 to 0.68, ps < .001. These correlations were comparable to the 6-year test-retest correlations of the 3 behavioral tasks we administered outside of the scanner, rs = 0.48 to 0.84, ps < .001. cEF, Updating-specific, and Shifting-specific factor scores for this assessment also showed strong correlations with those from the prior wave (based on 9 tests): rs = 0.796, 0.683, and 0.624, respectively, all ps < .001.
fMRI Group Average Activation
Group average maps for the main contrast of interest (i.e., demanding versus less demanding trials) for all three EF tasks and the three-way conjunction of these maps can be found in the left-hand column of Figure 1. The group average maps for all three tasks (Figures 1a–c) were very similar. The three-way conjunction of group average maps (Figure 1d) revealed clusters of peak activation common to all tasks and included classic frontoparietal and cingulo-opercular activations as well as default network deactivations.
Figure 1. fMRI results.
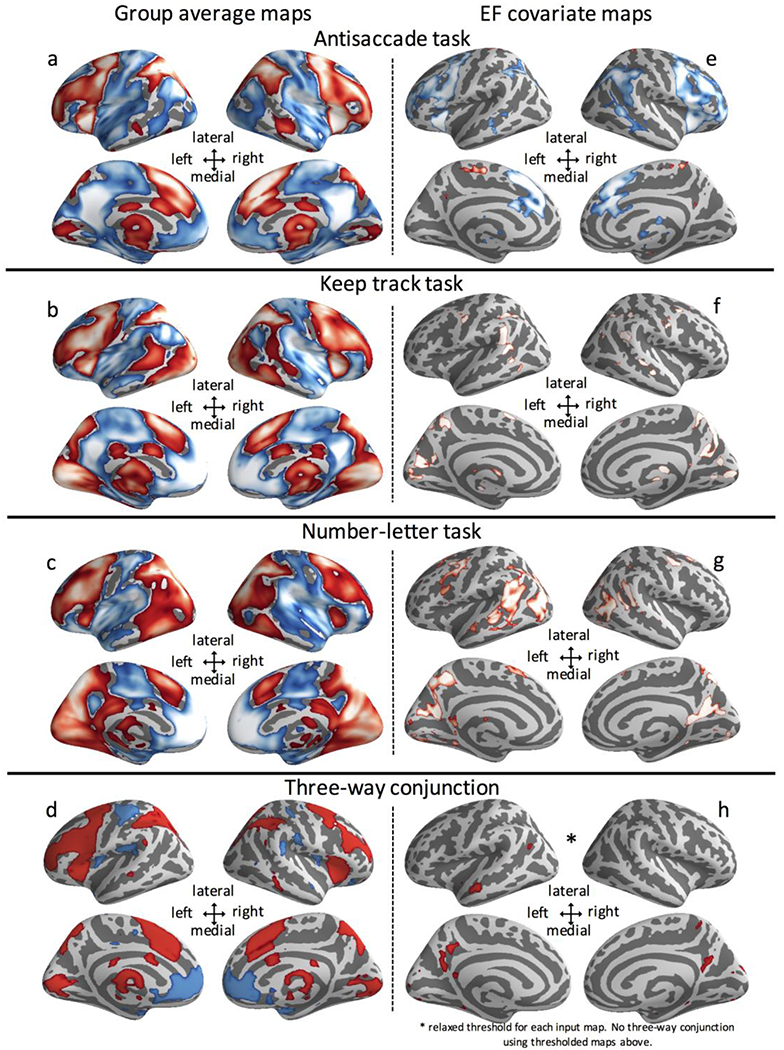
a-c. Group average activation for antisaccade, keep track, and number–letter tasks. d. Conjunction of group average map from all three tasks. e-g. Common Executive Functioning (cEF) covariate analyses. Individual differences maps for antisaccade, keep track, and number–letter tasks. h. Conjunction of individual differences maps for antisaccade, keep track, and number–letter tasks. Input maps are thresholded at pfdr < ∛0.05 = 0.368 rather than pfdr < 0.05 as in panels e, f, and g. red = positive activation or covariate association, blue = negative activation or covariate association
Because we tried to equate all contrasts in the main analysis based on the difficulty of the baseline conditions, we focused on the global switch cost contrast for the number–letter task based on the easier baseline condition of repeat trials in a single task context. However, we provide an analysis of the local switch cost contrast (switch versus repeat trials in the mixed context in the number–letter task) in a supplemental analysis for comparison to prior work (Figure S1). Activation for the local switch cost contrast was very similar to activation in the main contrast of switch (during mixed context) versus repeat (in single-task blocks), with additional sensory-somatomotor and insular activity in the latter.
A comparison of unthresholded or minimally thresholded maps could be a useful alternative to a conjunction analysis, similar to how meta-analysis of unthresholded maps has utility over meta-analysis of foci when investigating consistency in effects across many studies in fMRI meta-analysis (Salimi-Khorshidi, Smith, Keltner, Wager, & Nichols et al., 2009). The statistical maps (Table 2) showed moderate to strong correlations (rs = 0.39 - 0.77) when utilizing the subthreshold information (i.e., below the threshold for correcting for multiple comparisons). Although the group average activation maps were very similar overall, there are notable differences across the three group average maps, which were most pronounced in posterior cingulate cortex and the visual processing stream. The antisaccade task had more posterior cingulate cortex (PCC) deactivation and less visual activation than the other two tasks. The number–letter task had more visual activation and less PCC deactivation than the other tasks.
Table 2.
Spatial correlation (Pearson’s r) of all unthresholded z-statistic maps.
| a. | b. | c. | d. | e. | f. | g. | h. | i. | j. | k. | l. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLM | Group Average | a. | antisaccade | 1 | 0.65 | 0.39 | −0.68 | 0.34 | 0.15 | −0.05 | 0.08 | 0.13 | −0.17 | 0.24 | 0.09 |
| b. | keep track | 0.65 | 1 | 0.77 | −0.41 | 0.65 | 0.44 | 0.12 | 0.4 | 0.05 | −0.08 | 0.45 | −0.18 | ||
| c. | number-letter | 0.39 | 0.77 | 1 | −0.27 | 0.63 | 0.6 | 0.1 | 0.29 | 0.16 | −0.14 | 0.37 | −0.22 | ||
| cEF Covariate | d. | antisaccade | −0.68 | −0.41 | −0.27 | 1 | −0.21 | −0.27 | −0.11 | −0.15 | −0.2 | 0.15 | −0.06 | 0.01 | |
| e. | keep track | 0.34 | 0.65 | 0.63 | −0.21 | 1 | 0.6 | 0.25 | 0.32 | 0.04 | −0.2 | 0.34 | −0.21 | ||
| f. | number-letter | 0.15 | 0.44 | 0.6 | −0.27 | 0.6 | 1 | 0.33 | 0.37 | 0.08 | −0.14 | 0.18 | −0.29 | ||
| PPI | Group Average | g. | antisaccade | −0.05 | 0.12 | 0.1 | −0.11 | 0.25 | 0.33 | 1 | 0.73 | 0.23 | 0.11 | 0.14 | −0.35 |
| h. | keep track | 0.08 | 0.4 | 0.29 | −0.15 | 0.32 | 0.37 | 0.73 | 1 | 0.15 | 0.26 | 0.21 | −0.42 | ||
| i. | number-letter | 0.13 | 0.05 | 0.16 | −0.2 | 0.04 | 0.08 | 0.23 | 0.15 | 1 | 0.08 | 0 | 0.04 | ||
| cEF Covariate | j. | antisaccade | −0.17 | −0.08 | −0.14 | 0.15 | −0.2 | −0.14 | 0.11 | 0.26 | 0.08 | 1 | −0.02 | −0.08 | |
| k. | keep track | 0.24 | 0.45 | 0.37 | −0.06 | 0.34 | 0.18 | 0.14 | 0.21 | 0 | −0.02 | 1 | −0.15 | ||
| l. | number-letter | 0.09 | −0.18 | −0.22 | 0.01 | −0.21 | −0.29 | −0.35 | −0.42 | 0.04 | −0.08 | −0.15 | 1 |
Red = positive correlation, Blue = negative correlation. GLM = general linear model results; PPI = psychophysiological interaction analyses; cEF = Common Executive Functioning scores.
To explore whether there might be heterogeneity across tasks for different functional networks, we also looked at similarity of the statistic map after assigning voxels to one of 7 bins based on its functional network assignment derived from a commonly used parcellation (Yeo et al., 2011). This analysis allows us to assess whether correlation of group average statistic maps (Table 2) are driven by particular networks or are representative of a whole brain effect. For example, the overall correlation among the three tasks could be misleading if, for example, frontoparietal, dorsal attention, and ventral attention network activation were extremely correlated across all tasks but the default network was not. The distribution of voxel activations by functional network and correlation of the group average statistic maps by functional network is described in detail in Figure S2. This analysis predominantly revealed agreement in the correlation of per-network activations across the three task maps; however, the whole brain correlation (Table 2) of antisaccade and keep track maps may be suppressed because of heterogeneity across functional networks (e.g., there is a correlation of activity in the two tasks for all networks except the default network).
Is variability in cEF associated with an overlapping versus task-specific spatial patterns of activation across three tasks?
To investigate question 1, we used covariate models to quantify the relationship between cEF and change in activity from the less demanding to higher demanding task conditions. The cEF covariate results are shown in Figure 1e–g and described in Tables 3-5 for the antisaccade, keep track, and number–letter tasks, respectively. These maps were much less spatially consistent than the group average maps (Figure 1, left hand column).
Table 3. Composition of significant Individual differences clusters for keep track, number-letter, and antisaccade tasks.
Each significant individual difference cluster (columns) was composed of voxels from one to several functional subareas (rows). Region labels taken from Harvard-Oxford cortical atlas (top series of rows), Harvard-Oxford subcortical atlas (middle series of row), and Diedrichsen cerebellar atlas (bottom series of rows; Diedrichsen et al., 2009). To be included as a column, a threshold of 100 contiguous voxels was used. To be included as a row, a threshold of 50 voxels was used. Grey highlighted rows are regions with significant individual differences activations across all three tasks. Note, however, that antisaccade activations are negative whereas activations in the keep track and number–letter task are positive.
| Keep Track task | Number-letter task | Antisaccade task | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Frontal Pole | 4489 | ||||||||||||||||||||||||||||
| Insular Cortex | 615 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Superior Frontal Gyrus | 180 | 122 | 623 | 2952 | |||||||||||||||||||||||||
| Middle Frontal Gyrus | 217 | 220 | 84 | 204 | 740 | 3821 | |||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Inferior Frontal Gyrus, pars tri. | 509 | ||||||||||||||||||||||||||||
| Inferior Frontal Gyrus, pars oper. | 162 | 1302 | |||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Precentral Gyrus | 71 | 322 | 271 | 512 | 1406 | ||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Temporal Pole | 58 | ||||||||||||||||||||||||||||
| Superior Temporal Gyrus, ant. | 63 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Superior Temporal Gyrus, post. | 93 | 513 | 54 | 101 | |||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Middle Temporal Gyrus, post. | 306 | 174 | 379 | ||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Middle Temporal Gyrus, temp. | 124 | 634 | 668 | ||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Inferior Temporal Gyrus, ant. | 84 | ||||||||||||||||||||||||||||
| Inferior Temporal Gyrus, temp. | 58 | ||||||||||||||||||||||||||||
| Postcentral Gyrus | 55 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Superior Parietal Lobule | 73 | 110 | 381 | 265 | |||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Supramarginal Gyrus, ant. | 86 | 87 | 229 | ||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Supramarginal Gyrus, post. | 68 | 87 | 514 | 760 | 362 | 1024 | |||||||||||||||||||||||
| Angular Gyrus | 57 | 803 | 194 | 1211 | |||||||||||||||||||||||||
| Lateral Occipital Cortex, sup. | 73 | 165 | 716 | 2844 | 54 | 660 | 1290 | ||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Lateral Occipital Cortex, inf. | 1232 | ||||||||||||||||||||||||||||
| Intracalcarine Cortex | 685 | 231 | |||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Juxtapositional Lobule Cortex | 125 | 146 | 308 | ||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Paracingulate Gyrus | 1578 | ||||||||||||||||||||||||||||
| Cingulate Gyrus, ant. | 804 | ||||||||||||||||||||||||||||
| Cingulate Gyrus, post. | 233 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Precuneous Cortex | 2081 | 2833 | 54 | ||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Cuneal Cortex | 140 | 157 | |||||||||||||||||||||||||||
| Frontal Orbital Cortex | 995 | ||||||||||||||||||||||||||||
| Lingual Gyrus | 278 | 52 | 502 | ||||||||||||||||||||||||||
| Temporal Occipital Fusiform Cortex | 106 | 84 | |||||||||||||||||||||||||||
| Occipital Fusiform Gyrus | 187 | 710 | |||||||||||||||||||||||||||
| Frontal Operculum Cortex | 516 | ||||||||||||||||||||||||||||
| Supracalcarine Cortex | 98 | 137 | |||||||||||||||||||||||||||
| Occipital Pole | 107 | 372 | |||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Left Lateral Ventrical | 65 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Left Thalamus | 330 | 72 | 145 | ||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Left Caudate | 119 | 343 | |||||||||||||||||||||||||||
| Left Putamen | 217 | ||||||||||||||||||||||||||||
| Brain-Stem | 187 | 109 | 106 | 60 | |||||||||||||||||||||||||
| Right Lateral Ventricle | 56 | ||||||||||||||||||||||||||||
| Right Thalamus | 226 | 226 | |||||||||||||||||||||||||||
| Right Caudate | 57 | 344 | |||||||||||||||||||||||||||
| Right Putamen | 166 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Left I-IV | 835 | 63 | 105 | 343 | 635 | 843 | |||||||||||||||||||||||
| Right I-IV | 708 | 302 | 250 | 422 | |||||||||||||||||||||||||
| Left V | 622 | 160 | 222 | 383 | |||||||||||||||||||||||||
| Right V | 597 | 150 | 69 | 121 | |||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Left VI | 357 | 131 | |||||||||||||||||||||||||||
| Vermis VI | 196 | 100 | |||||||||||||||||||||||||||
| Right VI | 179 | 96 | |||||||||||||||||||||||||||
| Left Crus I | 172 | 74 | |||||||||||||||||||||||||||
| Vermis Crus I | 161 | 62 | |||||||||||||||||||||||||||
| Right Crus I | 155 | ||||||||||||||||||||||||||||
| Left Crus II | 114 | ||||||||||||||||||||||||||||
| Vermis Crus II | 102 | ||||||||||||||||||||||||||||
| Right Crus II | 68 | ||||||||||||||||||||||||||||
| Left VIIb | 60 | ||||||||||||||||||||||||||||
| Vermis VIIb | 58 | ||||||||||||||||||||||||||||
| Right VIIb | 57 | ||||||||||||||||||||||||||||
| Left VIIIa | 54 | ||||||||||||||||||||||||||||
| Vermis VIIIa | 52 | ||||||||||||||||||||||||||||
The three individual differences maps had no areas of three-way overlap based on our a priori analysis plan for conjunctions (overlap of the three FDR-corrected maps presented in Figure 2). Upon loosening the threshold for significance so the joint probability was less than 0.05 in the three-way conjunction (i.e., each individual map thresholded at pfdr < ∛0.05 = 0.368), there were several small areas of overlap. Across tasks, higher levels of cEF were associated with increased activation of bilateral precuneus, left posterior cingulate cortex, left lateral parietal cortex, and left anterior middle temporal gyrus (Figure 1h). These areas of weak overlap tend to be in the default mode network, supporting the fourth proposed mechanism for cEF individual differences (association with regions not typically during goal-directed behavior such as the default network). However, the directionality was such that higher cEF was associated with less deactivation of the default mode network during more demanding conditions.
Figure 2. Task-based functional connectivity results.
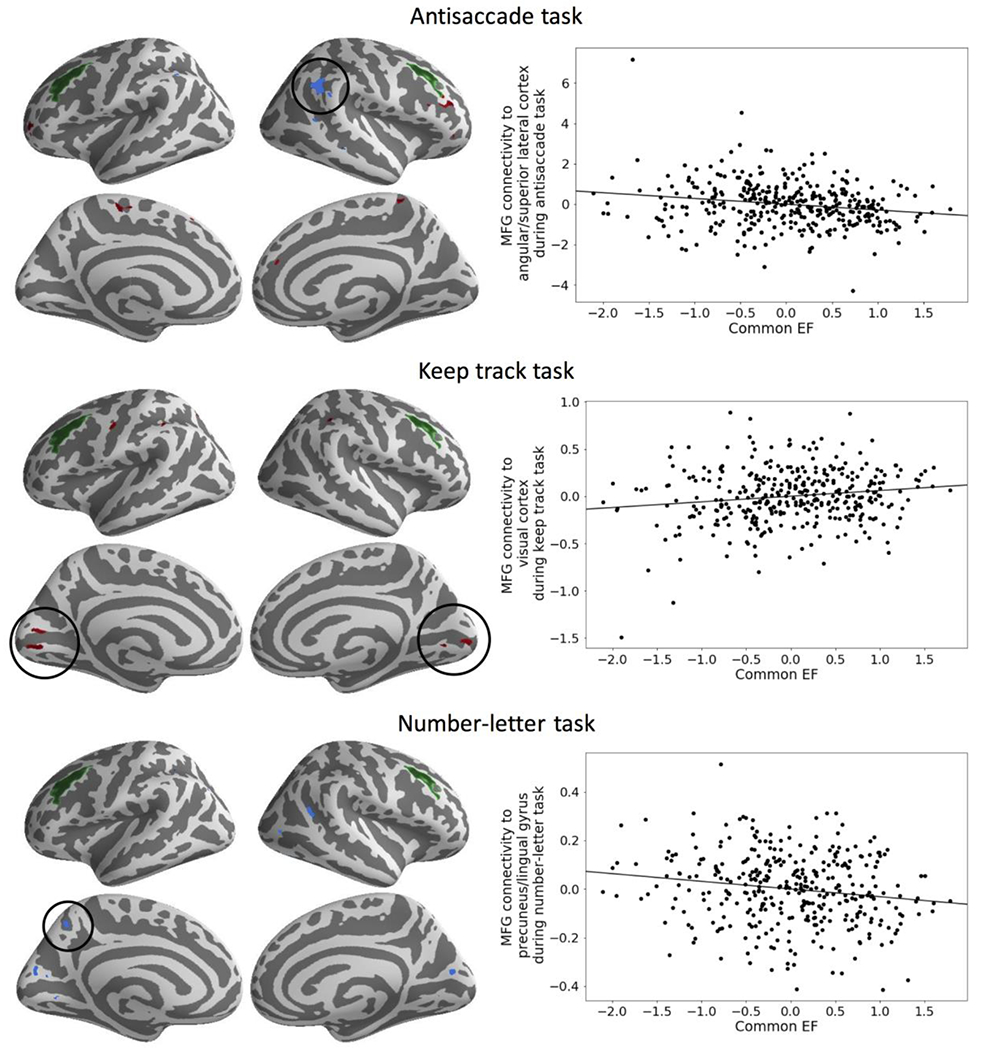
Individual differences in Common Executive Functioning (cEF) were associated with change in middle frontal gyrus (MFG; green) connectivity from the less demanding to the more demanding condition in each task. Connectivity result are masked by task activation cEF covariate maps and uncorrected for multiple comparisons. Connectivity beta values for the largest cluster for each task were extracted as examples. Scatterplots those connectivity betas versus cEF scores (residualized on age, sex, and scanner) are shown on the right for example clusters from maps on the left (black circles).
Although there were no regions in the three-way overlap of the FDR-corrected cEF individual differences maps, there were overlapping regions when ignoring directionality of effects. Upon absolute value transformation of the cEF covariate maps, clusters of overlap (Figure S5) included bilateral MFG, medial superior frontal gyrus, left angular/superior parietal cortex, and the cerebellum. These areas were all at the anatomical borders between major functional networks.
We were mindful that conjunctions are sensitive to thresholding decisions. To determine how similar the cEF covariate maps were, including all subthreshold information in the map and not just the information that exceeded our threshold for statistical significance, we correlated all pairwise combinations of the three maps as we did previously for the group average maps. The whole brain spatial correlation of unthresholded cEF covariate statistic maps for the three tasks is described overall in Table 2. Correlations between tasks were moderate-to-strong for all pairs of tasks (|r| = 0.21 - 0.60). However, these correlations varied in direction, with a positive correlation between the cEF-related pattern for keep track and category-switch, but negative correlations of those two patterns with the cEF-related pattern for antisaccade. The opposite direction of these correlations is inconsistent with a simple model in which higher cEF is associated with greater or less activation of a set of regions across different task contexts.
Additionally, to explore whether cEF-related activations show consistency across all three tasks for all networks, we calculated correlations between pairs of tasks after grouping voxels by functional network assignment as described by Yeo et al. (2011) (Figure S3). There appeared to be some heterogeneity: For example, although the whole brain correlation of antisaccade with keep track cEF covariate maps is only moderate overall, the cEF-related sensory-somatomotor network activation is more correlated than the overall correlation suggests.
A final question of interest was whether the brain areas that are active on average in the EF tasks are the same areas that are associated with individual differences in the cEF covariate. When comparing the cEF-covariate analysis maps to the group-average activation within each task, many areas involved in individual differences in cEF reside in areas active in the group on average. In fact, Table 2 and Figure S4a reveal the group average and cEF covariate maps are strongly correlated (|r| = 0.60 - 0.68) within task when examining the unthresholded statistic maps. As in the cross-task correlations, group average activation in antisaccade was negatively correlated with cEF covariate-related activation. That is, voxels positively activated in the group average analysis tended to have negative activations associated with cEF individual differences.
Due to task impurity, performance on any given EF task is driven by cEF as well as additional constructs. Therefore, a covariate statistic map based on performance on each task performed in the scanner should be a combination of cEF-covariate regions and additional task-specific regions. We derived covariate maps for in-scanner performance to compare them to the cEF maps as an exploratory look at regions relevant for task-specific individual differences (see Figure S6a–c). If the task-specific performance covariate maps are very similar to the cEF covariate maps, then cEF-related neural mechanisms explain most of the behavioral variation for that task. This exploratory analysis suggests the localization of cEF and task-specific performance effects is largely the same in the brain (for details see Supplementary Information and Figure S6c).
Is variability in cEF associated with connectivity of lateral prefrontal cortex across three tasks?
To investigate question 2, we evaluated PPI models. The PPI results presented below were not significant when conducting a whole-brain analysis and correcting for multiple comparisons across the brain, suggesting they should be interpreted as preliminary evidence of a connectivity-based basis of individual differences in cEF.
We measured changes in task-based connectivity of our a priori area of interest, MFG, from the less demanding to the more demanding condition in each task. We masked the PPI analysis results by the maps from Figure 1e–g to constrain our analysis to just those areas we previously demonstrated to be related to individual differences in cEF. For all three tasks, some of the same areas from the GLM cEF covariate results described above emerged in this targeted task-based functional connectivity analysis. These results provide some support for our hypothesis that lateral prefrontal cortex may have a role in biasing processing in task-specific areas (via the observed connectivity) even though lateral prefrontal cortex itself did not emerge as a predictor of cEF individual differences in the activation-based GLM analyses. Connectivity results and example scatterplots are provided in Figure 2.
For antisaccade, higher cEF was associated with increased MFG connectivity from the harder to easier condition to inferior MFG/inferior frontal gyrus, sensory cortex, frontal pole, medial frontal cortex, and decreased connectivity to lateral parietal cortex. For keep track, higher cEF was associated with increased MFG connectivity to visual cortex, motor cortex, and lateral parietal cortex. Finally, for number–letter, higher cEF was associated with decreased MFG connectivity to visual cortex, lateral parietal cortex, and precuneus. The spatial pattern of PPI and task activation covariate results was similar across the whole brain when considering all subthreshold (i.e., threshold for correction for multiple comparisons) information (Figure S4b).
Discussion
This study is the largest multi-task fMRI study (N=587) of EFs to date, and was uniquely designed to determine if there is a set of brain regions that are commonly engaged across EF tasks as a function of individual differences in cEF. We found robust group average activations within each of three task contexts as well as conjunction of the three tasks’ group average results. We also found robust activations associated with individual differences in cEF in each individual task. However, there was no significant three-way conjunction of these individual differences results. Of several possibilities (hypotheses 1a-1d in Introduction), our results for task activation analyses suggest the neural basis of cEF individual differences is spatially inconsistent (hypothesis 1c) and not restricted to frontal areas. Utilizing a lower threshold for statistical significance revealed default network activation may also be relevant to individual differences in cEF (hypothesis 1d). Although individual differences in activation of frontal maintenance areas were not consistently associated with cEF individual differences across all task contexts, preliminary results suggested task-based connectivity of lateral prefrontal cortex may be associated with individual differences in cEF across all task contexts (hypothesis 2b). However, because the connectivity results did not survive whole-brain correction for multiple testing, future work is required to more fully understand the role of prefrontal biasing in individual differences in cEF. Individual differences in cEF are primarily reflected in activation of task-specific areas.
Although our key finding, the lack of conjunction of the individual differences covariate maps, is a null result, it is striking in the context of this study. We scanned a very large sample, assessed cEF performance rigorously with a factor scores of 6 reliable tasks, found a robust conjunction of activation at the group-level, and found robust patterns of cEF-related activation differences within each task. Nevertheless, these robust cEF-related differences did not overlap across tasks, and in some cases reflected opposing patterns of associations. Similar null results have been briefly mentioned in prior reports: For example, Engelhardt et al. (2019) reported “no significant clusters of accuracy-correlated activity shared by the three tasks” (p. 486) in their fMRI study of three EF tasks in 117 children, although they did observe significant accuracy associations within each task. As unsatisfying as such a null result is, it is nevertheless informative. It challenges the somewhat prevalent, and very reasonable, assumption (evidenced by popular ROI approaches) that individual differences in cEF reflect variation in recruitment of the common cognitive control networks that are so strongly activated at the group level. Brain features that explain commonalities in EF performance across tasks remain to be discovered, and doing so will likely require alternative approaches to testing for spatial commonality, or alternative interpretations of task-specific associations.
Support for task-specific neural correlates
Our group-average results for each of the three tasks contained classic frontoparietal and cingulo-opercular activation as well as default network deactivations. This pattern is consistent with prior group-average results from single tasks (Duncan, 2010; Duncan & Owen, 2000; Kimberg, Aguirre, & D’Esposito, 2000; Luna et al., 2001; Wager, Jonides, & Reading, 2004; Wager & Smith, 2003), multi-task conjunctions (Collette et al., 2005; Engelhardt, Harden, Tucker-Drob, & Church, 2019), and meta-analyses of EF tasks (McKenna, Rushe, & Woodcock, 2017; Nee et al., 2013; Niendam et al., 2012; Owen, McMillan, Laird, & Bullmore, 2005). As such, it appears that our tasks successfully engaged brain regions typically associated with EFs.
Notably, however, individual differences in the extent to which these areas were activated was only weakly related to cEF ability. When considering only cEF-related activation that met or exceeded our threshold for statistical significance (accounting for multiple comparisons), there was no three-way overlap, suggesting the most powerful correlates of EF are task-specific activations. Differences in activation in association cortex were the dominant feature in cEF covariate maps for the keep track and number–letter task. In addition, both the antisaccade task and keep track task had frontal activations associated with individual differences. Although this pattern of results was unexpected, it is not incompatible with existing theories of the neural basis of EF, such as the multiple demand network (Duncan & Owen, 2000), the cascade of control model (Banich et al., 2000), and the hierarchical control model (Christoff & Gabrieli, 2000). These theories focus on neural machinery necessary to complete particular cognitive tasks rather than how individual differences in activations lead to differences in performance.
Our results suggest researchers interested in activation related to individual differences should be aware that task selection can critically affect the spatial pattern of results. Here we showed different EF tasks can even have individual differences activations in opposing directions. In the antisaccade task, high EF individuals were those who showed less activation in lateral frontal, superior medial, and anterior cingulate regions for the more demanding as compared to the less demanding condition. In the keep track task, higher cEF was associated with increased activation of frontal cortex for the more demanding compared to less demanding condition. A similar pattern was seen in the unthresholded maps: Although the covariate maps showed low to moderate correlations across tasks, the antisaccade cEF map was negatively correlated with the cEF maps for the other two tasks. When considering conjunctions regardless of directionality (i.e., in an absolute value analysis), we did see overlap in the fully corrected cEF covariate maps in several locations at the borders of the dorsal/ventral attention, frontoparietal, and default networks. In this case, activation in similar areas was associated with individual differences in cEF, but the directionality of the effect changed with different task demands. No prior work led us to predict this differential pattern. If this pattern is not specific to this speeded antisaccade task but instead reflects a general property of inhibitory tasks, future work will be needed to determine why inhibitory task contexts may lead to cEF-associated deactivations while other EF task contexts lead to cEF-associated activations.
Our results are also consistent with computational models of EF tasks. Computational models encode source-target relationships between frontal cortex maintenance functions (e.g., attractor dynamics; Hazy, Frank, & O’Reilly, 2007) and association cortex that encodes sensory and motor information relevant to the current goal (Banich, 2009; Miller & Cohen, 2001; Munakata et al., 2011; Posner & Driver, 1992). Relationships between sources and targets of control are often modified via changes in biasing, or connections between areas representing frontal cortex and posterior areas (Herd et al., 2014). As in such models, individual differences in biasing are reflected in task-based connectivity between frontal cortex and posterior areas as opposed to observing individual differences in task-set maintenance which would be detected by activation levels in GLM analyses. Consistent with this hypothesis that control is implemented via connectivity from frontal regions to posterior regions involved in goal-relevant processes, our task-based functional connectivity results indicated that individual differences in cEF were also related to the modulation of the spatially diverse task-specific regions by the same MFG region during each task, although these results were not significant at the whole-brain corrected level. Prior individual differences work supports the idea that MFG is involved in flexible biasing (Cole, Ito, & Braver, 2015; Depue, Orr, Smolker, Naaz, & Banich, 2015; Panikratova et al., 2020), perhaps due to its unique ability to fluidly connect to nearly all cortical areas (Cole et al., 2013; Ito et al., 2017).
Based on our results, future work might focus on connectivity of the lateral prefrontal cortex rather than exploring the complex spatial pattern of individual differences activations that may depend on task demands. However, connectivity of lateral prefrontal cortex may be only part of the story of individual differences. To make the best predictions about cognition using task-based activations, future studies may consider combining metrics from a priori areas such as lateral prefrontal cortex with information from across the whole brain. If a whole brain search is not desired, the current study suggests lateral parietal cortex, sensory cortex, and the default network are additionally involved in individual differences, and as such should be considered as regions of interest for future work.
The role of the default network in EF is particularly interesting given that the default network is typically associated with a variety of internally-directed functions (Andrews-Hanna, 2011) and that decrease in activation of this network is observed when participants are engaged in an externally-directed task (Spreng, Mar, & Kim, 2009). However, task-positive and task-negative networks can work synergistically under certain circumstances. For example, activation in the default mode network along with activation in the frontoparietal network can support goal-oriented behavior, as in autobiographical planning (Spreng et al., 2010). When we relaxed the threshold for conjunction significance for the input maps to a liberal threshold (so the probability of the product of significance across the three tasks was < 0.05) we also found evidence of common individual differences activation across all three tasks. In this relaxed conjunction analysis, we saw overlap across tasks for associations with cEF in bilateral precuneus, left posterior cingulate cortex, left lateral parietal cortex, and left anterior middle temporal gyrus. These regions span all three major subdivisions of the default network, the hub regions, the medial temporal lobe subsystem, and the dorsal medial prefrontal subsystem, which are involved in valuation of motivationally salient information, memory-based simulation functions, and introspection, respectively, among other functions (Andrews-Hanna, 2011). Our results suggest these regions are important for individual differences in cognitive ability. Specifically, higher cEF was associated with higher default network activity across the three EF tasks. One possibility is that high EF individuals could adaptively utilize the internal mentation functions of the default network highlighted above, such as memory-based simulation, to assist them in performance. However, there are other explanations. For example, high EF individuals may be able to tolerate more noise from internal mentation while performing EF tasks and hence exhibit more activation of these regions during task contexts than those with low EF. Future work is needed to test these hypotheses.
Strengths and Limitations
Our primary conjunction analyses assumed that activation in different brain areas reflects different functions, which may be invalid. Specifically, we searched for conjunction of individual differences as an indicator of seemingly centralized functions such as goal maintenance, assuming such maintenance happens in one location. Maintenance of antisaccade related goals and keep track related goals could be localized in different places; however, there is little external evidence to support this claim.
The current study reports an initial analysis of the neural basis of individual differences in cEF. The GLM and connectivity results presented here do not represent all the possible neural implementations of individual differences in cEF or all the possible ways to test the goal maintenance and biasing hypotheses posed in the current manuscript. For example, the tasks we used were designed primarily to isolate sources of executive function. Future work could, for example, utilize multi-voxel pattern analysis (Norman, Polyn, Detre, & Haxby, 2006) to isolate and track variability in the strength of targets of control to an extent we were not able to in the current study. Additionally, individual differences in cEF may manifest via alternative measures we did not test here. For example, in our prior resting-state work, network-to-network connectivity (Reineberg et al., 2018) as well as graph-theoretic properties (Reineberg & Banich, 2016) of several brain regions were associated with individual differences in executive functions. These and other measures could be considered in future task-based fMRI studies of individual differences.
A strength of our approach was the specialized design of the tasks to elicit individual differences. For example, we maintained the same temporal structure in the scanner that has been used in the past in a purely behavioral context. The time pressure adds difficulty that may not normally be present in scanning studies that have long inter-trial intervals. However, the speeded nature of the tasks means that our tasks may not be as comparable to prior imaging work in which individual differences were not the main focus. Arguing against this possibility, our group average activation results suggest the areas necessary to perform our tasks are highly comparable to what is found in the existing literature as summarized above, both in terms of the task-specific results, conjunctions, and meta-analyses. Thus, these versions of the tasks seem to be generally comparable to versions designed without regard to individual differences.
Finally, our experiment was not designed to differentiate between differences in strategy selection versus differences in neural mechanisms. Experimenter instructions only communicated the goals for each EF task but did not instruct individuals on how to implement the goals cognitively. High cEF individuals may adopt different strategies in the three EF tasks compared to their lower-performing counterparts. Differences in strategies across individuals are not out of the question and are a topic of interest in the aging literature, where older adults lower performance may be driven by adoption of less demanding (but less effective) cognitive strategies (Cabeza et al., 2018). Such differences are an issue for future exploration.
Conclusion
The current study is the first task-based activation study to examine the neural basis of individual differences in a cEF factor derived from performance on multiple EF tasks and in multiple task contexts. Although we found robust cEF-related activations in each task and subthreshold patterns of cEF-related activation were correlated across task, the peak cEF-related activations after correcting for multiple comparisons for each of three different EF tasks did not have any three-way conjunction. The role of the default network as observed in the analysis of individual differences in which we relaxed the significance threshold is a potentially novel mechanism to be explored in the future. cEF was not associated with the degree of lateral frontal activation in every task, although there was weak evidence for the hypothesis that cEF was associated with the functional connectivity of lateral prefrontal cortex to diverse task-specific areas, albeit at a much less rigorous standard of statistical significance when compared to the GLM analyses. Thus, there may be multiple contributors to good performance – quality of representations, default network activation to aid in internal mentation that may support task goals or task sub-processes, and strength of biasing towards domain-specific targets of control.
These insights would not be possible without this rich dataset from multiple fMRI tasks completed by a large sample of participants. Had we observed how individual differences in cEF or task performance predicted activity in one task, as is typically done in prior studies, we would have arrived at very different conclusions about the areas associated with individual differences, depending on which task we had chosen (e.g., potentially mis-localizing a locus of goal maintenance). Thus, although the results of this conjunction analysis are complex, this complexity advances the literature and may help resolve inconsistency in past studies with individual tasks.
Supplementary Material
Acknowledgements
This research was supported by NIH grants MH063207 (AER, NPF, MTB, TDW), AG046938 (NPF), DA046064 (NPF and TDW), DA051018 (MTB and NPF), and DA042742 (NPF).
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- Andrews-Hanna JR (2011). The brain’s default network and its adaptive role in internal mentation. The Neuroscientist, 1–20. 10.1177/1073858411403316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18(2), 89–94. 10.1111/j.1467-8721.2009.01615.x [DOI] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb AG, Wszalek T, … Magin R (2000). fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience, 12(6), 988–1000. [DOI] [PubMed] [Google Scholar]
- Braver TS, & Cohen JD (2000). On the control of control: The role of dopamine in regulating prefrontal function and working memory. In Monsel S & Driver J (Eds.), Control of cognitive processes: attention and performance XVIII (pp. 713–737). Cambridge, MA: MIT Press. 10.7551/mitpress/1481.003.0044 [DOI] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, … Rajah MN (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 19(11), 701–710. 10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, & Gabrieli JDE (2000). The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology, 28(2), 168–186. 10.3758/BF03331976 [DOI] [Google Scholar]
- Cole MW, Ito T, & Braver TS (2015). The Behavioral Relevance of Task Information in Human Prefrontal Cortex. Cerebral Cortex, 1–9. 10.1093/cercor/bhv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, & Braver TS (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16(9), 1348–1355. 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, & Salmon E (2005). Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping, 25(4), 409–423. 10.1002/hbm.20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu AO, O’Reilly JX, & Behrens TEJ (2016). Organizing conceptual knowledge in humans with a gridlike code. Science, 352(6292), 1464–1468. 10.1126/science.aaf0941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley RP, Reynolds CA, Wadsworth SJ, Rhea SA, & Hewitt JK (2019). The Colorado Twin Registry: 2019 Update. Twin Research and Human Genetics, 22(6), 707–715. 10.1017/thg.2019.50 [DOI] [PubMed] [Google Scholar]
- Depue BE, Orr JM, Smolker HR, Naaz F, & Banich MT (2015). The Organization of Right Prefrontal Networks Reveals Common Mechanisms of Inhibitory Regulation Across Cognitive, Emotional, and Motor Processes. Cerebral Cortex, 1–13. 10.1093/cercor/bhu324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, & Ramnani N (2009). A probabilistic MR atlas of the human cerebellum. NeuroImage, 46(1), 39–46. 10.1016/j.neuroimage.2009.01.045 [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, … Petersen SE (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812. 10.1016/j.neuron.2006.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Pont A, Rhee SH, Corley RP, Hewitt JK, & Friedman NP (2019). Rumination and executive functions: Understanding cognitive vulnerability for psychopathology. Journal of Affective Disorders, 256(April), 550–559. 10.1016/j.jad.2019.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2010). The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14(4), 172–179. 10.1016/j.tics.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, & Freer C (1996). Intelligence and the Frontal Lobe: The Organization of Goal-Directed Behavior. Cognitive Psychology, 30, 257–303. [DOI] [PubMed] [Google Scholar]
- Duncan J, & Owen AM (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23(10), 475–483. 10.1007/s11631-017-0212-0 [DOI] [PubMed] [Google Scholar]
- Engelhardt LE, Harden KP, Tucker-Drob EM, & Church JA (2019). The neural architecture of executive functions is established by middle childhood. NeuroImage, 185(September 2018), 479–489. 10.1016/j.neuroimage.2018.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences of the United States of America, 110(41), 16616–16621. 10.1073/pnas.1315235110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, & Hewitt JK (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology. General, 137(2), 201–225. 10.1037/0096-3445.137.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, & Dolan RJ (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6, 218–229. [DOI] [PubMed] [Google Scholar]
- Gustavson DE, Stallings MC, Corley RP, Miyake A, Hewitt JK, & Friedman NP (2017). Executive functions and substance abus: Relations in late adolescence and early adulthood. Journal of Abnormal Psychology, 126(2), 257–270. 10.1007/s11897-014-0247-z.Pathophysiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, & O’reilly RC (2007). Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 362(1485), 1601–1613. 10.1098/rstb.2007.2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C, Powell G, & Sumner P (2018). The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behavior Research Methods, 50(3), 1166–1186. 10.3758/s13428-017-0935-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd S. a, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, & Friedman NP (2014). A neural network model of individual differences in task switching abilities. Neuropsychologia, 62, 1–15. 10.1016/j.neuropsychologia.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kulkarni KR, Schultz DH, Mill RD, Chen RH, Solomyak LI, & Cole MW (2017). Cognitive task information is transferred between brain regions via resting-state network topology. Nature Communications, 8(1). 10.1038/s41467-017-01000-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar S, Hughes M, Fulham WR, Michie PT, & Karayanidis F (2010). The spatial and temporal dynamics of anticipatory preparation and response inhibition in task-switching. NeuroImage, 51(1), 432–449. 10.1016/j.neuroimage.2010.01.090 [DOI] [PubMed] [Google Scholar]
- Jessen F, Erb M, Klose U, Lotze M, Grodd W, & Heun R (1999). Activation of human language processing brain regions after the presentation of random letter strings demonstrated with event-related functional magnetic resonance imaging. Neuroscience Letters, 270(1), 13–16. 10.1016/S0304-3940(99)00453-X [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, & D’Esposito M (2000). Modulation of task-related neural activity in task-switching: An fMRI study. Cognitive Brain Research, 10(1–2), 189–196. 10.1016/S0926-6410(00)00016-1 [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, … Sweeney JA (2001). Maturation of widely distributed brain function subserves cognitive development. NeuroImage, 13(5), 786–793. 10.1006/nimg.2000.0743 [DOI] [PubMed] [Google Scholar]
- Mayr U, & Kliegl R (2000). Task-set switching and long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26(5), 1124–1140. 10.1037//0278-7393.26.5.1124 [DOI] [PubMed] [Google Scholar]
- McKenna R, Rushe T, & Woodcock KA (2017). Informing the Structure of Executive Function in Children: A Meta-Analysis of Functional Neuroimaging Data. Frontiers in Human Neuroscience, 11(April), 1–17. 10.3389/fnhum.2017.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Morris N, & Jones DM (1990). Memory updating in working memory: The role of the central executive. British Journd of Psychology, 81(1), 111–121. 10.1111/j.2044-8295.1990.tb02349.x [DOI] [Google Scholar]
- Morrison CL, Rhee SH, Smolker HR, Corley RP, Hewitt JK, & Friedman NP (2020). Genetic and Environmental Influences on Stressful Life Events and their Associations with Executive Functions in Young Adulthood : A Longitudinal Twin Analysis. Behavior Genetics, (0123456789). 10.1007/s10519-020-10017-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, & O’Reilly RC (2011). A unified framework for inhibitory control. Trends in Cognitive Sciences, 15(10), 453–459. 10.1016/j.tics.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, & Jonides J (2013). A meta-Analysis of executive components of working memory. Cerebral Cortex, 23(2), 264–282. 10.1093/cercor/bhs007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. 10.3758/s13415-011-0083-5.Meta-analytic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, & Haxby JV (2006). Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences, 10(9), 424–430. 10.1016/j.tics.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, & Bullmore E (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. 10.1002/hbm.20131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikratova YR, Vlasova RM, Akhutina TV, Korneev AA, Sinitsyn VE, & Pechenkova EV (2020). Functional connectivity of the dorsolateral prefrontal cortex contributes to different components of executive functions. International Journal of Psychophysiology, 151(January), 70–79. 10.1016/j.ijpsycho.2020.02.013 [DOI] [PubMed] [Google Scholar]
- Posner MI, & Driver J (1992). The neurobiology of selective attention. Current Opinion in Neurobiology, 2(2), 165–169. 10.1016/0959-4388(92)90006-7 [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Wager TD, & Nichols TE (2008). Inferring individual differences in fMRI: Finding brain regions with significant within subject correlation. Statistica Sinica, 18(4), 1483–1500. [Google Scholar]
- Reineberg AE, & Banich MT (2016). Functional connectivity at rest is sensitive to individual differences in executive function: A network analysis. Human Brain Mapping, 00. 10.1002/hbm.23219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg AE, Gustavson DE, Benca C, Banich MT, & Friedman NP (2018). The relationship between resting state network connectivity and individual differences in executive functions. Frontiers in Psychology, 9(SEP), 1–14. 10.3389/fpsyg.2018.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea S-A, Bricker JB, Wadsworth SJ, & Corley RP (2013). The Colorado Adoption Project. Twin Research and Human Genetics : The Official Journal of the International Society for Twin Studies, 16(1), 358–365. 10.1017/thg.2012.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea S-A, Gross A. a, Haberstick BC, & Corley RP (2006). Colorado twin registry. Twin Research and Human Genetics : The Official Journal of the International Society for Twin Studies, 9(6), 941–949. 10.1375/twin.9.6.941 [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Hager LD, & Heron C (1994). Prefrontal Cognitive Processes: Working Memory and Inhibition in the Antisaccade Task. Journal of Experimental Psychology: General, 123(4), 374–393. [Google Scholar]
- Rogers RD, & Monsell S (1995). Costs of a predictible switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124(2), 207–231. 10.1037/0096-3445.124.2.207 [DOI] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, & Nichols TE (2009). Meta-analysis of neuroimaging data: A comparison of image-based and coordinate-based pooling of studies. NeuroImage, 45(3), 810–823. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Schlichting ML, & Preston AR (2015). Memory integration: Neural mechanisms and implications for behavior. Current Opinion in Behavioral Sciences, 1, 1–8. 10.1016/j.cobeha.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, & Kim ASN (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. 10.1162/jocn.2008.21029 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, & Schacter DL (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage, 53(1), 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology. [Google Scholar]
- Wager TD, Jonides J, & Reading S (2004). Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage, 22(4), 1679–1693. 10.1016/j.neuroimage.2004.03.052 [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE, & Nichols TE (2005). Toward a taxonomy of attention shifting: Individual differences in fMRI during multiple shift types. Cognitive, Affective and Behavioral Neuroscience, 5(2), 127–143. 10.3758/CABN.5.2.127 [DOI] [PubMed] [Google Scholar]
- Wager TD, & Smith EE (2003). Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective & Behavioral Neuroscience, 3(4), 255–274. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15040547 [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester C-YC, Lacey SC, Nee DE, Franklin M, & Jonides J (2005). Common and unique components of response inhibition revealed by fMRI. NeuroImage, 27(2), 323–340. 10.1016/j.neuroimage.2005.01.054 [DOI] [PubMed] [Google Scholar]
- Wilcox RR, & Keselman HJ (2003). Modern robust data analysis methods: measures of central tendency. Psychological Methods, 8(3), 254–274. 10.1037/1082-989X.8.3.254 [DOI] [PubMed] [Google Scholar]
- Yarkoni T (2009). Big Correlations in Little Studies. Perspectives on Psychological Science, 4(3), 294–298. 10.1111/j.1745-6924.2009.01127.x [DOI] [PubMed] [Google Scholar]
- Yarkoni Tal, & Braver TS (2010). Cognitive neuroscience approaches to individual differences in working memory and executive control: Conceptual and methodological issues. Handbook of Individual Differences in Cognition: Attention, Memory, and Executive Control, 87–107. 10.1007/978-1-4419-1210-7_6 [DOI] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema DB (1963). Keeping track of several things at oncce. Human Factors : The Journal of the Human Factors and Ergonomics Society, 5(7), 7–17. 10.1177/001872086300500102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All unthresholded statistical maps will be made available in Neurovault (neurovault.org) upon publication. Further information and requests for data should be directed to the lead contact, Andrew Reineberg (andrew.reineberg@colorado.edu).


