Abstract
Current plant-based systems offer multiple advantages for monoclonal antibody (mAb) development and production beyond the traditional benefits of low cost and high scalability. Novel expression vectors have allowed the production of mAbs at high levels with unprecedented speed to combat current and future pandemics. Host glycoengineering has enabled plants to produce mAbs that have unique mammalian glycoforms with a high degree of homogeneity. These mAb glycovariants exhibit differential binding to various Fc receptors, providing a new way to optimize antibody effector function for improving mAb potency or safety. This review will summarize the status of anti-viral mAb development with plant-based systems. The preclinical and clinical development of leading plant-made mAb candidates will be highlighted. In addition, the remaining challenges and potential applications of this technology will be discussed.
Keywords: plant-made antibodies, monoclonal antibody, mAbs, virus, viral diseases, plantmade biologic
Introduction
Monoclonal antibodies (mAb) are the predominant growing class of biopharmaceutical products in recent years. Since the first approval of Muromonab-CD3 in 1985 [1], more than one hundred mAb-based drugs have been approved by the US food and drug administration (FDA) as of March 2021 [2]. Largely due to their high specificity, mAb-based drugs have revolutionized the pharmaceutical industry and achieved great financial success, creating a global market valued at greater than $100 billion for cancer therapy alone [3].
Recently, the FDA has issued emergency use authorization (EUA) of several mAbs for severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) treatment, highlighting the significance of antibody drugs in fighting infectious diseases [4]. Currently, there have been four mAbs approved by the FDA for the prevention or treatment of virus-caused infectious diseases [2]. In 1998, palivizumab, the first mAb against infectious disease, was licensed for the prevention of respiratory syncytial virus (RSV) infection, the most common cause for severe bronchiolitis in young children [5]. Palivizumab prevents RSV entry into host cells through specific binding to the RSV envelop fusion protein and inhibiting membrane fusion [6]. In 2018, Ibalizumab, a humanized immunoglobulin G4, was approved for clinical management of human immunodeficiency virus (HIV)-1 infection with multidrug resistance [7]. As a CD4-directed post-attachment inhibitor, this mAb binds to CD4 T cells and blocks the conformation changes required for HIV-1 entry [7]. In 2020, two antibody drugs, Inmazeb and Ebanga, were licensed for the treatment of Ebola virus (EBOV) infections. Inmazeb is a combination of three mAbs: atoltivimab, maftivimab, and odesivimab; all three of which can bind to the EBOV glycoprotein simultaneously to prevent virus entry [8]. Ebanga contains one mAb called ansuvimab, which also binds the EBOV glycoprotein and blocks its interaction with the host cell receptor [9]. In addition to blocking virus attachment or membrane fusion to prevent virus entry, increasing evidence shows that antibodies can provide significant therapeutic effects against viral infections through fragment crystallizable region (Fc) mediated effector functions, such as complement-dependent cytotoxicity (CDC), antibody-dependent cell cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP) [10–12]. For example, two of the mAbs in the EBOV drug, Inmazeb, and ansuvimab, can induce ADCC in addition to blocking virus entry [13, 14], indicating the importance of effector functions in the treatment of EBOV infection.
Almost all approved mAb drugs are produced in mammalian cell culture, which requires high-tech facilities, sophisticated bioreactors, expensive downstream processing, cold storage and transportation, and sterile delivery methods [15–18]. As a result, mAb drugs produced by current technology platforms are prohibitively expensive, for example, with an average annual price of $142,844 for cancer treatment [19], making them unaffordable for the majority of ordinary citizens in the world [20]. The high cost of current mAb drugs calls for the development of alternative production systems based on non-mammalian cells.
Plants as production host for mAbs
Antibody production requires the expression of at least two types of polypeptides and their proper assembly into a multimeric structure, as well as complex-type glycan modifications. Despite this complexity, plants have been shown to have the ability to produce mAbs since the first mAb made in tobacco in 1989 [21]. Since then, numerous mAbs and their structural variants including IgGs, secretory IgAs, pentameric IgMs, camelid nanobodies, tetravalent mAbs, bifunctional mAbs, recombinant immune-complex (RIC), single-domain fragments, single-chain variable fragments (scFv), and diabodies have been produced, several of which have entered human clinical trials [17, 22–24].
Low cost, high scalability, and low risk of human pathogen contamination are the hallmarks of plant-based systems for producing mAbs [15, 25, 26]. Unlike mammalian cell culture systems, plant biomass can be generated in greenhouses with simple mineral solutions. This eliminates the need for capital-prohibitive bioreactors and expensive culture media, resulting in significant cost savings associated with upstream processing of mAb production [17, 27], Production of mAbs in mammalian cells carries the inherent risk of contaminating drugs with animal pathogens, especially those that are unknown or uncharacterized. As plants rarely carry pathogens that are infectious to humans, such risk is greatly reduced in plant-produced mAbs. The ease of producing multiple hetero-subunit proteins and the unique characteristics of the cell wall provides plant cells with another advantage for mAb production and potentially a new route of drug delivery. Functional and protease-resistant secretory IgAs and pentameric IgMs which require up to four hetero-subunits have been produced successfully in plants [22, 28]. Since these mAbs are encapsulated inside the plant cell wall, they are protected from acids and enzymes in the stomach, allowing them to enter the gut lumen where they are enzymatically released by gut commensal bacteria [29]. This opens the possibility of combating gastrointestinal tract (GI) viral diseases via oral delivery of edible plant materials that encapsulate mAbs against GI viruses. Since many viruses use mucosal surfaces as sites of entry, plant-cell delivered mAbs can be used as both prophylactics to block viral entry, and therapeutics to treat viral infection. Prophylactic applications of mAbs to preventive viral infections would enroll larger populations with repeated dosing, thereby, demand large amounts of mAbs. In this case, plant-based production platforms become more attractive due to their ease of scale-up production without demanding prohibitive capital investment or the time-consuming development of new production processes [17].
Technological advances in transient expression and vector engineering offer plant-based systems the robustness, speed, and versatility that are unmatchable by mammalian cell cultures for mAb production. The development of “deconstructed” viral vector systems such as magnICON, geminiviral, and pEAQ vectors has allowed for a high and consistent levels of mAb expression in short time [30–34]. For example, mAbs can be produced within 1-2 weeks with magnICON-based transient systems at levels of up to 5 mg mAb per gram of leaf fresh weight (LFW) [35]. Since the deconstructed vectors are delivered into plants via Agrobacterium tumefaciens, a bacterium that naturally transfers a portion of its plasmid into plant cells [36–38], the transient systems retain the flexibility of nuclear gene expression while increasing production speed and yield. As a result, this type of transient expression is the system of choice for obtaining gram levels of mAbs for pre-clinical studies. As mAb-based drug discovery involves an iterative process of successive modifications and functional evaluation, the speedy nature of the plant transient system in producing gram-levels of mAb variants will greatly accelerate this process. In addition, the “bridge” versions of deconstructed viral vectors have also been developed, so that scale-up production of mAbs can be done in stable transgenic plants with the same speed and high yield as in transient expression [39, 40]. Therefore, current plant expression platforms based on deconstructed viral vectors offer advantages in all stages of mAb-drug development, from early candidate screening to the final stage of large-scale manufacturing.
Glycoengineering of plant hosts provides another advantage over the mammalian cell-based system for mAb development. The mAbs are N-linked glycosylated and their sugar moieties have a significant impact on their folding, serum and shelf half-life, and functionality [41]. Thus, one approach to enhance the potency, safety, and stability of mAbs is to alter protein-associated sugars to achieve the desirable properties. The challenge is to develop biological systems that can consistently produce mAbs with specific and homogeneous glycans on demand. In mammalian cells, there is a large diverse population of Golgi-located glycoenzymes, giving rise to more than 2000 different N-glycans generated by a few hundred enzymes in the secretory pathway [42]. For example, Chinese hamster ovary (CHO) cell-derived mAbs exhibit substantial glycan heterogeneity, precluding the ability to generate distinct glycoforms that could be used to enhance their efficacy [43]. Glycoengineering of CHO cells has only achieved modest success, especially in producing defined N-glycoforms with high degrees of homogeneity. In contrast, plant cells have a much smaller repertoire of glycoenzymes, and plant glycoproteins usually bear a single dominant N-glycan structure [41]. The inherent lack of N-glycan heterogeneity of plant-produced glycoproteins has turned out to be an advantage for plant cells as a host for generating mAbs with homogeneous glycans [43]. Glycoengineering of host plants by deleting or suppressing the expression of plant-specific glycan genes or/and inserting mammalian glycosylation genes has achieved remarkable success [44]. Studies have shown that mAbs produced in glycoengineered plants do not carry any plant-specific glycans, eliminating the concern for immunogenicity and the potential risk of adverse effects for plant-produced mAbs [45, 46]. Furthermore, mAbs made in glycoengineered plants usually carry a homogenous (>90%) N-glycan structure compared to the mixture of multiple glycans exhibited by the same mAbs produced in CHO cells [47–50]. A portfolio of plant lines has since been developed, each producing mAbs with a unique mammalian N-glycoform [44] (Fig. 1). The availability of such glycoengineered plant lines provides the opportunity to develop mAbs with defined and uniform carbohydrate moieties to enhance their efficacy and/or safety.
Figure. 1. Major N-glycan structures in wild-type and available glycoengineered N. benthamiana plant lines.
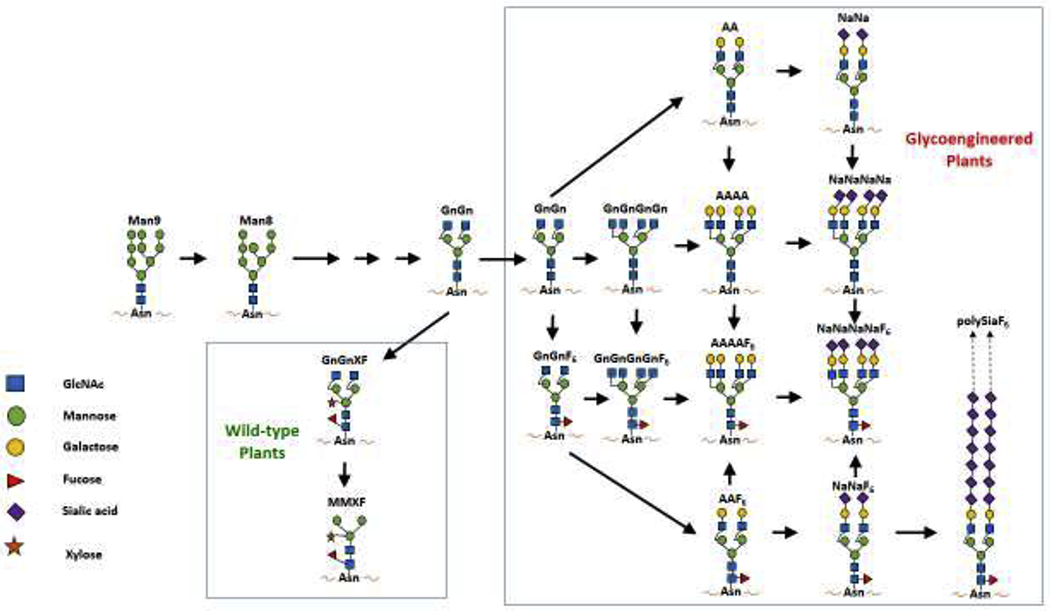
In wild-type plants, glycoproteins usually carry a single dominant N-glycan structure, GnGnXF or MMXF which contain plant-specific glycans of β1,2-xylose (X) and α1,3-fucose (F). By suppressing gene expression for xylosyl- and fucosyltransferases, plant-specific N-glycans can be eliminated from mAbs made in plants. Moreover, glycoengineered plants generated by introducing mammalian glycoenzymes to specific compartment of Golgi complex can overcome CHO cells’ inability to synthesize multi-antennary N-glycans, and can now produce mAbs with defined and homogeneous N-glycans on demand, including α1,6-fucosylated, bisected, tetra-antennary, bi-galactosylated, and bi-antennary sialylated complex N-glycoforms. (Glycan nomenclature according to Consortium of functional glycomics, see http://www.proglycan.com/upload/nomen_2007.pdf)
Leading candidates for plant-derived mAbs
Multiple mAbs and mAb derivatives against viral pathogens have been developed in various plant species. The initial studies aimed to examine mAb expression with different plant-expression platforms and to test their equivalency in physiochemical structure and both in vitro and in vivo functionality with their mammalian cell-produced counterparts. The success of glycoengineering in plants has allowed the paradigm shift of using plants to develop “biobetters” to improve the efficacy and/or safety of mAb-based drugs. These plant-made mAbs are in various stages of development ranging from research and development (R&D), preclinical testing, compassionate use in human patients, to Phase I human clinical trials (Table 1). Representative plant-made mAbs will be discussed in the following sections based on their developmental stages and disease targets.
Table 1:
Representative anti-viral mAbs made in plants at various developmental and clinical stages
| Product | Disease application | Plant host | In vivo efficacy | Development stage | References |
|---|---|---|---|---|---|
| IgG1 (2G12) | HIV prophylactic | Transgenic tobacco, corn endosperm, and N. benthamiana (transient expression) | Protection in mice, safety in humans | Phase I | [63, 64, 73] |
| IgG1 (ZMApp) | Ebola virus therapeutic | N. benthamiana (transient expression) | Protection in mice and rhesus macaques, safety and efficacy in humans | Phase I | [59, 60, 61] |
| IgG1 | Genital herpes (HSV-2)/HIV prevention | Transgenic Soybean N. benthamiana (transient expression) | Prophylactic protection in mice Safety and ex vivo activity in humans | Phase I | [116, 117] |
| IgG1 | West Nile virus prophylactic and therapeutic | N. benthamiana/Lettuce (transient expression) | Protection in mice | Pre-clinical | [47–50, 77] |
| IgG1 | Dengue virus therapeutic | N. benthamiana (transient expression) | Protection in mice | Pre-clinical | [86, 87] |
| IgG1 | Zika virus therapeutic | N. benthamiana (transient expression) | Protection in mice | Pre-clinical | [12] |
| IgG1 | Chikungunya virus therapeutic | N. benthamiana (transient expression) | Protection and reduced ankle inflammation in mice | Pre-clinical | [24, 79, 112] |
| IgG1 | Rabies virus prophylactic | Transgenic tobacco | Prophylactic protection in Hamster | Pre-clinical | [114] |
| IgG1 | Respiratory syncytial virus prophylactic and therapeutic | N. benthamiana (transient expression) | Prophylactic and therapeutic protection in cotton rats | Pre-clinical | [108] |
| RIC | Ebola virus vaccine | N. benthamiana (transient expression) | Protection in mice | Pre-clinical | [57, 58]] |
| IgG1 | SARS-CoV-2 cytokine storm prevention | N. benthamiana (transient expression) | Not reported | Research | [105] |
| IgG1 | SARS-CoV-2 therapeutic | N. benthamiana (transient expression) | Not reported | Research | [97, 99, 100] |
| IgG3 | SARS-CoV-2 therapeutic | N. benthamiana (transient expression) | Not reported | Research | [100] |
| IgA1 dimer | SARS-CoV-2 therapeutic | N. benthamiana (transient expression) | Not reported | Research | [99] |
RIC: recombinant immune complex, Pre-clinical: Efficacy demonstrated in animal models, Research: Efficacy only tested in vitro.
MAbs against Ebola virus
The Ebola outbreaks of 2014 attracted the attention of the public to plant-produced mAbs. Two critically ill American Ebola patients were given an experimental drug called ZMapp and quickly recovered [51]. ZMapp contains three chimeric mAbs produced in N. benthamiana plants. These mAbs can be produced quickly [52–54], and are highly efficacious in protecting mice and macaques against Ebola infection [55, 56]. Interestingly, a recombinant immune-complex vaccine based on these mAbs also protected mice from a lethal Ebola challenge [57, 58]. The potency of plant-derived mAbs is superior to that of their mammalian-produced counterparts, likely due to their homogenous GnGn mammalian glycan profile, which enhanced their binding to the Fc gamma receptor (FcγR) IIIA and possibly ADCC activity [55, 56]. ZMapp was used as an experimental drug in seven human patients during the 2014 Ebola outbreak. A subsequent study demonstrated that 100% of rhesus macaques can be successfully rescued by ZMapp even 5 days post a lethal Ebola challenge [59]. A phase I clinical trial was conducted in 2015 to assess the safety and efficacy of ZMapp. Although the trial did not reach its enrollment goal of 200 people due to the outbreak waning, 72 Ebola-infected subjects were enrolled, and, consequently, the results are not statistically significant. Nevertheless, ZMapp was found to be safe and well-tolerated, and patients who received ZMapp had a 40% lower risk of death [60]. During the 2018-19 Kivu Ebola outbreak in the Democratic Republic of the Congo, another randomized and controlled Phase I clinical trial was conducted for ZMapp along with three investigational agents. The results of this trial indicated that ZMapp lowered the mortality rate from ~ 70% to 49.7%, albeit the other candidates showed more superior efficacy [61]. Beyond the Ebola therapeutic target, the ZMapp clinical trials are also highly significant in several other aspects. It signifies the establishment of upstream and downstream processes for manufacturing plant-made mAbs that are compliant with current Good Manufacturing Practice (cGMP) regulations of the FDA. The clinical trial also helped drug approval agencies such as the FDA to navigate this new technology and pave a clearer regulatory pathway specific for plant-made mAbs. This should greatly streamline the approval of other plant-made mAbs.
MAbs against human immunodeficiency virus (HIV)
Despite the global efforts in curbing the HIV epidemic in the last few decades, thousands of newly infected cases are still reported daily in sub-Saharan Africa with 1.5 million new cases in 2020 [62]. Broadly neutralizing antibodies (bNAb) that can neutralize multiple HIV isolates are promising candidates for treating and preventing HIV infection. However, the implementation of bNAb treatment programs in resource-poor regions, where HIV is the most prevalent, is greatly challenged by the cost and scale-up capability associated with the CHO-based mAb production system. As a result, several bNAbs have been produced in various plant-based systems [63–67]. For example, 2G12 has been produced both in maize and in N. benthamiana [63, 64]. In general, the antigen specificity, affinity, and neutralization potency of plant-made 2G12 and other bNAbs are comparable to those of their mammalian cell-derived counterparts. However, bNAbs produced in glycoengineered plants with specific and homogeneous glycoforms appear to have enhanced FcγRIIIA-binding, neutralization, and/or ADCC activity [63, 67, 68]. In addition to glycoengineering, engineering of other posttranslational modification (PTM) pathways in plants also overcome the challenge of producing functional bNAbs that require specific mammalian-type PTM. For example, the expression of human tyrosyl protein sulfotransferase 1 in N. benthamiana allowed the proper tyrosine sulfation in the CDR H3 region of two bNAbs, preserving their broad neutralization activity against HIV [66, 69]. bNAbs and their fragments have also been fused to antiviral lectins to enhance their potency. For example, cyanovirin-N (CV-N) was fused to the C-terminus of bNAb b12 heavy chain (HC) and expressed in transgenic tobacco. Compared to b12 or cyanovirin alone, the fusion protein exhibited increased anti-HIV potency [70]. Similarly, a bispecific bNAb-lectin fusion protein composed of the antigen-binding fragment (Fab) of bNAb VRC01 and Avaren was expressed in N. benthamiana. The VRC01 Fab-Avaren fusion protein demonstrated more potent neutralizing activity compared to a cocktail of the two parent molecules [71]. Pharmacokinetics and preliminary safety of plant-produced HIV bNAbs have been investigated in nonhuman primate models [65, 72]. For example, VRC01 produced in N. benthamiana was formulated in the form of intravaginal rings and tested as a topical microbicide for preventing sexual HIV infection in a macaque model. The rings were found to sustain VRC01 levels in the range of 100 -1000 μg per gram of vaginal fluid for up to 21 days without causing any adverse safety indications [65]. Excitingly, a first-in-human, double-blind, placebo-controlled, randomized, dose-escalation phase I clinical trial was conducted for tobacco-produced 2G12 as a vaginal microbicide in a single dose up to 28mg in healthy female volunteers [73]. 2G12 was found to be safe as no major adverse events were identified during the trial and no specific anti-2G12 immunological changes were detected in serum or vaginal fluid after intravaginal administration of this mAb at any dosage [73]. Importantly, the clinical trial has allowed the identification of key regulatory issues specific to plant-made mAbs. Furthermore, cGMP-compliant procedures from seed bank establishment, to plant cultivation, to downstream processing have all been developed to address the key regulatory issues identified above [73]. The trial also helped the European regulatory agencies to establish specifications in identity, purity, and potency for plant-made mAbs acceptable for human applications [73]. Therefore, the successful completion of this clinical trial marks a significant milestone towards the eventual approval of the first plant-made mAb commercial product. Overall, the successful production and efficacy optimization of HIV bNAbs in plants supports the prospect of providing affordable mAb-based prophylactics and therapeutics to control HIV in resource-poor parts of the world.
MAbs against flaviviruses
The ease of producing mAbs that carry homogeneous and distinct mammalian glycans in glycoengineered plants offers opportunities to improve the efficacy and safety of mAb-based therapeutics against flaviviruses. West Nile virus (WNV) is a neurotropic virus that causes life-threatening WNV neuroinvasive diseases including encephalitis, meningitis, and acute flaccid paralysis [74, 75]. Our laboratory has developed a plant-derived mAb E16 that recognizes an epitope in domain III of WNV envelope protein [76]. A single dose of plant-made E16 protected mice from lethal infection of WNV, even given 4 days post-infection, when the virus has entered the brain [47–50]. Since most mAb therapeutics would have very limited efficacy in treating neuroinvasive diseases due to the incapability to cross the blood-brain barrier (BBB), we explored the possibility of using plant systems to produce more complexed antibody variants to facilitate their crossing of the BBB. Specifically, we designed a tetravalent E16 variant (Tetra E16) with two sets of Fabs [77]. Tetra E16 exhibits differential binding affinity to C1q and various FcγRs. Furthermore, Tetra E16 neutralizes WNV infection and protects mice from WNV lethal challenge equivalently as the parent E16 [77] (Fig. 2). These studies indicate plants are capable of producing complexed antibody variants, establishing the foundation to develop bispecific antibodies that can have BBB permeability [77] For example, bifunctional mAbs with a similar structure as the Tetra E16 but with one of the two sets of Fabs binding to a specific receptor (e.g., insulin receptor [78]) on the BBB may have the desired bi-functionality. That one set of Fabs would facilitate their transport into the brain and the other set of Fabs would retain its therapeutic activity against WNV in the brain (Fig. 3). Recently, we have demonstrated that such bifunctional mAb can be successfully expressed and assembled in plants with its specificity to each antigen preserved [79].
Figure 2. Plant-made E16 mAb variants protected mice against a lethal challenge of WNV infection.
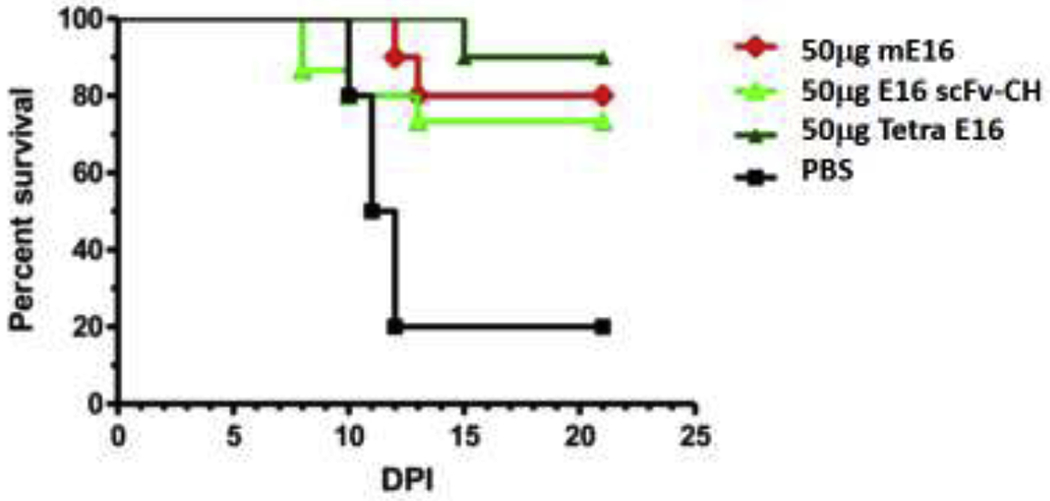
Wild type C57BL/6 mice were infected with 102 PFU of WNV. A single 50 μg dose of single-chain E16 (E16 scFv-CH or Tetra E16 was given to mice via an intraperitoneal route 4 days after infection. Mammalian cell-made E16 (mE16) was used as a positive control. Survival data was pooled from several independent experiments (n > 10 mice per dose) and analyzed by the log-rank test. (Adapted from reference [47] with permission)
Figure 3. Tetravalent E16 and bifunctional E16 design.
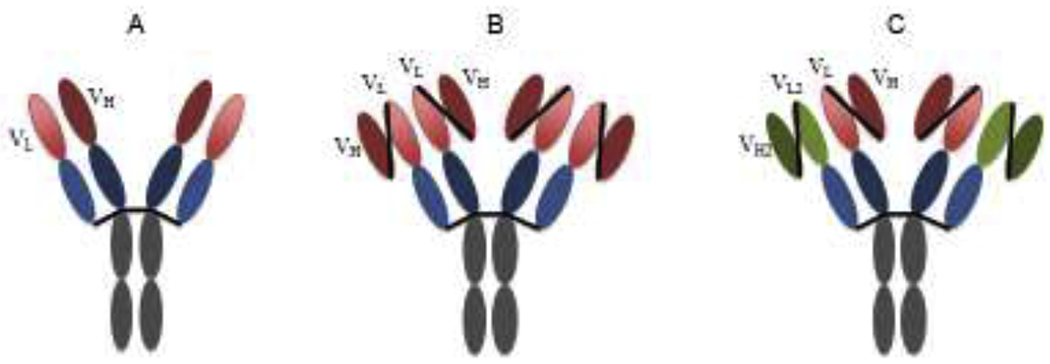
A. E16. B. Tetravalent E16. C. Bifunctional E16 that are designed to enhance its ability to cross the blood brain barrier. VL: variable region of light chain; VH: variable region of heavy chain; VL/VH: the first pair of antigen binding sites that bind and neutralize WNV. VL2/VH2: a second pair of antigen binding sites that bind to surface receptors on the endothelial cells of the BBB. This may enhance the permeability of the bifunctional antibody across the BBB via receptor-mediated transcytosis.
The four serotypes of dengue virus (DENV1-4) represent one of the largest global disease burdens with over 3 billion people at risk for infection [80]. DENV infections are prone to the development of antibody-dependent enhancement of infection (ADE). ADE occurs through the mechanism of immune-complex formation between the infecting flavivirus and the preexisting antibodies, including therapeutic mAbs, that bind to the virus but are non-neutralizing or at sub-neutralizing concentrations. Such immune complexes bind to FcγR-bearing cells, resulting in increased virus uptake and infection [81–83]. Therefore, ADE may render anti-flavivirus mAb treated subjects more at risk of developing severe disease, including life-threatening dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) [84, 85]. To address this major impediment, our laboratory used E60 mAb as a model to investigate if plants can help to overcome ADE by modulating mAb glycosylation. Various E60 glycoforms were produced in N. benthamiana and each was shown to carry a single expected N-glycoform with a high degree of homogeneity [86]. Furthermore, E60 glycovariants retain neutralizing potency against multiple DENV serotypes similar to the mammalian cell-made E60 [86]. Strikingly, plant-produced E60 glycovariants forego ADE activity, in contrast to mammalian E60 that exhibits strong ADE activity (Fig. 4) [86]. Our in vivo studies suggest that in contrast to CHO cell-produced E60 that has no therapeutic efficacy, plant-made E60 has gained potent post-exposure therapeutic activities in both lethal infection only and ADE mouse models [87]. These results indicate that by modulating N-glycans via plant glycoengineering, mAbs can be transformed from inactive binding antibodies into protective mAbs with potent in vivo efficacy even after the occurrence of ADE. This strategy is far more superior to the current approach of mutating the N-glycosylation recognition sequence used in CHO cells, as the latter method would completely remove all N-linked glycans from a mAb, causing total abolishment of mAb binding to all FcγRs and loss of effector function that may be required for the full therapeutic efficacy of a mAb [88–90]. Similarly, glycovariants of anti-Zika virus mAbs produced in plants also showed greatly reduced ADE activity in enhancing DENV infection [12]. Thus, N-glycan modulation by using glycoengineered plants has the potential to overcome ADE and increase the efficacy for mAb-based therapeutics against other ADE-prone viruses such as coronaviruses, paramyxoviruses, and lentiviruses [91].
Figure 4. Antibody-dependent enhancement of pE60 variants.
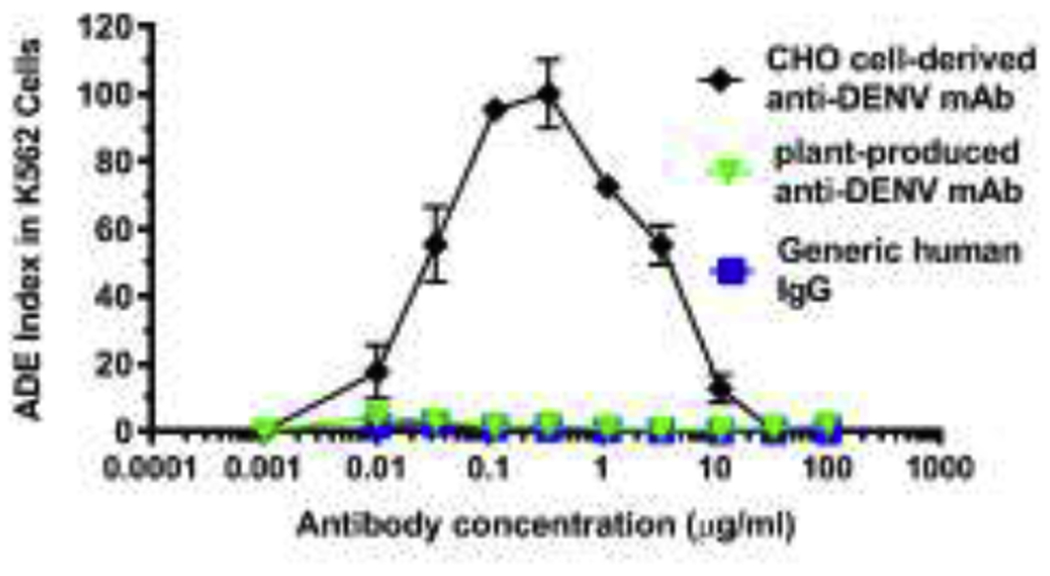
MAb glycovariants were serially diluted and mixed with DENV-2. The mAb and virus mixture was then added to FcγR-expressing K562 cells. Cells were fixed, permeabilized and stained with anti-DENV E antibody 4G2 48 hr later, and analyzed by flow cytometry for DENV infection of cells. (Adapted from reference [86] with permission)
MAbs against SARS-CoV-2
SARS-CoV-2 is the causal agent of coronavirus disease 2019 (COVID-19) and has infected over 255 million people, causing more than 5.1 million deaths globally since its emergence [92]. While vaccines remain the strategy of choice to curb the current COVID-19 pandemic, post-exposure therapeutics are also in critical demand to treat seriously ill COVID-19 patients and to prevent infection in certain vulnerable populations such as the unvaccinated or recently vaccinated high-risk patients. Neutralizing mAbs, especially those that target the spike (S) protein of SARS-CoV-2, have shown promising therapeutic potential in animal models and human patients [4]. The S protein of SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the target cell surface, thereby, facilitating cell binding and fusion [93]. MAbs targeting the S protein, therefore, can neutralize the virus by interfering in its ability to bind and fuse with the target host cell. Currently, there are more than 20 mAbs directed to the S protein that have entered clinical trials, and three mAb-based therapies have been granted EUA by the FDA for treating COVID-19 patients [4]. These therapies include two combinational therapies of bamlanivimab/etesevimab and casirivimab/imdevimab, as well as a monotherapy of sotrovimab [94–96]. Since the robust and rapid nature of plant transient expression allows for the production of mAbs at unprecedented speed, plants have been explored to quickly produce several types of mAbs against SARS-CoV-2 aiming to contribute to developing efficacious and affordable therapeutics to control the current COVID-19 pandemic. For example, two neutralizing mAbs against epitopes on the receptor-binding domain (RBD) of the S protein were expressed in N. benthamiana plants [97]. The mAbs were expressed in leaves within 4 days after the introduction of mAb-coding sequences, although the expression levels are relatively low [97]. The plant-made mAbs retain their RBD-binding specificity and neutralization activity. Similarly, expression of ScFv-Fc and camelid nanobodies has also been attempted in N. benthamiana [98]. A ground-breaking study came from Dr. Steinkellner’s laboratory, in which they examined and compared the neutralization potency of two N. benthamiana-produced anti-RBD mAbs in three formats: IgG1, IgA1 monomer, and IgA2 dimers [99]. While the three mAb variants showed similar binding to RBD, their neutralization potency against SARS-CoV-2 was significantly different, specifically in the order of IgG1< IgA1 monomer < IgA1 dimer, with IgA1 dimer having an up to 240-fold increased potency over the monomers [99]. In collaboration with our laboratory, Dr. Steinkellner’s group also compared the neutralization potency of the two anti-RBD mAbs in the four IgG subclasses [100]. IgG3 subtype was found to exhibit an up to 50-fold superior potency compared to the other subclasses [100]. These studies have revealed the importance of antibody multivalency and S protein cross-linking on the viral surface for neutralization potency and will have significant implications for COVID-19 vaccine and therapeutic development.
A characteristic seen in severe cases of COVID-19 appears to be a dysregulated immune response, resulting in cytokine storm and immunopathology [101]. Specifically, increased proinflammatory cytokine levels have a strong correlation with severe symptoms, and higher interleukin 6 (IL-6) levels are associated with non-survivors and patients needing mechanical ventilation [101–103]. One approach to curb the hyperinflammatory response is the inhibition of IL-6 signaling, as a large clinical trial using anti-IL-6 receptor (IL-6R) mAbs along with the use of corticosteroids on COVID-19 patients showed statistically significant benefit of the mAb treatment in improving patients’ clinical outcomes and survival [104]. To this end, our laboratory explored the feasibility of using plants to produce an anti-IL-6R mAb and examined its utility in reducing IL-6 signaling in a model, which simulates IL-6 induction during SARS-CoV-2 infection. N. benthamiana-produced anti-IL-6R mAb (pIL6RmAb) was shown to effectively inhibit IL-6 signaling in a cell-based model system. Furthermore, pIL6RmAb also suppressed IL-6 signaling that was induced by the exposure of human peripheral blood mononuclear cells to the spike protein of SARS-CoV-2 [105]. This study demonstrates the capacity of plants for producing functionally active mAbs that block cytokine signaling and implies their potential efficacy to curb cytokine storm in COVID-19 patients.
MAbs against other viruses
Plant-based mAb therapies against other viruses including RSV, chikungunya virus (CHIKV), rabies virus, and Herpes simplex virus-2 (HSV-2) have also been developed. Palivizumab is a neutralizing mAb targeted to the fusion protein of RSV that has been approved as prophylaxis for severe RSV infection in high-risk infants [106]. However, CHO cell-produced palivizumab is cost-prohibitive, which limits its application to the elderly who are also suffered from RSV-causing morbidity and mortality [107]. Encouragingly, plant-produced palivizumab was found to be equivalent in its efficacy both in vitro and in animal models [108]. Thus, plant-made palivizumab may provide an economical alternative to treat infants, as well as open up new markets for palivizumab.
Infection of CHIKV in humans can cause debilitating polyarthralgia that may persist for months to years and affects multiple joints [109]. The reemergence of CHIKV has resulted in millions of cases of severe, often chronic, arthralgia, and no therapeutics have been approved for human use [110]. MAbs against CHIKV envelope 1 and 2 protein (E1 and E2) have been shown to be protective against CHIKV infection in mouse models and Fc effector function has been implicated in their efficacy [111]. Our laboratory took the initiative to investigate if the efficacy of these mAbs can be improved by glycan modulation via using glycoengineered plants. Our results showed that an anti-CHIKV E1 mAb expressed in wild-type and glycoengineered N. benthamiana plants carried the unique N-glycans expected from those plant lines [24]. While both of the mAb glycovariants showed potent neutralization activity in vitro and efficacy in mice against CHIKV infection, their in vivo potency differed significantly, with the GnGn glycoform exhibiting superior potency in reducing viral load and in improving clinical symptoms [24, 112]. As CHIKV and DENV are co-circulating in the same parts of the world and co-infection with both viruses is likely, we also developed a bifunctional mAb in plants to meet the need for an effective therapeutic for patients in endemic areas. The plant-made bifunctional mAb showed the specific binding for both viruses and exhibited potent neutralization activity against both CHIKV and DENV [79]. These studies provide further examples of using glycoengineered plants to improve the efficacy of anti-viral mAbs via glycan modulation.
The current supply of prophylaxis for rabies virus is in the form of immunoglobulins from horses or humans immunized with rabies antigens [113]. The limited supply of such immunoglobulins and biosafety concerns for equine and human-derived products promoted the development of anti-rabies mAbs in plants. A mAb produced in transgenic tobacco leaves showed similar neutralizing potency and post-exposure prophylactic efficacy in hamsters compared to the equivalent mAb produced in hybridomas or anti-rabies human immunoglobulin [114]. This indicates that plant-derived mAbs are promising alternatives to address the supply and biosafety challenges of current post-exposure prophylaxis for treating rabies infection.
Genital herpes caused by HSV-2 is a global problem associated with significant morbidity and more than 491 million people worldwide are living with this incurable disease [115]. Furthermore, HSV-2 infection often the sexual transmission of HIV [115]. To explore the feasibility of developing inexpensive topical prophylaxis against HSV-2 infection, a transgenic soybean-produced anti-HSV glycoprotein B mAb was compared to the same mAb produced in mammalian cell culture for their in vitro properties as well as in vivo activity in a mouse model [116]. It was shown that the plant-produced mAb and its mammalian cell-derived counterpart had similar stability and diffusion rate in human cervical mucus and prevented vaginal HSV-2 infection similarly in a mouse model [116]. Notably, a N. benthamiana-produced mAb against HSV-2 (HSV8-N) was combined with an anti-HIV-1 mAb (VRC01-N), formulated into a vaginal film (MB66), and recently tested in a Phase I clinical trial to assess their safety, pharmacokinetics, and ex vivo efficacy in preventing HSV-2/HIV-1 infection [117]. The results from this trial indicated that repeated intravaginal applications of MB66 were safe and well-tolerated, and vaginal samples collected 24 hours post MB66 insertion significantly neutralized both HSV-2 and HIV-1 ex vivo [117] This clinical trial set a new milestone in the journey of plant-made mAbs to become approved anti-viral drugs and testified the feasibility of using plant-derived mAbs for topical applications.
Challenges and perspective
The successful production of full mAb and their variants in multiple defined human glycoforms with high yield, speed, enhanced scalability, and cost-effectiveness demonstrates plants’ potential as an attractive alternative platform for mAb development and production. However, there are remaining challenges that must be overcome to realize the full potential of plant-made mAbs. For example, despite the successes of large-scale production of several mAbs, large-scale downstream processing of mAbs from plant biomass remains challenging. Unlike mammalian cell-based platforms in which mAbs are usually secreted by cells into the cell culture media, full-length mAbs secreted by plant cells are retained in the extracellular space between the plasma membrane and the cell wall, a compartment known as apoplast [15]. Consequently, downstream processing of mAbs from plants usually requires tissue and cell disruption, which releases plant host proteins and proteases into the feed stream. Furthermore, plants usually produce abundant fibers and other solid debris and some Nicotiana species contain high levels of phenolics and alkaloids [118]. As a result, downstream processing is far more complex for plant-made mAbs. For example, clarification of plant extracts cannot be achieved simply by filtration as in the case of mammalian cells, and direct loading of plant extracts onto protein A column may cause resin fouling [119]. To overcome these challenges, several new strategies have been explored. An integrated method that combines two or more operations of extraction, bulk enrichment, purification, and concentration is one of these strategies. For example, our research has demonstrated that aqueous two-phase separation (ATPS) can combine separation, purification, and concentration in one operation. By using a plant-produced and unpurified hydrophobin-protein A fusion protein, we extracted and enriched plant-derived mAbs in one step to a comparable purity as those extracted and purified by conventional protein A chromatography [54, 120]. Another strategy is to avoid cell disruption and isolate mAbs directly from the apoplastic fluid by centrifugation [98]. While this method works for smaller mAb variants such as nanobodies and ScFvs, the sizes of full-length human mAbs are too large to pass the cell wall [15]. Nevertheless, attempts are being made to loosen the cell wall by treatment with a combination of chelators, pectinase, and cellulase aiming to allow extraction of full mAbs directly from apoplastic fluid [121]. An alternative approach from a different perspective is to further increase the expression levels of mAbs in plants. While transient expression with deconstructed viral vectors has allowed competitive levels of expression up to 5 g mAb per kilogram LFW [35], higher mAb expression levels will reduce the extraction volume and in turn, reduce consumable expenditure and the overall cost of downstream processing. The combination of these strategies is more than likely to resolve some of the challenges of downstream processing. However, further optimization of the explored technologies and/or the introduction of new processing methods are required to fully overcome this major impediment.
Other challenges for plant-made mAbs includes skepticism and lack of interest by industry experts and large pharmaceutical companies. ELEYSO™ is the only plant cell-made biologic approved by the FDA to date, but there are still no plant-made mAbs that have been licensed for human use [122]. The lack of a clear regulatory pathway in the past is partially responsible for the lingering skepticism and the inertia by large pharmaceutical companies, as regulatory agencies like FDA were uncertain about how to fit biologics made by this novel yet complex technology into their approval framework established mostly for biologics produced in mammalian cells. The approval of ELEYSO™ by the FDA along with the clinical development of ZMapp and 2G12 has cleared the regulatory pathway and slowly warmed up the interest of large pharmaceutical companies towards plant-made biologics including mAbs [122–124]. For example, Pfizer entered into an agreement to license the worldwide rights for commercializing ELEYSO™, and GlaxoSmithKline (GSK) is co-developing a plant-made COVID-19 vaccine with a Canadian plant-based biotech company [125]. These progresses should facilitate the commercial development of plant-made mAbs and streamline the approval of those that have shown safety in human clinical trials.
Since mAb titers ≥ 5.0 g per liter can be readily achieved in mammalian cell cultures [126], expression levels ≥ 5.0 g per kilogram LFW may be required for plant-made mAbs to compete with those derived from mammalian cells [119]. Thus, it is unlikely that plants will become the primary platform for mAb production in the foreseeable future. Instead, several special niches are waiting to be explored by this technology. For example, plants would be optimal to produce mAb biosimilars because they can produce large amounts of mAbs rapidly at a low cost. Similarly, plants should be a system of choice for producing mAbs with an extraordinarily large-quantity demand, such as prophylactic mAbs that are needed to cover a large population with repeated dosing requirements. Glycoengineered plants should be employed to develop and produce safer and more efficacious mAb biobetters due to their flexibility in producing mAbs with specific and homogeneous mammalian glycans and other PTMs that preferentially bind to various FcγRs, thereby, modulating mAb effector functions. As demonstrated by the development of several anti-SARS-CoV-2 mAbs in plants, the rapid nature of plant transient expression systems should be used more extensively to quickly produce various mAb glycovariants, isotype variants, and subtype variants to improve the efficacy and safety of prophylactic and therapeutic candidates in fighting the current COVID-19 and future potential pandemics [97, 127, 128]. The current priorities for the field of plant-made mAbs should be the focus on overcoming the challenges in downstream processing, actively engaging large pharmaceutical companies in co-developing new mAb drugs, and vastly expanding the approval pipeline of plant-made mAbs. We speculate that the first plant-made mAb-based antiviral drug should be approved within this decade.
Acknowledgements
The authors thank Josh Lesio for the critical reading of the manuscript. Relevant research in the author’s laboratory was supported in part by NIH-NIAID grants U01 AI075549 and R21/R33AI101329 to Qiang Chen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
conflict of interest
The author has no conflict of interest for the publication of this manuscript.
References
- [1].Smith SL, Ten years of Orthoclone OKT3 (muromonab-CD3): a review. J Transpl Coord, 1996. 6(3): p. 109–19; quiz 120-1. [DOI] [PubMed] [Google Scholar]
- [2].Society, A. 2021.
- [3].Lu R-M, et al. , Development of therapeutic antibodies for the treatment of diseases. Journal of Biomedical Science, 2020. 27(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Lu et al. provided a comprehensive review on the last market trends and predominant engineering technologies for monoclonal antibody-based drugs
- [4].Taylor PC, et al. , Neutralizing monoclonal antibodies for treatment of COVID-19. Nature Reviews Immunology, 2021. 21(6): p. 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Insightful discussion on lessons learned from past antibody therapies for viral infections and key issues that must be addressed for optimizing mAbs as therapy for SARS-CoV-2 infection.
- [5].Diseases, A.A.o.P.C.o.I., Updated guidance for palivizumab prophylaxis among Infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics, 2014. 134: p. e620–e638. [DOI] [PubMed] [Google Scholar]
- [6].Huang K, et al. , Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion. J Virol, 2010. 84(16): p. 8132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beccari MV, et al. , Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection. Antimicrob Agents Chemother, 2019. 63(6). [DOI] [PMC free article] [PubMed] [Google Scholar]; *Demonstrated the efficacy of the first mAb that has been approved to treat multidrug-resistant HIV-1 infection.
- [8].Atoltivimab, Maftivimab, and Odesivimab-ebgn. Am J Health Syst Pharm, 2021. 78(4): p. 279–281. [DOI] [PubMed] [Google Scholar]
- [9].Lee A, Ansuvimab: First Approval. Drugs, 2021. 81(5): p. 595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Documented the clinical results of the first approved mAb –based drug for treating Ebola virus infection in adults and pediatric patients.
- [10].Su B, et al. , Update on Fc-Mediated Antibody Functions Against HIV-1 Beyond Neutralization. Front Immunol, 2019. 10: p. 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Revealed the importance roles of antibody Fc effector functions in treating HIV infection.
- [11].van Erp EA, et al. , Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front Immunol, 2019. 10: p. 548. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Documented the contribution of antibody effector functions in fighting RSV infection.
- [12].Sun H, Chen Q, and Lai H, Development of Antibody Therapeutics against Flaviviruses. International Journal of Molecular Sciences, 2018. 19(1): p. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pascal KE, D. D, Trefry JC, et al. , Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in nonhuman primates. J Infect Dis, 2018. 218: p. S612–S626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corti D, et al. , Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science, 2016. 351(6279): p. 1339–42. [DOI] [PubMed] [Google Scholar]
- [15].Chen Q, Expression and manufacture of pharmaceutical proteins in genetically engineered horticultural plants, in Transgenic Horticultural Crops: Challenges and Opportunities - Essays by Experts, Mou B and Scorza R, Editors. 2011, Taylor & Francis Boca Raton, p. 83–124. [Google Scholar]
- [16].Chen Q, Turning a new leaf. European Biopharmaceutical Review 2011. 2(56): p. 64–68. [Google Scholar]
- [17].Chen Q and Lai H, Plant-derived monoclonal antibodies as human biologics for infectious disease and cancer in Plant-derived Pharmaceuticals: Principles and Applications for Developing Countries, Hefferon KL, Editor. 2014, CABI: Cryodon, UK. p. 42–75. [Google Scholar]
- [18].Klutz S, et al. , Cost evaluation of antibody production processes in different operation modes. Chemical Engineering Science, 2016. 141: p. 63–74. [Google Scholar]
- [19].Hernandez I, B. S, Patel A, Wolf C, Hospodar A, Sampathkumar A, Shrank W, Pricing of Monoclonal Antibody Therapies: Higher If Used for Cancer? The American Journal of Managed Care, 2018. 24(2): p. 109–112. [PubMed] [Google Scholar]
- [20].Walsh G, Biopharmaceutical benchmarks 2014. Nat Biotechnol, 2014. 32(10): p. 992–1000. [DOI] [PubMed] [Google Scholar]
- [21].Hiatt A, Cafferkey R, and Bowdish K, Production of antibodies in transgenic plants. Nature, 1989. 342(6245): p. 76–8. [DOI] [PubMed] [Google Scholar]
- [22].Loos A, et al. , Expression and glycoengineering of functionally active heteromultimeric IgM in plants. Proc Natl Acad Sci U S A, 2014. 111(17): p. 6263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Paul M, et al. , Characterization of a plant-produced recombinant human secretory IgA with broad neutralizing activity against HIV. mAbs, 2014. 6(6): p. 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hurtado J, et al. , In vitro and in vivo efficacy of anti-chikungunya virus monoclonal antibodies produced in wild-type and glycoengineered Nicotiana benthamiana plants. Plant Biotechnology Journal, 2020. 18(1): p. 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Demonstrated that the potency of anti-CHIKV mAbs can be enhanced by selecting a specific N-glycosylation via plant glycoengineering.
- [25].Chen Q, Zhang C, and Santi L, Plant-Made Biologics. BioMed Research International, 2014. 2014(DOI: 10.1155/2014/418064): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen Q and Davis K, The potential of plants as a system for the development and production of human biologics F1000Research, 2016. 5(912): p. doi: 10.12688/f1000research.8010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nandi S, et al. , Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs, 2016. 8(8): p. 1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wycoff KL, Secretory IgA Antibodies from Plants. Current Pharmaceutical Design, 2005. 11(19): p. 2429–2437. [DOI] [PubMed] [Google Scholar]
- [29].Kwon K-C and Daniell H, Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnology Journal, 2015. 13(8): p. 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen Q, et al. , Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Human Vaccines, 2011. 7(3): p. 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Klimyuk V, et al. , Production of recombinant antigens and antibodies in Nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. Curr Top Microbiol Immunol, 2014. 375: p. 127–54. [DOI] [PubMed] [Google Scholar]
- [32].Peyret H and Lomonossoff GP, When plant virology met Agrobacterium: the rise of the deconstructed clones. Plant Biotechnology Journal, 2015. 13(8): p. 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang Z, et al. , A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnology and Bioengineering, 2009. 103(4): p. 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lico C, Chen Q, and Santi L, Viral vectors for production of recombinant proteins in plants. Journal of cellular physiology, 2008. 216(2): p. 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bendandi M, et al. , Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Annals of Oncology, 2010. 21(12): p. 2420–2427. [DOI] [PubMed] [Google Scholar]
- [36].Leuzinger K, et al. , Efficient Agroinfiltration of Plants for High-level Transient Expression of Recombinant Proteins. Journal of Visualized Experiments, 2013(77): p. doi: 10.3791/50521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen Q and Lai H, Gene delivery into plant cells for recombinant protein production. BioMed Research International, 2014. 2014(DOI: 10.1155/2014/932161): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen Q, et al. , Agroinfiltration as an Effective and Scalable Strategy of Gene Delivery for Production of Pharmaceutical Proteins. Advanced Technolgy in Biology and Medicine, 2013. 1(1): p. 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Werner S, et al. , High-level recombinant protein expression in transgenic plants by using a double-inducible viral vector. Proceedings of the National Academy of Sciences of the United States of America, 2011. 108(34): p. 14061–14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dugdale B, et al. , In Plant Activation: An Inducible, Hyperexpression Platform for Recombinant Protein Production in Plants. The Plant Cell Online, 2013. 25(7): p. 2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Loos A and Steinkellner H, Plant glyco-biotechnology on the way to synthetic biology. Frontiers in Plant Science, 2014. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Varki A, Evolutionary Forces Shaping the Golgi Glycosylation Machinery: Why Cell Surface Glycans Are Universal to Living Cells. Cold Spring Harbor Perspectives in Biology, 2011. 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yang Z, et al. , Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotech, 2015. 33(8): p. 842–844. [DOI] [PubMed] [Google Scholar]
- [44].Chen Q, Glycoengineering of plants yields glycoproteins with polysialylation and other defined N-glycoforms. Proceedings of the National Academy of Sciences, 2016. 113(34): p. 9404–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schahs M, et al. , Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotechnol J, 2007. 5(5): p. 657–63. [DOI] [PubMed] [Google Scholar]
- [46].Strasser R, et al. , Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnology Journal, 2008. 6(4): p. 392–402. [DOI] [PubMed] [Google Scholar]
- [47].He J, et al. , Generation and Analysis of Novel Plant-Derived Antibody-Based Therapeutic Molecules against West Nile Virus. PLoS ONE, 2014. 9(3): p. e93541 DOI: 10.1371/journal.pone.0093541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lai H, et al. , Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnology Journal, 2014. 12(8): p. 1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].He J, et al. , A Novel System for Rapid and Cost-Effective Production of Detection and Diagnostic Reagents of West Nile Virus in Plants. Journal of Biomedicine and Biotechnology, 2012. 2012( 10.1155/2012/106783): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lai H, et al. , Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proceedings of the National Academy of Sciences of the United States of America, 2010. 107(6): p. 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Check Hayden E and Reardon S, Should experimental drugs be used in the Ebola outbreak? Nature (London), 2014. 10.1038/nature.2014.15698 [DOI] [Google Scholar]
- [52].Huang Z, et al. , High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnology and bioengineering, 2010. 106(1): p. 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lai H, et al. , Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnology Journal, 2012. 10(1): p. 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fulton A, et al. , Purification of monoclonal antibody against Ebola GP1 protein expressed in Nicotiana benthamiana. Journal of Chromatography A, 2015. 1389(0): p. 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zeitlin L, et al. , Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proceedings of the National Academy of Sciences of the United States of America, 2011. 108(51): p. 20690–20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Olinger GG, et al. , Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America, 2012. 109(44): p. 18030–18035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Phoolcharoen W, et al. , A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proceedings of the National Academy of Sciences of the United States of America, 2011. 108(51): p. 20695–20700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Phoolcharoen W, et al. , Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnology Journal, 2011. 9(7): p. 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Qiu X, et al. , Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature, 2014. 514(7520): p. 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Group PIW, et al. , A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection. The New England journal of medicine, 2016. 375(15): p. 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mulangu S, et al. , A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. New England Journal of Medicine, 2019. 381(24): p. 2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].UNAIDS/WHO, Global HIV & AIDS statistics — Fact sheet. AIDS by the numbers. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland, 2021. https://www.unaids.org/en/resources/fact-sheet(Accessed September 6, 2021). [Google Scholar]
- [63].Forthal DN, et al. , Fc-Glycosylation Influences Fcγ Receptor Binding and Cell-Mediated Anti-HIV Activity of Monoclonal Antibody 2G12. The Journal of Immunology, 2010. 185(11): p. 6876–6882. [DOI] [PubMed] [Google Scholar]
- [64].Rademacher T, et al. , Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc <i>N</i>-glycans. Plant Biotechnology Journal, 2008. 6(2): p. 189–201. [DOI] [PubMed] [Google Scholar]
- [65].Zhao C, et al. , Pharmacokinetics and Preliminary Safety of Pod-lntravaginal Rings Delivering the Monoclonal Antibody VRC01-N for HIV Prophylaxis in a Macaque Model. Antimicrobial agents and chemotherapy, 2017. 61(7): p. e02465–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Singh AA, et al. , Plant-based production of highly potent anti-HIV antibodies with engineered posttranslational modifications. Scientific Reports, 2020. 10(1): p. 6201. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Reported that mammalian-type sulfation of mAbs can be accomplished in plants by targeting mammalian sulfotransferase to a particular organelle.
- [67].Moore CM, et al. , Characterisation of a highly potent and near pan-neutralising anti-HIV monoclonal antibody expressed in tobacco plants. Retrovirology, 2021. 18(1): p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Strasser R, et al. , Improved Virus Neutralization by Plant-produced Anti-HIV Antibodies with a Homogeneous β1,4-Galactosylated N-Glycan Profile. Journal of Biological Chemistry, 2009. 284(31): p. 20479–20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Loos A, et al. , Glycan modulation and sulfoengineering of anti–HIV-1 monoclonal antibody PG9 in plants. Proceedings of the National Academy of Sciences, 2015. 112(41): p. 12675–12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sexton A, et al. , Design, expression, and characterization of a multivalent, combination HIV microbicide. The FASEB Journal, 2009. 23(10): p. 3590–3600. [DOI] [PubMed] [Google Scholar]
- [71].Seber Kasinger LE, et al. , A novel anti-HIV-1 bispecific bNAb-lectin fusion protein engineered in a plant-based transient expression system. Plant Biotechnology Journal, 2019. 17(8): p. 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Demonstrated the synergy between the VRC01 mAb and Avaren in a plant-made bispecific mAb-lection fusion molecule in treating HIV infection.
- [72].Rosenberg Y, et al. , Pharmacokinetics and Immunogenicity of Broadly Neutralizing HIV Monoclonal Antibodies in Macaques. PLOS ONE, 2015. 10(3): p. e0120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ma JKC, et al. , Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnology Journal, 2015. 13(8): p. 1106–1120. [DOI] [PubMed] [Google Scholar]
- [74].Alli A, et al. , Management of West Nile Encephalitis: An Uncommon Complication of West Nile Virus. Cureus, 2021. 13(2): p. e13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Petersen LR, Brault AC, and Nasci RS, West Nile virus: review of the literature. Jama, 2013. 310(3): p. 308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Oliphant T, et al. , Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med, 2005. 11(5): p. 522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].He J, et al. , Generation and analysis of novel plant-derived antibody-based therapeutic molecules against West Nile virus. PLoS One, 2014. 9(3): p. e93541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Boado RJ, et al. , Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnol Bioeng, 2007. 96(2): p. 381–91. [DOI] [PubMed] [Google Scholar]
- [79].Esqueda A, Jugler C, and Chen Q, Design and expression of a bispecific antibody against dengue and chikungunya virus in plants, in Methods in Enzymology. 2021, Academic Press. [DOI] [PubMed] [Google Scholar]
- [80].Murray NEA, Quam MB, and Wilder-Smith A, Epidemiology of dengue: past, present and future prospects. Clinical Epidemiology, 2013. 5: p. 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Morens DM, Antibody-dependent of enhancement of infection and the pathogenesis of viral disease. Clin Inf Dis, 1994. 19: p. 500–512. [DOI] [PubMed] [Google Scholar]
- [82].Taylor A, et al. , Fc receptors in antibody-dependent enhancement of viral infections. Immunological Reviews, 2015. 268(1): p. 340–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Takada A and Kawaoka Y, Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Reviews in Medical Virology, 2003. 13(6): p. 387–398. [DOI] [PubMed] [Google Scholar]
- [84].Rothman AL, Dengue: defining protective versus pathologic immunity. J Clin Invest, 2004. 113(7): p. 946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Halstead SB, Dengue. The Lancet, 2007. 370(9599): p. 1644–1652. [DOI] [PubMed] [Google Scholar]
- [86].Dent M, et al. , Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. Journal of General Virology, 2016. 97(12): p. 3280–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Chen Q, Lai H, and Hurtado J, Plant-derived antibodies and derivatives that reduce risk of antibody-dependent enhancement (ADE) of infection. 2021, US Patent 10,982,221.
- [88].Mehlhop E, et al. , Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe, 2009. 6(4): p. 381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Garcia G, et al. , Antibodies from patients with dengue viral infection mediate cellular cytotoxicity. J Clin Virol, 2006. 37(1): p. 53–7. [DOI] [PubMed] [Google Scholar]
- [90].Laoprasopwattana K, et al. , Antibody-dependent cellular cytotoxicity mediated by plasma obtained before secondary dengue virus infections: potential involvement in early control of viral replication. J Infect Dis, 2007. 195(8): p. 1108–16. [DOI] [PubMed] [Google Scholar]
- [91].Huisman W, M BEE., Rimmelzwaan GF, Gruters RA, Osterhaus ADME, Vaccine-induced enhancement of viral infections. Vaccine 2009. 27: p. 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].WHO, COVID-19 Dashboard. https://www.who.int/emergencies/diseases/novel-coronavirus-2019, 2021. Accessed [11/21/2021].
- [93].Lu R, et al. , Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London, England), 2020. 395(10224): p. 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gupta A, et al. , Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. New England Journal of Medicine, 2021. 385(21): p. 1941–1950. [DOI] [PubMed] [Google Scholar]; ** Demonstrated the clinical benefit of early treatment with neutralizing mAb in COVID-19 patents.
- [95].Gottlieb RL, et al. , Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA, 2021. 325(7): p. 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Showcased the efficacy of mAb combination therapy in COVID-19 patients with mild to moderate symptoms.
- [96].Weinreich DM, et al. , REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. The New England journal of medicine, 2021. 384(3): p. 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shanmugaraj B, et al. , Monoclonal Antibodies B38 and H4 Produced in Nicotiana benthamiana Neutralize SARS-CoV-2 in vitro. Frontiers in plant science, 2020. 11: p. 589995–589995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Diego-Martin B, et al. , Pilot Production of SARS-CoV-2 Related Proteins in Plants: A Proof of Concept for Rapid Repurposing of Indoor Farms Into Biomanufacturing Facilities. Frontiers in Plant Science, 2020. 11: p. 2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sun L, et al. , Increased in vitro neutralizing activity of SARS-CoV-2 IgA1 dimers compared to monomers and IgG. Proceedings of the National Academy of Sciences, 2021. 118(44): p. e2107148118. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Revealed the importance of multivalency for mAb efficacy against SARS-CoV-2.
- [100].Kallolimath S, et al. , Highly active engineered IgG3 antibodies against SARS-CoV-2. Proceedings of the National Academy of Sciences, 2021. 118(42): p. e2107249118. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Elucidated that the neutralizing potency of anti-SARS-CoV-2 mAbs can be greatly improved by switching to the IgG3 subtype and produced them in glycoengineering GnGn plants.
- [101].Chen G, et al. , Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of Clinical Investigation, 2020. 130: p. 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Costela-Ruiz VJ, et al. , SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine & Growth Factor Reviews, 2020. 54: p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Herold T, et al. , Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. Journal of Allergy and Clinical Immunology, 2020. 146: p. 128–136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Abani O, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial RECOVERY Collaborative Group*. in The Lancet. 2021. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jugler C, Sun H, and Chen Q, SARS-CoV-2 Spike Protein-Induced Interleukin 6 Signaling Is Blocked by a Plant-Produced Anti-Interleukin 6 Receptor Monoclonal Antibody. Vaccines, 2021. 9(11): p. 1365. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Demonstrated a plant-made mAb targeted to IL-6R can inhibit IL-6 signaling that is induced by the exposure to the spike protein of SARS-CoV-2.
- [106].Group*, I.-R.S., Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics, 1998. 102(3): p. 531–537. [PubMed] [Google Scholar]
- [107].Numa A, Outcome of respiratory syncytial virus infection and a cost-benefit analysis of prophylaxis. J Paediatr Child Health, 2000. 36(5): p. 422–7. [DOI] [PubMed] [Google Scholar]
- [108].Zeitlin L, et al. , Prophylactic and therapeutic testing of Nicotiana-derived RSV-neutralizing human monoclonal antibodies in the cotton rat model. mAbs, 2013. 5(2): p. 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Schilte C, et al. , Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS neglected tropical diseases, 2013. 7(3): p. e2137–e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Weaver SC and Forrester NL, Chikungunya: Evolutionary history and recent epidemic spread. Antiviral Research, 2015. 120: p. 32–39. [DOI] [PubMed] [Google Scholar]
- [111].Pal P, et al. , Development of a Highly Protective Combination Monoclonal Antibody Therapy against Chikungunya Virus. PLOS Pathogens, 2013. 9(4): p. e1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chen Q, et al. , Plant-produced mabs against chikungunya virus with enhanced effector function and efficacy. 2020, Application Application PCT/US2020/036136.
- [113].Wilde H, Tipkong P, and Khawplod P, Economic issues in postexposure rabies treatment. J Travel Med, 1999. 6(4): p. 238–42. [DOI] [PubMed] [Google Scholar]
- [114].Ko K, et al. , Function and glycosylation of plant-derived antiviral monoclonal antibody. Proc Natl Acad Sci U S A, 2003. 100(13): p. 8013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hook E.W.d., et al. , Herpes simplex virus infection as a risk factor for human immunodeficiency virus infection in heterosexuals [see comments]. J Infect Dis, 1992. 165(2): p. 251–5. [DOI] [PubMed] [Google Scholar]
- [116].Zeitlin L, et al. , A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nat Biotech, 1998. 16(13): p. 1361–1364. [DOI] [PubMed] [Google Scholar]
- [117].Politch JA, et al. , Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): A Phase I randomized trial. PLOS Medicine, 2021. 18(2): p. e1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Demonstrated the safety and ex vivo efficacy of a plant-made mAb-based film in preventing vaginal infection of HSV-1 and HIV-2 in a clinical setting.
- [118].Chen Q, Expression and Purification of Pharmaceutical Proteins in Plants. Biological Engineering, 2008. 1(4): p. 291–321. [Google Scholar]
- [119].Buyel JF, Twyman RM, and Fischer R, Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol Adv, 2015. 33(6 Pt 1): p. 902–13. [DOI] [PubMed] [Google Scholar]
- [120].Jugler C, Joensuu J, and Chen Q, Hydrophobin-Protein A Fusion Protein Produced in Plants Efficiently Purified an Anti-West Nile Virus Monoclonal Antibody from Plant Extracts via Aqueous Two-Phase Separation. International Journal of Molecular Sciences, 2020. 21(6): p. 2140. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Demonstrated the utility of using plant-made hydrophobin-protein A fusion molecule in a crude extract in purify plant-made mAbs via ATPS.
- [121].Couture M, Paquet D, and Vezina L-P, Method for recovering plant-derived proteins. 2017, Google Patents.
- [122].Traynor K, Taliglucerase alfa approved for Gaucher disease. Am J Health Syst Pharm, 2012. 69(12): p. 1009. [DOI] [PubMed] [Google Scholar]
- [123].Chen Q and Lai H, Plant-derived virus-like particles as vaccines. Human Vaccines & Immunotherapeutics, 2013. 9(1): p. 26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Paul MJ, Thangaraj H, and Ma JKC, Commercialization of new biotechnology: a systematic review of 16 commercial case studies in a novel manufacturing sector. Plant Biotechnology Journal, 2015. 13(8): p. 1209–1220. [DOI] [PubMed] [Google Scholar]
- [125].Medicago, Medicago to launch clinical trials for COVID-19 vaccine candidate with GSK’s pandemic adjuvant in Japan, https://medicago.com/en/media/, 2021. accessed November 22, 2021.
- [126].Shukla AA and Thömmes J, Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends in Biotechnology, 2010. 28(5): p. 253–261. [DOI] [PubMed] [Google Scholar]
- [127].Rosales-Mendoza S, Will plant-made biopharmaceuticals play a role in the fight against COVID-19? Expert opinion on biological therapy, 2020. 20(6): p. 545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]


