Abstract
Background & Aims:
The ‘gut-homing’ hypothesis suggests the pathogenesis of primary sclerosing cholangitis (PSC) is driven by aberrant hepatic expression of gut adhesion molecules and subsequent recruitment of gut-derived T-cells to the liver. However, inconsistencies lie within this theory including an absence of investigations and comparisons with other chronic liver diseases (CLD). Here we examine ‘the gut-homing theory’ in patients with PSC with associated inflammatory bowel disease (PSC-IBD) and across multiple inflammatory liver diseases.
Methods:
Expression of MAdCAM-1, CCL25 and E-Cadherin were assessed histologically and using RT-PCR on explanted liver tissue from CLD patients undergoing orthotopic liver transplantation and in normal liver. Liver mononuclear cells were isolated from explanted tissue samples and the expression of gut homing integrins and cytokines on hepatic infiltrating gut-derived T-cells was assessed using flow cytometry.
Results:
Hepatic expression of MAdCAM-1, CCL25 and E-Cadherin was upregulated in all CLDs compared to normal liver. There were no differences between disease groups. Frequencies of α4β7, αEβ7, CCR9 and GPR15 expressing hepatic T-cells was increased in PSC-IBD, but also in CLD controls, compared with normal liver. β7 expressing hepatic T-cells displayed an increased inflammatory phenotype compared with β7 negative cells, though this inflammatory cytokine profile was present in both the inflamed and normal liver.
Conclusions:
These findings refute the widely accepted ‘gut-homing’ hypothesis as the primary driver of PSC and indicate that aberrant hepatic recruitment of gut-derived T-cells is not unique to PSC, but is a pan-aetiological feature of CLD.
Keywords: primary sclerosing cholangitis, inflammatory bowel disease, chronic liver disease, mucosal addressin cellular adhesion molecule 1, alpha 4 beta 7 integrin, liver mononuclear cells, T helper cell
Introduction
The recruitment of T lymphocytes from the peripheral circulation to secondary lymphoid organs and peripheral tissues is a highly coordinated process enacted through sequential interactions between homing receptors and their respective ligands expressed on endothelial cells. Tissue-specific combinations of chemokine ligands and adhesion molecules create a molecular ‘address’ that is recognised by specific chemokine receptors and integrins expressed on lymphocytes. One of the most notable examples of tissue-specific recirculation of memory lymphocytes occurs within the gut. The gut molecular address consists of the chemokine ligand 25 (CCL25) and mucosal addressin cellular adhesion molecule 1 (MAdCAM-1), both of which regulate the recruitment of T lymphocytes to the gut through interaction with their cognate receptors: G protein coupled chemokine receptor 9 (CCR9) and alpha 4 beta 7 integrin (α4β7) (1). Under physiological conditions the expression of CCL25 and MAdCAM-1 are largely confined to gut associated lymphoid tissues. However, evidence of ectopic MAdCAM-1 and CCL25 expression was demonstrated in the liver of adults with chronic inflammatory liver disease, especially in primary sclerosing cholangitis (PSC), a condition frequently associated with inflammatory bowel disease (IBD), while their expression is absent in normal adult and foetal liver (2). The liver inflammatory lymphocytic infiltrate of patients with PSC has been reported to consist mainly of non-activated memory T-lymphocytes, a substantial proportion of which expressed the co-receptors α4β7 and/or CCR9 (3). Grant et al., extended this line of research showing hepatic MAdCAM-1 expression in a variety of chronic liver diseases (CLD), but particularly in patients with PSC and autoimmune hepatitis (AIH) (4). In addition, peripheral T lymphocytes from patients with PSC, ulcerative colitis (UC) and healthy controls adhered to histologically MAdCAM-1 positive PSC liver sections, indicating that the aberrant hepatic expression of MAdCAM-1 was functional. Further findings demonstrated that CCL25 could also be aberrantly expressed within the liver, particularly in PSC patients (increased hepatic CCL25 mRNA levels and positive histological expression) (5). Moreover, up to 20% of PSC hepatic T-cells expressed CCR9 compared with relatively low expression in primary biliary cholangitis (PBC) liver and non-diseased liver from organ donors (<2%) (5). It has been well documented that induction of gut tropism (expression of CCR9 and α4β7) on T lymphocytes requires the presence of retinoic acid (RA) (1). Gut dendritic cells (DCs) are a unique subset of DCs defined by their expression of retinol hydrogenases which metabolise retinol to RA giving them the ability to induce gut tropism upon T lymphocyte priming.
Elevations in the expression of CCR9 and α4β7 on hepatic T-cells in PSC patients raised the question whether the ability to induce the expression of gut tropic integrins on T-cells could also be induced extra-intestinally by liver DCs. Subsequent work reported that liver DCs isolated from normal and PSC livers were not able to induce or maintain expression of this gut tropic phenotype on T-cells. Activation by gut DCs, however, did imprint high levels of both CCR9 and α4β7 on T-cells, leading the authors to conclude that hepatic CCR9 and α4β7 expressing T-cells are indeed of gut origin (6).
Together these data prompted the proposal of a model of enterohepatic lymphocyte circulation in which long-lived gut activated memory T-cells recirculate through the liver and are recruited by aberrantly expressed gut adhesion molecules during hepatic inflammation, thereby providing a theoretical mechanistic explanation for the striking association between PSC and IBD, including how these related conditions often display an asynchronous onset and the observation of the former arising even in patients in whom the colon had been removed.
Recent data have challenged this model, with a number of studies reporting hepatic MAdCAM-1 expression and elevations in α4 and β7 gene expression in various non-autoimmune CLDs (7–9). Furthermore, there is a lack of response of PSC patients to the anti-α4β7 treatment Vedolizumab reported by several centres, including our own (10–13).
Here we appraise the gut-lymphocyte homing hypothesis in PSC with associated IBD (PSC-IBD), by characterising for the first time the hepatic expression of MAdCAM-1, CCL25 and E-Cadherin and the expression on both hepatic and peripheral T-cells of their cognate receptors α4β7, CCR9, αEβ7, and of the colonic homing receptor, GPR15, in paired blood and fresh liver tissue samples from patients with PSC-IBD or various other CLDs. Moreover, we define the inflammatory phenotype of gut-derived cells within the liver of patients with PSC-IBD, alcoholic liver disease (ALD) and in the normal liver.
Methods
Patients
Explanted liver tissue was collected from twenty-eight patients undergoing orthotopic liver transplantation (OLT) for CLD. Non-diseased, age matched control liver tissue was obtained from unused donor liver after organ reduction or split transplantation (n=4) or from patients undergoing partial hepatectomy for removal of colorectal metastases (n=2). Liver mononuclear cells (LMNC) were isolated from liver tissue from seventeen of these patients; non-alcoholic steatohepatitis (NASH, [n=3]), ALD, (n=7), PSC-IBD, (n=7) and from all non-diseased control samples (n=6). Peripheral blood samples were taken at the time of transplantation from ten CLD patients (NASH [n=3], ALD [n=3] and PSC-IBD [n=4) and from fourteen age matched healthy donors. For demographic and clinical details see supplementary material tables 1 and 2.
Immunohistochemistry
CLD explanted tissue sections obtained from PBC (n=2), ALD (n=8), NASH (n=6), chronic hepatitis C virus [HCV] (n=2), autoimmune hepatitis [AIH] (n=2), PSC-IBD (n=8) and non-diseased liver tissue (n=4) were collected for immunohistochemical analysis. In addition, diagnostic liver biopsy tissue sections obtained from PSC-IBD (n=6), AIH (n=5) and viral hepatitis (n=5) were also collected for comparative MAdCAM-1 immunoreactivity analysis. Samples were formalin fixed, paraffin embedded. Sections were subsequently deparaffinised in xylene, dehydrated in ethanol and re-hydrated in water. Following re-hydration, sections were incubated overnight at 4° C with rabbit polyclonal anti-human MAdCAM-1 (cat.# ab178549, Abcam), mouse monoclonal anti-human E-Cadherin (cat.# NCL-L-E-Cad, clone # 36B5, Leica) and polyclonal rabbit anti-human CD25 (clone # EPR12388), used at a dilution of 1:500, 1:50, and 1:250 respectively. Following endogenous peroxidase blocking with 3% H2O2, sections were incubated with goat anti-rabbit or goat anti-mouse (both from Vector Laboratories, Burlingame, CA) secondary antibodies, used at 1/1.000 and 1/400 respectively, for one hour at room temperature. After treatment with Vectastain Elite ABC kit (Vector Laboratories), ImmPACT DAB (Vector Laboratories) was applied and sections were examined by light microscopy.
RT-PCR
Expression of MAdCAM-1, CCL25, and E-Cadherin was determined by RT-PCR, as previously described(14), following total RNA extraction using TRIzol reagent (ThermoFisher Scientific) and mRNA reverse transcription using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer’s instructions. The primer sequences were as follows: MAdCAM-1: Forward, 5’-GTATTGCGC CGCTAGAGGTG-3’; Reverse, 5’ -CTGAACGCCACTTGT CCCTC-3’ CCL25: Forward, 5′-TTTGAAGACTGCTGCCTGG-3′; Reverse, 5′-GTCTTCTTCCTAACAAGCC-3′ E-Cadherin: Forward, 5′-CTGATGCTGATGCCCCCAATA-3′; Reverse, 5′-CAGTTTCTGCATCTTGCCAGG-3′
Cell isolation
Liver mononuclear cells (LMNC) were freshly isolated from explanted liver tissue or resected liver tissue according to established methodology (15). Briefly, approximately, 50–100g of liver tissue was placed in RPMI 1640 supplemented with 10% foetal bovine serum (FBS) and placed on ice for up to 4 hours prior to homogenisation. Liver was cut into 1cm3 pieces using a sterile No11 scalpel. Tissue cubes were then washed twice with cold RPMI 1640 before being placed into gentleMACS C Tubes (Miltenyi Biotec). C Tubes were then inserted into gentleMACS Tissue Disassociator (Miltenyi Biotec) and homogenised for 1 minute. Tissue homogenate was then diluted in cold RPMI 1640 and passed through a 63μm filter (Fisher Scientific). Homogenate was then further filtered through a 30μm filter (Miltenyi Biotec) before being centrifuged at 50xg (acceleration 5 and deceleration 3) at 20°C in order to separate larger cellular debris. Supernatant was then extracted and layered onto 15 ml Ficoll-Paque density gradient solution (1.077 g/ml) in 50ml sterile Falcon tube and centrifuged at 800xg for 20 minutes at 20°C without centrifuge braking enabled. Lymphocyte interface was then aspirated and filtered through a 20μm filter (pluriSelect) before being centrifuged once more. Cell pellet was then resuspended in 5ml of RPMI 1640 before cell counting. The viability of freshly isolated LMNCs consistently exceeded 90%.
Flow cytometry
Flow cytometry was performed following incubation of PBMCs and LMNCs with anti-human antibodies against cell surface antigens CD3 (clone # OKT3), CD4 (clone # RPA-T4), CD45RO (clone # UCHL1), CCR9 (clone # L053E8), GPR15 (clone # SA302A10), αE (clone # Ber-ACT8) all from Biolegend, San Diego, CA and to CD8 (clone # 1G1), CD127 (clone # 11A9), CD25 (clone # M-A251), Integrin β7 (clone # FIB504) and α4 (clone # HIL-7R-M21) from BD Biosciences Systems, San José, CA. Expression of intracellular cytokines was assessed following cell stimulation with eBiosciences cell stimulation cocktail 500X plus protein transport inhibitors at 37°C, 5% CO2 for 4 hours. Intracellular staining was conducted following cell fixation and permeabilisation with Cytofix/Cytoperm (BD Biosciences, San José, CA) and incubation with antibodies to human IFN-γ (clone # 4S.B3), TNF-α (clone # MAB11) and IL-17 (clone # BL168) all from eBioscience, San Diego, CA. Cell events were acquired on a BD LSRFortessa II and analysed using FlowJo 3 software (version 10, TreeStar, Ashland, OR). Positively stained cell populations were gated based on fluorescence-minus-one method. Compensation was adjusted using Ultra Comp eBeads™ Plus Compensation Beads (Invitrogen, Carlsbad, CA).
Statistics
Results are expressed as mean + SEM. Normality of variable distribution was assessed by Kolmogorov-Smirnov goodness-of-fit test. Comparisons were performed using parametric (paired or unpaired Student t test) or non-parametric (Wilcoxon signed-rank or Mann-Whitney test) tests according to data distribution. One-way ANOVA or Kruskal-Wallis test, followed by Tukey or Dunn multiple comparison tests, were used when comparing more than two sets of data. Statistical significance was defined as a p value <0.05 based on nominal p-values, and different levels were noted on graphs: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Correlation between variables was determined by Pearson correlation coefficient, if the data conformed to a normal distribution, or by Spearman rank correlation coefficient, if the data were not normally distributed. P values < 0.05 were considered significant. Statistical analysis was performed using GraphPad Prism, version 7.0a (GraphPad Software, San Diego, CA).
Results
Expression of gut adhesion molecules are elevated in the chronically inflamed liver
MAdCAM-1
Immunohistochemical detection of MAdCAM-1 in human liver samples revealed a variable pattern of expression. Positive MAdCAM-1 immunostaining was found on 6/8 PSC-IBD explanted liver sections but in none of the normal livers (0/4). Marked MAdCAM-1 expression, however, was also detected in 16/20 patients with other CLDs (Table 1). For a significant proportion (19/28) of the MAdCAM-1 positive explanted livers, MAdCAM-1 was detected on the endothelial cell lining of central veins and small vessels, though not all vessels in a given tissue section stained positive, indicating a patchy distribution of the MAdCAM-1 protein (Figure 1).
Table 1.
MAdCAM-1 staining in chronic liver disease.
| Disease | Endothelial vessels | Lymphoid aggregates | No staining |
|---|---|---|---|
| PSC-IBD (n=8) | 6/8 | 0/8 | 2/8 |
| PBC (n=2) | 1/2 | 2/2 | 0/2 |
| ALD (n=8) | 6/8 | 0/8 | 2/8 |
| NASH/NAFLD (n=6) | 4/6 | 0/6 | 2/6 |
| AIH (n=2) | 1/2 | 0/2 | 1/2 |
| HCV (n=2) | 2/2 | 2/2 | 0/2 |
| NL (n=4) | 0/4 | 0/4 | 4/4 |
PSC-IBD, primary sclerosing cholangitis with associated inflammatory bowel disease; PBC, primary biliary cholangitis; ALD, alcoholic liver disease; NASH, non-alcoholic steatohepatitis; NAFLD, non-alcoholic fatty liver disease; HCV, hepatitis C virus; NL, normal liver
Figure 1. Representative MAdCAM-1 immunoreactivity patterns in patients with chronic liver disease explants.
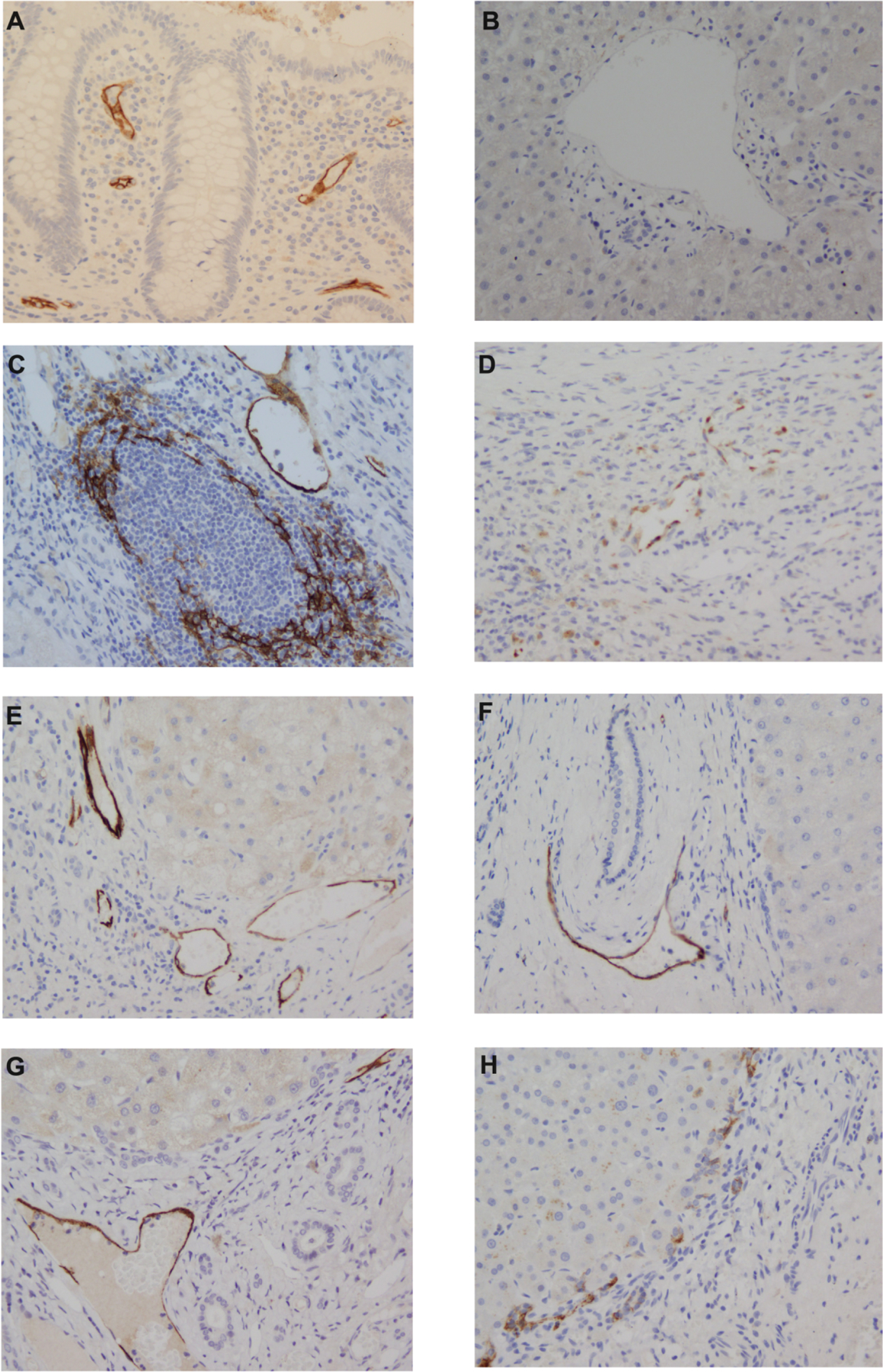
[A] Colon control tissue; [B] Normal liver [C] chronic hepatitis C; [D] primary sclerosing cholangitis; [E] alcoholic liver disease; [F] primary biliary cholangitis; [G] non-alcoholic steatohepatitis; [H] Autoimmune hepatitis). Vessel MAdCAM-1 staining is present in panels C-G; lymphoid aggregate MAdCAM-1 staining in panel C (HCV patient. Similar patterns were also observed in PBC sections). No staining is present in panel A.
Occasionally, MAdCAM-1 expression was seen within the hepatocyte cytoplasm, as well as in structures that had the morphology of dendritic cells. RT-PCR confirmed the histological findings, as MAdCAM-1 gene expression was significantly upregulated in CLD compared with normal liver tissue, the highest expression being found in PSC-IBD and ALD tissue (Figure 2A). Despite observing MAdCAM-1 positive immunoreactivity in multiple explanted CLD tissue sections, MAdCAM-1 staining was absent in all diagnostic liver biopsies (PSC-IBD 0/6, AIH 0/5 and chronic viral hepatitis (0/5) analyzed (Supplementary material figure 1).
Figure 2. E-Cadherin immunoreactivity patterns in control colon tissue, donor liver and in chronic liver disease explants.
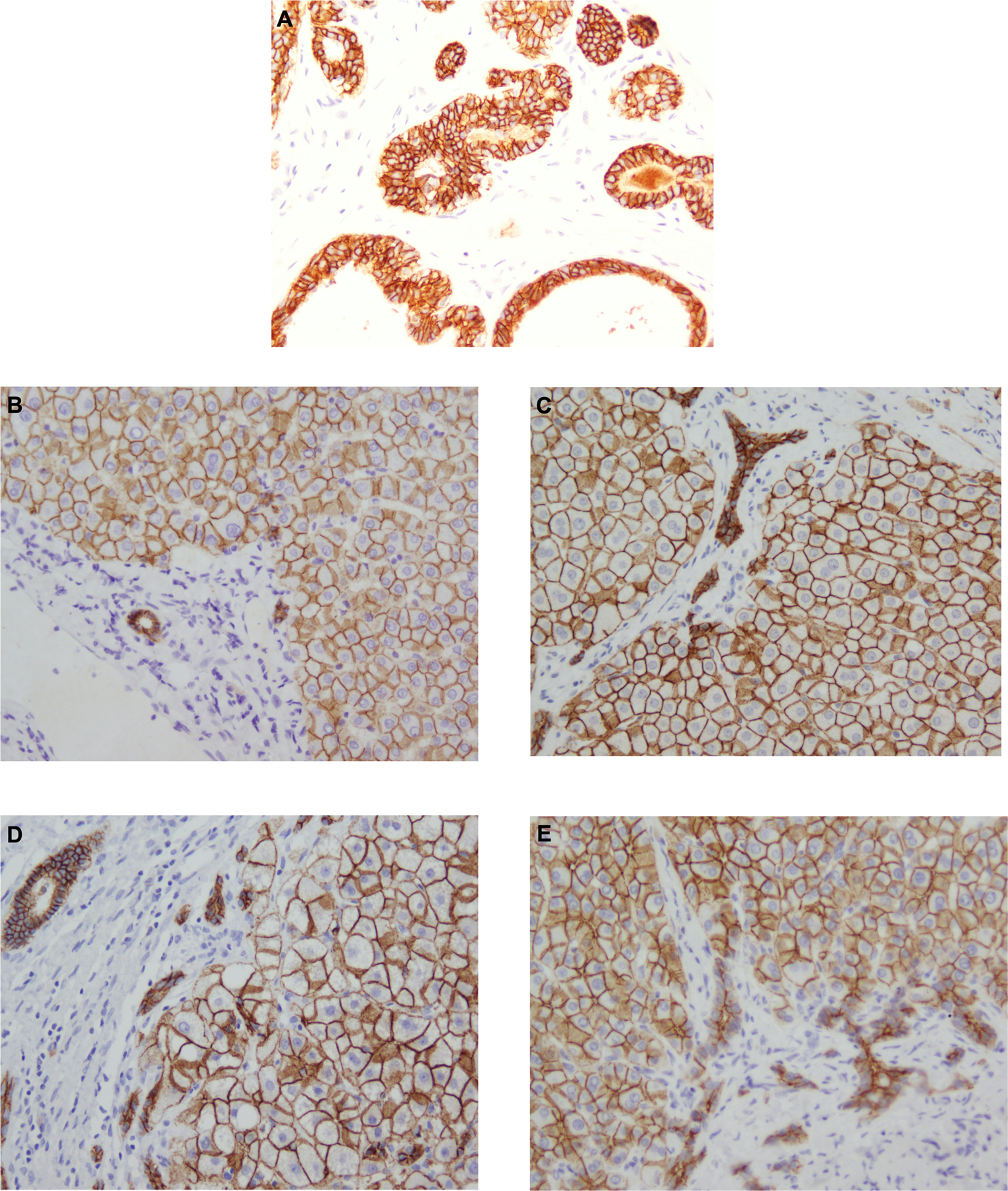
[A] colon, [B] normal liver [C] primary biliary cholangitis, [D] alcoholic liver disease; [E] primary sclerosing cholangitis. Positive immunostaining with strong membranous expression is present on hepatocytes and cholangiocytes in all sections. The pattern and intensity of expression was consistent throughout all chronic liver diseases and normal liver sections.
CCL25
On immunohistochemical staining, CCL25 immunoreactivity was negative in all PSC (n=7) and all PBC (n=2) liver tissue sections stained (Supplementary material figure 2.). Despite being unable to detect histological expression of CCL25, RT-PCR demonstrated significant upregulation in gene expression in explanted CLD tissue compared with normal tissue (Figure 3D). Similarly, to MAdCAM-1, CCL25 gene expression was highest in PSC-IBD and ALD patients (Figure 3B).
Figure 3. Expression of gut adhesion molecules is upregulated in chronic liver disease.
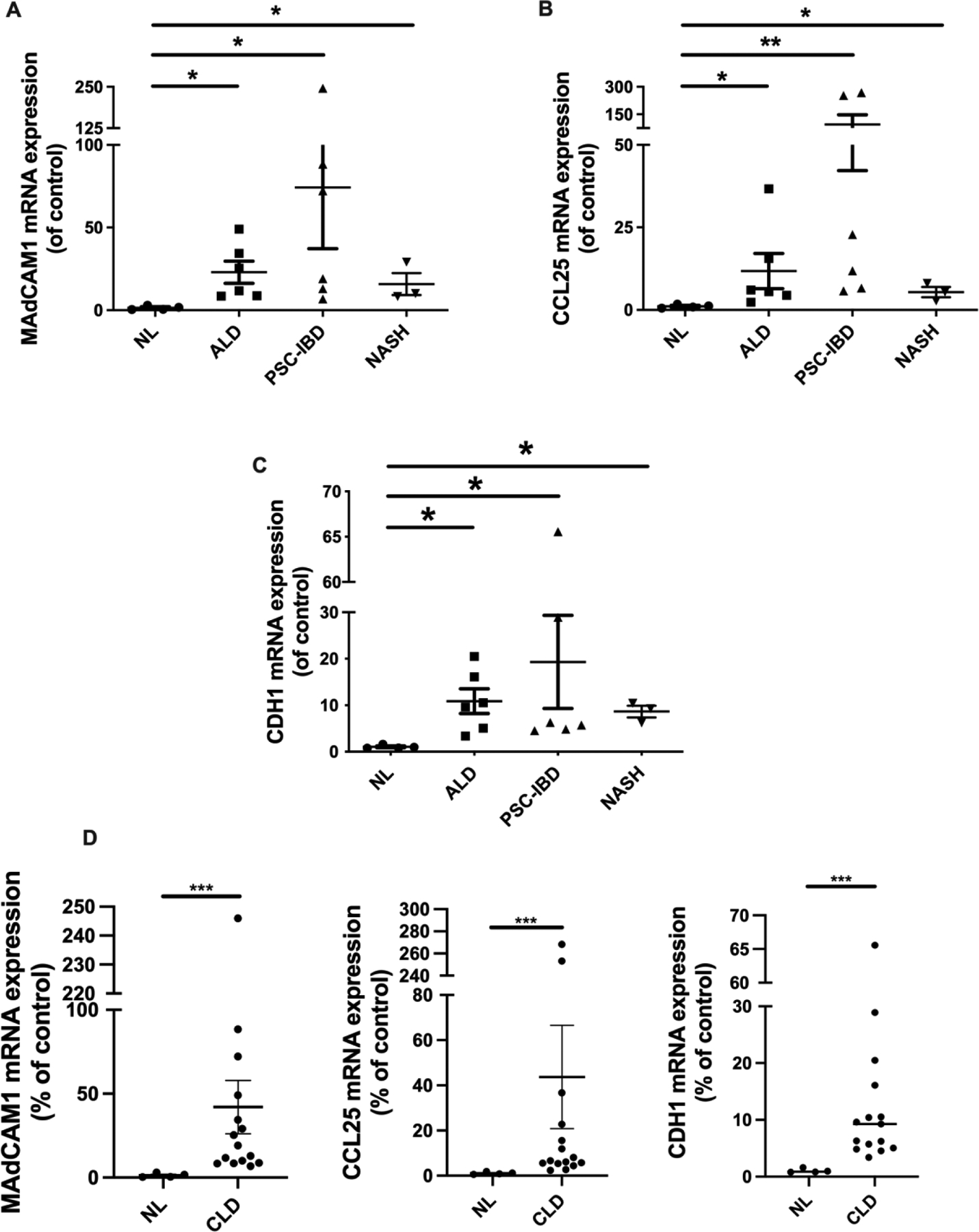
(A) MAdCAM-1, (B) CCL25 and (C) CDH1 (E-Cadherin) mRNA expression in normal liver, alcoholic liver disease (ALD), primary sclerosing cholangitis with inflammatory bowel disease (PSC-IBD) and non-alcoholic steatohepatitis (NASH). (D) MAdCAM-1 (left), CCL25 (centre) and CDH1 (right) mRNA expression in normal liver compared with combined chronic liver disease (CLD) groups (ALD, PSD-IBD and NASH). Results are expressed as Mean ± SEM: Expression is presented as a percentage deviation from the normal liver. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
E-Cadherin
Analysis of liver sections of explanted liver tissue stained for E-cadherin revealed strong immunoreactivity on all cases, with expression confined to hepatocyte and biliary epithelial cell membranes (Figure 2). We did not observe any difference in the staining intensity or pattern between disease groups, nor was there any observed between explanted liver sections and normal liver. Consistent with our histological findings, RT-PCR showed that CDH1 is highly expressed within the liver in CLD (Figure 3D), particularly in PSC-IBD patients (Figure 3C).
Gut integrin expression is increased on hepatic T-cells
Significant increases in the frequency of α4β7+ expressed on hepatic CD4+ T effector memory cells were observed in PSC-IBD, but also in NASH and ALD patients compared with normal liver (p<0.001, Figure 4B) with no differences among CLD groups. We also observed a significant reduction in peripheral α4β7 expressing CD4+ and CD8+ memory T-cells in patients undergoing OLT for CLD compared with healthy controls (Figure 4B, p<0.01). Despite observing significant increases in hepatic CD4+ T effector memory cells expressing α4β7 in CLD patients compared to healthy controls, there were no differences in CD8+ memory T-cells between normal liver and CLD (Figure 5A). In contrast to the findings on hepatic α4β7 expression, we observed significant increases in αEβ7 expression on CD8+ memory T-cells (Figure 5B, p<0.01), but not CD4+ T effector memory cells, in PSC-IBD and ALD patients when compared with normal liver. On both CD4+ and CD8+ T-cells there was a significantly increased expression of CCR9 in PSC-IBD patients when compared to normal liver (Figure 4D and 5C, p<0.01). Although not statistically significant, CCR9 expression on CD4+ and CD8+ T-cells was also elevated both in NASH and ALD with comparable levels to PSC-IBD patients. Peripheral T-cells showed significant reductions in CCR9 expression on CD4+ and CD8+ T-cells in patients undergoing OLT for CLD compared with healthy controls (Figure 4C, p<0.01).
Figure 4: Differential expression of gut homing integrins on hepatic and peripheral CD4+ T-cells in chronic liver disease.
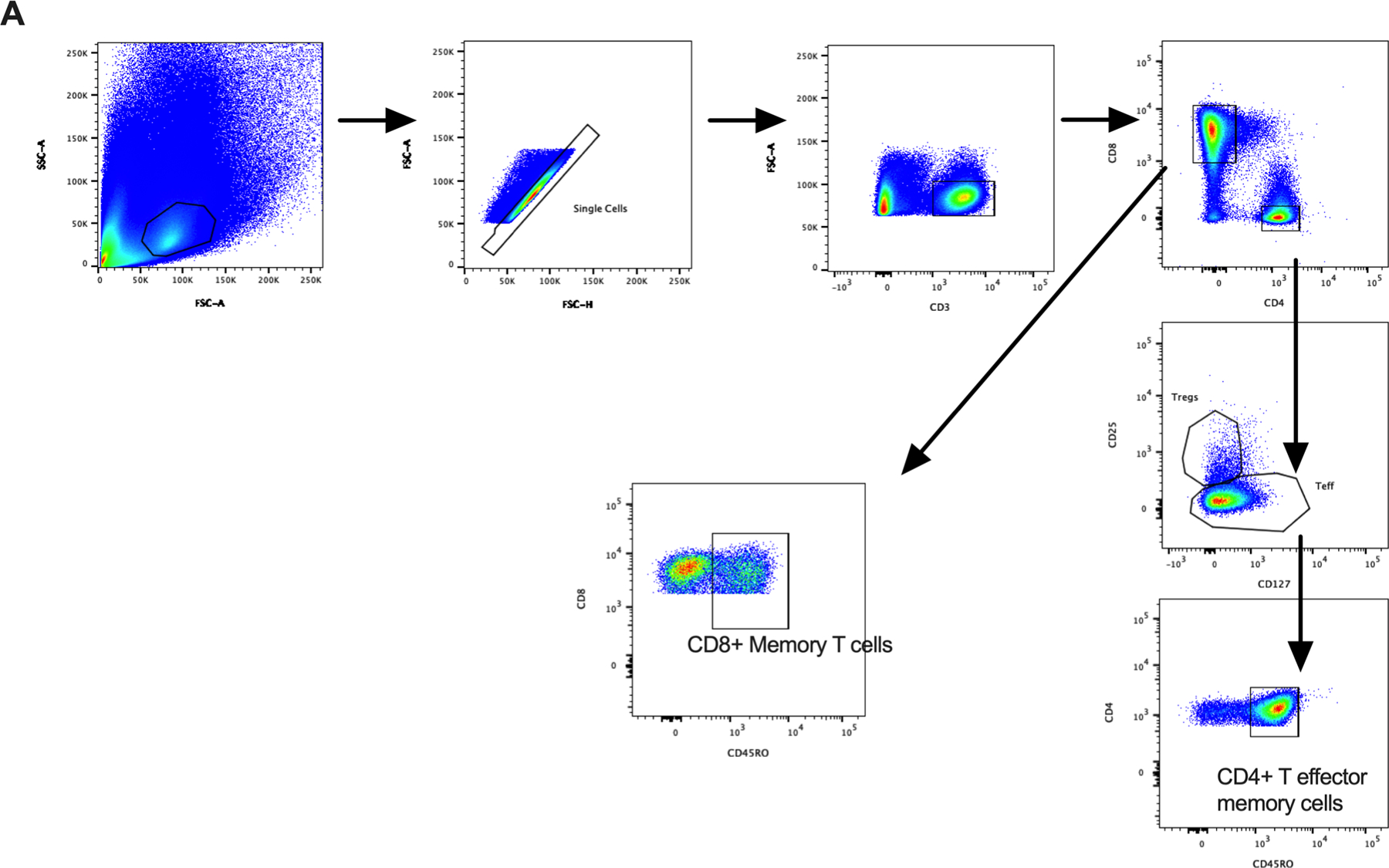
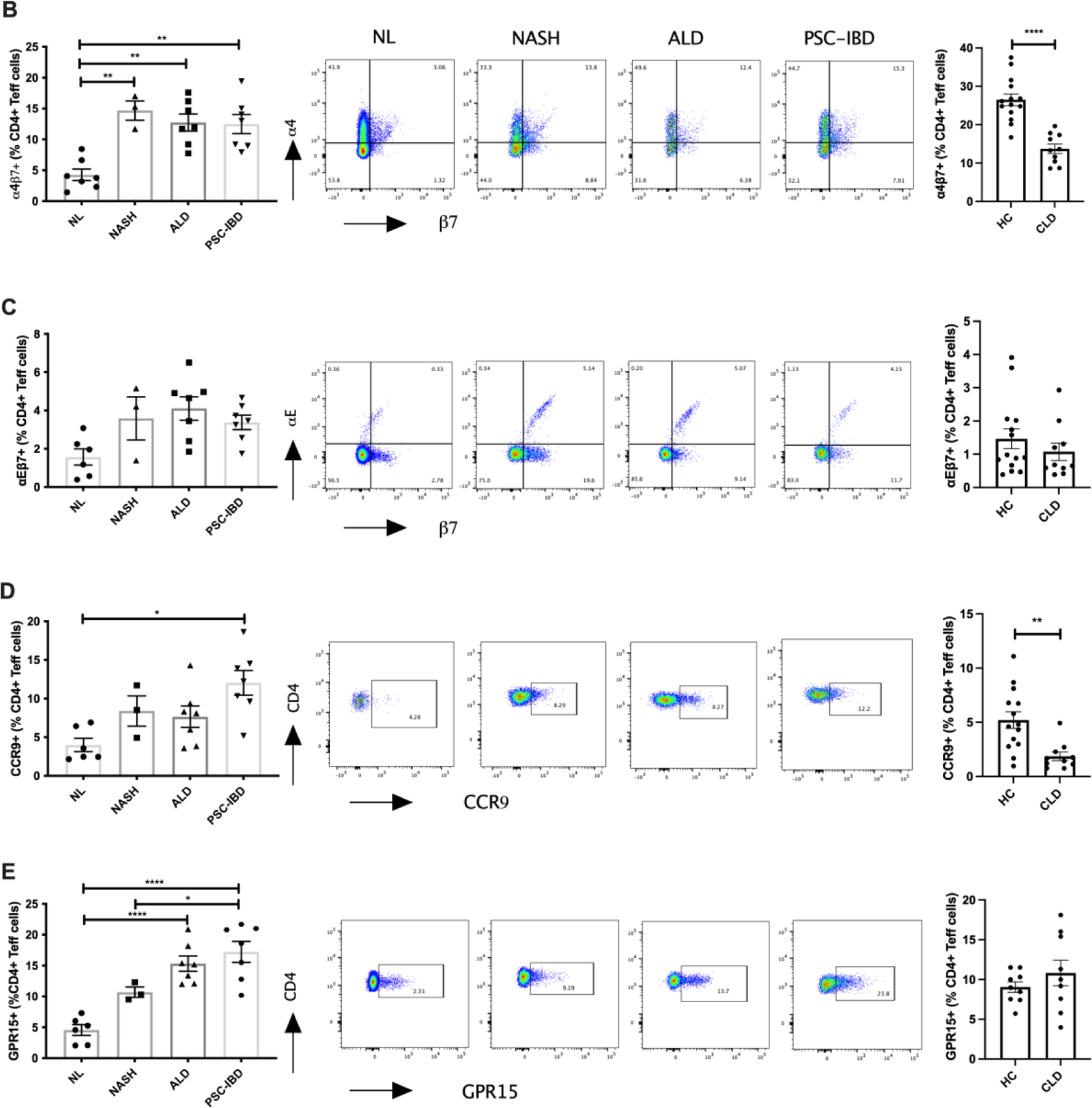
(A) Flow cytometry gating strategy for CD4+ T effector memory and CD8+ memory T-cells. Left panels: expression of gut homing integrins: (B) α4β7, (C) αEβ7, (D) CCR9 and (E) GPR15 on hepatic CD4+ T-cells (numbers expressed as a percentage of CD4+ T effector memory cells). Centre panels: Representative flow cytometry plots of gut homing integrins on hepatic CD4+ T-cells in normal liver (NL), non-alcoholic steatohepatitis (NASH), alcoholic liver disease (ALD) and primary sclerosing cholangitis with inflammatory bowel disease (PSC-IBD) (B-E). Right Panels (B-E): Differential expression of gut homing integrins on peripheral CD4+ T effector memory cells in NL and patients with chronic liver disease (CLD). Integrin positive cells were gated on and expressed as a percentage of CD3+CD4+CD127+CD45RO+ cells (T effector memory cells). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 001
Figure 5: Differential expression of gut homing integrins on hepatic and peripheral CD8+ T-cells in chronic liver disease.
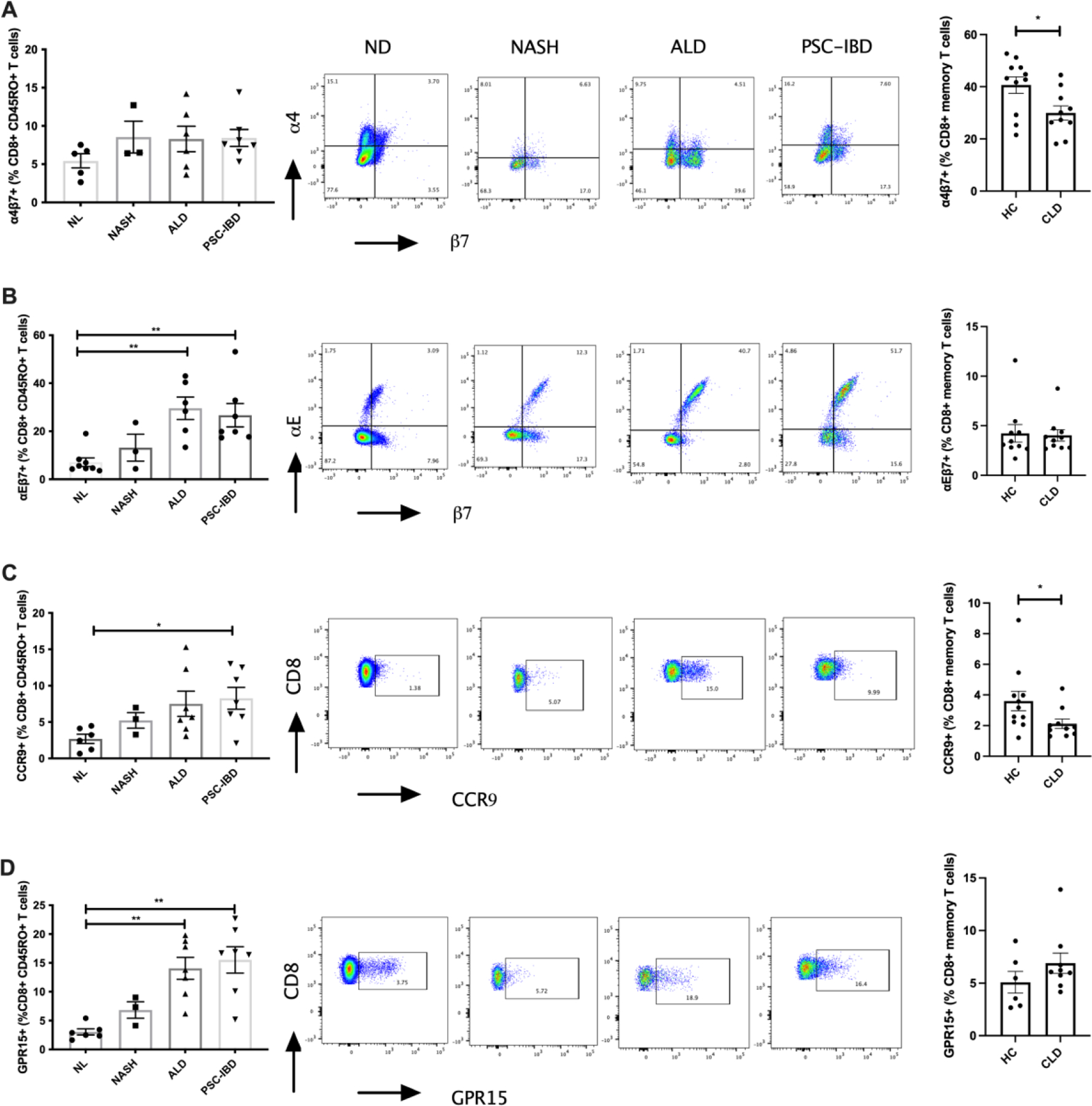
Left panels: expression of gut homing integrins: (A) α4β7, (B) αEβ7, (C) CCR9 and (D) GPR15 on hepatic CD8+ T-cells (numbers expressed as a percentage of CD8+ memory T-cells). Centre panels: Representative flow cytometry plots of gut homing integrins and on hepatic CD8+ T-cells in normal liver (NL), non-alcoholic steatohepatitis (NASH), alcoholic liver disease (ALD) and primary sclerosing cholangitis with inflammatory bowel disease (PSC-IBD). Cells were gated on CD3+CD8+CD45RO+ cells (memory T-cells). Right Panels (B-E): Differential expression of gut homing integrins on peripheral CD8+ memory T-cells in HC and CLD patients. *P ≤ 0.05
Like for CCR9 and β7 integrin, the expression of GPR15 was increased on hepatic T-cells in CLD. GPR15+ T-cells were found within the hepatic infiltrate with level of expression comparable between CD4+ and CD8+ T-cells for all disease groups and normal liver tissue (Figure 4E and 5D, p<0.01 p<0.0001). In ALD and PSC-IBD patients there was significantly increased GPR15 expression on both CD4+ and CD8+ T-cells compared with normal liver tissue. The level of expression was significantly greater in PSC-IBD patients on CD4+ T-cells when compared to NASH. Although, not significant, we also observed a trend towards increased expression on CD4+ and CD8+ T-cells in NASH patients compared with normal liver.
Hepatic infiltrating β7 expressing T-cells display an increased pro-inflammatory phenotype
We observed increased expression of TNF-α, IFN-γ, IL-17 and IFN-γ/IL-17 in hepatic memory CD4+ and CD8+ β7+ T-cells when compared with hepatic β7- T-cells (Figure 6A–D). This increased inflammatory phenotype was more marked within CD4+ T-cells with significant increases observed in IFN-γ, IL-17 and IFN-γ/IL-17 expression in β7+ versus β7- T-cells (Figure 6C–D). Despite the presence of IBD and cirrhosis, there were no differences in the inflammatory phenotype of CD4+ or CD8+ T-cells between PSC-IBD and ALD when compared to normal liver.
Figure 6: Gut-derived hepatic infiltrating memory T-cells are associated with increased Th1 and Th17 cytokine production.
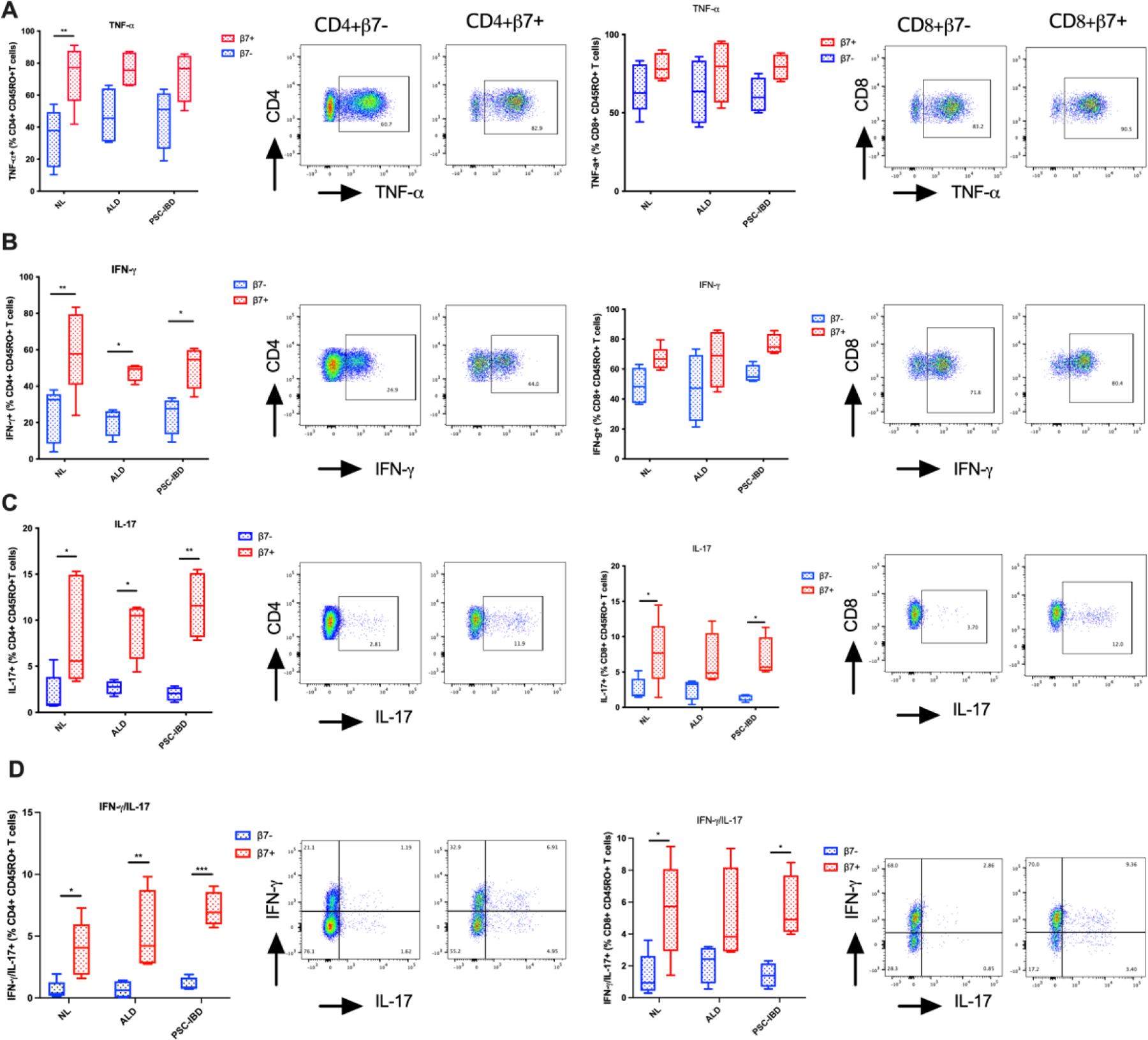
Expression of pro-inflammatory cytokines TNF-α (A), IFN-γ (B), IL-17 (C) and dual IFN-γ/IL-17 (D) and representative flow cytometry analyses between gut associated CD4+CD45RO+β7- and CD4+CD45RO+β7+ hepatic T-cells (Left Panels) and CD8+CD45RO+β7- and CD8+CD45RO+β7+ hepatic T-cells (Right Panels). Cytokines are expressed as a percentage of CD4+/CD8+CD45RO+β7-/β7+ hepatic T-cells. (*p<0.05; **p<0.01; ***p<0.001)
Discussion
Our data, demonstrating that expression of both MAdCAM-1 and CCL25 is upregulated not only in the liver of patients with PSC-IBD, but also in the liver of those with other CLDs, challenge the long-standing hypothesis formulated by Adams’ group that the pathogenesis of PSC-IBD is mediated by enterohepatic T-cell trafficking from the gut to the liver (4–6,16). In the original report, it had been postulated that an aberrant upregulation in hepatic expression of the gut specific adhesion molecules MAdCAM-1 and CCL25 was a feature largely confined to PSC-IBD patients, supporting the recruitment of pathogenic mucosal T-cells.
Early studies did report a strong expression of MAdCAM-1 and CCL25 in the PSC liver with minimal expression in other CLDs, lending support to the gut homing theory in the pathogenesis of PSC (4,5), but more recent reports challenged this notion, as hepatic MAdCAM-1 expression was observed in multiple CLDs (2,7,17). Consistent with the latter data are our findings in the present study, which is the first to investigate hepatic expression of both MAdCAM-1 and CCL25 and quantify expression of their cognate receptors CCR9 and α4β7, in addition to other colonic homing receptors αEβ7 and GPR15, on matched blood and tissue infiltrate in a sizeable group of patients with CLDs, including PSC-IBD. Not only do we describe positive hepatic MAdCAM-1 immunoreactivity in more than 75% of CLD patients regardless of aetiology, but we also show an increased expression of both CCL25 and MAdCAM-1 mRNA across all CLD groups compared to normal liver. In addition, by investigating paired tissue and blood samples, we show that expression of hepatic MAdCAM-1 and CCL25 induces tissue infiltration of α4β7 and CCR9 expressing CD4+ T-cells, which is not confined to PSC-IBD, but is present also in other CLDs, with a concomitant reduction in peripheral frequencies.
The importance of investigating not only patients with PSC, but also with other liver diseases at comparable stages of disease is illustrated by the discrepant reports in the literature. Henriksen et al. reported memory T-cells of common clonal origin in the gut and liver tissue of PSC patients, seemingly supporting the gut homing theory (16), but no other CLDs were investigated as controls, questioning the PSC specificity of their finding. More recently, a study did show elevations in hepatic MAdCAM-1 expression in PSC-IBD patients referred for liver transplantation compared with controls (PBC and HCV) (18). However, control samples were biopsies not taken at a comparable stage of disease being either diagnostic or resected samples, while comparisons between ‘short-term’ PSC, defined as disease duration of less than one year revealed no differences in MAdCAM-1 expression indicating the stage of disease is a strong determinate of expression. Supporting this notion, upon analysis of diagnostic liver biopsies we failed to identify MAdCAM-1 immunoreactivity in PSC-IBD and other CLDs, indicating aberrant hepatic MAdCAM-1 expression likely occurs in the later stages of CLD thereby, does not mediate hepatic inflammation via gut lymphocyte recruitment in the early stages of disease. In further agreement with our data, expression of MAdCAM-1 and α4 and β7 integrins were shown to be upregulated in the liver of patients with late-stage NASH (9); moreover hyperexpression of α4, αE and β7 integrins was reported in the liver of patients with severe alcoholic hepatitis compared to normal tissue (8). A recent report also showed no differences between PSC and other liver disease controls in CCR9 or β7 integrin gene expression in hepatic lymphocytes isolated by fine needle aspirate or in the expression of CCR9 on CD3+ cells in liver needle biopsies (19), in line with our observation that MAdCAM-1/α4β7 and CCL25/CCR9 mediated hepatic recruitment of T-cells is not unique to PSC-IBD.
To further understand whether gut-derived T-cell homing to the liver is a common feature of CLD, we investigated the hepatic expression of the homing receptor GPR15 and of αEβ7 integrin, which control trafficking and retention of T effector cells to the colon (20,21). As IBD in PSC patients is characterised predominantly by colonic inflammation, it is plausible to speculate that the hepatic PSC infiltrate should be rich in GPR15 and αEβ7 expressing lymphocytes, should the gut-homing theory prove true. Indeed, frequencies of both GPR15 and αEβ7 expressing T-cells were increased in the PSC-IBD liver compared to normal liver, but as for CCR9 and α4β7 expressing T-cells, elevations were also observed in other CLDs. In particular, the frequency of αEβ7 expressing CD8+ T-cells was high across disease groups, possibly because of the increased CDH1 gene expression observed by RT-PCR. An alternative possibility that should be addressed in future studies, is the role of transforming growth factor-beta (TGF-β), which participates in all stages of liver disease progression, from initial injury through inflammation and fibrosis, to cirrhosis and cancer (22), in downregulating α4 integrin and upregulating αE integrin, CD8+ T-cells being particularly responsive to TGF-β-mediated αE induction (23,24). There are no published data on GPR15, since, to our knowledge, this is the first study investigating its expression within the liver. However, a limitation of this work is the absence of data concerning the expression of its cognate receptor GPR15L, whose expression is reported to be absent or very weak in the human liver (25,26). It could be suggested that GPR15+ cells infiltrating the liver may have been recruited through α4β7/MAdCAM-1 interactions, since GPR15 cells enriched in the colon may also express α4β7. However, we found that <20% of GPR15+ T-cells expressed α4β7 (data not shown), suggesting that GPR15+ T-cells are likely to be recruited to the liver by hepatic expressed GPR15L, which similarly to other adhesion molecules, is upregulated in the liver in response to inflammation (7,27–29). The dense infiltration of both GPR15+ CD4+ and CD8+ T-cells we observed in the cirrhotic tissue suggests that GPR15L is indeed expressed within the liver, but the distribution and functional role of hepatic GPR15/GPR15L interactions in CLD require further investigation.
Expression of αEβ7 has been shown to characterise a more pro-inflammatory subset of CD4+ T-cells within the inflamed UC colon (20). It is thereby possible that the hepatic β7 expressing T-cells derived from an inflamed bowel, as is the case in PSC-IBD patients, could display a similarly enhanced inflammatory phenotype. We, therefore, investigated the inflammatory profile of hepatic β7+ versus β7- T-cells in CLD with IBD (PSC-IBD), without IBD (ALD) and normal liver. In all conditions, expression of β7 was associated with increased levels of Th1 (IFN-γ, TNF-α) and Th17 (IL-17) cytokines in both CD4+ and CD8+ memory T-cells compared with β7- T-cells, but the presence of IBD or CLD did not influence type and density of cytokine expression in hepatic β7+ T-cells, with normal liver displaying a cytokine profile similar to PSC-IBD and ALD patients. This, however, does not exclude an increased tissue inflammatory environment in CLD patients with or without concomitant IBD compared to health, as we have not quantified the relative abundance of β7 expressing T-cells within the different CLD livers. It is possible that the increased expression of MAdCAM-1 in the diseased liver is accompanied by the recruitment of a high number of α4β7+ T-cells and therefore enhanced inflammation. An increase in hepatic α4 and β7 integrin gene expression, has indeed been reported in both ALD and NASH (8,17,19), and increases in intestinal MAdCAM-1 expression are associated with infiltration of β7+ T-cells in the gut of IBD patients (20,24,30). Whether hepatic MAdCAM-1 mediated recruitment of inflammatory β7 expressing T-cells contributes to fibrogenesis and disease progression in CLD remains to be defined. In this context it is of interest that in murine models of both NASH and concanavalin A induced hepatitis, MAdCAM-1 ablation resulted in reduced oxidative stress and inflammation (31,32), and that in the NASH model, blockade of α4β7 attenuated hepatic inflammation and fibrosis (17), implicating a pathogenic role for hepatic MAdCAM-1/α4β7 mediated cell recruitment in CLD. Further understanding of the mechanisms and in particular, identification of the time of hepatic MAdCAM-1 induction throughout the course of CLD could pave the way for the use of targeted anti-integrin therapies, as used in IBD, with the aim of blocking hepatic MAdCAM-1/α4β7 mediated cell recruitment and inhibiting the progression of CLD.
In summary, we have demonstrated that aberrant hepatic expression of MAdCAM-1 and CCL25 and subsequent infiltration of α4β7 and CCR9 expressing T-cells into the liver is a common feature of CLD, questioning previous reports suggesting that this phenomenon is confined to PSC-IBD. The functional relevance of gut-derived hepatic infiltrating T-cells displaying an inflammatory phenotype and their role in the progression of liver damage in CLD with or without IBD remains unknown and requires further investigation.
Supplementary Material
Grant support:
This work has been supported by the King’s College Hospital Alex Mowat Charity and PSC Partners.
Footnotes
Conflict of Interest: None
References
- 1.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004; [DOI] [PubMed] [Google Scholar]
- 2.Hillan KJ, Hagler KE, MacSween RN, Ryan AM, Renz ME, Chiu HH, et al. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver [Internet]. 1999. Dec [cited 2019 May 15];19(6):509–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10661685 [DOI] [PubMed] [Google Scholar]
- 3.Ponsioen CY, Kuiper H, Ten Kate FJ, Van Milligen De Wit M, Van Deventer SJ, Tytgat GN. Immunohistochemical analysis of inflammation in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 1999; [DOI] [PubMed] [Google Scholar]
- 4.Grant A, Lalor PF, Hübscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology [Internet]. 2001. May [cited 2019 May 15];33(5):1065–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11343233 [DOI] [PubMed] [Google Scholar]
- 5.Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hübscher SG, et al. Hepatic Endothelial CCL25 Mediates the Recruitment of CCR9 + Gut-homing Lymphocytes to the Liver in Primary Sclerosing Cholangitis. J Exp Med [Internet]. 2004. Dec 6 [cited 2019 May 15];200(11):1511–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15557349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee-Turner L, Villablanca EJ, et al. Gut Homing Receptors on CD8 T Cells Are Retinoic Acid Dependent and Not Maintained by Liver Dendritic or Stellate Cells. Gastroenterology [Internet]. 2009;137(1):320–9. Available from: 10.1053/j.gastro.2009.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ala A, Brown D, Khan K, Standish R, Odin JA, Fiel MI, et al. Mucosal Addressin Cell Adhesion Molecule (MAdCAM-1) Expression Is Upregulated in the Cirrhotic Liver and Immunolocalises to the Peribiliary Plexus and Lymphoid Aggregates. Dig Dis Sci [Internet]. 2013. Sep 10 [cited 2019 May 15];58(9):2528–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23839340 [DOI] [PubMed] [Google Scholar]
- 8.Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen B, Micic D, Gibson PR, Yarur A, Bellaguarda E, Corsello P, et al. Vedolizumab in patients with concurrent primary sclerosing cholangitis and inflammatory bowel disease does not improve liver biochemistry but is safe and effective for the bowel disease. Aliment Pharmacol Ther. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse CS, Loftus EV, Raffals LE, Gossard AA, Lightner AL. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2018; [DOI] [PubMed] [Google Scholar]
- 12.Arijs I, De Hertogh G, Lemmens B, Van Lommel L, de Bruyn M, Vanhove W, et al. Effect of vedolizumab (anti-α4β7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut [Internet]. 2018. Jan [cited 2019 May 15];67(1):43–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27802155 [DOI] [PubMed] [Google Scholar]
- 13.Pavlidis P, Graham J, Gulati S, Dubois P, Heneghan M, Joshi D, et al. Letter: vedolizumab for autoimmune liver disease associated inflammatory bowel disease. Alimentary Pharmacology and Therapeutics. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Liberal R, Grant CR, Yuksel M, Graham J, Kalbasi A, Ma Y, et al. Regulatory T-cell conditioning endows activated effector T cells with suppressor function in autoimmune hepatitis/autoimmune sclerosing cholangitis. Hepatology. 2017;66(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liaskou E, Jeffery LE, Trivedi PJ, Reynolds GM, Suresh S, Bruns T, et al. Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriksen EKK, Jørgensen KK, Kaveh F, Holm K, Hamm D, Olweus J, et al. Gut and liver T-cells of common clonal origin in primary sclerosing cholangitis-inflammatory bowel disease. J Hepatol. 2017; [DOI] [PubMed] [Google Scholar]
- 17.Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Krijger M, Visseren T, Wildenberg ME, Hooijer GKJ, Verstegen MMA, van der Laan LJW, et al. Characterization of gut-homing molecules in non-endstage livers of patients with primary sclerosing cholangitis and inflammatory bowel disease. J Transl Autoimmun. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch K, Curion F, Yeung H-Y, Fryer E, Slater A, Ferry H, et al. PS-127-Analysis of liver infiltrating lymphocytes in primary sclerosing cholangitis by surface antigen and single cell RNAseq. J Hepatol. 2019; [Google Scholar]
- 20.Lamb CA, Mansfield JC, Tew GW, Gibbons D, Long AK, Irving P, et al. αEβ7 Integrin Identifies Subsets of Pro-Inflammatory Colonic CD4+ T Lymphocytes in Ulcerative Colitis. J Crohns Colitis [Internet]. 2017;11(5):610–20. Available from: https://academic.oup.com/ecco-jcc/article-lookup/doi/10.1093/ecco-jcc/jjw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen LP, Pan J, Dinh TT, Hadeiba H, O’hara E, Ebtikar A, et al. Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat Immunol. 2015;16(2):207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, et al. TGF-β signalling and liver disease. FEBS J. 2016; [DOI] [PubMed] [Google Scholar]
- 23.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. J Immunol. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zundler S, Schillinger D, Fischer A, Atreya R, López-Posadas R, Watson A, et al. Blockade of αeβ7 integrin suppresses accumulation of CD8 + and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut. 2017; [DOI] [PubMed] [Google Scholar]
- 25.Suply T, Hannedouche S, Carte N, Li J, Grosshans B, Schaefer M, et al. A natural ligand for the orphan receptor GPR15 modulates lymphocyte recruitment to epithelia. Sci Signal. 2017; [DOI] [PubMed] [Google Scholar]
- 26.Ocón B, Pan J, Dinh TT, Chen W, Ballet R, Bscheider M, et al. A mucosal and cutaneous chemokine ligand for the lymphocyte chemoattractant receptor GPR15. Front Immunol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular Adhesion Protein-1 Mediates Adhesion and Transmigration of Lymphocytes on Human Hepatic Endothelial Cells. J Immunol. 2002; [DOI] [PubMed] [Google Scholar]
- 28.Salmi S, Jalkanen DH, Adams PF, Lalor S, Edwards G, Mcnab M, et al. Cells Lymphocytes on Human Hepatic Endothelial Adhesion and Transmigration of Vascular Adhesion Protein-1 Mediates Vascular Adhesion Protein-1 Mediates Adhesion and Transmigration of Lymphocytes on Human Hepatic Endothelial Cells. J Immunol [Internet]. 2002. [cited 2017 Nov 28];169:983–92. Available from: http://www.jimmunol.org/content/169/2/983 [DOI] [PubMed] [Google Scholar]
- 29.Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LSK, et al. Epithelial Inflammation Is Associated with CCL28 Production and the Recruitment of Regulatory T Cells Expressing CCR10. J Immunol. 2006; [DOI] [PubMed] [Google Scholar]
- 30.Fischer A, Zundler S, Atreya R, Rath T, Voskens C, Hirschmann S, et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drescher HK, Schippers A, Clahsen T, Sahin H, Noels H, Hornef M, et al. β7-Integrin and MAdCAM-1 play opposing roles during the development of non-alcoholic steatohepatitis. J Hepatol. 2017; [DOI] [PubMed] [Google Scholar]
- 32.Schippers A, Hübel J, Heymann F, Clahsen T, Eswaran S, Schlepütz S, et al. MAdCAM-1/α4β7 Integrin-Mediated Lymphocyte/Endothelium Interactions Exacerbate Acute Immune-Mediated Hepatitis in Mice. Cell Mol Gastroenterol Hepatol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


