Abstract
Background.
Cystic fibrosis (CF)-specialized nutrition care strives to meet normal infant growth, but the relationship of dietitian assessments to weight outcomes is unknown. We characterize nutrition management for inadequate weight gain and assess association of dietitian assessments and center-level weight-for-age Z-scores (WAZ).
Methods.
We used encounter data from 226 infants across 28 US CF Centers from the Baby Observational Nutritional study between January, 2012 through December, 2017. We identified dietitian assessments and consensus guideline-recommended responses to inadequate weight gain: calorie increases, pancreatic enzyme replacement therapy (PERT) increases, or shortened time to next visit. We compared center assessments by funnel plot and summarize median WAZ by center.
Results.
Of 2,527 visits, 808 (32%) visits had identified inadequate weight gain, distributed in 216 infants. Assessments occurred in 1953 visits (77%), but varied widely between centers (range 17% - 98%). For inadequate weight gain, most and least common responses were calorie increase (64%) and PERT increase (21%). Funnel plot analysis identified 4 high-performers for frequent dietitian assessments (range 92% - 98%) and 4 under-performers (range 17% - 56%). High-performers treated inadequate weight gain more often with adequate calories (24/30, 80% v. 12/23, 52%) and closer follow up (104/164, 63% v. 60/120, 49%) compared to under-performers. Three of 4 high-performing sites met center nutrition goals for positive median WAZ at 2 years old unlike 3 under-performers (WAZHigh 0.33 v. WAZLow −0.15), despite similar patient characteristics.
Conclusion:
We characterized multicenter variation in dietitian assessments, identifying opportunities to improve care delivery to target early nutrition outcomes.
Keywords: Medical nutrition therapy, guideline adherence, newborn, pediatric, patient registry, weight surveillance
1.1. Introduction
Nutrition management for infants with cystic fibrosis (CF) is critical to optimize rapid growth (1–3). Importantly, children who met and maintained goal weight and growth indices in the first year of life had the highest lung function at ages 6–7 years (4). Early aggressive nutrition management may improve lifelong heath trajectories.
Evidence-based guidelines and expert consensus support standards for early nutrition management in CF including frequent assessments (1, 2, 5–7). Registered dietitians (RD) monitor nutrition measures and respond to inadequate gains. However, there is limited evidence for whether and how these assessments improve outcomes (7).
The Baby Observational Nutritional Study (BONUS) was a prospective, observational study of 226 infants with CF from 28 US CF Foundation-accredited centers (8). On average, the cohort weight at 12 months of age was similar to the general population, an overall improvement compared to historical controls who were not universally diagnosed by newborn screening. The cohort’s success in weight correction was thought to result from early access to interdisciplinary care and aggressive nutrition management as recommended by the CF Foundation (CFF) Evidence-Based Guidelines for Management of Infants with CF (1). BONUS presents an opportunity to assess a contemporary state of nutrition care delivery and use of CFF infant management guidelines.
The purpose of this study was to characterize guideline-recommended nutrition management in response to inadequate weight gain for infants with CF and assess the association of center-level weight-for age z score (WAZ) at 2 years of age and frequency of RD assessments. We determined frequency of RD assessments and response to visits with inadequate weight gain. Specific responses determined were for calorie intake changes, pancreatic enzyme replacement therapy (PERT) dose increases, and shortened time interval to the next clinic visit. We hypothesized that centers with consistent dietitian assessments would have higher median WAZ at 2 years of age.
1.2. Methods
1.2.1. Data Sources and Study Population
BONUS was a well-described prospective cohort of infants with CF enrolled before 3.5 months of age and followed for the first year of life (8). Study visits coincided with routine clinic visits per CFF infant care guidelines (1). At all visits, weight and length measurements were assessed by a trained and certified RD or designee with standard training for technical competence (9). Parents/legal guardians completed a questionnaire for 3 days at home prior to each study visit which included a diet diary to calculate a calorie count and PERT dose. The research coordinator or RD was expected to review the questionnaire with families at the subsequent visit.
Infants who participated in BONUS were also registered in the CFF Patient Registry (CFFPR). CFFPR is a robust registry with data entered across encounters by CF centers (10). We used CFFPR to capture longitudinal encounter data in the second year of life beyond the original study period of BONUS. Data were analyzed from CF clinic visits from January 1, 2012 through December 31, 2017, excluding any inpatient visits. The Seattle Children’s Hospital Institutional Review Board reviewed and approved this study.
1.2.2. Definition of Inadequate Weight Gain and Selection of Guideline-recommended Responses
CFF infant management guidelines present an algorithm to identify and respond to infants with inadequate weight gain (1). From this algorithm, we defined inadequate weight gain as grams per day that did not meet age and gender specified norms (1). Weight change was calculated as the difference in weight obtained at two consecutive visits over the days between visits.
Frequency of RD assessments were summarized as both part of routine care, that is any clinic visit regardless of weight gain status and in response to inadequate weight gain. Four interventions were defined as a response to inadequate weight gain: (a) assessment for adequate calorie intake for age, (b) advancement of caloric density of feedings, (c) changes to PERT dose to the higher end of dosing range, and (d) shortened interval to follow up CF clinic visit (Table 1). These response interventions were chosen a priori for analysis through review of guidelines and a survey of pediatric CF physicians from the CF Learning Network (1, 2, 11, 12) (eMethods in Supplement).
Table 1.
Practice definitions
| Practice Definition | Inclusion Criteria | Ages at visit included | Patients Eligible (No.) | Visits Eligible (No.) |
|---|---|---|---|---|
| Dietitian Assessment | ||||
| Patients documented as seen by a dietitian at or subsequent visit to inadequate weight gain | At least 1 inadequate weight gain visit | 0–24 months | 216 | 808 |
| Calorie Intake Assessment | ||||
| Calorie intake at visit subsequent to inadequate weight gain should be greater than minimum age-expected calories in formula-fed infants. | • Exclusively formula fed • Available calorie count at visit subsequent to inadequate weight gain |
0–12 months | 110 | 145 |
| Calorie Density Increase | ||||
| Formula calorie density should be reported as maintains or greater than at least 24 kcal/30 mL in subsequent visit to inadequate weight gain. | Formula calorie density in kcal/30 mL reported in CFFPR in visit subsequent to inadequate weight gain | 0–24 months | 101 | 161 |
| PERT Dose Increase | ||||
| PERT dose increases to greater than 300 units lipase/kg body weight compared to prior enzyme dose in subsequent visit to inadequate weight gain. | • Pancreatic insufficient (PI) as defined by fecal elastase (FE) <200 ug/g or 2 CFTR variants associated with pancreatic insufficiency • PERT dose recorded at visit subsequent to inadequate weight gain |
0–12 months | 152 | 270 |
| Follow up clinic visit timing | ||||
| Subsequent visit to inadequate weight gain seen sooner than routine follow up for agea: | At least 1 inadequate weight gain visit | 0–24 months | 216b | 808 |
| 0–6 months old <3.5 weeks (26 days) |
142 | 216 | ||
| 6–12 months old < 6 weeks (43 days) |
100 | 133 | ||
| 12–24 months old < 12 weeks (85 days) |
203 | 459 | ||
Current recommendations for routine follow-up: monthly for infants < 6 months of age; every 2 months for infants 6–12 months of age; every 3 months for children 12–24 months of age
Patients eligible at more than one age group for follow up
1.2.3. Visit analysis
BONUS was a one-year study. Longitudinal follow up for this cohort was written in the consent to be obtained through CFFPR. We chose a 24-month end point beyond the original study to use longitudinal CFFPR data when available to extend our understanding of nutrition care delivery and outcomes over the two years of life. Eligible patients and number of visits that met inclusion criteria varied per practice (Table 1). We determined frequency of guideline use as a proportion of eligible visits that met defined criteria. Measures for calorie intake assessment and PERT dosages were obtained from parent diet diaries and only available during the BONUS study period in the first year of life. Infants could contribute more than 1 visit to the analysis.
1.2.4. Center analysis
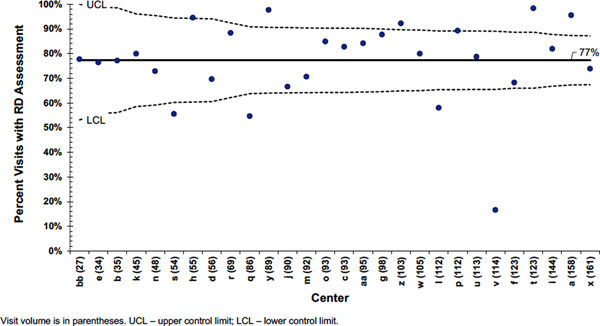
To assess the impact of RD assessments, we determined performance differences in assessment frequency across centers. We compared centers through funnel plot analysis based on statistical process control charts, specifically p-charts (13–16). Funnel plots determine where significant differences between center performance exist, taking into account differences in sample size (13–16). The center line represents the mean percentage of RD assessments for any clinic visit. Upper and lower control limits are defined as three sigma or approximately 99.8% confidence intervals (14, 15). Three performance groups for frequent RD assessments were determined: (a) high, exceeding the upper control limit (UCL), (b) low, below the lower control limit (LCL), and (c) mid, within control limits (16).
To identify patterns for medical nutrition therapy associated with frequent RD assessments, we determined the count and frequency of responses to inadequate weight gain visits across the three performance groups. To assess for an association of RD care delivery on center weight goal, we summarized the annualized median World Health Organization (WHO) WAZ at 24–27 months for patients at each center (17). An aggregate median of all the center-level WHO WAZ scores for each RD care delivery group is reported.
1.2.5. Patient analysis
Patient data were analyzed using descriptive statistics; medians and interquartile ranges (IQR) for continuous variables and counts and proportions for categorical variables. Patient characteristics across groups defined by levels of RD care delivery were compared using chi-square test for categorical variables and ANOVA or non-parametric equivalent for continuous variables. A two-sided p-value of <0.05 was considered statistically significant.
Analyses were performed using Stata v16 (Stat Corp Inc). Control charts were created using software package QI-charts V.2.0.22 (Process Improvement Products, USA) in Microsoft Excel Office 365 (Microsoft, USA).
1.3. Results
1.3.1. Study sample
Among infants who had at least one visit with inadequate weight gain in the first 2 years of life, approximately half were female, and the majority were Caucasian and had private insurance (eTable 1 in Supplement). Median age of diagnosis was 8 days old (IQR 2–18) and median birth weight was 3.2 kg (IQR 2.9–3.5) (eTable 1 in Supplement). The median number of total visits was 13 visits (1).
1.3.2. Inadequate weight gain visits
Overall, 2,527 visits were available to calculate average change in weight per day from the prior visit. During the first year of life,178 infants contributed at least 1 inadequate weight gain visit. Of 1385 visits during study participation in BONUS, inadequate weight gain was identified in 349 visits (25.2%). After BONUS participation completed, 459 visits of 1142 available visits (40.2%) in the second year of life had inadequate weight gain. In total, 216 infants contributed 808 (31.9%) visits that had inadequate weight gain. Over the first two years, 139 infants (64.4%) contributed 2 to 4 inadequate weight gain visits (eFigure 1 in Supplement). There were 125 infants who had two consecutive inadequate weight gain events.
1.3.3. RD assessments
Infants were commonly seen by an RD regardless of weight gain status. Among all 2527 visits, 1953 visits (77.3%) had an RD assessment at that visit or the subsequent visit. For 808 visits with identified inadequate weight gain, 720 visits (89.1%) had an RD assessment at or just subsequent to that visit (Table 2). In comparison, 1233 of 1719 normal weight visits (71.7%) had an RD assessment, suggesting some priority response for inadequate gain. RD assessments were the most frequent response to visits with inadequate weight gain, a pattern that minimally changed from year 1 to 2 (Table 2).
Table 2.
Proportion of practices for visits with inadequate weight gain over ages 1 and 2 years
| Guideline Practice | Proportion Eligible Visits Met Guideline 0–12 months No. (%) | Proportion Eligible Visits Met Guideline 12–24 months No. (%) | Total Proportion Eligible Visits Met Guideline No. (%) | |
|---|---|---|---|---|
| Dietitian Assessment | 316/349 (90.5%) | 404/459 (88.0%) | 720/808 (89.1%) | |
| Intake Above Minimum Calories for Age | 93/145 (64.1%) | --a | 93/145 (64.1%) | |
| Calorie Density Increased | 92/143 (64.3%) | 13/18 (72.2%) | 105/161 (65.2%) | |
| Pancreatic Enzyme Dose Increased | 57/270 (21.1%) | -- a | 57/270 (21.1%) | |
| Shortened Interval to Next Clinic Visit | <6 months | 6–12 months | 361/459 (78.7%) | 462/808 (57.2%) |
| 34/216 (15.7%) | 67/133 (50.4%) | |||
No calorie intake or pancreatic enzyme dose data available for year 2.
1.3.4. Calorie intake assessment practice
We limited analysis to exclusively formula-fed infants and to the first year of life, when these data were being collected at every visit as part of BONUS because total caloric intake could not be calculated for breast fed infants (110/178 infants, 62%). We identified 93 visits (64%) among formula-fed infants that had a caloric intake at or above the minimum calories per day expected for age (Table 2). Of the remaining 52 visits (36%) in which average daily calorie intake recorded were below minimum expected calories, all occurred in the first 6 months of age.
1.3.5. Calorie-dense formula use practice
In the first year of life, 41% visits (143/349) with inadequate weight gain had documentation of use of calorie-density in CFFPR. Documentation for calorie-dense formula was missing for most visits with inadequate weight in the second year of life (441/459 visits, 96%) (Table 2). Among the available 143 visits following a visit with inadequate weight gain in the first year only, formula calorie density was increased from or maintained at greater than 24 kcal per 30mL in 92 (64.1%) visits (Table 2).
1.3.6. PERT dose increase practice
We identified 152 infants with pancreatic insufficiency (PI) who contributed 270 inadequate weight gain encounters in first year of life (Table 1). PERT dose was increased by at least 300 lipase units/kg/dose subsequent to identified inadequate weight gain in 57 (21.1%) visits (Table 2). None of these visits were at maximum dose per meal or by day (1). The PERT dose was lower in 180 (66.7%) subsequent visits suggesting weight gain in the interval without an immediate prior change in enzyme dose. Of the 180 visits, 19 visits (10.6%) were already at maximum dose by meal or by day. Thirty-three visits (12.2%) were found to have a minimally increased dose between 0–300 lipase units/kg/dose suggesting dose differences from weight fluctuations and not a prescribed increase in lipase units by change in number of dosed capsules. In one of these 33 visits, the PERT dose was at the maximum meal or daily dose.
1.3.7. Follow up clinic visit timing
The median time interval between clinic visits met recommended routine visit time for all age groups (<6 months: 28 days (IQR 26–35); 6–12 months: 56 days (39–63); 12–24 months: 70 days (49–91). However, a visit after inadequate weight gain did not occur frequently in closer follow up for infants <6 months old (15.7%) when routine visits are recommended monthly (Table 2).
1.3.8. Center Comparison for RD Assessments and Guideline Practice Patterns
The average number of inadequate weight gain visits per center was 32% of visits (SD 8%). There was no specific pattern in the distribution of number of patients or inadequate weight gain visits across sites (eFigure 2 in the Supplement). That is, there was no tendency for a center with more patients to have proportionally more inadequate weight gain visits.
There was a wide range for RD assessments to occur at every visit across CF centers (range 16.7%–98.4%) (Figure 1). Four centers had a significantly lower performance for frequency of dietitian assessments, occurring at most for 58% of visits (Table 3). In contrast, 4 centers demonstrated high reliability to provide RD assessments at over 90% of all patient visits (Figure 1). Mid-performance centers on average provided RD assessments at 79% of visits.
Figure 1. Funnel plot for center comparison of dietitian (RD) assessments.
Table 3.
Weight-for-age Z (WAZ) score at 2 years by performance group for registered dietitian (RD) assessments
| Centers with High Performance for RD Assessments | No. enrolled patients | No. visits | No. (%) of visits with RD Assessment | Annualized Median WAZ 24–27 months (IQR)a | Overall Median WAZ for Performance Group |
|---|---|---|---|---|---|
| a | 11 | 158 | 151 (96%) | −0.2 (−0.8, 0.2) | 0.33 |
| t | 11 | 123 | 121 (98%) | 0.45 (−0.25, 0.95) | |
| z | 9 | 103 | 95 (92%) | 0.2 (0, 0.7) | |
| y | 8 | 89 | 87 (98%) | 0.6 (−0.1,1.3) | |
| Centers with Low Performance for RD Assessments | |||||
| v | 10 | 114 | 19 (17%) | −0.1 (−0.7, 0.3) | −0.15 |
| l | 11 | 112 | 65 (58%) | 0.1 (−0.1, 0.5) | |
| q | 8 | 86 | 47 (55%) | −0.2 (−1.4, 0) | |
| s | 5 | 54 | 30 (56%) | −0.35 (−0.9, −0.1) |
IQR – interquartile range
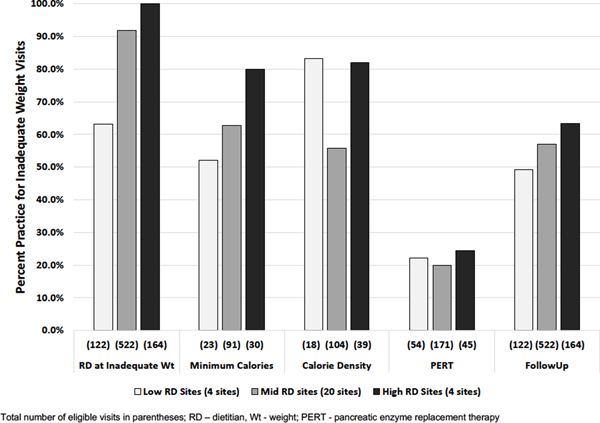
High performance centers responded to inadequate weight gain visits more often with greater than minimum calories for age compared to sites with less frequent assessments (24/30, 80% v. 12/23, 52%) (Figure 2). Similarly, these sites with frequent RD assessments responded more often to inadequate weight gain visits with closer follow up (104/164, 63% v. 60/120, 49%) (Figure 2). Mid-performance centers had intermediate levels of response for these two practices. In contrast, increase in PERT dose subsequent to inadequate weight gain was not common for any performance group. We were unable to interpret increases in formula caloric density given the amount of missing data.
Figure 2. Guideline-recommended practices for inadequate weight gain by performance group for dietitian assessments.
1.3.9. Frequent RD Assessments and Center-level WAZ at 24–27 months old
Median WHO WAZ at 24–27 months was variable across centers (eFigure 3 in Supplement) ranging from −0.4 to +0.9. Of the 4 centers that had a high performance for frequent RD assessments, 3 had a positive or normal center-level WHO WAZ with an overall group median of +0.33 (Table 3). In contrast, 3 of 4 centers with infrequent RD assessments had a negative WHO WAZ with a group median of −0.15 (Table 3). The mid-performing centers (n=20) had a median WAZ similar to high-performing centers (+0.30) with 4 centers with negative median WAZ.
Characteristics of sex, race, ethnicity, birth weight, and proportion of pancreatic insufficient patients were not statistically different among patients categorized by RD center performance groups (eTable 2 in Supplement). A total of 26 patients (11.5%) had a prior history of meconium ileus in the cohort. Although there were similar proportions of meconium patients represented in sites with high (20.5%) and low (20.6%) frequencies for RD-supported visits, mid-performing sites had 7.2% of meconium ileus patients (p=0.013). Small numbers of meconium ileus patients limit further analysis to determine associations of this difference with WAZ. Median age at diagnosis was significantly different for high, mid, and low performing sites at 18 days (IQR 11–26), 11 days (IQR 4–20), and 16 days (IQR 0–29) respectively (p=0.02) (eTable2 in Supplement). High performing sites also had a significantly higher median number of clinic visits per patient compared to mid or low performing sites (13 vs. 11 visits, p=0.006).
1.4. Discussion
In this retrospective multicenter study of infants, we identified a high prevalence of clinic visits in which patients presented with inadequate weight gain and found inconsistent use of guideline-based nutrition interventions. We found substantial variation in frequency of dietitian assessments across centers. In centers with the highest performance of consistent dietitian assessments, common responses to inadequate weight gain included adequate calories for age or closer follow up. Further, infants from the highest performing centers had an overall positive median WAZ at 2 years old, normal with the general population in contrast to infants from sites with less frequent assessments.
Our study is the first, to our knowledge, to examine the role of frequent dietitian assessments and guideline use to manage infant nutrition. BONUS provided detailed data to characterize targeted RD care delivery for infants in a contemporary cohort across several pediatric centers. The BONUS protocol included features such as calorie counts prior to visits and encouraged dietitians to work with the research team to obtain anthropometric measures and review this information with patients (8). Consistent review of detailed calories and measures could bias toward more recognition and response to inadequate weight gain. Despite these features, we identified variation in dietitian assessments and a pattern for incremental increase in use of some guidelines with more frequent RD care.
A key finding of our study is nearly a third of clinic visits from this cohort had insufficient daily weight change from the prior visit. This proportion was similarly distributed across centers, indicating that nutritional surveillance and management should remain an important part of comprehensive CF care. Notably, inadequate weight gain was identified at one quarter of visits in the first year of life when the frequency of recommended follow-up is more intensive; 40% of visits in the second year of life had identified inadequate weight gain. A major difference in the second year of life is the extension of follow up time to quarterly visits. A shortened time to follow up as seen in the first year of life may support earlier recognition and management of inadequate weight gain. Surveillance for inadequate weight events could be systematically augmented as a strategy to trigger infant assessments. A study of infants with hypoplastic left heart syndrome found sites that incorporated use of specific red flags such as failure to gain 20 grams per day between palliative surgeries more commonly had positive WAZ changes in their patients, and subsequently the same group demonstrated success in improving nutrition outcomes across sites in implementation of a care bundle incorporating weight surveillance (18, 19).
Benchmarking for ideal lung function and nutrition outcomes is an established and important method to identify key practices and guide the CF model of care (20, 21). A prior benchmarking project in the US CF Care Center Network described early and aggressive management of clinical declines as a key practice for centers with ideal outcomes (21). Our study corroborates this practice for the CF infant population and identifies variation in delivery for timely nutrition management. Deficits in delivery of service factors to pediatric patients are known health care quality issues (22). Although this retrospective study was not able to describe barriers to more frequent RD assessments, care access through telehealth has changed for families and CF programs during the COVID-19 pandemic (23, 24). This study presents a pre-pandemic characterization of frequent dietetic reviews and an opportunity for studies to investigate potential impacts of telehealth visits on nutrition care delivery.
Multiple studies confirm the association of future lung health and nutrition outcomes, particularly if growth outcomes are achieved early in life (4, 25–27). Early diagnosis by newborn screening has significantly improved the long-term health outcomes for patients with CF since its widespread implementation (3). CF-specialized dietitians are an important structure of interdisciplinary quality care to sustain these outcomes from diagnosis (28). The US Preventive Services Task Force rates services A or B for evidence-informed screening and preventive care, emphasizing the importance for studies to demonstrate the health impact of RD care delivery and increase reimbursement options through the Affordable Care Act (29).
Insufficient evidence to guide optimal PERT dosing has been well-described in the literature (30, 31). In prior analysis from the BONUS cohort, high median PERT dose in patients and across centers was not associated with improved growth parameters (32). Our study adds to uncertainty of best practices for PERT dosing. We found increases in PERT dose were present as a treatment response to only 1 of 5 underweight visits regardless of frequency of nutrition assessments. It remains unknown if consistent dose changes to underweight events would improve nutrition outcomes. Future investigations should consider care delivery guidance such as timing of dose changes.
Our analysis is limited by missing data. Calorie counts were not available in the second year after BONUS completed. In addition, the CFFPR although a valuable tool, did not have complete data for calorie density. We acknowledge that these types of important nutrition measures may be difficult to collect. Further work is required to understand the challenges of this data collection and identify processes to obtain these measures more consistently.
This study has additional limitations. We measured guideline use in response to inadequate weight gain between encounters. Other measures such as attained weight (33) or weight-for-length percentile may be more sensitive to trigger treatment responses. We selected surveillance for weight change between visits to follow recommendations from infant guidelines (1). Additionally, weight changes between assessments are easily identified and actionable by both home-based or in-clinic assessments, important care delivery considerations in current and future state following the COVID-19 pandemic (34). Second, we are not able to describe reasons for why a guideline was not used or what organizational or patient-facing barriers may be present to perform more frequent dietetic reviews. This study, however takes an initial step to characterize processes for nutrition care delivery and underscores future improvement opportunities. In addition, care delivery by phone and email work were not assessed in this study, potentially underestimating active nutrition management. We anticipate future studies will capitalize on recently added CFF Patient Registry fields for phone and video encounters to estimate the potential impact of these encounters. Lastly, this is an observational study, and we are cautious to not overinterpret the modest differences in WAZ outcomes or imply causality. We present individual center data across high and low performance groups.
1.5. Conclusions
Using a robust, nutrition-focused observational study, we identified opportunities to provide timely and effective use of guidelines for medical nutrition management. Specialized dietitians play an important role for care delivery. These findings may inform clinician and care center decision-making and structures of care delivery to promote goal weight outcomes for infants with CF.
Supplementary Material
Highlights:
A third of visits for 216 infants had inadequate weight gain from the prior visit.
High RD-supported sites gave infants high calories more than less RD-supported sites.
Centers with the most frequent RD visits more likely had goal center weight-for-age.
1.6. Acknowledgements
The authors thank Dr. D. Borowitz for her review of this manuscript, S. Casey, RD, for her perspectives on this study, and the contributions of the care teams and patient and family partners from the Cystic Fibrosis Learning Network.
The authors also acknowledge the following principal investigators and study site coordinators who participated in the Baby Observational and Nutrition Study (BONUS): A. Stecenko, M. Schechter, and E. Hunter (Emory University, Atlanta, Georgia);W. Hoover, H. Hathorne, and K. Brand (University of Alabama at Birmingham); A. Filbrun and M. Linn (University of Michigan, Ann Arbor); D. Borowitz and N. Caci (Women and Children’s Hospital of Buffalo, Buffalo, New York); B. McWilliams and I. Brazil (Children’s Medical Center of Central Texas, Austin); J. Heubi and M. Bushman (Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio); S. McColley and A. Bowen (Chicago Lurie Children’s Hospital, Chicago, Illinois); K. McCoy and K. Sakellaris (Nationwide Children’s Hospital, Columbus, Ohio); P. Sharma, C. Cannon, and A. Hebert (University of Texas Southwestern Medical Center, Dallas); S. Sagel and M. Anthony (Children’s Hospital Colorado, Denver); M. Dyson and H. Urbanek (Cook Children’s Medical Center, Fort Worth, Texas); S. Millard and C. Gile (Helen DeVos Women and Children’s Center, Grand Rapids, Michigan); G. Graff and L. Allwein (Hershey Medical Center, Hershey, Pennsylvania); P. Hiatt and N. Schaap (Baylor College of Medicine, Houston, Texas); N. Krupp and L. Bendy (Riley Hospital for Children, Indianapolis, Indiana); R. Ahrens and M. Teresi (University of Iowa, Iowa City); A. Berlinski and L. L. Ramsey (Arkansas Children’s Hospital, Little Rock); J. McNamara and M. Johnson (Minnesota Children’s Hospital and Clinics, Minneapolis); R. Brown and P. Berry (Vanderbilt Children’s Hospital, Nashville, Tennessee); N. Mehdi, J. Royall, and D. Thomas (Children’s Hospital of Oklahoma, Oklahoma City); R. Rubenstein and E. Donnelly (Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania); D.Weiner, S. Hurban, and E. Hartigan (Children’s Hospital of Pittsburgh and University of Pittsburgh, Pittsburgh, Pennsylvania); M. Powers and K. Simmons (Oregon Health & Sciences University, Portland); B. Chatfield and H. Oldroyd (University of Utah, Salt Lake City); L. Hoffman and S. McNamara (Seattle Children’s Hospital, Seattle, Washington); J. Wooldridge and A. Cooper (Cardinal Glennon Children’s Hospital and St Louis University, St Louis, Missouri); A. Faro and D. Rodgers (St Louis Children’s Hospital, St Louis, Missouri); C. Fortner and V. Suttmore (SUNY Upstate Medical University, Syracuse); J. Hocevar-Trnka, M. Kloster, M. Skalland, and A. Fowler (Cystic Fibrosis Foundation Therapeutics Development Network Coordinating Center, Seattle Children’s Research Institute, Seattle, Washington).
1.7 Funding Sources
TO was supported by Agency for Healthcare Quality and Research (1K12HS026393-01) and the Cystic Fibrosis Foundation (ONG20A0-KB). This study was also supported by the Cystic Fibrosis Foundation (SEID19AB0, BONUS11KO, COGEN18YZ), and NIH (UL1 TR002319, P30DK089507, R01DK095738).
Abbreviations:
- CF
Cystic fibrosis
- WAZ
weight-for-age Z-scores
- PERT
pancreatic enzyme replacement therapy
- RD
Registered dietitian
- BONUS
Baby Observational Nutritional Study
- CFF
CF Foundation
- CFFPR
CFF Patient Registry
- UCL
upper control limit
- LCL
lower control limit
- WHO
World Health Organization
- IQR
interquartile ranges
Footnotes
Declaration of interest: Dr. Ong has received honoraria from Cincinnati Children’s Hospital Medical Center to serve on the Cystic Fibrosis Foundation CF Learning Network Leadership Team. The other authors report no conflict of interest.
1.8 References
- 1.Cystic Fibrosis F, Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 Suppl):S73–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahiri T, Hempstead SE, Brady C, Cannon CL, Clark K, Condren ME, et al. Clinical Practice Guidelines From the Cystic Fibrosis Foundation for Preschoolers With Cystic Fibrosis. Pediatrics. 2016;137(4). [DOI] [PubMed] [Google Scholar]
- 3.VanDevanter DR, Kahle JS, O’Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: A review of disease manifestation, progression, and response to early treatment. J Cyst Fibros. 2016;15(2):147–57. [DOI] [PubMed] [Google Scholar]
- 4.Sanders DB, Fink A, Mayer-Hamblett N, Schechter MS, Sawicki GS, Rosenfeld M, et al. Early Life Growth Trajectories in Cystic Fibrosis are Associated with Pulmonary Function at Age 6 Years. J Pediatr. 2015;167(5):1081–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turck D, Braegger CP, Colombo C, Declercq D, Morton A, Pancheva R, et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr. 2016;35(3):557–77. [DOI] [PubMed] [Google Scholar]
- 6.van der Haak N, King SJ, Crowder T, Kench A, Painter C, Saxby N, et al. Highlights from the nutrition guidelines for cystic fibrosis in Australia and New Zealand. J Cyst Fibros. 2020;19(1):16–25. [DOI] [PubMed] [Google Scholar]
- 7.McDonald CM, Alvarez JA, Bailey J, Bowser EK, Farnham K, Mangus M, et al. Academy of Nutrition and Dietetics: 2020 Cystic Fibrosis Evidence Analysis Center Evidence-Based Nutrition Practice Guideline. J Acad Nutr Diet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung DH, Heltshe SL, Borowitz D, Gelfond D, Kloster M, Heubi JE, et al. Effects of Diagnosis by Newborn Screening for Cystic Fibrosis on Weight and Length in the First Year of Life. JAMA Pediatr. 2017;171(6):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coburn-Miller C, Casey S, Luong Q, Cameron N, Hocevar-Trnka J, Leung DH, et al. Standardization of Research-Quality Anthropometric Measurement of Infants and Implementation in a Multicenter Study. Clin Transl Sci. 2015;8(4):330–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173–9. [DOI] [PubMed] [Google Scholar]
- 11.Martiniano SL, Daines CL, Dellon EP, Esther CR Jr., Muhlebach MS, Ong T, et al. Highlights from the 2018 North American cystic fibrosis conference. Pediatr Pulmonol. 2019;54(7):941–8. [DOI] [PubMed] [Google Scholar]
- 12.Britto MT, Fuller SC, Kaplan HC, Kotagal U, Lannon C, Margolis PA, et al. Using a network organisational architecture to support the development of Learning Healthcare Systems. BMJ Qual Saf. 2018;27(11):937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med. 2005;24(8):1185–202. [DOI] [PubMed] [Google Scholar]
- 14.Provost L, Murray S. The Health Care Data Guide: Learning from Data for Improvement. San Francisco: Jossey-Bass; 2011. [Google Scholar]
- 15.Kunadian B, Dunning J, Roberts AP, Morley R, Twomey D, Hall JA, et al. Cumulative funnel plots for the early detection of interoperator variation: retrospective database analysis of observed versus predicted results of percutaneous coronary intervention. BMJ. 2008;336(7650):931–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulman J, Spiegelhalter DJ, Parry G. How to interpret your dot: decoding the message of clinical performance indicators. J Perinatol. 2008;28(9):588–96. [DOI] [PubMed] [Google Scholar]
- 17.Group. WMGRS. WHO Child Growth Standards: Length/height-for-age, weight-for age, weight-for length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. 312 p. [Google Scholar]
- 18.Anderson JB, Iyer SB, Schidlow DN, Williams R, Varadarajan K, Horsley M, et al. Variation in growth of infants with a single ventricle. J Pediatr. 2012;161(1):16–21 e1; quiz e2–3. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JB, Beekman RH 3rd, Kugler JD, Rosenthal GL, Jenkins KJ, Klitzner TS, et al. Use of a learning network to improve variation in interstage weight gain after the Norwood operation. Congenit Heart Dis. 2014;9(6):512–20. [DOI] [PubMed] [Google Scholar]
- 20.Schechter MS. Benchmarking to improve the quality of cystic fibrosis care. Curr Opin Pulm Med. 2012;18(6):596–601. [DOI] [PubMed] [Google Scholar]
- 21.Boyle MP, Sabadosa KA, Quinton HB, Marshall BC, Schechter MS. Key findings of the US Cystic Fibrosis Foundation’s clinical practice benchmarking project. BMJ Qual Saf. 2014;23 Suppl 1:i15–i22. [DOI] [PubMed] [Google Scholar]
- 22.Mangione-Smith R, DeCristofaro AH, Setodji CM, Keesey J, Klein DJ, Adams JL, et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357(15):1515–23. [DOI] [PubMed] [Google Scholar]
- 23.Jaclyn D, Andrew N, Ryan P, Julianna B, Christopher S, Nauman C, et al. Patient and family perceptions of telehealth as part of the cystic fibrosis care model during COVID-19. J Cyst Fibros. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins RC, Davis J, NeSmith A, Bailey J, Powers MR, Chaudary N, et al. Favorable Clinician Acceptability of Telehealth as Part of the Cystic Fibrosis Care Model during the COVID-19 Pandemic. Ann Am Thorac Soc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162(3):530–5 e1. [DOI] [PubMed] [Google Scholar]
- 26.Sanders DB, Zhang Z, Farrell PM, Lai HJ, Wisconsin CFNSG. Early life growth patterns persist for 12years and impact pulmonary outcomes in cystic fibrosis. J Cyst Fibros. 2018;17(4):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earnest A, Salimi F, Wainwright CE, Bell SC, Ruseckaite R, Ranger T, et al. Lung function over the life course of paediatric and adult patients with cystic fibrosis from a large multi-centre registry. Sci Rep. 2020;10(1):17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Services CMS. Affordable Care Act Implementation FAQs-Set 12 2013 [12/8/2020]. This set of FAQs addresses limitations on cost-sharing under the ACA and coverage of preventive services under the ACA.]. Available from: https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12#fn5. [Google Scholar]
- 30.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H, Clinical Practice Guidelines on G, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–9. [DOI] [PubMed] [Google Scholar]
- 31.Borowitz D, Gelfond D, Maguiness K, Heubi JE, Ramsey B. Maximal daily dose of pancreatic enzyme replacement therapy in infants with cystic fibrosis: a reconsideration. J Cyst Fibros. 2013;12(6):784–5. [DOI] [PubMed] [Google Scholar]
- 32.Gelfond D, Heltshe SL, Skalland M, Heubi JE, Kloster M, Leung DH, et al. Pancreatic Enzyme Replacement Therapy Use in Infants With Cystic Fibrosis Diagnosed by Newborn Screening. J Pediatr Gastroenterol Nutr. 2018;66(4):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heltshe SL, Borowitz DS, Leung DH, Ramsey B, Mayer-Hamblett N. Early attained weight and length predict growth faltering better than velocity measures in infants with CF. J Cyst Fibros. 2014;13(6):723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombo C, Burgel PR, Gartner S, van Koningsbruggen-Rietschel S, Naehrlich L, Sermet-Gaudelus I, et al. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med. 2020;8(5):e35–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.