Abstract
Inter-individual differences can inform treatment procedures and—if accounted for—have the potential to significantly improve patient outcomes. However, when studying brain anatomy, these inter-individual variations are commonly unaccounted for, despite reports of differences in gross anatomical features, cross-sectional, and connectional anatomy. Brain connections are essential to facilitate functional organization and, when severed, cause impairments or complete loss of function. Hence, the study of cerebral white matter may be an ideal compromise to capture inter-individual variability in structure and function. We reviewed the wealth of studies that associate cognitive functions and clinical symptoms with individual tracts using diffusion tractography. Our systematic review indicates that tractography has proven to be a sensitive method in neurology, psychiatry, and healthy populations to identify variability and its functional correlates. However, the literature may be biased, as the most commonly studied tracts are not necessarily those with the highest sensitivity to cognitive functions and pathologies. Additionally, the hemisphere of the studied tract is often unreported, thus neglecting functional laterality and asymmetries. Finally, we demonstrate that tracts, as we define them, are not correlated with one, but multiple cognitive domains or pathologies. While our systematic review identified some methodological caveats, it also suggests that tract–function correlations might still be a promising tool in identifying biomarkers for precision medicine. They can characterize variations in brain anatomy, differences in functional organization, and predicts resilience and recovery in patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00429-021-02382-w.
Keywords: Variability, Tractography, White matter, Patients, Cognition, Personalized medicine, Biomarker
Introduction
Stopping in a busy street to observe passers-by, one cannot help but notice that people are physically different. This diversity in appearance, but also opinions, creativity, and morals has enabled technological innovation and helped create a rich society. Individuals of different races, ethnicities, religious beliefs, socioeconomic status, language, and geographical origins make up this diverse community. Ever-evolving changes in our genome and adaptation to environmental factors have contributed to a range of genotypes (the variability in genetic code) and phenotypes (the variability in observable traits, e.g., eye color) and the interaction between them (White and Rabargo-Smith 2011). In the medical world, studying these inter-individual differences has led to the new disciplines of personalised and precision medicine. Inter-individual differences can help inform treatment procedures, and accounting for them has already improved patient outcomes and saved lives.
However, when turning to differences in brain anatomy, these inter-individual variations are relatively understudied (Glasser et al. 2016). It is often assumed that we share the same organization of cognitive functions and underlying brain anatomy (Caramazza 1986; Goldin et al. 2008; Greene et al. 2004; Johnson-Frey 2004; Treu et al. 2020; Linden 2020). For this reason, results from brain mapping studies are often depicted as group averages on template brains where inter-individual variability is considered an irrelevant deviation from the mean or a pathological change. In contrast, neuroanatomical studies often report variability in individual brain structures (e.g., Sachs 1892; Ono et al. 1990; Rademacher et al. 1993; Amunts et al. 1999; Caspers et al. 2006; Fornito et al. 2008), psychologists assume a Gaussian distribution of cognition and behavior (Seghier and Price 2018), and clinicians report differences in susceptibility to disorders and recovery (Forkel et al. 2014a, b; Forkel et al. 2020). Although the existence of structural and functional variability is known (e.g. Hirsch 1963; Ono et al. 1990), the ability to study inter-individual variability across large populations and consider structural variability as having functional correlates has emerged only recently. This development has been made possible through the availability of unique datasets with critical sample sizes and advances in computing power (Braver et al. 2010; Kanai and Rees 2011; Dubois and Adolphs 2016). The structure and function of the brain varies greatly between individuals and neuroimaging is sensitive to capture both sources of variability (Lerch et al. 2017; Gordon et al. 2017; Grasby et al. 2020; Tavor et al. 2016). On a structural level, measures of cortical surface area and thickness show hemispheric asymmetries that vary within the population (Kong et al. 2018). Brain morphology is also variable with half of the population having an additional gyrus, the paracingulate gyrus, in at least one hemisphere, for example (Fornito et al. 2008). Even primary cortical regions, such as the motor, auditory, and visual cortices, are subject to anatomical variations (Uylings et al. 2005; Caulo et al. 2007; Leonard et al. 1998; Eichert et al. 2020) and associative cortical regions have variable cytoarchitectonic boundaries (Amunts et al. 1999). This body of literature indicates that a large amount of structural variability exists in primary cortical areas and associative cortices. Still, it is as yet unclear how observable behavior and cognitive measures relate to these structural alterations.
There is increasing interest in understanding the brain’s structure–function relationship in light of inter-individual variability. Recent evidence has identified anatomical variations that are linked to differences in cognition and clinical outcomes (Forkel et al. 2020; Harrison et al. 2020; Johnson et al. 2020; Taebi et al. 2020; Munsell et al. 2020; Wang et al. 2021). The neurosurgical literature is also increasing our understanding through mapping cognitive–anatomical variability in single case series during pre-, post-, and intrasurgical imaging assessments (e.g., Vanderweyen et al. 2020). This multi-modal brain mapping approach is able to reveal variation but also ‘atypical cases’, meaning the patients that do not fit expected assumptions of associations between brain areas or connections and deficit in certain cognitive domains. In the surgical setting, transcranial magnetic stimulation for presurgical planning (e.g., Giampiccolo et al. 2020; Mirchandani et al. 2020), deep brain stimulation (e.g., Calabrese 2016; Akram et al. 2017), and direct electrical cortical stimulation during awake surgery (e.g., Puglisi et al. 2019; Middlebrooks et al. 2020) have been aided by the consideration of inter-individual variability in white matter tracts estimated with tractography. However, there has not yet been a systematic attempt to capture this variability in connections across the entire brain and associate white matter phenotypes with cognitive profiles and clinical dimensions. It is, therefore, high time we included inter-individual variability and revisited the drawing board of neurology and psychiatry.
Clinical cases and mapping of inter-individual differences are beginning to explain the observed variance in cognitive and behavioral measures. As such, a better understanding of variability is crucial to explain differences in human abilities and disabilities and improve our clinical models and predictions (Seghier and Price 2018). While the cerebral white matter may not be a functional agent per se (see Innocenti et al. 2017; Rockland 2020), it constrains the brain’s functional organization (Bouhali et al. 2014; Thiebaut de Schotten et al. 2017; Takemura and Thiebaut de Schotten 2020) and leads to cognitive impairment or complete loss of function when severed (Geschwind et al. 1965a, b). Hence, mapping white matter variability may be a useful surrogate measure to capture inter-individual differences in structure and function. Diffusion tractography has become an established non-invasive quantitative method to study connectional anatomy in the living human brain over the past 15 years (for reviews, see Assaf et al. 2017; Jbabdi and Johanson-Berg 2011). Tractography has been employed as a neuroimaging biomarker to link white matter phenotypes, meaning inter-individual variations in white matter networks, to cognition. These white matter phenotypes are a product of an environment–genotype interaction as has been demonstrated for the language and limbic networks (Su et al. 2020; Budisaljevic et al. 2015, 2016). Consequently, white matter networks are subject to variations over the lifespan and can change with training (Scholz et al. 2009; Thiebaut de Schotten et al. 2012; Lebel et al. 2019; Vanderauwera et al. 2018). Tractography has been shown to be highly sensitive in capturing these variations, which can be associated with inter-individual differences in neuropsychological measures in the healthy population (e.g., Catani et al. 2007; Thiebaut de Schotten et al. 2011a, b; Howells et al. 2018) and clinical groups (e.g., Forkel et al. 2014a, b; Forkel et al. 2020; Thompson et al. 2017; Pacella et al. 2019; Alves et al. 2021). Therefore, tractography can be used to study variability in the human brain and map functional white matter correlates.
Identifying consistent trends in the diffusion tractography literature may be a crucial step in mapping white matter phenotypes and their impact on cognition, and hence, a systematic review is timely. We focus here on studies that describe significant correlations between structural and continuous cognitive measures in healthy adults, and psychiatric and neurological patients. For structure, we focus on volumetric or microstructural (e.g., fractional anisotropy and mean diffusivity) measures of white matter tracts that can be extracted from tractography reconstructions and voxel-wise measurements. We concentrate on neuropsychological tests in healthy volunteers and clinical scales in pathological populations to estimate cognitive–behavioral measures and clinical symptom severity. In this review, we summarize dimensional differences (i.e., correlations) between structural white matter connectivity (i.e., volumetric or microstructural) and cognition as a step to support consideration of inter-individual variability in neuroscience studies.
Methods
We undertook a systematic review of published journal articles that correlated measures derived from white matter tractography with cognition or clinical symptoms, following PRISMA guidelines (Liberati et al. 2009).
The resources obtained from this study and created for this data are made available as supplementary material: https://github.com/StephForkel/PhenotypesReview.git.
Data sources
A title/abstract search in MEDLINE and Scopus (which includes most of the EMBASE database, https://www.elsevier.com/solutions/embase-biomedical-research) was conducted. The search term ‘tractography’ returned a total of 5303 in PubMed and 7204 results in Scopus. We hence restricted our search (conducted on February 25th, 2020) to the following strings: (predictor OR "correlat*" OR regression OR “assoc*”) AND (tractography). Additional filters were applied to include only human adult studies published in English as final stage peer-reviewed articles in scientific journals. The search returned 1333 results on PubMed and 2380 results on Scopus, yielding 3713 records. There were no internal duplicates within each database, and we excluded 1224 external duplicates between the databases. After removing duplicates from these lists, a total of 2489 results were screened.
Data screening and eligibility
Figure 1 summarizes the following workflow. During the screening, we applied further exclusion criteria leading to the exclusion of pediatric studies, non-human studies, pure methodological papers without behavioral correlates, correlations between tractography and physiological rather than behavioral measures (e.g., heart rate), non-brain studies (e.g., cranial nerves, spine, muscle), graph theory and tract-based spatial statistics (TBSS) studies, and case studies or mini-series (less than 10 participants/patients) and papers that reported no significant correlation. All studies reporting variability of white matter tracts using tractography that described a significant association with continuous cognitive measures, clinical symptom severity, and/or continuous recovery were included. After screening of the abstracts, we retained a list of 466 research papers. Full-text screening further identified studies that fulfilled the exclusion criteria defined above. This led to a total of 326 studies included in the final analysis.
Fig. 1.
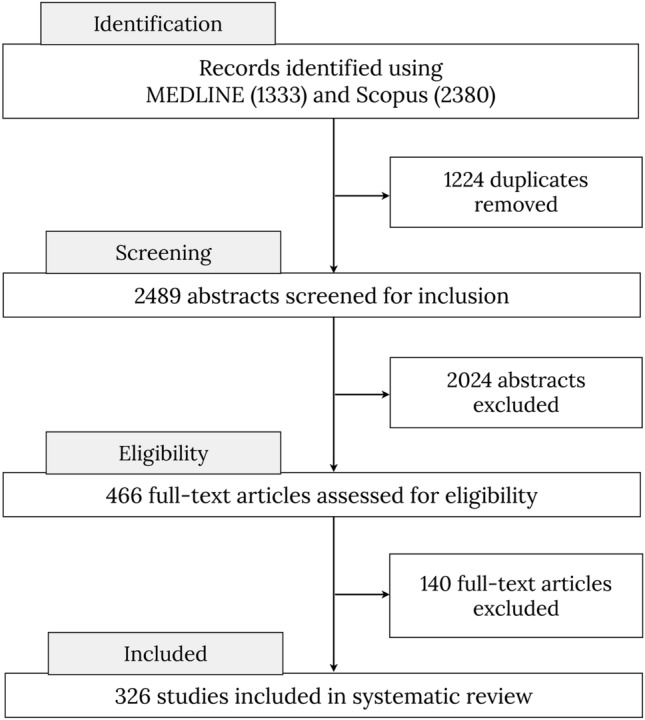
PRISMA flowchart
Study quality
The QUADAS quality assessment tool (Whiting et al. 2003) was adapted for the review to document the steps taken by each paper to avoid bias and justify and validate the protocols. The following criteria were used to rate publications: (1) sufficient detail provided to reproduce the protocol, (2) clearly defined white matter tracts, and (3) the groups, cognitive measures, or clinical characteristics were reported.
Data extraction
The following information was collected from the records: year of publication, group (e.g., healthy participants vs degeneration vs psychiatric vs neurological vs neurodevelopmental), sample sizes, left/right/unspecified hemisphere, tractography indices, label of white matter tracts, clinical symptoms, behavior and/or cognitive domain, differential neuropsychological measures (e.g., Trail Making test), and finally the interaction between white matter tracts and neuropsychological assessments. The labeling of the groups (healthy participants vs degeneration vs psychiatric vs neurological vs neurodevelopmental) was aligned to current diagnostic criteria and categories of disease (DSM-5, IDC-11). The coding of the parameters is available from the supplementary material online (https://github.com/StephForkel/PhenotypesReview). As an example, a study in neurological patients measuring motor functions and the left corticospinal tract would be coded as: neurological*motor*CST_lh.
Data synthesis and analyses
In the synthesis of this dataset, we summarized degeneration, and neurosurgical and common neurological symptoms as the neurological group. Similarly, the psychiatric group included adult neurodevelopmental and psychiatric studies. We also synthesized clinical symptoms, behavior, and/or cognitive domains using the following terms: we limited the taxonomy of cognitive domains to the terms that are currently widely accepted in the literature, including attention, executive functions, language, memory, and reward. Terms defining behavioral domains included addiction, auditory, visual and motor behavior, sleep, mood, and social measures (e.g., theory of mind). To assess the sensitivity of tracts to clinical measures, we also included a “symptoms” dimension corresponding to neurological and psychiatric severity measures.
According to domains, the classification of the correlations was replicated three times by SJF, PF, and HH, and in case of disagreements, a consensus was reached. From these terms, we could extract variables of interest such as the number of correlations per tract (i.e., sensitivity or how likely a tract can correlate), but also the number of studies reporting significant correlations for that tract (i.e., popularity or how often studies report a significant correlation for that tract). A differentiation between sensitivity and popularity was made because many studies tested multiple associations between a tract and several cognitive measures. Therefore, if a study investigated the relationship between a tract and multiple functions, the sensitivity measure would increase with each significant correlation; however, the study would only be counted once for the popularity value. Subsequently, we also investigated the number of correlations reported according to the domains of interest (i.e., specificity to cognitive domains: attention, executive functions, language, memory, and reward; behavioral domains: addiction, auditory, visual and motor behavior, sleep, mood, social; and symptoms severity).
Results
The number of correlations and studies per tract
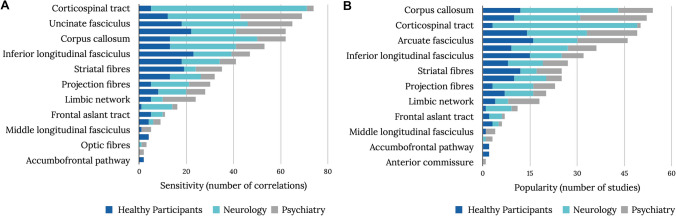
A total of 25 individual white matter tracts were reported to correlate with performance on neuropsychological tests and clinical symptoms (Fig. 2). Among these, certain tracts were more commonly correlated with cognitive-behavioral measures (Fig. 2A). We report here the number of studies that described correlations (Fig. 2A) and the number of correlations per tract (Fig. 2B). Showing this difference is essential, as some studies reported more than one tract correlation. Notably, commonly reported tracts (i.e., sensitivity) were not always those that were most systematically studied (i.e., popularity) indicated by the different number of studies per tract (Fig. 2B).
Fig. 2.
Frequencies of reported correlations (A) and the number of studies (B) per tract in each group (i.e., healthy participants, neurology, and psychiatry). A high number of correlations indicate a high tract sensitivity, and the number of studies represents tract popularity
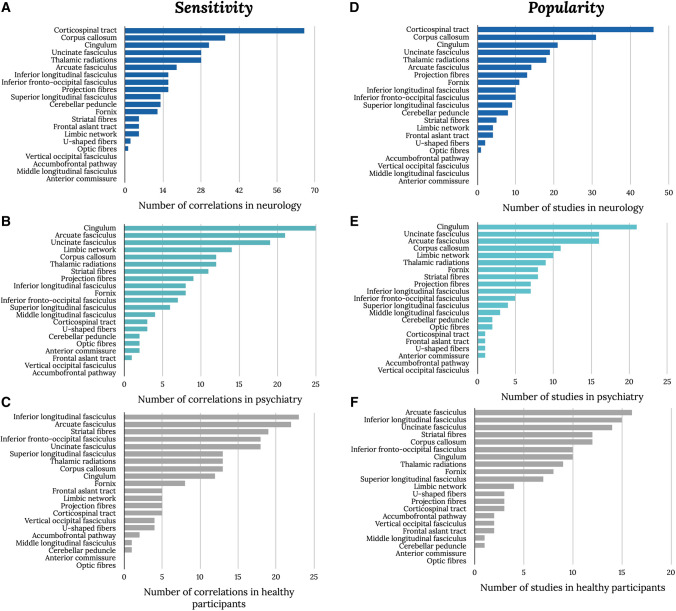
Our review demonstrates that most results were reported for patients with neurological (45%) or psychiatric (29%) pathologies rather than controls (25%) (Fig. 2). Additionally, the most studied tracts that were reported to correlate with cognitive measures vary for each group. For example, most correlations reported in healthy participants were with the inferior longitudinal fasciculus, arcuate fasciculus, and striatal fibers (Fig. 3A). In the neurological groups, correlations were mainly reported with the corticospinal tract, the corpus callosum, and the cingulum (Fig. 3B). The cingulum, arcuate, and uncinate fasciculus were the most prominent tracts to correlate with psychiatric symptoms (Fig. 3C). The most correlated (i.e., sensitivity), however, does not mean the most commonly studied tracts (i.e., popularity) and might point toward a bias in the literature to focus on ‘target’ tracts rather than systematically studying the whole white matter. In healthy participants, the most ‘popular’ tracts were the arcuate, inferior longitudinal, and uncinate fasciculi (Fig. 3D). For the neurological group, the most studied tracts were also the most sensitive tracts, namely the corticospinal tract, corpus callosum, and cingulum (Fig. 3E). In the psychiatric group, the most sensitive and popular tracts were the cingulum, arcuate fasciculus, and uncinate (Fig. 3F).
Fig. 3.
“Tract bias” in the literature. Tract sensitivity (A–C) and tract popularity (D–F) in healthy participants, neurological group, and psychiatric group. The number of correlations per tract is defined as ‘sensitivity’ or how likely a tract can correlate, whereas ‘popularity’ is defined as the number of studies reporting significant correlations for that tract. (Data shown are the same as Fig. 2 split by group)
The number of correlations per cognitive domain
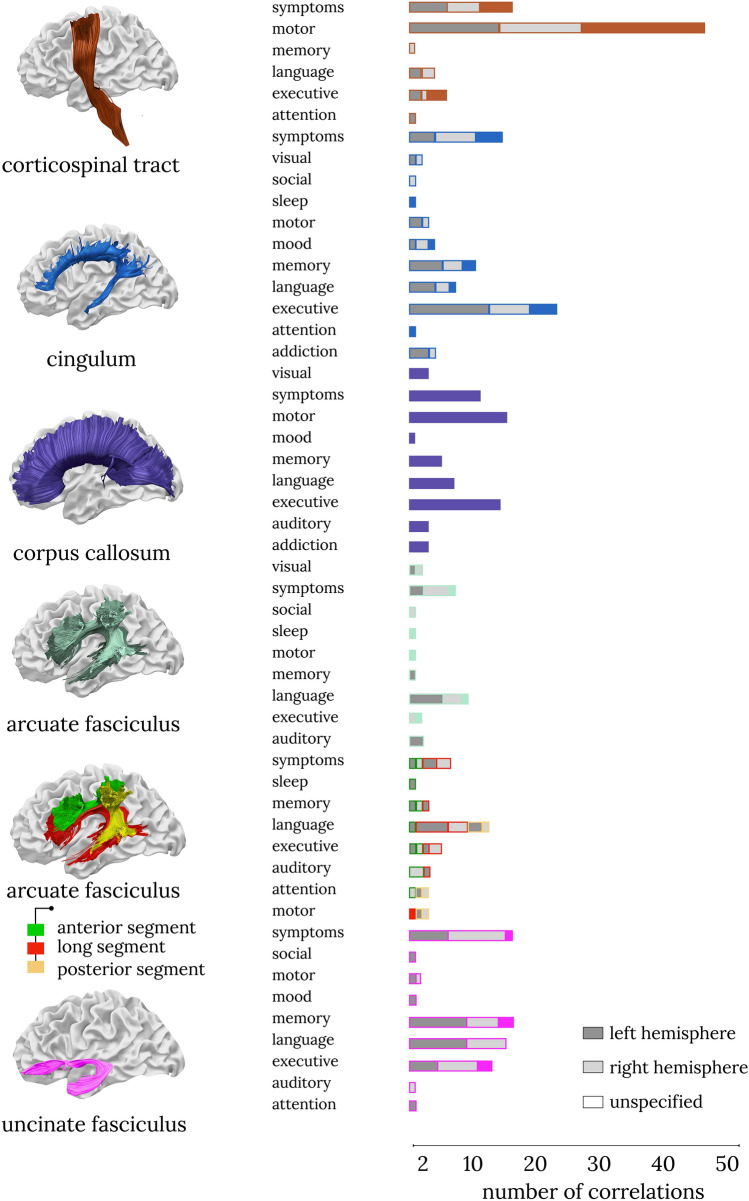
The analysis of correlations between tracts and cognitive domains showed no one-to-one correspondence between a white matter tract and a domain (Fig. 4). The tracts that had the highest number of correlations (i.e., selectivity) with one domain were the corticospinal tract with the motor domain and the cingulum with executive functions (Fig. 4). Additional figures showing all correlations per domain for all tracts are available in the supplementary material (https://github.com/StephForkel/PhenotypesReview.git). This summary also shows that the extent of a tract’s selectivity to one domain is often related to the diversity of the tract’s projections. For instance, the corpus callosum, which projects to most of the brain’s surface (Karolis et al. 2019), is associated with most domains. Association tracts such as the arcuate fasciculus were also reported to be involved in several domains, but the most common association for this pathway was with language measures. When separating the arcuate into its subdivisions (Catani 2005), this showed that its fronto-temporal segment was driving the domain specificity of the arcuate with language. In contrast, correlations with the anterior and posterior segments of the arcuate fasciculus were usually with aspects of the memory and attention domain (Fig. 4).
Fig. 4.
Specificity for tract × domain correlations. The number of correlations between cognitive, behavioral, or clinical assessments and white matter tracts demonstrates that the concept of ‘one tract-one function’ does not hold. The figure shows the most studied tracts as identified by the current study. The other tract-domain correlations are available as additional figures (see Additional file 1)
Hemispheric specialization
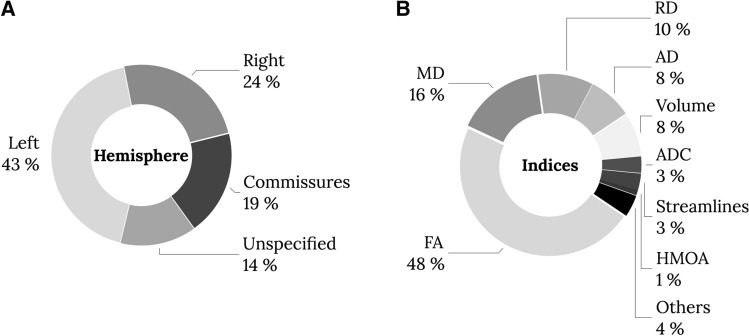
Hemispheric specialization was inconsistently reported in this literature, with as many as 14% of studies not specifying if their correlations were with a domain are for the left or right hemisphere tracts (Fig. 5A). Among the 326 papers assessed, a total of 674 tract–function correlations were extracted with a p value reported of 0.05 or below. Within this data pool, an equal number of studies specified their results for the left (37.38%) and the right hemisphere (35.01%), while the remaining results (n = 186) were unspecified (14%) or described commissural connections (19%) that cannot be attributed to either hemisphere. When looking at the distribution of significant correlations with cognitive measures, it was evident that correlations with the left hemisphere are more commonly reported—or more commonly studied (Fig. 5A). This is essential when studying association or projection fibres, given the strong structural and functional lateralisation of the brain for some tracts and cognitive functions (e.g., Thiebaut de Schotten et al. 2011a, b; Koralis et al. 2019).
Fig. 5.
Summary of reporting of tract-domain correlations for each hemisphere of the brain (A) and diffusion indices (B). The results show that a greater number of correlations between tractography results in the left hemisphere and cognitive–behavioral measures exist in the literature and that studies with significant correlations most commonly use fractional anisotropy (FA). MD mean diffusivity, RD radial diffusivity, AD axial diffusivity, ADC apparent diffusion coefficient, HMOA hindrance modulated orientational anisotropy
Diffusion indices
A multitude of diffusion imaging methods have been developed and applied to the living brain. The first model to be widely applied to the study of the healthy living brain and pathological groups was diffusion tensor imaging (DTI). Using this model, various average properties of tissues within each voxel could be characterized by diffusion indices, including fractional anisotropy (FA), mean diffusivity (MD), number of streamlines, and voxels intersected by streamlines as a proxy of volume (mm3 or cm3). Volumetric measures accounted for 11% of the tract-domain correlations in the studied literature (Fig. 5). These indices are computed using the diffusivity in each voxel, have been associated with microstructural properties, and are used to indicate axonal damage or degeneration (Le Bihan and Breton 1985; Le Bihan 1995; Pierpaoli and Basser 1996; Beaulieu 2002a, b; Ciccarelli et al. 2008; Afzali et al. 2021). Each index was extracted from the 326 studies and the results highlight that some measures are more commonly reported than others (Fig. 5). Below we briefly discuss the meaning of these indices and their prevalence in the literature.
The quantitative apparent diffusion coefficient map (ADC) is a primary sequence in acute stroke imaging due to its sensitivity to early ischemic changes. The lesion cascade typically induces cytotoxic oedema which results in a quick drop in ADC by 30–50% (Moseley 1990). ADC comprises about 3% of the correlational diffusion tractography literature. Another clinically useful surrogate measure of diffusion deficits is mean diffusivity (MD = (λ1 + λ2 + λ3)/3), a measure independent of tissue directionality and more prominently used in the literature at 16% (Fig. 5). This map does not offer anatomical details, but is sensitive to diffusion abnormalities, such as acute ischemic lesions (Lythgoe et al. 1997). The diffusivity measured along the principle axis (λ1) is referred to as axial diffusivity (also called longitudinal or parallel diffusivity as diffusivity is parallel to the axonal fibers, AD = λ1). While, the diffusivity perpendicular to the fibers is calculated from the mean along the two perpendicular directions.
This average of diffusivities along the two orthogonal axes (λ2, λ3) is denoted as perpendicular or radial diffusivity (RD = (λ2 + λ3)/2). Together, axial and radial indices make up 18% of the literature (Fig. 5). The application of these measure is however still debated, as the direction and magnitude of the eigenvalue/eigenvector system relies on physical measures that are sensitive to noise and may be influenced by the estimated tensor ellipsoid and underlying pathologies (Wheeler-Kingshott and Cercignani 2009). As such, changes in axial diffusivity measurements, for example, could be related to intra-axonal composition, while radial diffusivity may be more sensitive to changes in membrane permeability and myelin density (Song et al. 2002). The most prominently used diffusion measure is fractional anisotropy (FA) which comprises 48% of the literature (Fig. 5). The variance of all three eigenvectors (ν1–3) about their mean is normalized by the overall magnitude of the tensor and is referred to as fractional anisotropy (Jones 2008, 2009). The resulting FA index represents the fraction of the tensor that can be attributed to anisotropic diffusion (i.e., unequal diffusivity along directions) as the deviation from isotropy (i.e., equal diffusivity in all directions). FA designates free diffusivity (i.e., unhindered isotropic) with a value of 0, and constrained diffusion (i.e., anisotropic along one axis only) with the value of 1. Despite its wide adoption in the healthy and clinical literature, DTI and its voxel-wise measurements have their limitations (Dell’Acqua and Tournier 2019; Schilling et al. 2019; Meiher-Hein et al. 2017). Over the past years, advanced diffusion models have moved toward diffusion and fiber orientation density functions (fODF) to capture the complexity of white matter organization using tract-specific measurements. One such measure is the apparent fiber density also referred to as hindrance modulated orientational anisotropy (HMOA) (Dell’Acqua et al. 2013; Dell’Acqua and Tournier 2019), which currently comprise about 1% of the correlational diffusion tractography literature.
Together, these in vivo diffusion-based measurements allow connectional anatomy to be defined at different scales in health and disease. However, given the sensitivity of these diffusion indices to tissue characteristics and brain lesions (e.g., in the presence of oedema), they have to be interpreted carefully.
Discussion
Over the course of the last 15 years, there have been over 300 studies in human adults showing significant correlations between white matter tracts and cognitive measures. These correlations demonstrate how important it can be to consider inter-individual differences in healthy participants and brain pathologies (e.g., neurological and psychiatric disorders). Our systematic review of this literature demonstrates that tractography is commonly used to study inter-individual variability and is a sensitive method to test neurology, psychiatry, and healthy volunteers. Second, there may be a “tract bias” in the literature, as tracts that are commonly studied (high popularity) are not necessarily those that have the highest number of significant correlations (high specificity) for a given cognitive function or clinical symptoms. Finally, our review clearly shows that tracts, as we define them, are never correlated with only one cognitive domain.
Our investigation collated tract–function correlations across neurological, psychiatric, and healthy populations. Most tract-domain correlations in the literature were identified in studies of pathological groups rather than healthy participants (Fig. 3). There are several possible explanations for this. This predominance may originate from the broader dispersion of data points associated with pathologies (i.e., more variability). As the presence of pathology causes higher variability in both anatomy and cognitive/clinical test scores, these variations are more likely to be detected by linear correlations. Another explanation for the high number of tract-domain correlations in clinical groups is that differences between healthy participants are often considered to be noise which can mean they are reduced during data processing (Kanai and Rees 2011). While noise may contribute to the difference observed in controls, it is now clearly established that diffusion tractography can capture inter-individual differences that reflect some of the variations in the anatomy and functioning of the brain (e.g., Powell et al. 2006; Vernooij et al. 2007; Lazari et al., preprint). An alternative hypothesis could be that current neuroimaging or cognitive and behavioral tests are not sensitive enough to systematically disentangle noise from real variability in healthy participants (Braver et al. 2010; Rousselet and Pernet 2012). The latter may be improved using finer-grained cognitive measures, higher resolution data, and better anatomical tract definitions.
Our review identified a total of twenty-five studied tracts that were significantly correlated with cognitive measures in healthy participants or symptom severity in patients. The precise number of white matter tracts in the human brain remains unknown and variable estimates originate from the use of different methods. Most atlases suggest that twenty-six tracts can be reliably identified with most tractography methods (Mori et al. 2005, 2009; Lawes et al. 2008; Catani and Thiebaut de Schotten 2008; Thiebaut de Schotten et al. 2011a, b; Rojkova et al. 2016). Some recent atlases further identify additional intralobar connections (Catani et al. 2012, 2017; Guevara et al. 2012, 2020). This review reported some additional tracts that have not yet been incorporated into atlases, including the accumbofrontal tract and the vertical occipital fasciculus (Martínez-Molina et al. 2019; Rigoard et al. 2011; Vergani et al. 2014, 2016; Yeatman et al. 2013), while other tracts have not yet been widely used in the literature and therefore do not feature in this review (e.g., medial occipital longitudinal tract; Beyh et al. 2021). Our results also highlight a bias in the literature toward studying specific tracts that have very well-established functions (e.g., corticospinal tract) or are easy to dissect in clinical groups (e.g., cingulum). The omission of other tracts does, of course, not mean that they are functionally irrelevant as shown by our sensitivity measure. For example, a high number of correlations were identified for the corticospinal tract and motor functions as can be expected. However, there were also some significant correlations with other tracts not typically associated with motor functions (e.g., arcuate fasciculus and uncinate fasciculus). It could thus be that some tract–function relationships are still poorly understood. Some may have non-linear or indirect relationships with function, for which correlational approaches are not appropriate. Furthermore, understudied tracts may be more challenging to reconstruct due to limited anatomical guidelines or available algorithms (e.g., U-shaped fibers, Attar et al. 2020; Mandelstam 2012; Maffei et al. 2019a, b).
For the most sensitive, or commonly correlated, tracts, several functions were reported. Our results show that even the corticospinal tract that is primarily studied within the motor domain (62.21% of correlations, Fig. 4) showed a non-uniform functional profile. For instance, some studies reported correlations between the corticospinal tract and executive functions (8.11%) and language/speech processes (5.4%). For other tracts, the correlations were even more diverse. For example, the cingulum correlated with psychiatric symptom severity (20.29%), memory (14.49%), and language measures (10.14%). These results highlight hierarchical organization of brain function, with some tracts recruited for many functions, whereas others may have a more specific functional role (Pandya and Yeterian 1990). While the number of associations is likely to be biased by several factors including prior hypotheses that a given tract is involved in a specific function, a recent study mapped a total of 590 cognitive functions, as defined by a meta-analysis of activations derived from fMRI paradigms, onto a white matter atlas (Thiebaut de Schotten et al. 2020). This functional atlas of white matter demonstrated that one tract is relevant for multiple functions. Another possible interpretation of this finding is that human-ascribed definitions of white matter tracts are too coarse to be specific to only one given function. For example, segmenting the arcuate fasciculus into three components in line with early work (Catani et al. 2005) shows correlations with more domain specificity than correlations with the entire arcuate fasciculus. This may call for finer-grained white matter divisions or data-driven approaches to identify segments of white matter that may be related to specific functions (see, for example, Foulon et al. 2018; Nozais et al. 2021).
We also show differential patterns between healthy participants and pathological groups. One such example is the size of the uncinate fasciculus that has primarily been associated with memory in healthy aging (Sasson et al. 2013), with psychopathy in psychiatric studies (e.g., Craig et al. 2009), and language in neurological studies (e.g., D’Anna et al. 2016). Similarly, the size of the arcuate fasciculus has been implicated in learning new words in healthy participants (Lopez-Barroso et al. 2013), which supports the role of the arcuate fasciculus as the mediator between the temporal–parietal–frontal cortices and the neural substrate for the phonological loop (Baddley et al. 1998; Catani et al. 2005; Baddley 2007; Buchsbaum and D’Eposito 2008; see Baddeley and Hitch 2019 for a recent review on the phonological loop). Recently, this hypothesis was supported by intraoperative direct cortical stimulation in neurosurgical patients (Duffau et al. 2008; Papagano et al. 2017). In psychiatric and neurological patients, damage to the arcuate fasciculus was associated with auditory hallucinations in schizophrenia (Catani et al. 2011), aphasia severity in stroke (Forkel et al. 2014a, b), and repetition deficits in primary progressive aphasia patients (Forkel et al. 2020). Therefore, the functions associated with a tract might not purely be a product of the cortical regions connected by white matter but instead rely on the interplay of one region with another. When pathology is introduced into this delicate network, differential patterns of symptoms may reflect the variable impact on brain regions within such a network. Furthermore, the pathophysiological mechanisms vary across pathologies and have different long-range effects on connected regions (e.g., Catani et al. 2005; Catani and Ffytche 2005).
There are limitations associated with tractography that may have influenced the studies summarized in the current work. We set out to systematically review tract–function correlations irrespective of these limitations, to identify broad patterns; however, it is essential to caveat this by stating what tractography can and cannot do when interpreting results. While tractography has proven useful for research and clinical applications, interpretation of voxel-based indices presents challenges (Dell’Acqua and Tournier 2019). When considering the resolution of diffusion data, for example, diffusion indices are averaged across and within voxels, which may mask meaningful changes. For research purposes, the voxel size is typically 2*2*2 mm, while the voxel sizes are often larger for clinical acquisitions leading to even lower spatial resolution. As such, a research acquisition with an 8mm3 voxel is likely to contain an inhomogeneous sample of tissue classes, intra- and extracellular space, and axons of different densities and diameters. This multi-tissue composition within voxels can pose challenges for the study of projection and commissural fibers and afflict tractography reconstructions with false-positive and false-negative reconstructions. A recent preprint also looked at the commonly reported index of FA and demonstrated that multi-modal approaches can help detect white matter behavior relationships that are not detected with FA alone (Lazari et al. 2020). This study also raised the notion of the need for sufficiently powered samples to detect changes in myelin in relation to behavior.
The diffusion signal itself is also inhomogeneous across the brain. As a result, areas such as the orbitofrontal cortex and anterior temporal cortex are often distorted. Methodological advances partially correct for these distortions (e.g., TOPUP, Andersson et al. 2003) and disentangle some of these components to reconstruct crossing fibers and extract tract-specific measurements (see Dell’Acqua and Tournier 2019). However, most studies included in this review used diffusion tensor algorithms rather than advanced algorithms and indices (see Fig. 5, HMOA 1% of studies). While recent research studies have the methodological means to mitigate such distortions (e.g., Andersson et al. 2003), most current clinical studies still suffer from these limitations, potentially explaining the lack of tract-domain specificity.
Another source of inconsistencies originates from incoherent reporting of the anatomy. For example, many studies did not specify which hemisphere was studied or collapsed their white matter across both hemispheres and correlated the averaged anatomy with cognitive and behavioral measures. Collapsing measurements from anatomical features across both hemispheres might prove problematic for white matter tracts that are subject to more considerable inter-individual variability and subsequently might get over- and underrepresented in each hemisphere (e.g., Catani et al. 2007; Thiebaut de Schotten et al. 2011a, b; Rojkova et al. 2016; Croxson et al. 2018; Howells et al. 2018, 2020). Furthermore, while the concept of a strict hemispheric dichotomy might be seen as overly simplistic (e.g., Vingerhoets 2019), splitting the measurements by hemisphere may reveal useful insights and higher specificity into the contribution of either side to a measured cognitive behavior or disorder (Floris and Howells 2018). Another limitation comes from inconsistencies in the classification of white matter tracts. For instance, the superior longitudinal fasciculus (SLF) was often considered in its entirety without specifying which branch was studied. When branches were specified, a variety of terminologies were used, including the three branches (SLFI-III, Thiebaut de Schotten et al. 2011a, b), or a lobar-based segmentation into SLFtp (temporal projections) and SLFpt (parietal projections) (e.g., Nakajima et al. 2019). Another example is the arcuate fasciculus that was sometime considered in its entirety and sometimes split into several branches (e.g., Catani et al. 2005; Kaplan et al. 2010). Perhaps due to early anatomical descriptions where the terminology was used interchangeably and incorrectly, we are still faced with a body of literature that uses the terms SLF and arcuate interchangeably. This confusion may have come about in the literature, as the SLF system was not easily dissected in the human brain using either post-mortem methods or tractography due to crossing fibers. In fact, this fronto-parietal network was first described in the monkey brain and only subsequently identified in the human brain using diffusion imaging (Makris et al. 2005) and diffusion tractography (Thiebaut de Schotten et al. 2011a, b). While there is some overlap between both networks, such as the SLF-III and the anterior segment of the arcuate fasciculus, the other branches and segments are distinct. From an anatomical and etymological perspective, the superior longitudinal fasciculus should be ascribed solely to fronto-parietal connections (i.e., “superior and longitudinal”; Thiebaut de Schotten et al. 2011a, b), whereas the arcuate fasciculus should be considered fronto-temporal connections (i.e., ‘arching’ around the Sylvian fissure; Catani et al. 2005). Recent attempts have synthesized this literature, suggesting using the term superior longitudinal system (SLS) to include the arcuate fasciculus stricto sensu and the three branches of the SLFs in one multilobar fiber system (Mandonnet et al. 2018; Vavassori et al. 2021). Another controversy in the literature is the differentiation between the posterior segment of the arcuate fasciculus and the vertical occipital fasciculus (Martino and Garcia-Porrero 2013; Bartsch et al. 2013; Bullock et al. 2019; Weiner et al. 2017). The anatomy of the VOF has been verified using tractography (Yeatman et al. 2014; Keser et al. 2016; Briggs et al. 2018; Schurr et al. 2019; Panesar et al. 2019), post-mortem dissections (Vergani et al. 2014; Gungor et al. 2017; Palejwala et al. 2020), and comparative anatomy (Takemura et al. 2017). These descriptions were scrutinized against historical post-mortem descriptions from Wernicke (Yeatman et al. 2014), Sachs (Vergani et al. 2014) and the Dejerine's (Bugain et al. 2021). Anatomically, the vertical occipital fiber system and the posterior arcuate segment are distinct bundles in terms of their trajectories and cortical terminations. More precisely, the vertical occipital fasciculus projections are in the occipital lobe and the posterior segment of the arcuate fasciculus projections are in the posterior temporal and parietal lobes (Weiner et al. 2017). Another example is the differential and synonymous use of the terminologies external capsule (Rilling et al. 2012), external/extreme fiber complex (Mars et al. 2016), inferior fronto-occipital fasciculus (Forkel et al. 2014a, b; Hau et al. 2016), and inferior occipitofrontal fasciculus (Kier et al. 2004). The difference in terminology is owed mainly to the description of these tracts using different methods (Forkel et al. 2014a, b) and some consensus is certainly needed to improve consistency in the literature (Maier-Hein et al. 2017; Mandonnet et al. 2018; Vavassori et al. 2021). Another tract that appears under two terminologies in the literature is the medial occipital longitudinal tract (MOLT) relevant for visuospatial processing (Beyh et al. 2021). This tract has previously been referred to as the ‘sledge runner’ (Vergani et al. 2014).
Additionally, to harvest the inter-individual variability results, this review focused on continuous cognitive and clinical measures obtained from correlations to associate them with white matter phenotypes. As such, we did not separate distinct structural subtypes (e.g., Ferreira et al. 2020; Forkel et al. 2020) and did not take different diffusion matrices (e.g., fractional anisotropy vs mean diffusivity) or tractography algorithms (e.g., tensor vs HARDI) into account. Some of these parameters may be more sensitive and specific than others as discussed above.
However, some measures were underrepresented in our systematic review which prevented any valid comparison (Fig. 5). Finally, while correlational research indicates that there may be a relationship between two variables (e.g., structure and function), it cannot assume causality and prove that one variable causes a change in another variable (e.g., Rousselet and Pernet 2012). This means that from the correlation data reviewed in this study, it is impossible to determine whether anatomical variability is driving behavior or if the anatomy results from an expressed behavior (i.e., directionality problem). In addition, it is also not possible to know whether a third factor mediates the changes in both variables and that the two variables are in fact not related (i.e., third variable problem). Future studies using correlational tractography may benefit from exploring other statistical frameworks such as Bayesian methods to get closer to establishing causal relationships between variables (Pacella et al. 2019). Systematic reviews cannot answer all clinically relevant questions as they are retrospective research projects and as such subject to bias. One bias that we were not able to mitigate in this review was the positive publication bias. We did not report negative correlations as they are not coherently reported in the literature. This review aimed to systematically evaluate and summarize current knowledge and going forward new initiatives such as registered reports should help reduce the positive publication bias associated with the clinical–anatomical correlation method (for an example, see Lazari et al. 2020). Functional white matter atlases (Thiebaut de Schotten et al. 2020) can also help to decode cognitive networks and individual assessment battery correlations (Talozzi et al. 2021).
In conclusion, acknowledging and objectively quantifying the magnitude of variability between each of us, particularly when it comes to brain anatomy (i.e. neurovariability), will potentially have a far-reaching impact on clinical practice. While some methodological refinement is needed in the field of white matter tractography (e.g., Dell'Acqua and Catani 2012; Wasserthal et al. 2018; Maier-Hein et al. 2017; Grisot et al. 2021), preliminary evidence indicates that differences in white matter phenotypes are beginning to explain disease progression and differential symptom presentations (Forkel et al. 2020). Variability in structural brain connections can also shed light on why current invasive and non-invasive treatments and therapies help some but not all patients (Lunven et al. 2019; Parlatini et al. 2021; Sanefuji et al. 2017). These findings are encouraging, as we move toward more personalised approaches to medicine. With the improvements suggested in this systematic review, tract–function correlations could be a useful adjunct in studies predicting resilience and recovery in patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank our reviewers and the Groupe d'imagerie neurofonctionnelle (GIN) whiteboard team for helpful discussions.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 101028551 (SJF) and the European Research Council (ERC) Consolidator grant agreement No. 818521 (MTdS).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afzali M, Pieciak T, Newman S, Garifallidis E, Özarslan E, Cheng H, Jones DK. The sensitivity of diffusion MRI to microstructural properties and experimental factors. J Neurosci Methods. 2021;347:108951. doi: 10.1016/j.jneumeth.2020.108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram H, Sotiropoulos SN, Jbabdi S, Georgiev D, Mahlknecht P, Hyam J, Foltynie T, Limousin P, De Vita E, Jahanshahi M, Hariz M, Ashburner J, Behrens T, Zrinzo L. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson's disease. Neuroimage. 2017;158:332–345. doi: 10.1016/j.neuroimage.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves PN, Fonseca AC, Silva DP, Andrade MR, Pinho-e-Melo T, Thiebaut de Schotten M, Martins IP. Unravelling the neural basis of spatial delusions after stroke. Ann Neurol. 2021;89:1181–1194. doi: 10.1002/ana.26079. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings H, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Johansen-Berg H, Thiebaut de Schotten M. The role of diffusion MRI in neuroscience. NMR Biomed. 2017 doi: 10.1002/nbm.3762. [DOI] [PubMed] [Google Scholar]
- Attar FM, Kirilina E, Haenelt D, Pine KJ, Trampel R, Edwards L, et al. Mapping short association fibers in the early cortical visual processing stream using in vivo diffusion tractography. Cereb Cortex. 2020 doi: 10.1093/cercor/bhaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD. Working memory, thought, and action. Oxford: Oxford University Press; 2007. p. 215. [Google Scholar]
- Baddeley AD, Hitch GJ. The phonological loop as a buffer store: An update. Cortex. 2019;112:91–106. doi: 10.1016/j.cortex.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Gathercole S, Papagno C. The Phonological loop as a language learning device. Psychol Rev. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Geletneky K, Jbabdi S. The temporo-parietal fiber intersection area and wernicke perpendicular fasciculus. Neurosurgery. 2013;73:E381–E382. doi: 10.1227/01.neu.0000430298.25585.1d. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beyh A, Dell’Acqua F, Sestieri C, Caulo M, Zappala G, Cancemi D, De Santiago RF, Della Sala S, Ffytche D, Catani M. The missing pathway in current visuospatial processing models. bioRxiv. 2021 doi: 10.1101/2021.06.08.442147. [DOI] [Google Scholar]
- Bouhali F, Thiebaut de Schotten M, Pinel P, Poupon C, Mangin JF, Dehaene S, Cohen L. Anatomical connections of the visual word form area. J Neurosci. 2014;34(46):15402–15414. doi: 10.1523/JNEUROSCI.4918-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cole MW, Yarkoni T. Vive les differences! Individual variation in neural mechanisms of executive control. Curr Opin Neurobiol. 2010;20(2):242–250. doi: 10.1016/j.conb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs RG, Conner AK, Sali G, Rahimi M, Baker CM, Burks JD, Glenn CA, Battiste JD, Sughrue ME. A connectomic atlas of the human cerebrum-chapter16: tractographic description of the vertical occipital fasciculus. Oper Neurosurg. 2018;15(1):S456–S461. doi: 10.1093/ons/opy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugain M, Dimech Y, Torzhenskaya N, et al. Occipital Intralobar fasciculi: a description, through tractography, of three forgotten tracts. Commun Biol. 2021;4:433. doi: 10.1038/s42003-021-01935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, D'Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20(5):762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Budisavljevic S, Dell'Acqua F, Rijsdijk FV, Kane F, Picchioni M, McGuire P, et al. Age related differences and heritability of the Perisylvian language networks. J Neurosci. 2015;35(37):12625–12634. doi: 10.1523/JNEUROSCI.1255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budisavljevic S, Kawadler JM, Dell'Acqua F, Rijsdijk FV, Kane F, Picchioni M, et al. Heritability of the limbic networks. Soc Cogn Affect Neurosci. 2016;5:746–757. doi: 10.1093/scan/nsv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock D, et al. Associative white matter connecting the dorsal and ventral posterior human cortex. Brain Struct Funct. 2019;224:2631–2660. doi: 10.1007/s00429-019-01907-8. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Diffusion tractography in deep brain stimulation surgery: a review. Front Neuroanat. 2016;10:45. doi: 10.3389/fnana.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A. On drawing inferences about the structure of normal cognitive systems from the analysis of patterns of impaired performance: the case for single-patient studies. Brain Cogn. 1986;5(1):41–66. doi: 10.1016/0278-2626(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Catani M, Ffytche D. The rises and falls of disconnection syndromes. Brain. 2005;128(10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones D, Ffytche D. Perisylvian language network of the human brain. Ann Neurol. 2004;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MPG, Husain M, Pugliese L, Mesulam M-M, Murray RM, et al. Symmetries in human brain language pathways correlate with verbal recall. PNAS. 2007;104(43):17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M, Craig MC, Forkel SJ, Kanaan R, Picchioni M, Toulopoulou T, Shergill S, Williams S, Murphy DG, McGuire P. Altered integrity of Perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol Psychiatry. 2011;70(12):1143–1150. doi: 10.1016/j.biopsych.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P, et al. Short frontal lobe connections of the human brain. Cortex. 2012;48(2):273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Robertsson N, Beyh A, Huynh V, de Santiago RF, Howells H, et al. Short parietal lobe connections of the human and monkey brain. Cortex. 2017;97:339–357. doi: 10.1016/j.cortex.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Caulo M, Briganti C, Mattei PA, Perfetti B, Ferretti A, Romani GL, et al. New morphologic variants of the hand motor cortex as seen with MR imaging in a large study population. Am J Neuroradiol. 2007;28(8):1480–1485. doi: 10.3174/ajnr.A0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Catani M, Johansen-Berg H, Clark CA, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7(8):715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- Craig M, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, et al. Altered connections on the road to psychopathy. Mol Psychiatry. 2009;14(10):946–53, 907. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Forkel SJ, Cerliani L, Thiebaut de Schotten M. Structural variability across the primate brain: a cross-species comparison. Cereb Cortex. 2018;28(11):3829–3841. doi: 10.1093/cercor/bhx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna L, Mesulam MM, Thiebaut de Schotten M, Dell'Acqua F, Murphy D, Wieneke C, et al. Frontotemporal networks and behavioral symptoms in primary progressive aphasia. Neurology. 2016;86(15):1393–1399. doi: 10.1212/WNL.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua F, Catani M. Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol. 2012;25(4):375–383. doi: 10.1097/WCO.0b013e328355d544. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua F, Tournier JD. Modelling white matter with spherical deconvolution: how and why? NMR Biomed. 2019;32(4):e3945. doi: 10.1002/nbm.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua F, Simmons A, Williams SC, Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp. 2013;34(10):2464–2483. doi: 10.1002/hbm.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Adolphs R. Building a science of individual differences from fMRI. Trends Cogn Sci. 2016;20(6):425–443. doi: 10.1016/j.tics.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109(3):461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Eichert N, Watkins KE, Mars RB, Petrides M. Morphological and functional variability in central and subcentral motor cortex of the human brain. BioRxiv. 2020 doi: 10.1101/2020.03.17.995035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D, Nordberg A, Westman E. Biological subtypes of Alzheimer disease: a systematic review and meta-analysis. Neurology. 2020;94(10):436–448. doi: 10.1212/WNL.0000000000009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris D, Howells H. Atypical structural and functional motor networks in autism. Prog Brain Res. 2018;238:207–248. doi: 10.1016/bs.pbr.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Dell'Acqua F, Kalra L, Murphy DG, Williams SC, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral Perisylvian language networks. Brain. 2014;137(Pt 7):2027–2039. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Kawadler JM, Dell'Acqua F, Danek A, Catani M. The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex. 2014;56:73–84. doi: 10.1016/j.cortex.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Rogalski E, Drossinos Sancho N, D'Anna L, Luque Laguna P, Sridhar J, Dell'Acqua F, et al. Anatomical evidence of an indirect pathway for word repetition. Neurology. 2020;94(6):e594–e606. doi: 10.1212/WNL.0000000000008746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Wood SJ, Whittle S, Fuller J, Adamson C, Saling MM, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum Brain Mapp. 2008;29(2):222–236. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnéhun S, Levy R, Rosso C, Urbanski M, et al. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. GigaScience. 2018;7(3):giy004. doi: 10.1093/gigascience/giy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88(3):585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Giampiccolo D, Howells H, Bährend I, Schneider H, Raffa G, Rosenstock T, Vergani F, Vajkoczy P, Picht T. Preoperative transcranial magnetic stimulation for picture naming is reliable in mapping segments of the arcuate fasciculus. Brain Commun. 2020;2(2):fcaa158. doi: 10.1093/braincomms/fcaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson ER, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC. A multi-modal parcellation of the human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt Drazen C, Gratton C, Sun H, et al. Precision functional mapping of individual human brains. Neuron. 2017;95:791–807.e7. doi: 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasby K, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, et al. The genetic architecture of the human cerebral cortex. Science. 2020;367(6484):eaay6690. doi: 10.1126/science.aay6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44(2):389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Grisot G, Haber SN, Yendiki A. Diffusion MRI and anatomic tracing in the same brain reveal common failure modes of tractography. Neuroimage. 2021;239:118300. doi: 10.1016/j.neuroimage.2021.118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara P, Duclap D, Poupon C, Marrakchi-Kacem L, Fillard P, Le Bihan D, et al. Automatic fiber bundle segmentation in massive tractography datasets using a multi subject bundle atlas. Neuroimage. 2012;61(4):1983–1099. doi: 10.1016/j.neuroimage.2012.02.071. [DOI] [PubMed] [Google Scholar]
- Guevara M, Guevara P, Román C, Mangin JF. Superficial white matter: a review on the dMRI analysis methods and applications. Neuroimage. 2020 doi: 10.1016/j.neuroimage.2020.116673. [DOI] [PubMed] [Google Scholar]
- Güngör A, Baydin S, Middlebrooks EH, Tanriover N, Isler C, Rhoton AL., Jr The white matter tracts of the cerebrum in ventricular surgery and hydrocephalus. J Neurosurg. 2017;126(3):945–971. doi: 10.3171/2016.1.JNS152082. [DOI] [PubMed] [Google Scholar]
- Hau J, Sarubbo S, Perchey G, Crivello F, Zago L, Mellet E, Jobard G, Joliot M, Mazoyer BM, Tzourio-Mazoyer N, Petit L. Cortical terminations of the inferior fronto-occipital and uncinate fasciculi: anatomical stem-based virtual dissection. Front Neuroanat. 2016;10:58. doi: 10.3389/fnana.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Bijsterbosch JD, Segerdahl AR, Fitzgibbon SP, Farahibozorg SR, Duff EP, et al. Modelling subject variability in the spatial and temporal characteristics of functional modes. Neuroimage. 2020;222:117226. doi: 10.1016/j.neuroimage.2020.117226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. Behavior genetics and individuality understood. Science. 1963;142(3598):1436–1442. doi: 10.1126/science.142.3598.1436. [DOI] [PubMed] [Google Scholar]
- Howells H, Thiebaut de Schotten M, Dell'Acqua F, Beyh A, Zappalà G, Leslie A, Simmons A, et al. Frontoparietal tracts linked to lateralized hand preference and manual specialization. Cereb Cortex. 2018;28(7):2482–2494. doi: 10.1093/cercor/bhy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells H, Puglisi G, Leonetti A, Vigano L, Fornia L, Simone L, et al. The role of left fronto parietal tracts in hand selection: evidence from neurosurgery. Cortex. 2020 doi: 10.1016/j.cortex.2020.03.018. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Network causality, axonal computations, and Poffenberger. Exp Brain Res. 2017;235:2349–2357. doi: 10.1007/s00221-017-4948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Johansen-Berg H. Tractography: where do we go from here? Brain Connect. 2011;1(3):169–183. doi: 10.1089/brain.2011.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends Cogn Sci. 2004;8(2):71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Duffley G, Anderson DN, Ostrem JL, Welter ML, Baldermann JC, et al. Structural connectivity predicts clinical outcomes of deep brain stimulation for Tourette syndrome. Brain. 2020;143(8):2607–2623. doi: 10.1093/brain/awaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44(8):936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Jones DK (2009) Gaussian modeling of the diffusion signal in diffusion MRI Editor(s): Heidi Johansen-Berg, Timothy E.J. Behrens, pp 37–54. 10.1016/B978-0-12-374709-9.00003-1
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12(4):231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Naeser MA, Martin PI, Ho M, Wang Y, Baker E, Pascual-Leone A. Horizontal portion of arcuate fasciculus fibers track to pars opercularis, not pars triangularis, in right and left hemispheres: a DTI study. Neuroimage. 2010;52(2):436–444. doi: 10.1016/j.neuroimage.2010.04.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolis V, Corbetta M, Thiebaut de Schotten M. The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nat Commun. 2019;10:1417. doi: 10.1038/s41467-019-09344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keser Z, Ucisik-Keser FE, Hasan KM. Quantitative mapping of human brain vertical-occipital fasciculus. J Neuroimaging. 2016;26(2):188–193. doi: 10.1111/jon.12268. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25(5):677–691. [PMC free article] [PubMed] [Google Scholar]
- Kong XZ, Mathias SR, Guadalupe T, ENIGMA Laterality Working Group, Glahn DC, Franke B, et al (2018) Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. PNAS 115(22): E5154–E5163 [DOI] [PMC free article] [PubMed]
- Lawes IN, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, et al. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 2008;39(1):62–79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Lazari A, et al. Heterogeneous relationships between white matter and behaviour. bioRxiv. 2020 doi: 10.1101/2020.12.15.422826. [DOI] [Google Scholar]
- Le Bihan D, Breton E. Imagerie de diffusion in vivo par re résonance magnétique nucléaire. Comptes-Rendus de l'Académie des Sciences. 1985;93(5):27–34. [Google Scholar]
- Lebel C, Treit S, Beaulieu C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019;32(4):e3778. doi: 10.1002/nbm.3778. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Puranik C, Kuldau JM, Lombardino LJ. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl's gyrus: where is it? Cereb Cortex. 1998;8(5):397–406. doi: 10.1093/cercor/8.5.397. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Van Der Kouwe AJW, Raznahan A, Paus T, Johansen-Berg H, Miller KL, Smith SM, Fischl B, Sotiropoulos SN. Studying neuroanatomy using MRI. Nat Neurosci. 2017;20:314–326. doi: 10.1038/nn.4501. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Linden D. Unique: the new science of human individuality. New York: BasicBooks; 2020. [Google Scholar]
- López-Barroso D, Catani M, Ripollés P, Dell'Acqua F, Rodríguez-Fornells A, de Diego BR. Word learning is mediated by the left arcuate fasciculus. Proc Natl Acad Sci U S A. 2013;110(32):13168–13173. doi: 10.1073/pnas.1301696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunven M, Rode G, Bourlon C, Duret C, Migliaccio R, Chevrillon E, et al. Anatomical predictors of successful prism adaptation in chronic visual neglect. Cortex. 2019;120:629–641. doi: 10.1016/j.cortex.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Lythgoe MF, Busza AL, Calamante F, Sotak CH, King MD, Bingham AC, Williams SR, Gadian DG. Effects of diffusion anisotropy on lesion delineation in a rat model of cerebral ischemia. Magn Reson Med. 1997;38(4):662–668. doi: 10.1002/mrm.1910380421. [DOI] [PubMed] [Google Scholar]
- Maffei C, Sarubbo S, Jovicich J. A missing connection: a review of the macrostructural anatomy and tractography of the acoustic radiation. Front Neuroanat. 2019;13:27. doi: 10.3389/fnana.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei C, Sarubbo S, Jovicich J. Diffusion-based tractography atlas of the human acoustic radiation. Sci Rep. 2019;9:4046. doi: 10.1038/s41598-019-40666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein KH, Neher PF, Houde JC, Côté MA, Garyfallidis E, Zhong J, Chamberland M, Yeh FC, Lin YC, Ji Q, Reddick WE, Glass JO, Chen DQ, Feng Y, Gao C, Wu Y, Ma J, He R, Li Q, Westin CF, Deslauriers-Gauthier S, González JOO, Paquette M, St-Jean S, Girard G, Rheault F, Sidhu J, Tax CMW, Guo F, Mesri HY, Dávid S, Froeling M, Heemskerk AM, Leemans A, Boré A, Pinsard B, Bedetti C, Desrosiers M, Brambati S, Doyon J, Sarica A, Vasta R, Cerasa A, Quattrone A, Yeatman J, Khan AR, Hodges W, Alexander S, Romascano D, Barakovic M, Auría A, Esteban O, Lemkaddem A, Thiran JP, Cetingul HE, Odry BL, Mailhe B, Nadar MS, Pizzagalli F, Prasad G, Villalon-Reina JE, Galvis J, Thompson PM, Requejo FS, Laguna PL, Lacerda LM, Barrett R, Dell'Acqua F, Catani M, Petit L, Caruyer E, Daducci A, Dyrby TB, Holland-Letz T, Hilgetag CC, Stieltjes B, Descoteaux M. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 2017;8(1):1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam SA. Challenges of the anatomy and diffusion tensor tractography of the meyer loop. Am J Neuroradiol. 2012;33:1204–1210. doi: 10.3174/ajnr.A2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Sarubbo S, Petit L. The nomenclature of human white matter association pathways: proposal for a systematic taxonomic anatomical classification. Front Neuroanat. 2018;12:94. doi: 10.3389/fnana.2018.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Foxley S, Verhagen L, Jbabdi S, Sallet J, Noonan MP, et al. The extreme capsule fiber complex in humans and macaque monkeys: a comparative diffusion MRI tractography study. Brain Struct Funct. 2016;221(8):4059–4071. doi: 10.1007/s00429-015-1146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Molina N, Mas-Herrero E, Rodríguez-Fornells A, Zatorre RJ, Marco-Pallarés J. White matter microstructure reflects individual differences in music reward sensitivity. J Neurosci. 2019;39(25):5018–5027. doi: 10.1523/JNEUROSCI.2020-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, García-Porrero JA. Wernicke perpendicular fasciculus and vertical portion of the superior longitudinal fasciculus. Neurosurgery. 2013;73:E382–E383. doi: 10.1227/01.neu.0000430303.56079.0e. [DOI] [PubMed] [Google Scholar]
- Middlebrooks EH, Domingo RA, Vivas-Buitrago T, Okromelidze L, Tsuboi T, Wong JK, Eisinger RS, Almeida L, Burns MR, Horn A, Uitti RJ, Wharen RE, Jr, Holanda VM, Grewal SS. Neuroimaging advances in deep brain stimulation: review of indications, anatomy, and brain connectomics. AJNR Am J Neuroradiol. 2020;41(9):1558–1568. doi: 10.3174/ajnr.A6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirchandani AS, Beyh A, Lavrador JP, Howells H, Dell'Acqua F, Vergani F. Altered corticospinal microstructure and motor cortex excitability in gliomas: an advanced tractography and transcranial magnetic stimulation study. J Neurosurg. 2020;1:1–9. doi: 10.3171/2020.2.JNS192994. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Mori S, Oishi K, Faria AV. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol. 2009;22(4):362–369. doi: 10.1097/WCO.0b013e32832d954b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, Wendland MF, Weinstein PR. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990;14(2):330–346. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- Munsell BC, Gleichgerrcht E, Hofesmann E, Delgaizo J, McDonald CR, Marebwa B, et al. Personalised connectome fingerprints: Their importance in cognition from childhood to adult years. Neuroimage. 2020;221:117122. doi: 10.1016/j.neuroimage.2020.117122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R, Kinoshita M, Shinohara H, Nakada M. The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging Behav. 2019 doi: 10.1007/s11682-019-00187-4. [DOI] [PubMed] [Google Scholar]
- Nozais V, Forkel SJ, Foulon C, et al. Functionnectome as a framework to analyse the contribution of brain circuits to fMRI. Commun Biol. 2021;4:1035. doi: 10.1038/s42003-021-02530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. Stuttgart: Thieme; 1990. [Google Scholar]
- Pacella V, Foulon C, Jenkinson PM, Scandola M, Bertagnoli S, Avesani R, et al. Anosognosia for hemiplegia as a tripartite disconnection syndrome. Elife. 2019;8:e46075. doi: 10.7554/eLife.46075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palejwala AH, O'Connor KP, Pelargos P, Briggs RG, Milton CK, Conner AK, Milligan TM, O'Donoghue DL, Glenn CA, Sughrue ME. Anatomy and white matter connections of the lateral occipital cortex. Surg Radiol Anat. 2020;42(3):315–328. doi: 10.1007/s00276-019-02371-z. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cerebral cortex: implications for brain evolution and function. New York: The Guilford Press; 1990. [Google Scholar]
- Panesar SS, Belo JTA, Yeh FC, Fernandez-Miranda JC. Structure, asymmetry, and connectivity of the human temporo-parietal aslant and vertical occipital fasciculi. Brain Struct Funct. 2019;224(2):907–923. doi: 10.1007/s00429-018-1812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno C, Comi A, Riva M, Bizzi A, Vernice M, Casarotti A, Fava E, Bello L. Mapping the brain network of the phonological loop. Hum Brain Mapp. 2017;38(6):3011–3024. doi: 10.1002/hbm.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlatini V, Radua J, Whickers R, Maltezos S, Sanefuji M, Dell’Acqua F et al (2021) The anatomy of attentive brain networks predicts response to stimulant treatment in adults with attention deficit hyperactivity disorder (ADHD), under review
- Powell HWR, Parker GJM, Alexander D, Symms MR, Boulby PA, Wheeler-Kingshott CAM, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Puglisi G, Howells H, Sciortino T, Leonetti A, Rossi M, Conti Nibali M, Gabriel Gay L, Fornia L, Bellacicca A, Viganò L, Simone L, Catani M, Cerri G, Bello L. Frontal pathways in cognitive control: direct evidence from intraoperative stimulation and diffusion tractography. Brain. 2019;142(8):2451–2465. doi: 10.1093/brain/awz178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, Caviness VS, Jr, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3(4):313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- Rigoard P, Buffenoir K, Jaafari N, Giot JP, Houeto JL, Mertens P, et al. The accumbofrontal fasciculus in the human brain: a microsurgical anatomical study. Neurosurgery. 2011;68(4):1102–1111. doi: 10.1227/NEU.0b013e3182098e48. [DOI] [PubMed] [Google Scholar]
- Rilling J, Glasser M, Jbabdi S, Andersson J, Preuss T. Continuity, divergence, and the evolution of brain language pathways. Front Evol Neurosci. 2012;3:11. doi: 10.3389/fnevo.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS. What we can learn from the complex architecture of single axons. Brain Struct Funct. 2020 doi: 10.1007/s00429-019-02023-3. [DOI] [PubMed] [Google Scholar]
- Rojkova K, Volle E, Urbanski M, Humbert F, Dell'Acqua F, Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct Funct. 2016;221(3):1751–66. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Pernet CR. Improving standards in brain-behavior correlation analyses. Front Hum Neurosci. 2012;6:119. doi: 10.3389/fnhum.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs H (1892) Das Hemisphaerenmark des menschlichen Grosshirns. I Der Hinterhauptlappen. Leipzig: Georg Thieme Verlag
- Sanefuji M, Craig M, Parlatini V, Mehta M, Murphy DG, Catani M, et al. Double dissociation between the mechanism leading to impulsivity and inattention in attention deficit hyperactivity disorder: a resting-state functional connectivity study. Cortex. 2017;86:290–302. doi: 10.1016/j.cortex.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Tate M, De Benedictis A, Merler S, Moritz-Gasser S, Herbet G, Duffau H. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. Neuroimage. 2020;205:116237. doi: 10.1016/j.neuroimage.2019.116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tararasch R, Assaf Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front Neurosci. 2013;7:32. doi: 10.3389/fnins.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling KG, Nath V, Hansen C, Parvathaneni P, Blaber J, Gao Y, Neher P, Aydogan DB, Shi Y, Ocampo-Pineda M, Schiavi S, Daducci A, Girard G, Barakovic M, Rafael-Patino J, Romascano D, Rensonnet G, Pizzolato M, Bates A, Fischi E, Thiran JP, Canales-Rodríguez EJ, Huang C, Zhu H, Zhong L, Cabeen R, Toga AW, Rheault F, Theaud G, Houde JC, Sidhu J, Chamberland M, Westin CF, Dyrby TB, Verma R, Rathi Y, Irfanoglu MO, Thomas C, Pierpaoli C, Descoteaux M, Anderson AW, Landman BA. Limits to anatomical accuracy of diffusion tractography using modern approaches. Neuroimage. 2019;185:1–11. doi: 10.1016/j.neuroimage.2018.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr R, Filo S, Mezer AA. Tractography delineation of the vertical occipital fasciculus using quantitative T1 mapping. Neuroimage. 2019;202:116121. doi: 10.1016/j.neuroimage.2019.116121. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Interpreting and utilising intersubject variability in brain function. Trends Cogn Sci. 2018;22(6):517–530. doi: 10.1016/j.tics.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Su M, Thiebaut de Schotten M, Zhao J, Song S, Zhou W, Gong G, et al. Influences of the early family environment and long-term vocabulary development on the structure of white matter pathways: a longitudinal investigation. Dev Cogn Neurosci. 2020;42:100767. doi: 10.1016/j.dcn.2020.100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taebi A, Kiesow H, Vogeley K, Schilbach L, Bernhardt BC, Bzdok D. Population variability in social brain morphology for social support, household size and friendship satisfaction. Soc Cogn Affect Neurosci. 2020;15(6):635–647. doi: 10.1093/scan/nsaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H, Thiebaut de Schotten M. Perspectives given by structural connectivity bridge the gap between structure and function. Brain Struct Funct. 2020;225:1189–1192. doi: 10.1007/s00429-020-02080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H, Pestilli F, Weiner KS, Keliris GA, Landi SM, Sliwa J, Ye FQ, Barnett MA, Leopold DA, Freiwald WA, Logothetis NK, Wandell BA. Occipital white matter tracts in human and macaque. Cereb Cortex. 2017;27(6):3346–3359. doi: 10.1093/cercor/bhx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talozzi L, Forkel SJ, Pacella V, Nozais V, Nachev P, Corbetta M, Thiebaut De Schotten M. (preprint) Large scale disconnectome prediction of individual symptom severity in chronic stroke
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TB, Jbabdi S. Task-free MRI predicts individual differences in brain activity during task performance. Science. 2016;352(6282):216–220. doi: 10.1126/science.aad8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Shallice T. Identical, similar or different? Is a single brain model sufficient? Cortex. 2017;86:172–175. doi: 10.1016/j.cortex.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, et al. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. 2011;54(1):49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex. 2012;24(4):989–995. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Foulon C, Nachev P. Brain disconnections link structural connectivity with function and behaviour. Nat Commun. 2020;11(1):5094. doi: 10.1038/s41467-020-18920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Murphy D, Dell'Acqua F, Ecker C, McAlonan G, Howells H, et al. Impaired communication between the motor and somatosensory homunculus is associated with poor manual dexterity in autism spectrum disorder. Biol Psychiatry. 2017;81(3):211–219. doi: 10.1016/j.biopsych.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treu S, Strange B, Oxenford S, Neumann WJ, Kühn A, Li N, Horn A. Deep brain stimulation: imaging on a group level. Neuroimage. 2020;219:11708. doi: 10.1016/j.neuroimage.2020.117018. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Rajkowska G, Sanz-Arigita E, Amunts K, Zilles K. Consequences of large interindividual variability for human brain atlases: converging macroscopical imaging and microscopical neuroanatomy. Anat Embryol. 2005;210(5–6):423–431. doi: 10.1007/s00429-005-0042-4. [DOI] [PubMed] [Google Scholar]
- Vanderauwera J, De Vos A, Forkel SJ, Catani M, Wouters J, Vandermosten M, Ghesquière P. Neural organization of ventral white matter tracts parallels the initial steps of reading development: a DTI tractography study. Brain Lang. 2018;183:32–40. doi: 10.1016/j.bandl.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Vanderweyen DC, Theaud G, Sidhu J, Rheault F, Sarubbo S, Descoteaux M, Fortin D. The role of diffusion tractography in refining glial tumor resection. Brain Struct Funct. 2020;225(4):1413–1436. doi: 10.1007/s00429-020-02056-z. [DOI] [PubMed] [Google Scholar]
- Vavassori L, Sarubbo S, Petit L. Hodology of the superior longitudinal system of the human brain: a historical perspective, the current controversies, and a proposal. Brain Struct Funct. 2021;226(5):1363–1384. doi: 10.1007/s00429-021-02265-0. [DOI] [PubMed] [Google Scholar]
- Vergani F, Mahmood S, Morris CM, Mitchell P, Forkel SJ. Intralobar fibres of the occipital lobe: a post mortem dissection study. Cortex. 2014;56:145–156. doi: 10.1016/j.cortex.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Vergani F, Martino J, Morris C, Attems J, Ashkan K, DellʼAcqua F. Anatomic connections of the subgenual cingulate region. Neurosurgery. 2016;79(3):465–472. doi: 10.1227/NEU.0000000000001315. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G. Phenotypes in hemispheric functional segregation? Perspectives and challenges. Phys Life Rev. 2019;30:1–18. doi: 10.1016/j.plrev.2019.06.002. [DOI] [PubMed] [Google Scholar]
- Wang Q, Akram H, Muthuraman M, Gonzalez-Escamilla G, Sheth SA, Oxenford S, et al. Normative vs. patient-specific brain connectivity in deep brain stimulation. Neuroimage. 2021;224:117307. doi: 10.1016/j.neuroimage.2020.117307. [DOI] [PubMed] [Google Scholar]
- Wasserthal J, Neher P, Maier-Hein KH. TractSeg—fast and accurate white matter tract segmentation. Neuroimage. 2018;183:239–253. doi: 10.1016/j.neuroimage.2018.07.070. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Yeatman JD, Wandell BA. The posterior arcuate fasciculus and the vertical occipital fasciculus. Cortex. 2017;97:274–276. doi: 10.1016/j.cortex.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About "axial" and "radial" diffusivities. Magn Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- White D, Rabago-Smith M. Genotype–phenotype associations and human eye color. J Hum Genet. 2011;56:5–7. doi: 10.1038/jhg.2010.126. [DOI] [PubMed] [Google Scholar]
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;10(3):25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 2013;125(2):146–155. doi: 10.1016/j.bandl.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Weiner KS, Pestilli F, Rokem A, Mezer A, Wandell BA. The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc Natl Acad Sci U S A. 2014;111(48):E5214–E5223. doi: 10.1073/pnas.1418503111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.