Abstract
Objective:
1) Describe the progression of diabetes mellitus over time in an observational study of Wolfram syndrome, a rare, genetic, neurodegenerative disorder which often includes diabetes mellitus and is typically diagnosed during childhood or adolescence. 2) Determine whether C-peptide could be used as a marker of diabetes progression in interventional trials for Wolfram syndrome.
Methods:
N=44 (25F/19M) participants with genetically-confirmed Wolfram syndrome attended the Washington University Wolfram Research Clinic annually from 2010–2019. Medical history, physical examinations, blood sampling, and questionnaires were used to collect data about diabetes mellitus and other components of Wolfram syndrome. Beta-cell function was assessed by determination of C-peptide during a mixed meal tolerance test. Random coefficients models evaluated the rate of progression of C-peptide over time, and power analyses were used to estimate the number of subjects needed to detect a change in C-peptide decline during an intervention trial.
Results:
93.2% of patients had diabetes mellitus. Mean HbA1c across all study visits was 7.9%. C-peptide significantly decreased with increasing duration of diabetes mellitus (p<0.0001); an optimal break point in C-peptide decline was identified to occur between 0.1 and 2.3 years after diabetes mellitus diagnosis. Twenty patients per group (active vs. control) were estimated to be needed to detect a 60% slowing of C-peptide decline during the first 2.3 years following diabetes diagnosis.
Conclusion:
C-peptide declines over time in Wolfram syndrome and could potentially be used as a marker of diabetes progression in interventional studies for Wolfram syndrome, especially within the first 2 years after diabetes diagnosis.
Keywords: DIDMOAD, Neurodegenerative Disease, C-Peptide, Hb A1c
Introduction
Wolfram syndrome (OMIM #222300), also known as DIDMOAD (Diabetes Insipidus, Diabetes Mellitus, Optic Atrophy, Deafness), is a rare, genetic, neurodegenerative disorder estimated to affect 1 in 770,000 to 1 in 500,000 people, and it is typically diagnosed during childhood or adolescence1–3. Wolfram syndrome is most often caused by mutations in the WFS1 gene, located on chromosome 4p16.1, which results in increased endoplasmic reticulum stress and subsequent cell death, with pancreatic beta-cells and neurons being particularly susceptible4–5. Wolfram syndrome is typically characterized by some combination of diabetes mellitus, diabetes insipidus, optic atrophy, hearing defects, urological dysfunction, and neurodevelopmental abnormalities, with widely varying phenotypes across patients1,6. Recent studies from our cohort and others have shown progressive changes in hearing7, vision8,9, metabolites10, and brain development/neurodegeneration11; however, little remains known about the course of progression of diabetes mellitus, which has been shown to affect over 98% of patients with Wolfram syndrome6.
Not only are descriptions of diabetes mellitus-related symptoms within Wolfram syndrome important alone, but determining how they change longitudinally, could potentially identify reliable markers of disease progression for use in intervention studies for Wolfram syndrome. The longitudinal Washington University Wolfram Syndrome Research Clinic study has already found two markers of Wolfram syndrome progression: visual acuity9 and regional brain volumes (e.g., thalamus, ventral pons)11. However, diabetes mellitus is of particular interest as a marker of progression as it is usually the earliest and most consistent feature of Wolfram syndrome to be recognized6, typically has an abrupt clinical presentation with clear signs and symptoms, and has a clear and easily determined diagnosis based on commonly available blood tests (e.g., glucose, Hemoglobin A1c; HbA1c). Further, diabetes mellitus is the Wolfram syndrome feature most well-defined mechanistically and there is a depth of knowledge about how WFS1 mutations affect the beta-cell. These characteristics could be particularly advantageous compared to other measures of disease progression, such as vision loss or neurodegenerative measures, which may require more expensive and time-intensive testing, and may have a more insidious onset1,7,12. Thus, in addition to describing diabetes mellitus measures over time, the current study also aimed to determine whether C-peptide, a measure of beta-cell function, could potentially be used as a marker of early progression in intervention trials for Wolfram syndrome.
Methods
Participants
Subjects were identified through the Washington University International Wolfram Syndrome Registry, the Pediatric Diabetes Clinic at St. Louis Children’s Hospital, or direct referral. Requirements for enrollment included genetically confirmed WFS1 disease-associated mutations and age less than 30 years old at time of enrollment. These criteria were established to select subjects most likely to have a progression of disease rapid enough to be detected over several years. Enrolled subjects were seen at an annual Washington University Wolfram Research Clinic held each summer from 2010 to 2019 (excluding 2018). The study was approved by the Human Research Protection Office at Washington University in St. Louis and informed consent was obtained prior to testing for all participants. For children under age 18, parents/guardians provided written consent and children provided assent.
Assessments
Overall, subjects provided medical, family, and surgical history; had a physical examination (by BAM or NHW); had vision, hearing, smell identification, taste, cognition, psychological, balance, gait, and motor neurological function evaluations; and, if eligible, had neuroimaging (MRI). Subsets of these data have been previously published7,9,11,13–25. However, we focus here on diabetes mellitus-related tests and questionnaires outlined in detail below. Data were managed in REDCap, a web-based electronic database housed in the Institute for Informatics of Washington University School of Medicine26.
Diabetes Mellitus Measures
Descriptive data for diabetes mellitus including age of diabetes onset and medications were obtained via interviews and reference to medical records at the clinic visits. To assess beta-cell function, subjects underwent a mixed meal tolerance test (MMTT) on one morning of each annual research clinic that they attended. The night before the MMTT, subjects fasted from midnight until the start of the test at 8AM and adjusted their insulin dosing as needed based on self-glucose monitoring to avoid hypoglycemia. The mixed meal consisted of 6 ml/kg (maximum 360 ml) of BOOST Original (Nestle, Vevey, Switzerland), providing 1 g/kg carbohydrate, 0.25 g/kg protein, and 0.1 g/kg fat. C-peptide, HbA1c, and glucose levels were drawn at time 0 (fasting; before the BOOST), and C-peptide and glucose were drawn 30 minutes after the mixed meal (stimulated; after the BOOST). The higher of the pre- and post-meal C-peptide values (peak C-peptide) was used for all data analyses, with the reasoning that many subjects presented for the MMTT with some elevation in their fasting glucose and thus with a pre-existing stimulus for C-peptide secretion. If a subject’s fasting glucose exceeded 250 mg/dL (13.9 mmol/l), BOOST was not given, but fasting C-peptide and HbA1c were still collected. In these cases, the baseline C-peptide (when blood glucose exceeded 250 mg/dL (13.9 mmol/l) was considered the peak C-peptide for purposes of data analysis. Similar assays were used for C-peptide over time; however, the samples were analyzed on different systems across the longitudinal study as there were equipment updates at the Washington University Core Laboratory for Clinical Studies. From 2010–2012 samples were run using Immulite C-peptide kits on a Siemens Immulite 1000; in 2013, samples were run by electrochemiluminecense using Roche Elecsys C-peptide kits on a Roche Elecsys 2010 analyzer; and from 2014–2019, C-peptide was analyzed by electrochemiluminecense using Roche Elecsys C-peptide kits on a Roche e601 analyzer. Most were analyzed promptly using fresh samples, with the exception of the 2019 clinic where n=33 of the samples were frozen for approximately 6 weeks and analyzed in batch.
A standard length (4-hour) MMTT was considered, but it was not thought to be in the subjects’ best interest as they are medically and psychologically fragile, often with diabetes insipidus in addition to diabetes mellitus, and carrying out the full-length MMTT could result in subjects being extremely hyperglycemic for a more extended time period. The standard MMTT also could have led to complications with the interpretation of other data collected during the research clinic days, particularly brain MRI data, cognitive data, and detailed neurologic testing that is subject to effects of dehydration and hyperosmolarity. Similarly, while an effort was made to standardize the insulin dosing the night prior to the MMTT, hospital admission overnight was not feasible and insulin dose adjustment on an outpatient basis using a standardized approach was not acceptable in a majority of subjects; thus, pre-MMTT insulin doses were determined on an individual basis based on consultation with one of the authors (BAM) to avoid both hypo- and hyperglycemia during the night.
Statistical Methods
Descriptive statistics were calculated using SPSS Version 26 and Microsoft Excel. Kaplan-Meier survival estimates were obtained, and a Log-rank test was conducted to determine if age of onset curves differed between males and females. Random coefficients models were conducted to relate HbA1c to age, and C-peptide to diabetes mellitus duration only in subjects with a diabetes mellitus diagnosis. One participant with diabetes mellitus refused blood draw and, thus, did not have C-peptide measures. One patient developed diabetes mellitus after enrollment in the study; only data collected after this patient’s diabetes mellitus diagnosis were included in the random coefficients models.
To better define the pattern of change in beta-cell function as determined by C-peptide, a piecewise random slope model based on a grid search method was conducted to determine the breakpoint at which C-peptide decline slows (SAS Version 9.4). Power analyses using the estimated annual rate of change in C-peptide were performed to determine the sample size needed to detect a significant effect of an intervention on beta-cell function after the diagnosis of diabetes mellitus. Extensive simulations were conducted to determine the sample size to achieve at least 80% power to detect a 50% or 60% reduction in mean annual rate of decline in beta-cell function (as determined by peak C-peptide) at a significance level of 0.05. We assumed that a trial would measure the outcome every six months during a 3-year follow-up. Mean trajectories were simulated using the random coefficients model assuming mean annual rate of progression in the placebo group estimated from the Wolfram syndrome group data and a random slope distributed N (0, δ2) and homoscedastic error distributed N (0, Ɛ2) with both δ2 and Ɛ2 estimated from the Wolfram syndrome group data. For this sample size estimate, means of C-peptide measures were assumed to be equal at baseline for the two groups. We assumed equal allocation between the two groups (treatment vs. placebo) and 1000 simulated trials were analyzed with empirical power calculated. The log scale of C-peptide was used for all mixed model analyses to meet the normality assumption. All statistical tests were two-sided and significance was set at p<0.05.
Results
Participants
A total of n=44 participants (25F/19M) were enrolled and attended at least one research clinic between 2010 and 2019, with attendance ranging from 1 to 8 visits (n=5 had < 2 visits; n=18 had 2–4 visits; n=12 had 4–6 visits; n=9 had > 6 visits). Descriptive statistics for the study sample are outlined in Table 1.
Table 1.
Demographic and age of disease onset data for diabetes mellitus and Wolfram syndrome; IQR=interquartile range; q1=25%; q3=75%.
| Variable | n | Descriptive statistics |
|---|---|---|
| Age at first visit (mean ± SD, range) | 44 | 13.5 ± 5.9, 4.8–27.2 |
| Age at most recent visit (mean ± SD, range) | 44 | 18.5 ± 6.5, 4.8–33.7 |
| Ethnicity | ||
| Non-Hispanic White | 29 | 65.9% |
| Hispanic White | 2 | 4.5% |
| Non-White Hispanic | 13 | 29.5% |
| Full DIDMOAD† phenotype | 21 | 47.7% |
| Age of Wolfram syndrome diagnosis (mean ± SD, range) | 43‡ | 11.0 ± 4.9, 2.7–25.4 |
| Median Wolfram syndrome age of diagnosis (IQR q1-q3) | 43‡ | 10.1 (7.1–14.3) |
| Diabetes mellitus age of diagnosis (mean ± SD, range) | 41 | 5.8 ± 2.7, 2.3–14.0 |
| Median diabetes mellitus age of diagnosis (IQR q1-q3) | 41 | 5.0 (4.4–6.7) |
| Difference between Wolfram and diabetes mellitus age of diagnosis (mean ± SD, range) | 40‡ | 5.2 ± 4.9, − 2.81–17.16 |
| Median difference between Wolfram and diabetes mellitus age of diagnosis (IQR q1-q3) | 40‡ | 3.7 (1.1–9.6) |
DIDMOAD=carries a diagnosis of all the following Wolfram features: diabetes insipidus (DI), diabetes mellitus (DM), optic atrophy (OA), and deafness (D).
One participant with type 1 diabetes had missing data for age of Wolfram syndrome diagnosis.
Diabetes Mellitus Measures
A total of 93.2% (n=41; 23F/18M) of Wolfram syndrome subjects had diabetes mellitus at time of enrollment (n=40; 22F/18M; 90.9%) or developed it during the study (n=1, 1F; 2.3%). Three participants had not been diagnosed with diabetes mellitus as of their last clinic visit (two females, ages 12 and 15, and one male, age 16) and there was no significant difference in frequency of diabetes mellitus diagnosis across sex (Chi2=1.27, df=1 p=0.72). There was also no significant difference in age of diabetes mellitus onset between males and females (Chi2=1.21, df=1, p=0.27).
At their most recent clinic visit, 75.6% of subjects with diabetes mellitus (n=31) were taking rapid-acting insulin (e.g., insulin aspart, insulin lispro, and insulin glulisine) either alone with pump therapy or in combination with long-acting insulin with multiple daily injections, 9.8% (n=4) were taking a mix of short- and intermediate-acting insulin (e.g., insulin regular and NPH), 2.4% (n=1) were taking a mix of rapid-acting and intermediate-acting insulin (e.g., insulin lispro and NPH), and the type of insulin used was unknown for 12.2% (n=5) of participants. The mean HbA1c and C-peptide levels are outlined in Table 2. There was no statistically significant linear relationship between HbA1c and age (t=0.99, df=32 p=0.33) and HbA1c across visits did not differ by sex (F=1.38, df= 85, p=0.24) or age at diabetes mellitus onset (F=0.40, df=85, p=0.53).
Table 2.
Diabetes Mellitus measures in patients with Wolfram syndrome and Diabetes Mellitus.
| Mean (SD) | n | Range | |
|---|---|---|---|
| Overall Fasting C-peptide across all visits | 0.37 (0.35) | 158 | 0.01–1.97 |
| Overall Stimulated C-peptide across all visits | 0.76 (0.68) | 88 | 0.13–3.62 |
| HbA1c at first clinic visit | 7.9 (1.43) | 40 | 5.9–12.4 |
| HbA1c across all visits | 7.9 (1.34) | 157 | 5.4–13.8 |
There was a significant linear relationship between peak C-peptide (log scale) and diabetes mellitus duration, such that peak C-peptide (log scale) decreased over time (Akaike Information Criterion= 363.6, F=64.77, df= 38, p< 0.0001). For each year of diabetes mellitus, peak C-peptide declined by 0.1360 ng/ml and the rate of change in peak C-peptide did not differ by sex (F=2.40, df= 121, p=0.12) or age of diabetes mellitus onset (F=1.42, df=121, p=0.24). The piecewise random slope model fit the data better than the linear model when there was a break point in peak C-peptide decline between 0.1 (Akaike Information Criterion=355.4) and 2.3 (Akaike Information Criterion=363.5) years after diabetes mellitus onset. The estimated slope of decline before 2.3 years of diabetes mellitus was significantly different from 0 (t=−2.00, df= 122, p=0.048) with peak C-peptide levels declining 0.37 ng/ml for each year of diabetes mellitus. The estimated slope of decline after 2.3 years was also significantly different from 0 (t=−7.10, df=122, p<0.0001), and peak C-peptide levels declined 0.13ng/ml for each year of diabetes mellitus. Using the model fit statistic Akaike Information Criterion any time point between 0.1 and 2.3 years could be the optimal breakpoint.
However, there was no significant difference in slope before and after the breakpoint period (p>0.05). Further, all of the estimated slopes after the breakpoint period had a 95% confidence interval below 0 suggesting no clear detection of a plateau in C-peptide levels after the breakpoint period (i.e., C-peptide levels still continued to decline). The relationship between C-peptide and diabetes duration is depicted in Figure 1. The n=3 subjects not diagnosed with diabetes mellitus (not included in the C-peptide analyses or Figure 1) had normal, nondiabetic, fasting C-peptide levels (range= 1.36 – 4.34 ng/ml)27.
Figure 1.
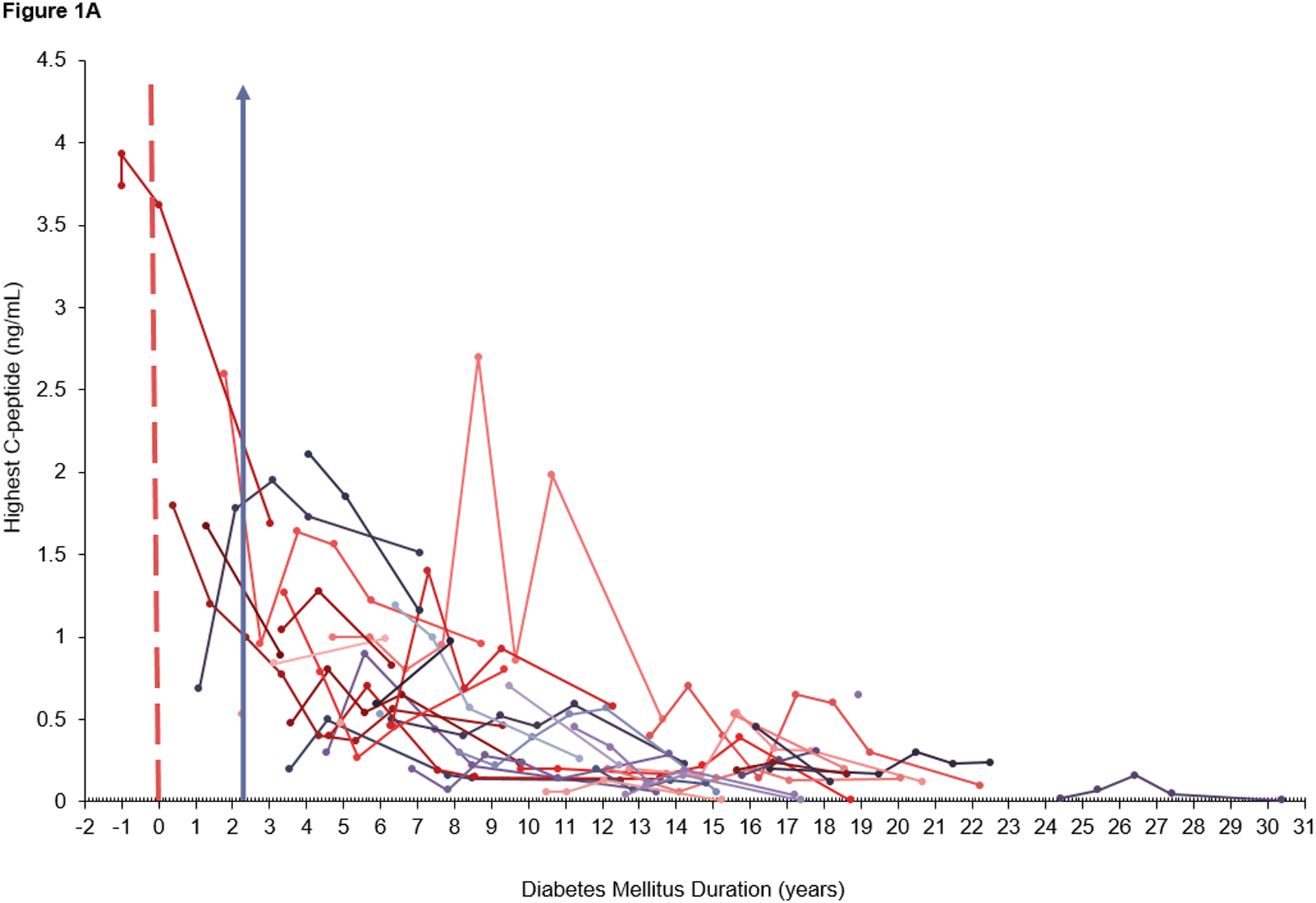
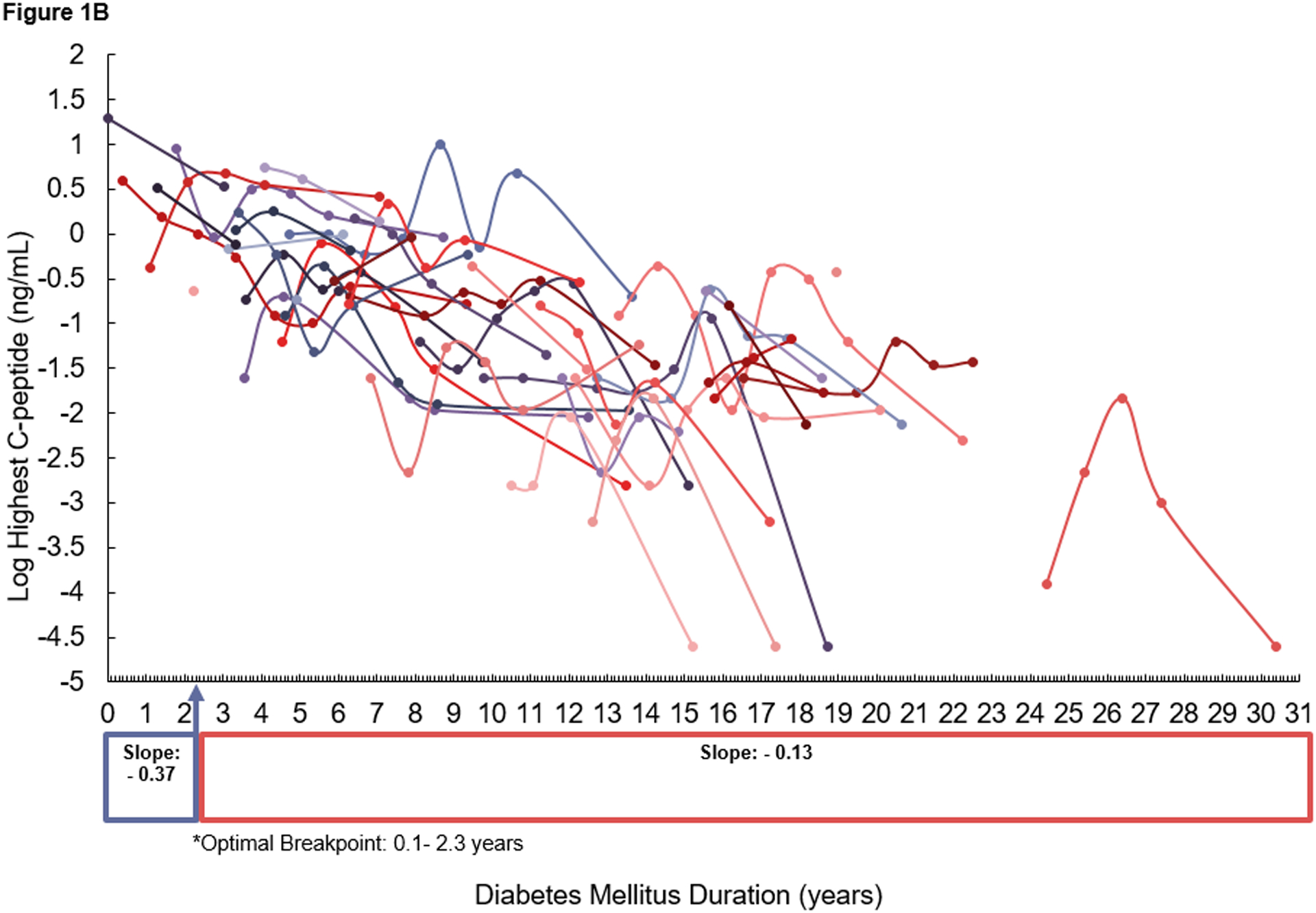
Scatterplot of A) raw data for peak C-peptide and B) log-transformed data for peak C-peptide, over time (measured as diabetes duration at current clinic) in patients with diabetes mellitus. Each trajectory profile represents an individual participant’s peak C-peptide levels over time. Each dot represents an individual data point for peak C-peptide measured at a participant’s clinic visit with a line connecting data points for that participant over the duration of their diabetes mellitus. A) The dashed red line represents the time point at which diabetes began. The data to the left of the red line represents C-peptide levels for the participant who developed diabetes mellitus during the study. Pre-diabetes mellitus diagnosis data was not included in the random coefficients model. A&B) The purple arrow represents the optimal breakpoint (2.3 years) at which the slope for C-peptide decline changes.
Power Analysis for Sample Size Considerations
Power analysis simulations over the entire range of peak C-peptide estimated that it would require 190 and 130 subjects per group (treatment vs. placebo) to detect a 50% and 60% slowing of the annual decline rate of C-peptide, respectively, assuming a constant rate of annual decline. Given that the purpose of the power analysis was to determine how many subjects would be needed to detect a slowing of C-peptide in an intervention trial, simulations were conducted to determine the number of subjects needed before the optimal breakpoint period for C-peptide, which was between 0.1 and 2.3 years after diabetes mellitus diagnosis. For enrollment in a trial within 2.3 years after diabetes mellitus diagnosis, it was estimated that 30 and 20 subjects per group (treatment vs. placebo) would be required to detect a 50% and 60% slowing of the annual decline rate of C-peptide during the first 2.3 years of diabetes mellitus, respectively. A total of n=14 participants (34%) with both Wolfram syndrome and diabetes mellitus in the current sample had a diagnosis of Wolfram syndrome within 2.3 years of receiving a diabetes mellitus diagnosis; of these, n=8 were enrolled within 2.3 years of receiving a diabetes mellitus diagnosis. An additional n=1 participant (2%) had a diagnosis of Wolfram syndrome before a diabetes mellitus diagnosis.
Discussion
This study first aimed to describe the course of diabetes mellitus in Wolfram syndrome. A large majority of our sample with Wolfram syndrome had diabetes mellitus (93.2%), with an average HbA1c of 7.9%. C-peptide clearly declined in patients with Wolfram syndrome with an optimal break point in decline between 0.1 and 2.3 years after diabetes diagnosis. However, interestingly, there was no clear statistically significant plateau at which C-peptide no longer changed after reaching an optimal breakpoint (i.e., C-peptide continued to decline). This is in contrast to type 1 diabetes mellitus populations described in the literature, which have been shown to have an exponential fall in C-peptide measured via urinary C-peptide creatinine ratio (UCPCR) over 7 years followed by a stable plateau in C-peptide levels28. Thus, C-peptide could potentially decline more rapidly in Wolfram syndrome patients, but residual beta-cell function may persist longer than in patients with type 1 diabetes mellitus; however, given the different methodologies used between studies, future research is needed to directly compare C-peptide decline in Wolfram syndrome and type 1 diabetes using identical methodology.
The current study also aimed to determine if beta-cell function could be used as a marker of diabetes progression in patients with Wolfram syndrome. Power analyses estimated that in subjects beginning an intervention trial within 2.3 years of diagnosis of diabetes mellitus, a sample size of only 30–20 per group would be needed to detect a 50%−60% slowing in the rate of C-peptide decline. A total of n=14 (34%) participants with both Wolfram syndrome and diabetes mellitus in the current sample had a diagnosis of Wolfram syndrome within 2.3 years of a diabetes mellitus diagnosis.
Given these results, there are numerous considerations to be made when considering beta-cell function as a marker of disease progression in interventional trials for Wolfram syndrome. First, lower differences in the rate of C-peptide decline may also be meaningful and more feasible (e.g., 25% slowing); however, it would require more subjects which may be challenging given the rarity of the syndrome. The recruitment challenges for an interventional trial using beta-cell function also highlight the crucial importance of collaborative efforts across national and international centers that have expertise with Wolfram syndrome. However, the sample size required for C-peptide is much more feasible than those calculated for other Wolfram syndrome manifestations such as hearing loss, which was shown to require 75 subjects with sensorineural hearing loss to detect a 50% slowing7. Third, the results highlight the importance of early diagnosis of Wolfram syndrome. Not only would early diagnosis help in using beta-cell function as a marker of diabetes progression, but early diagnosis of monogenetic forms of diabetes could help families and health care teams develop treatment and support plans tailored to improve quality of life for the patient. Thus, endocrinologists should further evaluate youth with autoimmune negative diabetes to determine any underlying causes that might require a unique treatment plan for the patient (e.g., skills training programs for those with vision or hearing loss). Lastly, it is important to note that even if an intervention slows the progression of diabetes, it may or may not be relevant for the more devastating neurological features of Wolfram syndrome.
There are study limitations to be considered. The sample size was limited given the rarity of the disease. In addition, it is likely that many patients with Wolfram syndrome may not manifest other features of the syndrome for years after the initial diagnosis of diabetes mellitus and, therefore, may not be diagnosed with Wolfram syndrome until after the first few years of diabetes mellitus (e.g., only n=8 in the current sample were recruited within 2.3 years after diabetes mellitus diagnosis). Second, the inclusion criteria may also have biased the sample given that subjects had to have the resources and ability to travel to Washington University in St. Louis for participation. The sample also includes a younger population given that early-onset subjects were specifically recruited for the clinic to increase the likelihood of a more detectable progression and description of the natural history of Wolfram syndrome over time; therefore, the results may not be generalizable to older populations. It should be noted that the median age of onset of diabetes mellitus in our cohort was 5–6 years old and there was minimal C-peptide remaining after 8–10 years of diabetes mellitus; thus, few adults with Wolfram syndrome will still have adequate C-peptide to use as a marker in a clinical trial. Lastly, given that the current study only measured HbA1c once annually at the clinic, a comprehensive measure of cumulative glycemic exposure over time could not be obtained and could potentially be a determinant of the rate of C-peptide decline.
Overall, the current study suggests that beta-cell function could potentially be a useful marker of progression if restricted to Wolfram syndrome subjects early in the course of diabetes mellitus. This could have important implications for Wolfram syndrome as researchers seek to develop novel pharmacological treatments to improve or slow the progression of the condition.
Acknowledgements:
The authors would like to thank The Washington University Wolfram Study Group, Samantha Ranck for study coordination, and Heather Lugar, Tasha Doty, Anagha Narayanan for data management at Washington University in St. Louis.
Funding:
National Institute of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01HD070855); Research reported in this publication was also supported by the Washington University Institute of Clinical and Translational Sciences (UL1TR002345) from the National Center for Advancing Translational Sciences (NCATS) of the NIH, the National Center for Research Resources (NCRR) Clinical and Translational Science Award (UL1RR024992), Washington University Diabetes Research Center (DK020579), Washington University Biomedical Research Training in Drug Abuse training grant (T32DA007261), Washington University Transdisciplinary Postdoctoral Training Program in Obesity and Cardiovascular Disease training grant (T32HL130357), National Center for Advancing Translational Sciences of the National Institutes of Health (KL2 TR002346). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. Further funding includes: Snow Foundation, George Decker and Julio V. Santiago Pediatric Diabetes Research Fund; Mallinckrodt Institute of Radiology; McDonnell Center for Systems Neuroscience.
Footnotes
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest Disclosure: All authors declare no conflict of interest.
Ethics Approval Statement: The study was approved by the Human Research Protection Office at Washington University in St. Louis and informed consent was obtained prior to testing for all participants. For children under age 18, parents/guardians provided written consent and children provided assent.
References
- 1-.Barrett TG, Bundey SE, Macleod AF. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 1995;346:1458–1463 [DOI] [PubMed] [Google Scholar]
- 2-.Wolfram Syndrome [article online], 2015. Available from https://ghr.nlm.nih.gov/condition/wolfram-syndrome. Accessed 14 September 2020
- 3-. https://rarediseases.org/rare-diseases/wolfram-syndrome/
- 4-.Collier DA, Barrett TG, Curtis D, Macleod A, Arranz MJ, Maassen JA, Bundey S. Linkage of Wolfram syndrome to chromosome 4p16.1 and evidence for heterogeneity. Am J Hum Genet 1996;59:855–863 [PMC free article] [PubMed] [Google Scholar]
- 5-.Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Crock P, Rogers D, Mikuni M, Kumashiro H, Higashi K, Sobue G, Oka Y, Permutt MA. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 1998;20:143–148 [DOI] [PubMed] [Google Scholar]
- 6-.de Heredia ML, Cleries R, Nunes V. Genotypic classification of patients with Wolfram syndrome: insights into the natural history of the disease and correlation with phenotype. Genet Med. 2013;15:497–506 [DOI] [PubMed] [Google Scholar]
- 7-.Karzon R, Narayanan A, Chen L, Lieu JE, Hershey T. Longitudinal hearing loss in Wolfram syndrome. Orphanet J Rare Dis. 2018;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8-.Zmyslowska A, Fendler W, Waszczykowska A, Niwald A, Borowiec M, Jurowski P, Mlynarski W. Retinal thickness as a marker of disease progression in longitudinal observation of patients with Wolfram syndrome. Acta Diabetol 2017;54:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9-.Hoekel J, Narayanan A, Rutlin J, Lugar H, Al-Lozi A, Hershey T, Tychsen L. Visual pathway function and structure in Wolfram syndrome: patient age, variation and progression. BMJ Open Opthalmol 2018;3:e000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10-.Zmyslowska A, Ciborowski M, Borowiec M, Fendler W, Pietrowska K, Parfieniuk E, Antosik K, Pyziak A, Waszczykowska A, Kretowski A, Mlynarski W. Serum metabolic fingerprinting identified putatively annotated sphinganine isomer as a biomarker of Wolfram syndrome. J Proteome Res 2017;16: 4000–4008 [DOI] [PubMed] [Google Scholar]
- 11-.Lugar HM, Koller JM, Rutlin J, Eisenstein SA, Neyman O, Narayanan A, Chen L, Shimony JS, Hershey T. Evidence for altered neurodevelopment and neurodegeneration in Wolfram syndrome using longitudinal morphometry. Sci Rep 2019;9:6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12-.Eljamel S, Ghosh W, De Stone S, Griffiths A, Barrett T, Thompson R. A cost of illness study evaluating the burden of Wolfram syndrome in the United Kingdom. Orphanet J Rare Dis 2019;141:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13-.Hershey T, Lugar HM, Shimony JS, Rutlin J, Koller JM, Perantie DC, Paciorkowski AR, Eisenstein SA, Permutt MA, Washington University Wolfram Study Group. Early brain vulnerability in Wolfram syndrome. PLoS One 2012;7:e40604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14-.Nguyen C, Foster ER, Paciorkowski AR, Paciorkowski AR, Viehoever A, Considine C, Bondurant A, Marshall BA, Hershey T. Reliability and validity of the wolfram unified rating scale (WURS). Orphanet J Rare Dis 2012;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15-.Marshall BA, Permutt MA, Paciorkowski A, Hoekel J, Karzon R, Wasson J, Viehover A, White NH, Shimony JS, Manwaring L, Austin P, Hullar TE, Hershey T, Washington University Wolfram Study Group. Phenotypic characteristics of early Wolfram syndrome. Orphanet J Rare Dis 2013;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16-.Pickett KA, Duncan RP, Hoekel J, Marshall B, Hershey T, Earhart GM, Washington University Wolfram Study Group. Early presentation of gait impairment in Wolfram Syndrome. Orphanet J Rare Dis 2012;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17-.Pickett KA, Duncan RP, Paciorkowski AR, Permutt MA, Marshall B, Hershey T, Earhart GM, Washington University Wolfram Study Group. Balance impairment in individuals with Wolfram syndrome. Gait Posture 2012;36:619–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18-.Hoekel J, Chisholm SA, Al-Lozi A, Hershey T, Tychsen L, Washington University Wolfram Study Group. Ophthalmologic correlates of disease severity in children and adolescents with Wolfram syndrome. J AAPOS 2014;18:461–5 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19-.Bischoff AN, Reiersen AM, Buttlaire A, Al-Lozi A, Doty T, Marshall BA, Hershey T, Washington University Wolfram Syndrome Study Research Study Group. Selective cognitive and psychiatric manifestations in Wolfram Syndrome. Orphanet J Rare Dis 2015;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20-.Alfaro R, Doty T, Narayanan A, Lugar H, Hershey T, Pepino MY. Taste and smell function in Wolfram syndrome. Orphanet J Rare Dis 2020;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21-.Licis A, Davis G, Eisenstein SA, Lugar HM, Hershey T. Sleep disturbances in Wolfram syndrome. Orphanet J Rare Dis 2019;14:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22-.Stone S, Abreu D, Mahadevan J, Asada R, Kries K, Graf R, Marshall BA, Hershey T, Urano F. Pancreatic stone protein/regenerating protein is a potential biomarker for endoplasmic reticulum stress in beta cells. Sci Rep 2019;9:5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23-.Rove KO, Vricella GJ, Hershey T, Thu MH, Lugar HM, Vetter J, Marshall BA, Austin PF. Lower urinary tract dysfunction and associated pons volume in patients with Wolfram syndrome. J Urol 2018;200:1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24-.Doty T, Foster ER, Marshall B, Ranck S, Hershey T. The effects of disease-related symptoms on daily function in Wolfram Syndrome. Transl Sci Rare Dis 2017;2:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25-.Lu S, Kanekura K, Hara T, Mahadevan J, Spears LD, Oslowski CM, Martinez R, Yamazaki-Inoue M, Toyoda M, Neilson A, Blanner P, Brown CM, Semenkovich CF, Marshall BA, Hershey T, Umezawa A, Greer PA, Urano F. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc Natl Acad Sci USA 2014;111:E5292–E5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26-.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27-.Leighton E, Sainsbury CAR, Jones GC. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther 2017;8:475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28-.Shields BM, McDonald TJ, Oram R, Hill A, Hudson M, Leete P, Pearson ER, Richardson SJ, Morgan NG, Hattersley AT, TIGI Consortium. C-peptide decline in type 1 diabetes has two phases: an initial exponential fall and a subsequent stable phase. Diabetes Care 2018;41:1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]


