Abstract
Hyaloklossia Labbé, 1896 (Apicomplexa: Sarcocystidae) is a renal coccidium that infects anuran species. The genus consists of two species: H. lieberkuehni, recorded from Pelophylax kl. esculentus, Pelophylax ridibundus, and Rana temporaria in Europe; and H. kasumiensis, recorded from Pelophylax porosus porosus in Japan. However, there have been no reports of Hyaloklossia in the other anurans in Japan. On June 2021, we examined a total of 58 adult frogs comprising 2 P. p. porosus, 23 Pelophylax nigromaculatus, 8 Rana japonica, 3 Glandirana rugosa (Ranidae), 13 Fejervarya kawamurai (Dicroglossidae), and 9 Buergeria buergeri (Rhacophoridae) for infection by Hyaloklossia. Microscopic examination of kidney tissues revealed a high infection incidence of 47.8% (11/23) in P. nigromaculatus, but the other frog species were negative for Hyaloklossia. Morphological and molecular analyses using nuclear ribosomal and mitochondrial genes confirmed the infective species as H. kasumiensis. This is a new host record for H. kasumiensis.
Keywords: Apicomplexa, Coccidia, Hyaloklossia, Pelophylax, Anuran, Kidney
Graphical abstract
Highlights
-
•
Hyaloklossia genus contains two parasitic coccidian species that infect anurans.
-
•
A total of 58 frogs of 3 families, 5 genera, and 6 species were examined for renal coccidian parasites in Japan.
-
•
We found that 47.8% of Pelophylax nigromaculatus hosted Hyaloklossia kasumiensis.
-
•
All other frog species examined did not harbour Hyaloklossia infections.
-
•
This is the first description of H. kasumiensis in Pelophylax nigromaculatus.
1. Introduction
The coccidian Hyaloklossia Labbé, 1896 comprises species that parasitizes anurans. For over 120 years, the genus was considered monotypic: containing only a single species, H. lieberkuehni (Labbé, 1894), reported from Pelophylax spp. (Anura: Ranidae) in Europe (Modrý et al., 2001; Duszynski et al., 2007; Duszynski, 2021). Recently, a second species, Hyaloklossia kasumiensis Tokiwa, Chou, Tochigi, Katayama et Duszynski, 2021, was described from Japan in East Asia (Tokiwa et al., 2021). Genetically, Hyaloklossia belongs to the family Sarcocystidae and forms a sister group to other coccidia of medical and veterinary importance: Toxoplasma and Neospora in subfamily Toxoplasmatinae, Cystoisospora in subfamily Cystoisosporinae (Modrý et al., 2001; Oborník et al., 2002; Šlapeta et al., 2003; Chou et al., 2020, 2021; Tokiwa et al., 2021). The morphological features of disporocystic and tetrasporozoic oocysts without Stieda bodies of Hyaloklossia are similar to those of other related species of the family Sarcocystidae with multi-host (i.e., indirect, heteroxenous, or facultatively heteroxonous) life cycle, with carnivorous animals as the definitive hosts. However, it is believed that all Hyaloklossia species use only a single-host (homoxenous) life cycle, with anuran species as the definitive hosts (Laveran and Mesnil, 1902; Duszynski et al., 2007). Hyaloklossia is thus a unique taxon with interesting aspects for studying the phylogenetics of Sarcocystidae; however, they are largely documented to be present in Pelophylax species in Europe, and have not been studied in endemic anurans other than P. p. porosus in Japan (Tokiwa et al., 2021). In this study, coccidian parasites in the kidney tissues of anuran species in Japan were investigated to obtain basic knowledge about Hyaloklossia infection. Namely, this study aimed to investigate infection status and infection rate in six anuran species in Japan and examine Hyaloklossia lineages using molecular analyses.
2. Materials and methods
2.1. Sample collection and analyses
A total of 58 frogs were used in this study. They consisted of 23 black-spotted pond frogs, Pelophylax nigromaculatus (Hallowell, 1861); two Tokyo Daruma pond frogs, P. porosus porosus (Cope, 1868); three wrinkled frogs, Glandirana rugosa (Temminck et Schlegel, 1838); and eight Japanese brown frogs, Rana japonica Boulenger, 1879 of the family Ranidae; 13 Japanese rice frogs, Fejervarya kawamurai (Djong et al., 2011) of the family Dicroglossidae; and nine Kajika frogs, Buergeria buergeri (Temminck et Schlegel, 1838) of the family Rhacophoridae. All frogs were collected on June 2021. The details of the frogs used in this study are listed in Table 1. Captured frogs were placed in laundry net bags and transported to Nippon Veterinary and Life Science University under refrigeration. All frogs were anesthetized by direct immersion in 0.3% isoflurane solution and then euthanized by cervical disruption. After measuring the length and weight of the frogs, their kidney tissue was collected and examined. All procedures were approved by the Ethical Committee for the Care and Use of Laboratory Animals at Nippon Veterinary and Life Science University (No. 2021S-45).
Table 1.
Details of the six anuran species used in this study.
| Hosts | Localities |
No. of examined | Body size |
|||
|---|---|---|---|---|---|---|
| City, Prefecture | Habitat | Latitude and longitude | Length (cm) | Weight (g) | ||
| Ranidae | ||||||
| Pelophylax nigromaculatus | Takashima, Shiga | Paddy field | 35°22′ N, 135°53′ E | 14 | 3.7–5.8 (4.7 ± 0.5) | 5.0–20.0 (10.7 ± 4.1) |
| Tadami, Fukushima | Paddy field | 37°21′ N, 139°18′ E | 9 | 4.2–7.0 (5.7 ± 0.8) | 9.3–34.4 (17.2 ± 8.0) | |
| Pelophylax porosus porosus | Kamisu, Ibaraki | Paddy field | 35°51′ N, 140°40′ E | 2 | 5.0–5.9 (5.5) | 10.9–13.8 (12.4) |
| Glandirana rugosa | Takashima, Shiga | Paddy field | 35°22′ N, 135°53′ E | 3 | 4.5–5.8 (5.2 ± 0.5) | 14.0–20.0 (17.3 ± 2.5) |
| Rana japonica | Mobara, Chiba | Paddy field | 35°28′ N, 140°18′ E | 8 | 2.5–3.4 (3.0 ± 0.4) | 3.0–5.0 (4.1 ± 0.9) |
| Dicroglossidae | ||||||
| Fejervarya kawamurai | Moriyama, Shiga | Paddy field | 35°6′ N, 135°58′ E | 7 | 3.5–4.1 (3.8 ± 0.2) | 3.6–10.0 (5.9 ± 2.3) |
| Kamisu, Ibaraki | Paddy field | 35°51′ N, 140°40′ E | 6 | 3.5–5.0 (4.2 ± 0.6) | 5.4–13.8 (8.1 ± 2.8) | |
| Rhacophoridae | ||||||
| Buergeria buergeri | Takashima, Shiga | River | 35°23′ N, 135°51′ E | 9 | 3.8–4.5 (4.1 ± 0.2) | 4.0–5.0 (4.6 ± 0.5) |
2.2. Morphological examination
Approximately half of each kidney tissue sample was homogenized using a BioMasher (Nippi, Japan), and the other half was dissected under an SZX61 stereomicroscope (Olympus, Japan). The right kidney was preserved in 10% neutral buffered formalin or 2% potassium dichromate solution. These samples were examined for the presence of sporocysts and oocysts under a BX53 optical microscope (Olympus). Photomicrographs were captured using a DP27 photomicroscope (Olympus). For measurements, ImageJ2 software (Rueden et al., 2017) was used to analyze prerecorded images captured at 1000 × magnification. The size values are reported in micrometers and are given as a range followed by the mean and standard deviation in parentheses.
Abbreviations used throughout this article are standardized (Wilber et al., 1998; Tokiwa et al., 2021); Oocyst characteristics: length (L), width (W), their ratio (L/W), micropyle (M), micropyle cap (MC), and oocyst residuum (OR); sporocyst characteristics: length (L), width (W), their ratio (L/W), Stieda body (SB), substieda body (SSB), sporocyst residuum (SR), and sporozoites (SZ).
2.3. DNA extraction, polymerase chain reaction (PCR), and sequencing
Genomic DNA was extracted from all sporocyst-positive samples (n = 11). A sporocyst mass detected in the kidney tissue was collected using a glass Pasteur pipette under an SZX16 stereomicroscope (Olympus) and used for DNA extraction. Genomic DNA was extracted from the mass using Qiagen Power Soil DNA Isolation Kit (Qiagen, Germany) according to the specified procedure with a prolonged vortex time of 20 min with the TissueLyser LT (Qiagen). The sample obtained was used as a PCR template. Double-distilled water was used as negative control.
Two genetic loci, nuclear small subunit ribosomal RNA (18S) and mitochondrial cytochrome c oxidase subunit I (cox1), were amplified using specific primers (Table 2). PCR was performed using 20 μL reaction volumes, each containing 0.2 μL of TaKaRa ExTaq polymerase (TaKaRa, Japan), 2 μL of 10 × buffer, 1.6 μL of dNTPs (2.5 mM each), 0.2 μL of each primer (50 μM), 1.0 μL of the template, and 14.8 μL of double-distilled water. The thermocycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 45–60 °C for 30 s, and extension at 72 °C for 1 min. Final extension was performed at 72 °C for 10 min (for 18S) or 5 min (for cox1), followed by a hold step at 4 °C.
Table 2.
Primers used in this study.
| Primers | Gene | Direction | Sequence (5′–3′) | References |
|---|---|---|---|---|
| EF | 18S | Forward | GAAACTGCGAATGGCTCATT | Kvicerová et al. (2008) |
| FR | Reverse | CTTGCGCCTACTAGGCATTC | Kvicerová et al. (2008) | |
| Sdae_COX1_260F | cox1 | Forward | GATCTTTATGTTYTTRATGCC | Ogedengbe et al. (2016) |
| SR5 | Reverse | TAGGTATCATGTAACGCAATATCCAT | Gjerde (2013) |
The PCR products were separated using 1.5% agarose gel electrophoresis and visualized under an LE transilluminator after staining with GR green (Bio Craft, Japan). The size of the PCR products were estimated by comparison with a 100 bp DNA plus DNA ladder (Maestrogen, Taiwan). The PCR products were sequenced by Macrogen Corp., Japan using ABI 3730xl DNA Analyzer (Thermo Fisher Scientific, USA) and the same PCR primers.
2.4. Genetic analysis
The obtained 18S and cox1 sequences were separately aligned using MAFFT with Q–INS–I (Katoh and Standley, 2013). Sequence similarity was analyzed using the BLASTn program available on the website of the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/Blast.cg).
Phylogenetic trees were constructed using MEGA 11 software (Tamura et al., 2021) with cox1 sequence data of sarcocystids, obtained from the International Nucleotide Sequence Databases (INSD; GenBank/DDBJ/EMBL). Sarcocystis rileyi (accession no. KT184389) was used as an outgroup for rooting the resulting trees. Phylogenetic trees were reconstructed using the neighbor-joining (NJ) and maximum likelihood (ML) methods. The best-fit DNA evolution model was estimated for each dataset individually using the Akaike information criterion and determined to be the Tamura-Nei model with gamma distribution. The bootstrap values for NJ and ML were obtained from 1500 to 1000 replicates, respectively.
3. Results and discussions
During our survey of renal coccidia in frogs, Hyaloklossia associated with two different stages of development were found in 11 of 14 P. nigromaculatus collected in a paddy in Shiga Prefecture, Japan (Table 3). Nine P. nigromaculatus from Fukushima and the other frog species were negative for Hyaloklossia infection.
Table 3.
Summary of the 11 Hyaloklossia-positive adult Pelophylax nigromaculatus from Shiga, Japan.
| Host ID | Sex | Length (cm) | Weight (g) | Hyaloklossia Stage |
|---|---|---|---|---|
| PEL24 | Male | 4.9 | 11 | Sporocyst |
| PEL26 | Male | 4.5 | 12 | Sporocyst |
| PEL27 | Male | 4.9 | 10 | Sporocyst |
| PEL28 | Male | 4.6 | 15 | Sporocyst, oocyst |
| PEL29 | Male | 4.3 | 8 | Sporocyst, oocyst |
| PEL30 | Female | 4.1 | 9 | Sporocyst |
| PEL31 | Female | 4.3 | 6 | Sporocyst |
| PEL32 | Female | 3.7 | 6 | Sporocyst |
| PEL33 | Female | 5.0 | 5 | Sporocyst |
| PEL34 | Male | 4.9 | 10 | Sporocyst |
| PEL36 | Male | 4.3 | 8 | Sporocyst |
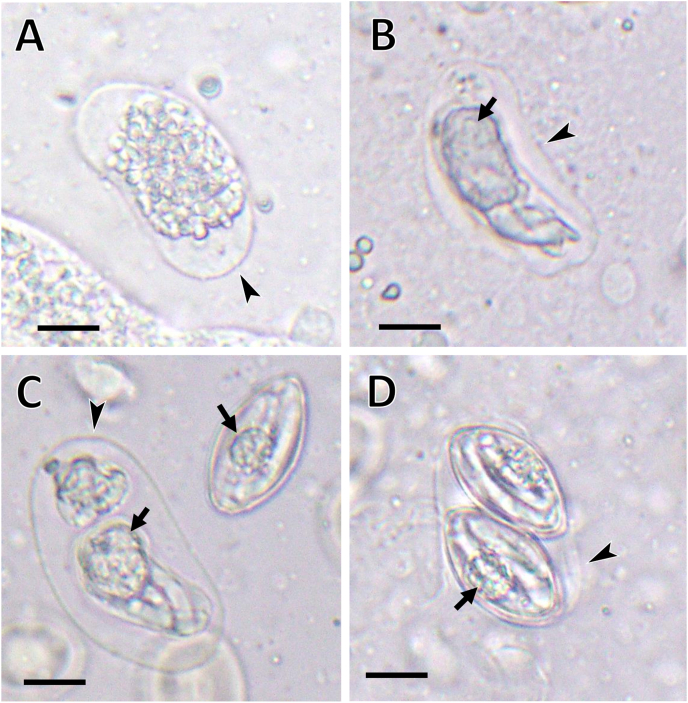
The morphological characteristics of Hyaloklossia found in P. nigromaculatus were as follows. Immature oocysts (Fig. 1A–C) were bean-shaped or elongated-ovoidal, and measured L × W (n = 20): 32.3–41.5 (39.3 ± 2.9) × 18.6–23.4 (21.3 ± 1.6); L/W ratio: 1.6–2.0 (1.9 ± 0.1). Their walls were very thin and transparent. The sporont (Fig. 1A) was spheroidal with a granular cytoplasm and measured 15.9–27.8 (24.8 ± 3.3) × 15.6–19.8 (17.9 ± 1.7). Sporoblasts (Fig. 1B and C) were elongated and showed primordia of SR and SZ, but walls were very thin. The mature oocysts (Fig. 1D) were elongate and dedicate, susceptible to deformation, and measured L × W (n = 11): 35.9–42.3 (37.9 ± 1.7) × 18.6–22.5 (21.9 ± 1.3). They contained two sporocysts each. Sporocysts (Fig. 1C and D) were broadly spindle-shaped with a smooth, single layered wall and measured L × W (n = 30): 24.2–28.0 (26.2 ± 1.5) × 13.2–16.1 (14.5 ± 1.0); L/W ratio: 1.6–2.0 (1.8 ± 0.1). SB, SSB, and PSB were all absent. Sporocysts had four SZ each and an SR with a round, granular body and measured, L × W (n = 10): 6.8–10.2 (8.4 ± 0.9) × 6.5–8.5 (7.6 ± 0.7). Based on the size of its sporocyst and SR the present species was identified as H. kasumiensis. The original description of H. kasumiensis oocysts was based on a small number obtained from tissue sections of the P. p. porosus kidney (Tokiwa et al., 2021), and thus, the measurements were not accurate. This is the first detailed description of both the mature and immature oocysts of H. kasumiensis. However, the oocysts were highly polymorphic, and it was difficult to differentiate them from those of H. lieberkuehni reported from P. kl. esculentus (Laveran and Mesnil, 1902; Kazubski and Grabda-Kazubska, 1973). Furthermore, fresh kidney samples should be used for morphological observation of sporocysts; preservation in 10% formalin fixed or potassium dichromate is not recommended, as it might cause sample denaturation.
Fig. 1.
Light microscopy of Hyaloklossia oocysts in the kidney of Pelophylax nigromaculatus. A. Immature oocyst showing the sporont with granular cytoplasm that does not fill the space inside the oocyst completely. B. An immature oocyst showing the sporoblast with very thin wall. C. An immature oocyst (left) and mature sporocyst (right). D. A mature oocyst with two sporocysts. Arrowhead and arrows indicate oocyst wall and sporocyst residuum, respectively. Scale bar = 5 μm.
The species-level identification of Hyaloklossia was strongly supported by genetic analysis of its 18S sequence. Partial fragments of 18S sequences (1472 bp) of eight of the 11 samples were successfully amplified and sequenced, with 100% identity to each other. A representative sequence was deposited in the DNA Data Bank of Japan (DDBJ) under the accession no. LC669718. When compared with available sequences in INSD, the 18S sequence of Hyaloklossia from P. nigromaculatus in this study matched with 100% identity (1449/1449 bp) with H. kasumiensis (accession no. LC602188) reported from P. p. porosus in Ibaraki, Japan. Furthermore, the sequence identity of H. lieberkuehni (accession no. AF298623) from P. kl. esculentus from Czech Republic was 99.73% (1468/1472) when compared to the sequence data obtain in the current study. The homology compared to coccidia of the other members of Sarcocystidae was less than 97.56%.
The cox1 (872 bp) sequences were obtained from the same eight samples. One sample (PEL27) was suspected to be simultaneously infected by more than one lineage of Hyalokossia based on superimposed double nucleotide peaks on the sequence electroprograms (Supplemental Fig. 1). Multiple alignment of the other seven sequences of Hyaloklossia from P. nigromaculatus and the reference sequence of H. kasumiensis (accession no. LC602189) from P. p. porosus revealed that the GC content ranged from 37.4% to 37.7%, and 868 bp (99.5%) of these sequences were conserved, and the four sites (0.08%) were polymorphic, generating three different haplotypes. These haplotypes were designated as Hk1, Hk2, and Hk3, respectively (Table 4). Hk1 was designed for H. kasumiensis from P. p. porosus (type host) in Ibaraki, Japan. Hk2 and Hk3 were identified in four (PEL24, PEL26, PEL30, and PEL32) and three (PEL28, PEL29, and PEL34) samples of P. nigromaculatus, respectively. The cox1 sequences were deposited in the DDBJ under the accession numbers LC669719 (Hk2) and LC669720 (Hk3). Phylogenetic relationships of the three cox1 haplotypes of H. kasumiensis and related taxa are shown in Fig. 2. Topologies were consistent for both the NJ and ML trees. For brevity, we present only the NL tree and include bootstrap values for ML. Four independent clades were present in the tree, of which the Hyaloklossia clade became monophyletic with Cystoisospora + Nephroisospora clade; within the Hyaloklossia clade, Hk2 diverged first, and then Hk1 and Hk3 formed a monophyletic lineage.
Table 4.
Nucleotide differences of cox1 sequences (872-bp) of Hyaloklossia kasumiensis from and Pelophylax nigromaculatus and Pelophylax porosus porosus.
| Haplotypes | Host (sample ID) | Accession no. | Sequence positions |
|||
|---|---|---|---|---|---|---|
| 232 | 233 | 375 | 548 | |||
| Hk1 | Ppp (P01) | LC602189 | G | A | A | C |
| Hk2 | Pn (PEL24, PEL26, PEL30, PEL32) | LC669719 | T | T | A | A |
| Hk3 | Pn (PEL28, PEL29, PEL34) | LC669720 | G | A | C | C |
| Mixed | Pn (PEL27) | – | G | A | A/C | A/C |
Ppp: P. p. porosus, Pn: P. nigromaculatus.
Fig. 2.
Phylogenetic tree of based on cox1 sequences of Hyaloklossia and related species belonging to Toxoplasmatinae (Toxoplasma, Neospora, Hammondia, Heydornia), Cystososporinae (Cystoisospora) and Eumonosporinae (Eumonospora) constructed using the neighbor joining method. The nodes are labeled using support from the bootstrap values obtained for the neighbor joining (left) and maximum likelihood (right) methods. Pn: Pelophylax nigromaculatus.
Lieberkühn (1854) was first to discover coccidium in the kidney of P. kl. esculentus. Labbé (1894) later described these coccidian species reported by Lieberkühn (1854) as Klossia lieberkühni. However, this was soon reclassified in a new genus Hyaloklossia (Labbé, 1896). Several researchers have reported on the wide spread occurrence of H. lieberkuehni in ranids of P. kl. esculentus, P. ridibundus and R. temporaria in Europe (Laveran and Mesnil 1902; Nöller, 1923; Walton, 1949; Kazubski and Grabda-Kazubska 1973; Vojtková 1976; Modrý et al., 2001). Recently, Hyaloklossia infections were reported in P. p. porosus, an endemic species of Japan, and described it as H. kasumiensis (Tokiwa et al., 2021). Pelophylax nigromaculatus, which was found to be infected by Hyaloklossia in this study, is the second definitive host of H. kasumiensis. However, P. nigromaculatus collected in Shiga showed a high infection rate of 78.6% (11/14; 95% CI 51.7%–93.2%), whereas Pelophylax spp. from Fukushima (n = 9) and Ibaraki (n = 2), which are adjacent to each other and located on the east side of Japan, were negative (95% CI 0%–30.0%). The low infection rates in these areas are similar to the result of the previous study of P. p. porosus in Ibaraki (Tokiwa et al., 2021), the infection rate of H. kasumiensis was 11% (1/9; 95% CI 0%–45.7%).
The life cycle of Hyaloklossia is not well understood. According to Laveran and Mesnil (1902), Hyaloklossia sporocysts are ingested orally by omnivorous tadpoles, and sporozoites released in the intestinal tract pass through the bloodstream to invade and multiply in the lungs, kidneys, spleen, liver, and adipose tissue. Of these, merozoites that reach the kidneys undergo sporogony. However, Nöller (1913, 1923) believed that the endogenous stages reported in extraintestinal organs other than the kidneys by Laveran and Mesnil (1902) were those of Lankesterella minima (Apicomplexa: Eimeriorina), and those of Hyaloklossia are limited to the kidney. More importantly, Hyaloklossia has a homoxenous lifecycle and is transmitted orally to omnivorous tadpoles via their sporocysts shed in frog urine. Thus, infection is more likely to occur in water bodies such as paddy fields and water-tight ponds; so, the host habitat may have made a difference in the prevalence of Hyaloklossia infection.
The infectivity of Hyaloklossia in other frogs is not well understood. In the present study, we were unable to identify any other Hyaloklossia-infected individuals among G. rugosa (Ranidae), F. kawamurai (Dicroglossidae), and B. buergeri (Rhacophoridae). In particular, G. rugosa was collected from the same paddy field in Shiga where high Hyaloklossia infection rates were observed in P. nigromaculatus, indicating that H. kasumiensis is highly adapted to Pelophylax. Hyaloklossia-like organisms have been reported in the kidneys of the Northern leopard frog Lithobates pipiens (Ranidae) in the USA and yellow-bellied toad Bombina variegata (Bombinatoridae) in Bulgaria (Golemasky and Miceva, 1975; Levine and Nye, 1977). This implies the existence of undescribed Hyaloklossia species in other frog taxa, and it is necessary to continuously survey anurans in the field and clarify host specificity through infection experiments.
Two Pelophylax species are found in Japan: P. nigromaculatus and P. porosus. The former is widespread in East Asia, including most of Japan, and the latter is endemic to Japan and comprises two subspecies: P. p. porosus distributed in eastern Japan and P. p. brevipodus distributed in western Japan (Matsui and Maeda, 2018). In the present study, in terms of intraspecific cox1 sequence diversity in H. kasumiensis, haplotype Hk1 was recorded from P. p. porosus in Ibaraki and Hk2 and Hk3 from P. nigromaculatus in Shiga. To clarify whether this diversity is due to host species or geographical factors, continuous investigation of the infection status and molecular characteristics of H. kasumiensis in P. nigromaculatus in East Asia and of P. p. brevipodus in western Japan is needed.
In conclusion, we reported a second definitive host for H. kasumiensis: the black-spotted pond frog P. nigromaculatus. Understanding the host-parasite relationships of Hyaloklossia species may be key to unraveling the phylogeny of Sarcocystidae.
Funding
This work was supported by Japan Society for the Promotion of Science KAKENHI grant number 21K06323 (TT), the internal research grant of the National Museum of Nature and Science, Tokyo (NY), and the commissioned research grant from the Division of Environmental Affairs, Kamisu City Office, Ibaraki Prefecture (NY).
Declaration of competing interest
No conflict of interest declared by any author.
Acknowledgments
The authors would like to thank Dr. Noe Matsushima (Toho University) and Mrs. Kazuko Watanabe for their cooperation in collecting frogs, and Dr. Atsushi Tominaga (University of the Ryukyus) and Dr. Tomoaki Nakada (Nippon Veterinary and Life Science University) for their advice on sampling design. We also wish to thank Editage for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2022.02.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Superimposed double nucleotide peaks at two positions for cox1 sequences on Sanger sequencing electrograms. Both forward and reverse polymerase chain reaction products revealed multiple Hyaloklossia haplotype infections in Pelophylax nigromaculatus (PEL27) from Shiga, Japan.
References
- Chou S., Tokiwa T., Hadano S., Izawa N., Ueda M., Kojima A., Ike K. Resurrection of the genus Eumonospora (Apicomplexa: Sarcocystidae) for Caryospora species without Stieda body. Parasitol. Int. 2020;77 doi: 10.1016/j.parint.2020.102101. [DOI] [PubMed] [Google Scholar]
- Chou S., Izawa N., Ike K., Tokiwa T. Detection of Eumonospora henryae (Apicomplexa: Sarcocystidae) from Falco columbarius (Falconiformes: Aves): comparison of host-parasite phylogram and comments on the family Sarcocystidae Poche, 1913. Int. J. Parasitol. 2021;14:75–83. doi: 10.1016/j.ijppaw.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duszynski D.W., Bolek M.G., Upton S.J. Coccidia (Apicomplexa: Eimeriidae) of amphibians of the world. Zootaxa. 2007;1667:1–77. [Google Scholar]
- Duszynski D.W. Biodiversity of the Coccidia (Apicomplexa: Conoidasida) in vertebrates: what we know, what we do not know, and what needs to be done. Folia Parasitol. 2021;68 doi: 10.14411/fp.2021.001. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013;43:579–591. doi: 10.1016/j.ijpara.2013.02.004. 2013. [DOI] [PubMed] [Google Scholar]
- Golemansky V., Miceva V. Studies upon the protozoan parasitic fauna on amphibia in Bulgaria. I. Bombina variegata (L.) Acta Zoologica Bulgaria. 1975;1:23–32. [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazubski S.L., Grabda-Kazubska B. Isospora lieberkuehni (Labbé, 1896) and I. neos Yakimoff and Gousseff, 1936 (Eimeriidae) in frogs in Poland. Acta Parasitol. Pol. 1973;21:3–8. [Google Scholar]
- Kvicerová J., Pakandl M., Hypsa M. Phylogenetic relationships among Eimeria spp. (Apicomplexa, Eimeriidae) infecting rabbits: evolutionary significance of biological and morphological features. J. Parasitol. 2008;135:443–452. doi: 10.1017/S0031182007004106. [DOI] [PubMed] [Google Scholar]
- Labbé A. Recherches zoologiques, cytologiques et biologiques sur les parasites endoglobulaires du sang vertebrés. Arch. Zool. Exp. Gen. (Ser. III). 1894;2:55. 258. [Google Scholar]
- Labbé A. Recherches zoologiques, cytologiques et biologiques sur les coccidies. Arch. Zool. Exp. Gen. (Ser. III). 1896;4:517–654. [Google Scholar]
- Laveran M.A., Mesnil F. Sur la coccidie trouvée dans le rein de la Rana esculenta et sur l'infection generale qu'elle produit. C. R. Acad., Sci. Paris. III. 1902;135:82–87. [Google Scholar]
- Levine N.D., Nye R.R. A survey of blood and other tissue parasites of leopard frogs Rana pipiens in the United States. J. Wildl. Dis. 1977;13:17–23. doi: 10.7589/0090-3558-13.1.17. [DOI] [PubMed] [Google Scholar]
- Lieberkühn N. Über die psorospermien. Arch. Anat. Physiol. wiss. Med. 1854:1–24. [Google Scholar]
- Matsui M., Maeda N. Bun-ichi Sogo Shuppan; Tokyo, Japan: 2018. Encyclopedia of Japanese Frogs. [Google Scholar]
- Modrý D., Šlapeta J.R., Jirků M., Obornik M., Lukeš J., Koudela B. Phylogenetic position of a renal coccidium of the European green frogs, ‘Isospora’ liberkuehni Labbé, 1894 (Apicomplexa: Sarcocystidae) and its taxonomic implications. Int. J. Syst. Evol. Microbiol. 2001;51:767–772. doi: 10.1099/00207713-51-3-767. [DOI] [PubMed] [Google Scholar]
- Nöller W. Die Blutprotozoen des Wasserfrosches und ihre Übertragung. I. Teil. Arch. Protistenkd. 1913;31:169–240. [Google Scholar]
- Nöller W. Zur kenntnis eines nierencoccids. Der entwicklungskreis des coccids der wasserfroschniere [Isospora lieberkühni (Labbé, 1894)] Arch. Protistenkd. 1923;47:101–108. [Google Scholar]
- Oborník M., Jirků M., Slapeta J.R., Moderý D., Koudela B., Lukes J. Notes on coccidian phylogeny, based on the apicoplast small subunit ribosomal DNA. Parasitol. Res. 2002;88:360–363. doi: 10.1007/s00436-001-0557-4. [DOI] [PubMed] [Google Scholar]
- Ogedengbe M.E., Ogedengbe J.D., Whale J.C., Elliot K., Juárez-Estrada M.A., Barta J.R. Molecular phylogenetic analyses of tissue coccidian (Sarcocystidae; Apicomplexa) based on nuclear 18S rDNA and mitochondrial COI sequences confirms the paraphyly of the genus Hammondia. Parasitol Open. 2016;2:2e. doi: 10.1017/pao.2015.7. [DOI] [Google Scholar]
- Rueden C.T., Schindelin J., Hiner M.C., Dezonia B.E., Walter A.E., Arena E.T., Eliceiri K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šlapeta J.R., Modrý D., Votýpka J., Jirků M., Lukeš J., Koudela B. Evolutionary relationships among cyst-forming coccidia Sarcocystis spp. (Alveolata: Apicomplexa: Coccidea) in endemic African tree vipers and perspective for evolution of heteroxenous life cycle. Mol. Phylogenet. Evol. 2003;27:464–475. doi: 10.1016/s1055-7903(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa T., Chou S., Tochigi Y., Katayama K., Duszynski D.W. Hyaloklossia Labbé, 1896 (Alveolata: Apicomplexa) in frogs: description of a new species and proposing a new subfamily to accommodate these enigmatic parasites. Int. J. Parasitol. Parasites Wildl. 2021;15:199–207. doi: 10.1016/j.ijppaw.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtková L. Prvoci (Protozoa) obojživelníků ČSSR. Scr. Facutlatis Sci. Nat. Univ. Purkynianae Brun. Biol. 1976;5(6):177–210. [Google Scholar]
- Walton A.C. Parasites of the Ranidae (Amphibia). XIX. J. Parasitol. 1949;35(Suppl. 101):39–40. [Google Scholar]
- Wilber P.G., Duszynski D.W., Upton S.J., Seville R.S., Corliss J.O. A revision of the taxonomy and nomenclature of the Eimeria spp. (Apicomplexa: Eimeriidae) from rodents in the Tribe Marmotini (Sciuridae) Syst. Parasitol. 1998;39:113–135. doi: 10.1023/A:1005914010087. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Superimposed double nucleotide peaks at two positions for cox1 sequences on Sanger sequencing electrograms. Both forward and reverse polymerase chain reaction products revealed multiple Hyaloklossia haplotype infections in Pelophylax nigromaculatus (PEL27) from Shiga, Japan.