Highlights
-
•
Focal epilepsy from oligodendrogliomas can be very treatment resistant.
-
•
IDH1/2 mutation can lower seizure threshold by D2HG production.
-
•
Ivosidenib, an IDH1 inhibitor, significantly improved seizures in our patient.
-
•
In our patient, seizure improvement was seen with stable tumor appearance on MRI.
Keywords: Epilepsy, Seizure, Oligodendroglioma, Glioma, Ivosidenib, IDH1 mutation
Abstract
Compared to high grade gliomas, low grade gliomas such as oligodendrogliomas are often more epileptogenic. Epilepsy develops in 70–90% of patients with oligodendrogliomas and 40% of these are resistant to anti-seizure medications and surgery [3]. IDH1/2 mutation is one defining feature of oligodendrogliomas and confers improved prognosis when found in astrocytomas [7]. One possible etiology of the high rate of epileptogenicity in oligodendrogliomas is D-2-Hydroxyglutarate (D2HG), an oncometabolite seen in IDH mutation [8]. D2HG can mimic the effect of glutamate at the NMDA receptor and increase the seizure risk [11]. In this case report, we present a patient with drug resistant focal epilepsy from IDH1 mutant oligodendroglioma with markedly improved seizure frequency after starting Ivosidenib, an IDH1 inhibitor, in the absence of any changes to traditional anti-seizure medications. Our case suggests the possibility that IDH1 inhibitors may help reduce seizure burden in patients with difficult to control epilepsy from IDH1 mutant oligodendrogliomas. This is significant because we show that a targeted cancer therapy is able to improve seizure frequency through a unique pathway, and suggests that research into similar targeted, precision medicine therapies in brain lesions associated with epilepsy may be beneficial.
1. Introduction
Oligodendroglioma is a rare glioma that has a more favorable prognosis than other types of gliomas [1]. Prior to the 2016 revised WHO classification of CNS tumors, the 5-year survival rate for oligodendroglioma and anaplastic oligodendroglioma was 81.3% and 56.6% respectively [1]. Standard treatment includes maximal safe resection followed by observation or chemotherapy and radiation therapy depending on the completeness of resection and grade of the tumor. Chemotherapy regimens can include Temozolomide or Procarbazine, Lomustine, Vincristine (PCV) and treatment decisions are often individualized [2]. Epilepsy develops in 70–90% of patients with oligodendrogliomas, and management includes anti-seizure medications (ASM) and tumor resection. However, drug-resistant epilepsy occurs in 40% of oligodendrogliomas, despite surgery and ASM [3]. Chemotherapy agents are also well-established treatment for seizures in LGG. Temozolomide has been associated with up to 50% seizure reduction in LGG [4].
In 2016, the revised WHO classifications of CNS tumors redefined IDH mutated gliomas based on molecular genetic markers to include astrocytomas (IDH-mutant, ATRX-mutant, 1p/19q-intact) or oligodendrogliomas (IDH-mutant, ATRX-wildtype and 1p/19q-codeleted) rather than purely based on histological phenotype, thus eliminating oligoastrocytomas from the classification [5]. IDH-wild type gliomas are classified distinctly. Subsequent updates of the WHO classification system are expected to further build on and retain these changes. However, the revised classification system retained the previously held histopathology basis for tumor grading (grade 1 to 4) [6].
Isocitrate dehydrogenases (IDH) are key Krebs cycle enzymes that include isoforms IDH1, IDH2 and IDH3 and catalyze the transformation of isocitrate into alpha-ketoglutarate (a-KG). IDH1 is located in the cytoplasm and peroxisomes while IDH2 and 3 are located in the mitochondrial matrix. IDH mutations are common in malignancies and often involve an arginine residue that is crucial for the recognition of isocitrate [7]. Acquiring an IDH mutation results in significant metabolic reprogramming and depletion of a-KG for D-2-Hydroxyglutrate (D2HG) [8]. D2HG can promote tumor growth via multiple mechanisms such as DNA and histone hypermethylation and by inhibiting a-KG dependent enzymes [8]. IDH mutation is emerging as an important independent prognostic and predictive biomarker for clinical outcomes [9]. This mutation is also emerging as a significant therapeutic target of interest for these tumors [9].
In gliomas, IDH mutation is associated with increased seizure risk [10]. D2HG, a product of the IDH mutation, can mimic glutamate's action (an excitatory neurotransmitter) at the NMDA receptors thus lowering the seizure threshold [11]. There are currently two drugs approved for IDH mutant acute myeloid leukemia: Ivosidenib (IDH1 inhibitor) and Enasidenib (IDH2 inhibitor). Ivosidenib an oral agent is now approved for the treatment of newly-diagnosed acute myeloid leukemia (AML) with a susceptible IDH1 mutation and in adults with previously treated, locally advanced or metastatic, cholangiocarcinoma with an IDH1 mutation. Although not currently approved for treatment of IDH-mutant gliomas, it has demonstrated meaningful efficacy in reducing tumor growth in non-enhancing glioma in an open label, phase 1 study with favorable safety profile. [12].
Here we report a patient with drug resistant epilepsy due to a oligodendroglioma with improved seizure control after being started on Ivosidenib.
2. Case report
Consent was obtained from the patient and this case report was reviewed by the institutional IRB and determined to not represent human research. Our patient is a 73-year-old male with a history of left insular oligodendroglioma grade II diagnosed in 1998. He received treatment with 4 cycles of PCV. In 2006, MRI showed progression of disease with worsening speech. The patient declined radiation and was started on Temozolomide which he tolerated for 5 cycles prior to stopping due to allergic response. In 2010, he underwent surgery with pathology showing oligodendroglioma without anaplastic features. Immunohistochemical stains were performed on formalin-fixed, paraffin embedded tissue, using a biotin-free protocol that included appropriate positive and negative controls with the following results: GFAP: Positive, Vimentin: Negative, Ki-67: <2%, p53: Negative. Fluorescent in situ hybridization (FISH) was performed with Abbott probes showing loss of both 1p and 19q chromosomes. The patient was being monitored off therapy with brain MRI every 6 months that demonstrated slow interval progression of non-enhancing tumor by 2016. At the time, his specimen from 2010 was sent for molecular testing and found to be positive for mutation R132H in IDH1 gene. While there is evidence that radiation therapy can reduce seizure burden in low grade glioma patients, the patient did not think that surgical interventions or radiation therapy were within his goals of care [13].
He had focal epilepsy that was difficult to control since 2017. In 2016, prior to the noted small interval progression, he was having highly stereotyped 10–20 second episodes. He immediately recognized they were characterized by an epigastric rising sensation associated with a strange aftertaste progressing to expressive aphasia with preserved awareness and intact receptive language. Following a seizure, he had persistent aphasia for 1–2 minutes followed by return to baseline. After several seizures in a day, he would experience fatigue. He has never had focal to bilateral tonic-clonic seizures. He underwent a 23-hour ambulatory EEG without video in 2020 that showed left fronto-temporal slowing and breach rhythm. No epileptiform discharges or seizures were seen. His typical events were not associated with any clear change on scalp-recorded EEG. The clinical interpretation was focal aware seizures that were scalp EEG negative. This was thought to be due to the deep location of the tumor, and seizures were thought to arise from an insular-limbic network. ASM trials included: phenytoin, carbamazepine, levetiracetam, and lamotrigine, alone and in combination. Since his slow interval progression in 2017, his brain imaging shows a non-enhancing tumor which has been stable from 2018 to 2021.
Despite apparent tumor stability, his seizures progressively increased in frequency but not severity. By February 2021, he was consistently having 4–6 seizures per day on levetiracetam 1500 mg twice daily and lamotrigine 225 mg twice daily. A levetiracetam level was 44.2 mcg/ml (range: 36.1 – 70 mcg/ml for peak level) and lamotrigine level was 9.7 mcg/ml (range: 2.5–15 mcg/ml). There is emerging data from a recent trial showing that the first-in-class oral IDH1 inhibitor demonstrated reduced growth in non-enhancing tumors with a favorable side effect profile [12]. We discussed with the patient that there is also anecdotal evidence of improvement in seizure frequency from this medication. Seizure frequency is an outcome measure for a currently enrolling study of newly diagnosed patients at our institution. The patient agreed to start Ivosidenib with the intent to reduce his seizures frequency as well as to reduce growth of his non-enhancing oligodendroglioma. He was unable to enroll in ongoing trials for Ivosidenib due to previous treatments for disease progression. This medication was approved by his insurance for off-label use and he was started on Ivosidenib 500 mg daily after baseline CMP, CBC, EKG was obtained. He maintained a careful seizure diary prior to initiation of the drug. He tolerated the medication well with the only side effect being constipation for which he takes an over the counter medication. He did not experience any QT prolongation or LFT abnormalities.
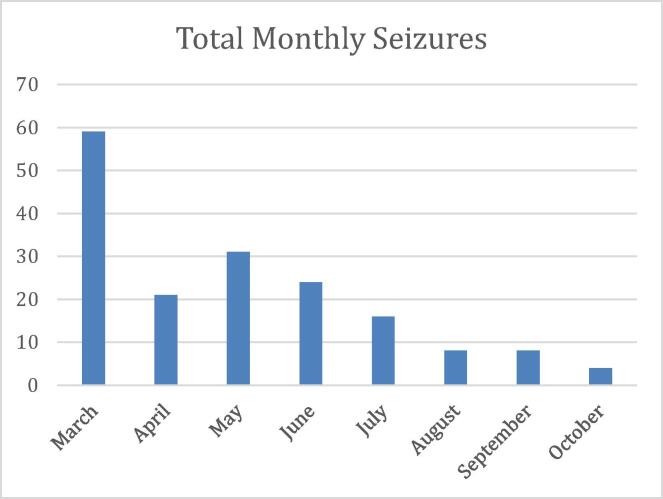
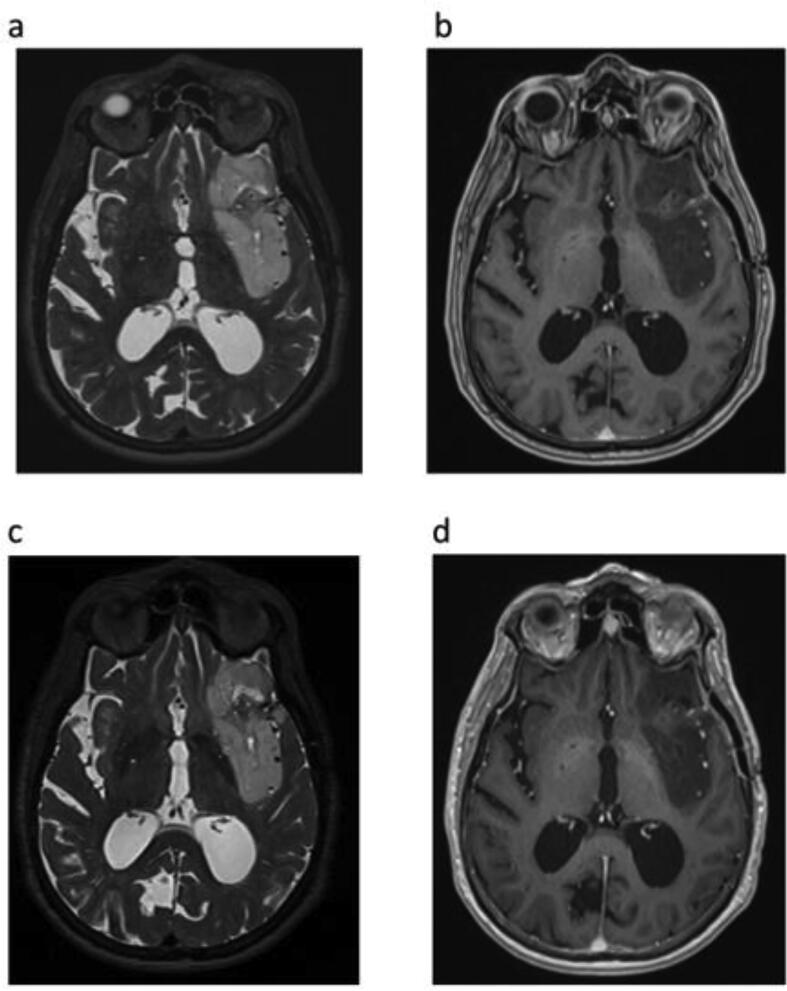
His seizure frequency decreased substantially after starting Ivosidenib with no change in his ASM regimen. He was started on Ivosidenib on March 15Th, 2021 and after three months, his seizure frequency decreased from 4-6 seizures per day to 0–1 per day. Seizure diary data is summarized in Table 1 and Fig. 1. Total seizures per month decreased from 59 seizures a month prior to starting Ivosidenib to currently 4 seizures a month. His seizure semiology and duration were unchanged when they did occur. Recently, he went 10 days without any seizures. An MRI using a brain tumor protocol with and without contrast was obtained 5 months after Ivosidenib was started and is included in Fig. 2. This post-treatment MRI was unchanged in size demonstrating the hyperintense non-enhancing mass.
Table 1.
Total Monthly seizures.
| Time | Total seizures |
|---|---|
| March | 59 |
| April | 21 |
| May | 31 |
| June | 24 |
| July | 16 |
| August | 8 |
| September | 8 |
| October | 4 |
Fig. 1.
Total monthly seizures.
Fig. 2.
Brain MRI With and Without Contrast tumor protocol of our patient. (a) and (b) demonstrating a hyperintense mass on T2 that is non-enhancing on T1 post contrast respectively centered within the left frontal/insular cortical region on 1/29/2021 prior to starting Ivosidenib. (c) and (d) demonstrating unchanged size of hyperintense non-enhancing mass on T2 and T1 post contrast axial view respectively on 8/9/21 after starting Ivosidenib.
3. Discussion
Precision medicine is the therapeutic approach of targeting specific molecular or genetic mechanisms responsible for the pathophysiology of a disease. However, due to the complex nature of genotype-phenotype correlations in seizures, there are very limited options for precision medicine within the field of epilepsy [14]. We present a case where off-label Ivosidenib improved drug-resistant focal epilepsy due to an oligodendroglioma in the absence of any changes to traditional anti-seizure medications. Post-treatment brain MRI tumor protocol demonstrated stable T2 signal changes of the non-enhancing mass. This suggests that Ivosidenib may potentially reduce seizures through a unique mechanism.
IDH1/2 mutation is one defining feature of oligodendrogliomas and confers improved prognosis when found in astrocytoma [7]. Oligodendrogliomas are particularly notorious for inducing seizures compared to other gliomas, and focal epilepsy from oligodendrogliomas can be very treatment resistant [3], [15]. In addition to cortical dysfunction from the tumor itself, IDH mutations can also potentially lower the seizure threshold through a unique pathway in which the product of IDH1/2 mutations (D2HG) may stimulate tumor growth as well as mimic glutamate, an excitatory neurotransmitter, thereby promoting seizures [11]. Preclinical data previously demonstrated that Ivosidenib produces strong inhibition of D2HG production in brain tumor samples using an orthotopic mouse xenograft model of human IDH1 mutant glioma [16]. In a phase I open-label study, Ivosidenib demonstrated CNS penetration and lowered D2HG in treated tumors compared to the control group [17]. This suggests that targeted IDH1 inhibitors may help manage seizures in patients with IDH1 mutant glioma and epilepsy [10]. There is a recent case report that shows Ivosidenib can be a safe treatment in a patient with glioblastoma and reduce seizures as well [18]. Due to the dramatic response in our patient’s seizure frequency, this reduction in D2HG could potentially explain how IDH1 inhibitors exert their anti-seizure effects in patients with focal epilepsy from IDH1 mutant oligodendroglioma.
4. Conclusion
The inhibition of D2HG production by IDH1 inhibitors is a possible mechanism for seizure improvement in our patient. This suggests that Ivosidenib may potentially be a safe treatment and help improve seizure control in patients with drug resistant epilepsy from non-enhancing oligodendrogliomas through a unique mechanism. There is ongoing research using IDH1 inhibitors in patients with gliomas examining long-term outcome as well as seizure control. This suggests that it may be possible to improve seizures by targeting specific molecular–genetic markers, however, further research involving other molecular–genetic markers in brain lesions associated with epilepsy will be beneficial.
Ethical approval
This case report was reviewed by the Oregon Health & Science University institutional IRB and determined to not represent human research. This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)-compliant manner.
Statement of informed consent
Written informed consent was obtained including permission for publication of all photographs and images included.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Submission declaration
This work has not been published previously. it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Anh Huan Vo, Email: ahv2019@outlook.com.
Prakash Ambady, Email: ambady@ohsu.edu.
David Spencer, Email: spencerd@ohsu.edu.
References
- 1.Ostrom Q.T., Gittleman H., Liao P., Vecchione-Koval T., Wolinsky Y., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morshed R.A., Young J.S., Hervey-Jumper S.L., Berger M.S. The management of low-grade gliomas in adults. J Neurosurg Sci. 2019;63:450–457. doi: 10.23736/S0390-5616.19.04701-5. [DOI] [PubMed] [Google Scholar]
- 3.Kerkhof M., Benit C., Duran-Pena A., Vecht C.J. Seizures in oligodendroglial tumors. CNS Oncol. 2015;4(5):347–356. doi: 10.2217/cns.15.29. Epub 2015 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koekkoek J.A.F., Dirven L., Heimans J.J., Postma T.J., Vos M.J., Reijneveld J.C., et al. Seizure reduction in a low-grade glioma: more than a beneficial side effect of temozolomide. J Neurol Neurosurg Psychiatry. 2015;86(4):366–373. doi: 10.1136/jnnp-2014-308136. [DOI] [PubMed] [Google Scholar]
- 5.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 6.Wen P.Y., Packer R.J. The 2021 WHO classification of tumors of the central nervous system: clinical implications. Neuro Oncol. 2021;23(8):1215–1217. doi: 10.1093/neuonc/noab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirozzi C.J., Yan H. The implications of IDH mutations for cancer development and therapy. Nat Rev Clin Oncol. 2021;18(10):645–661. doi: 10.1038/s41571-021-00521-0. Epub 2021 Jun 15. [DOI] [PubMed] [Google Scholar]
- 8.Han S., Liu Y., Cai S.J., Qian M., Ding J., Larion M., et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580–1589. doi: 10.1038/s41416-020-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkalp Z., Karamchandani J., Das S. IDH mutation in glioma: new insights and promises for the future. JAMA Neurol. 2014;71(10):1319–1325. doi: 10.1001/jamaneurol.2014.1205. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y., Mao Q., Wang X., Liu Y., Mao Y., Zhou Q., et al. An analysis of 170 glioma patients and systematic review to investigate the association between IDH-1 mutations and preoperative glioma-related epilepsy. J Clin Neurosci. 2016;31:56–62. doi: 10.1016/j.jocn.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Chen H., Judkins J., Thomas C., Wu M., Khoury L., Benjamin C.G., et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. doi: 10.1212/WNL.0000000000003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellinghoff I.K., Ellingson B.M., Touat M., Maher E., De La Fuente M.I., Holdhoff M., et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J Clin Oncol. 2020;38(29):3398–3406. doi: 10.1200/JCO.19.03327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koekkoek J.A., Kerkhof M., Dirven L., Heimans J.J., Reijneveld J.C., Taphoorn M.J. Seizure outcome after radiotherapy and chemotherapy in low-grade glioma patients: a systematic review. Neuro Oncol. 2015 Jul;17(7):924–934. doi: 10.1093/neuonc/nov032. Epub 2015 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demarest S.T., Brooks-Kayal A. From molecules to medicines: the dawn of targeted therapies for genetic epilepsies. Nat Rev Neurol. 2018;14(12):735–745. doi: 10.1038/s41582-018-0099-3. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowski A.F., Blakeley J. Clinical Management of Seizures in Patients With Low-Grade Glioma. Semin Radiat Oncol. 2015;25(3):219–224. doi: 10.1016/j.semradonc.2015.02.009. Epub 2015 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolay B., Narayanaswamy R., Aguado E., et al. EXTH-59. The IDH1 mutant inhibitor AG-120 shows strong inhibition of 2-HG production in an orthotopic IDH1 mutant glioma model in vivo. Neuro Oncol. 2017;19(Suppl. 6) vi86–vi86. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C38&q=The+IDH1+mutant+inhibitor+AG-120+shows+strong+inhibition+of+2-HG+production+in+an+orthotopic+IDH1+mutant+glioma+model+in+vivo&btnG= [Google Scholar]
- 17.Mellinghoff I.K., Cloughesy T.F., Wen P.Y., et al. A phase 1, open-label, perioperative study of AG-120 and AG-881 in recurrent IDH1 mutant, low-grade glioma: Results from cohort 1. J Clin Oncol. 2019;37(15_suppl abstr):2003. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C38&q=%3A+A+phase+1%2C+open-label%2C+perioperative+study+of+AG-120+and+AG-881+in+recurrent+IDH1+mutant%2C+low-grade+glioma%3A+Results+from+cohort+1&btnG= [Google Scholar]
- 18.Tejera D., Kushnirsky M., Gultekin S.H., Lu M., Steelman L., de la Fuente M.I. Ivosidenib, an IDH1 inhibitor, in a patient with recurrent, IDH1-mutant glioblastoma: a case report from a Phase I study. CNS Oncol. 2020;9(3):CNS62. doi: 10.2217/cns-2020-0014. Epub 2020 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]