Abstract
Adiponectin can participate in the regulation of glucose and lipid metabolism, energy regulation, immune response, resistance to inflammation, oxidative stress, and apoptosis. Studies in rodents demonstrated that the small molecule compound adiponectin receptor agonist AdipoRon could activate the adiponectin receptor and played the same biological role as adiponectin. To explore the influence and regulation of AdipoRon on lipid metabolism disorder in Huoyan goose liver, in this study, goslings were fed a high-fat diet and then administered different dosages of AdipoRon. Subsequently, goose body weight, liver index, liver histopathological changes, blood glucose, blood and liver lipid, biochemical indexes related to liver function and oxidative stress, and the expression levels of genes related to lipid metabolism, inflammation, apoptosis, and autophagy, adiponectin and its receptors, key molecules of adiponectin involved signal pathway, and transcription factors in the liver, were detected using H&E and Oil red O staining, ELISA, and qRT-PCR methods. The results indicated that AdipoRon could alter the expression of lipid metabolism-related genes, inflammatory factors, apoptosis and autophagy genes, and adiponectin and its receptor genes in liver tissues through signaling pathways such as AMPK and p38 MAPK, as well as the involvement of transcription factors such as PPARα, PPARγ, SIRT1, and FOXO1, reduce the lipid content in blood and liver tissues of geese fed high-fat diets, improve liver antioxidant capacity, regulate apoptosis and autophagy of hepatocytes, and reduce liver inflammatory injury. Our study suggests that AdipoRon has a protective effect on fatty liver injury in goslings fed a high-fat diet.
Key words: AdipoRon, liver, protection, high-fat diet, Goose
INTRODUCTION
Adiponectin is a cytokine secreted by adipose tissue which is involved in the regulation of various physiological functions, molecular and cellular events such as sugar and lipid metabolism, energy regulation, immune response, as well as resistance to inflammation, oxidative stress and apoptosis (Fang and Judd, 2018). Adiponectin could exert biological functions by initiating a series of downstream signaling events in various target tissues such as skeletal muscle, liver, heart, kidney and pancreas mainly through its receptors AdipoR1 and AdipoR2. Reports found that Adiponectin and its receptors could be expressed in tissues such as pituitary gland, hypothalamus, liver, skeletal muscle, ovary, spleen, and kidney of birds such as chickens and geese (Maddineni, 2005; Cao et al., 2015). The liver is the main target organ of adiponectin. In the liver tissue, adiponectin binds to its specific receptor and can induce the activation of a series of signaling pathways, exerting its regulatory carbohydrate and lipid metabolism, and antioxidative stress effects (Gamberi et al., 2018).
At present, there are many studies on adiponectin, but because the monomeric form of adiponectin cannot play its role, the structure of synthetic adiponectin polymers is unstable, and adiponectin in vivo requires a high plasma concentration, these limits the direct application of adiponectin in clinical medicine (Wu et al., 2019). During the process of studying the mechanism of adiponectin, it was found that AdipoR1 and AdipoR2 play an important role in regulating glucose and lipid metabolism, inflammation and oxidative stress in the body. Therefore, another strategy that can replace adiponectin is to find agonists that can activate AdipoR1 and AdipoR2 receptors, and thus performing the same biological role as adiponectin. AdipoRon is a small molecule compound obtained by Professor MikiOkada-Iwabu, at the Institute of Pharmaceutical Research, University of Tokyo, Japan. AdipoRon has been found to bind to AdipoR1 and AdipoR2 in rodents, showing similar effects as adiponectin (Okada-Iwabu et al., 2013).
The liver is the primary site of lipid metabolism for birds. The lipids of poultry, especially waterfowl (ducks and geese), are mainly derived from endogenous hepatic synthesis (Wei et al., 2021). Abnormalities in one or more metabolic pathways such as fatty acid synthesis and esterification, fatty acid transport, fatty acid oxidation, lipolysis, and utilization in the liver can lead to lipid metabolism disorders, excessive accumulation of triglycerides and free fatty acids in hepatocytes leads to steatosis, increased fatty acid oxidation produces excessive ROS causing oxidative stress and inflammatory response, and fatty liver disease occurs. Fatty liver disease in poultry occurs frequently in young meat-type and egg-laying poultry, resulting in excessive liver fat deposition, reduced liver function, decreased egg production rate, and increased mortality. With the rapid development of intensive breeding in poultry industry, there are more and more factors causing fatty liver disease in poultry. Based on the biological effects of adiponectin and its receptor agonists in the regulation of lipid metabolism, antioxidative stress and inflammation, efficient related clinical drugs (such as adiponectin agonists, etc.) can be developed through various strategies such as pharmacology, medicinal chemistry, and biochemistry in the future to increase the expression level of adiponectin and its receptors in the liver, prevent the occurrence of fatty liver disease or alleviate its harm.
In this study, goslings were fed with diet, and then treated with different doses of adiponectin receptor agonist AdipoRon. In order to investigate the effect and regulation of AdipoRon on abnormal liver metabolism in geese, metabolic parameters such as goose body weight and liver index, as well as liver histopathological changes, blood and liver adiponectin content, blood glucose, blood lipids, liver function, and oxidative stress-related biochemical indicators, the expression level of liver metabolism-related genes, inflammatory factors, apoptosis and autophagy-related genes, key molecules, and transcription factors of lipid-related signaling pathways were detected by pathology, ELISA, quantitative real-time PCR (qRT-PCR), western blot and other methods. The results provided some theoretical basis for further exploration of the application of AdipoRon in regulating geese lipid metabolism and preventing and treating liver-related diseases.
MATERIALS AND METHODS
Ethics Statement
All experimental procedures in this study were approved by the animal welfare committee of the College of Animal Science and Veterinary Medicine of Shenyang Agricultural University (No.202006006) and performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (State Science and Technology Commission in China, 1988).
Animals and Diets
Ninety one-day-old healthy Huoyan geese (half male and half female) were obtained from a goose breeding farm located in Changtu county, Liaoning province, China. Geese were randomly divided into 5 groups, designated as blank control group (B), high-fat diet group (M), low-dose AdipoRon group (AL), medium-dose AdipoRon group (AM), and high-dose AdipoRon group (AH), with 3 replicates per group and 6 geese per replicates. Six geese in each replicate were placed in a pen (1.5 × 1.3 m2). All geese in 15 pens were raised in a temperature and humidity-controlled animal facility with windows and concrete floors covered by clean rice bran. Room temperature was maintained at 32−33°C at 1−3-day-old, 30−31°C at 4−7-day-old, 27−28°C at 8−14-day-old, 24−26°C at 15−21-day-old, 20−22°C from 21-day-old until the end of experiment. Relative humidity was maintained at approximately 65% throughout the whole experimental period. A 24-h light was used on the first day, followed by a 1-h reduction every 2 d, and natural light was used starting on the 4th wk. The geese were permitted ad libitum access to the diet and water. The basal diet for goose was purchased from Shenyang Aitejie Feed Co., LTD (Shenyang, China). The composition and nutrient levels of basal diet are shown in Table S1. The high-fat diet was supplemented with 5% lard tallow based on the basal diet.
Experimental Design and Sample Collection
After 1 wk acclimatization period (fed with basal diet), geese (7-day-old) in group B were continued to be fed with basal diet, while geese (7-day-old) in groups M, AL, AM, and AH were fed with high-fat diet. After 25 d of feeding, geese (32-day-old) in group M were intraperitoneally injected with 1 mL of saline every day for 3 d, while geese (32-day-old) in AL, AM, and AH groups were intraperitoneally injected with 1 mL of low concentration (0.1 mg /mL), medium concentration (0.5 mg /mL), and high concentration (1 mg /mL) of AdipoRon (purchased from MedChemExpress, Monmouth Junction, NJ) solution for 3 d, respectively. At the end of the experiment (35-day-old), following overnight fasting, body weight was monitored using electronic balance (SH-HL-C3201, Accuracy 0.1g, Beijing North Shouheng Electronic Technology Co., Ltd, Beijing, China), and then the geese were killed by severing the jugular vein. Blood samples were collected from the wing vein into vacuum blood collection tubes and centrifuged at 3,000 rpm for 5 min at 4°C, and the obtained serum was stored at −20°C until analysis. The livers were excised, rinsed with saline, weighed using above mentioned electronic balance, and separated into 4 portions. One portion of liver tissue was fixed in 4% formaldehyde for Hematoxylin and Eosin (H&E) Staining. The other portion of liver tissue was embedded in a package with OCT (optimal cutting temperature compound) for Oil Red O Staining. Another portion of liver tissue was stored at −20°C refrigerator for biochemistry analysis. The remaining liver tissue samples were snap-frozen immediately in liquid nitrogen and stored at −80°C refrigerator for molecular analysis. The body and liver weight were utilized to calculate the liver index using the following equation: Liver index = liver weight/body weight × 100%.
Biochemical Analysis
The levels of serum glucose (GLU), triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL-C), low density lipoprotein (LDL-C), free fatty acid (FFA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were measured using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Liver samples were thawed at 4°C, minced in an ice-water bath, and pre-cooled normal saline was added at a 1:9 (weight/volume) dilution, and then homogenized, the homogenates were centrifuged at 4,000 rpm for 10 min at 4°C, and the supernatants were collected for biochemical assay. The levels of triglyceride (TG), total cholesterol (TC), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and malondialdehyde (MDA) in liver were determined using commercially available kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions.
Hematoxylin and Eosin (H&E) Staining
Liver tissue samples were fixed in 4% formaldehyde solution for 24 h, embedded in paraffin, cut on a microtome into sections with a thickness of 4 μm. Then, tissue sections were stained with hematoxylin and eosin (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China) using the routine procedure. Afterward, the stained sections were observed for histopathological changes and photographed using the Optical microscope (Nikon Eclipse Ti-SR, Japan).
Oil Red O Staining
The OCT embedded liver tissue samples were cut into frozen liver slices of 10-μm thickness. The slices were stained with Oil Red O solution (Beijing Solarbio Science & Technology Co., Ltd). After differentiated with isopropanol and washed with distilled water, then re-stained with hematoxylin. The stained sections were visualized and imaged with microscope (Nikon Eclipse CI-L). The lipid droplet area was measured using Image-Pro Plus 6.0 software. The relative percentage of lipid droplet area was calculated according to the following equation: Relative lipid droplet area (%) = lipid droplet area/tissue area × 100%.
Quantitative Real-Time PCR
Total RNA was extracted from liver tissues samples using Trizol reagent (TaKaRa, Dalian, China) according to the manufacturer's protocol. The RNA purity and concentration were determined using NanoDrop 8000 spectrophotometry (NanoDrop, Thermo Scientific, Waltham, MA). First-strand cDNA was synthesized using the FastQuant RT Kit (with gDNase) (TIANGEN, Beijing, China) according to the manufacturer's instructions. The primers for target and internal reference genes (listed in Table S2) were designed and synthesized by Sangon Biotech Co., LTD (Shanghai, China). The quantitative real-time PCR was performed in the Bio-Rad iQ5 Real-time PCR Detection System (BIO-RAD, Hercules, CA) using 2 × SYBR Premix Ex Taq II (Takara) as the detection dye. Each 25 µL reaction volume contained 1 μL 10 µM (each) forward and reverse primers, 12.5 μL 2 × SYBR Premix Ex Taq II (Takara), and 2 μL cDNA products, and the final volume was adjusted using PCR-water. The procedure included one cycle of 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 10 s and annealing and extension at 55°C for 30 s. At the end of the amplification, a melting curve was run to ensure that only a single specific product was amplified. Each sample was repeated 3 times. The relative expression of targeted gene was calculated using the 2−△△Ct method. The blank control group was used as calibrator (relative expression = 1), and β-actin was designated as an internal reference gene.
Statistical Analysis
The results were presented as the mean ± standard error (SE). All data were analyzed via one-way ANOVA followed by least significant difference (LSD) post-hoc test using SPSS 19.0 for Windows (SPSS Inc. Chicago, IL). The P < 0.05 and P < 0.01 were considered to be significant difference, respectively.
RESULTS
Metabolic Parameters
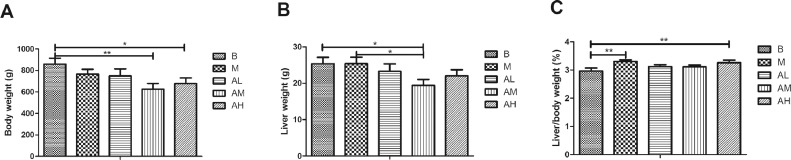
As shown in Figure 1, the body weight and liver weight of geese in the 3 AdipoRon treatment groups was lower than that in the control group and high-fat diet group, among them, the medium-dose AdipoRon group and high-dose AdipoRon group was significantly lower than the control group (P < 0.01, P < 0.05). The liver index of geese in high-fat diet group was significantly higher than that in the control group (P < 0.01), and that in the 3 AdipoRon treatment groups was lower than that in the high-fat diet group (P > 0.05). These results suggest that AdipoRon can reduce goose body weight, liver weight, and improve liver index to some extent.
Figure 1.
Effect of AdipoRon on body weight, liver weight and liver index of geese. * indicate the significant difference (P < 0.05); ** indicate the extremely significant difference (P < 0.01). (A) Body weight; (B) liver weight; (C) liver index. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group and high-dose AdipoRon group are denoted by the B, M, AL, AM and AH, respectively.
Pathological Changes of Liver
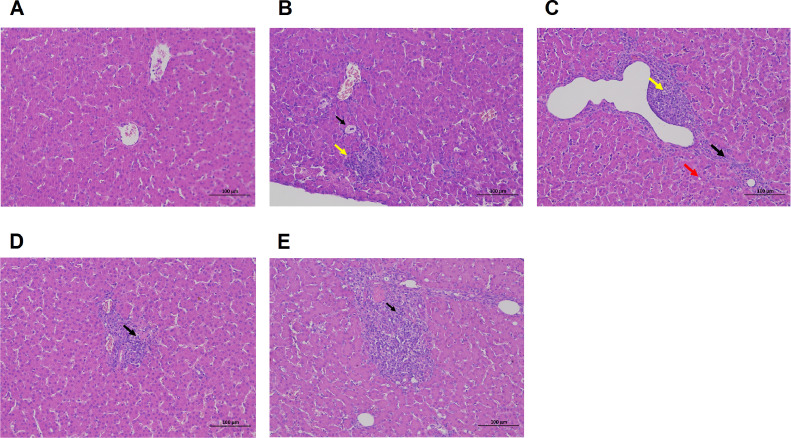
The results of H.E staining of liver tissues of geese are shown in Figure 2. The capsule of liver tissues in the control group was composed of dense connective tissue rich in elastic fibers with uniform thickness, the hepatocytes were round and plump, the liver plates were arranged regularly and neatly, and the sinusoids were not significantly dilated or compression; no significant abnormalities in the portal area between adjacent hepatic lobules; no obvious inflammatory changes. In the high-fat group, there were few hepatocyte ballooning-like degeneration, cell swelling, nucleus in the middle, cytoplasm vacuolating, mild connective tissue hyperplasia (black arrow) around the local portal area, and rare lymphocyte infiltration (yellow arrow). In the low-dose AdipoRon group, multiple desmoplasia (black arrows) accompanied by lymphocytic infiltration (yellow arrows) were observed in the liver tissue, and multiple sites of mild steatosis of hepatocytes, smaller round vacuoles (red arrows) were seen in the cytoplasm. In the medium-dose AdipoRon group, the hepatocyte morphology and structure were normal; rare focal lymphocyte infiltration and desmoplasia (black arrow). In the high-dose AdipoRon group, the hepatocyte morphology and structure were normal, and there were focal lymphocyte infiltration and desmoplasia (black arrows) around the portal areas. The results show that AdipoRon can alleviate the fatty pathological injury in liver tissue of goose.
Figure 2.
Effect of AdipoRon on histopathological changes of geese liver (H.E staining 200 ×). (A) Blank control group; (B) high-fat diet group; (C) low-dose AdipoRon group; (D) medium-dose AdipoRon group; (E) high-dose AdipoRon group. Lymphocytic infiltration was marked by yellow arrows, desmoplasia was marked by black arrows, and smaller round vacuoles was marked by red arrows.
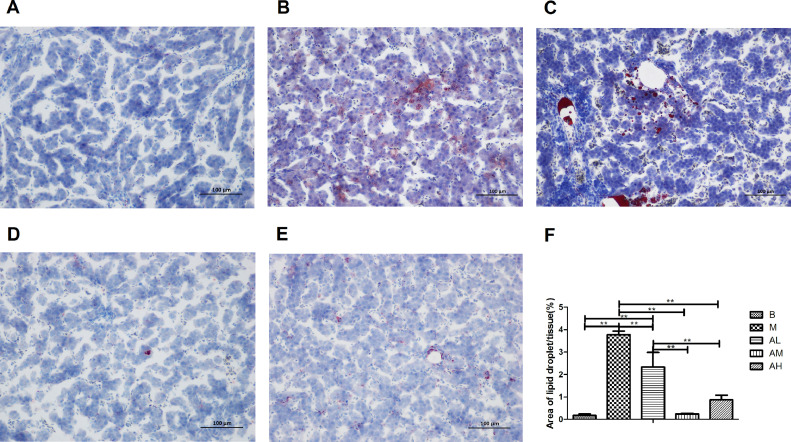
The results of oil red O staining of liver tissue are shown in Figure 3. The number of lipid droplets in the high-fat diet group was significantly more than that in the control group, and the relative lipid droplet area was significantly greater than that in the control group (P < 0.01). The number of lipid droplets in the 3 AdipoRon treatment groups was less than that in the high-fat diet group, and the relative lipid droplet area was smaller than that in the high-fat diet group (P < 0.01). These results indicate that AdipoRon treatment can reduce lipid deposition in liver tissue of goose.
Figure 3.
Effect of AdipoRon on lipid deposition of goose liver (Oil Red O staining, 200 ×). (A) Blank control group; (B) high-fat diet group; (C) low-dose AdipoRon group; (D) medium-dose AdipoRon group; (E) high-dose AdipoRon group; (F) analysis results of lipid droplet area stained with Oil red O.
Lipid and Glucose Content in Serum and Liver
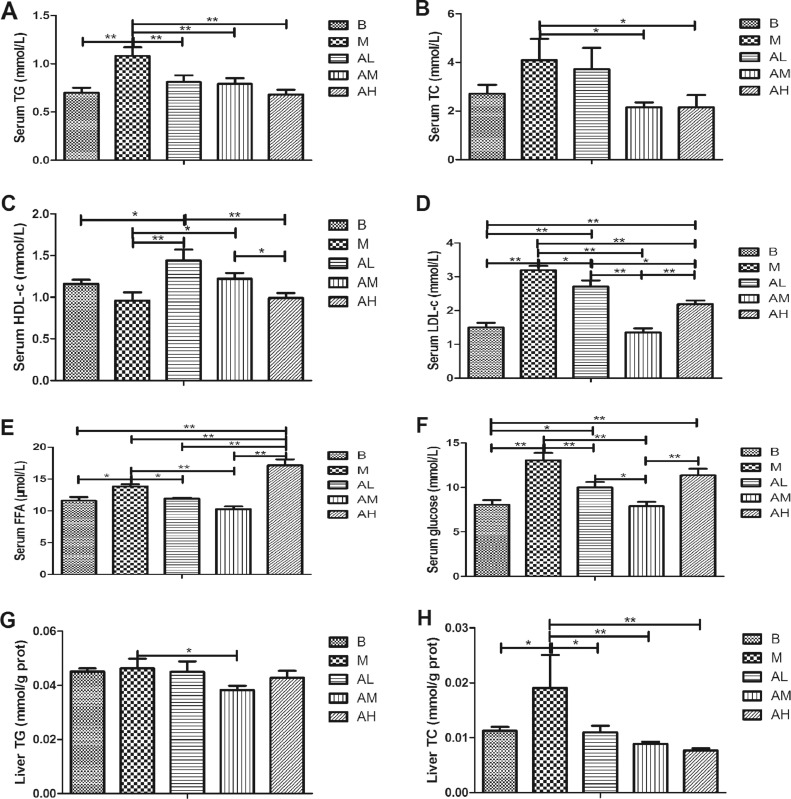
As shown in Figure 4A, the serum TG level of high-fat diet group was significantly higher than that of the control group (P < 0.01). All 3 AdipoRon groups were significantly lower than those in high-fat diet group (P < 0.01).
Figure 4.
Effect of AdipoRon on lipid and glucose content in serum and liver of geese. (A) Serum TG; (B) serum TC; (C) serum HDL-c; (D) serum LDL-c; (E) serum FFA; (F) serum glucose; (G) liver TG; (H) liver TC. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group, and high-dose AdipoRon group are denoted by the B, M, AL, AM, and AH, respectively. Abbreviations: HDL-C, high density lipoprotein; LDL-C, low density lipoprotein; TC, total cholesterol; TG, triglyceride.
As shown in Figure 4B, the serum TC level of geese in high-fat diet group was higher than that in the control group (P > 0.05). The three AdipoRon groups were all lower than the high-fat diet group. Among them, the difference between the medium-dose AdipoRon group and the high-dose AdipoRon group with the high-fat diet group was statistically significant (P < 0.05).
As shown in Figure 4C, the serum HDL-C level of high-fat diet group was lower than that of the control group (P > 0.05). The three AdipoRon groups were higher than the high-fat diet group. Among them, the low-dose and medium-dose AdipoRon group was significantly different with the high-fat diet group (P < 0.01, P < 0.05).
As shown in Figure 4D, the level of serum LDL-C in the high-fat diet group was significantly higher than that in the control group (P < 0.01). Low-dose and medium-dose AdipoRon group was significantly lower than high-fat diet group (P < 0.05, P < 0.01).
As shown in Figure 4E, the level of serum FFA in the high-fat diet group was significantly higher than that in the control group (P < 0.05). Low-dose and medium-dose AdipoRon group was significantly lower than high-fat diet group (P < 0.05, P < 0.01).
As shown in Figure 4F, the blood glucose level of high-fat diet group was significantly higher than that of the control group (P < 0.01). The three AdipoRon groups were all lower than the high-fat diet group, and especially the low-dose and medium-dose AdipoRon groups were significantly lower than the high-fat diet group (P < 0.01).
As shown in Figure 4G, the liver TG content of goose in the high-fat diet group was slightly higher than that in the control group (P > 0.05). All of the 3 AdipoRon groups were lower than the high-fat diet group, and there was significant difference between the medium-dose AdipoRon group and the high-fat diet group (P < 0.05).
As shown in Figure 4H, the liver TC content goose in the high-fat diet group was significantly higher than that in the control group (P < 0.05). All the 3 AdipoRon groups were lower than the high-fat diet group, and the difference was statistically significant (P < 0.05, P < 0.01).
These results suggest that AdipoRon can inverse the increase of lipid (TG, TC, LDL-C, and FFA) and glucose content in blood and liver of goose caused by high-fat diet, and increase the level of HDL-C in blood.
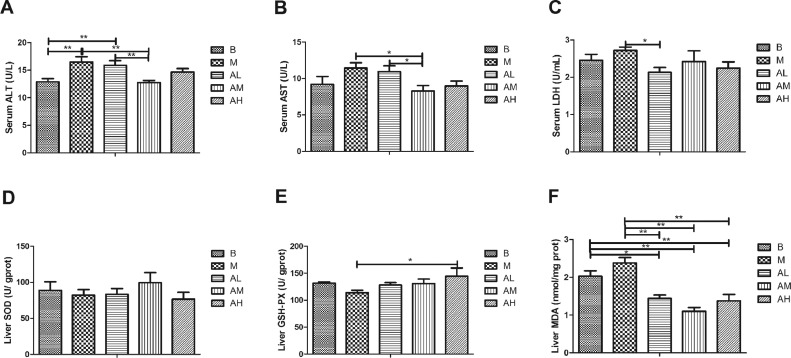
Biochemical Indexes Related to Liver Function and Oxidative Stress
As shown in Figure 5A, the activity of serum ALT in the high-fat diet group was significantly higher than that in the control group (P < 0.01). All of the 3 AdipoRon groups were lower than the high-fat diet group, and the difference between the medium-dose AdipoRon group and the high-fat diet group was significant (P < 0.01). As shown in Figure 5B, the serum AST activity in high-fat diet group was higher than that in control group (P > 0.05). All of the 3 AdipoRon groups were lower than the high-fat diet group, and there was significant difference between the medium-dose AdipoRon group and the high-fat diet group (P < 0.05). As shown in Figure 5C, the serum LDH activity in the high-fat diet group was higher than that in the control group (P > 0.05). All of the 3 AdipoRon groups were lower than the high-fat diet group. Among them, the difference between the low-dose AdipoRon group and the high-fat diet group was statistically significant (P < 0.05). These results indicate that AdipoRon can decrease the activity of enzymes related to goose liver function damage and improve the liver injury caused by high-fat diet.
Figure 5.
Effect of AdipoRon on biochemical indices related to liver function and oxidative stress in geese. (A) Serum ALT; (B) serum AST; (C) serum LDH; (D) liver SOD; (E) liver GSH-PX; (F) liver MDA. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group, and high-dose AdipoRon group are denoted by the B, M, AL, AM, and AH, respectively. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GSH-Px, glutathione peroxidase; LDH, lactate dehydrogenase; MDA, malondialdehyde; SOD, superoxide dismutase.
As shown in Figure 5D, the liver SOD activity of goose in the high-fat diet group was lower than that in the control group (P > 0.05). Low-dose and medium-dose AdipoRon groups were higher than high-fat diet group, but the difference was not statistically significant (P > 0.05). As shown in Figure 5E, the liver GSH-Px activity of goose in the high-fat diet group was lower than that in the control group (P > 0.05). All of the 3 AdipoRon groups were higher than the high-fat diet group, and the difference between the high-dose AdipoRon group and the high-fat diet group was significant (P < 0.05). As shown in Figure 5F, the liver MDA content of goose in the high-fat diet group was higher than that in the control group. All the 3 AdipoRon groups were significantly lower than those in high-fat diet group (P < 0.01, P < 0.05). The above results suggest that AdipoRon can increase the activity of antioxidant enzymes and alleviate oxidative stress in liver tissue of goose.
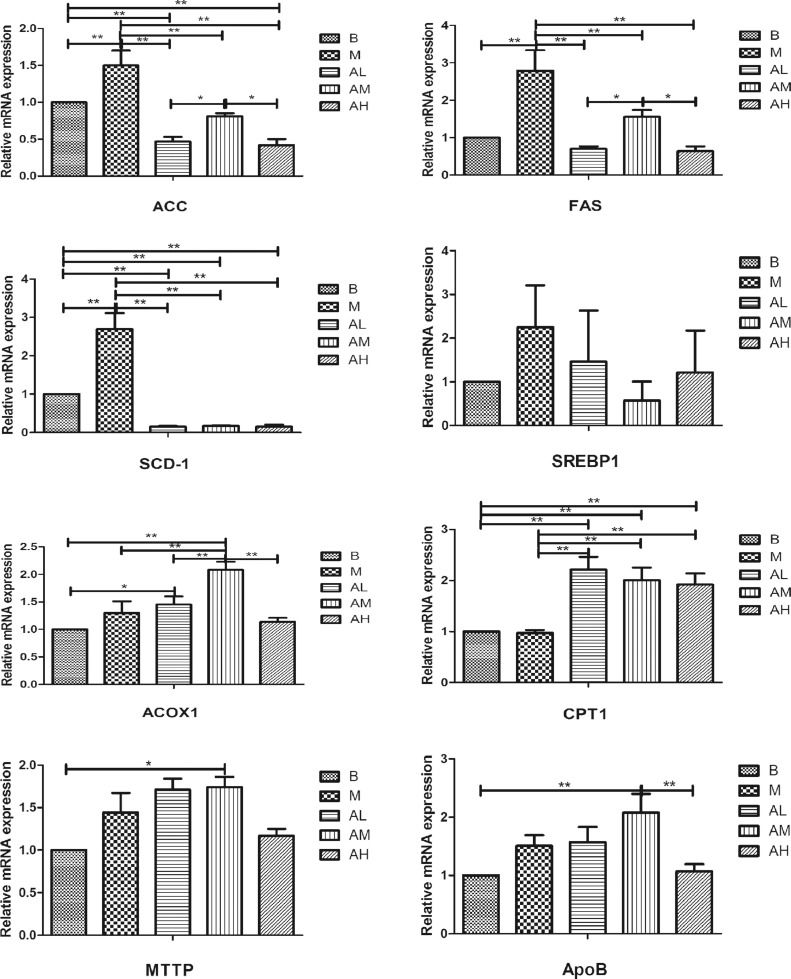
Expression of Genes Related to Lipid Metabolism
As shown in Figure 6, for genes related to lipid synthesis, the mRNA expression level of ACC, FAS and SCD-1 genes in liver tissue of geese in high-fat diet group was extremely significantly higher than that in the control group (P < 0.01). All the 3 AdipoRon groups were significantly lower than those in high-fat diet group (P < 0.01). The mRNA expression level of SREBP1 in liver tissue of geese in high-fat diet group was higher than that in control group (P > 0.05). All of the 3 AdipoRon groups were lower than those in high-fat diet group (P > 0.05). These results suggest that AdipoRon can decrease the mRNA expression levels of fatty acid synthesis-related genes (FAS, ACC, and SREBP-1) and fatty acid desaturation related enzymes (SCD-1) in goose liver tissues.
Figure 6.
Effect of AdipoRon on the mRNA expression of lipid metabolism related genes in geese livers. Total RNA was extracted using Trizol reagent from liver tissues of goose, and complementary DNA was synthesized using cDNA synthesis kit from the total RNA. Quantitative real-time PCR was done with cDNA, primers, and SYBR PCR Premix solution. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group and high-dose AdipoRon group are denoted by the B, M, AL, AM, and AH, respectively. Y-axis represents the fold change of mRNA expression level which presented in AU (arbitrary units). Values of bars are expressed as mean ± SE (n = 9). The data were analyzed by one-way ANOVA followed by LSD post-hoc test. * indicate P < 0.05; ** indicate P < 0.01.
For genes related to fatty acid oxidation, the mRNA expression level of ACOX1 in liver tissue of geese in high-fat diet group was higher than that in the control group (P > 0.05). Low-dose and medium-dose AdipoRon group was significantly higher than high-fat diet group (P < 0.05). The mRNA expression level of CPT1 in liver tissues of geese in high-fat diet group was not significantly different from that in control group, and the 3 AdipoRon groups were significantly higher than those in high-fat diet group and control group (P < 0.01). These results indicate that AdipoRon can increase the mRNA expression of fatty acid oxidation-related genes (ACOX1 and CPT1) in goose liver tissue.
For genes related to lipid transport, the mRNA expression level of apoB in liver tissue of geese in high-fat diet group was higher than that in the control group (P > 0.05). Low-dose and medium-dose AdipoRon group was higher than high-fat diet group, but the difference was not significant (P > 0.05). The mRNA expression level of MTTP in liver tissue of geese in high-fat diet group was higher than that in control group (P > 0.05). The low-dose and medium-dose AdipoRon groups were both higher than the high-fat diet group (P > 0.05), the high-dose AdipoRon group was lower than the high-fat diet group (P > 0.05). These results suggest that the appropriate dose of AdipoRon can increase the mRNA expression level of genes involved in lipid (TG) transport to some extent.
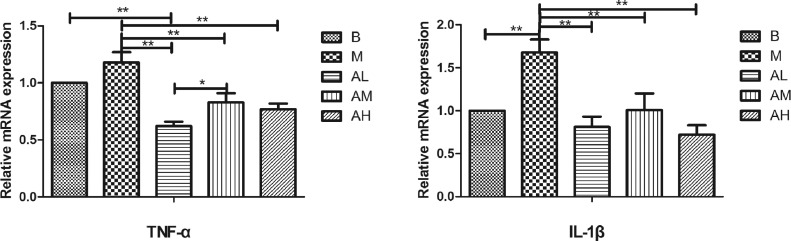
Expression of Inflammatory Factors
As shown in Figure 7, the mRNA expression level of TNF-α in liver tissue of geese in high-fat diet group was higher than that in the control group (P > 0.05). All of the three AdipoRon groups were significantly lower than that of the high-fat diet group (P < 0.01). The mRNA expression level of IL-1β in liver tissue of geese in high-fat diet group was significantly higher than that of control group (P < 0.01). All the 3 AdipoRon groups were significantly lower than those in high-fat diet group (P < 0.01). These results suggest that AdipoRon may reduce the expression levels of proinflammatory factor TNF-α and IL-1β mRNA in liver tissue, thereby alleviating the inflammatory response of liver tissue.
Figure 7.
Effect of AdipoRon on the mRNA expression of inflammatory factors in geese livers. Total RNA was extracted using Trizol reagent from liver tissues of goose, and complementary DNA was synthesized using cDNA synthesis kit from the total RNA. Quantitative real-time PCR was done with cDNA, primers, and SYBR PCR Premix solution. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group, and high-dose AdipoRon group are denoted by the B, M, AL, AM, and AH, respectively. Y-axis represents the fold change of mRNA expression level which presented in AU (arbitrary units). Values of bars are expressed as mean ± SE (n = 9). The data were analyzed by one-way ANOVA followed by LSD post-hoc test. * indicate P < 0.05; ** indicate P < 0.01.
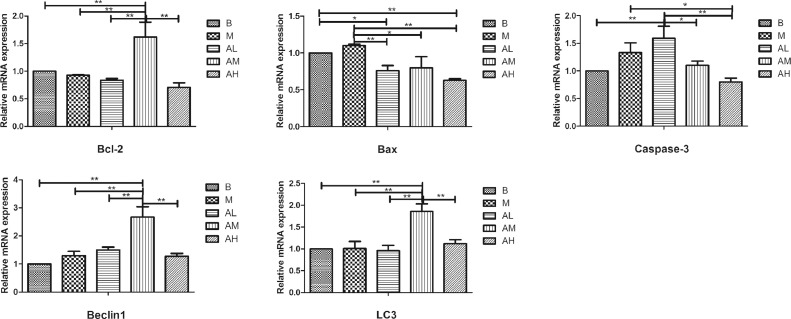
Expression of Genes Related to Apoptosis and Autophagy
As shown in Figure 8, the mRNA expression level of bcl-2 in liver tissue of geese in high-fat diet group was lower than that in the control group (P > 0.05). There was no significant difference between low-dose AdipoRon group and high-fat diet group (P > 0.05). The medium-dose AdipoRon group was significantly higher than high-fat diet group and control group (P < 0.01). The mRNA expression level of Bax in liver tissue of geese in high-fat diet group was higher than that in control group (P > 0.05). All of the three AdipoRon groups were significantly lower than the high-fat diet group (P < 0.05, P < 0.01). The mRNA expression level of caspase-3 in liver tissue of geese in high-fat diet group was higher than that in control group (P>0.05). The low-dose AdipoRon group was higher than the high-fat diet group (P > 0.05). The medium-dose and high-dose AdipoRon group was nonsignificantly and significantly lower than the high-fat diet group (P > 0.05, P < 0.05), respectively. The mRNA expression level of Beclin1 in liver tissue of geese in high-fat diet group was higher than that in control group (P > 0.05). Low-dose and medium-dose AdipoRon group was nonsignificantly and significantly higher than high-fat diet group and control group (P > 0.05, P < 0.01), respectively. For the mRNA expression level of LC3, there was no significant difference between the high-fat diet group and low-dose group with the control group (P > 0.05). Medium-dose and high-dose AdipoRon group was significantly and nonsignificantly higher than high-fat diet group and control group (P < 0.01, P > 0.05), respectively. These results suggest that the appropriate dose of AdipoRon can increase the mRNA expression levels of antiapoptotic gene Bcl-2 and autophagy-related genes Beclin1 and LC3, and decrease the mRNA expression levels of proapoptotic genes Bax and caspase-3.
Figure 8.
Effect of AdipoRon on the mRNA expression of apoptosis and autophagy related genes in geese livers. Total RNA was extracted using Trizol reagent from liver tissues of goose, and complementary DNA was synthesized using cDNA synthesis kit from the total RNA. Quantitative Real-time PCR was done with cDNA, primers, and SYBR PCR Premix solution. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group, and high-dose AdipoRon group are denoted by the B, M, AL, AM, and AH, respectively. Y-axis represents the fold change of mRNA expression level which presented in AU (arbitrary units). Values of bars are expressed as mean ± SE (n = 9). The data were analyzed by one-way ANOVA followed by LSD post-hoc test. * indicate P < 0.05; ** indicate P < 0.01.
Expression of Adiponectin and Adiponectin Receptors
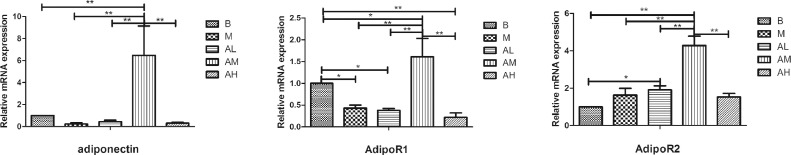
As shown in Figure 9, the mRNA expression level of adiponectin in liver tissue of geese in high-fat diet group was lower than that in the control group (P > 0.05). All of the 3 AdipoRon groups were higher than the high-fat diet group, and the medium-dose AdipoRon group was significantly higher than the high-fat diet group (P < 0.01). The mRNA expression level of AdipoR1 in liver tissue of geese in high-fat diet group was significantly lower than that in the control group (P < 0.05). There was no significant difference between low-dose AdipoRon group and high-fat diet group (P > 0.05). The medium-dose AdipoRon group was significantly higher than the high-fat diet group (P < 0.01) and the control group (P < 0.05), and the high-dose AdipoRon group was lower than the high-fat diet group (P > 0.05), and significantly lower than the control group (P < 0.01). The mRNA expression level of AdipoR2 in goose liver of high-fat diet group was higher than that of the control group (P > 0.05). Low-dose AdipoRon group was higher than high-fat diet group (P > 0.05), medium-dose AdipoRon group was significantly higher than other groups (P < 0.01), and there was no significant difference between high-dose AdipoRon group and high-fat diet group (P > 0.05). These results indicate that AdipoRon can increase the mRNA expression of adiponectin and its receptor gene in goose liver.
Figure 9.
Effect of AdipoRon on the mRNA expression of adiponectin and adiponectin receptor genes in geese livers. Total RNA was extracted using Trizol reagent from liver tissues of goose, and complementary DNA was synthesized using cDNA synthesis kit from the total RNA. Quantitative real-time PCR was done with cDNA, primers, and SYBR PCR Premix solution. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group, and high-dose AdipoRon group are denoted by the B, M, AL, AM, and AH, respectively. Y-axis represents the fold change of mRNA expression level which presented in AU (arbitrary units). Values of bars are expressed as mean ± SE (n = 9). The data were analyzed by one-way ANOVA followed by LSD post-hoc test. * indicate P < 0.05; ** indicate P < 0.01.
Expression of Key Genes and Transcription Factors Related to Adiponectin Signal Pathway
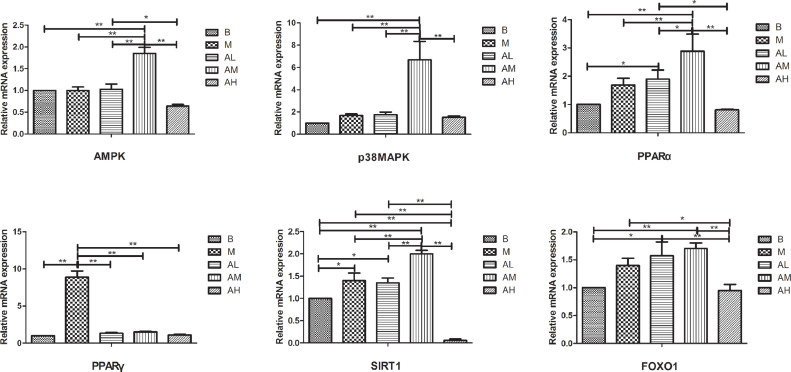
As shown in Figure 10, the mRNA expression level of AMPK in the high-fat diet group, the control group and low-dose AdipoRon group was no significant difference (P > 0.05). Medium-dose AdipoRon group was significantly higher than high-fat diet group and control group (P < 0.01), and high-dose AdipoRon group was lower than high-fat diet group and control group (P > 0.05). Compared with the 3 AdipoRon groups, the medium-dose AdipoRon group was significantly higher than the low-dose and high-dose AdipoRon groups (P < 0.01), and the high-dose group was significantly lower than the low-dose group (P < 0.05).
Figure 10.
Effect of AdipoRon the expression of adiponectin related signaling pathway molecules and transcription factors in liver of geese fed a high-fat diet. Total RNA was extracted using Trizol reagent from liver tissues of goose, and complementary DNA was synthesized using cDNA synthesis kit from the total RNA. Quantitative real-time PCR was done with cDNA, primers, and SYBR PCR Premix solution. The blank control group, high-fat diet group, low-dose AdipoRon group, medium-dose AdipoRon group, and high-dose AdipoRon group are denoted by the B, M, AL, AM and AH, respectively. Y-axis represents the fold change of mRNA expression level which presented in AU (arbitrary units). Values of bars are expressed as mean ± SE (n = 9). The data were analyzed by one-way ANOVA followed by LSD post-hoc test. * indicate P < 0.05; ** indicate P < 0.01.
The mRNA expression level of p38 MAPK in liver tissue of geese in high-fat diet group was higher than that in control group, but the difference was not significant (P > 0.05). Low-dose AdipoRon group was higher than high-fat diet group (P > 0.05), medium-dose AdipoRon group was significantly higher than other groups (P < 0.01), and there was no significant difference between high-dose AdipoRon group and high-fat diet group (P > 0.05).
The mRNA expression level of PPARα in liver tissues of geese in high-fat diet group was higher than that in control group, the difference was not significant (P > 0.05). Low-dose AdipoRon group was higher than high-fat diet group (P > 0.05), medium-dose AdipoRon group was significantly higher than high-fat diet group and control group (P < 0.01), and high-dose AdipoRon group was lower than high-fat diet group and control group, the difference was not statistically significant (P > 0.05).
The mRNA expression level of PPARγ in liver tissues of geese in high-fat diet group was significantly higher than that in control group (P < 0.01). All the three AdipoRon groups were significantly lower than those in high-fat diet group (P < 0.01).
The mRNA expression level of SIRT1 in liver tissues of geese in high-fat diet group was higher than that in the control group (P < 0.05). There was no significant difference between low-dose AdipoRon group and high-fat diet group (P > 0.05), but it was significantly higher than that in the control group (P < 0.05). The medium-dose AdipoRon group was significantly higher than other groups (P < 0.01), and the high-dose AdipoRon group was significantly lower than the high-fat diet group and the control group (P < 0.01).
The mRNA expression level of FoxO1 in liver tissue of geese in high-fat diet group was higher than that in control group, but the difference was not statistically significant (P > 0.05). Low-dose and medium-dose AdipoRon group were higher than high-fat diet group, but the difference was not statistically significant (P > 0.05), whereas significantly higher than control group (P < 0.05, P < 0.01). High-dose AdipoRon group was significantly lower than high-fat diet group (P < 0.05). When comparing the 3 AdipoRon groups, the high-dose AdipoRon group was significantly lower than the other 2 groups (P < 0.01).
These results indicate that AdipoRon can alter the expression of key genes and transcription factors related to adiponectin signal pathway.
DISCUSSION
Effects of AdipoRon on Body Weight, Liver Index, and Blood Glucose Levels of Goose Fed a High-Fat Diet
Adiponectin has been reported to inhibit food intake, stimulate energy consumption and lead to weight loss. Intraventricula injection of adiponectin could increase energy expenditure and reduce body weight by upregulating hypothalamic corticotrophin-releasing hormone (CRH) (Kusminski et al., 2007). Long-term administration of adiponectin protein could reduce the body weight of mice fed with high-fat diet (Fruebis et al., 2001). Similar with these previous reports, in our current experiment, AdipoRon was found to reduce goose body weight, liver weight, and liver index.
AMPK is an important serine/threonine protein kinase. AMPK activation can promote glucose transport, accelerate glycolysis and inhibit triglyceride synthesis (Kahn et al., 2005; Zhang et al., 2015). Adiponectin could activate AMPK, and activated AMPK reduced endogenous glucose production and accelerated glycolysis in liver of mice by inhibiting the transcription of phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase; Combs et al., 2001). Adiponectin receptor agonist AdipoRon has also been observed to have the same effect. Report indicated that AdipoRon could increase insulin sensitivity and improve glucose tolerance in mice (Okada-Iwabu et al., 2015). Oral administration of AdipoRon to mice fed a high-fat diet could significantly activate the AdipoR1/AMPK signal pathway, induce AMPK phosphorylation in liver tissue, decrease the expression of gluconeogenesis related genes in liver tissue, inhibit the synthesis of endogenous glucose, and reduce blood glucose level (Okada-Iwabu et al., 2013). In our animal experiment, feeding high-fat diet increased the blood glucose level of goose, while treatment with appropriate dose of AdipoRon upregulated the mRNA expression of AMPK in liver tissue and decreased the blood glucose level.
Effects of AdipoRon on Lipid Metabolism of Goose Fed a High-Fat Diet
Liver is the main organ of lipid metabolism in poultry. Lipid metabolism is a complex process, including fatty acid and TG synthesis, fatty acid oxidation, VLDL-TG assembly, and secretion. Changing the state and rate of TG synthesis, transport and fatty acid oxidation will lead to abnormal lipid accumulation in liver. Feeding high-fat diet can cause excessive fatty acid production in poultry, which exceeds the metabolic needs of the body, resulting in increased TG deposition in the liver and increased FFA level in the blood circulation, leading to oxidative stress in hepatocytes and fatty liver disease.
The production process of newborn fat in poultry depends on the activities of ACC, FAS, SCD-1, and other enzymes. ACC catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, providing acetyl for fatty acid synthesis. FAS can directly catalyze acetyl-CoA and malonyl-CoA to generate long-chain palmitoyl-CoA (Mourot et al., 2000). SCD-1 is a rate-limiting enzyme that catalyzes the conversion of saturated long-chain fatty acids to monounsaturated fatty acids, thus plays a key role in the synthesis of TG (Hodson and Fielding, 2013). De novo fatty acid synthesis in the liver of SCD-1 knockout mice was inhibited, triglyceride content was significantly reduced, fat oxidation was enhanced, and liver steatosis did not develop when fed with high-fat diet (Dobrzyn and Ntambi, 2005). Especially, the rate of de novo fatty acid synthesis is mainly regulated at the transcriptional level. Sterol regulatory element binding protein 1 (SREBP1) is the determinant of transcription factors related to fat synthesis and can regulate the expression of ACC, FAS, and SCD-1 genes (Edwards et al., 2000). Overexpression of SREBP1 will cause disorder of lipid metabolism, induce the increase of lipid production, and cause lipid accumulation in liver and other tissues (Sekiya et al., 2007). AMPK can regulate the expression of adipogenesis-related genes, such as FAS, ACC, and SCD-1, through the transcription factor SREBP1, thereby regulating the synthesis of fatty acids in the liver (Stoeckman and Towle, 2002). As a member of the protein serine/threonine kinase family, p38MAPK can inhibit the transcription of SREBP1, downregulate the expression of its downstream key genes for fat synthesis, thus inhibiting fat synthesis in the liver (Xiong et al., 2007). Adiponectin was demonstrated to activate AMPKα and p38MAPK through its receptor, inhibit the expression of SREBP1 gene and regulate the process of fat synthesis (Awazawa et al., 2009). In vitro experiments showed that AdipoRon could inhibit the expression of fatty acid synthesis genes FAS and ACC in myostatin induced mouse liver cell line FL83B (Liu et al., 2019). In addition, the nuclear receptor superfamily ligand-activated transcription factor PPARγ, can directly participate in lipid metabolism and affect lipid synthesis and metabolism in the liver. Specific overexpression of PPARγ, resulted in high expression of genes related to lipid synthesis in liver hepatocytes, significantly increased TG content in liver, and steatosis in hepatocytes (Yu et al., 2004). Knocking out the PPARγ gene in obese mice, the lipid content in hepatocytes was significantly decreased and the degree of hepatic steatosis was alleviated (Morán-Salvador et al., 2011). JT003, an adiponectin receptor agonist, it could reduce PPARγ and inhibit lipid production by activating PI3K/Akt signaling pathway (Xu et al., 2020). The biochemical test results of our study showed that feeding high-fat diets increased the content of TG, TC, FFA, LDL-C in blood and TG and TC in liver of geese. The oil red O staining results also showed that feeding high-fat diet increased lipid deposition in goose liver tissue, while the appropriate concentration of AdipoRon reversed the increase of lipid content in the body. Further investigations showed that AdipoRon at an appropriate concentration could upregulate the mRNA expression of AMPK and p38MAPK, the key molecules of adiponectin signaling pathway, and downregulate the mRNA expression of genes related to fatty acid and TG synthesis such as SREBP1, FAS, ACC, SCD-1, and PPARγ in liver tissue, thereby reducing fat synthesis and deposition in liver of goslings fed high-fat diet.
Fatty acid β-oxidation in poultry is mainly carried out in organelles such as mitochondria, peroxisomes, and microsomes. CPT-1, located in the outer mitochondrial membrane, is the rate-limiting enzyme that regulates the oxidation of long-chain fatty acids (Schreurs et al., 2010). ACOX is a rate-limiting enzyme that catalyzes the β-oxidation of very long-chain fatty acids in peroxisome. The nuclear receptor transcription factor PPARα can promote the oxidation of fatty acids by upregulating the transcription and expression of these key fatty acid β-oxidation genes (ACOX and CPT-1) in mitochondria, peroxisomes and microsomes. Adiponectin has been found to promote fatty acid oxidation by activating AMPKα, p38MAPK, and PPARα signaling pathways and increasing the transcription of its downstream target genes including CPT-1 and ACOX (Yoon et al., 2006). Treating high-fat diet fed mice with AdipoRon can activate the AdipoR2/PPARα signaling pathway in liver tissues, increase the expression of fatty acid oxidation-related genes, such as ACOX1, promote fatty acid oxidation, and reduce plasma TG and FFA concentrations (Okada-Iwabu et al., 2013). Interestingly, our study showed that the appropriate dose of AdipoRon could increase the mRNA expression of AMPK, p38MAPK, PPARα, CPT-1, and ACOX genes related to fatty acid oxidation in goose liver tissue.
Synthesized TG in poultry liver is combined with ApoB, secreted from liver cells as the form of VLDL, and transported to extrahepatic tissues through the blood circulation for utilization. ApoB is the main structural protein for the assembly and transport of liver VLDL. Its expression level is correlated with the level of liver VLDL assembly and transport, and is the rate-limiting enzyme of hepatic VLDL generation (Nguyen et al., 2008). MTTP is an endoplasmic reticulum lumen protein involved in TG transport and VLDL assembly (Tietge et al., 1999). MTTP can catalyze the transfer of lipids into the newly generated apolipoprotein ApoB to form VLDL precursor particles. Inhibition of MTTP activity can disrupt the assembly and secretion of VLDL-TG, which in turn leads to the accumulation of TG in the liver (Hussain et al., 2003). Activated p38MAPK is able to affect the transport of TG by altering the gene expression level of MTTP (Au et al., 2003). Notably, our qRT-PCR results also showed that AdipoRon at an appropriate dose could increase the mRNA expression levels of p38MAPK, as well as the lipid transport-related genes ApoB and MTTP in goose liver tissue. It suggests that AdipoRon may affect the assembly and secretion of VLDL-TG.
Taken together, our study suggest that AdipoRon can inhibit the synthesis of fatty acids and TG in the liver, promote the β-oxidation of fatty acids, and enhance the transport of TG from the liver to peripheral tissues, thereby alleviating liver lipid deposition induced by feeding high-fat diet.
Effects of AdipoRon on Antioxidant Stress in Liver of Geese Fed a High-Fat Diet
Oxidative stress caused by lipid peroxidation is a major factor responsible for liver injury. The occurrence of oxidative stress is usually caused by the imbalance between ROS production and antioxidant capacity. When TG and FFA produced by feeding high-fat diets accumulate in hepatocytes, the β-oxidation of fatty acids in mitochondria increases, a large amount of acetyl-CoA is generated, the TCA cycle (tricarboxylic acid cycle) is strengthened, and the production of mitochondrial ROS increases, causing oxidative stress (Chavez-Tapia et al., 2012). Oxidative stress induced by excessive ROS can be detoxified by a variety of antioxidant enzymes. SOD is an important antioxidant enzyme and responsible for catalyzing the disproportionation of superoxide into hydrogen peroxide and dioxygen. GSH-Px mainly removes lipid peroxides and protects the structure and function of cell membranes. MDA is the lipid peroxide generated during peroxidation, which is the main index for detecting the degree of lipid peroxidation and can indirectly reflect the degree of cellular oxidative damage (Fridovich, 1986). Adiponectin has a negative regulatory effect on the signal pathway that generates ROS, and can counteract the excessive accumulation of intracellular oxidation products, thereby preventing the generation of oxidative stress. In liver cells, adiponectin activates the PPARα signaling pathway by binding to its receptor to reduce AOX-1 activity and reduce the level of intracellular ROS (Neumeier et al., 2006). Adiponectin can also participate in inducing the production of SOD 1 and catalase through AdipoR2, and increase the activity of ROS detoxification enzymes (Yamauchi et al., 2007).
Study has shown that AdipoRon also has antioxidative stress properties. In the study of ethanol-induced gastric ulcer model in mice, the application of AdipoRon could increase the activities of GSH and SOD through the AMPK pathway, enhance the ability of cells to resist oxidative stress, and reduce gastric injury (Zatorski et al., 2021). In mice with nephropathy induced by iopromide injection, AdipoRon could activate the AMPK pathway, reverse the decrease in SOD and GSH-Px content and the increase in MDA content in the kidney tissue caused by the contrast agent, and then play its role in regulating oxidative stress (Gu et al., 2020). Our current study showed that feeding high-fat diets could increase the content of MDA and decrease the activity of SOD in liver tissues, whereas, the appropriate dose of AdipoRon could ameliorate these changes, indicating that AdipoRon could improve the antioxidant capacity of the liver and alleviate liver injury caused by oxidative stress.
Effects of AdipoRon on Liver Function and Inflammatory Response of Geese Fed a High-Fat Diet
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are the biochemical markers reflecting liver function impairment. Under normal physiological conditions, ALT exists in the cytoplasm, and AST exists in the cytoplasm and mitochondria of hepatocyte. When steatosis occurs in the liver, TG accumulates excessively in hepatocytes, forcing hepatocyte enlargement and damage, and a large amount of transaminase spills. LDH is a glycolytic enzyme present in the cytoplasm, and its activity is significantly increased when liver metabolism is severely abnormal (Kunde et al., 2005). In addition, when liver function is impaired and lipid metabolism is abnormal, excessive accumulation of TG in the liver will be toxic to liver cells, and a large number of inflammatory factors such as TNF-α and IL-1β will be released, thereby inducing inflammation. As an important proinflammatory factor, TNF-α can damage cellular mitochondrial respiratory function, lead to lipid peroxidation and cause liver inflammation, and directly mediate hepatocyte necrosis and apoptosis (Xu et al., 2019). In vitro and in vivo experiments have found that adiponectin can improve liver steatosis and alleviate inflammation by inhibiting the expression of proinflammatory factors such as TNF-α and IL-1β in liver tissue, and inducing the production of anti-inflammatory factors, such as IL-10 (Hui et al., 2004; Kim et al., 2017).
Several works have also investigated whether adiponectin receptor agonists have anti-inflammatory effects. In mice with acute hepatitis induced by lipopolysaccharide/D-galactose and CCl4, AdipoRon can reduce the elevation of serum ALT and AST, inhibit the production of TNF-α and IL-1β, reduce the inflammatory pathological changes in liver tissue, improve liver injury, and increase the survival rate of mice (Xiao and Zhang, 2019; Sha et al., 2020). In mice fed a high-fat diet, oral administration of AdipoRon also reduced the expression of proinflammatory cytokines such as TNF-α and IL-6 in liver tissues, thus exerting anti-inflammatory effects (Okada-Iwabu et al., 2013). Our study showed that feeding high-fat diet resulted in pathological changes such as hepatocyte ballooning degeneration, cell swelling, connective tissue proliferation around the portal area, and lymphocyte infiltration in goose liver tissue, accompanied by increased serum ALT, AST, and LDH activities, as well as increased mRNA expression levels of proinflammatory factors TNF-α and IL-1β in liver tissue. AdipoRon at appropriate dose could alleviate inflammatory pathological changes in liver tissue, reduce serum ALT, AST, and LDH activities, and downregulate the expression of proinflammatory factors TNF-α and IL-1β. These results suggest that AdipoRon can ameliorate the liver inflammation caused by high-fat diet in geese.
Effects of AdipoRon on the Expression of Apoptosis and Autophagy-Related Genes in Liver Tissue of Geese Fed a High-fat Diet
The increase of saturated fatty acids in the liver can activate hepatocyte apoptosis and promote the development of fatty liver injury. The process of cell apoptosis can be precisely regulated by genes such as Bcl-2, Bax, and caspase. The proapoptotic gene Bax promote the apoptotic response by increasing the permeability of mitochondria and releasing apoptosis-related enzymes, while Bcl-2, as a homologous gene of Bax, antagonizes Bax and has the function of antiapoptosis (Finucane et al., 1999). Hepatocyte apoptosis is also regulated by both the exogenous pathway of caspase and the endogenous pathway regulated by Bcl-2 family genes, both of which ultimately activate caspase-3, the final executor of apoptosis (Kanda et al., 2018). Study demonstrated that adiponectin could inhibit cell apoptosis through a variety of signaling pathways (Sun and Chen, 2010). Adiponectin can ameliorate D-galactosamine/lipopolysaccharide (LPS) (GalN/LPS) induced liver injury in KK-Ay obese mice by reducing hepatocyte apoptosis and necrosis (Masaki et al., 2004). In HepG2 cells, a hepatoma cell line, adiponectin can reduce free fatty acid-induced CD95/Fas expression and CD95/Fas-mediated apoptosis (Wedemeyer et al., 2009). Consistent with the antiapoptotic activity of adiponectin, in lipopolysaccharide/D-galactose-induced acute hepatitis mice, the adiponectin receptor agonist AdipoRon was observed to inhibit inhibit the activation of caspase in liver tissue, inhibit caspase-3 cleavage, restore the Bcl-2/Bax ratio, reduce the number of TUNEL-positive cells, and exert its antiapoptosis properties, thereby protecting the liver (Xiao and Zhang, 2019).
Autophagy is activated as a cellular adaptive mechanism that prevents cellular damage by liver injury (Yan et al., 2017). Autophagy is a complex biological process involving multiple steps, and each step is coordinated by specific genes, such as Beclin-1 and LC3. Among them, Beclin-1 plays a key role in initiating the autophagic process, and LC3 is an important component of autophagosomes. Several studies have demonstrated that adiponectin is associated with autophagy in many types of cells (Habeeb et al., 2011; Gamberi et al., 2016). Adiponectin can significantly restore ethanol-induced inhibition of autophagy-related genes (Beclin-1 and LC3B), as well as autophagosome formation, and prevented ethanol-induced apoptosis in primary rat hepatocytes and human hepatoma cell line (HepG2) by activating the AMPK/FOXO3A pathway (Nepal and Park, 2013). Activation of autophagy by administration of adiponectin can alleviate or ameliorate liver diseases such as alcoholic and nonalcoholic fatty liver disease, liver fibrosis, and viral hepatitis, suggesting that activation of the autophagic process may be a common mechanism by which adiponectin exerts protective effects against these various forms of acute and chronic liver injury (Gamberi et al., 2018).
Our present study revealed that the appropriate dose of AdipoRon could upregulate the mRNA expression of antiapoptotic gene Bcl-2, autophagy-related genes Beclin-1 and LC3, and downregulate the mRNA expression of proapoptotic genes Bax and caspase-3 in goose liver tissue. Thus, it is speculated that AdipoRon can regulate the apoptosis and autophagy process of goose hepatocytes by altering the expression of apoptosis and autophagy genes.
Effects of AdipoRon on the Expression of Adiponectin and Its Receptors, Related Signaling Pathway Molecules and Transcription Factors in Liver of Geese Fed a High-Fat Diet
Plasma adiponectin concentrations are associated with a variety of factors that regulate adiponectin gene expression and secretion. Studies have shown that adiponectin gene expression and blood adiponectin concentration are negatively correlated with obesity, and that adiponectin mRNA expression and plasma adiponectin concentration are reduced in obese mice and humans compared with normal controls (Swarbrick and Havel, 2008). High-fat diet and the resulting obesity can not only reduce plasma adiponectin concentrations but also downregulate adiponectin receptor expression levels (Arita et al., 1999; Tsuchida et al., 2004). In addition, when inflammation occurs in the liver, macrophages activate proinflammatory factors (e.g., TNF-α, IL-1β, IL-6) and nitric oxide (NO), which in turn inhibit the transcription of adiponectin mRNA and the expression of adiponectin protein (Buechler et al., 2011). The biological function of adiponectin is initiated by binding to its receptors AdipoR1 and AdipoR2. Both AdipoR1 and AdipoR2 are expressed in the liver and have physiological functions in regulating glucose and lipid metabolism, inflammation, and oxidative stress in vivo. The small molecule compound adiponectin receptor agonist AdipoRon is able to specifically bind and activate AdipoR1 and AdipoR2, exerting the same effects as adiponectin (Okada-Iwabu et al., 2013). Our results showed that the mRNA expression of adiponectin and its receptors in liver of geese fed a high-fat diet was significantly decreased. Whereas, appropriate doses of AdipoRon increased the mRNA expression levels of adiponectin and its receptors AdipoR1 and AdipoR2, especially the expression of AdipoR2. Thus, it is speculated that AdipoRon can activate the signaling pathway of goose liver adiponectin and its receptor, thus exerting its multifaceted physiological functions.
Silent information regulator 1 (SIRT1) is a NAD+ dependent protein deacetylase that deacetylates and modifies a variety of target proteins, thereby participating in the regulation of various biological functions including glucose and lipid metabolism. SIRT1 plays a beneficial role in regulating hepatic lipogenesis and fatty acid β-oxidation, alleviating hepatic oxidative stress, and preventing high-fat diet induced fatty liver disease by deacetylating some transcriptional regulators (Ding et al., 2017). SIRT1 can reduce fatty acid production by activating AMPK, and inhibiting the activities of fatty acid synthesis-related enzymes ACCα and FAS (Lan et al., 2008). Over-expression of SIRT1 reduces acetylation of SREBP-1 and downregulates the transcriptional activity of SREBP-1, which in turn reduces the expression levels of SREBP-1 mediated related lipogenic genes, such as FAS, SCD1 and ACC, and protects the liver against high-fat or high-sugar diet induced hepatic steatosis (Ponugoti et al., 2010). Over-expression of SIRT1 also enhances the transcriptional activity of PPARα via deacetylation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), promote the transcription of PPARα signaling pathway-related genes ACOX and CPT-1, and stimulate fatty acid β-oxidation in the liver. In contrast, downregulation or deletion of SIRT1 leads to impaired PPARα signaling, reduced fatty acid oxidation, and aggravated hepatic steatosis and inflammation (Colak et al., 2014). In SIRT1 transgenic mice, SIRT1 over-expression increased the expression of the antioxidant protein MnSOD and protected mice from high-fat-induced hepatic oxidative stress and fatty liver development (Pfluger et al., 2008). SIRT1 gene deletion significantly increased ROS levels in liver tissue, leading to severe hepatic oxidative stress and fatty liver disease (Yin et al., 2014).
Forkhead transcription factor O (FOXO) family genes are also able to participate in regulating glucose and lipid metabolism and oxidative stress. During hepatocyte lipid metabolism, FOXO1 can inhibit lipogenesis by inhibiting the expression of SREBP-1 and its downstream genes involved in fatty acid synthesis (Xiong et al., 2013). FOXO1 can promote the synthesis and secretion of very low-density lipoprotein (VLDL) in hepatocytes and increase the degradation of TG through upregulation of MTTP and ApoB expression (Zhang et al., 2006). In transgenic mice, FOXO1 activity is increased due to specific transgenesis, thereby upregulating MTTP expression and thus promoting VLDL production (Kamagate et al., 2008). FOXO1-knockout mice have elevated TG and TC in the liver and develop significant hepatic steatosis and liver injury (Pan et al., 2017). Noteworthily, FOXO1 is a downstream molecule of SIRT1. SIRT1 can regulate FOXO1 transcriptional activity by deacetylating FOXO1 (Hori et al., 2013). In the study of diet-induced nonalcoholic fatty liver disease, activation of SIRT1 deacetylates and activates the transcriptional activity of FOXO1, stimulating high expression of FOXO1 downstream antioxidant genes, and thus resisting oxidative stress in the liver (Feige et al., 2008). Adiponectin can activate the SIRT1 gene by elevating the NAD+/NADH ratio through its receptor (Iwabu et al., 2010). The adiponectin/adiponectin receptor signaling pathway was also found to activate the AMPK/SIRT1 pathway, positively regulate Catalase and SOD genes, and thereby reduces tissue oxidative stress responses (Iwabu et al., 2015). Our current study showed that appropriate doses of AdipoRon increased the mRNA expression levels of FOXO1 and SIRT1in geese liver tissue. Thus, it is suggested that adiponectin receptor agonist AdipoRon might be involved in the regulation of liver-related physiological activities by activating the SIRT1/FOXO1 pathway.
In summary, AdipoRon, an adiponectin receptor agonist, may alter the expression of lipid metabolism-related genes, inflammatory factors, apoptosis and autophagy genes, and adiponectin and its receptor genes in liver tissues through signaling pathways such as AMPK and p38 MAPK, as well as the involvement of transcription factors such as PPARα, PPARγ, SIRT1, and FOXO1, reduce the lipid content in blood and liver tissues of geese fed a high-fat diet, improve liver antioxidant capacity, regulate apoptosis and autophagy of hepatocytes, and reduce liver inflammatory injury. This study suggests that AdipoRon can protect geese from fatty liver injury caused by high-fat diet to some extent.
ACKNOWLEDGMENTS
This project was supported by the Scientific Research Funding Project of the Education Department of Liaoning Province (Grant No. LJKZ0668) and the National Natural Science Foundation of China (Grant No. 32072810).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101708.
Appendix. Supplementary materials
Table S1 Composition and nutrient levels of basal diet for goose (air-dry basis).
Table S2 Primers used for quantitative real-time PCR analysis.
References
- Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., Kuriyama H., Nishida M., Yamashita S., Okubo K., Matsubara K., Muraguchi M., Ohmoto Y., Funahashi T., Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Au W.-S., Kung H.-F., Lin M.C. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes. 2003;52:1073–1080. doi: 10.2337/diabetes.52.5.1073. [DOI] [PubMed] [Google Scholar]
- Awazawa M., Ueki K., Inabe K., Yamauchi T., Kaneko K., Okazaki Y., Bardeesy N., Ohnishi S., Nagai R., Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem. Biophys. Res. Commun. 2009;382:51–56. doi: 10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- Buechler C., Wanninger J., Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World. J. Gastroenterol. 2011;17:2801–2811. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Li J., Luo L., Li X., Liu M., Gao M., Yin Y., Luan X. Molecular cloning and expression analysis of adiponectin and its receptors (AdipoR1 and AdipoR2) in the hypothalamus of the Huoyan goose during different stages of the egg-laying cycle. Reprod. Biol. Endocrinol. 2015;13:87. doi: 10.1186/s12958-015-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Tapia N.C., Rosso N., Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:20. doi: 10.1186/1471-230X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak Y., Yesil A., Mutlu H.H., Caklili O.T., Ulasoglu C., Senates E., Takir M., Kostek O., Yilmaz Y., Yilmaz Enc F., Tasan G., Tuncer I. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J. Gastrointest. Liver. Dis. 2014;23:311–319. doi: 10.15403/jgld.2014.1121.233.yck. [DOI] [PubMed] [Google Scholar]
- Combs T.P., Berg A.H., Obici S., Scherer P.E., Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R.B., Bao J., Deng C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017;13:852–867. doi: 10.7150/ijbs.19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyn A., Ntambi J.M. The role of stearoyl-CoA desaturase in the control of metabolism. Prostaglandins. Leukot. Essent. Fatty. Acids. 2005;73:35–41. doi: 10.1016/j.plefa.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Edwards P.A., Tabor D., Kast H.R., Venkateswaran A. Regulation of gene expression by SREBP and SCAP. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids. 2000;1529:103–113. doi: 10.1016/s1388-1981(00)00140-2. [DOI] [PubMed] [Google Scholar]
- Fang H., Judd R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018;8:1031–1063. doi: 10.1002/cphy.c170046. [DOI] [PubMed] [Google Scholar]
- Feige J.N., Lagouge M., Canto C., Strehle A., Houten S.M., Milne J.C., Lambert P.D., Mataki C., Elliott P.J., Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell. Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Finucane D.M., Bossy-Wetzel E., Waterhouse N.J., Cotter T.G., Green D.R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fruebis J., Tsao T.S., Javorschi S., Ebbets-Reed D., Erickson M.R., Yen F.T., Bihain B.E., Lodish H.F. Vol. 98. 2001. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice; pp. 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberi T., Modesti A., Magherini F., D'Souza D.M., Hawke T., Fiaschi T. Activation of autophagy by globular adiponectin is required for muscle differentiation. Biochim. Biophys. Acta. 2016;1863:694–702. doi: 10.1016/j.bbamcr.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Gamberi T., Magherini F., Modesti A., Fiaschi T. Adiponectin signaling pathways in liver diseases. Biomedicines. 2018;6:1–16. doi: 10.3390/biomedicines6020052. , 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D., Shi Y., Gong Z., Xia T., Ren H., He D., Yang J., Han Y., Zeng C. AdipoRon, an adiponectin receptor agonist, protects contrast-induced nephropathy by suppressing oxidative stress and inflammation via activation of the AMPK pathway. Clin. Exp. Nephrol. 2020;24:989–998. doi: 10.1007/s10157-020-01944-2. [DOI] [PubMed] [Google Scholar]
- Habeeb B.S., Kitayama J., Nagawa H. Adiponectin supports cell survival in glucose deprivation through enhancement of autophagic response in colorectal cancer cells. Cancer. Sci. 2011;102:999–1006. doi: 10.1111/j.1349-7006.2011.01902.x. [DOI] [PubMed] [Google Scholar]
- Hodson L., Fielding B.A. Stearoyl-CoA desaturase: rogue or innocent bystander? Prog. Lipid. Res. 2013;52:15–42. doi: 10.1016/j.plipres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Hori Y.S., Kuno A., Hosoda R., Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One. 2013;8:e73875. doi: 10.1371/journal.pone.0073875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J.M., Hodge A., Farrell G.C., Kench J.G., Kriketos A., George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- Hussain M.M., Shi J., Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid. Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- Iwabu M., Yamauchi T., Okada-Iwabu M., Sato K., Nakagawa T., Funata M., Yamaguchi M., Namiki S., Nakayama R., Tabata M., Ogata H., Kubota N., Takamoto I., Hayashi Y.K., Yamauchi N., Waki H., Fukayama M., Nishino I., Tokuyama K., Ueki K., Oike Y., Ishii S., Hirose K., Shimizu T., Touhara K., Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Iwabu M., Okada-Iwabu M., Yamauchi T., Kadowaki T. Adiponectin/adiponectin receptor in disease and aging. NPJ. Aging. Mech. Dis. 2015;1:15013. doi: 10.1038/npjamd.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell. Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kamagate A., Qu S., Perdomo G., Su D., Kim D.H., Slusher S., Meseck M., Dong H.H. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 2008;118:2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Matsuoka S., Yamazaki M., Shibata T., Nirei K., Takahashi H., Kaneko T., Fujisawa M., Higuchi T., Nakamura H., Matsumoto N., Yamagami H., Ogawa M., Imazu H., Kuroda K., Moriyama M. Apoptosis and non-alcoholic fatty liver diseases. World J. Gastroenterol. 2018;24:2661–2672. doi: 10.3748/wjg.v24.i25.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Kim E.H., Pun N.T., Chang J.H., Kim J.A., Jeong J.H., Choi D.Y., Kim S.H., Park P.H. Globular adiponectin inhibits lipopolysaccharide-primed inflammasomes activation in macrophages via autophagy induction: the critical role of AMPK signaling. Int. J. Mol. Sci. 2017;18:1–21. doi: 10.3390/ijms18061275. , 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunde S.S., Lazenby A.J., Clements R.H., Abrams G.A. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–656. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- Kusminski C.M., McTernan P.G., Schraw T., Kos K., O'Hare J.P., Ahima R., Kumar S., Scherer P.E. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- Lan F., Cacicedo J.M., Ruderman N., Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.H., Pan J.P., Bauman W.A., Cardozo C.P. AdipoRon prevents myostatin-induced upregulation of fatty acid synthesis and downregulation of insulin activity in a mouse hepatocyte line. Physiol. Rep. 2019;7:e14152. doi: 10.14814/phy2.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddineni S. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146:4250–4256. doi: 10.1210/en.2005-0254. [DOI] [PubMed] [Google Scholar]
- Masaki T., Chiba S., Tatsukawa H., Yasuda T., Noguchi H., Seike M., Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- Morán-Salvador E., López-Parra M., García-Alonso V., Titos E., Martínez-Clemente M., González-Périz A., López-Vicario C., Barak Y., Arroyo V., Clària J. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB. J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- Mourot J., Guy G., Lagarrigue S., Peiniau P., Hermier D. Role of hepatic lipogenesis in the susceptibility to fatty liver in the goose (Anser anser) Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2000;126:81–87. doi: 10.1016/s0305-0491(00)00171-1. [DOI] [PubMed] [Google Scholar]
- Nepal S., Park P.H. Activation of autophagy by globular adiponectin attenuates ethanol-induced apoptosis in HepG2 cells: involvement of AMPK/FoxO3A axis. Biochim. Biophys. Acta. 2013;1833:2111–2125. doi: 10.1016/j.bbamcr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Neumeier M., Weigert J., Schäffler A., Weiss T.S., Schmidl C., Büttner R., Bollheimer C., Aslanidis C., Schölmerich J., Buechler C. Aldehyde oxidase 1 is highly abundant in hepatic steatosis and is downregulated by adiponectin and fenofibric acid in hepatocytes in vitro. Biochem. Biophys. Res. Commun. 2006;350:731–735. doi: 10.1016/j.bbrc.2006.09.101. [DOI] [PubMed] [Google Scholar]
- Nguyen P., Leray V., Diez M., Serisier S., Bloc'h J.Le, Siliart B., Dumon H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr (Berl). 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- Okada-Iwabu M., Yamauchi T., Iwabu M., Honma T., Hamagami K., Matsuda K., Yamaguchi M., Tanabe H., Kimura-Someya T., Shirouzu M., Ogata H., Tokuyama K., Ueki K., Nagano T., Tanaka A., Yokoyama S., Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- Okada-Iwabu M., Iwabu M., Ueki K., Yamauchi T., Kadowaki T. Perspective of small-molecule adipoR agonist for type 2 diabetes and short life in obesity. Diabetes. Metab. J. 2015;39:363–372. doi: 10.4093/dmj.2015.39.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhang Y., Kim H.G., Liangpunsakul S., Dong X.C. FOXO transcription factors protect against the diet-induced fatty liver disease. Sci. Rep. 2017;7:44597. doi: 10.1038/srep44597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger P.T., Herranz D., Velasco-Miguel S., Serrano M., Tschöp M.H. Vol. 105. 2008. Sirt1 protects against high-fat diet-induced metabolic damage; pp. 9793–9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponugoti B., Kim D.H., Xiao Z., Smith Z., Miao J., Zang M., Wu S.Y., Chiang C.M., Veenstra T.D., Kemper J.K. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs M., Kuipers F., van der Leij F.R. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010;11:380–388. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- Sekiya M., Yahagi N., Matsuzaka T., Takeuchi Y., Nakagawa Y., Takahashi H., Okazaki H., Iizuka Y., Ohashi K., Gotoda T., Ishibashi S., Nagai R., Yamazaki T., Kadowaki T., Yamada N., Osuga J., Shimano H. SREBP-1-independent regulation of lipogenic gene expression in adipocytes. J. Lipid. Res. 2007;48:1581–1591. doi: 10.1194/jlr.M700033-JLR200. [DOI] [PubMed] [Google Scholar]
- Sha M., Gao Y., Deng C., Wan Y., Zhuang Y., Hu X., Wang Y. Therapeutic effects of AdipoRon on liver inflammation and fibrosis induced by CCl4 in mice. Int. Immunopharmacol. 2020;79 doi: 10.1016/j.intimp.2019.106157. [DOI] [PubMed] [Google Scholar]
- Stoeckman A.K., Towle H.C. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J. Biol. Chem. 2002;277:27029–27035. doi: 10.1074/jbc.M202638200. [DOI] [PubMed] [Google Scholar]
- Sun Y., Chen X. Effect of adiponectin on apoptosis: proapoptosis or antiapoptosis? Biofactors. 2010;36:179–186. doi: 10.1002/biof.83. [DOI] [PubMed] [Google Scholar]
- Swarbrick M.M., Havel P.J. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab. Syndr. Relat. Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietge U.J., Bakillah A., Maugeais C., Tsukamoto K., Hussain M., Rader D.J. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid. Res. 1999;40:2134–2139. [PubMed] [Google Scholar]
- Tsuchida A., Yamauchi T., Ito Y., Hada Y., Maki T., Takekawa S., Kamon J., Kobayashi M., Suzuki R., Hara K., Kubota N., Terauchi Y., Froguel P., Nakae J., Kasuga M., Accili D., Tobe K., Ueki K., Nagai R., Kadowaki T. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J. Biol. Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- Wedemeyer I., Bechmann L.P., Odenthal M., Jochum C., Marquitan G., Drebber U., Gerken G., Gieseler R.K., Dienes H.P., Canbay A. Adiponectin inhibits steatotic CD95/Fas up-regulation by hepatocytes: therapeutic implications for hepatitis C. J. Hepatol. 2009;50:140–149. doi: 10.1016/j.jhep.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Wei R., Han C., Deng D., Ye F., Gan X., Liu H., Li L., Xu H., Wei S. Research progress into the physiological changes in metabolic pathways in waterfowl with hepatic steatosis. Br. Poult. Sci. 2021;62:118–124. doi: 10.1080/00071668.2020.1812527. [DOI] [PubMed] [Google Scholar]
- Wu X., Qiu W., Hu Z., Lian J., Liu Y., Zhu X., Tu M., Fang F., Yu Y., Valverde P., Tu Q., Chen J. An adiponectin receptor agonist reduces type 2 diabetic periodontitis. J. Dent. Res. 2019;98:313–321. doi: 10.1177/0022034518818449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W.Z., Zhang L. Adiponectin receptor agonist AdipoRon relieves endotoxin-induced acute hepatitis in mice. Chin. Med. J. (Engl). 2019;132:2438–2445. doi: 10.1097/CM9.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Tao R., DePinho R.A., Dong X.C. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PLoS One. 2013;8:e74340. doi: 10.1371/journal.pone.0074340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Collins Q.F., An J., Lupo E., Liu H.-Y., Liu D., Robidoux J., Liu Z., Cao W. p38 Mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J. Biol. Chem. 2007;282:4975–4982. doi: 10.1074/jbc.M606742200. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhao Q., Song N., Yan Z., Lin R., Wu S., Jiang L., Hong S., Xie J., Zhou H., Wang R., Jiang X. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat. Commun. 2020;11:5807. doi: 10.1038/s41467-020-19668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Ge C., Qin Y., Gu T., Lv J., Wang S., Ma Y., Lou D., Li Q., Hu L., Nie X., Wang M., Huang P., Tan J. Activated TNF-α/RIPK3 signaling is involved in prolonged high fat diet-stimulated hepatic inflammation and lipid accumulation: inhibition by dietary fisetin intervention. Food. Funct. 2019;10:1302–1316. doi: 10.1039/c8fo01615a. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Nio Y., Maki T., Kobayashi M., Takazawa T., Iwabu M., Okada-Iwabu M., Kawamoto S., Kubota N., Kubota T., Ito Y., Kamon J., Tsuchida A., Kumagai K., Kozono H., Hada Y., Ogata H., Tokuyama K., Tsunoda M., Ide T., Murakami K., Awazawa M., Takamoto I., Froguel P., Hara K., Tobe K., Nagai R., Ueki K., Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Yan S., Huda N., Khambu B., Yin X.M. Relevance of autophagy to fatty liver diseases and potential therapeutic applications. Amino. Acids. 2017;49:1965–1979. doi: 10.1007/s00726-017-2429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Hu M., Liang X., Ajmo J.M., Li X., Bataller R., Odena G., Stevens S.M., Jr., You M. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology. 2014;146:801–811. doi: 10.1053/j.gastro.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M.J., Lee G.Y., Chung J.J., Ahn Y.H., Hong S.H., Kim J.B. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- Yu S., Viswakarma N., Batra S.K., Rao M.Sambasiva, Reddy J.K. Identification of promethin and PGLP as two novel up-regulated genes in PPARgamma1-induced adipogenic mouse liver. Biochimie. 2004;86:743–761. doi: 10.1016/j.biochi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Zatorski H., Salaga M., Zielińska M., Majchrzak K., Binienda A., Kordek R., Małecka-Panas E., Fichna J. AdipoRon, an orally active, synthetic agonist of AdipoR1 and AdipoR2 receptors has gastroprotective effect in experimentally induced gastric ulcers in mice. Molecules. 2021;26:1–15. doi: 10.3390/molecules26102946. , 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wang C., Zhao H., Zhao C., Chen Y., Wang Y., McClain C., Feng W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J. Nutr. Biochem. 2015;26:337–344. doi: 10.1016/j.jnutbio.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Patil S., Chauhan B., Guo S., Powell D.R., Le J., Klotsas A., Matika R., Xiao X., Franks R., Heidenreich K.A., Sajan M.P., Farese R.V., Stolz D.B., Tso P., Koo S.H., Montminy M., Unterman T.G. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Composition and nutrient levels of basal diet for goose (air-dry basis).
Table S2 Primers used for quantitative real-time PCR analysis.