Abstract
The poultry industry contributes significantly to bridging the nutritional gap in many countries because of its meat and eggs products rich in protein and valuable nutrients at a cost less than other animal meat sources. The natural antibiotics alternatives including probiotics, prebiotics, symbiotics, organic acids, essential oils, enzymes, immunostimulants, and phytogenic (phytobiotic) including herbs, botanicals, essential oils, and oleoresins are the most common feed additives that acquire popularity in poultry industry following the ban of antibiotic growth promoters (AGPs). They are commonly used worldwide because of their unique properties and positive impact on poultry production. They can be easily mixed with other feed ingredients, have no tissue residues, improve feed intake, feed gain, feed conversion rate, improve bird immunity, improve digestion, increase nutrients availability as well as absorbability, have antimicrobial effects, do not affect carcass characters, decrease the usage of antibiotics, acts as antioxidants, anti-inflammatory, compete for stress factors and provide healthy organic products for human consumption. Therefore, the current review focuses on a comprehensive description of different natural antibiotic growth promoters’ alternatives, the mode of their action, and their impacts on poultry production.
Key words: antibiotics, organic additives, poultry, performance, health
INTRODUCTION
Organic farming is one of the fastest expanding agricultural sectors in the United States. Since the 1990s, demand for organically farmed commodities, including animal products, has increased, with American customers driving the market. Organic food sales in the United States are predicted to have surpassed $35 billion in 2014, up from $28.4 billion in 2012. (USDA-ERS, 2009, 2014; Greene, 2013; Salim et al., 2018). Since the demand for organic foods out-grew supplies in the last few years, the U.S. retailers imported several billion dollars’ worths of organic foods to the American market (Crandall et al., 2009). Among the organic animal products, avian meat and eggs are available widely across the country and are well-accepted by the consumers (Agricultural Marketing Resource, 2013; Ponnampalam et al., 2019). The latest NASS NASS 2010 survey indicated that the sale of organic poultry meat and eggs fetched ∼$350 million in 2008 (NASS, NASS 2010). However, even with 9 million certified broilers, 5.5 million certified layer hens and 400,000 certified organic turkeys (NASS, NASS 2010; Agricultural Marketing Resource, 2013), the industry has not met the increasing demand for organic poultry. This underscores the tremendous opportunity for the growth of the organic poultry sector in the coming years, as predicted by the Organic Trade Association (Greene, 2013; Wan et al., 2019). Although there is significant scope for expanding organic poultry, concerns over the safety of organic meat and eggs potentially contaminated with foodborne pathogens could curtail the opportunity (Mogelonsky, 2008; El Jeni et al., 2021).
Raising broiler chickens is a significant part of Egyptian poultry production (El Nagar and Ibrahim, 2021; Salem and Attia, 2021). This situation warrants rapid response to identify alternative and applicable antimicrobial intervention methods of pathogen control in organic poultry (El-Shall et al., 2022). The National Organic Program (NOP) restricts antibiotics, hormones, herbicides, and pesticides from organic agricultural activities to safeguard the environment, people, and animals, thereby potentially improving the sector's sustainability (Fanatico, 2008; Zhang et al., 2021).
In addition, the consumers of organic feed generally tend to perceive the products as safer alternatives due to less/no added preservatives/chemicals and choose them for their families. In line with NOP compliance, naturally derived enzymes, antioxidants, and botanicals can be used in organic poultry farming to combat infections, improve growth and enhance the quality of the products. Vaccines are allowed against many different diseases as Marek's disease virus, Newcastle disease virus, infectious bronchitis virus, Mycoplasma, and coccidia (Setta et al., 2018; Marouf et al., 2020, 2021; El-Naggar et al., 2022).
Probiotics are the beneficial bacteria that can fight pathogens in the gastrointestinal tract of chickens and could also improve general health and disease prevention in birds.
In severe infections where antibiotics are used for treatment, birds cannot be marketed as organic (Fanatico, 2008; Yang et al., 2009). However, the lack of sufficient and reliable research data on the methods as mentioned earlier on improving the microbiological quality of organically raised poultry is a hindering agent. In addition, supporting the inadequacy of the current techniques, several studies have indicated the presence of similar levels of pathogen contamination in organic and commercial poultry products (Sato et al., 2004; Cui et al., 2005; Stone et al., 2013; Noormohamed and Fakhr, 2014). This situation presents a unique challenge for the organic sector to advise its beneficiaries, including producers and processors, on potential antimicrobials that can render their products safe from infectious agents. In addition, as per the NOP standards, poultry must have outdoor access, an environment that may have pathogenic organisms such as Salmonella, Clostridia, and Campylobacter (Abd El-Hack et al., 2021a; Salem et al., 2021). Similar factors that could pose a challenge to safe organic poultry production include the use of slow-growing breeds and minimal slaughtering facilities – both potentially increase the propensity for pathogen contamination in the products (Cui et al., 2005). This review article highlights the different types of organic alternatives for AGPs, their mode of action and their impact on the poultry industry.
Feed Additives
Different feed additives, including biologically synthesized nanoparticles, probiotics, prebiotics, synbiotics, herbal extract, essential oils, organic acids, enzymes, essential amino acids, etc., have been widely used to substitute the use of AGPs (Reda et al., 2020; Sheiha et al., 2020; Abd El-Ghany et al., 2021; El-Saadony et al., 2021a,b,c; Reda et al., 2021a). Common feed additives include antibiotics, probiotics, prebiotics, and enzymes (Wenk, 2000; Abd El-Hack et al., 2021b). Like all other feed additives, antibiotics are expected to increase host health and production performance (Dibner and Richards, 2005). Although the exact physiological mechanisms of feed additives are unclear, the health effects are focused on the gut (Dibner and Richards, 2005; Krysiak et al., 2021). Probiotics are valuable gut microbe species that can colonize the gut, and prebiotics is indigestible oligosaccharides that can be utilized by the useful gut microbes (Wenk, 2000; Kulshreshtha et al., 2014; Murate et al., 2015). Endogenous enzymes such as carbohydrases and proteases are used to increase the digestibility of feed in the gut (Wenk, 2000; Sharma et al., 2005).
Phytogenic feed additives (PFAs) are another promising type of alternative. This phytogenic class contains four main subgroups: herbs, botanicals, essential oils, and oleoresins (Abdelnour et al., 2020a,b; Ogbuewu et al., 2020). These herbal products (cinnamon, ginger, pepper, turmeric, etc.) positively affect bird performance and health due to their antimicrobial, antioxidant, immune-boosting, and gut manipulation properties (Yakhkeshi et al., 2011; Abd El‐Hack et al., 2021c,d).
Antibiotic Additives
Many infectious diseases, viral, bacterial, parasitic, and fungal, threatenpoultry and animal production (Abd El Hamid et al., 2019; Salem et al., 2019; Morsy et al., 2020; Yousry et al., 2020; Attia and Salem, 2022). The use of an antibiotic as a growth promoter began in the 1940s with the discovery of growth responses from the presence of Streptomyces aureofaciens in a monogastric diet (Hashemi and Davoodi, 2010). When used as a feed additive in the diet, subtherapeutic antibiotic levels are present in the range of about 2.5 to 50 ppm (Hashemi and Davoodi, 2010). Due to antibiotic supplementation in the diet, broilers' growth rate and overall productiveness have increased in the last 50 yr (Tajodini et al., 2015).
As some antibiotic growth promoters (AGPs) are not absorbed, the mechanism of antibiotics is most likely in the gut with interactions with the microflora. Thinning of the intestinal wall villi and the total gut wall and reducing opportunistic pathogens by competitive exclusion have been associated with the use of AGPs (Dibner and Richards, 2005). In an antibiotic comparison study, male and female broilers fed Bacitracin methylene di-salicylate, Virginiamycin, and a control diet. Miles et al. (2006) found that antibiotic-fed birds were heavier at 7 wk than the control birds and their muscularis interna in the duodenum was thinner. At 3 and 7 wk, the antibiotic-fed birds had a lighter GIT weight than the control birds. Resistance and residue problems have increased the negative consumer perception of growth-promoting antibiotic use in animal diets. One study examining antibiotic resistance tested 58 Salmonella enterica serovar Heidelberg isolates and found that 72% were resistant to at least one antibiotic and 24% of the isolates were resistant to 8 or more antimicrobials (Hoffman-Pennesi and Wu 2010). In 1997, the World Health Organization (WHO) announced that antibiotic resistance was a global public health problem (Tajodini et al., 2015).
The WHO promotes a proactive approach to reduce the need and use of AGPs in animals, especially within the classes used in humans (Dibner and Richards 2005). There is accumulated evidence of a correlation between antibiotics used as a growth-promoting feed additive and antibiotic resistance in humans and animals. However, in 2009, the American Veterinary Medical Association (AVMA) cited no established direct link between antimicrobials used in animals and subsequent resistance in humans (Hoffman-Pennesi and Wu, 2010). Besides the potential for resistance, antibiotics also can leave a residue in the meat (Khan and Naz, 2013). In 1986, Sweden became the first country to ban the use of AGPs (Dibner and Richards, 2005; Wenk, 2000). On January 1, 2006, the European Union banned growth-promoting antibiotics (Kulshreshtha et al., 2014; Hafeez et al., 2016). Due to the potential and realized problems occurring with the use of growth-promoting antibiotics and consumer pressure, the need for an alternative is essential for the poultry industry.
Natural Alternatives for AGPs
The three main options for lowering our dependency on antibiotics in animals are creating antibiotic-free alternatives that stimulate growth by improving feed conversion efficiency. Promoting animal condition by proper rearing methods might be difficult to accomplish in most situations (Adams, 1999). Growth promoters have been demonstrated to work best under the most adverse settings, such as animals in poor health and living in filthy conditions. The need for growth stimulants may be eliminated if the environment is improved through reduced overcrowding and improved infection control procedures (Prescott et al., 2000; Chervonova, 2021). Antibiotic growth promoters and the emergence of antibiotic resistance are inextricably linked. Enzymes, probiotics, prebiotics, herbs, amino acids, immunostimulants, organic acids, bacteriocins, and phytotherapeutic plants have been investigated as antibiotics growth promotors' alternatives (Figure 1; Langout, 2000; Abou-Kassem et al., 2021a; Arif et al., 2021). There is growing concern about developing antibiotic-resistant bacteria strains because of exposure to these subtherapeutic antibiotic dosages.
Figure 1.
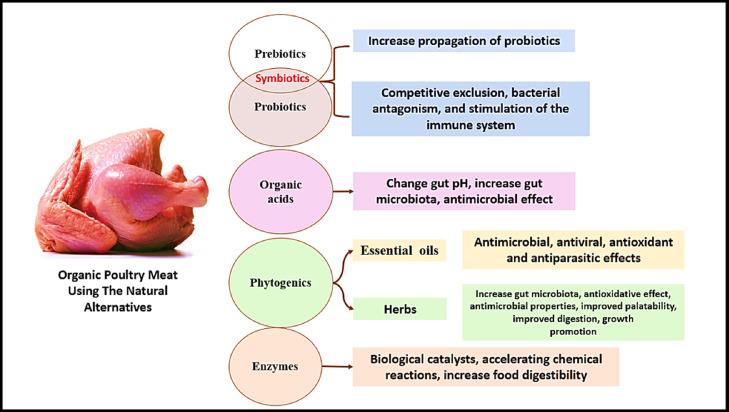
Most common natural alternatives used for production of organic poultry meat.
Prebiotic and Probiotic Additives
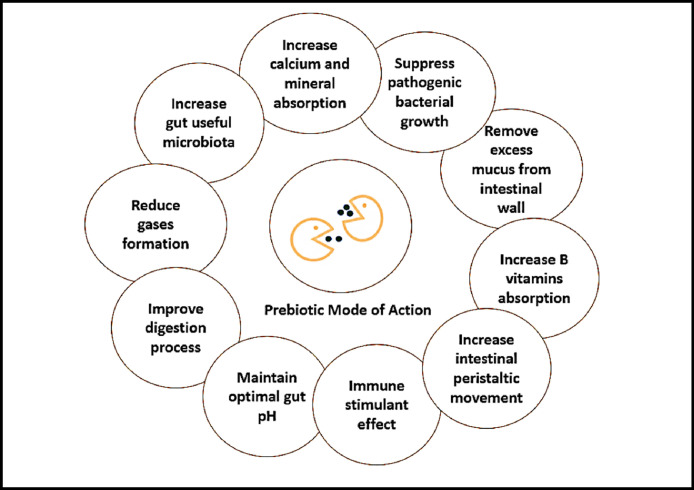
Prebiotics, probiotics and symbiotics (prebiotic and probiotic combination; Figure 2) are alternative feed additives used to control pathogens in the gut and increase bird performance (Murate et al., 2015; Peralta-Sánchez et al., 2019). Prebiotics are oligosaccharides indigestible by the host animal but are utilized by specific populations of gut microorganisms. Prebiotics enable already present microorganisms to increase their numbers, reduce pathogenic bacteria, increase digestibility, increase minerals and vitamins absorbability, maintain optimal intestinal pH, and maximize nutrients utilization (Figure 3; Wenk, 2000; Kulshreshtha et al., 2014; Murate et al., 2015; Mazanko et al., 2018).
Figure 2.
The use of probiotics, prebiotics and symbiotics for organic poultry meat production.
Figure 3.
Prebiotics’ mode of action.
Prebiotics can affect host health in several ways, such as the production of metabolites like lactic acid, modification of microbial metabolism, and increased cell integrity of the epithelium (Neupane et al., 2019; Abd El-Hack et al., 2021d, Abd El-Hack et al., 2021c; Yaqoob et al., 2021). Unlike prebiotics, probiotics are microorganisms that can alter the host health by colonizing the host GIT and providing a more balanced microbiota (Wenk, 2000; Khan and Naz, 2013; Murate et al., 2015). Probiotics can be yeast, bacteria, or fungi based and, unlike antibiotics, do not have the potential to leave a residue (Khan and Naz, 2013). Lactic acid bacteria, which produce antifungal metabolites, are common probiotic bacteria (Londero et al., 2014).
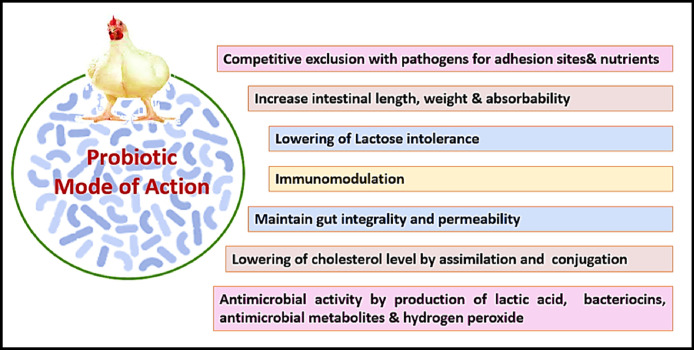
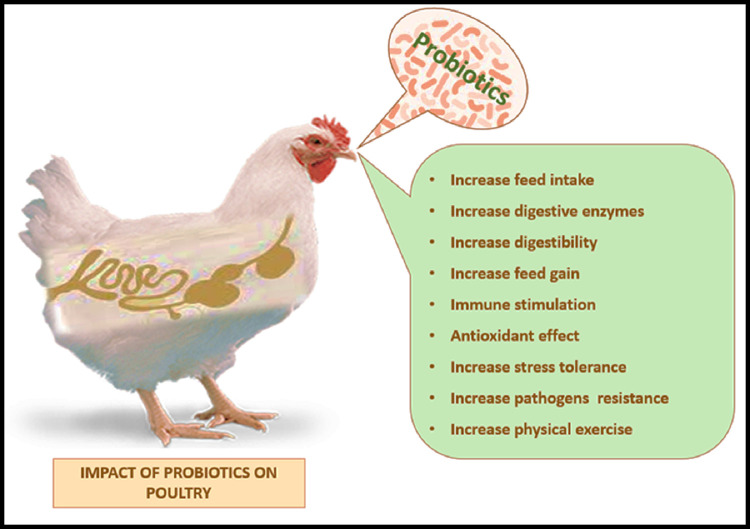
Probiotics, also known as direct-fed microbial (DFMs), are classified by FAO/WHO (2001) as “live microorganisms which when administered in adequate amounts confer a health benefit on the host.” Probiotics specific to broilers include the genera Lactobacillus, Streptococcus, Bacillus, Bifidobacterium, Enterococcus, Aspergillus, Candida, and Saccharomyces (Kabir, 2009). The beneficial effects of probiotics include improved performance, modulating the intestinal microbiota, inhibiting pathogens, improved intestinal integrity, immunomodulation, and improving microbiological and sensory characteristics of broiler meat (Figure 4, Figure 5; Alagawany et al., 2021a; El-Saadony et al., 2021d).
Figure 4.
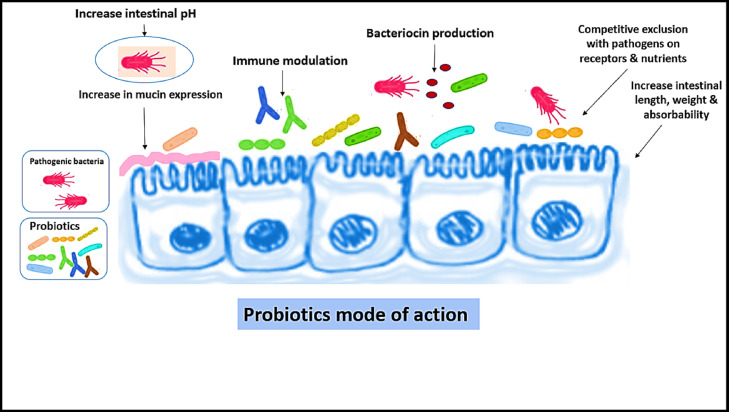
Probiotics’ mode of action.
Figure 5.
Impacts of probiotics on chickens.
Essential characteristics of a probiotic include being resistant to bile and acid, being strain-specific, having no side effects, reducing the number of pathogenic microorganisms and can withstand feed processing (St˛eczny and Kokoszy´ nski, 2020). The most common probiotic mode of action is by lowering the gut pH through the volatile fatty acids and organic acids produced during the probiotic product breakdown (Khan and Naz, 2013; Al-Fatah, 2020). Pathogenic bacteria such as Salmonella and E. coli strains cannot grow well below a certain pH (Wenk, 2000; Khan and Naz, 2013; Hidayat et al., 2019; Swelum et al., 2021). Furthermore, probiotics effectively control pathogen levels in the gut is through the competitive exclusion of adhesion areas along the gut lining. Immune system stimulation, nutrient competition and reduced levels of toxin-producing β-glucosidase and β-glucuronidase in the intestines and feces are other mechanisms probiotics utilize for pathogen protection (Khan and Naz, 2013).
Research on polysaccharides in seaweed for use as a prebiotic supplement has seemed promising due to its indigestibility by the host digestive enzymes and its strain specificity for beneficial microbes. Supplementation with seaweed increased the acetic acid, propionic acid, n-butyric acid, and i-butyric acid in the cecum by significant levels while decreasing the prevalence of Clostridium perfringens (Kulshreshtha et al., 2014). In a study done by Murate et al. (2015) on probiotic, prebiotic, and symbiotic supplementation, broilers and layers were challenged with Salmonella enterica serovar Enteritidis. Significant decreases in infected birds were found in the layers with the prebiotic treatment but not the control treatments. There was no effect between the probiotic, prebiotic and symbiotic control treatments in the broiler birds concerning Salmonella infection.
At early postinfection, there was a significant reduction in Salmonella counts in the broiler and layer birds receiving the prebiotic compared to the control diet. However, by d 14 and 21, there were no significant differences in the Salmonella count (Murate et al., 2015; Michel et al., 2019). For decades, it has been custom to use anticoccidials in poultry feed to control coccidiosis (Dalloul and Ritzi, 2016). Consumer concerns over drug residues in their food products and concerns about multidrug-resistant parasites and bacteria have driven the modern research community to search for alternative methods. Vaccines have a positive outcome; however, they are expensive and must be carefully administered to be effective and not cause unwanted harm. Immunomodulators such as probiotics have been at the forefront of worldwide research (Dalloul and Ritzi, 2016).
Probiotic's Modes of Action
Probiotics help maintain a healthy balance in the gut to promote proper health, performance, and defense against enteric diseases (Dalloul, 2017; Abd El‐Hack et al., 2020). Probiotics function through 3 main mechanisms: competitive exclusion, bacterial antagonism, and stimulation of the immune system (Ohimain and Ofongo, 2012). As soon as a chick hatches, gut microbiota begins to establish within hours, so early administration of probiotics has a better chance of improving gut microbial profiles (Timmerman et al., 2006).
A chick would typically establish a gut microbiota via contact with adult chickens’ feces. However, probiotic supplementation is beneficial in a hatchery due to lack of contact with grown chickens (Kabir, 2009). Probiotics can competitively exclude pathogenic strains of bacteria by establishing in the gut, taking up the space and nutrients in the digestive tract that pathogens would otherwise use to colonize. Many probiotic species are antagonistic to bacterial pathogens by producing antimicrobial substances and secretions that lower pH in the gut, which inhibit pathogenic growth (Hume, 2011). Short-chain fatty acids (SCFA) produced by beneficial bacteria have been shown to increase host defense peptide gene expression (Sunkara et al., 2011, 2012), and in high concentrations, disrupt proton motive force and metabolic reactions in bacteria when small, nonionized acids cross bacterial membranes and then dissociate into protons and anions in the cytoplasm.
The protons lead to acidification of intracellular compartments where proton motive force occurs, disturbing metabolic reactions, and anions disrupt osmotic balance (Sun and O'Riordan, 2013). Bacteriocins, small peptides or proteins secreted by beneficial bacteria, kill closely related strains of bacteria by forming pores in the cell membrane and disrupting enzyme function. Beneficial Lactobacillus spp. is known for secreting lactic acid, which lowers intestinal pH and inhibits pathogenic growth (Figure 6; Travers et al., 2011). The vital functions of probiotics to stimulate the immune system are discussed below.
Figure 6.
Probiotic effect on intestinal villi & their mode of action.
Probiotics, Performance, and Intestinal Development
A healthy gut is linked with the proper performance of poultry (Getachew, 2016; Hargis et al., 2021). Probiotics can boost performance by helping to establish a healthy gut environment. In chickens, body weight gain (BWG) and feed conversion ratio (FCR) have been improved with probiotic supplementation (Sen et al., 2012; Popova, 2017). A study by Mountzouris et al. (2007) demonstrated performance results at the same level as the AGPs avilamycin.
Other studies have shown no improvement in performance due to probiotic supplementation (Rahimi et al., 2011; Wolfenden et al., 2011; Getachew, 2016). This could be explained by the strain of probiotic bacteria not having an effect, the viability being compromised during feed preparation, or improper dosage levels. Other factors that can affect probiotic efficacy are diet, overall health and age of birds, interactions with other additives in the feed, or stress factors such as temperature and stocking density (Patterson and Burkholder, 2003; Mountzouris et al., 2007; Cox and Dalloul, 2015). Probiotics have also shown the potential to improve gut development and makeup. They have resulted in an increased length of intestinal villi and a decrease in crypt depth (Samanya and Yamauchi, 2002; Marković, 2009).
Longer villi length correlates to a larger surface area for absorbing nutrients. Crypt sites are the location of enterocyte proliferation, and shallow crypts indicate less need for epithelial cell turnover, taking energy that would go toward cell turnover and placing it toward animal growth (Marković, 2009). Another example of probiotics benefiting gut health was demonstrated when the mycotoxin deoxynivalenol (DON) was added to feed (Awad et al., 2006). DON usually damages the gut by reducing the villus height and width in the duodenum and jejunum, whereas probiotic treatment alleviated the deleterious effects of villus reduction (Awad et al., 2006).
Probiotics and Innate Immunity
Innate immune responses are non-specific defenses consisting of physical, chemical, and cellular barriers (Applegate et al., 2010). Physical barriers include epithelial cells (enterocytes), chemical barriers include secretions or mucus or antibacterial peptides (defensins, lysozymes), and cellular barriers include phagocytes and macrophages that engulf bacteria and present it to the adaptive immune system (Applegate et al., 2010). The avian heterophil is equivalent to the mammalian neutrophil.
These cells play an essential role in the first line of defense, phagocytosing pathogens, then using oxidative burst and degranulation to destroy them. Probiotics have been shown to enhance oxidative burst and degranulation, thus protecting against pathogens (Harmon, 1998; Duskaev et al., 2020). Heterophils isolated from probiotic-supplemented broilers displayed increased oxidative burst and degranulation (Farnell et al., 2006; Stringfellow et al., 2011). Macrophages phagocytose pathogens, process them intracellularly, and present antigens to the adaptive immune system cells, playing a role in lymphocyte differentiation. Broiler chicks fed a commercially available Lactobacillus-based product had increased macrophages in the ileum and cecum. Still, macrophage levels were reduced after exposure to the pathogen Salmonella enteritidis and supplementation with probiotics one hour after the challenge. Competitive exclusion by the probiotics could cause a decrease in macrophages after the challenge, reducing the pathogen load in the intestine (Higgins et al., 2007a; Price et al., 2020). Probiotics play a role in enhancing innate immune responses and the competitive exclusion of pathogens in the gut.
Probiotics and Adaptive Immunity
The adaptive immune system mounts a specific defense to foreign antigens, consisting of T cells and B cells that produce antigen-specific antibodies (Applegate et al., 2010; Xiang et al., 2019). It is the second line of defense and essential to protection against reinfection (Dalloul, 2017). Supplementation with probiotics increased antibody titers to sheep red blood cells and Newcastle disease virus and infectious bursal disease, both important avian viral diseases (Apata, 2008; Karimi Torshizi et al., 2010). A study involving oral gavage female broilers found that probiotic treated birds had increased natural antibody production and increased levels of IgA responsive to tetanus toxoid and increased IgG and IgM antibodies reactive to tetanus toxoid and alpha-toxin (Haghighi et al., 2006).
Mountzouris et al. (2009) compared probiotic treatments to the AGP avilamycin. They determined that both resulted in lower plasma IgA and IgG levels and decreased intestinal IgA against Salmonella enteritidis compared to a challenging control. Reduced antibody levels may indicate enhanced clearing and recovery of the disease. Eimeria-infected birds supplemented with probiotics produced more Eimeria-specific antibodies than controls (Lee et al., 2007a,b; Elkhouly et al., 2016). Probiotic supplementation has been shown to increase the number of intestinal intraepithelial lymphocytes expressing the cell surface markers CD3, CD4, and CD8 (Dalloul et al., 2003; Noujaim et al., 2008).
A study by Karimi Torshizi et al. (2010) challenged birds with 1- chloro-2, 4-dinitrobenzene (DNCB) and birds supplemented with a water application of probiotics displayed enhanced immune response. Birds challenged with a PHA-M (phytohemagglutinin-M) injection were supplemented with feed or water applied probiotics and showed enhanced immune response as measured by increased skin thickness. Different strains of probiotics can have other effects on cytokine production, as indicated by studies using Lactobacillus and Bacillus-based probiotics that modulated the levels of proinflammatory cytokines (IL-1β, IL-6, IL-17a, IL-18), Th1 cytokines (IFN-γ, IL-2, IL-12), and Th2 cytokines (IL-4, IL-10, IL-13) (Dalloul et al., 2005; Brisbin et al., 2010; Lee et al., 2010). The strain of probiotics used can profoundly affect the immune response, as measured by lymphocyte and cytokine production.
Probiotics and Host Defense Against Pathogens
Pathogens such as Eimeria spp., Clostridium perfringens, Campylobacter jejuni and Salmonella enteritidis are making a comeback in modern poultry and rabbit production due to the ban on the prophylactic use of antibiotics (Khelfa et al., 2012, 2012a,b; Dalloul and Ritzi, 2016; Jiang et al., 2017). These diseases can have devastating effects on the intestines, leading to reduced performance and death. Probiotics can improve gut health and reduce the impacts of common enteric disorders. Reduced oocyst shedding has been observed in probiotic-fed chicks that were later infected with E. acervulina or E. tenella (Dalloul et al., 2003, 2005; Lee et al., 2007a,b). The severity of lesions from E. maxima infected broilers was reduced in probiotic supplemented treatments (Lee et al., 2010). Ritzi et al. (2014) conducted studies on feed vs. water application of probiotics in assessing anticoccidial effects.
It was found that a water-based probiotic led to lower duodenal and jejunal lesion scores and those birds intermittently given water-based probiotic treatment shed fewer oocysts. The subsequent study concluded that probiotics in conjunction with vaccination lead to additional benefits, as seen by improved performance and reduced lesion scores when challenged birds with Eimeria (Ritzi et al., 2016). Further, probiotics given to birds on embryonic d 18 had reduced disease severity after being challenged with Eimeria (Pender et al., 2016). Other pathogens, such as Salmonella, Campylobacter jejuni, and Clostridium perfringens, were reduced by probiotic supplementation as well (Saint-Cyr et al., 2016). A study by Revolledo et al. (2009) found that probiotic supplementation reduced colonization of Salmonella in the ceca, liver, and spleen of broiler chicks. Necrotic enteritis induced by C. perfringens was partially alleviated as indicated by reduced lesion scores, mortality, and levels of C. perfringens (McReynolds et al., 2009).
Intracellular pathogens such as Salmonella enteritidis and Campylobacter jejuni were reduced by probiotic supplementation (Higgins et al., 2007b; Ghareeb et al., 2012). Reducing pathogens in the gut can aid in protection against secondary infections that are often associated with the primary disease; for example, Eimeria infection often leads to necrotic enteritis by C. perfringens (Chapman et al., 2002).
Phytogenic Feed Additives and Poultry
Antibiotic-growth promoters have been used to improve poultry performance for decades; however, recent bans in the European Union (EU) on these products and concerns over microbial resistance and health effects in humans have led to increased interest in natural alternatives (Alcicek et al., 2004).
A broad category of feed additives commonly researched in poultry is phytogenic (PFAs), intended to improve gut health and function (Alcicek et al., 2004). PFAs are plant-derived compounds such as essential oils, spices, safe natural compounds, and herbs intended to provide a health benefit when added to feed (Puvača et al., 2013; Ashour et al., 2021). Typical examples are rosemary derivatives, oregano, thyme, sage, cinnamon, citrus, pepper, and anise (Mountzouris, 2016). The efficacy of phytogenics can depend on numerous factors, including composition, inclusion level in the feed, bird genetics, and feed composition (Ferdous et al., 2019).
According to Mountzouris (2016), optimal gut function, animal health, and performance are linked and can be achieved when healthy gut microbiota, dietary factors, gut mucosa, and immune responses are balanced and work together to eliminate pathogens, improve nutrient absorption, and modulate inflammation (Koutsos and Arias, 2006; Choct, 2009; Applegate et al., 2010; Mountzouris, 2016). The efficacy of plants depends on the type and amount of plant secondary metabolites or phytochemicals contained in the plant. These components act on the GIT of the host animal and help to improve the microbial community, which in turn helps resist colonization and take over from pathogenic microorganisms (El-Saadony et al., 2021e; El-Saadony et al., 2021f).
The antimicrobial property of phytogenic feed additives is due to the bioactive components such as tannic acid and saponins. Tannic acid can promote iron deprivation, and saponins can bind with sterols to cause microorganism damage and cell destruction (Hashemi and Davoodi, 2011). There are many examples of positive findings with PFA supplementation. The impact of nutrient density and PFA supplementation (quillaja, anise, and thyme) on the growth and performance of meat ducks was investigated by Gheisar et al. (2015). Authors found that it was possible to recover some growth performance, meat quality, and nutrient digestibility loss that resulted from the lower nutrient density diet by supplementing the diet with a PFA (Gheisar et al., 2015). This study highlights the positive effects of PFA's with reduced nutrient diets. A study with a phytogenic compound containing thyme and star anise was supplemented at 150, 750, and 1,500 mg/kg in the diet.
The PFA was not found to affect the body weight positively, body weight gain, or feed conversion intake compared to the non-supplemented control diet. Although there were no positive production findings, as supplementation increased, the apparent ileal digestibility of crude ash, crude protein, crude fat, and phosphorous and calcium absorption increased linearly. The birds fed the highest level of supplementation had the highest level of nutrient digestibility (Amad et al., 2011).
Modes of Action
PFAs may promote a healthy gut and improve performance through various mechanisms: antioxidative and antimicrobial properties, improved palatability, improved digestion, growth promotion, and improved gut health (Alcicek et al., 2004). Studies on palatability are inconclusive, but the PFAs may improve feed quality due to antioxidative properties and slow bacterial and fungal growth (Lambert et al., 2001; Soliman and Badeaa, 2002; Burt, 2004).
Phytogenic (Phytobiotic), Avian Performance, and Intestinal Effects
Phytogenic or phytobiotic feed additives have had varying effects on performance. Some studies demonstrated no differences in various performance parameters. One study used essential oregano oil at 50 or 100 mg/kg in a wheat-soybean meal diet with Cobb broilers, and there was a difference in body weight (BW) or FCR from controls (Botsoglou et al., 2002; Grashorn, 2010). Another study using female Cobb broilers found that supplementation with thymol, cinnamaldehyde, and commercial preparation had no effects on feed intake (FI), BWG, or FCR (Lee et al., 2003; Ren et al., 2019).
Other studies later demonstrated the benefits of various PFAs in poultry feed given to different breeds of broiler chickens. Two studies involving different diets, the first diet included carboxyl methyl cellulose to corn to increase intestinal viscosity. The adverse effects on BWG were partially offset (Lee et al., 2004a) while the second diet was rye-based to suppress weight gain, but the cinnamaldehyde partially offset the reduced weight gain over the first 2 wk (Lee et al., 2004b). Eimeria-infected Cobb birds fed wheat soybean meal with oregano supplementation at 300mg/kg had better BWG and FCR, although they were lower than the group provided a coccidiostat (Giannenas et al., 2003).
In another 6-wk study by Mountzouris et al. (2011), using different inclusion levels of PFA, no significant differences were seen during the starter and grower phases in BW, BWG, FI, or FCR; however, during the finisher phase, BWG increased linearly, FI decreased linearly, and FCR improved linearly with the PFA inclusion level. Cumulatively, the addition of PFA improved BWG, FI, and FCR (Mountzouris et al., 2011). Similarly, another study demonstrated no significant difference in performance parameters until the finisher phase. BWG increased linearly, FI was reduced quadratically, and overall FCR was improved quadratically with increasing PFA inclusion level (Paraskeuas et al., 2017). Soltan et al. (2008) demonstrated varying outcomes, depending on the PFA concentration, emphasizing the importance of composition in a feed additive. Anise seeds were included in a corn-soybean meal diet at 0.5 to 0.75 g/kg fed to Hubbard broilers for 6 wk.
Overall, these treatments improved BWG with no effect on FI or FCR. The highest inclusion rate of anise seed was 1.5 g/kg and reduced growth performance (Soltan et al., 2008). A proper balance of ingredients and concentrations of PFAs can lead to improved performance. The importance of concentration has been documented in several studies. Bolukbasi and Erhan (2007) added thyme to layer diets at 0, 0.1, 0.5, and 1% and observed improved FCR, egg production, and reduced fecal E. coli levels in the 0.1 and 0.5% concentrations. Another study evaluated three different levels of Azadirachta indica (Indian lilac) dried leaf meal at 0, 1.25, 2.5, and 5.0 g/kg of feed, resulting in better body weight, FCR, and dressing percent in the 2.5 g/kg diet, but no improvement in higher or lower amounts (Ansari et al., 2011). Jamroz et al. (2006) divided broilers into corn-fed or wheat and barley-fed diets, with or without carvacrol, cinnamaldehyde, and capsicum oleoresin plant extracts to investigate the effect of diet composition on the efficacy of PFAs. The diet's significant effect was observed by shorter jejunal villi height and shallower crypt depth in broilers fed corn and PFAs, while no difference was observed in the wheat and barley diet supplemented with PFAs. Both corn and wheat/barley diets that had PFAs had improved FCR. The range of efficacy varies depending on the type of plant used, type of basal diet, chicken breed, and age. Phytogenic feed additives may improve digestibility by increasing enzyme action and mucus production in the gut. Broilers had greater trypsin, lipase, and amylase activity (Lee et al., 2003; Jamroz et al., 2005).
Older broiler chickens began receiving PFAs at 41 d of age and exhibited a 38 to 46% increase in lipase activity (Jamroz et al., 2005). Another study reported increased thickness in the jejunum and stomach and increased mucus production, signifying a protective effect against pathogen colonization (Jamroz et al., 2006). The effects on gut microflora indicate PFAs have antimicrobial properties. Many phytogenic mixtures have shown antimicrobial activity against foodborne pathogens, including Salmonella typhimurium, S. enteritidis, E. coli 0157:H7, Shigella dysenteria, Listeria monocytogenes, Bacillus cereus, Pseudomonas aeruginosa, and Staphylococcus aureus (Lambert et al., 2001; Burt, 2004; Chorianopoulos et al., 2004; Penalver et al., 2005; Si et al., 2006).
Speculation as to the mechanism of these actions is credited toward PFAs phenolic structures (Burt, 2004; Penalver et al., 2005; Si et al., 2006). Phenolics can create disturbances in bacterial cytoplasmic membranes, disrupt the proton motive force, electron flow, active transport, and coagulate cell contents (Lambert et al., 2001; Burt, 2004). These studies above indicate that PFAs may be able to control the growth of pathogens in the gut; however, subsequent studies involving birds result in various outcomes. One commercial blend of essential oils could lower E. coli levels and increase beneficial Lactobacillus levels (Jamroz et al., 2005). Another study using a mixture of 5 herbs had no significant effect on cecal or excreta microbial populations (Cross et al., 2007). Mountzouris et al. (2011) demonstrated no difference in microbial populations by d 14 or d 28, but variations by d 42 when linear increases in cecal aerobes, Clostridium, Lactobacillus, Bifidobacterium, and Gram +ve cocci were observed in direct relation to PFA inclusion levels.
Phytogenics and Innate Immunity
The innate immune system acts as the first line of defense against disease. A critical barrier to pathogens in the gut is mucus production by goblet cells in the epithelial layer. Mucus coats the epithelial cells lining the length of the gut, protecting them from pathogenic colonization (Mountzouris, 2016). Mucins are glycoproteins that can be produced, stored, and secreted by goblet cells. A study by Tsirtsikos et al. (2012) isolated mucin from d 14 broilers fed a control diet, AGP (avilamycin) diet, or PFA treatments at increasing levels of 80, 125, and 150 mg/kg diet. In the mucin, increased mannose in the ileum and increased galactose in the duodenum of birds fed PFA compared to the negative control.
Mannose and galactose are oligosaccharides that have been associated with increased response to enteric disease (Mountzouris, 2016). A separate trial used a different mixture of PFA and found a trend for increased ileal MUC2 gene expression in PFA-treated birds at d 42. Those birds also exhibited a trend towards lower spleen iNOS levels, indicating an anti-inflammatory effect on the innate immune system (Paraskeuas et al., 2016). The same study substituted corn for wheat, and contrastingly, there were no observed differences in ileal MUC2 or spleen iNOS between treatments (Paraskeuas et al., 2016).
Phytogenics and Adaptive Immunity
The immune system strives to maintain homeostasis between immunostimulation and immunosuppression (Applegate et al., 2010). In human literature, phytogenics have been shown to have immunostimulatory activity, as is the case with ginseng stimulating lymphocyte activity and increased cytokine production of IL-1, IL-6, IL-12, IL-6, TNF-α, and interferon-γ (Tan and Vanitha, 2004). Anti-inflammatory properties of Ginko biloba have been noted in human studies, demonstrating how its flavonoids and terpenes mediate the production of proinflammatory cytokines (Li, 2000). The study by Paraskeuas et al. (2016) increased ileal IgA levels and a trend towards a decrease in spleen IL-18 of d 42 broilers fed a PFA mixture. The same study using wheat instead of corn observed no differences in ileal IgA or spleen IL-18.
Essential Oils
Essential oil is classified as the plant essence or benzene or terpene derivatives obtained through water and/or steam distillation (El-Tarabily et al., 2021; Abd El-Hack et al., 2022a,b,c). Essential oils are valuable due to their antimicrobial, antiviral, antioxidant and antiparasitic capabilities. Many plant sources of essential oils have been studied for feed additive efficacy. Tecnaroma Herbal Mix PL essential oil blend, containing herbs such as thyme, basil, and oregano, was supplemented in 100-g increments from 100 to 500 in broiler chickens in a study by Khattak et al. (2014). The addition of essential oil positively impacted avian performance and carcass characteristics (Khattak et al., 2014). Essential oils can reduce the levels of pathogens such as gram-negative E. coli and Salmonella bacteria in the gut.
To test the antioxidant capabilities of specific phytogenic products, the oxygen radical absorbance capacity is determined. In a study by Hoffman-Pennesi and Wu (2010), thyme oil was found to be a better antioxidant than cinnamon bark oil and caeffic acid was found to have the highest antioxidant capability. However, the feed conversion efficiency of broilers was not affected by the supplementation of thymol and thyme oil (Hoffman-Pennesi and Wu, 2010). With phenolic OH groups, certain PFAs have antioxidant qualities which promote a reduction in hydroxyl peroxide formation (Gheisar et al., 2015). The area of PFAs is still relatively new and debated. There have been many conflicting results from different studies. The quality and quantity of the herbs is an important component that can affect study outcomes/findings. The quality can be affected by plant age, when it was harvested, how it was extracted, and what other ingredients are combined to produce the additive (Hashemi and Davoodi, 2010).
Mode of Action
In broiler production, essential oils demonstrate the potential for curative treatment in various situations. Essential oils improve poultry production by increasing the activity of digestive enzymes, lowering the number of fermentation products, lowering the number of pathogens, improving nutrient digestion, enhancing intestinal accessibility of important nutrients, and enhancing antioxidant capacity and immune function (Brenes and Roura, 2010; Alagawany et al., 2021b; Abd El-Hack et al., 2022a).
The authors’ research focused on applying the aforementioned essential oil, compounds, and other newly screened compounds to control pathogens in organic poultry. The feed conversion ratio measures how well feed mass is converted to body mass over a set period. Cinnamon powder, which includes cinnamaldehyde, has been demonstrated to help broilers enhance their FCR (Hernandez et al., 2004). These molecules have shown not only antibacterial effect against Salmonella and Campylobacter, but also on several other economically important and hazardous infectious agents such as Clostridium jejuni, E. coli O157: H7 and Listeria monocytogenes on several nonpoultry food matrices (Baskaran et al., 2013; Upadhyay et al., 2013; Mooyottu et al., 2014). Since these compounds are safe for animals and humans and are environment-friendly, we explore their potential in organic poultry production for pre-harvest and post-harvest applications. A part from salmonellosis and campylobacteriosis, the organic industry faces challenges with coccidiosis, clostridia infections, internal and external parasites, and high mortality from diseases for which the potential of essential oils and their compounds must be explored. Moreover, economic considerations for including these compounds in organic poultry feeds must be determined (Darre et al., 2014; Bortoluzzi et al., 2019).
Organic Acids
Organic acids are organic substances that have an acidic pH. Carboxylic acids such as lactic acid, propionic acid, acetic acid, formic acid, sorbic acid, citric acid, oxalic acid, uric acid, and butyric acid are the most prominent types (Dibner and Buttin, 2002). Organic acids are not antibiotics, but when used in conjunction with excellent nutrition, management, and biosecurity procedures, they can help poultry maintain intestinal health, improving livability, feed conversion ratios, weight gain, live weight, and immunological responses (Adil et al., 2011).
Organic Acids' Effects on Broiler Performance and Intestinal Health
Organic acids' antibacterial activity is linked to pH decrease and their tendency to dissociate, making them reluctant proton donors in aqueous solution, producing weak acids. A weak acid's dissociation is pH-dependent; therefore, the antibacterial activity improves with a lower pH value (Hajati, 2018; Jadhao et al., 2019). Organic acids are lipid-soluble in their undissociated form, easily transferring them into microbial cells via passive and carrier-mediated pathways.
In the alkaline condition, the organic acid releases the proton H+, lowering intracellular pH that changes microbial metabolism by blocking the function of crucial microbial enzymes, forcing the bacterial cell to expend energy to export surplus protons H+, resulting in starving death. In bacteria, acid-sensitive proteins and DNA can be denatured by protons H+ (Khan and Iqbal, 2016). Lactic acid bacteria may grow at a lower pH than other bacteria like E. coli and Salmonella, making them more resistant to organic acids. Gram-positive bacteria (such as Lactobacilli) have a high intracellular potassium content protecting them against acid anions (Russell and Diez-Gonzalez, 1998; Araujo et al., 2019).
Organic acids suppress pathogenic intestinal bacteria due to their antimicrobial activity, resulting in less bacterial competition for available nutrients, lower levels of harmful bacterial metabolites, improved protein and energy digestibility, and improved avian performance (Baurhoo et al., 2007). Supplementation of organic acids affects the histological structure of the gastrointestinal tract resulting in increased villus length, improving the absorptive ability of the intestinal mucosa; consequently, better nutrient absorption, maximizing nutrient utilization and improving growth performance were obtained. Lowered pH values in the gut encourage the growth of beneficial bacteria and suppress the growth of harmful bacteria, which thrive at a higher pH. Also, these acid anions join with calcium, phosphorus, magnesium, and zinc, thus enhancing their digestibility. Pepsin activity is increased because of the lower gastric pH. The peptides produced by pepsin proteolysis stimulate the release of hormones such as gastrin and cholecystokinin, which regulate protein digestion and absorption (Lan et al., 2005; Araujo et al., 2019). Acidomix FG, a micro-granulated feed acidifier based on formic acid, lactic acid, fumaric acid, and ammonium format, is an example of commercially available organic acids and is considered as an unprotected organic acid mixture which is active in the foregut as it is neutralized by bile. Avimatrix consists of 3.0% calcium format and 49.0% benzoic acid. It is considered a protected organic acid mixture active in the midgut as it dissociates in the alkaline pH within the jejunum.
Activate WD is an unprotected organic acid that contains methionine hydroxyl analogue (HMTBa), formic acid, and propionic acid. It works as a water sanitizer and affects the upper intestine by reducing stomach pH, boosting pepsin activity, and improving protein digestion. Low pH promotes the growth of acid-tolerant beneficial bacteria and inhibits the growth of acid-labile harmful pathogens.
Application of Organic Acids in Poultry Production
Both prophylactic and therapeutic antibiotics have been used in the poultry industry to treat gastrointestinal diseases. Because of the difficulties associated with antibiotics in food animal production, efforts have been made to employ the commensal intestinal microbiota to manage pathogenic microorganisms. This includes stabilizing the microbiota at required levels or modifying those using natural alternatives (Lan et al., 2005).
Prebiotics, enzymes, acidifiers, herbs, essential oils, and immunomodulators (Figure 7). Organic acids have long been used to combat bacterial and fungal pollution in poultry feeds. Citric, propionic, fumaric, lactic, formic, and benzoic acids are the most prevalent organic acids in animal nutrition. They efficiently reduce pathogenic bacterial infections caused by Salmonella, Campylobacter, Clostridium perfringens, and others (Banupriya et al., 2016). Therefore, organic acids can alternate AGPs in chickens (Chaveerach et al., 2001; Montonya et al., 2011; Abdelrazek et al., 2016).
Figure 7.
Types of the natural alternatives for antibiotics and their mode of action.
Herbal Alternatives
Herbal extracts and spices play a significant role in improving both productivity and health condition of avian species (El-Saadony et al., 2021g). Positive impacts of plant extracts or active substances in bird feed may involve the stimulation of appetite and increase feed intake, enhance the endogenous digestive enzyme production, stimulate immunity and have antiviral, antibacterial, anthelminthic and antioxidant activities. Isoprene derivatives, flavonoids, glucosinolates and other herbal metabolites may affect the physiological and chemical function of the birds gut. The stabilizing impact on the intestinal microbiome may be accompanied by intermediate nutrient metabolism (El-Saadony et al., 2021h; Reda et al., 2021b; Saad et al., 2021a,b,c).
Many different pathogens threaten poultry and animal production resulting in severe economic losses (Attia et al., 2021, 2021a; Hegazy et al., 2021; Salem et al., 2021a, 2022; Soliman et al., 2021). Due to the limitation on using some antibiotics, dangerous residual effects, and cost-effectiveness, the use of herbal feed additives is gaining traction in poultry production. Herbs, spices, and their extracts (botanicals) can be used for various purposes (Suganya et al., 2016; Abou-Kassem et al., 2021b). They have antibacterial, coccidiostats, anthelmintic properties, and promote feed intake, stimulate the immune response, and act as antioxidants. Nutmeg, Cinnamon, Cloves, Cardamom, Coriander, Cumin, Anise, Celery, Parsley, Fenugreek, Capsicum, Pepper, Horseradish, Mustard, Ginger, Garlic, Onion, Rosemary, Thyme, Mint, Shatavari, Jivanti, Shatavari, and turmeric are the most common herbal feed additives used in poultry feed (Muanda et al., 2011; Suganya et al., 2016).
Modes of Action
Herbs or botanicals have a great impact on poultry production. They improve bird feed intake, increase feed digestibility, and have antibacterial, antiviral, anthelmintic, coccidiostats, anti-inflammatory, antioxidant, etc., immunostimulant effects (El Tazi et al., 2014). Some herbs have Pro-oxidative action as Cardamom, Sage, Verbena, Coriander, Estragon, and Eucalyptus. While, Clove, Cinnamon, Laurel, Almond bitter, Rosemary Thyme, Peppermint, Pepper, Nutmeg, and Mint are considered effective antioxidative agents (Suganya et al., 2016).
Herbs or Botanicals as a Potent Immunostimulant
Some herbs feed additives are rich in vitamin C and carotenoids, which plays a severe role in bird immune response, and they stimulate the activity of lymphocytes, macrophages, interferons, and natural killer cells (NK) (Frankic et al., 2009; Suganya et al., 2016)
Enzymes
Purpose and History of Enzymes
Enzymes are biological catalysts that can speed up chemical reactions. Enzymes are protein molecules with significant consequences for their stability during the production of high-temperature feeds (pelleting) and transit through the alimentary tract (Ravindran, 2013). All animals use enzymes to digest feed. Supplementing the feed with particular enzymes improves the nutritional value of feed ingredients, increasing digestion efficiency (Llamas-Moya et al., 2019). Ultimately, feed enzymes are used to improve feed efficiency, reduce feed cost, and create a better environment by reducing the volume of manure produced and phosphorus and nitrogen content excreted (Barletta, 2010).
Enzymes can be categorized into several classes. They can be categorized by the substrate they act upon and their origin. The enzymes classified by the substrates they act upon are phytases, phytate degrading enzymes; proteases, fiber, and starch degrading enzymes; and proteases, which consist of protein degrading enzymes. The second area of categorization of enzymes is the origin from which the enzymes are derived, including exogenous and endogenous sources. Research regarding enzymes in poultry diets has been ongoing since the 1920s. The first report of an enzyme product used in poultry diets was known as Protozyme, which derived from the fungus Aspergillus oryzae (Ravindran, 2013). Adding an enzyme cocktail containing xylanase and ß-glucanase to a un pelleted poultry diet containing rye and wheat at various inclusion levels resulted in a significant increase in body weight gain and feed intake (Pettersson and Aman, 1989).
Exogenous Enzymes
The addition of exogenous enzymes has become the standard to improving digestibility and efficiency of nutrient utilization (Ravindran, 2013). According to Pariza and Cook (2010), to digest food, all animals need enzymes, which are either created by the animal or by bacteria in the digestive tract. The digestive system, on the other hand, isn't perfect. Therefore, supplementing the animal feed with appropriate enzymes improves digestive efficiency (Munir and Maqsood, 2013).
Enzyme supplementation helps to reduce the amount of nutrient excretion, which, if ignored, can result in extra cost to the farmer, feed supplier, and the environment (Sheppy, 2010; Ali and Abdelaziz, 2018). Exogenous enzymes are essential to the reduction of feed cost. Because feed accounts for most production costs, enzymes can reduce costs by poorly absorbing other nutrients. When the price of corn, wheat, fat, and inorganic P increases, the use of enzymes in feed becomes more economically attractive, providing a more significant return on investment (Barletta, 2010). Phytase is an enzyme that hydrolyzes phytate to inositol and inorganic phosphate (Bilal et al., 2015). On a more in-depth level, phytase is known as Myo-inositol hexakisphosphate phosphohydrolase. Poultry needs to have dietary phosphorus (P) for maintenance and growth. Therefore, an adequate amount must be included in the diet. Even with enough total P in the diet, a portion of the total P comes from cereal grains and this P exists in a form that poultry cannot digest.
The majority of P, about 60%, is not accessible to non-ruminants because it is associated with phytate. Phytate binds to many dietary cations as Cu, Zn, Ca, Fe, Mg, Mn protein, fat, and vitamins, resulting in serious reductions in nutrient availability (Bohn et al., 2008). In the current market, trends have shown that hydrolytic enzymes have emerged as feed supplements to improve the digestion and absorption of poorly available nutrients, such as dietary phytate (Barletta, 2010). Based on Iqbal et al. (1994), non-ruminant animals can only digest phytate due to the lack of significant endogenous phytase activity and low microbial population in the upper portion of the digestive tract.
The main sites of phytate degradation by phytases are the forestomach (crop, proventriculus, gizzard) in poultry, where there is little degradation in the distal gastrointestinal tract (Selle et al., 2010). Phytases can be divided into two classes, depending on the carbon site of hydrolysis of phytic acid. The 3-phytase begins hydrolysis at the three positions of the myo-inositol ring, while the 6-phytase begins hydrolysis at position 6 of the ring (Dersjant-Li et al., 2015). Furthermore, phytases have different origins of expression, pH optima and temperature optima. The most common origins of phytase used in poultry feeds include Aspergillus niger, Escherichia coli, and Buttiauxella spp. Optimal pH and temperature range are essential factors affecting the feed's phytase activity. Naves et al. (2012) examined three commercials microbial phytase products, and results show that A. oryzae exhibited optimal activity at pH 4.0 and temperature 40°C. The second expression, A. niger exhibited maximal activity at pH 5.0 and 45°C. Lastly, S. cerevisae presented its highest activity at pH 4.5 and temperature between 50 and 60°C. The researchers ultimately advised A. niger and S. cerevisae exhibited the highest in-vitro activities that correspond with the optimal physiological conditions of broiler chickens.
This finding would allow a higher rate of hydrolysis of phytate. Phytase is expressed as phytase units (FTU). Ballam et al. (1984) and Edwards and Veltmann (1983) reported that broiler chickens fed a corn-soybean meal (SBM) based diet had phytate-P utilization between 10 and 53% – adding exogenous phytase increased the P availability to 65%.
However, there have often been studies where the addition of phytase to diets has been inconsistent. The inconsistency in the relationship between microbial phytase and P digestibility can be linked to dietary phytate level, feed composition, or the Ca:P ratio in broilers of all ages. High levels of Ca in poultry diets (Scheideler and Sell, 1987) decrease the availability of P absorbed. Calcium can form insoluble complexes with phytate-P, ultimately obstructing the phytase activity (Angel et al., 2002).
Calcium-phytate complexes are mainly formed in the small intestine, where it adversely influences the efficacy of mucosal phytase. While these studies (Scheideler and Sell, 1987) reported Ca negatively impacted P digestibility, the influence of Ca on phytase tends to be positive. In essence, the use of exogenous phytase in non-ruminant animals’ diets such as poultry is important because phytase can cleave the phosphate groups from phytate, complex cations, and proteins, which increases availability (Kies et al., 2006). Nelson et al. (1968) were the first to show that a phytase could hydrolyze phytate-P in diets. The use of phytase significantly increased after it was included in several publications after Nelson et al. (1968) and Rojas and Scott (1969) reported their results.
Since the Nelson et al. (1968) publication, phytase has been relied upon heavily to increase P availability and performance while reducing P excretion. To achieve success in poultry production, there must be a reduction of cost and waste output while increasing animal performance. Phosphorus excretion plays a vital role in poultry production because environmental pollution and waste of nutrients raise production costs (Martins et al., 2013). Still, it also reduces feed cost because P is one of the most important expensive nutrients in poultry diets. Assuena et al. (2009) reported that the highest phytase inclusion level resulted in the lowest P excreted. It was also stated that any phytase inclusion level over 250 FTU compromised live broiler performance. The results presented contradict other studies (Lan et al., 2002).
In the previously mentioned literature, phytase nutritional matrix results were not considered in the formulation of experimental diets, which Assuena et al. (2009) attribute the difference in results. Because of phytase, researchers and nutritionists have made phosphorus available to nonruminants in a once unavailable way. Carbohydrases are fiber and starch degrading enzymes. In poultry, carbohydrates are known as non-starch polysaccharides (NSP) degrading enzymes because they degrade the NSP in feed ingredients. Because of the degradation, xylanase and β-glucanase are common carbohydrates supplemented to the diet to improve performance and digestibility. Xylanase is an enzyme that originates from the hemicellulosic polysaccharide, xylan. Xylan, a polysaccharide, comprises units of D-xylose, a pentose sugar. Xylans make up 30 to 35% of the cell wall material of annual plants; therefore, it is considered an integral part of animal feed. Xylanase has been used as a feed additive for over 20 yr in poultry diets, primarily to improve feed conversion ratio and weight gain (Bedford and Partridge, 2001).
According to a study done by Conte et al. (2003), xylanase inclusion improved broilers’ feed conversion ratio, but there was no influence on other performance parameters measured. Soluble NSPs cause highly viscous digesta and poor litter quality (Ward, 1996). The jejunal and ileal digesta viscosity could be affected by age and adaptation to the experimental diets (Petersen et al., 1999). Arabinoxylans and β-glucans are the essential antinutritive NSP in cereal grains. While arabinoxylans are more prevalent in wheat and rye diets and β-glucans in barley, β-glucans are more susceptible to bacterial degradation through the intestinal tract (Knudsen, 2014; Gonzalez-Ortiz et al., 2017). Because of the issues related to NSPs, xylanase supplementation has been added to diets to decrease litter issues, such as wet litter, and increase nutrient uptake.
Wu and Ravindran (2004) completed a study and reported that supplemented xylanase significantly lowered feed efficiency in whole wheat and ground wheat-based diets. Also, feed intake was significantly reduced (P < 0.001) in whole wheat-based diets with supplemented xylanase compared to the control and ground wheat-based diets with supplemented xylanase. Cowieson et al. (2010) reported an improvement in DE content (3.2%) when broiler corn-SBM-based diets were supplemented with xylanase. Additionally, Aftab (2012) reported a ME improvement, from 2.2 to 5.3%, in broiler corn-SBM diets supplemented with xylanase. While this study by Cowieson et al. (2010) showed a positive effect of xylanase, this enzyme does not always prove to have a positive effect on digestibility, growth, or performance. Ideally, xylanase is used to break down NSPs and reduce the viscosity of digesta to improve pre-cecal nutrient digestibility (Bedford, 2000).
However, nutrient digestibility improvement does not always explain the effects of enzyme supplementation on performance. β-glucanase is another common carbohydrase supplement in poultry diets. High levels of β-glucans have been reported to reduce the nutritional value of barley in non-ruminants by increasing the viscosity of the intestinal fluid (Burnett, 1966; Adeola and Cowieson, 2011).
White et al. (1983) states that the increase in viscosity likely interferes with the digestive process by blocking the enzyme-substrate association. Increased viscosity also reduces the rate at which the released nutrients approach the mucosal surface for absorption. When poultry diets are supplemented with β-glucanase, the nutritional value of barley can increase significantly. Campbell et al. (1989) fed broiler chickens barley-based diets with or without supplemented β-glucanase and the results show that chickens fed the diet with supplemented β-glucanase were 35% heavier and 15% more efficient in feed utilization.
When xylanase and glucanase are combined in poultry diets, it has also been reported to improve digestibility and performance. Mathlouthi (2002) conducted a study to determine if supplementation of xylanase and glucanase improved conjugated bile acid fraction in intestinal contents in broilers fed corn or rye-based diet. Adding these exogenous enzymes to the rye-based diet improved weight gain, feed intake, feed efficiency, and decreased water intake. The digestibility of nutrients and AME were also significantly increased.
CONCLUSIONS
The natural antibiotics growth promoter alternatives are recommended to use on a large scale as safe and healthy alternatives that have a positive immune-modulating effect, enhance productivity, enhance digestion, improve nutrients availability, increase absorbability, improve intestinal health, reduce the incidence of antibiotic resistance, and help obtain nutritious, useful, and safe organic chicken meat for human consumption.
Acknowledgments
ACKNOWLEDGMENTS
The authors extend their appreciation to the deanship of Scientific Research at King Khalid University, Abha KSA, for supporting this work under grant number (R.G.P.2/61/42).
Author contributions: All authors were equally contributed to writing this review article. All authors reviewed and approved the final version of the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
References
- Abd El Hamid M.I., Abd El-Moaty D.A.M., El-Sergany E.F., Salem H.M., El-Sawy H., Abbas A.M. Utility of molecular biology tools for identification and characterization of Egyptian Riemerella anatipestifer duck isolates. Int. J. Vet. Sci. 2019;8:335–341. [Google Scholar]
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 2021;77:1001–1025. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Ghanima M.M.A.…El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Nahed A., Saad A.M., Salem H.M.…El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives–a comprehensive review. Poult. Sci. 2021 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Gado A.R., Nader M.M., Saad A.M., El-Tahan A.M., Taha A.E., Salem H.M., El-Tarabily K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: an updated overview. Poult. Sci. 2021 doi: 10.1016/j.psj.2021.101684. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S.…Abdel-Moneim A.M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al- Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdelrazek H.M., Abuzead S.M., Ali S.A., El-Genaidy H.M., Abdel-Hafez S.A. Effect of citric and acetic acid water acidification on broiler's performance with respect to thyroid hormones levels. Adv. Anim. Vet. Sci. 2016;4:271–278. [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.063. Accessed November 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Adams C. Nottingham University Press; Nottingham, UK: 1999. Nutricines: Food Components in Health and Nutrition. [Google Scholar]
- Adeola O., Cowieson A.J. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Adil S., Banday M.T., Bhat G.A., Mir M.S. Alternative strategies to antibiotic growth promoters–a review. Vet. Med. J. 2011;6:76. [Google Scholar]
- Aftab U. Exogenous carbohydrase in corn-soy diets for broilers. Worlds Poult. Sci. J. 2012;68:447–463. [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcicek A.H.M.E.T., Bozkurt M., Çabuk M. The effect of a mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S. Afr. J. Anim. Sci. 2004;34:217–222. [Google Scholar]
- Al-Fatah M.A. Probiotic modes of action and its effect on biochemical parameters and growth performance in poultry. Iran. J. Appl. Anim. Sci. 2020;10:9–15. [Google Scholar]
- Ali N., Abdelaziz M. Effect of feed restriction with supplementation of probiotic with enzymes preparation on performance, carcass characteristics and economic traits of broiler chickens during finisher period. Egypt. J. Nutr. Feed. 2018;21:243–254. [Google Scholar]
- Amad A.A., Männer K., Wendler K.R., Neumann K., Zentek J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011;90:2811–2816. doi: 10.3382/ps.2011-01515. [DOI] [PubMed] [Google Scholar]
- Angel R., Tamim N.M., Applegate T.J., Dhandu A.S., Ellestad L.E. Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 2002;11:471–480. [Google Scholar]
- Ansari J., Khan S.H., ul Haq A., Yousaf M. Effect of the levels of Azadirachta indica dried leaf meal as phytogenic feed additive on the growth performance and haemato-biochemical parameters in broiler chickens. J. Appl. Anim. Res. 2011;40:336–345. [Google Scholar]
- Apata D.F. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture of Lactobacillus bulgaricus. J. Sci. Food Agric. 2008;88:1253–1258. [Google Scholar]
- Applegate T.J., Klose V., Steiner T., Ganner A., Schatzmayr G. Probiotics and phytogenics for poultry: myth or reality? J. Appl. Poult. Res. 2010;19:194–210. [Google Scholar]
- Araujo R.G.A.C., Polycarpo G.V., Barbieri A., Silva K.M., Ventura G.a, Polycarpo V.C.C. Performance and economic viability of broiler chickens fed with probiotic and organic acids in an attempt to replace growth-promoting antibiotics. Braz. J. Poult. Sci. 2019;21:02. [Google Scholar]
- Arif M., Baty R.S., Althubaiti E.H., Ijaz M.T., Fayyaz M., Shafi M.E., Albaqami N.M., Alagawany M., Abd El-Hack M.E., Taha A.E., Salem H.M., El-Tahan A.M., Elnesr S.S. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.002. Accessed November 11, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.M.E., El-Saadony M.T., Osman A.O., Abou Sayed-Ahmed E.T., Albaqami N.M., Shafi M.E., Taha A.E., Abd El-Hack M.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Assuena V., Junqueira O.M., Duarte K.F., Laurentiz A.C., Filardi R.S., Sgavioli S. Effect of dietary phytase supplementation on the performance, bone densitometry, and phosphorus and nitrogen excretion of broilers. Rev. Bras. Cienc. Avic. 2009;11:25–30. [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int. J. Trop. Insect Sci. 2022;42:733–740. [Google Scholar]
- Attia M.M., Soliman S.M., Salaeh N.M.K., Salem H.M., Alkafafy M.Mohamed, Saad A.M., El-Saadony M.T., El-Gameel S.M. Evaluation of immune responses and oxidative stress in donkeys: immunological studies provoked by Parascaris equorum infection. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Böhm J., Razzazi-Fazeli E., Ghareeb K., Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult. Sci. 2006;85:974–979. doi: 10.1093/ps/85.6.974. [DOI] [PubMed] [Google Scholar]
- Ballam G.C., Nelson T.S., Kirby L.K. Effect of fiber and phytate source and of calcium and phosphorus level on phytate hydrolysis in the chick. Poult. Sci. 1984;63:333–338. doi: 10.3382/ps.0630333. [DOI] [PubMed] [Google Scholar]
- Banupriya S., Kathirvelan C., Joshua P.Patric. Significance of feed acidification in poultry feed. Int. J. Sci. Environ. Technol. 2016;5:1596–1599. [Google Scholar]
- AgMRC., (Agricultural Marketing Resource Center). 2013. Organic poultry profile. Accessed Mar. 2015. http://www.agmrc.org/commodities__products/livestock/poultry/organic-poultry-profile-625/
- Barletta, A. 2010. Enzymes in farm animal nutrition, M. R. Bedford and G. G. Partridge (eds.) Pages 1–11 in Enzymes in Farm Animal Nutrition, 2nd ed. CABI Publishing, Oxfordshire, UK.
- Baskaran S.A., Upadhyay A., Kollanoor-Johny A., Upadhyaya I., Mooyottu S., Amalaradjou M.A.R., Schreiber D., Venkitanarayanan K. Efficacy of plant-derived antimicrobial wash treatments for reducing enterohaemorrhagic Escherichia coli O157: H7 on apples. J. Food Sci. 2013;78:1399–1404. doi: 10.1111/1750-3841.12174. [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Letellier A., Zhao X., Ruiz-Feria C.A. Cecal populations of lactobacilli and bifidobacteria and Escherichia coli populations after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poult. Sci. 2007;86:2509–2516. doi: 10.3382/ps.2007-00136. [DOI] [PubMed] [Google Scholar]
- Bedford M.R. Exogenous enzymes in monogastric nutrition – their current value and future benefits. Anim. Feed Sci.Tech. 2000;86:1–13. [Google Scholar]
- Bilal T., Atis S., Keser O. The effects of microbial phytase on serum calcium and phosphorus levels and alkaline phosphatase activities in broilers fed diets containing different levels of phosphorus. Acta Sci. Vet. 2015;43:1327. [Google Scholar]
- Bohn L., Meyer A.S., Rasmussen S.K. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B. 2008;9:165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolukbasi S.C., Erhan M.K. Effect of dietary thyme (thyme vulgaris) on laying hens’ performance and Escherichia coli (E. coli) concentration in feces. Int. J. Nat. Engin. Sci. 2007;1:55–58. [Google Scholar]
- Bortoluzzi C., Vieira B.S., de Paula Dorigam J.C., Menconi A., Sokale A., Doranalli K., Applegate T.J. Bacillus subtilis DSM 32315 supplementation attenuates the effects of Clostridium perfringens challenge on the growth performance and intestinal microbiota of broiler chickens. Microorganisms. 2019;7:71. doi: 10.3390/microorganisms7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsoglou N.A., Christaki E., Fletouris D.J., Florou-Paneri P., Spais A.B. Effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002;62:259–265. doi: 10.1016/s0309-1740(01)00256-x. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed Sci. Technol. 2010;158:1–14. [Google Scholar]
- Brisbin J.T., Gong J., Parvizi P., Sharif S. Effects of Lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010;17:1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett G.S. Studies of viscosity as the probable factor involved in the improvement of certain barleys for chickens by enzyme supplementation. Br. Poult. Sci. 1966;7:55–75. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods – a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Campbell G.L., Rossnagel B.G., Classen H.L., Thacker P.A. Genotypic and environmental differences in extract viscosity of barley and their relationship to its nutritive-value for broiler-chickens. Anim. Feed Sci. Tech. 1989;26:221–230. [Google Scholar]
- Chapman H.D., Cherry T.E., Danforth H.D., Richards G., Shirley M.W., Williams R.B. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int. J. Parasitol. 2002;32:617–629. doi: 10.1016/s0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Chaveerach P., Keuzenkamp D.A., Urlings H.A., Lipman L.J., Van Knapen F. In vitro study on the effect of organic acids on Campylobacter jejuni/coli populations in mixtures of water and feed. Poult. Sci. 2001;81:621–628. doi: 10.1093/ps/81.5.621. [DOI] [PubMed] [Google Scholar]
- Chervonova I. Influence of probiotic and prebiotic on meat quality of broiler chicken carcasses. BIO Web Conf. 2021;32:04009. [Google Scholar]
- Choct M. Managing gut health through nutrition. Br. Poult. Sci. 2009;50:9–15. doi: 10.1080/00071660802538632. [DOI] [PubMed] [Google Scholar]
- Chorianopoulos, N., E. Kalpoutzakis, N. Aligiannis, S. Mitaku, George-John Nychas, and S. A. Haroutounian. 2004. Essential oils of satureja, origanum, and thymus species: chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 52: 8261-8267. [DOI] [PubMed]
- Conte A.J., Teixeira A.S., Fialho E.T., Schoulten N.A., Bertechini A.G. Effect of phytase and xylanase on the performance and bone characteristics of broiler chicks fed diets with rice bran. Braz. J. Anim. Sci. 2003;32:1147–1156. [Google Scholar]
- Cowieson A.J., Bedford M.R., Ravindran V. Interactions between xylanase and glucanase in maize-soy-based diets for broilers. Bri. Poult. Sci. 2010;51:246–257. doi: 10.1080/00071661003789347. [DOI] [PubMed] [Google Scholar]
- Cox C.M., Dalloul R.A. Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef. Microbes. 2015;6:45–52. doi: 10.3920/BM2014.0062. [DOI] [PubMed] [Google Scholar]
- Crandall P.G., Seideman S., Ricke S.C., O'bryan C.A., Fanatico A.F., Rainey R. Organic poultry: consumer perceptions, opportunities and regulatory issues. J. Appl. Poult. Res. 2009;18:795–802. [Google Scholar]
- Cross D.E., McDevitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, digestibilities and gut microflora in chickens 7 to 28 d of age. Br. Poult. Sci. 2007;4:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Cui S., Ge B., Zheng J., Meng J. Prevalence and antimicrobial resistance of Campylobacter spp. and Salmonella serovars in organic chickens from Maryland retail stores. Appl. Environ. Microbiol. 2005;71:4108–4111. doi: 10.1128/AEM.71.7.4108-4111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul R.A. Burleigh Dodds Science Publishing; Sawston, Cambridge, UK: 2017. Chapter 6: Achieving sustainable production of poultry meat, volume 3: health and welfare [Ed. T.J. Applegate] Understanding and Enhancing Immune Systems in Poultry. [Google Scholar]
- Dalloul R.A., Lillehoj H.S., Shellem T.A., Doerr J.A. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult. Sci. 2003;82:62–66. doi: 10.1093/ps/82.1.62. [DOI] [PubMed] [Google Scholar]
- Dalloul R.A., Ritzi M.M. Probiotics: a modern solution to coccidiosis and necrotic enteritis. Pages 117–123 in Proceedings of the 2016 World Nutrition Forum; Erber Ag, Austria; 2016. [Google Scholar]
- Dalloul Rami A., Lillehoj Hyun S., Tamim Nada M., Shellem Timothy A., Doerr John A. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based product. Comp. Immunol. Microbiol. Infect. Dis. 2005;28:351–361. doi: 10.1016/j.cimid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Darre M.J., Kollanoor-Johny A., Venkitanarayanan K., Upadhyaya I. Practical implications of plant-derived antimicrobials in poultry diets for the control of Salmonella enteritidis. J. Appl. Poult. Res. 2014;23:1–5. [Google Scholar]
- Dersjant-Li Y., Awati A., Schulze H., Partridge G. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015;95:878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J.J., Buttin P. Use of organic acid as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002;11:453–463. [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Duskaev G., Rakhmatullin S., Kvan O. Effects of Bacillus cereus and coumarin on growth performance, blood biochemical parameters, and meat quality in broilers. Vet. World. 2020;13:2484–2492. doi: 10.14202/vetworld.2020.2484-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards H.M., Veltmann J.R. The role of calcium and phosphorus in the etiology of tibial dyschondroplasia in young chicks. J. Nut. 1983;113:1568–1575. doi: 10.1093/jn/113.8.1568. [DOI] [PubMed] [Google Scholar]
- El Jeni R., Dittoe D.K., Olson E.G., Lourenco J., Corcionivoschi N., Ricke S.C., Callaway T.R. Probiotics and potential applications for alternative poultry production systems. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Tazi S.M.A., Mukhtar M.A., Mohamed K.A., Tabidi M.H. Effect of using black pepper as natural feed additive on performance and carcass quality of broiler chicks. Global Adv. Res. J. Agri. Sci. 2014;4:108–113. [Google Scholar]
- Elkhouly M., Khairy M., Abd-El Alim A., Ali A. Effect of phytobiotics, probiotics and toltrazuril on chicken coccidiosis. Zagazig Vet. J. 2016;44:214–223. [Google Scholar]
- El-Naggar M.S., Ibrahim H.M., Salem H.M., Marouf S. A novel locally prepared inactivated bivalent mycoplasma vaccine for chicken flocks in Egypt. Adv. Anim. Vet. Sci. 2022;10:55–61. [Google Scholar]
- El-Saadony M.T., Saad A.M., Elakkad H.A., El-Tahan A.M., Alshahrani O.A., Alshilawi M.S., El-Sayed H., Amin S.A., Ahmed A.I. Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4°C through controlling chemical and microbial fluctuations. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.08.092. Accessed November 11, 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28:4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A., Dhama K., Abdel-Latif H.M.R. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69 [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021;37:1–23. [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28:4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Nagar, A., A. Ibrahim. 2021. Case study of the Egyptian poultry sector. Poultry in the 21st Century. Accessed Nov. 2021. https://www.fao.org/AG/AGAInfo/home/events/bangkok2007/docs/part1/1_6.pdf. Accessed November 30, 2021
- Fanatico A. 2008. Organic poultry production in the United States. National Sustainable Agriculture Information Service. Accessed Nov. 2021. http://sd.appstate.edu/sites/sd.appstate.edu/files/organicpoultry.pdf.
- Farnell M.B., Donoghue A.M., Solis De Los Santos F., Blore P.J., Hargis B.M., Tellez G., Donoghue D.J. Upregulation of oxidative burst and degranulation in chicken heterophils stimulated with probiotic bacteria. Poult. Sci. 2006;85:1900–1906. doi: 10.1093/ps/85.11.1900. [DOI] [PubMed] [Google Scholar]
- Ferdous M.F., Arefin M.S., Rahman M.M., Ripon M.M.R., Rashid M.H., Sultana M.R.…Rafiq K. Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. Adv. Vet. Anim. Res. 2019;6:409–415. doi: 10.5455/javar.2019.f361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankič T., Voljč M., Salobir J., Rezar V. Use of herbs and spices and their extracts in animal nutrition. Acta Agric. Slov. 2009;92:95–102. [Google Scholar]
- Getachew T. A review on the effects of probiotic supplementation in poultry performance and cholesterol levels of egg and meat. J. World Poult. Res. 2016;6:31–36. [Google Scholar]
- Ghareeb K., Awad W.A., Mohnl M., Porta R., Biarnes M., Böhm J., Schatzmayr G. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 2012;91:1825–1832. doi: 10.3382/ps.2012-02168. [DOI] [PubMed] [Google Scholar]
- Gheisar M.M., Im Y.W., Lee H.H., Choi Y.I., Kim I.H. Inclusion of phytogenic blends in different nutrient density diets of meat-type ducks. Poult. Sci. 2015;94:2952–2958. doi: 10.3382/ps/pev301. [DOI] [PubMed] [Google Scholar]
- Giannenas, Ilias, P. Florou-Paneri, M. Papazahariadou, E. Christaki, N. A. Botsoglou, A. B. Spais. 2003. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella.Arch. Anim. Nutr. 57: 99-106. [DOI] [PubMed]
- Gonzalez-Ortiz G., Sola-Oriol D., Martinez-Mora M., Perez J.F., Bedford M.R. Response of broiler chickens fed wheat-based diets to xylanase supplementation. Poult. Sci. 2017;96:2776–2785. doi: 10.3382/ps/pex092. [DOI] [PubMed] [Google Scholar]
- Grashorn M.A. Use of phytobiotics in broiler nutrition—an alternative to infeed antibiotics. J. Anim. Feed Sci. 2010;19:338–347. [Google Scholar]
- Greene, C. 2013. Growth patterns in the U.S. organic industry. USDA Economic Research Service. Accessed Mar. 2015. http://www.ers.usda.gov/amberwaves/2013-october/growth-patterns-in-the-us-organic-industry.aspx#.VPdRlvnF98E
- Hafeez A., Männer K., Schieder C., Zentek J. Effect of supplementation of phytogenic feed additives (powdered vs. encapsulated) on performance and nutrient digestibility in broiler chickens. Poult. Sci. 2016;95:622–629. doi: 10.3382/ps/pev368. [DOI] [PubMed] [Google Scholar]
- Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Zhou H., Sanei B.…Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajati H. Application of organic acids in poultry nutrition. Int. J. Avian Wildl. Biol. 2018;3:324–329. [Google Scholar]
- Hargis, B., G. Tellez, J. D. Latorre, & R. Wolfenden. 2021. Compositions, probiotic formulations and methods to promote digestion and improve nutrition in poultry. U.S. Patent No. 10,959,447. Washington, DC: U.S. Patent and Trademark Office.
- Harmon B.G. Avian heterophils in inflammation and disease resistance. Poult. Sci. 1998;77:972–977. doi: 10.1093/ps/77.7.972. [DOI] [PubMed] [Google Scholar]
- Hashemi S.R., Davoodi H. Phytogenics as new class of feed additive in poultry industry. J. Anim. Vet. Adv. 2010;9:2295–2304. [Google Scholar]
- Hashemi S.R., Davoodi H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011;35:169–180. doi: 10.1007/s11259-010-9458-2. [DOI] [PubMed] [Google Scholar]
- Hegazy M.I., Hegazy A.M., Saad A.M., Salem H.M., El-Tahan A.M., El-Saadony M.T., Soliman S.M., Taha A.E., Alshehri M.A., Ahmed A.E., Swelum A.A. Biologically active some microorganisms have the potential to suppress mosquito larvae (Culex pipiens, Diptera: Culicidae) Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F., Madrid J., Garcia V., Orengo J., Megias M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Hidayat M.N., Kiramang K., Gunawan F. Total of Escherichia coli excreta broiler given Enterococcus sp. as probiotics candidate of poultry. Chalaza J. Anim. Husb. 2019;4:6–12. [Google Scholar]
- Higgins J.P., Higgins S.E., Vicente J.L., Wolfenden A.D., Tellez G., Hargis B. Temporal effects of lactic acid bacteria probiotic culture on Salmonella in neonatal broilers. Poult. Sci. 2007;86:1662–1666. doi: 10.1093/ps/86.8.1662. [DOI] [PubMed] [Google Scholar]
- Higgins S.E., Erf G.F., Higgins J.P., Henderson S.N., Wolfenden A.D., Gaona-Ramirez G., Hargis B.M. Effect of probiotic treatment in broiler chicks on intestinal macrophage numbers and phagocytosis of Salmonella Enteriditis by abdominal exudate cells. Poult. Sci. 2007;86:2315–2321. doi: 10.3382/ps.2007-00123. [DOI] [PubMed] [Google Scholar]
- Hoffman-Pennesi D., Wu C. The effect of thymol and thyme oil feed supplementation on growth performance, serum antioxidant levels, and cecal Salmonella population in broilers. J. Appl. Poult. Res. 2010;19:432–443. [Google Scholar]
- Hume M.E. Historic perspective: prebiotics, probiotics, and other alternatives to antibiotics. Poult. Sci. 2011;90:2663–2669. doi: 10.3382/ps.2010-01030. [DOI] [PubMed] [Google Scholar]
- Iqbal T.H., Lewis K.O., Cooper B.T. Phytase activity in the human and rat small intestine. Gut. 1994;35:1233–1236. doi: 10.1136/gut.35.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhao G.M., Sawai D.H., Rewatkar H.N., Kolhe R.P., Bansod A.P., Nandeshwar J.D. Effect of organic acids with probiotic supplementation on immunity and blood biochemical status of broiler chicken. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:1952–1959. [Google Scholar]
- Jamroz D., Wiliczkiewicz A., Wertelecki T., Orda J., Skorupińska J. Use of active substances of plant origin in chicken diets based on maize and domestic grains. Br. Poult. Sci. 2005;46:485–493. doi: 10.1080/00071660500191056. [DOI] [PubMed] [Google Scholar]
- Jamroz D., Wertelecki T., Houszka M., Kamel C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Ani. Physiol. Ani. Nutr. 2006;90:255–268. doi: 10.1111/j.1439-0396.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- Jiang T., Li H.S., Han G.G., Singh B., Kang S.K., Bok J.D.…Cho C.S. Oral delivery of probiotics in poultry using pH-sensitive tablets. J. Microbiol. Biotechnol. 2017;27:739–746. doi: 10.4014/jmb.1606.06071. [DOI] [PubMed] [Google Scholar]
- Kabir S.M.L. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi Torshizi M.A., Moghaddam A.R., Rahimi S.H., Mojgani N. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 2010;51:178–184. doi: 10.1080/00071661003753756. [DOI] [PubMed] [Google Scholar]
- Khan R.U., Naz S. The applications of probiotics in poultry production. Worlds Poult. Sci. J. 2013;69:621–632. [Google Scholar]
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016;44:359–369. [Google Scholar]
- Khattak F., Ronchi A., Castelli P., Sparks N. Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult. Sci. 2014;93:132–137. doi: 10.3382/ps.2013-03387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelfa D.E., Abd El-Ghany W.A., Salem H.M. Serological and molecular typing of Clostriduim Perfringens and Its toxins recovered from weaned rabbit's flocks in Egypt. Life Sci. J. 2012;9:2263–2271. [Google Scholar]
- Khelfa D.E., Abd El-Ghany W.A., Salem H.M. The effect of Clostridium difficile experimental infection on the health status of weaned rabbits. J. Appl. Sci. Res. 2012;8:4672–4677. [Google Scholar]
- Khelfa D.E., Abd El-Ghany W.A., Salem H.M. Recent status of clostridial enteritis affecting early weaned rabbits in Egypt. Life Sci. J. 2012;9:2272–2279. [Google Scholar]
- Kies A.K., De Jonge L.H., Kemme P.A., Jongbloed A.W. Interaction between protein, phytate, and microbial phytase. In vitro studies. J. Agric. Food Chem. 2006;54:1753–1758. doi: 10.1021/jf0518554. [DOI] [PubMed] [Google Scholar]
- Knudsen K.E.B. Fiber and non-starch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014;93:2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Koutsos E.A., Arias V.J. Intestinal ecology: interactions among the gastrointestinal tract, nutrition and the microflora. J. Appl. Poult. Res. 2006;15:161–173. [Google Scholar]
- Krysiak, K., D. Konkol, and M. Korczyński. 2021. Overview of the use of probiotics in poultry production. Animals, 11, 1620. [DOI] [PMC free article] [PubMed]
- Kulshreshtha G., Rathgeber B., Stratton G., Thomas N., Evans F., Critchley A.…Prithiviraj B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. 2014;93:2991–3001. doi: 10.3382/ps.2014-04200. [DOI] [PubMed] [Google Scholar]
- Lambert, R. J. W., P. N. Skandamis, P. J. Coote, G-JE Nychas. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91:453-462. [DOI] [PubMed]
- Lan G.Q., Abdullah N., Jalaludin S., Ho Y.W. Efficacy of supplementation of a phytase-producing bacterial culture on the performance and nutrient use of broiler chickens fed corn-soybean meal diets. Poult. Sci. 2002;81:1522–1532. doi: 10.1093/ps/81.10.1522. [DOI] [PubMed] [Google Scholar]
- Lan Y., Verstegen M.W.A., Tamminga S., Williams B.A. The role of the commensal gut microbial community in broiler chicken. Worlds Poult. Sci. J. 2005;61:95–104. [Google Scholar]
- Langout P. New additives for broiler chickens. Feed mix special: alternatives to antibiotics. Br. J. Nut. 2000;9:24–27. [Google Scholar]
- Lee K.W., Everts H., Kappert H.J., Wouterse H., Frehner M., Beynen A.C. Cinnamaldeheyde but not thymol, counteracts the carboxymethyl cellulose induced growth depression in female broiler chickens. Int. J. Poult. Sci. 2004;3:608–612. [Google Scholar]
- Lee K.W., Everts H., Kappert H.J., Van Der Kuilen J., Lemmens A.G., Frehner M., Beynen A.C. Growth performance, intestinal viscosity, fat digestibility and plasma cholesterol in broiler chickens fed a rye-containing diet without or with essential oil components. Int. J. Poult. Sci. 2004;3:613–618. [Google Scholar]
- Lee K.W., Lee S.H., Lillehoj H.S., Li G.X., Jang S.I., Babu U.S., Park M.S., Kim D.K., Lillehoj E.P., Neumann A.P., Rehberger T.G. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010;89:203–216. doi: 10.3382/ps.2009-00418. [DOI] [PubMed] [Google Scholar]
- Lee K-W., Everts H., Kappert H.J., Frehner M., Losa R., Beynen A.C. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Dalloul R.A., Park D.W., Hong Y.H., Lin J.J.L. Influence of Pediococcus -based probiotic on coccidiosis in broiler chickens. Poult. Sci. 2007;86:63–66. doi: 10.1093/ps/86.1.63. [DOI] [PubMed] [Google Scholar]
- Lee S., Lillehoj H.S., Park D.W., Hong Y.H., Lin J.J. Effects of Pediococcus – and Saccharomyces -based probiotic (MitoMax) on coccidiosis in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 2007;30:261–268. doi: 10.1016/j.cimid.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Li X.Y. Immunomodulating components from Chinese medicines. Pharm. Biol. 2000;38:33–40. doi: 10.1076/phbi.38.6.33.5961. [DOI] [PubMed] [Google Scholar]
- Llamas-Moya S., Girdler C.P., Shalash S.M.M., Atta A.M., Gharib H.B., Morsy E.A., Salim H., Awaad M.H.H., Elmenawey M. Effect of a multi-carbohydrase containing a-galactosidase enzyme on the performance, carcass yield, and humoral immunity of broilers fed corn–soybean meal–based diets of varying energy density. J. Appl. Poult. Res. 2019;29:142–151. [Google Scholar]
- Londero A., León Peláez M.A., Diosma G., De Antoni G.L., Abraham A.G., Garrote G.L. Fermented whey as poultry feed additive to prevent fungal contamination. J. Sci. Food Agric. 2014;94:3189–3194. doi: 10.1002/jsfa.6669. [DOI] [PubMed] [Google Scholar]
- Marković R. Effect of different growth promoters on broiler performance and gut morphology. Arch. Med. Vet. 2009;41:163–169. [Google Scholar]
- Marouf S., Moussa I.M., Salem H., Sedeik M., Elbestawy A., Hemeg H.A., Dawoud T.M., Mubarakb A.S., Mahmouda H., Alsubki R.A., Bahkali A.H. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: Phenotypic and genotypic characterization. J. King Saud Univ. Sci. 2020;32:2263–2268. [Google Scholar]
- Marouf S., Khalf M.A., Alorabi M., El-Shehawi A.M., El-Tahan A.M., Abd El-Hack M.E., El-Saadony M.T., Salem H.M. Mycoplasma gallisepticum: a devastating organism for the poultry industry in Egypt. Poult. Sci. 2021;101 doi: 10.1016/j.psj.2021.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins B.A.B., Borgatti L.M.D.O., Souza L.W.D.O., Robassini S.L.D.A., Albuquerque R.D. Bioavailability and poultry fecal excretion of phosphorus from soybean-based diets supplemented with phytase. Rev. Bras. Zootec. 2013;42:174–182. [Google Scholar]
- Mathlouthi N. Xylanase and beta-glucanase supplementation improve conjugated bile acid fraction in intestinal contents and increase villus size of small intestine wall in broiler chickens fed a rye-based diet. J. Anim. Sci. 2002;80:2773–2779. doi: 10.2527/2002.80112773x. [DOI] [PubMed] [Google Scholar]
- Mazanko M.S., Gorlov I.F., Prazdnova E.V., Makarenko M.S., Usatov A.V., Bren A.B., Chistyakov V.A., Tutelyan A.V., Komarova Z.B., Mosolova N.I., Pilipenko D.N. Bacillus probiotic supplementations improve laying performance, egg quality, hatching of laying hens, and sperm quality of roosters. Probiotics Antimicrob. Proteins. 2018;10:367–373. doi: 10.1007/s12602-017-9369-4. [DOI] [PubMed] [Google Scholar]
- McReynolds J., Waneck C., Byrd J., Genovese K., Duke S., Nisbet D. Efficacy of multistrain direct-fed microbial and phytogenic products in reducing necrotic enteritis in commercial broilers. Poult. Sci. 2009;88:2075–2080. doi: 10.3382/ps.2009-00106. [DOI] [PubMed] [Google Scholar]
- Michel M.A., Revidatti F.A., Fernández R.J., Sindik M.L., Sanz P., Hernandez-Velasco X.…Tellez-Isaias G. Combination of a Lactobacillus-based probiotic and organic acids decrease egg to chick weight loss and reduce Salmonella spp. counts in the litter of commercial broiler breeders. Food Nutr. Sci. 2019;10:1011–1020. [Google Scholar]
- Miles R.D., Butcher G.D., Henry P.R., Littell R.C. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 2006;85:476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- Mogelonsky, M. 2008. Organic food and drink – April 2008. Accessed Dec. 2021. http://www.preparedfoods.com/articles/106350-article-organic-food-and-drink-april-2008.
- Montonya, A., S. Young, C. Hofacre, H. Cholick, M. Quiroz, J. Dibner, R. Berghaus, S. Thayer. 2011. Use of an organic acid (Activate® wd) as field intervention to reduce Salmonella spp. in broiler chickens. Salmonella control in Poultry. M. Anaya, ed. Accessed Feb. 2022. https://en.engormix.com/poultry-industry/articles/salmonella-control-in-poultry-t34884.htm.
- Mooyottu S., Kollanoor-Johny A., Flock G., Bouillaut L., Upadhyay A., Sonenshein A.L., Venkitanarayanan K. Carvacrol and trans-cinnamaldehyde reduce Clostridium difficile toxin production and cytotoxicity in vitro. Int. J. Mol. Sci. 2014;15:4415–4430. doi: 10.3390/ijms15034415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy E.A., Salem H.M., Khattab M.S., Hamza D.A., Abuowarda M.M. Encephalitozoon cuniculi infection in farmed rabbits in Egypt. Acta Vet. Scand. 2020;62:11. doi: 10.1186/s13028-020-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountzouris K.C., Balaskas C., Xanthakos I., Tzivinikou A., Fegeros K. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella Enteriditis. Br. Poult. Sci. 2009;50:467–478. doi: 10.1080/00071660903110935. [DOI] [PubMed] [Google Scholar]
- Mountzouris, K. C., P. Tsirtsikos, E. Kalamara, Schatzmayr Nitsch, G. Schatzmayr, K. Fegeros. 2007. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 86:309-317. [DOI] [PubMed]
- Mountzouris K.C., Paraskevas V., Tsirtsikos P., Palamidi I., Steiner T., Schatzmayr G., Fegeros K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Ani. Feed Sci. Technol. 2011;168:223–231. [Google Scholar]
- Mountzouris K.C. Phytogenic and probiotic feed additives for broilers: evidence for growth performance links with gut performance indices. Pages 107-116 in Proceedings of the 2016 World Nutrition Forum. Pages 107-116 in Proceedings of the 2016 World Nutrition Forum; Austria; Erber Ag; 2016. [Google Scholar]
- Muanda F., Koné D., Dicko A., Soulimani R., Younos C. Phytochemical composition and antioxidant capacity of three malian medicinal plant parts. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1093/ecam/nep109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir K., Maqsood S. A review on role of exogenous enzyme supplementation in poultry production. Emir. J. Food Agric. 2013;25:66–80. [Google Scholar]
- Murate L.S., Paião F.G., de Almeida A.M., Berchieri Jr A., Shimokomaki M. Efficacy of prebiotics, probiotics, and synbiotics on laying hens and broilers challenged with Salmonella enteritidis. J. Poult. Sci. 2015;52:52–56. [Google Scholar]
- Naves L.de P., Corrêa A.D., Bertechini A.G., Gomide E.M., dos Santos C.D. Effect of pH and temperature on the activity of phytase products used in broiler nutrition. Rev. Bras. Ciência Avícola. 2012;14:181–185. [Google Scholar]
- Nelson T.S., Shieh T.R., Wodzinski R.J., Ware J.H. The availability of phytate phosphorus in soybean meal before and after treatment with a mold phytase. Poult. Sci. 1968;47:1842–1848. doi: 10.3382/ps.0471842. [DOI] [PubMed] [Google Scholar]
- Neupane D., Nepali D.B., Devkota N., Sharma M.P., Kadaria I.P. Effect of probiotics on production and egg quality of dual-purpose chicken at Kathmundu in Nepal. Bangladesh J. Anim. Sci. 2019;48:29–35. [Google Scholar]
- Noormohamed A., Fakhr M.K. Prevalence and antimicrobial susceptibility of Campylobacter spp. in Oklahoma conventional and organic retail poultry. Open Microbiol. J. 2014;8:130–137. doi: 10.2174/1874285801408010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noujaim J.C., Filho R.L.A., Lima E.T., Okamoto A.S., Amorim R.L., Torres Neto R. Detection of T lymphocytes in intestine of broiler chicks treated with Lactobacillus spp. and challenged with Salmonella enterica serovar Enteritidis. Poult. Sci. 2008;87:927–933. doi: 10.3382/ps.2007-00476. [DOI] [PubMed] [Google Scholar]
- Ogbuewu I.P., Okoro V.M., Mbajiorgu C.A. Meta-analysis of the influence of phytobiotic (pepper) supplementation in broiler chicken performance. Trop. Anim. Health Prod. 2020;52:17–30. doi: 10.1007/s11250-019-02118-3. [DOI] [PubMed] [Google Scholar]
- Ohimain E.I., Ofongo R.T.S. The effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora: a review. Int. J. Anim. Vet. Adv. 2012;4:135–143. [Google Scholar]
- Bedford M.R., Partridge G.G. CABI; Wallingford, UK: 2001. Enzymes in Farm Animal Nutrition. CABI. [Google Scholar]
- Paraskeuas V., Fegeros K., Hunger C., Theodorou G., Mountzouris K.C. Dietary inclusion level effects of a phytogenic characterized by menthol and anethole on broiler growth performance, biochemical parameters including total antioxidant capacity and gene expression. An. Prod. Sci. 2016;57:33–41. [Google Scholar]
- Paraskeuas V., Fegeros K., Palamidi I., Hunger C., Mountzouris K.C. Growth performance, nutrient digestibility, antioxidant capacity, blood biochemical biomarkers and cytokines expression in broiler chickens fed different phytogenic levels. An. Nutr. 2017;3:114–120. doi: 10.1016/j.aninu.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariza M.W., Cook M. Determining the safety of enzymes used in animal feed. Regul. Toxico. Pharmacol. 2010;56:332–342. doi: 10.1016/j.yrtph.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Penalver P., Huerta B., Borge C., Astorga R., Romero R., Perea A. Antimicrobial activity of five essential oil against animal origin strains of the Enterobacteriaceae family. Acta Path. Micro. Im. B. 2005;113:1–6. doi: 10.1111/j.1600-0463.2005.apm1130101.x. [DOI] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Benef. Microbes. 2016;7:699–705. doi: 10.3920/BM2016.0080. [DOI] [PubMed] [Google Scholar]
- Peralta-Sánchez J.M., Martín-Platero A.M., Ariza-Romero J.J., Rabelo-Ruiz M., Zurita-González M.J., Baños A.…Martínez-Bueno M. Egg production in poultry farming is improved by probiotic bacteria. Front. Microbiol. 2019;10:1042. doi: 10.3389/fmicb.2019.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.T., Wiseman J., Bedford M.R. Effects of age and diet on the viscosity of intestinal contents in broiler chicks. Br. Poult. Sci. 1999;40:364–370. doi: 10.1080/00071669987467. [DOI] [PubMed] [Google Scholar]
- Pettersson D., Aman P. Enzyme supplementation of a poultry diet containing rye and wheat. Br. J. Nut. 1989;62:139–149. doi: 10.1079/bjn19890014. [DOI] [PubMed] [Google Scholar]
- Ponnampalam E.N., Bekhit A.E.D., Bruce H., Scollan N.D., Muchenje V., Silva P., Jacobs J.L. In: Pages 17–44 in Sustainable Meat Production and Processing. Galanakis C.M., editor. Academic Press; London, UK: 2019. Chapter 2—Production strategies and processing systems of meat: current status and future outlook for innovation—a global perspective. Pages 17–44 in Sustainable Meat Production and Processing. [Google Scholar]
- Popova T. Effect of probiotics in poultry for improving meat quality. Curr. Opin. Food Sci. 2017;14:72–77. [Google Scholar]
- NASS. 2010. “National Agricultural Statistics Service”. Accessed Nov. 2021. http://www.agcensus.usda.gov/Publications/2007/Online_Highlights/Organics/organics_1_10.pdf.
- Prescott, John Francis, J. Desmond Baggot, and Robert D. Walker. 2000. Antimicrobial Therapy in Veterinary Medicine (3rd ed.). (J. F. Steeve Giguère, Ed.) Iowa, IA: Iowa State University Press.
- Price P.T., Gaydos T.A., Berghaus R.D., Baxter V., Hofacre C.L., Sims M.D. Salmonella enteritidis reduction in layer ceca with a Bacillus probiotic. Vet. World. 2020;13:184–187. doi: 10.14202/vetworld.2020.184-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvača N., Stanaćev V., Glamočić D., Lević J., Perić L., Milić D. Beneficial effects of phytoadditives in broiler nutrition. Worlds Poult. Sci. J. 2013;69:27–34. [Google Scholar]
- Rahimi S., Kathariou S., Grimes J.L., Siletzky R.M. Effect of direct-fed microbials on performance and Clostridium perfringens colonization of turkey poults. Poult. Sci. 2011;90:2556–2562. doi: 10.3382/ps.2011-01342. [DOI] [PubMed] [Google Scholar]
- Ravindran V. Feed enzymes: the science, practice, and metabolic realities. J. Appl. Poult. Res. 2013;22:628–636. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilization, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Vahjen W., Dadi T., Saliu E.-M, Boroojeni F.G., Zentek J. Synergistic effects of probiotics and phytobiotics on the intestinal microbiota in young broiler chicken. Microorganisms. 2019;7:684. doi: 10.3390/microorganisms7120684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revolledo L., Ferreira C.S.A., Ferreira A.J.P. Prevention of Salmonella Typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult. Sci. 2009;88:734–743. doi: 10.3382/ps.2008-00410. [DOI] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., Van-Heerden K., Mohnl M., Barrett N.W., Dalloul R.A. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet. Res. 2016;47:111. doi: 10.1186/s13567-016-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Rojas S.W., Scott J.L. Factors affecting the nutritive value of cottonseed meal as a protein source in chick diets. Poult. Sci. 1969;48:819–835. doi: 10.3382/ps.0480819. [DOI] [PubMed] [Google Scholar]
- Russell J.B., Diez-Gonzalez F. The effects of fermentation acids on bacterial growth. Adv. Microb. Physiol. 1998;39:205–234. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT-Food Sci. Technol. 2021;148 [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28:5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr M.J., Guyard-Nicodème M., Messaoudi S., Chemaly M., Cappelier J.M., Dousset X., Haddad N. Recent advances in screening of anti-Campylobacter activity in probiotics for use in poultry. Front. Microbiol. 2016;7:553. doi: 10.3389/fmicb.2016.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Khattab M.S., Yehia N., Abd El-Hack M.E., El-Saadony M.T., Alhimaidi A.R., Swelum A.A., Attia M.M. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021;41:2549–2554. [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Morsy E.A., Hassanen E.I., Shehata A.A. Outbreaks of myxomatosis in Egyptian domestic rabbit farms. World Rabbit Sci. 2019;27:85–91. [Google Scholar]
- Salem H.M., Yehia N., Al-Otaibi S., El-Shehawi A.M., Elrys A.A.M.E., El-Saadony M.T., Attia M.M. The prevalence and intensity of external parasites in domestic pigeons (Columba livia domestica) in Egypt with special reference to the role of deltamethrin as insecticidal agent. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim H.M.D., Shahidul Huque K., Kamaruddin K.M., Haque Beg A. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018;101:52–75. doi: 10.3184/003685018X15173975498947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanya M., Yamauchi K. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002;133:95–104. doi: 10.1016/s1095-6433(02)00121-6. [DOI] [PubMed] [Google Scholar]
- Sato K., Bartlett P.C., Kaneene J.B., Downes F.P. Comparison of prevalence and antimicrobial susceptibilities of Campylobacter spp. isolates from organic and conventional dairy herds in Wisconsin. Appl. Environ. Microbiol. 2004;70:1442–1447. doi: 10.1128/AEM.70.3.1442-1447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheideler S.E., Sell J.L. Utilization of phytate phosphorus in laying hens as influenced by dietary phosphorus and calcium. Nutr. Rep. Int. 1987;35:1073–1081. [Google Scholar]
- Selle P.H., Bedford M.R. 2nd ed. CABI Publishing; Oxfordshire, UK: 2010. Patridge. Enzymes in Farm Animal Nutrition; p. 169. [Google Scholar]
- Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Kim H.S., Ryu M.H., Kwon I.K., Chae B.J. Effect of supplementation of Bacillus subtilis LS 1–2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Abdel Satar Arafa A. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8:1–8. [Google Scholar]
- Sharma K.K., Kapoor M., Kuhad R.C. In vivo enzymatic digestion, in vitro xylanase digestion, metabolic analogues, surfactants and polyethylene glycol ameliorate laccase production from Ganoderma sp. Lett. Appl. Microbiol. 2005;41:24–31. doi: 10.1111/j.1472-765X.2005.01721.x. [DOI] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd-El-Hack M.E., Khafaga A.F., Metwally K.A.…El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppy C. Enzymes in Farm Animal Nutrition. CABI Publishing; Oxfordshire, UK: 2010. pp. 1–10. [Google Scholar]
- Si W., Gong J., Tsao R., Zhou T., Yu H., Poppe C., Johnson R., Du Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006;100:296–305. doi: 10.1111/j.1365-2672.2005.02789.x. [DOI] [PubMed] [Google Scholar]
- Soliman K.M., Badeaa R.I. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002;40:1669–1675. doi: 10.1016/s0278-6915(02)00120-5. [DOI] [PubMed] [Google Scholar]
- Soliman S.M., Attia M.M., Al-Harbi M.S., Saad A.M., El-Saadony M.T., Salem H.M. Low host specificity of Hippobosca equina infestation in different domestic animals and pigeon. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltan M.A., Shewita R.S., El-Katcha M.I. Effects of diary anise seeds supplementation on growth performance, immune response, carcass traits and some blood parameters of broiler chickens. Int. J. Poult. Sci. 2008;7:1078–1088. [Google Scholar]
- St˛eczny K., Kokoszy´ nski D. Effect of probiotic preparations (EM) on productive characteristics, carcass composition, and microbial contamination in a commercial broiler chicken farm. Anim. Biotechnol. 2020;32:1–8. doi: 10.1080/10495398.2020.1754841. [DOI] [PubMed] [Google Scholar]
- Stone, Diana, Margaret Davis, Katherine Baker, Tom Besser, Rohini Roopnarine, and Ravindra Sharma. 2013. MLST genotypes and antibiotic resistance of Campylobacter spp. isolated from poultry in Grenada. Biomed Res. Int. 2013:794643. [DOI] [PMC free article] [PubMed]
- Stringfellow K., Caldwell D., Lee J., Mohnl M., Beltran R., Schatzmayr G., Fitz-Coy S., Broussard C., Farnell M. Evaluation of probiotic administration on the immune response of coccidiosis vaccinated broilers. Poult. Sci. 2011;90 doi: 10.3382/ps.2010-01026. [DOI] [PubMed] [Google Scholar]
- Suganya T., Senthilkumar S., Deepa K., Muralidharan J., Gomathi G., Gobiraju S. Herbal feed additives in poultry. Int. J. Sci. Environ. Technol. 2016;5:1137–1145. [Google Scholar]
- Sun Y., O'Riordan M.X.D. Regulation of bacterial pathogenesis by intestinal short chain fatty acids. Adv. Appl. Microbiol. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara L.T., Achanta M., Schreiber N.B., Bommineni Y.R., Dai G., Jiang W., Lamont S., Lillehoj H.S., Beker A., Teeter R.G., Zhang G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6:e27225. doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara Lakshmi T., Jiang W., Zhang G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS One. 2012;7:e49558. doi: 10.1371/journal.pone.0049558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O., Alhotan R., Suliman G.M.…Abd El-Hack M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajodini M., Saeedi H.R., Moghbeli P. Use of black pepper, cinnamon, and turmeric as feed additives in the poultry industry. Worlds Poult. Sci. J. 2015;71:175–183. [Google Scholar]
- Tan B.K.H., Vanitha J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: a review. Curr. Med. Chem. 2004;11:1423–1430. doi: 10.2174/0929867043365161. [DOI] [PubMed] [Google Scholar]
- Timmerman H.M., Veldman A., Van den Elsen E., Rombouts F.M., Beynen A.C. Mortality and growth performance of broilers given drinking water supplemented with chicken specific probiotics. Poult. Sci. 2006;85:1383–1388. doi: 10.1093/ps/85.8.1383. [DOI] [PubMed] [Google Scholar]
- Travers, Marie-Agnès, I. Florent, L. Kohl, Ph. Grellier. 2011. Probiotics for the control of parasites: an overview. J. Parasitol. Res. 2011:610769. [DOI] [PMC free article] [PubMed]
- Tsirtsikos P., Fegeros K., Kominakis A., Balaskas C., Mountzouris K.C. Modulation of intestinal mucin composition and mucosal morphology by dietary phytogenic inclusion level in broilers. Animal. 2012;6:1049–1057. doi: 10.1017/S1751731111002680. [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Ananda Baskaran S., Mooyottu S., Karumathil D., Venkitanarayanan K. Inactivation of Listeria monocytogenes on frankfurters by plant derived antimicrobials alone or in combination with hydrogen peroxide. Int. J. Food Microbiol. 2013;163:114–118. doi: 10.1016/j.ijfoodmicro.2013.01.023. [DOI] [PubMed] [Google Scholar]
- USDA-ERS, 2009. Emerging issues in the U.S. organic industry. Accessed Mar. 2015. http://www.ers.usda.gov/media/155923/eib55_1_.pdf
- USDA-ERS, 2014. Organic market review. Accessed Mar. 2015. http://www.ers.usda.gov/topics/natural-resources-environment/organic-agriculture/organicmarket-overview.aspx
- Wan M.L.Y., Forsythe S.J., El-Nezami H. Probiotics interaction with foodborne pathogens: a potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019;59:3320–3333. doi: 10.1080/10408398.2018.1490885. [DOI] [PubMed] [Google Scholar]
- Ward N.E. Watt Publishing Co; Blairstown, NJ: 1996. Intestinal Viscosity, Broiler Performance Poultry Digest. [Google Scholar]
- Wenk C. Recent advances in animal feed additives such as metabolic modifiers, antimicrobial agents, probiotics, enzymes and highly available minerals. Rev. Asian-Aus. J. Anim. Sci. 2000;13:86–95. [Google Scholar]
- White W.B., Bird H.R., Sunde M.L., Marlett J.A., Prentice N.A., Burger W.C. Viscosity of beta-glucan as a factor in the enzymatic improvement of barley for chicks. Poult. Sci. 1983;62:853–862. [Google Scholar]
- Wolfenden R.E., Pumford N.R., Morgan M.J., Shivaramaiah S., Wolfenden A.D., Pixley C.M., Green J., Tellez G., Hargis B.M. Evaluation of selected direct-fed microbial candidates on live performance and Salmonella reduction in commercial turkey brooding houses. Poult. Sci. 2011;90:2627–2631. doi: 10.3382/ps.2011-01360. [DOI] [PubMed] [Google Scholar]
- Wu Y.B.B., Ravindran V. Influence of whole wheat inclusion and xylanase supplementation on the performance, digestive tract measurements and carcass characteristics of broiler chickens. Anim. Feed Sci. Tech. 2004;116:129–139. doi: 10.1080/00071660410001730888. [DOI] [PubMed] [Google Scholar]
- Xiang Q., Wang C., Zhang H., Lai W., Wei H., Peng J. Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals. 2019;9:1110. doi: 10.3390/ani9121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakhkeshi S., Rahimi S., Gharib Naseri K. The effects of comparison of herbal extracts, antibiotic, probiotic and organic acid on serum lipids, immune response, git microbial population, intestinal morphology and performance of broilers. J. Med. Plant Res. 2011;10:80–95. [Google Scholar]
- Yang Y., Iji P.A., Choct M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 2009;65:97–114. [Google Scholar]
- Yaqoob M., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry C., Zikry P.M., Salem H.M., Basalious E.B., Gazayerly El- O.N. Integrated nanovesicular/self-nanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020:801–814. doi: 10.1007/s13346-020-00716-5. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang R., Jia H., Zhu Z., Li H., Ma Y. Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life Sci. 2021;16:311–322. doi: 10.1515/biol-2021-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]