Abstract
Background
Ertapenem, cefepime, imipenem, ofloxacin, ceftazidime, clarithromycin, cefaclor, levofloxacin, linezolid, moxifloxacin, azithromycin, piperacillin–tazobactam, trimethoprim–sulfamethoxazole, metronidazole, ciprofloxacin, and cefuroxime are known to be associated with delirium. Other antibiotics may also lead to delirium, but no study has systemically compared delirium associations for many available antibiotics.
Objective
The objective of this study was to evaluate the association between delirium and antibiotics using the FDA Adverse Event Reporting System (FAERS).
Methods
FAERS reports from January 1, 2004 to December 31, 2018 were included in the study. Reporting odds ratios (RORs) and corresponding 95% confidence intervals (95% CI) for the association between antibiotics and delirium were calculated. An association was considered to be statistically significant when the lower limit of the 95% CI was greater than 1.0.
Results
A total of 10,015,622 reports (including 16,982 delirium reports) were considered, after inclusion criteria were applied. Statistically significant delirium RORs (95% CI) for antibiotics were: ertapenem 21.07 (16.38–27.10), cefepime 9.8 (6.37–15.09), imipenem 9.68 (6.75–13.89), ofloxacin 7.73 (4.00–14.92), ceftazidime 6.09 (2.73–13.62), clarithromycin 5.34 (4.37–6.53), cefaclor 5.32 (1.71–16.58), ampicillin–sulbactam 4.49 (2.13–9.45), levofloxacin 4.47 (3.88–5.16), linezolid 4.33 (3.28–5.72), moxifloxacin 3.51 (2.81–4.38), azithromycin 2.76 (2.09–3.64), piperacillin–tazobactam 2.41 (1.47–3.93), trimethoprim–sulfamethoxazole 2.36 (1.61–3.47), metronidazole 1.85 (1.31–2.60), ciprofloxacin 1.83 (1.44–2.33), and cefuroxime 1.81 (1.03–3.20).
Conclusion
This study found statistically significant increased risk of reporting delirium with ertapenem, cefepime, imipenem, ofloxacin, ceftazidime, clarithromycin, cefaclor, ampicillin–sulbactam, levofloxacin, linezolid, moxifloxacin, azithromycin, piperacillin–tazobactam, trimethoprim–sulfamethoxazole, metronidazole, ciprofloxacin, and cefuroxime.
Key Points
| This study identifies previously unknown delirium reporting association with ampicillin–sulbactam. |
| Ertapenem had the highest delirium reporting association among antibiotics evaluated in the study. |
| Older patients had a higher delirium Reporting Odds Ratio than younger patients among most of the antibiotic classes analyzed. |
Introduction
Delirium is diagnosed in the presence of a disturbance in attention and awareness that develops over a short period of time, represents a change from baseline, tends to fluctuate, has an additional cognitive disturbance (e.g., memory deficit, disorientation, language, visuospatial ability, or perception), all of which are not better explained by other diagnoses (e.g., coma) [1]. Delirium is associated with higher mortality rates, affects up to 50% of hospitalized elderly patients, and costs more than $164 billion per year in the United States [2]. Delirium may be nothing more than a marker of severity of illness likened to a cough for pneumonia. Treatment of pneumonia resolves the cough like treatment of sepsis may resolve delirium. This point was proven in multiple trials of critically ill patients including BRAIN-ICU, which showed ICU delirium is associated with long-term cognitive impairment, and MIND-USA, which showed delirium treatment in those with hypoactive delirium does not improve outcome [3, 4].
Many antibiotics are known to be associated with delirium. Overall quality of evidence for many reports is very low. Multiple case reports have reported on ertapenem-induced delirium [5–7]. A case of hypoactive delirium and another case of encephalopthy as adverse effects of imipenem were reported [8, 9]. A case report demonstrated a case of delirium with hallucinations likely caused by a combination of diphenhydramine and linezolid [10]. Delirium was also reported to be induced by clarithromycin monotherapy or a combination of clarithromycin and fluoxetine [11–13]. Delirium was associated with azithromycin in two geriatric patients treated for lower respiratory tract infection [14]. There are at least 6 published case reports of delirium associated with the use of azithromycin, including one pediatric patient, and 12 published case reports of delirium associated with the use of clarithromycin [15]. Four fluoroquinolone antibiotics, which were ofloxacin, levofloxacin, moxifloxacin, and ciprofloxacin, were all reported to be associated with delirium [16–19]. Cefepime use was associated with increased likelihood and duration of acute encephalopathy (presence of delirium or depressed level of consciousness in the absence of deep sedation) in critically ill patients in a retrospective case–control study [20]. A nested cohort study concluded that first, second, and third-generation cephalosporins doubled the odds of delirium [21]. A case report reported higher-level gait disorder and nocturnal delirium likely induced by trimethoprim–sulfamethoxazole [22]. In another case report, metronidazole and levofloxacin induced delirium in a chronic kidney disease patient [23]. Delirium was also reported to be induced by piperacillin–tazobactam in a peritoneal dialysis patient [24].
Few studies have looked at antibiotics and compared their associations with delirium. The objective of this study was to evaluate the association between delirium and antibiotics using FDA Adverse Event Reporting System (FAERS).
Methods
Data Source
FAERS is a publicly available database composed of adverse event reports that were submitted to United States Food and Drug Administration (FDA) [25]. FAERS data contain drug information (drug name, active ingredient, route of administration, the drug’s reported role in the event) and reaction information. Healthcare professionals, consumers or manufacturers may submit reports. The types of data found in FAERS include reason for administration, specific reaction, seriousness, outcome, gender, event data, patient age, who sent the report, reporter type, country, whether or not it was reported to the manufacturer and whether or not it was published. Quarterly Data Files were used to obtain FAERS data. The Institutional Review Board (IRB) of The University of South Carolina determined that this study is not human subjects research (IRB number: Pro00101342).
Study Design
FAERS data from January 1, 2004 to December 31, 2018 were included in the study. Some adverse event reports were submitted multiple times with updated information. Therefore, duplicate reports were removed by case number, with the most recent submission included in the study.
Drug Exposure Definition
Each antibiotic was identified in FAERS by generic and brand names listed in the Drugs@FDA Database [26]. Antibiotics with less than three delirium Adverse Drug Reaction (ADR) reports were excluded from this data analysis [27]. All reports were included, regardless of route of administration and the drug’s reported role in the event. Antibiotics included in the study were ertapenem, imipenem, meropenem, linezolid, clarithromycin, azithromycin, erythromycin, ofloxacin, levofloxacin, moxifloxacin, ciprofloxacin, cefepime, ceftazidime, cefaclor, cefuroxime, cephalexin, ceftriaxone, trimethoprim–sulfamethoxazole, metronidazole, ampicillin-sulbactam, piperacillin–tazobactam, amoxicillin–clavulanate, amoxicillin, nitrofurantoin, daptomycin, vancomycin, minocycline, and clindamycin. Antibiotic classes included in the study were carbapenems, macrolides, fluoroquinolones, cephalosporins, and penicillin combinations.
Adverse Drug Reaction Definition
FAERS defines ADRs using Preferred Terms (PT) from the Medical Dictionary for Regulatory Activities (MedDRA) [28]. Preferred Term “Delirium” was used to identify delirium cases.
Statistical Analysis
A disproportionality analysis was conducted by computing Reporting Odds Ratios (ROR) and corresponding 95% confidence intervals (95%CI) for the association between delirium and each antibiotic class or individual antibiotic [29]. ROR was calculated as the ratio of the odds of reporting delirium versus all other ADRs for a given drug, compared with this reporting odds for all other drugs present in FAERS [29]. An association was considered to be statistically significant if the lower limit of 95% CI was above 1.0 [29]. A subgroup analysis was performed on patients who were 65 years or older and patients less than 65 years old. Data analysis was performed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA) and SAS 9.4 (SAS Institute, Cary, NC).
Results
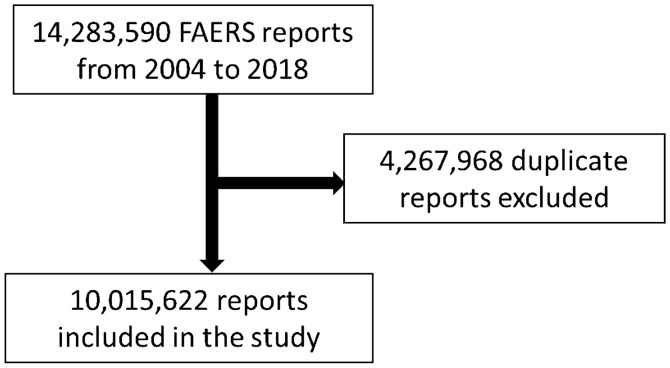
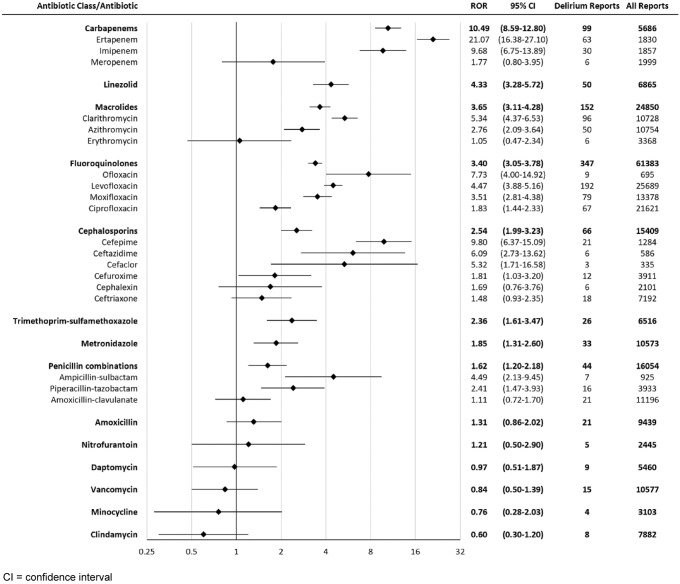
A total of 10,015,622 reports (including 16,982 delirium reports) were considered, after inclusion criteria were applied (Fig. 1). The number of cases for each drug and drug class is listed in Fig. 2. Statistically significant delirium RORs (95% CI) for antibiotic classes were: carbapenems 10.49 (8.59–12.80), macrolides 3.65 (3.11–4.28), fluoroquinolones 3.40 (3.05-3.78), cephalosporins 2.54 (1.99–3.23), and penicillin combinations 1.62 (1.20–2.18).
Fig. 1.
Flow diagram for inclusion and exclusion of the FAERS reports
Fig. 2.
Reporting odds ratios (ROR) for delirium with antibiotics
Statistically significant delirium RORs (95% CI) for antibiotics were: ertapenem 21.07 (16.38–27.10), cefepime 9.8 (6.37–15.09), imipenem 9.68 (6.75–13.89), ofloxacin 7.73 (4.00–14.92), ceftazidime 6.09 (2.73–13.62), clarithromycin 5.34 (4.37–6.53), cefaclor 5.32 (1.71–16.58), ampicillin–sulbactam 4.49 (2.13–9.45), levofloxacin 4.47 (3.88–5.16), linezolid 4.33 (3.28–5.72), moxifloxacin 3.51 (2.81–4.38), azithromycin 2.76 (2.09–3.64), piperacillin–tazobactam 2.41 (1.47–3.93), trimethoprim–sulfamethoxazole 2.36 (1.61–3.47), metronidazole 1.85 (1.31–2.60), ciprofloxacin 1.83 (1.44–2.33), and cefuroxime 1.81 (1.03–3.20) (Fig. 2).
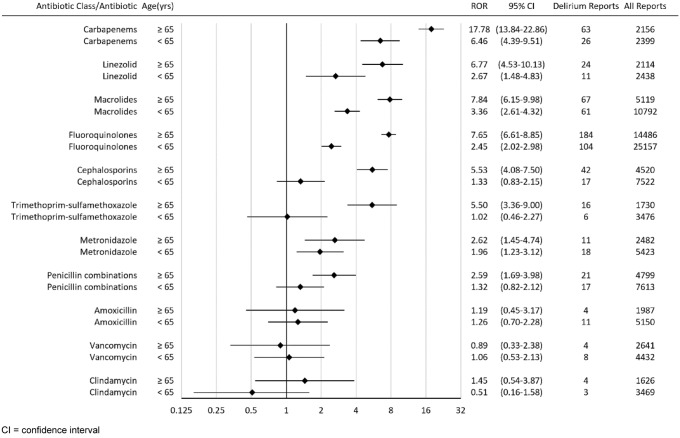
Among patients who took carbapenems, linezolid, macrolides, fluoroquinolones, cephalosporins, trimethoprim–sulfamethoxazole, metronidazole, penicillin combinations, and clindamycin, patients who were 65 years or older had a higher delirium ROR than those less than 65 years old (Fig. 3). Among patients who took amoxicillin and vancomycin, patients who were 65 years or older had a lower delirium ROR than those less than 65 years old (RORs were not statistically significant considering that the lower limit of 95% CI was below 1.0).
Fig. 3.
Reporting odds ratios (RORs) for delirium with antibiotics stratified by age
Discussion
Our results indicated significant delirium reporting associations (from strongest to weakest) with the following antibiotic classes: carbapenems, macrolides, fluoroquinolones, cephalosporins, and penicillin combinations (Fig. 2).
Our results demonstrated significant delirium reporting associations (from strongest to weakest) with the following antibiotics: ertapenem, cefepime, imipenem, ofloxacin, ceftazidime, clarithromycin, cefaclor, ampicillin–sulbactam, levofloxacin, linezolid, moxifloxacin, azithromycin, piperacillin–tazobactam, trimethoprim–sulfamethoxazole, metronidazole, ciprofloxacin, and cefuroxime (Fig. 2). Our results were in agreement with previously known delirium associations with ertapenem, cefepime, imipenem, ofloxacin, ceftazidime, clarithromycin, cefaclor, levofloxacin, linezolid, moxifloxacin, azithromycin, piperacillin–tazobactam, trimethoprim–sulfamethoxazole, metronidazole, ciprofloxacin, and cefuroxime [5–24]. Ampicillin–sulbactam was found to be associated with delirium in FAERS, which was not reported in the literature.
Cefepime is known to be able to induce neurotoxicity due to its ability to cross the blood–brain barrier and to exhibit concentration-dependent competitive gamma-aminobutyric acid (GABA) antagonism [30–32]. The signs and symptoms of cefepime-induced neurotoxicity included altered mental status, reduced consciousness, confusion, myoclonus, aphasia, agitation, and seizures [32]. Many of the signs and symptoms of cefepime-induced neurotoxicity correlated well with signs and symptoms of delirium [1]. Therefore, it is not surprising to find the significant association between cefepime and delirium in FAERS.
Carbapenem antibiotics may have a higher neurotoxic potential than that of penicillins and cephalosporins [33]. Indeed, in our study, carbapenem antibiotic class had the highest delirium ROR among all antibiotic classes included in the study (Fig. 1). This may not be a class-wide effect and certain carbapenems may be more offensive (e.g., imipenem) than others (e.g., meropenem). Prior studies have suggested that cefepime may have a tenfold greater risk for neurotoxicity than meropenem [34, 35]. Neurotoxicity of carbapenems was also thought to be caused by an interaction with GABA receptors [33]. The association between imipenem and delirium found in FAERS may be due to this mechanism.
Although ampicillin–sulbactam-induced delirium was not reported in the literature, ampicillin–sulbactam is a beta-lactam, which has the potential to induce neurotoxicity via interactions with GABA receptors [31]. The association between ampicillin–sulbactam and delirium found in FAERS may be explained by this mechanism. It is interesting that ampicillin–sulbactam was associated with delirium given its minimal penetration across the blood–brain-barrier. Prior reports have stated its penetrance is 1% of the plasma concentration and only increases in the setting of inflamed meninges [36].
Although a case of meropenem-associated delirium was reported in the literature, our study did not find statistically significant ROR for meropenem and delirium. The low number of meropenem delirium reports in FAERS may be the reason of statistical insignificance (Fig. 2).
In the subgroup analysis, the delirium ROR rank order in both subgroups (< 65 years old and ≥ 65 years old) were similar to that in all patients. Our results showed that older patients had a higher delirium ROR than younger patients among most of the antibiotic classes analyzed (Fig. 3). Carbapenems, macrolides, fluoroquinolones, cephalosporins, and trimethoprim–sulfamethoxazole had significantly greater associations with delirium in older patients than those in younger patients. It is known that delirium risk is higher in older patients [1]. To note, ciprofloxacin and trimethoprim–sulfamethoxazole were also included in the American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults [37]. Ciprofloxacin was in the Beers Criteria for tendon rupture and hyperkalemia, but not for delirium.
Limitations
A causal relationship between a drug and an ADR cannot be determined by FAERS. Significant bias may occur because of the spontaneous and voluntary reporting of ADRs. Media attention and recent publication of an ADR in the literature might affect the reporting behaviors. The association between a drug and an ADR is confounded by comorbidities and concomitant drugs. According to FDA, information submitted has not been verified by a medical professional. FAERS data may be submitted by healthcare professionals, consumers and manufacturers. Source of submission must be considered. Some information is missing or incomplete in FAERS. There have been cases in which age was not reported or drug names were misspelled. FDA does not receive reports for every adverse event or medication error that occurs with a product. In addition, ROR only investigates an increased risk of ADR reporting and not risk of ADR occurrence in absolute terms [38]. Some of the confidence intervals are quite wide such as cefaclor 5.32 (1.71–16.58) because of the low number of cefaclor ROR reports for delirium. An additional limitation of this study is the reliance on “delirium” as the search term in the FAERS database. Terms such as delirium, encephalopathy, acute confusional state, acute brain dysfunction and acute brain failure are often used synonymously and inappropriately in the literature [39]. Without the ability to determine how delirium was diagnosed, it is possible that numerous cases were misclassified and could have altered the findings. Despite the limitations, FAERS has a large sample size and is suitable for discovering a signal of new and rare drug-ADR associations.
Conclusion
This study found statistically significant increased risk of reporting delirium with ertapenem, cefepime, imipenem, ofloxacin, ceftazidime, clarithromycin, cefaclor, ampicillin–sulbactam, levofloxacin, linezolid, moxifloxacin, azithromycin, piperacillin–tazobactam, trimethoprim–sulfamethoxazole, metronidazole, ciprofloxacin, and cefuroxime. Results obtained from FAERS should be interpreted with caution in the context of data limitations. Antibiotic stewardship is needed to prevent delirium and to improve health outcomes.
Acknowledgements
No funding was sought for this research study. Dr. Frei was supported, in part, by a NIH Clinical and Translational Science Award (National Center for Advancing Translational Sciences, UL1 TR001120 and UL1 TR002645) while the study was being conducted. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the authors’ affiliated institutions.
Declarations
Funding
No funding was sought for this research study. Dr. Frei was supported, in part, by a NIH Clinical and Translational Science Award (National Center for Advancing Translational Sciences, UL1 TR001120 and UL1 TR002645) while the study was being conducted. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest
Dr. Frei’s institution has received grants for research from AstraZeneca Pharmaceuticals in the last 36 months.
Ethics approval
The Institutional Review Board (IRB) of The University of South Carolina determined that this study is not human subjects research (IRB number: Pro00101342).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Codes used during the current study are available from the corresponding author on reasonable request.
Author contributions
Study concept and design: all authors. Statistical analysis: CT. Interpretation of data: all authors. Drafting of the manuscript: CT. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: CT.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, Malhotra A, Owens RL, Feinstein DJ, Khan B. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veillette JJ, Van Epps P. Ertapenem-induced hallucinations and delirium in an elderly patient. Consult Pharm. 2016;31(4):207–214. doi: 10.4140/TCP.n.2016.207. [DOI] [PubMed] [Google Scholar]
- 6.Apodaca K, Baker J, Bin-Bilal H, Raskin Y, Quinn DK. Ertapenem-induced delirium: a case report and literature review. Psychosomatics. 2015;56(5):561–566. doi: 10.1016/j.psym.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Lin H, Chew ST. Status epilepticus and delirium associated with ertapenem in a very elderly patient with chronic kidney disease and silent ischaemic cerebrovascular disease. Drug Saf Case Rep. 2015;2(1):19. doi: 10.1007/s40800-015-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Africa BL, Carthen D, Arabelo H. A4 Delirious because of treatment: an adverse effect of imipenem. J Am Geriatr Soc. 2012;60:S18. [Google Scholar]
- 9.Fernández-Torre JL, Velasco M, Gutiérrez R, Fernández-Sampedro M. Encephalopathy secondary to imipenem therapy. Clin EEG Neurosci. 2004;35(2):100–103. doi: 10.1177/155005940403500210. [DOI] [PubMed] [Google Scholar]
- 10.Serio RN. Acute delirium associated with combined diphenhydramine and linezolid use. Ann Pharmacother. 2004;38(1):62–65. doi: 10.1345/aph.1D018. [DOI] [PubMed] [Google Scholar]
- 11.Ozsoylar G, Sayin A, Bolay H. Clarithromycin monotherapy-induced delirium. J Antimicrob Chemother. 2007;59(2):331. doi: 10.1093/jac/dkl480. [DOI] [PubMed] [Google Scholar]
- 12.Mermelstein HT. Clarithromycin-induced delirium in a general hospital. Psychosomatics. 1998;39(6):540–542. doi: 10.1016/S0033-3182(98)71287-3. [DOI] [PubMed] [Google Scholar]
- 13.Pollak PT, Sketris IS, MacKenzie SL, Hewlett TJ. Delirium probably induced by clarithromycin in a patient receiving fluoxetine. Ann Pharmacother. 1995;29(5):486–488. doi: 10.1177/106002809502900506. [DOI] [PubMed] [Google Scholar]
- 14.Cone LA, Padilla L, Potts BE. Delirium in the elderly resulting from azithromycin therapy. Surg Neurol. 2003;59(6):509–511. doi: 10.1016/S0090-3019(03)00065-X. [DOI] [PubMed] [Google Scholar]
- 15.Pejčić AV. Delirium associated with the use of macrolide antibiotics: a review. Int J Psychiatry Clin Pract. 2020;1–14. 10.1080/13651501.2020.1828933 [DOI] [PubMed]
- 16.Odeh M, Kogan Y, Paz A, Elias N. Delirium induced by levofloxacin. J Clin Neurosci. 2019;66:262–264. doi: 10.1016/j.jocn.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 17.Tasleem H, Viswanathan R. Moxifloxacin-induced delirium with hallucinations. Psychosomatics. 2011;52(5):472–474. doi: 10.1016/j.psym.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Jay GT, Fitzgerald JM. Ciprofloxacin-induced delirium. Ann Pharmacother. 1997;31(2):252. doi: 10.1177/106002809703100221. [DOI] [PubMed] [Google Scholar]
- 19.Fennig S, Mauas L. Ofloxacin-induced delirium. J Clin Psychiatry. 1992;53(4):137–138. [PubMed] [Google Scholar]
- 20.Singh TD, O'Horo JC, Day CN, Mandrekar J, Rabinstein AA. Cefepime is associated with acute encephalopathy in critically ill patients: a retrospective case-control study. Neurocrit Care. 2020;33:695–700. doi: 10.1007/s12028-020-01035-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grahl JJ, Stollings JL, Rakhit S, Person AK, Wang L, Thompson JL, et al. Antimicrobial exposure and the risk of delirium in critically ill patients. Crit Care. 2018;22(1):337. doi: 10.1186/s13054-018-2262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dakin LE. Probable trimethoprim/sulfamethoxazole-induced higher-level gait disorder and nocturnal delirium in an elderly man. Ann Pharmacother. 2009;43(1):129–133. doi: 10.1345/aph.1L295. [DOI] [PubMed] [Google Scholar]
- 23.Velickovic-Radovanovic R, Catic-Dordevic A, Dinic K, Radivojevic J, Zikic O, Cvetkovic T, et al. Metronidazole- and levofloxacin-induced psychotic disorders in chronic kidney patient. Eur J Hosp Pharm. 2019;26(6):347–349. doi: 10.1136/ejhpharm-2018-001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong MK, Siu YP, Yung CY, Kwan TH. Piperacillin/tazobactam-induced acute delirium in a peritoneal dialysis patient. Nephrol Dial Transplant. 2004;19(5):1341. doi: 10.1093/ndt/gfh048. [DOI] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. FDA Adverse Event Reporting System (FAERS). 2020. https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. Accessed 1 Oct 2020.
- 26.Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 1 Oct 2020.
- 27.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–486. doi: 10.1002/pds.677. [DOI] [PubMed] [Google Scholar]
- 28.MedDRA MSSO. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 23.1. 2020. https://admin.new.meddra.org/sites/default/files/guidance/file/SMQ_intguide_23_1_English.pdf. Accessed 1 Oct 2020.
- 29.Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 30.Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care. 2013;17(6):1–6. doi: 10.1186/cc13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother. 2008;42(12):1843–1850. doi: 10.1345/aph.1L307. [DOI] [PubMed] [Google Scholar]
- 32.Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017;21(1):276. doi: 10.1186/s13054-017-1856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norrby SR. Neurotoxicity of carbapenem antibacterials. Drug Saf. 1996;15(2):87–90. doi: 10.2165/00002018-199615020-00001. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka A, Takechi K, Watanabe S, Tanaka M, Suemaru K, Araki H. Comparison of the prevalence of convulsions associated with the use of cefepime and meropenem. Int J Clin Pharm. 2013;35(5):683–687. doi: 10.1007/s11096-013-9799-3. [DOI] [PubMed] [Google Scholar]
- 35.Chaïbi K, Chaussard M, Soussi S, Lafaurie M, Legrand M. Not all β-lactams are equal regarding neurotoxicity. Crit Care. 2016;20(1):1–2. doi: 10.1186/s13054-016-1522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearney BP, Aweeka FT. The penetration of anti-infectives into the central nervous system. Neurol Clin. 1999;17(4):883–900. doi: 10.1016/S0733-8619(05)70171-7. [DOI] [PubMed] [Google Scholar]
- 37.BY the American Geriatrics Society Beers Criteria Update Expert Panel American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 38.Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72(6):905–908. doi: 10.1111/j.1365-2125.2011.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slooter AJ, Otte WM, Devlin JW, Arora RC, Bleck TP, Claassen J, Duprey MS, Ely EW, Kaplan PW, Latronico N, Morandi A. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46(5):1020–1022. doi: 10.1007/s00134-019-05907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]