Abstract
Background:
Lower limb bone stress injury (BSI) of the pelvis, femur, and tibia is prevalent in collegiate track and field distance runners. Bone mineral density (BMD), body composition (BComp), and anthropometric parameters before initial collegiate injury have not been compared between runners with BSI and their noninjured counterparts.
Purpose:
To characterize bone health in relation to BComp and anthropometric measurements from total-body dual x-ray absorptiometry (DXA) scans in collegiate male and female distance runners before BSI and develop BMD prediction models.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
Distance runners (N = 79) from a single university track and field team were retrospectively enrolled into this study. The runners completed a DXA scan during the fall season (August-November) and participated in sport activities before the scan. Three months after scanning, electronic medical records were reviewed for the occurrence of BSI. An independent-sample t test was used to compare BMD (total and regional [spine, pelvis, and legs]), BComp (% body fat, fat mass, and lean mass), and anthropometric measurements (shoulder width and leg, arm, and trunk length) between runners with versus without BSI (included subgroup analysis by sex). Multiple linear regression with stepwise removal was used to determine variables most predictive of BMD.
Results:
Of the 79 enrolled participants (42 male, 37 female), 18 runners (22.8%; 11 female, 7 male) sustained a lower limb BSI. Compared with the noninjured group, injured runners had lower total and regional BMD (P < .001 for all) and shorter leg and arm lengths (P < .05 for both), whereas injured male runners had lower fat mass and injured female runners had lower lean mass in the legs (P < .05 for both). Injured runners’ age-matched total BMD Z score (-0.1 ± 0.6) was considered clinically normal. BComp and anthropometric measures were predictive of total and regional BMD (P < .05; R 2 = 0.64-0.80; percentage error = 3.8%-4.8%).
Conclusion:
The DXA scans of injured runners prior to incidence indicated lower BMD compared with noninjured runners. Shorter limb lengths, lower fat mass (male), and lower leg lean mass (female) may also be indicative of risk. Certain BComp and anthropometric measures were predictive of BMD.
Keywords: bone, collegiate, distance, DXA, injury, runners
Bone stress injury (BSI) is common among collegiate track and field distance runners. 30,33,38 When compared with all male and female collegiate athletes, distance runners sustain the most BSI annually, 33 with lower limb BSI of the femur, tibia, and pelvis being more prevalent than in other track and field athletes. 5 These more common lower limb injuries in distance runners are likely due to the repetitive impact forces inherent in the sport in combination with extrinsic and intrinsic factors. An important extrinsic factor is an increase in running activity or training intensity and can include a sudden change in running frequency, mileage, pace, or terrain, which can commonly occur throughout a collegiate season that comprises cross-country, indoor, and outdoor competition. 20 Intrinsic factors for BSI development in runners can be related to bone health and body composition (ie, fat and lean mass) such as low body fat percentage, low muscle mass, and low bone mineral density (BMD); thus, when exposed to sport-related activity during a competitive season, there is a greater likelihood of injury. 5,30 Therefore, accounting for these respective intrinsic risk factors, collectively, during a competitive collegiate season is of critical importance for identifying risk and making appropriate training or nutritional modifications.
Dual x-ray absorptiometry (DXA) is an accurate, reliable technique for measuring and quantifying total and regional lean mass, fat mass, and BMD in athletes 6 and is used commonly among athletic populations. 7,10 Despite the accuracy and popularity of DXA in addition to previous evidence linking its measures of BMD to BSI in athletic populations, 2,17,22,37 minimal research has been conducted to examine this relationship within collegiate running athletes. 4,9 However, Tenforde et al 36 recently reported an association between BSI risk and lumbar spine BMD in female collegiate athletes, thus further supporting its potential utility of DXA for tracking sport-specific bone health in specialized athletic populations such as collegiate distance runners. Also, during a competitive season, it is still unknown within this respective specialized sport population whether total and regional BMD measures are indicative of BSI risk during a competitive season and whether current risk assessment standards (ie, Z score/t score) based on general population norms are appropriate for this athletic population. Given the high-impact nature of distance running combined with, at times, reduced energy availability (ie, difference in energy intake and exercise energy expenditure in relation to fat-free mass) in these athletes, 27 it is possible that different risk metrics within bone and body composition measures specific to lower limb BSI are needed in this specialized population of athletes. 16 Therefore, identifying population-specific risk for low BMD and BSI are of important clinical value with regard to injury prevention.
Importantly, many institutions and athletes do not have readily available access to DXA screening. Therefore, identifying intrinsic factors associated with BMD and BSI risk that can be assessed in the clinic would be of considerable value with regard to screening. Barrack et al 1 and Lambert et al 21 have both recently used regression modeling and identified several demographic and anthropometric variables that were highly predictive of BMD in male adolescent athletes and professional ballet performers (a group also commonly afflicted by BSIs), respectively. However, it was acknowledged that the prediction models developed were likely highly specific to the type of athletes observed. Thus, if similar modeling techniques were utilized for collegiate distance runners (which may be applicable to a wider range of athletes), the development of novel screening equations would be of great value for high throughput screening and risk monitoring, particularly for institutions without readily available access to DXA. Given the high prevalence of lower limb BSIs in collegiate distance runners, often paired with prolonged recovery trajectories, 28 those identified to be at risk could be flagged for either further diagnostic evaluation by a clinician or close monitoring by athletic training staff, or be provided nutritional counseling/intervention. 12
In light of previous observations and a clinical need in this unique population, the purpose of this study was to characterize DXA-derived BMD, body composition, and skeletal dimensions in collegiate male and female distance runners prior to lower limb BSI status (ie, injured or noninjured) during a competitive season as well as provide indices of risk specific to the total body DXA assessment. We hypothesized that (1) collegiate distance runners with a lower limb BSI will exhibit lower BMD relative to runners without injury; (2) total and regional body composition (ie, fat and lean) as well as skeletal dimension differences would be observed between respective BSI status groups; and (3) measures of body composition and skeletal dimensions could be used to develop prediction models for regional and total BMD.
Methods
Study Design
This was a retrospective case-control study that was conducted at a university athletic training facility during the fall (August-November) 2013-2019 cross-country seasons. Included were male and female distance running athletes who were members of a National Collegiate Athletic Association (NCAA) Division I-A track and field team. Study measurements included a total-body DXA scan, weight, height, and body mass index (BMI). Within the 3 months following study measurement, the electronic medical records for all participants were reviewed for incidence of a diagnosed lower limb BSI. Because the study participants received multiple fall season scan measurements throughout their collegiate career, the inclusion DXA scan for the participants with a lower limb BSI was the scan before the first injury of their collegiate career, while we used the last scan of the eligible fall season for participants with no injury during their collegiate career. The study protocol received institutional review board approval, and written informed consent was obtained from the athletes prior to participation.
Patients
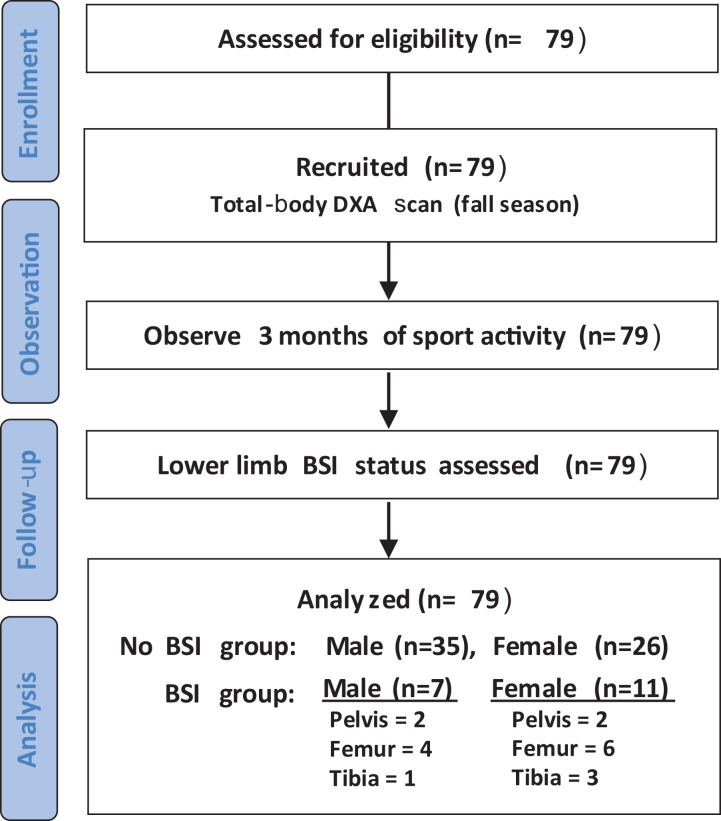
Overall, 79 male and female distance runners were assessed for study eligibility (Figure 1). Inclusion criteria included participation in sport-related activity prior to and after DXA scan for 3 months as well as scans only prior to the first collegiate lower limb BSI. Exclusion criteria included any bone-related injury or recovery from such injury at the time of the DXA scan that prohibited prospective sport-related activities as well as scans prior to reoccurring lower limb BSI.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. BSI, bone stress injury; DXA, dual x-ray absorptiometry.
DXA and Anthropometric Analyses
All DXA scans were collected on a Lunar iDXA device (GE Healthcare) located within the university athletic department’s sport performance center. The scans were analyzed using enCORE software (Version 14.10.022; GE Medical Systems Lunar). The DXA scan measured total bone mass, total-body BMD, spine BMD, pelvis BMD, leg BMD (femoral and tibial), percentage body fat (%BF), fat mass, fat-free mass, and lean mass (overall and for legs, trunk, and arms).
Skeletal dimensions (shoulder width, trunk length, arm length, leg length, and leg/trunk length ratio) were also obtained from the total-body DXA scan and analyzed using ImageJ (National Institutes of Health) analysis software, 32 using similar methods to those conducted by Stanelle et al. 34 Shoulder width was measured between the widest point of each shoulder. Trunk length was measured at the widest point on the pelvis to the top of the clavicle. Arm length was the sum of lengths (ie, top to end) of the humerus and radius, while leg length was measured as the distance between the widest point on the pelvis to bottom center of the distal tibia. All skeletal dimensions completed within each study participant scan were derived from the midsagittal plane that was the clearest in identifying predefined bony landmarks for respective lengths.
Age- and sex-matched Z scores were calculated for BMD measures using general population norms extracted from the National Health and Nutrition Examination Survey database. 24 The Z scores were defined as the difference between the participant’s BMD and the BMD of an age-matched reference group divided by the general population standard deviation. 18 Similar to the diagnostic criteria for the spine, femoral neck, and forearm, criteria for total and regional low BMD were defined as follows: osteopenia, Z score less than -1; osteoporosis, Z score less than -2.5. 23
Lower Limb BSI
The workup of a suspected sport-related lower limb BSI was made based on a history of increasing localized pain with exercise (especially in a setting of recent increase in exercise duration or intensity) and localized tenderness on examination by the team physician. Although the initial imaging modality may have been plain radiograph, for the purposes of this study, BSI was confirmed only when the clinical suspicion was confirmed by magnetic resonance imaging (MRI) showing Fredericson grade changes of at least 2, as documented in each study participant’s electronic medical records. 18 All lower limb BSIs that were documented and included within the analyses occurred after the fall-season DXA scan; sport-related; and noted as stress fracture or stress reaction within the pelvis, femoral neck, femoral shaft, or tibia. The date of lower limb BSI was determined from the date of clinical assessment when imaging was ordered, since completion of MRI generally occurred within 1 week of the respective assessment.
Statistical Analysis
Data were analyzed using SPSS statistics software (SPSS Statistics; IBM). An independent-sample t test was used to compare BMD, body composition, skeletal dimensions, and demographic variables between BSI status (ie, with or without) among all athletes and within male and female athletes compared separately (critical alpha was set at α = .05 for all comparisons). For all significant comparisons, effect size was calculated using Cohen d statistics and interpreted as follows: 0.0-0.09, negligible; 0.10-0.29, small; 0.30-0.49, moderate; 0.5-0.69, large; and greater than 0.7, very large. Next, multiple linear regression with stepwise removal was used to predict bone mineral content and BMD (total body, legs, spine, pelvis) using height, weight, BMI, body composition, sex, and skeletal dimensions, whereby final variables included in each prediction model were based on the highest adjusted R 2 with the lowest level of variance inflation as well as a minimum of 10 observations (athletes) per number of regression coefficients included in each model.
Results
Table 1 shows the characteristics of the 79 study participants, overall and by sex. Male and female athletes were significantly different in height, weight, and all BComp; BMD; and skeletal dimensions (P < .01 for all). Only age and BMI were not statistically different between the sexes. BSI frequency for all athletes was 22.8%. Differences between injured male (16.7%) and female (29.7%) frequencies did not reach statistical significance.
TABLE 1.
Characteristics of the Included Athletes a
| All Athletes (N = 79) | Men (n = 42) | Women (n = 37) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Height, cm | 172.9 ± 0.3 | 178.2 ± 1.8 | 166.9 ± 2.0 | <.001 |
| Weight, kg | 61.2 ± 1.6 | 65.7 ± 1.8 | 56.2 ± 1.7 | <.001 |
| Age, years | 20.0 ± 0.3 | 20.2 ± 0.4 | 19.7 ± 0.5 | .203 |
| BMI, kg/m2 | 20.7 ± 0.3 | 20.9 ± 0.4 | 20.5 ± 0.5 | .253 |
| Body composition | ||||
| %BF | 15.9 ± 1.3 | 11.4 ± 0.7 | 21.0 ± 1.2 | <.001 |
| Fat mass, kg | 9.6 ± 0.7 | 7.5 ± 0.5 | 11.9 ± 0.9 | <.001 |
| Fat-free mass, kg | 51.7 ± 1.9 | 58.8 ± 0.8 | 44.3 ± 1.3 | <.001 |
| Skeletal dimensions, cm | ||||
| Leg length | 94.8 ± 1.3 | 98.0 ± 1.5 | 91.1 ± 1.4 | <.001 |
| Arm length | 55.6 ± 0.7 | 57.4 ± 0.7 | 53.4 ± 0.9 | <.001 |
| Trunk length | 46.7 ± 0.7 | 48.1 ± 0.9 | 45.1 ± 0.7 | <.001 |
| Shoulder width | 38.9 ± 0.5 | 40.5 ± 0.5 | 37.0 ± 0.5 | <.001 |
| Bone densitometry | ||||
| Total-body BMD, g/cm2 | 1.22 ± 0.02 | 1.26 ± 0.03 | 1.17 ± 0.03 | <.001 |
| Total bone mass, kg | 2.50 ± 0.09 | 2.65 ± 0.13 | 2.32 ± 0.11 | <.001 |
| Leg BMD, g/cm2 | 1.33 ± 0.03 | 1.40 ± 0.03 | 1.26 ± 0.04 | <.001 |
| Spine BMD, g/cm2 | 1.07 ± 0.03 | 1.10 ± 0.03 | 1.02 ± 0.04 | .003 |
| Pelvis BMD, g/cm2 | 1.13 ± 0.03 | 1.17 ± 0.04 | 1.09 ± 0.03 | .002 |
| BSI frequency, % | 22.8 | 16.7 | 29.7 | .133 |
a Data are presented as means ± 95% CI. Bolded P values indicate statistically significant difference between men and women (P < .05). %BF, percentage body fat; BMD, bone mineral density; BMI, body mass index; BSI, bone stress injury.
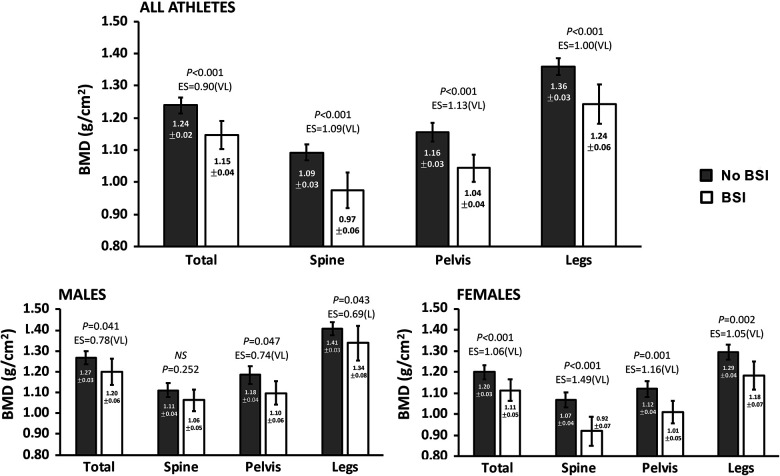
Comparison of BMD Between Athletes With and Without BSI
Figure 2 shows the BMD characteristics compared between athletes with versus without a lower limb BSI. Overall, those in the no-BSI group had significantly higher BMD overall as well as in the spine, pelvis, and legs (P < .001 for all). In male athletes, the no-BSI group had significantly higher overall, pelvis, and leg BMD (P < .05), while no difference was observed in spine BMD compared with the BSI group. Within female runners, the no-BSI group had significantly higher BMD in all measures compared with the BSI group (P < .01 for all).
Figure 2.
Bone mineral density (BMD) comparison. Data are presented as means ± 95% CI for athlete BMD with long BSI or no long BSI (no BSI). Type I error set at α = .05. For each significant comparison, Cohen d effect sizes are interpreted as 0.0-0.09, negligible; 0.10-0.29, small; 0.30-0.49, moderate; 0.50-0.69, large (L); and >0.7, very large (VL). BSI, bone stress injury; EF, effect size; NS, not significant.
Predictors of Bone Mass and BMD
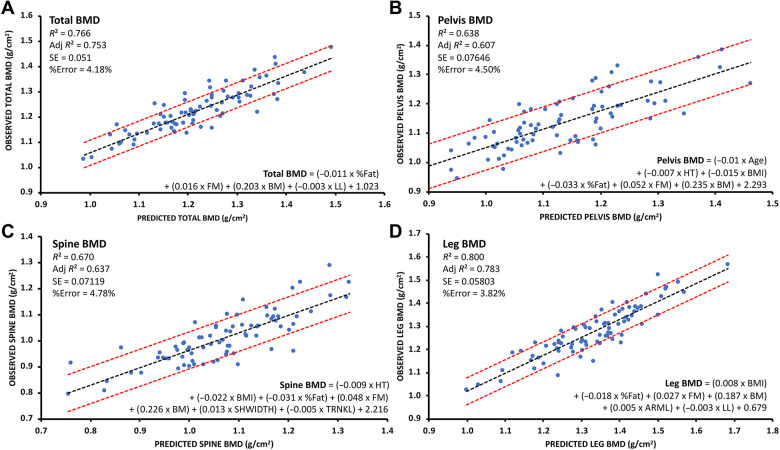
Multiple linear regression analysis revealed that BComp and anthropometric measures were predictive of total bone mass (bone mass = [0.046 × age] + [0.024 × weight] + [0.014 × %BF] + [-0.017 × arm length] + [0.017 × shoulder width] + [-0.009 × trunk length] + [0.037 × leg length] −2.867; P < .05; R 2 = 0.61; percentage error, 11.01%). In addition to predicted bone mass, other BComp and anthropometric measures (age, height, weight, BMI, fat mass, %BF, bone mass, limb length, shoulder width, trunk length, and arm length) were predictive of total and regional BMD (P < .05; R 2 = 0.64-0.80; percentage error, 3.8% to 4.8%) (Figure 3).
Figure 3.
Graphs showing R 2, adj R 2, SE, and %error for results of multiple linear regression analysis to predict (A) total, (B) pelvis, (C) spine, and (D) leg bone mineral density (BMD). Red dashed lines on either side of the line of best fit indicate SE. Adj, adjusted; ARML, arm length (cm); BM, total bone mass (kg); BMI, body mass index; FM, fat mass (kg); HT, height; LL, leg length (cm); SHWIDTH, shoulder width (cm); TRNKL, trunk length (cm).
Comparison of Athletes With and Without BSI
A physiologic comparison of athletes with and without long-bone BSIs is shown in Table 2. No significant differences were found in demographic characteristics between the BSI and no-BSI groups. BComp values in male runners showed fat mass to be significantly lower in BSI compared with no BSI, whereas in female runners, leg lean mass in the BSI group was significantly lower versus no BSI. Among all athletes, the BSI group had significantly shorter arm and leg limb lengths as well as a lower leg/trunk ratio compared with the no-BSI group.
TABLE 2.
Physiologic Comparison Between the BSI and no-BSI Groups a
| All Athletes | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BSI (n = 18) |
No BSI (n = 61) |
P (ES b ) | BSI (n = 7) |
No BSI (n = 35) |
P (ES b ) | BSI (n = 11) |
No BSI (n = 26) |
P (ES b ) | |
| Demographics | |||||||||
| Height, cm | 169.8 ± 4.1 | 173.9 ± 2.0 | NS | 176.7 ± 6.1 | 178.5 ± 1.8 | NS | 165.3 ± 3.6 | 167.6 ± 2.4 | NS |
| Weight, kg | 58.6 ± 3.8 | 62.0 ± 1.8 | NS | 65.1 ± 5.6 | 65.8 ± 1.9 | NS | 54.4 ± 3.1 | 57.0 ± 2.0 | NS |
| Age, years | 19.5 ± 0.7 | 20.1 ± 0.4 | NS | 20.4 ± 1.5 | 20.1 ± 0.5 | NS | 19.0 ± 0.5 | 20.1 ± 0.6 | NS |
| BMI, kg/m2 | 20.6 ± 0.7 | 20.7 ± 0.4 | NS | 21.2 ± 0.4 | 20.8 ± 0.5 | NS | 20.3 ± 1.0 | 20.5 ± 0.6 | NS |
| Body composition | |||||||||
| %BF | 17.2 ± 3.1 | 15.5 ± 1.4 | NS | 9.9 ± 1.8 | 11.7 ± 0.7 | NS | 21.8 ± 2.1 | 20.7 ± 1.5 | NS |
| Fat mass, kg | 9.8 ± 1.6 | 9.5 ± 0.8 | NS | 6.4 ± 0.7 | 7.7 ± 0.5 | .041 (1.01 [VL]) |
11.9 ± 1.5 | 11.9 ± 1.1 | NS |
| Fat-free mass, kg | 48.8 ± 4.6 | 52.6 ± 2.0 | NS | 58.8 ± 5.8 | 58.1 ± 1.7 | NS | 42.5 ± 2.3 | 45.1 ± 1.5 | NS |
| Lean mass, kg | 46.6 ± 4.5 | 49.9 ± 1.9 | NS | 56.4 ± 5.6 | 55.4 ± 1.6 | NS | 40.3 ± 2.1 | 42.73 ± 1.5 | NS |
| Legs lean mass, kg | 15.5 ± 1.2 | 17.1 ± 0.7 | .042 (.53 [L]) |
18.6 ± 2.0 | 18.8 ± 0.6 | NS | 13.5 ± 0.7 | 14.7 ± 0.6 | .035 (0.82 [VL]) |
| Trunk lean mass, kg | 23.1 ± 2.3 | 24.3 ± 0.9 | NS | 28.1 ± 2.8 | 26.8 ± 0.8 | NS | 19.9 ± 1.2 | 20.9 ± 0.8 | NS |
| Arms lean mass, kg | 4.9 ± 0.6 | 5.5 ± 0.3 | NS | 6.2 ± 0.9 | 6.4 ± 0.3 | NS | 4.0 ± 0.3 | 4.2 ± 0.2 | NS |
| Skeletal dimensions | |||||||||
| Leg length, cm | 91.9 ± 2.6 | 95.6 ± 1.4 | .015 (0.67 [L]) |
96.0 ± 3.9 | 98.4 ± 1.6 | NS | 89.3 ± 2.4 | 91.9 ± 1.6 | NS |
| Arm length, cm | 53.8 ± 1.5 | 56.1 ± 0.8 | .006 (0.74 [VL]) |
56.3 ± 1.9 | 57.7 ± 0.7 | NS | 52.2 ± 1.5 | 53.9 ± 1.0 | NS |
| Trunk length, cm | 46.6 ± 1.5 | 46.8 ± 0.8 | NS | 48.9 ± 2.4 | 48.0 ± 1.0 | NS | 45.1 ± 1.0 | 45.1 ± 0.8 | NS |
| Shoulder width, cm | 37.9 ± 1.3 | 39.1 ± 0.6 | NS | 40.7 ± 1.2 | 40.5 ± 0.6 | NS | 36.2 ± 1.0 | 37.3 ± 0.6 | NS |
| Leg/trunk ratio | 1.98 ± 0.03 | 2.05 ± 0.05 | .027 (0.60 [L]) |
1.97 ± 0.08 | 2.06 ± 0.05 | NS | 1.98 ± 0.06 | 2.04 ± 0.04 | NS |
a Data are presented as mean ± 95% CI. BF, body fat; BMI, body mass index; BSI, bone stress injury; ES, effect size; L, large effect size; NS, not significant; VL, very large effect size.
b Cohen d effect sizes: 0.0-0.09 = negligible; 0.10-0.29 = small; 0.3-0.49 = moderate; 0.5-0.69 = large; >0.7, very large.
Table 3 shows male and female athlete BMD relative to population norms by age-matched Z scores. In comparison with age-matched Z score within both male/female BSI and no-BSI groups, observed total, spine, and leg BMD values were clinically normal and not indicative of osteopenia (Z < -1) or osteoporosis (Z < -2.5) relative to general population norms. However, pelvis BMD Z score in both male (-1.2 ± 0.3) and female (-1.6 ± 0.3) runners in the BSI group were less than -1 and met the criteria for osteopenia.
TABLE 3.
Athlete BMD Relative to Population Norms by Age-Matched Z Score a
| Male | Female | |||
|---|---|---|---|---|
| BSI | No BSI | BSI | No BSI | |
| Total body | -0.1 ± 0.6 | 0.5 ± 0.3 | -0.1 ± 0.6 | 0.9 ± 0.3 |
| Spine | 0.7 ± 0.4 | 1.1 ± 0.2 | -0.4 ± 0.7 | 1.0 ± 0.4 |
| Pelvis | -1.2 ± 0.3 | -0.9 ± 0.2 | -1.6 ± 0.3 | -0.9 ± 0.3 |
| Legs | 0.0 ± 0.6 | 0.5 ± 0.2 | 0.2 ± 0.7 | 1.2 ± 0.4 |
a Data are presented as mean ± 95% CIfor age-matched Z scores among male and female athletes with (BSI) and without (No BSI) the presence of long BSI. Age-matched Z score is defined as the difference between the study participant’s BMD and an age-matched reference group divided by general population SD. 18 Similar to the diagnostic criteria for spine, femoral neck, and forearm, criteria for total and regional low BMD was defined as follows: osteopenia, Z score < -1; osteoporosis, Z score < -2.5. 18 BMD, bone mineral density; BSI, bone stress injury.
Discussion
To our knowledge, our investigation is the first to examine bone health, BComp, and anthropometric (ie, skeletal dimension) parameters in collegiate distance runners within 3 months prior to, or in the absence of, a sport-related lower limb BSI during a competitive season. The results indicated that the injured male and female distance runners have significantly lower total and regional BMD (Figure 2) as well as unique sex-specific tissue (ie, fat and lean) differences and shorter limb lengths compared with noninjured distance runners (Table 2). Also, certain body composition, skeletal dimensions, and demographics were predictive of total and regional BMD within a 3% to 5% error range (R 2 = 0.61-0.78) in this sport population.
These findings provide insight into bone, BComp, and anthropometric characteristics of at-risk athletes within a specialized collegiate sport population known to have a high prevalence of lower limb BSIs. The data can be used to contribute to new normative BMD benchmarks to assess injury risk as well as potentially provide, after future research to establish reliability, a tool to assess BMD for those without readily available access to DXA technology using our novel prediction equations. For example, health practitioners may be able to use such benchmarks to identify lower limb BSI at-risk runners in (1) university athletic programs without accessibility to a DXA, (2) preseason use before undergoing in-season, sport-related running activities, and (3) continual monitoring throughout the fall and spring competitive seasons.
BSI in Distance Runners
In our study sample, within 3 months of a fall-season DXA scan during a competitive season, we observed a mean lower limb BSI incidence rate of 22.8%, with respective incidences in our female distance runners trending higher compared with male runners. These BSI rates are similar to sports medicine clinics reporting up to 20%, and track and field teams, which range between 10% and 31%. 12,13 Also, our rates align with other collegiate track and field distance runner injury data stating that annual BSI rates can be higher than 20%. 12,38 Moreover, previous research in track and field athletes notes that female runners have higher incidences of BSI compared with their male counterparts with recent findings supporting these trends and reporting that female distance runners are associated with greater BSI occurrences. 15,30 Thus, our study sample’s BSI incidence rates are like previous findings, suggesting our data are representative of a common injury within this specialized sport population.
Our first important finding was that total and regional measures of BMD were lower in distance runners prior to first collegiate lower limb BSI than in athletes who were without injury. Low BMD is an intrinsic risk factor for a BSI and, when combined with extrinsic risk factors such as rapid change in frequency, mileage, pace, or terrain as observed during a competitive season, it can increase the likelihood of injury. 30,33 However, when assessing surrogates of bone strength like BMD via DXA to determine their relationship to BSI risk, the findings remain unclear. 4,5,9 This may be because, when compared with inactive controls, running activity and running athletes are generally associated with equivalent or slightly greater BMD, 33 as observed within our study sample, in which the lower total-body BMD of the injured athletes compared with age-matched Z scores were not indicative of low BMD (ie, Z score of ≤ -1) 31 relative to general population standards. We find it likely that comparing total-body BMD measures with general population norms may be an inappropriate determinant of injury risk in this highly active athletic population exposed to elevated physiological stresses. Therefore, the data presented here may assist in setting normative benchmarks specific to this sport population in efforts to improve risk assessment and mitigate potential lower limb BSI. For example, based on the present data, it may be desirable for collegiate distance runners to stay above the lower bound confidence intervals provided for those within the no-BSI group (Figure 2).
Sex-Based and Demographic Differences
Our BComp measures indicated sex-specific tissue differences (ie, fat and lean) in runners prior to injury in comparison with noninjured runners. For example, in our female runners, prior to a lower limb BSI, leg lean mass was lower than in female runners who did not sustain a respective BSI (Table 2). This finding of lower limb lean mass in women has been previously reported among White track and field athletes and identified to be a significant risk factor for BSI. 5 In our injured male runners, total-body fat mass was lower than in noninjured male runners. According to recent normative DXA data within NCAA Division I-A male distance runners, mean total-body fat mass was 7.9 ± 2.1 kg and similar to the mean noninjured male runners in our cohort (7.7 ± 0.5 kg). 10 However, our injured male runners’ total-body fat mass was lower, at 6.4 ± 0.7 kg. Thus, these observed sex-specific differences of lower fat or lean tissue content between the BSI and no-BSI groups may be indicative of a unique response within each sex during the competitive season, possibly related to reduced energy availability. Given the significant daily energy requirements for this sport activity during a competitive collegiate season, 25 insufficient energy intake, whether intentional or unintentional, 3 that subsequently limits available energy pools for normal physiological functions, 29 may also alter tissue quantities differently between male and female runners. For example, when energy availability ([energy intake – energy expended during exercise]/lean mass) is low in female runners, a greater preference may look to preserve fat tissue levels and support other vital physiological functions such as menstruation instead of preserving other metabolically costly tissue such as lean muscle. 31 In male runners, however, low energy availability may preferentially preserve lean tissue until body fat levels become too low, as has been observed in other male athlete sport populations. Previous research in nonrunning male athletes reports a loss in muscle during times of low energy availability and extremely low DXA body fat levels (ie, 4%-5%). 11 Our injured male runners’ %BF levels were 9.9%, thus potentially not low enough to perturb lean mass levels, but potentially low enough to influence bone health. 35 Nonetheless, further research related to distance runners, energy availability status, and sex-specific tissue changes is needed to confirm these observations as well as to ascertain whether these respective tissue changes are associated with altered bone health.
Skeletal Dimensions
A novel finding within this study was the significant difference in limb lengths between injured and noninjured distance runners (Table 2). More specifically, arm and leg limb lengths were shorter as well as smaller leg/trunk ratio within the runners who were injured. Biomechanical factors related to static alignment and anatomic issues such as leg-length discrepancy and smaller calf circumference are reported to contribute to BSI 5,19 ; however, our findings suggest limb length and skeletal dimensions may also contribute, or be related, to BSI. Since the etiology of a BSI is attributed largely to repeated mechanical loading, we find it possible that an increased frequency of foot contacts across a given distance in runners with shorter lower limbs may influence BSI risk and injury occurrence. 14,39 Furthermore, other extrinsic risk factors for BSI, such as running on hard training surfaces or running in older footwear that impairs proper shock absorption, could be exacerbated in runners with shorter limb lengths resulting from these greater foot strike frequencies. 14 Thus, due to the required weekly distances completed throughout a competitive season, limb length may be another important factor to consider in collegiate distance runners at risk for a lower limb BSI.
Noninvasively Predicting BMD in Collegiate Distance Runners
A second novel finding was that certain BComp and anthropometric measures were predictive of total bone mass and BMD. Recently, Lambert et al 21 demonstrated in elite male and female ballet dancers that several demographic and anthropometric measures (sex, height, weight, age, fat mass, lean mass, and %BF) were predictive of BMD. In this study, multiple linear regression similarly revealed that bone mass could be predicted using a combination of demographics (age, weight), BComp (%BF), and skeletal dimensions (shoulder width, trunk length, arm length, leg length) and then be used, in addition to other BComp and anthropometric measures, to predict total and regional BMD (Figure 3). It is understood that not every athletics program has access to DXA; however, our findings suggest simple BComp and anthropometric measures can provide an effective screening tool for health practitioners to identify runners with at-risk BMD values without formal DXA assessments at the beginning of a competitive season. This scenario could promote a proactive approach and provide athletes resources for additional follow-up evaluations in efforts to mitigate a lower limb BSI incidence.
Such field predictions may be of significant benefit among all competitive levels of collegiate athletics where DXA screening is impractical, cost-prohibitive, or unavailable. However, we note that the equations developed in this study for bone mass and BMD were specific to the population studied and should not be used for screening in other sport populations, as external validation studies are required to determine each model’s utility among other athletic populations with demographics and physiological characteristics akin to our study sample.
Limitations
This study is not without limitations. First, while the studied university track and field distance running team comprised athletes of mixed ethnicities, the distance runners in the study consisted only of White runners. However, Bulathsinhala et al 8 previously identified Whites to be at greatest risk for BSI in a cohort of 1.3 million United States Army soldiers. Regardless, as BMD differences have been reported between different ethnicities, further research will be required to establish BMD normative benchmarks for BSI risk and to determine the degree to which ethnicity should be incorporated in future BMD prediction models. Second, some our BMD prediction models include a predicted total bone mass; thus, additional variance is introduced within our total and regional BMD prediction models. Despite this known variability (∼11% percentage error for a single regression coefficient), we still observed significant correlations and relatively low percentage errors (<5%) for each model. Therefore, while not intended as diagnostic measures for osteoporosis or osteopenia (as with site-specific spine, hip, and wrist scans), these models remain sufficient as screening tools to assess risk and need for further evaluation (particularly when no other screening tools are available).
As a third limitation, this study was retrospective and observational in nature, with no additional data collection outside of the single fall-season total-body DXA scan and 3-month injury follow-up. Although all runners participated in the same progressive training program, sport-specific training/running loads, dietary intake, and menstrual history data were not recorded. However, it was estimated through sport coach interaction that their seasonal training volumes and intensities were comparable with those previously reported by Kurz et al 20 among NCAA Division I-A collegiate runners. Regardless, future studies will be required to determine how those factors, including race history, may be cross-referenced with the intrinsic risk factors identified here to assist in mitigating injury risk. Furthermore, future research must examine how each identified parameter specifically influences BSI risk and occurrence as well as validate their predictive value. For example, in recreational runners, it has been well-documented that increased lower extremity BSI rates are associated with increasing running distances beyond 32 km per week. 26 Last, randomized controlled trials are necessary to determine the effect various nutrition and training interventions have on these markers of bone and body composition health within this sport population.
Conclusion
The study findings highlight the need for normative BMD benchmarks within this specialized sport population to better assess at-risk athletes for a lower limb BSI. Lower, sex-specific, fat (male) and leg lean (female) tissue may also be indicative of lower limb bone injury risk as well as shorter limbs and smaller stature. These findings provide additional normative benchmarks unique to body composition and skeletal dimensions for at-risk athletes. Future research will be required to determine if these prediction models are applicable to other sport populations with similar physiological characteristics.
Acknowledgment
The authors acknowledge and thank Kansas Athletics Inc, particularly Natasha Hansen and the Performance Nutrition Program, and the student-athletes for the support of this research.
Footnotes
Final revision submitted August 20, 2021; accepted October 4, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: P.C.M. has received education payments from Arthrex and Smith & Nephew, consulting fees from Smith & Nephew, and honoraria and nonconsulting fees from Vericel. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of Kansas.
References
- 1. Barrack MT, Fredericson M, Tenforde AS, Nattiv A. Evidence of a cumulative effect for risk factors predicting low bone mass among male adolescent athletes. Br J Sports Med. 2017;51(3):200–205. doi:10.1136/bjsports-2016-096698 [DOI] [PubMed] [Google Scholar]
- 2. Barrack MT, Gibbs JC, De Souza MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad—related risk factors: a prospective multisite study of exercising girls and women. Am J Sports Med. 2014;42(4):949–958. doi:10.1177/0363546513520295 [DOI] [PubMed] [Google Scholar]
- 3. Beermann BL, Lee DG, Almstedt HC, McCormack WP. Nutritional intake and energy availability of collegiate distance runners. J Am Coll Nutr. 2020;39(8):747–755. doi:10.1080/07315724.2020.1735570 [DOI] [PubMed] [Google Scholar]
- 4. Bennell K, Crossley K, Jayarajan J, et al. Ground reaction forces and bone parameters in females with tibial stress fracture. Med Sci Sports Exerc. 2004;36(3):397–404. doi:10.1249/01.mss.0000117116.90297.e1 [DOI] [PubMed] [Google Scholar]
- 5. Bennell KL, Malcolm SA, Thomas SA, et al. Risk factors for stress fractures in track and field athletes: a twelve-month prospective study. Am J Sports Med. 1996;24(6):810–818. doi:10.1177/036354659602400617 [DOI] [PubMed] [Google Scholar]
- 6. Bilsborough JC, Greenway K, Opar D, Livingstone S, Cordy J, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821–1828. doi:10.1080/02640414.2014.926380 [DOI] [PubMed] [Google Scholar]
- 7. Buehring B, Krueger D, Libber J, et al. Dual-energy X-ray absorptiometry measured regional body composition least significant change: effect of region of interest and gender in athletes. J Clin Densitom. 2014;17(1):121–128. doi:10.1016/j.jocd.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 8. Bulathsinhala L, Hughes JM, McKinnon CJ, et al. Risk of stress fracture varies by race/ethnic origin in a cohort study of 1.3 million US army soldiers. J Bone Miner Res. 2017;32(7):1546–1553. 10.1002/jbmr.3131 [DOI] [PubMed] [Google Scholar]
- 9. Crossley K, Bennell KL, Wrigley T, Oakes BW. Ground reaction forces, bone characteristics, and tibial stress fracture in male runners. Med Sci Sports Exerc. 1999;31(8):1088–1093. doi:10.1097/00005768-199908000-00002 [DOI] [PubMed] [Google Scholar]
- 10. Dengel DR, Keller KA, Stanforth PR, Oliver JM, Carbuhn A, Bosch TA. Body composition and bone mineral density of Division 1 collegiate track and field athletes: a Consortium of College Athlete Research (C-CAR) Study. J Clin Densitom. 2020;23(2):303–313. doi:10.1016/j.jocd.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 11. Fagerberg P. Negative consequences of low energy availability in natural male bodybuilding: a review. Int J Sport Nutr Exerc Metab. 2018;28(4):385–402. doi:10.1123/ijsnem.2016-0332 [DOI] [PubMed] [Google Scholar]
- 12. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309–325. doi:10.1097/RMR.0b013e3180421c8c [DOI] [PubMed] [Google Scholar]
- 13. Fricker P, Purdam C. Stress fractures of the femoral shaft in athletes---more common than expected: a new clinical test. Am J Sports Med. 1995;23(3):372. doi:10.1177/036354659502300323 [DOI] [PubMed] [Google Scholar]
- 14. Harrast MA, Colonno D. Stress fractures in runners. Clin Sports Med. 2010;29(3):399–416. doi:10.1016/j.csm.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 15. Hollander K, Rahlf AL, Wilke J, et al. Sex-specific differences in running injuries: a systematic review with meta-analysis and meta-regression. Sports Med. 2021;51(5):1011–1039. doi:10.1007/s40279-020-01412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hutson MJ, O’Donnell E, Brooke-Wavell K, Sale C, Blagrove RC. Effects of low energy availability on bone health in endurance athletes and high-impact exercise as a potential countermeasure: a narrative review. Sports Med. 2021;51(3):391–403. doi:10.1007/s40279-020-01396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelsey JL, Bachrach LK, Procter-Gray E, et al. Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007;39(9):1457–1463. doi:10.1249/mss.0b013e318074e54b [DOI] [PubMed] [Google Scholar]
- 18. Kijowski R, Choi J, Shinki K, Del Rio AM, De Smet A. Validation of MRI classification system for tibial stress injuries. AJR Am J Roentgenol. 2012;198(4):878–884. doi:10.2214/ajr.11.6826 [DOI] [PubMed] [Google Scholar]
- 19. Korpelainen R, Orava S, Karpakka J, Siira P, Hulkko A. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001;29(3):304–310. doi:10.1177/03635465010290030901 [DOI] [PubMed] [Google Scholar]
- 20. Kurz MJ, Berg K, Latin R, Degraw W. The relationship of training methods in NCAA Division I cross-country runners and 10,000-meter performance. J Strength Cond Res. 2000;14:196–201. doi:10.1519/00124278-200005000-00013 [Google Scholar]
- 21. Lambert BS, Cain MT, Heimdal T, et al. Physiological parameters of bone health in elite ballet dancers. Med Sci Sports Exerc. 2020;52(8):1668–1678. doi:10.1249/mss.0000000000002296 [DOI] [PubMed] [Google Scholar]
- 22. Lauder TD, Dixit S, Pezzin LE, Williams MV, Campbell CS, Davis GD. The relation between stress fractures and bone mineral density: evidence from active-duty army women. Arch Phys Med Rehabil. 2000;81(1):73–79. doi:10.1016/s0003-9993(00)90225-9 [DOI] [PubMed] [Google Scholar]
- 23. Leib ES, Lewiecki EM, Binkley N, Hamdy RC. Official positions of the International Society for Clinical Densitometry. J Clin Densitom. 2004;7(1):1–6. doi:10.1385/jcd:7:1:1 [DOI] [PubMed] [Google Scholar]
- 24. Looker AC, Borrud LG, Hughes JP, Fan B, Shepherd JA, Sherman M. Total body bone area, bone mineral content, and bone mineral density for individuals aged 8 years and over: United States, 1999-2006. Vital Health Stat 11. 2013;253:1–78. [PubMed] [Google Scholar]
- 25. Ludbrook C, Clark D. Energy expenditure and nutrient intake in long-distance runners. Nutr Res. 1992;12(6):689–699. doi:10.1016/S0271-5317(05)80566-4 [Google Scholar]
- 26. Macera CA. Lower extremity injuries in runners: advances in prediction. Sports Med. 1992;13(1):50–57. doi:10.2165/00007256-199213010-00005 [DOI] [PubMed] [Google Scholar]
- 27. Melin AK, Heikura IA, Tenforde A, Mountjoy M. Energy availability in athletics: health, performance, and physique. Int J Sport Nutr Exerc Metab. 2019;29(2):152–164. doi:10.1123/ijsnem.2018-0201 [DOI] [PubMed] [Google Scholar]
- 28. Miller TL, Jamieson M, Everson S, Siegel C. Expected time to return to athletic participation after stress fracture in Division I collegiate athletes. Sports Health. 2018;10(4):340–344. doi:10.1177/1941738117747868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad---Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014;48(7):491–497. doi:10.1136/bjsports-2014-093502 [DOI] [PubMed] [Google Scholar]
- 30. Nattiv A. Stress fractures and bone health in track and field athletes. J Sci Med Sport. 2000;3(3):268–279. doi:10.1016/s1440-2440(00)80036-5 [DOI] [PubMed] [Google Scholar]
- 31. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. American College of Sports Medicine position stand: the female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–1882. doi:10.1249/mss.0b013e318149f111 [DOI] [PubMed] [Google Scholar]
- 32. Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18(1):529. doi:10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scofield KL, Hecht S. Bone health in endurance athletes: runners, cyclists, and swimmers. Curr Sports Med Rep. 2012;11(6):328–334. doi:10.1249/JSR.0b013e3182779193 [DOI] [PubMed] [Google Scholar]
- 34. Stanelle ST, Crouse SF, Heimdal TR, Riechman SE, Remy AL, Lambert BS. Predicting muscular strength using demographics, skeletal dimensions, and body composition measures. Sports Med Health Sci. 2021;3(1):34–39. doi:10.1016/j.smhs.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taguchi M, Moto K, Lee S, Torii S, Hongu N. Energy intake deficiency promotes bone resorption and energy metabolism suppression in Japanese male endurance runners: a pilot study. Am J Mens Health. 2020;14(1):15579 88320905251. doi:10.1177/1557988320905251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tenforde AS, Carlson JL, Sainani KL, et al. Lower trabecular bone score and spine bone mineral density are associated with bone stress injuries and triad risk factors in collegiate athletes. PM R. 2020;13:945–953. doi:10.1002/pmrj.12510 [DOI] [PubMed] [Google Scholar]
- 37. Tenforde AS, Parziale AL, Popp KL, Ackerman KE. Low bone mineral density in male athletes is associated with bone stress injuries at anatomic sites with greater trabecular composition. Am J Sports Med. 2018;46(1):30–36. doi:10.1177/0363546517730584 [DOI] [PubMed] [Google Scholar]
- 38. Tenforde AS, Nattiv A, Barrack MT, et al. Distribution of bone stress injuries in elite male and female collegiate endurance runners. Med Sci Sports Exerc. 2015;47(5 suppl):905.25207929 [Google Scholar]
- 39. Zadpoor AA, Nikooyan AA. The relationship between lower-extremity stress fractures and the ground reaction force: a systematic review. Clin Biomech (Bristol, Avon). 2011;26(1):23–28. doi:10.1016/j.clinbiomech.2010.08.005 [DOI] [PubMed] [Google Scholar]