Abstract
Different pathophysiologic mechanisms are involved in the initiation, development, and outcome of dry eye disease (DED). Animal models have proven valuable and efficient in establishing ocular surface microenvironments that mimic humans, thus enabling better understanding of the pathogenesis. Several dry eye animal models, including lacrimal secretion insufficiency, evaporation, neuronal dysfunction, and environmental stress models, are related to different etiological factors. Other models may be categorized as having a multifactorial DED. In addition, there are variations in the methodological classification, including surgical lacrimal gland removal, drug-induced models, irradiation impairment, autoimmune antibody-induced models, and transgenic animals. The aforementioned models may manifest varying degrees of severity or specific pathophysiological mechanisms that contribute to the complexity of DED. This review aimed to summarize various dry eye animal models and evaluate their respective characteristics to improve our understanding of the underlying mechanism and identify therapeutic prospects for clinical purposes.
Keywords: dry eye, animal model, lacrimal gland, tear deficiency, evaporative, environmental stress, translational research, DED
Introduction
Dry eye disease (DED) is one of the most common ocular surface disorders affecting millions of people worldwide (1–3). Its symptoms range from irritation and light sensitivity to blindness in severe cases (4–6). The tear film is the most important component of the ocular surface in maintaining microenvironment stability and providing lubrication to the cornea, thus maintaining its refractive function (7–9). Factors that affect the production or quality of tear film may lead to DED; these include but are not limited to lacrimal gland impairment, inflammation, infection, systemic autoimmune conditions, and environmental stress (10–14). The pathogenesis of DED is complicated, and much remains unknown. Animal models of DED are essential to better investigate the mechanisms underlying this multifactorial condition, explore potential therapeutic targets, and identify factors that can accurately predict prognosis. Conventionally, DED is classified into two categories, namely tear-deficient and evaporative (15). Recent evidence from a translational research using animal models demonstrated that tear film dysfunction involves multiple risk factors, with ocular surface inflammation an important component, where the resident immune cells initiate and propagate alterations in the ocular surface microenvironment (16, 17). Herein, we reviewed various dry eye animal models from published literature according to different modeling methods. We will discuss their therapeutic targets and relative advantages and disadvantages in this translational research.
Aqueous Deficient Dry Eye Types
Lacrimal Gland Excision/Radiation Models
The surgical removal of the lacrimal gland in mice and rats is the most commonly reported model in DED research. Generally, the main procedures for mice lacrimal gland excision are as follows: after anesthetic administration, an incision is made to expose the extraorbital and/or intraorbital lacrimal gland with the aid of stereoscopic microscopy, and glands are generally excised and removed using micro-forceps and Vannas scissors without injuring surrounding blood vessels and nerves. All excisions are made ipsilaterally, usually on the right side, and only a sham surgery with cutaneous incision is performed on the contralateral side simultaneously (18, 19).
Principally, the excision of the primary lacrimal gland immediately reduces aqueous tear secretion. Compromised tear production manifesting with a reduced Schirmer's test score is the major outcome (20). This model can also be applied to other animals, such as dogs, rabbits, cats, and monkeys (21–24). The aforementioned models have demonstrated reduced Schirmer's test values and decreased basal tear production. However, vast differences in ocular surface anatomy and physiology between these models lead to inconsistency in the extent of affecting tear production, the duration of ocular surface complications, and reversibility. This warrants the selection of an appropriate animal specific to the needs of each study. For example, according to studies on the rat and mouse dry eye model (19, 25), the excision of the extra-orbital lacrimal gland reduces the tear volume and results in significant corneal epitheliopathy. However, decreased tear production does not necessarily manifest signs of ocular surface disease (26). Contrarily, the severity of dry eye symptoms may vary between genders. Mecum et al. (18) reported on a gender predilection that female mice were more susceptible to lacrimal gland excision-induced corneal damage. Regarding conjunctival changes resulting from the lacrimal gland removal, the evidence seems to vary among different animal modes. Stevenson et al. (19) reported increased T helper 17-cell frequencies in the conjunctiva and draining lymph nodes after extraorbital lacrimal gland removal in female C57BL/6 mice over 14 days. In contrast, Maitchouk et al. (21) concluded that the main lacrimal gland removal is not related to keratoconjunctivitis sicca.
External beam radiation therapy is a risk factor for DED following the treatment of head and neck cancers (27–29). Thus, it can be used to induce DED in animals. Hakim et al. (30) reported on a rabbit dry eye model using 15 Gy external beam radiation, which resulted in the functional impairment of the lacrimal gland and reduced tear production. Rocha et al. (31) introduced serial radiation doses at 8 or 6 Gy, delivered over 5 consecutive days, and successfully induced dry eye syndrome in mice. They observed a considerably lower production of tear secretion in the radiation group as well as a reduction in the epithelial thickness of the cornea, the absence of basal epithelium, and the thickening of corneal stroma at 10 days. However, these additional differences were transient and disappeared at 56 days post-radiation. Thus, radiation-induced dry eye models are reproducible and involve reversible alterations that provide a platform for mechanistic research into the treatment and prognosis of DED (32). The nuclear factor of activated T cells 5 plays a potential role, whereas α-lipoic acid exerts a protective effect on this radiation-induced model (33). However, compared with the previous simple lacrimal gland removal method, this method requires special radioactive equipment and poses certain risks and collateral damage.
Blockage of Lacrimal Gland-Associated Nerve Pathways
The lacrimal nerve displays extensive sympathetic innervation, thus influencing tear production and composition (34). Therefore, researchers can develop aqueous-deficient models by blocking the afferent or efferent nerves associated with the lacrimal gland. Parasympathetic blockers are the first and foremost drugs. As a competitive antagonist of muscarinic acetylcholine receptors, atropine blocks the action of acetylcholine, thereby suppressing the function of the parasympathetic nervous system. In an albino rabbit dry eye model (35), atropine eye drops considerably reduced tear production and Schirmer's test scores within 2 days of onset. Aicher et al. (36) subcutaneously injected male Sprague-Dawley rats with 0.1% methyl atropine (1 mg/kg) twice daily for 2 days. Methyl atropine considerably reduced basal tear production compared with pretreatment baselines. This dry eye model can be implemented rapidly and easily; however, it is relatively simple and does not represent complicated pathophysiological processes in human dry eye syndrome.
Another method involves the use of neurturin-deficient (NRTN−/−) transgenic mice. Neurturin is a neurotrophic factor that regulates neuronal survival and function (37). Neurturin-deficient mice develop ocular surface inflammation similar to that observed in human DED. This transgenic mouse model substantially decreased aqueous tear production, tear fluorescein clearance, and corneal sensation (38). Moreover, it provided researchers with a method for better understanding the association between lacrimal gland denervation and ocular surface inflammation in DED. Nonetheless, its disadvantages are that the use and verification of this model are time-consuming.
Autoimmune Disease-Associated Dry Eye Models
A specific class of mice shares common characteristics with autoimmune DED, owing to the presence of a specific mutation or gene-editing technique. They release autoreactive lymphocytes to attack their lacrimal glands, consequently resulting in tear secretion deficiency. They can be categorized as experimental autoimmune disease-associated dry eye models (Table 1).
Table 1.
Autoimmune disease-associated dry eye models.
| Models | Modeling method | Modeling genre | Sex preference | Pathogenesis | Detectable serum antibodies | Pathogenic effects | References |
|---|---|---|---|---|---|---|---|
| NOD mouse | Spontaneously developed | SS-like | Male | CD4+ Th1 cell infiltration of the lacrimal gland | Anti-thyroid Ab Anti-ss-A/Ro Anti-b adrenergic R Ab Anti-a-fodrin Ab Anti-M3 muscarinic R Ab |
Pancreas, submandibular, and thyroid gland | (39) |
| NOD.B10.H2b mice | NOD mutant with an altered MHC region | SS-like | Male | Did not develop autoimmune diabetes, but displays lacrimal T-cell infiltration | Similar with NOD mouse | Similar to NOD mouse | (40) |
| MRL-1 pr/1pr mouse | Mutated Fas antigen | 2nd-SS. | Female | Serological manifestations characteristic of SS and exhibit lacrimal gland infiltration, predominantly by CD4– T cells | Anti-ds DNA Ab Anti-ss DNA Ab Anti-gp70 Ab Rheumatoid factor Anti-SA/Ro Anti-SS-B/La |
Sialadenitis Dacryoadenitis Kidney Joints blood vessels |
(41) |
| Id3-deficient mice | Gene knockout | pSS-like | ND | T-cell-dominant lymphocyte infiltration in both lacrimal and salivary glands | Anti-SSA/Ro Anti-SSB/La antibodies |
Reduced abilities to secrete tears and saliva | (42) |
| NFS/sld mouse | Spontaneous autosomal recessive mutation | pSS-like | Female | Lymphocytic infiltrates in exocrine glands are dominated by CD4+ T cells, with fewer CD8+ T cells and B cells | Anti-a-fodrin Ab | Inflammatory changes in the submandibular, parotid, and lacrimal glands | (43) |
| 3d-Tx NFS/sld mouse | Thymectomy of NFS/sld mice at 3 days of age | pSS-like | Female | Thymectomy impairs the expansion of regulatory T cells | ND | Severe than NFS/sld mouse | (44) |
| CD25KO mice | Interleukin 2 receptor alpha gene knockout | SS-like disease | ND | Worsening of corneal surface parameters and an increase of CD4+ T cell infiltrating the cornea | Anti-RBC antibody | Age-dependent SS-like autoimmune lacrimal-keratoconjunctivitis, dacryoadenitis, and corneal epithelial disease | (45, 46) |
| PD knock-in mouse | p65 S276D knock-in mice | KCS or SS-like | ND | Dependent on NF-κB; TNFR1-independent corneal inflammation | ND | Genetic and independent of decreased lacrimal function; Dacryoadenitis | (47) |
| IQI/Jic mouse | CR-derived inbred strain | 2nd-SS disease | Female | Focal lymphocyte infiltration and tissue destruction in the salivary glands (SG) and LG | Antinuclear autoantibodies | Salivary and lacrimal glands, pancreas, and lungs dysfunction | (48, 49) |
| Aly/aly mouse | Spontaneous autosomal recessive mutation | SS-like disease | No predilection | Chronic inflammatory cell (CD4+ T cell) infiltration in multiple organs | No detectable autoantibodies against nuclear components or salivary gland proteins | Cell infiltration in multiple organs, including the salivary and lacrimal glands, pancreas, skin, bones and lungs | (50) |
| TGF-b1 Knock out mouse | Gene knockout | SS-like disease | ND | Mainly CD4+ T cells infiltration | ND | Heart, lung, pancreas, lacrimal, salivary, and submandibular gland | (51, 52) |
KCS, keratoconjunctivitis sicca; ND, not determined; pSS, primary Sjögren's syndrome; and 2nd-SS, secondary Sjögren's syndrome.
Sjögren's syndrome is a systemic autoimmune disease that causes secretory gland dysfunction (53). Several genetically modified mice have been used to mimic Sjögren's syndrome in dry eye studies. The non-obese diabetic (NOD) mouse model is mostly used for type 1 diabetes mellitus (54). NOD mice are susceptible to the spontaneous development of autoimmune insulin-dependent diabetes mellitus (55). Moreover, it facilitates investigating the influence of autoimmune processes on dry eye syndrome (56, 57). Lymphocytic infiltration leads to the degradation of extracellular matrix structures in the lacrimal gland of NOD mice (58). Ju et al. (39) reported that NOD mouse lacrimal glands displayed increased lymphocytic infiltration. Furthermore, they demonstrated substantially increased expression of major histocompatibility (MHC) II and interferon-γ in the lacrimal gland at 12 and 20 weeks. However, this model demonstrated a higher incidence of dacryoadenitis in male NOD mice than in females (59), thus suggesting the spontaneous autoimmune response may be modulated by sex steroids, particularly testosterone. Autoimmune lesions in this model involve autoreactive Th1 cell secretions, including interleukin (IL)-10 and IL-12. Sjögren's syndrome in the NOD mouse model is an interleukin-4, time-dependent, antibody isotype-specific autoimmune disease (60). Recently, Robinson et al. reported on a NOD-derived murine model (40), where NOD.B10.H2b mice, comprising MHC congenic to NOD, exhibited exocrine gland lymphocytic infiltration typical of Sjögren's syndrome-like disease and dysfunction observed in NOD mice, but without diabetes. Thus, the NOD.B10.H2b mouse model is considered interesting for studying primary Sjögren's syndrome.
The MRL/1pr mouse is a model of autoimmune arteritis, antiphospholipid syndrome, and systemic lupus erythematosus-like autoimmune syndromes (61–63). The lpr gene is a mutated Fas antigen that leads to lymphoproliferative disease (64). This model demonstrates anti-Ro/Sjögren's-syndrome type A and anti-La/Sjögren's syndrome type B autoantibody production, a characteristic manifestation of Sjögren's syndrome, besides exhibiting lacrimal gland infiltration, predominantly by CD4+ T cells (41). Unlike the NOD mouse model, the extent of inflammation is considerably greater in the lacrimal glands of female MRL/lpr mice than that in males (65). Furthermore, MRL/lpr mice develop glomerulonephritis, which is classic in systemic lupus erythematosus but rare in Sjögren's syndrome (66). Therefore, the MRL /1pr mouse model is usually considered for secondary Sjögren's syndrome.
The inhibitor of DNA binding 3 (Id3) is an immediate early response gene involved in growth regulation and T-cell receptor-mediated T cell selection during its development (42). Id3-deficient mice develop lymphocyte infiltration in the lacrimal and salivary glands, reducing tear and saliva secretion and detectable anti-Ro and anti-La antibodies in the mouse serum. Similar to the clinical manifestations of primary Sjogren's syndrome, Id3-deficient mice serve as useful dry eye models for primary Sjogren's syndrome.
Conjunctival changes were also observed in autoimmune disease-related dry eye models. Using an autoimmune disease model mouse, BXSB/MpJ-Yaa, Hiraishi et al. (67) observed that goblet cell density in the conjunctiva epithelium decreased at 20 and 28 weeks compared to at 8 weeks. Wang et al. (68) reported spontaneous Sjögren-Like lacrimal keratoconjunctivitis in germ-free C57BL/6 mice, with significant goblet cell loss compared to conventional mice.
Researchers have reported other models, including the New Zealand Black and New Zealand White mouse (30, 35), the NFS/sld mouse (44), the IQI/Jic mouse (48, 69), the CD25 knockout mouse (45, 46), and the transforming growth factor β1 knockout mouse (51, 52). These autoimmune models affect the function of the lacrimal gland, eventually resulting in inadequate tear production. Depending on the characteristics of the specific model, they can be applied to different scenarios. Of all autoimmune dry eye models, the NOD mouse (39, 40) and the MRL-1 pr/1pr mouse model (41) are most commonly reported in scientific literature.
Evaporative Dry Eye Models
Environmental Stress-Induced Dry Eye Models
In addition to decreased tear production, DED can occur despite normal tear secretion in the context of significant environmental stressors. Numerous models utilize changes in the external environment, ventilation, humidity, or the forced exposure of the ocular surface to simulate evaporative DED.
Dursun et al. (70) introduced a desiccating environment by placing mice in a hood with a continuous airflow blower, with a flow rate of 300 ft/min at 7 s for 1 h, thrice per day for 4 days. Mice placed in the blower hood manifested the most severe ocular surface disease. Simsek et al. (71) used a model in which BALB/c male mice were exposed to an air fan inside a small compartment for 5 h per day for 3 days. The external environment considerably decreased the tear volume and increased corneal fluorescein and lissamine green staining scores. Moreover, the corneal subbasal nerve density was substantially damaged following exposure. Contrarily, several studies have introduced dry eye mouse models induced by air pollution particulate matter (PM), such as PM10 and urban particulate matter (UPM). PM10 impairs tear film function and destructs the structural organization of the ocular surface in mice. The topical administration of PM10 in mice induces ocular surface changes, similar to those induced by DED in humans (72). UPM exposure induces apoptosis in the corneal epithelium and decreases the number of goblet cells in the conjunctiva. Moreover, it affects the stability of the tear film by disrupting its mucin-4 layer (73). The advantage of these models, which simulate the real environment, is highly relevant to the development of environmentally-induced ocular surface diseases, including DED.
In another model, researchers introduced a lid retractor to prevent blinking. This model can be used in a short time and is easy to implement. It enables testing preventive and therapeutic strategies for DED (74). However, minimal changes in the ocular surface during a short preparation time limit its application in studying dry eye syndrome.
Other models exposed animals to low-humidity environments and continuous airflow. Chen et al. (75) established a murine model of DED using an intelligently controlled environmental system that maintained low humidity. Animals exposed to this environment exhibited decreased aqueous tear production, increased corneal fluorescein staining, and marked thinning and accelerated desquamation of the apical corneal epithelium compared with control eyes. The dry eye environment supposedly upregulated apoptosis on the ocular surface. Furthermore, biological and morphological changes in this model were similar to those in human DED. Barabino et al. (76) developed a controlled-environment chamber and confirmed that low humidity could substantially alter tear secretion, goblet cell density, and related ocular surface signs. Moreover, Nakamura et al. (77) combined a low-humidity environment, continuous airflow, and jogging board treatment, which mimicked both mental and physical stress, to induce abnormal tear dynamics and superficial punctate keratopathy, similar to that in humans.
Meibomian Gland Dysfunction Models
Meibomian glands produce lipids, which are important components of the tear film (78). In physiological states, they prevent or lessen tear film evaporation, serve as the superficial protective layer, and stabilize the tear film by lowering surface tension. Lipid deficiency can lead to dryness of the ocular surface, damage to the conjunctiva and corneal epithelium, and an imbalance of the ocular surface microenvironment (79).
Jester et al. (80) introduced a meibomian gland dysfunction (MGD) model in 34 albino rabbits by topically applying 2% epinephrine twice daily, over 6 months to 1 year. Sixty-eight (56%) rabbits developed signs of MGD. The development and progression of MGD in rabbits appeared to correlate with increasing stratification and keratinization of the meibomian gland duct epithelium. Mishima et al. reported another rabbit MGD model by squeezing out meibomian gland contents and cauterizing the lid margin. Thus, a protective oily film layer could not form over the eyes of the treated animals, eventually leading to rapid tear evaporation. In another rabbit MGD model that used light cautery on meibomian gland orifices, researchers observed increased tear osmolarity in the presence of normal lacrimal gland function and ocular surface abnormalities, similar to that in keratoconjunctivitis sicca (81).
Other types of MGD models comprise transgenic mouse models, including X-linked anhidrotic-hypohidrotic ectodermal dysplasia (Tabby), apolipoprotein C1 transgenic mice, and ACAT-1-/- mice (82, 83). The meibomian glands were absent or abnormal in the aforementioned mice (Table 2). Tabby mice sequentially developed corneal epithelial defects, central corneal stromal edema, and corneal neovascularization 8–16 weeks following birth (91). Despite reduced tear film breakup time and tear evaporation times, tear secretion remained normal. This model is useful for identifying novel therapeutic agents for evaporative DED.
Table 2.
Transgenic models of meibomian gland dysfunction.
| Models | Effect results | References |
|---|---|---|
| ACAT-1–/– | Similar to dry eye syndrome in humans | (83) |
| TRAF6–/– | Defective development of epidermal appendixes | (84) |
| K14-Noggin | Replacement of meibomian glands in eyelids with hair | (85) |
| Smad4CKO | Hair follicles in place of the meibomian glands | (86) |
| Barx2–/– | Defective lacrimal gland morphogenesis, defects in meibomian gland | (87) |
| Klf5CN | Defective eyelids with malformed meibomian glands, the abnormal cornea loss of conjunctival goblet cells | (88) |
| Fatp4–/– | Abnormal development of both sebaceous glands and meibomian glands, specialized sebaceous glands of the eyelids | (89) |
| Fgfr2CKO | Significant meibomian gland acinar atrophy and clinical manifestations of MGD | (90) |
MGD, meibomian gland dysfunction.
Chemical-Induced Dry Eye Models
Researchers have used chemical substances, drugs, or biological agents to develop a class of lacrimal gland injury models. The scopolamine-induced dry eye model is most commonly used. Simsek et al. (92) assessed morphological changes in the corneal subbasal nerve plexus in wild-type mice following exposure to scopolamine. They observed decreased tear volume and shortened tear film breakup time (TFBUT). Confocal microscopy revealed substantially lower mean corneal subbasal nerve fiber density and reflectivity in the scopolamine-treated groups. Furthermore, the mean tortuosity and mean dendritic cell density were considerably higher in this model. Viau et al. (93) induced dry eye symptoms using scopolamine in 6-week-old female Lewis rats. Scopolamine was delivered via subcutaneously implanted osmotic pumps. TNF-α, IL-1β, and IL-6 mRNA levels increased with scopolamine treatment in both the conjunctiva and ex-orbital lacrimal glands. All animals exhibited unilateral or bilateral keratitis after 17 days. The scopolamine-induced dry eye model could serve as a stable model of moderate severity for dry eye studies.
Benzalkonium chloride (BAK) is the most frequently used preservative in eye drops. Researchers have consistently demonstrated its toxic effects on the ocular surface (94–96). It causes tear film instability, the loss of goblet cells, conjunctival squamous metaplasia and apoptosis, the disruption of the corneal epithelium barrier, and damage to deeper ocular tissues (97). In a rabbit BAK toxicity model, researchers demonstrated damage to the conjunctiva-associated lymphoid tissue via the topical application of BAK (98). In addition, Pauly et al. (99) developed a technical model that closely resembled the human ocular surface environment. They used fluorescence techniques conjugated with confocal microscopy on a 3-D reconstructed corneal epithelial model and observed an increase in apoptotic cells from the superficial to the deeper layers.
Botulinum toxin (BTX) is a potent toxin widely used in modern medicine (100) and has been used in various dry eye models. Park et al. (101) developed a mouse tear-deficient dry eye model without lacrimal gland inflammation by injecting BTX-B into the lacrimal gland. This model could effectively induce dry eye in mice 2 and 4 weeks following injection. The lacrimal structures were adequately maintained without significant T lymphocyte infiltration. Moreover, there are reports of a BTX-A-induced DED model in male C57BL/6 mice (102), which has also been demonstrated to be stable.
Researchers have also used other agents to establish different mouse, rat, or rabbit models, such as the injection of human recombinant interleukin-1 and concanavalin A into lacrimal glands and the oral administration of finasteride. Table 3 summarizes the chemical-, biological agent-, and drug-induced dry eye animal models.
Table 3.
A summary of chemical-, biological agent-, and drug-induced dry eye animal models.
| Models | Modeling method | Demonstrated outcomes | References |
|---|---|---|---|
| C57BL/6 female mice | Subcutaneous injection of scopolamine hydrobromide, an exposure to an air draft, and 30% ambient humidity | Tear-deficiency dry eye | (103) |
| Male Sprague-Dawley rats | Topically administered 10% N-acetylcysteine | Mucin-deficiency dry eye | (104) |
| Rabbit | Topically administered 0.1% benzalkonium chloride | Both aqueous tear and mucin deficiency | (105) |
| Rabbit | Burned with 50% trichloroacetic acid | Conjunctival goblet cells damage | (22) |
| Rabbit | Trichloroacetic acid-treated and/or the removal of nictitating membrane | Stable KCS model, similar to surgical LG removal | (106) |
| Rat | Subcutaneous implantation of scopolamine micro-osmotic pumps | Moderate dry eye | (93) |
| Wistar rats | Bilateral ovariectomy in female rats and oral finasteride; both male and female rats challenged | Tear deficiency Androgen deficiency dry eye |
(107) |
| CBA/J mice | BTX-B injection into the LG | Mimic those in humans with non-SS, KCS | (108) |
| CBA/J mice | Transconjunctival injection of BTX-B into the lacrimal gland | Dry eye model without significant inflammatory cells infiltration | (109) |
| Female Wistar rats | Androgen deficiency dry eye model induced by finasteride | Androgen deficiency dry eye | (110) |
| Female BALB/c mice | A single injection of interleukin-1α into the lacrimal gland | A severe, but reversible inflammatory response in lacrimal gland acinar epithelial cells | (111, 112) |
BTX, botulinum toxin; KCS, keratoconjunctivitis sicca; LG, lacrimal gland; and SS, Sjögren's syndrome.
Discussion
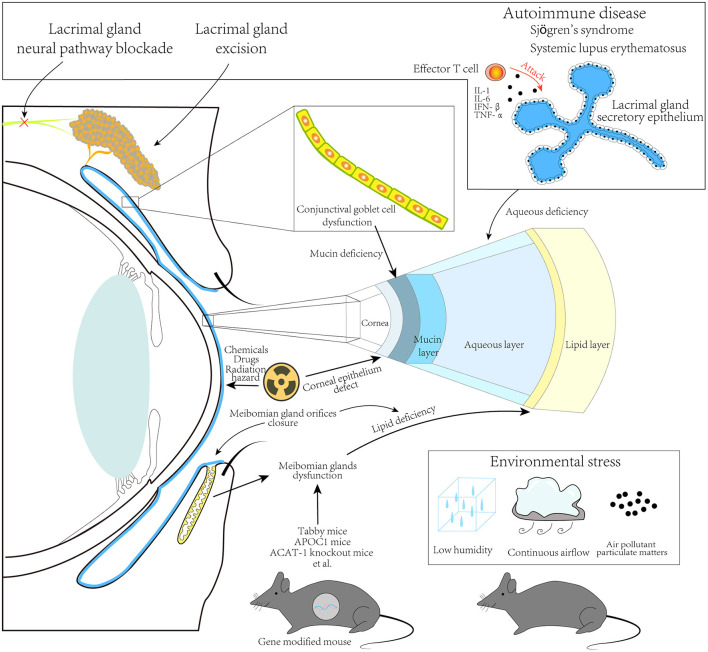
DED is a common ocular disorder that threatens the quality of life. Its symptoms vary from mild to severe, which are considered public health issues (113–115). The pathophysiologic mechanisms of DED comprise multiple factors that not only involve ocular surface inflammatory processes but are also related to systemic conditions (116, 117). Dry eye animal models for interpreting the underlying mechanisms are indispensable. We reviewed articles on such models, assessed their types, principles, and characters (Figure 1), and discussed their potential value for DED. A miscellaneous set of dry eye models has been created and provided to researchers. Their manifestations and severities vary according to the specific pathophysiological mechanism. Researchers should consider an appropriate model according to their objective.
Figure 1.
Principles for animal dry eye modeling. A schematic showing approaches to developing animal dry eye models, including major methods used in recently published studies, as described in this review. Lacrimal glands excision, neural pathway blocking, and autoimmune disease models, including Sjögren's syndrome and systemic lupus erythematosus, have been developed by targeting the lacrimal gland. These result in an aqueous deficiency in the tear film. Conjunctival goblet cell damage can result in mucin deficiency in the tear film. Further, chemicals, drugs, and radiation hazards mainly cause corneal epithelium damage. Some gene-modified mice can present with dysfunction or direct damage of the Meibomian glands, resulting in lipid deficiency in the tear film. Environmental stress due to changing humidity, controlling airflow, and/or introduction of air pollution particulate matter, could also be significant in animal dry eye modeling.
The surgical removal of lacrimal glands, chemical- or drug-induced lacrimal damage, and challenging environments are convenient and effective methods for achieving aqueous deficiency and evaporative dry eye models. Generally, aqueous deficiency and evaporative models are the major classifications (15). Researchers have developed models based on these classifications according to different implementation methods (Table 4). They share common traits, including rapid symptom occurrence, short implementation time, and reproducibility in different animals. Moreover, they decrease tear secretion production, which is ideal for assessing the efficacy of different therapies. However, these models have different prerequisites. Specifically, a mouse model using lacrimal gland excision could induce severe aqueous deficiency; More dedicated surgical techniques are required. In addition, lacrimal gland removal may not fully reproduce the complete clinical dry eye phenotype and systemic diseases associated with DED, including Sjögren's syndrome, rheumatoid arthritis, and systemic lupus erythematosus because it is merely artificial. For chemical-induced models, the use of 1.0% atropine sulfate eye drops in albino rabbits rapidly provided the required symptoms on day 2 of treatment. In addition, Schirmer test scores, corneal fluorescein staining, and Ferning tests confirmed dry eye signs (35). However, the observations were conducted only until day 5, without additional data.
Table 4.
Dry eye animal models.
| Animal models | Type | Species | Procedure | Experimental days | Effects to dry eye examinations | Pros | Cons | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tear secretion volume | Tear film breakup time | CFS | ||||||||
| Lacrimal gland excision | Aqueous deficiency | C57BL/6 WT mice | Exorbital and intraorbital lacrimal gland excision | 14 days | ↓16.6% | ND | ↑↑↑ | Inducing severe aqueous deficiency model | More surgically invasive | (118) |
| Radiation-induced model | Aqueous deficiency | female New Zealand rabbit | Radiation 15 Gy | 3 days | ↓ | ND | ND | Objective surrogate parameters for radiogenic dysfunction | Requires special radioactive equipment | (30) |
| Drug-induced | Aqueous deficiency | Male New Zealand albino rabbits | 1.0% atropine sulfate eye drop | 2 days | ↓↓ | ND | ↑ | Producing the required symptoms rapidly | Only observed for 5 days, no longer time observation data | (35) |
| Drug-induced | Lacrimal Gland Denervation | Male Sprague-Dawley rats | 192-IgG-saporin was microinjected into the lacrimal gland | 3–4 weeks | No changes | ND | ND | Useful for exploring the mechanism underlying corneal hypoalgesia. | Microsurgery requirements; Normal basal tear production | (36) |
| Autoimmune model | Aqueous deficiency | NOD mice | Derived from the outbred Jcl:ICR line of mice | 10–14 weeks | ↓↓ | 61.43% 1~2 second | ↑ | Ideal model for autoimmune related DED | Discrepancies between preclinical studies and clinical outcomes | (54, 56, 119, 120) |
| Autoimmune model | Aqueous deficiency | MRL/lpr mice | Derived from the MRL/n mouse strain | 16–18 weeks (female) 18–20 (male) |
↓ | ND | ND | A pivotal model for neurological SLE | Lack of data in the literature | (121, 122) |
| Autoimmune model | Aqueous deficiency | Id3-deficient mice | Gene knockout | 8 weeks | ↓ | ND | ND | Ideal primary Sjogren's syndrome model | Technical challenges in gene knockout | (42) |
| Autoimmune model | Aqueous deficiency | IQI/Jic mice | Developed from outbred ICR mice | At least 9 months | ND | ND | ND | Model for secondary Sjogren's syndrome model | The age of onset limited its application | (48, 69) |
| Environmental stress | Evaporative dry eye model | Balb/c male mice | Exposed to an air fan 5 hours a day for 3 days | 3 days | ↓↓↓ | ↓↓↓ | ↑↑↑ | Promising model to study the ocular surface and corneal nerve changes | Only male mice and acute alterations were assessed | (71) |
| Environmental stress | Evaporative dry eye mode | Rabbit | Eyes were held open with an eye specula | 1–3 h | ND | ND | ↑↑ | Simply and short-term to implement | Not suitable for mechanism research | (74) |
| Meibomian gland dysfunction | Evaporative dry eye mode | New Zealand rabbit | Meibomian gland orifices were closed by electrical coagulation or light cautery | 1–14 days | ↓↓↓ (Observed on day 1–3) | ↓ (On day 3, 7, 14) | No difference | Suitable for MGD related dry eye research | Biochemistry and biophysics differences between rabbit and human meibum | (81, 123) |
CFS, corneal fluorescein staining; DED, dry eye disease; ICR, imprinting control region; MGD, meibomian gland dysfunction; ND, not determined; and SLE, systemic lupus erythematosus.
↑, increased; ↓, decreased.
Evaporative DED models were developed by changing the feeding environment or imposing environmental stress on different animals. Animals were kept in a low-humidity environment or continuous airflow chambers for hours or days, which decreased tear film production, shortened the breakup time, and increased corneal fluorescence staining. The aforementioned environmental stress models are economical and easy to implement. Moreover, some studies focused on air pollution particulate matters, such as PM2.5, PM10 exposure, or simulating office working environments (72, 124, 125). These studies are valuable for investigating environmental factors of DED and the evaluation of related dry eye medicine therapies.
Evaporative dry eye models can be achieved by Meibomian gland dysfunction. One type involves the closure of the Meibomian gland orifices using electrical coagulation or light cautery. Lipid layer deficiency indirectly leads to aqueous layer evaporation. Usually, this type of model is combined with a low-humidity environment or rapid airflow to enhance evaporation. The abovementioned dysfunction of the Meibomian gland could provide tools for evaporative DED research; however, biochemical and biophysical differences between animal and human meibum limit its application. Other MGD models comprise transgenic models, such as ACAT-1, TRAF6, and Barx2 knockout mice, which exhibit the abnormal development of meibomian glands (83, 84, 87). These models are technically challenging, time-consuming, and expensive.
The short TFBUT-type dry eye model, with or without decreased tear secretion, highlights the importance of tear film stability in DED. Tear film stability is considered one of the important factors for understanding DED (126). Zhang Y et al. (127) developed a murine model based on graft-vs.-host disease (GVHD). Shimizu et al. (128) evaluated TFBUT in this GVHD-related model and observed significant differences in TFBUT, tear secretion, and corneal fluorescein scores between the syngeneic and GVHD groups from 9 to 12 weeks of age. Carpena-Torres et al. (129) reported on the topical instillation of 0.2% benzalkonium chloride for 5 consecutive days for establishing a dry eye model. The results demonstrated a significant difference in TFBUT before and after instillation; however, there was no difference in tear secretion. Different dry eye models are essential for understanding short TFBUT-type DED. Researchers have demonstrated a higher incidence of short TFBUT and concomitant keratoconjunctivitis in patients with thyroid eye disease (130, 131). Thus, these models facilitate understanding the etiology of short TBUT-type DED, particularly for patients clinically diagnosed with DED and normal tear secretion.
Dry eye models developed from autoimmune diseases provide insight into the immunopathogenic mechanisms of DED. NOD mice and MRL/lpr mice are typical systemic autoimmune disease models of Sjögren's syndrome, presenting multiple organ inflammatory lesions, including lacrimal gland damage that eventually results in aqueous deficiency. Both demonstrated significant lymphocytic infiltration of CD4+ T cells in the lacrimal gland. Dacryoadenitis revealed a higher incidence in male NOD mice than female mice. Compared with human Sjögren's syndrome, which is predominantly associated with female predilection, tear secretion was not profound in mouse models (57). MRL/lpr mice displayed more severe lacrimal gland inflammation in females than in males (65). Moreover, researchers observed a significant Th2 T cell response in the lacrimal gland of MRL/lpr mice, thus suggesting a mechanism different from the NOD mouse model (132). Nevertheless, these two models have been used for decades, with mature technology and sufficient research support, which are ideal dry eye models for DED.
This review summarized several major types of dry eye animal models and discussed their advantages and disadvantages in interpreting DED. This study had several limitations. It involved a limited number of animal species. The results of animal models differ considerably from human clinical manifestations. Moreover, they exhibit anatomical differences in the lacrimal gland system. Despite the variety of dry eye animal models, there are no widely homogeneous criteria for evaluating abnormalities of the ocular surface and the quality of tear film, thus making a model-wise comparison difficult.
Conclusion
Researchers have used several dry eye animal models in translational research, each focusing on different pathophysiological mechanisms of DED. Of these models, lacrimal gland excision is the easiest and most practical method, and it is widely used in dry eye research. However, it is relatively simple as it only reflects the aqueous deficiency component of DED. Several dry eye models, such as NOD mice, MRL/lpr mice, and specific transgenic models, provide researchers with a better understanding of the underlying mechanisms of DED and ideas for the development of novel biological treatments. Models based on environmental stress and drug toxicity may be more feasible for studies on real-world risk factors for DED. However, DED in humans is usually multifactorial in nature and involves complicated pathophysiological and immune responses. Therefore, no single dry eye animal model can serve as the best tool for research.
Author Contributions
TI: conceptualization and funding acquisition. TI and JZ: methodology, validation, and project administration. JZ: data curation and writing—original draft preparation. TI and KS: writing—review and editing. FC and AM: supervision. YO, KFujio, TH, KN, YA, KFujim, AY, MM, AM-I, KH, MK, HS, AE, and YM: review and suggestion. All authors have read and agreed to the publishing of the final version.
Funding
This research was supported by JSPS KAKENHI Grant Numbers 20K09810 (TI), 20KK0207 (TI), 20K22985 (KFujim), 21K16884 (KFujim), and 21K20996 (HS). JZ was the recipient of a fellowship provided by the Japan-China Sasakawa Medical Fellowship program (Grant Number. 2018314).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank the staff of the Laboratory of Molecular and Biochemical Research at the Research Support Center of the Juntendo University Graduate School of Medicine for their technical assistance.
References
- 1.Uchino M. What we know about the epidemiology of dry eye disease in Japan. Invest Ophthalmol Vis Sci. (2018) 59:DES1–6. 10.1167/iovs.17-23491 [DOI] [PubMed] [Google Scholar]
- 2.Osae AE, Gehlsen U, Horstmann J, Siebelmann S, Stern ME, Kumah DB, et al. Epidemiology of dry eye disease in Africa: the sparse information, gaps and opportunities. Ocul Surf. (2017) 15:159–68. 10.1016/j.jtos.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 3.Donthineni PR, Das AV, Basu S. Dry eye disease in children and adolescents in India. Ocul Surf. (2020) 18:777–82. 10.1016/j.jtos.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 4.Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. (2014) 157:799–806. 10.1016/j.ajo.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inomata T, Iwagami M, Nakamura M, Shiang T, Yoshimura Y, Fujimoto K, et al. Characteristics and risk factors associated with diagnosed and undiagnosed symptomatic dry eye using a smartphone application. JAMA Ophthalmol. (2020) 138:58–68. 10.1001/jamaophthalmol.2019.4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhadva P, Alexander A, McClellan AL, McManus KT, Seiden B, Galor A. The impact of conjunctivochalasis on dry eye symptoms and signs. Invest Ophthalmol Vis Sci. (2015) 56:2867–71. 10.1167/iovs.14-16337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. (1998) 17:565–96. 10.1016/S1350-9462(98)00004-4 [DOI] [PubMed] [Google Scholar]
- 8.Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res. (2004) 23:449–74. 10.1016/j.preteyeres.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Willshire C, Bron AJ, Gaffney EA, Pearce EI. Basal tear osmolarity as a metric to estimate body hydration and dry eye severity. Prog Retin Eye Res. (2018) 64:56–64. 10.1016/j.preteyeres.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 10.Rhee MK, Mah FS. Inflammation in dry eye disease: how do we break the cycle? Ophthalmology. (2017) 124:S14–9. 10.1016/j.ophtha.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Miguel A, Teson M, Martin-Montanez V, Enriquez-de-Salamanca A, Stern ME, Gonzalez-Garcia MJ, et al. Clinical and molecular inflammatory response in sjogren syndrome-associated dry eye patients under desiccating stress. Am J Ophthalmol. (2016) 161:133–41. 10.1016/j.ajo.2015.09.039 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Dana R. Autoimmunity in dry eye disease - an updated review of evidence on effector and memory Th17 cells in disease pathogenicity. Autoimmun Rev. (2021) 20:102933. 10.1016/j.autrev.2021.102933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceribelli A, Cavazzana I, Cattaneo R, Franceschini F. Hepatitis C virus infection and primary sjogren's syndrome: a clinical and serologic description of 9 patients. Autoimmun Rev. (2008) 8:92–4. 10.1016/j.autrev.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. (2017) 124:S20–6. 10.1016/j.ophtha.2017.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007) . Ocul Surf. (2007) 5:75–92. 10.1016/S1542-0124(12)70081-2 [DOI] [PubMed] [Google Scholar]
- 16.Inomata T, Hua J, Nakao T, Shiang T, Chiang H, Amouzegar A, et al. Corneal tissue from dry eye donors leads to enhanced graft rejection. Cornea. (2018) 37:95–101. 10.1097/ICO.0000000000001400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua J, Inomata T, Chen Y, Foulsham W, Stevenson W, Shiang T, et al. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci Rep. (2018) 8:7059. 10.1038/s41598-018-25384-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mecum NE, Cyr D, Malon J, Demers D, Cao L, Meng ID. Evaluation of corneal damage after lacrimal gland excision in male and female mice. Invest Ophthalmol Vis Sci. (2019) 60:3264–74. 10.1167/iovs.18-26457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson W, Chen Y, Lee SM, Lee HS, Hua J, Dohlman T, et al. Extraorbital lacrimal gland excision: a reproducible model of severe aqueous tear-deficient dry eye disease. Cornea. (2014) 33:1336–41. 10.1097/ICO.0000000000000264 [DOI] [PubMed] [Google Scholar]
- 20.Nemet A, Belkin M, Rosner M. Transplantation of newborn lacrimal gland cells in a rat model of reduced tear secretion. Isr Med Assoc J. (2007) 9:94–8. [PubMed] [Google Scholar]
- 21.Maitchouk DY, Beuerman RW, Ohta T, Stern M, Varnell RJ. Tear production after unilateral removal of the main lacrimal gland in squirrel monkeys. Arch Ophthalmol. (2000) 118:246–52. 10.1001/archopht.118.2.246 [DOI] [PubMed] [Google Scholar]
- 22.Li N, Deng X, Gao Y, Zhang S, He M, Zhao D. Establishment of the mild, moderate and severe dry eye models using three methods in rabbits. BMC Ophthalmol. (2013) 13:50. 10.1186/1471-2415-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helper LC, Magrane WG, Koehm J, Johnson R. Surgical induction of keratoconjunctivitis sicca in the dog. J Am Vet Med Assoc. (1974) 165:172–4. [PubMed] [Google Scholar]
- 24.McLaughlin SA, Brightman AH, Helper LC, Primm ND, Brown MG, Greeley S. Effect of removal of lacrimal and third eyelid glands on Schirmer tear test results in cats. J Am Vet Med Assoc. (1988) 193:820–2. [PubMed] [Google Scholar]
- 25.Skrzypecki J, Tomasz H, Karolina C. Variability of dry eye disease following removal of lacrimal glands in rats. Adv Exp Med Biol. (2019) 1153:109–15. 10.1007/5584_2019_348 [DOI] [PubMed] [Google Scholar]
- 26.Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. (2004) 45:1641–6. 10.1167/iovs.03-1055 [DOI] [PubMed] [Google Scholar]
- 27.Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Severe dry-eye syndrome following external beam irradiation. Int J Radiat Oncol Biol Phys. (1994) 30:775–80. 10.1016/0360-3016(94)90348-4 [DOI] [PubMed] [Google Scholar]
- 28.Jeganathan VS, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. (2011) 79:650–9. 10.1016/j.ijrobp.2010.09.056 [DOI] [PubMed] [Google Scholar]
- 29.Bhandare N, Moiseenko V, Song WY, Morris CG, Bhatti MT, Mendenhall WM. Severe dry eye syndrome after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys. (2012) 82:1501–8. 10.1016/j.ijrobp.2011.05.026 [DOI] [PubMed] [Google Scholar]
- 30.Hakim SG, Schroder C, Geerling G, Lauer I, Wedel T, Kosmehl H, et al. Early and late immunohistochemical and ultrastructural changes associated with functional impairment of the lachrymal gland following external beam radiation. Int J Exp Pathol. (2006) 87:65–71. 10.1111/j.0959-9673.2006.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocha EM, Cotrim AP, Zheng C, Riveros PP, Baum BJ, Chiorini JA. Recovery of radiation-induced dry eye and corneal damage by pretreatment with adenoviral vector-mediated transfer of erythropoietin to the salivary glands in mice. Hum Gene Ther. (2013) 24:417–23. 10.1089/hum.2012.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris DL, Yamaguchi T, Hamrah P. A novel murine model of radiation keratopathy. Invest Ophthalmol Vis Sci. (2018) 59:3889–96. 10.1167/iovs.18-24567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H, Yoo WS, Jung JH, Jeong BK, Woo SH, Kim JH, et al. Alpha-lipoic acid ameliorates radiation-induced lacrimal gland injury through NFAT5-dependent signaling. Int J Mol Sci. (2019) 20:5691. 10.3390/ijms20225691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding C, Walcott B, Keyser KT. Sympathetic neural control of the mouse lacrimal gland. Invest Ophthalmol Vis Sci. (2003) 44:1513–20. 10.1167/iovs.02-0406 [DOI] [PubMed] [Google Scholar]
- 35.Burgalassi S, Panichi L, Chetoni P, Saettone MF, Boldrini E. Development of a simple dry eye model in the albino rabbit and evaluation of some tear substitutes. Ophthalmic Res. (1999) 31:229–35. 10.1159/000055537 [DOI] [PubMed] [Google Scholar]
- 36.Aicher SA, Hermes SM, Hegarty DM. Denervation of the lacrimal gland leads to corneal hypoalgesia in a novel rat model of aqueous dry eye disease. Invest Ophthalmol Vis Sci. (2015) 56:6981–9. 10.1167/iovs.15-17497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. (1999) 22:253–63. 10.1016/S0896-6273(00)81087-9 [DOI] [PubMed] [Google Scholar]
- 38.Song XJ, Li DQ, Farley W, Luo LH, Heuckeroth RO, Milbrandt J, et al. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. (2003) 44:4223–9. 10.1167/iovs.02-1319 [DOI] [PubMed] [Google Scholar]
- 39.Ju Y, Janga SR, Klinngam W, MacKay JA, Hawley D, Zoukhri D, et al. NOD and NOR mice exhibit comparable development of lacrimal gland secretory dysfunction but NOD mice have more severe autoimmune dacryoadenitis. Exp Eye Res. (2018) 176:243–51. 10.1016/j.exer.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson CP, Yamachika S, Bounous DI, Brayer J, Jonsson R, Holmdahl R, et al. A novel NOD-derived murine model of primary Sjogren's syndrome. Arthritis Rheum. (1998) 41:150–6. [DOI] [PubMed] [Google Scholar]
- 41.Schrader S, Mircheff AK, Geerling G. Animal models of dry eye. Dev Ophthalmol. (2008) 41:298–312. 10.1159/000131097 [DOI] [PubMed] [Google Scholar]
- 42.Li H, Dai M, Zhuang Y, A T. cell intrinsic role of Id3 in a mouse model for primary sjogren's syndrome. Immunity. (2004) 21:551–60. 10.1016/j.immuni.2004.08.013 [DOI] [PubMed] [Google Scholar]
- 43.Hayashi Y, Kojima A, Hata M, Hirokawa K. A new mutation involving the sublingual gland in NFS/N mice. partially arrested mucous cell differentiation. Am J Pathol. (1988) 132:187–91. [PMC free article] [PubMed] [Google Scholar]
- 44.Haneji N, Hamano H, Yanagi K, Hayashi Y. A new animal model for primary Sjogren's syndrome in NFS/sld mutant mice. J Immunol. (1994) 153:2769–77. [PubMed] [Google Scholar]
- 45.De Paiva CS, Hwang CS, Pitcher JD3rd, Pangelinan SB, Rahimy E, Chen W, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. (2010) 49:246–58. 10.1093/rheumatology/kep357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahimy E, Pitcher JD3rd, Pangelinan SB, Chen W, Farley WJ, Niederkorn JY, et al. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. (2010) 177:744–53. 10.2353/ajpath.2010.091116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong J, Jimi E, Zeiss C, Hayden MS, Ghosh S. Constitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory disease. Genes Dev. (2010) 24:1709–17. 10.1101/gad.1958410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saegusa J, Kubota H. Sialadenitis in IQI/Jic mice: a new animal model of Sjogren's syndrome. J Vet Med Sci. (1997) 59:897–903. 10.1292/jvms.59.897 [DOI] [PubMed] [Google Scholar]
- 49.Jung JY, Saegusa J, Nakayama H, Doi K. Comparative study on picryl chloride (PCL)-induced contact dermatitis in female IQI/Jic and BALB/c mice. Exp Anim. (2004) 53:89–96. 10.1538/expanim.53.89 [DOI] [PubMed] [Google Scholar]
- 50.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. (1994) 24:429–34. 10.1002/eji.1830240224 [DOI] [PubMed] [Google Scholar]
- 51.McCartney-Francis NL, Mizel DE, Frazier-Jessen M, Kulkarni AB, McCarthy JB, Wahl SM. Lacrimal gland inflammation is responsible for ocular pathology in TGF-beta 1 null mice. Am J Pathol. (1997) 151:1281–8. [PMC free article] [PubMed] [Google Scholar]
- 52.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. (1992) 359:693–9. 10.1038/359693a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramos-Casals M, Brito-Zeron P, Siso-Almirall A, Bosch X. Primary sjogren syndrome. BMJ. (2012) 344:e3821. 10.1136/bmj.e3821 [DOI] [PubMed] [Google Scholar]
- 54.Reed JC, Herold KC. Thinking bedside at the bench: the NOD mouse model of T1DM. Nat Rev Endocrinol. (2015) 11:308–14. 10.1038/nrendo.2014.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. (1992) 51:285–322. 10.1016/S0065-2776(08)60490-3 [DOI] [PubMed] [Google Scholar]
- 56.Xiao W, Wu Y, Zhang J, Ye W, Xu GT. Selecting highly sensitive non-obese diabetic mice for improving the study of Sjogren's syndrome. Graefes Arch Clin Exp Ophthalmol. (2009) 247:59–66. 10.1007/s00417-008-0941-1 [DOI] [PubMed] [Google Scholar]
- 57.Humphreys-Beher MG, Hu Y, Nakagawa Y, Wang PL, Purushotham KR. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjogren's syndrome. Adv Exp Med Biol. (1994) 350:631–6. 10.1007/978-1-4615-2417-5_105 [DOI] [PubMed] [Google Scholar]
- 58.Schenke-Layland K, Xie J, Magnusson M, Angelis E, Li X, Wu K, et al. Lymphocytic infiltration leads to degradation of lacrimal gland extracellular matrix structures in NOD mice exhibiting a sjogren's syndrome-like exocrinopathy. Exp Eye Res. (2010) 90:223–37. 10.1016/j.exer.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi M, Ishimaru N, Yanagi K, Haneji N, Saito I, Hayashi Y. High incidence of autoimmune dacryoadenitis in male non-obese diabetic (NOD) mice depending on sex steroid. Clin Exp Immunol. (1997) 109:555–61. 10.1046/j.1365-2249.1997.4691368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao J, Killedar S, Cornelius JG, Nguyen C, Cha S, Peck AB. Sjogren's syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. (2006) 26:90–103. 10.1016/j.jaut.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 61.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. clinical and immunopathological manifestations in several strains. J Exp Med. (1978) 148:1198–215. 10.1084/jem.148.5.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith HR, Hansen CL, Rose R, Canoso RT. Autoimmune MRL-1 pr/1pr mice are an animal model for the secondary antiphospholipid syndrome. J Rheumatol. (1990) 17:911–5. [PubMed] [Google Scholar]
- 63.Moyer CF, Reinisch CL. Vasculitis in MRL/1 pr mice: model of cell-mediated autoimmunity. Toxicol Pathol. (1989) 17:122–8. 10.1177/019262338901700108 [DOI] [PubMed] [Google Scholar]
- 64.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. (1992) 356:314–7. 10.1038/356314a0 [DOI] [PubMed] [Google Scholar]
- 65.Toda I, Sullivan BD, Rocha EM, Da Silveira LA, Wickham LA, Sullivan DA. Impact of gender on exocrine gland inflammation in mouse models of Sjogren's syndrome. Exp Eye Res. (1999) 69:355–66. 10.1006/exer.1999.0715 [DOI] [PubMed] [Google Scholar]
- 66.Killian M, Batteux F, Paul S. The MRL/lpr mouse model: an important animal model for systemic sjogren syndrome and polyautoimmunity. J Rheumatol. (2020) 47:157. 10.3899/jrheum.190820 [DOI] [PubMed] [Google Scholar]
- 67.Hiraishi M, Masum MA, Namba T, Otani Y, Elewa YH, Ichii O, et al. Histopathological changes in tear-secreting tissues and cornea in a mouse model of autoimmune disease. Exp Biol Med. (2020) 245:999–1008. 10.1177/1535370220928275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C, Zaheer M, Bian F, Quach D, Swennes AG, Britton RA, et al. Sjogren-like lacrimal keratoconjunctivitis in germ-free mice. Int J Mol Sci. (2018) 19:565. 10.3390/ijms19020565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takada K, Takiguchi M, Konno A, Inaba M. Spontaneous development of multiple glandular and extraglandular lesions in aged IQI/Jic mice: a model for primary sjogren's syndrome. Rheumatology. (2004) 43:858–62. 10.1093/rheumatology/keh209 [DOI] [PubMed] [Google Scholar]
- 70.Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. (2002) 43:632–8. [PubMed] [Google Scholar]
- 71.Simsek C, Kojima T, Dogru M, Tsubota K. Alterations of murine subbasal corneal nerves after environmental dry eye stress. Invest Ophthalmol Vis Sci. (2018) 59:1986–95. 10.1167/iovs.17-23743 [DOI] [PubMed] [Google Scholar]
- 72.Li J, Tan G, Ding X, Wang Y, Wu A, Yang Q, et al. A mouse dry eye model induced by topical administration of the air pollutant particulate matter 10. Biomed Pharmacother. (2017) 96:524–34. 10.1016/j.biopha.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 73.Song SJ, Hyun SW, Lee TG, Park B, Jo K, Kim CS. New application for assessment of dry eye syndrome induced by particulate matter exposure. Ecotoxicol Environ Saf. (2020) 205:111125. 10.1016/j.ecoenv.2020.111125 [DOI] [PubMed] [Google Scholar]
- 74.Fujihara T, Nagano T, Nakamura M, Shirasawa E. Establishment of a rabbit short-term dry eye model. J Ocul Pharmacol Ther. (1995) 11:503–8. 10.1089/jop.1995.11.503 [DOI] [PubMed] [Google Scholar]
- 75.Chen W, Zhang X, Zhang J, Chen J, Wang S, Wang Q, et al. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol Vis Sci. (2008) 49:1386–91. 10.1167/iovs.07-0744 [DOI] [PubMed] [Google Scholar]
- 76.Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. (2005) 46:2766–71. 10.1167/iovs.04-1326 [DOI] [PubMed] [Google Scholar]
- 77.Nakamura S, Shibuya M, Nakashima H, Imagawa T, Uehara M, Tsubota K. D-beta-hydroxybutyrate protects against corneal epithelial disorders in a rat dry eye model with jogging board. Invest Ophthalmol Vis Sci. (2005) 46:2379–87. 10.1167/iovs.04-1344 [DOI] [PubMed] [Google Scholar]
- 78.Butovich IA. Lipidomics of human Meibomian gland secretions: chemistry, biophysics, and physiological role of meibomian lipids. Prog Lipid Res. (2011) 50:278–301. 10.1016/j.plipres.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. (2004) 2:149–65. 10.1016/S1542-0124(12)70150-7 [DOI] [PubMed] [Google Scholar]
- 80.Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction. II the role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. (1989) 30:936–45. [PubMed] [Google Scholar]
- 81.Gilbard JP, Rossi SR, Heyda KG. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. (1989) 96:1180–6. 10.1016/S0161-6420(89)32753-9 [DOI] [PubMed] [Google Scholar]
- 82.Jong MC, Gijbels MJ, Dahlmans VE, Gorp PJ, Koopman SJ, Ponec M, et al. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J Clin Invest. (1998) 101:145–52. 10.1172/JCI791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yagyu H, Kitamine T, Osuga J, Tozawa R, Chen Z, Kaji Y, et al. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J Biol Chem. (2000) 275:21324–30. 10.1074/jbc.M002541200 [DOI] [PubMed] [Google Scholar]
- 84.Naito A, Yoshida H, Nishioka E, Satoh M, Azuma S, Yamamoto T, et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci U S A. (2002) 99:8766–71. 10.1073/pnas.132636999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. (2004) 164:1099–114. 10.1016/S0002-9440(10)63197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang J, Dattilo LK, Rajagopal R, Liu Y, Kaartinen V, Mishina Y, et al. FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate. Development. (2009) 136:1741–50. 10.1242/dev.034082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsau C, Ito M, Gromova A, Hoffman MP, Meech R, Makarenkova HP. Barx2 and Fgf10 regulate ocular glands branching morphogenesis by controlling extracellular matrix remodeling. Development. (2011) 138:3307–17. 10.1242/dev.066241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kenchegowda D, Swamynathan S, Gupta D, Wan H, Whitsett J, Swamynathan SK. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol. (2011) 356:5–18. 10.1016/j.ydbio.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin MH, Hsu FF, Miner JH. Requirement of fatty acid transport protein 4 for development, maturation, and function of sebaceous glands in a mouse model of ichthyosis prematurity syndrome. J Biol Chem. (2013) 288:3964–76. 10.1074/jbc.M112.416990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reneker LW, Wang L, Irlmeier RT, Huang AJW. Fibroblast growth factor receptor 2 (FGFR2) is required for meibomian gland homeostasis in the adult mouse. Invest Ophthalmol Vis Sci. (2017) 58:2638–46. 10.1167/iovs.16-21204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang YC, Li S, Chen X, Ma B, He H, Liu T, et al. Meibomian gland absence related dry eye in ectodysplasin a mutant mice. Am J Pathol. (2016) 186:32–42. 10.1016/j.ajpath.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 92.Simsek C, Kojima T, Nagata T, Dogru M, Tsubota K. Changes in murine subbasal corneal nerves after scopolamine-induced dry eye stress exposure. Invest Ophthalmol Vis Sci. (2019) 60:615–23. 10.1167/iovs.18-26318 [DOI] [PubMed] [Google Scholar]
- 93.Viau S, Maire MA, Pasquis B, Gregoire S, Fourgeux C, Acar N, et al. Time course of ocular surface and lacrimal gland changes in a new scopolamine-induced dry eye model. Graefes Arch Clin Exp Ophthalmol. (2008) 246:857–67. 10.1007/s00417-008-0784-9 [DOI] [PubMed] [Google Scholar]
- 94.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. (2007) 17:341–9. 10.1177/112067210701700311 [DOI] [PubMed] [Google Scholar]
- 95.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. (2002) 86:418–23. 10.1136/bjo.86.4.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galletti JG, Gabelloni ML, Morande PE, Sabbione F, Vermeulen ME, Trevani AS, et al. Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol. (2013) 6:24–34. 10.1038/mi.2012.44 [DOI] [PubMed] [Google Scholar]
- 97.Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. (2010) 29:312–34. 10.1016/j.preteyeres.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 98.Liang H, Baudouin C, Labbe A, Riancho L, Brignole-Baudouin F. Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study. PLoS ONE. (2012) 7:e33913. 10.1371/journal.pone.0033913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pauly A, Meloni M, Brignole-Baudouin F, Warnet JM, Baudouin C. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: early detection of toxic damage. Invest Ophthalmol Vis Sci. (2009) 50:1644–52. 10.1167/iovs.08-2992 [DOI] [PubMed] [Google Scholar]
- 100.Jankovic J. Botulinum toxin: state of the art. Mov Disord. (2017) 32:1131–8. 10.1002/mds.27072 [DOI] [PubMed] [Google Scholar]
- 101.Park CY, Zhuang W, Lekhanont K, Zhang C, Cano M, Lee WS, et al. Lacrimal gland inflammatory cytokine gene expression in the botulinum toxin B-induced murine dry eye model. Mol Vis. (2007) 13:2222–32. [PubMed] [Google Scholar]
- 102.Kim DW, Lee SH, Ku SK, Cho SH, Cho SW, Yoon GH, et al. Transduced PEP-1-FK506BP ameliorates corneal injury in Botulinum toxin A-induced dry eye mouse model. BMB Rep. (2013) 46:124–9. 10.5483/BMBRep.2013.46.2.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi W, Lee JB, Cui L, Li Y, Li Z, Choi JS, et al. Therapeutic efficacy of topically applied antioxidant medicinal plant extracts in a mouse model of experimental dry eye. Oxid Med Cell Longev. (2016) 2016:4727415. 10.1155/2016/4727415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li X, Kang B, Woo IH, Eom Y, Lee HK, Kim HM, et al. Effects of topical mucolytic agents on the tears and ocular surface: a plausible animal model of mucin-deficient dry eye. Invest Ophthalmol Vis Sci. (2018) 59:3104–14. 10.1167/iovs.18-23860 [DOI] [PubMed] [Google Scholar]
- 105.Xiong C, Chen D, Liu J, Liu B, Li N, Zhou Y, et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. (2008) 49:1850–6. 10.1167/iovs.07-0720 [DOI] [PubMed] [Google Scholar]
- 106.Chen ZY, Liang QF, Yu GY. Establishment of a rabbit model for keratoconjunctivitis sicca. Cornea. (2011) 30:1024–9. 10.1097/ICO.0b013e3181f1b0fc [DOI] [PubMed] [Google Scholar]
- 107.Singh S, Moksha L, Sharma N, Titiyal JS, Biswas NR, Velpandian T. Development and evaluation of animal models for sex steroid deficient dry eye. J Pharmacol Toxicol Methods. (2014) 70:29–34. 10.1016/j.vascn.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 108.Zhu L, Shen J, Zhang C, Park CY, Kohanim S, Yew M, et al. Inflammatory cytokine expression on the ocular surface in the botulium toxin B induced murine dry eye model. Mol Vis. (2009) 15:250–8. [PMC free article] [PubMed] [Google Scholar]
- 109.Suwan-apichon O, Rizen M, Rangsin R, Herretes S, Reyes JM, Lekhanont K, et al. Botulinum toxin B-induced mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. (2006) 47:133–9. 10.1167/iovs.05-0380 [DOI] [PubMed] [Google Scholar]
- 110.Li K, Zhang C, Yang Z, Wang Y, Si H. Evaluation of a novel dry eye model induced by oral administration of finasteride. Mol Med Rep. (2017) 16:8763–70. 10.3892/mmr.2017.7754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. (2008) 49:4399–406. 10.1167/iovs.08-1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. (2007) 84:894–904. 10.1016/j.exer.2007.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clayton JA. Dry eye. N Engl J Med. (2018) 378:2212–23. 10.1056/NEJMra1407936 [DOI] [PubMed] [Google Scholar]
- 114.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. (2017) 15:334–65. 10.1016/j.jtos.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 115.Inomata T, Shiang T, Iwagami M, Sakemi F, Fujimoto K, Okumura Y, et al. Changes in distribution of dry eye disease by the new 2016 diagnostic criteria from the Asia dry eye society. Sci Rep. (2018) 8:1918. 10.1038/s41598-018-19775-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. (2012) 31:271–85. 10.1016/j.preteyeres.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inomata T, Nakamura M, Iwagami M, Shiang T, Yoshimura Y, Fujimoto K, et al. Risk factors for severe dry eye disease: crowdsourced research using dryeyerhythm. Ophthalmology. (2019) 126:766–8. 10.1016/j.ophtha.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 118.Shinomiya K, Ueta M, Kinoshita S. A new dry eye mouse model produced by exorbital and intraorbital lacrimal gland excision. Sci Rep. (2018) 8:1483. 10.1038/s41598-018-19578-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. (2005) 23:447–85. 10.1146/annurev.immunol.23.021704.115643 [DOI] [PubMed] [Google Scholar]
- 120.Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, et al. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. (2008) 30:212–21. 10.1016/j.jaut.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verhagen C, Rowshani T, Willekens B, van Haeringen NJ. Spontaneous development of corneal crystalline deposits in MRL/Mp mice. Invest Ophthalmol Vis Sci. (1995) 36:454–61. [PubMed] [Google Scholar]
- 122.Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model. Autoimmun Rev. (2014) 13:963–73. 10.1016/j.autrev.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 123.Eom Y, Han JY, Kang B, Hwang HS, Lee HK, Kim HM, et al. Meibomian glands and ocular surface changes after closure of meibomian gland orifices in rabbits. Cornea. (2018) 37:218–26. 10.1097/ICO.0000000000001460 [DOI] [PubMed] [Google Scholar]
- 124.Tan G, Li J, Yang Q, Wu A, Qu DY, Wang Y, et al. Air pollutant particulate matter 2. 5 induces dry eye syndrome in mice. Sci Rep. (2018) 8:17828. 10.1038/s41598-018-36181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim Y, Choi YH, Kim MK, Paik HJ, Kim DH. Different adverse effects of air pollutants on dry eye disease: Ozone, PM2.5, and PM10. Environ Pollut. (2020) 265:115039. 10.1016/j.envpol.2020.115039 [DOI] [PubMed] [Google Scholar]
- 126.Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. (2017) 124:S4–13. 10.1016/j.ophtha.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. J Immunol. (2002) 168:3088–98. 10.4049/jimmunol.168.6.3088 [DOI] [PubMed] [Google Scholar]
- 128.Shimizu E, Ogawa Y, Yazu H, Aketa N, Yang F, Yamane M, et al. “Smart Eye Camera”: An innovative technique to evaluate tear film breakup time in a murine dry eye disease model. PLoS ONE. (2019) 14:e0215130. 10.1371/journal.pone.0215130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carpena-Torres C, Pintor J, Huete-Toral F, Martin-Gil A, Rodriguez-Pomar C, Martinez-Aguila A, et al. Efficacy of artificial tears based on an extract of artemia salina containing dinucleotides in a rabbit dry eye model. Int J Mol Sci. (2021) 22:11999. 10.3390/ijms222111999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alanazi SA, Alomran AA, Abusharha A, Fagehi R, Al-Johani NJ, El-Hiti GA, et al. An assessment of the ocular tear film in patients with thyroid disorders. Clin ophthalmol. (2019) 13:1019–26. 10.2147/OPTH.S210044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takahashi Y, Lee PAL, Vaidya A, Kono S, Kakizaki H. Tear film break-up patterns in thyroid eye disease. Sci Rep. (2021) 11:5288. 10.1038/s41598-021-84661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jabs DA, Lee B, Whittum-Hudson JA, Prendergast RA. Th1 versus Th2 immune responses in autoimmune lacrimal gland disease in MRL/Mp mice. Invest Ophthalmol Vis Sci. (2000) 41:826–31. 10.1097/00003226-200011002-00086 [DOI] [PubMed] [Google Scholar]