Abstract
Background
A Delphi consensus was conducted to evaluate the influence of single nucleotide polymorphisms (SNPs) in genes encoding gonadotropin and gonadotropin receptors on clinical ovarian stimulation outcomes following assisted reproductive technology (ART) treatment.
Methods
Nine experts plus two Scientific Coordinators discussed and amended statements plus supporting references proposed by the Scientific Coordinators. The statements were distributed via an online survey to 36 experts, who voted on their level of agreement or disagreement with each statement. Consensus was reached if the proportion of participants agreeing or disagreeing with a statement was >66%.
Results
Eleven statements were developed, of which two statements were merged. Overall, eight statements achieved consensus and two statements did not achieve consensus. The statements reaching consensus are summarized here. (1) SNP in the follicle stimulating hormone receptor (FSHR), rs6166 (c.2039A>G, p.Asn680Ser) (N=5 statements): Ser/Ser carriers have higher basal FSH levels than Asn/Asn carriers. Ser/Ser carriers require higher amounts of gonadotropin during ovarian stimulation than Asn/Asn carriers. Ser/Ser carriers produce fewer oocytes during ovarian stimulation than Asn/Asn or Asn/Ser carriers. There is mixed evidence supporting an association between this variant and ovarian hyperstimulation syndrome. (2) SNP of FSHR, rs6165 (c.919G>A, p.Thr307Ala) (N=1 statement): Few studies suggest Thr/Thr carriers require a shorter duration of gonadotropin stimulation than Thr/Ala or Ala/Ala carriers. (3) SNP of FSHR, rs1394205 (−29G>A) (N=1 statement): Limited data in specific ethnic groups suggest that A/A allele carriers may require higher amounts of gonadotropin during ovarian stimulation and produce fewer oocytes than G/G carriers. (4) SNP of FSH β-chain (FSHB), rs10835638 (−211G>T) (N=1 statement): There is contradictory evidence supporting an association between this variant and basal FSH levels or oocyte number. (5) SNPs of luteinizing hormone β-chain (LHB) and LH/choriogonadotropin receptor (LHCGR) genes (N=1 statement): these may influence ovarian stimulation outcomes and could represent potential future targets for pharmacogenomic research in ART, although data are still very limited.
Conclusions
This Delphi consensus provides clinical perspectives from a diverse international group of experts. The consensus supports a link between some variants in gonadotropin/gonadotropin receptor genes and ovarian stimulation outcomes; however, further research is needed to clarify these findings.
Keywords: single nucleotide polymorphisms (SNPs), genetics, genetic variants, gonadotropins, FSHB/FSHR, LHB/LHCGR, ovarian stimulation and response, assisted reproductive technology (ART)
Introduction
Infertility is a significant global health problem and socioeconomic burden, affecting 15% of childbearing-age individuals worldwide and with an increasing prevalence over the last two decades (1). Assisted reproduction technology (ART) has provided a critical tool for addressing reproductive challenges in men and women (2–5). However, ART remains an area with unmet clinical needs. According to the International Committee for Monitoring Assisted Reproductive Technology (ICMART), the global in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) combined delivery rates per fresh aspiration and frozen embryo transfer cycles in 2013 were 24.2% and 22.8%, respectively, with a cumulative delivery rate per aspiration of 30.4% (6).
Delivery rate is closely associated with the number of oocytes retrieved during ovarian stimulation (7). This relationship is even more evident considering how frozen cycles contribute to cumulative live birth rate (8). In this sense, the goal is to safely retrieve the highest number of mature oocytes in order to get the highest percentage of delivery rate per initiated ovarian stimulation cycle for ART treatment.
A critical step of ART is ovarian stimulation using gonadotropins, the aim of which is to obtain an optimum number of mature oocytes without the risk of ovarian hyperstimulation syndrome (OHSS) (9). Responses to gonadotropin stimulation are highly variable and dependent on individual patient factors. The prediction of ovarian response is critical to enable optimal and individualized management of ovarian stimulation. Current ovarian stimulation protocols use several parameters to predict ovarian response and optimize the dose of gonadotropins accordingly, including age, body mass index, ovarian reserve tests such as anti-Müllerian hormone (AMH), antral follicular count (AFC), endocrine status and baseline serum follicle stimulating hormone (FSH) (10–12). In particular, AMH and AFC are widely considered the best predictors of ovarian potential (13–16). However, ovarian reserve cannot fully explain the individual response to ovarian stimulation. For instance, a subgroup of women with normal ovarian reserve but suboptimal or poor response (hypo-responders) have been described (17–19). These patients have an “unexpected” reduced response to ovarian stimulation and are characterized by low prognosis to ART (17, 20, 21). The mechanisms underlying this unexpected ovarian resistance to ovarian stimulation are not fully understood, but it is believed that an individual’s genetics play a significant role (17, 22).
Several gene association studies have identified specific single nucleotide polymorphisms [SNPs ( Box 1 )] of gonadotropins and their receptors that could influence ovarian response (26–28). These include SNPs of the FSH receptor (FSHR) gene, FSH β-chain (FSHB) gene, luteinizing hormone β-chain (LHB) gene and LH/choriogonadotropin receptor (LHCGR) gene ( Table 1 ). The identification of genetic variants that are able to predict ovarian response could pave the way to tailor ovarian stimulation on the basis of individual genotype profile. Unfortunately, these data are still controversial and, at times, contradictory or limited.
Box 1. Single Nucleotide Polymorphisms (SNPs).
SNPs can occur in both non-coding and coding sequences of genes.
SNPs in non-coding regions appear to affect transcription as well as non-coding RNAs that can also influence gene expression (23).
-
SNPs in coding regions can be synonymous or nonsynonymous.
Synonymous SNPs do not produce altered coding sequences, since some amino acids are coded for by more than one three-base-pair codon. Although these are often referred to as ‘silent’ polymorphisms, they can still affect the function of a gene (24).
Nonsynonymous SNPs can be missense (a single change of nucleotide base resulting in a change in amino acid) or nonsense (a point mutation in a sequence of DNA that results in premature termination of protein synthesis and in a truncated and usually non-functional protein product (25).
Specific SNPs are catalogued by researchers and databases according to a non-redundant accession number, the reference SNP cluster identifier (rs).
Table 1.
SNPs identified in gonadotropin isoforms and gonadotropin receptors.
| SNP | Gene | Nucleotide change | Amino acid substitution | Molecular characteristics: Homozygous vs heterozygous states | Clinical relevance: Homozygous vs heterozygous states |
|---|---|---|---|---|---|
| rs6165* | FSHR | c.919G>A | p.Thr307Ala | Amino acid substitution results in a change from a polar to a nonpolar, hydrophobic amino acid and removal of a potential O-linked glycosylation site (29, 30). | Higher number of retrieved oocytes (31) and MII oocytes (32), and shorter duration of ovarian stimulation in Ala/Ala carriers versus Thr/Thr or Ala/Thr carriers (31). |
| rs6166* | FSHR | c.2039A>G | p.Asn680Ser | Amino acid substitution results in a potential phosphorylation site in the intracellular domain of the receptor (29, 30). | Higher basal gonadotropin levels, higher r-hFSH consumption, decreased estradiol levels on the day of hCG administration, lower number of retrieved oocytes and a higher incidence of hypo-responders in Ser/Ser versus Asn/Asn carriers (33–37). Asn/Asn carriers at an increased risk of OHSS versus Ser/Ser carriers (38). |
| rs1394205 | FSHR | −29G>A | N/A | SNP in the promoter region of the FSHR, upstream of the translational initiation codon (39). | Reduced expression of FSHR and lower basal FSH levels in AA homozygotes versus GG homozygotes or AG heterozygotes (31, 39). |
| rs2293275 | LHCGR | c.935A>G | p.Asn312Ser | Amino acid substitution is located near a glycosylation site (40) | Higher r-hFSH and r-hLH consumption and reduced sensitivity to LH with Ser/Ser carriers versus Asn/Asn carriers (41, 42). Significantly higher live birth rate with Ser/Ser carriers versus Asn/Asn or Asn/Ser carriers (p=0.043) (40). |
| rs1800447 (v-betaLH) | LHB | c.82T>C | p.Trp8Arg | v-betaLH amino acid substitution results in an extra glycosylation site in the β subunit, leading to a second oligosaccharide side-chain to Asn13. The v-betaLH homozygous variant has an elevated bioactivity in vitro but with a significantly shorter half-life versus wild type LHB (43). | Characterized by a less active form of LH that does not adequately support FSH activity during ovarian stimulation (43). |
| rs34349826 (v-betaLH) | LHB | c.104A>G | p.Ile35Thr | Associated with elevated testosterone levels in women withPCOS (44). | |
| rs10835638 | FSHB | 211G>T | N/A | SNP in the promoter of FSHB influences gene transcription (30) | Poor response to ovarian stimulation, higher basal FSH/LH levels, lower AFC, and lower retrieved numbers of oocytes, MII oocytes and embryos with GT heterozygotes versus GG wild type (45–47) |
*rs6165 and rs6166 are in linkage disequilibrium, apart from in some African populations (30, 31). Linkage disequilibrium is defined as the non-random association of alleles of different loci that are inherited co-ordinately ( Box 2 ).
A, adenine; AFC, antral follicle count; Ala, alanine; Arg, arginine; Asn, asparagine; c., coding DNA sequence; C, cytosine; FSH, follicle-stimulating hormone; FSHB, follicle-stimulating hormone beta subunit; FSHR, follicle-stimulating hormone receptor; G, guanine; Ile, isoleucine; LH, luteinizing hormone LHB, luteinizing hormone β-chain; LHCGR, luteinizing hormone/choriogonadotropin receptor; MII, metaphasis II; N/A, not applicable; p., protein-level amino acid sequence; PCOS, polycystic ovary syndrome; r-hLH, recombinant human luteinizing hormone; r-hFSH recombinant human follicle-stimulating hormone; Ser, serine; SNP, single nucleotide polymorphism; T, thymine; Trp, tryptophan; Thr, threonine.
The aim of the current Delphi Consensus study was to generate a series of literature-supported consensus statements regarding the most relevant genetic variants of gonadotropin and gonadotropin receptors involved in ovarian response.
Assessment of Statements According to Delphi Consensus Process
Role of the Sponsor
The Delphi consensus was coordinated by a healthcare consulting and training company (Sanitanova Srl, Milan, Italy). The consensus concept was initiated and funded by Merck KGaA, Darmstadt, Germany. The sponsor was involved early in the process, defining the overarching topic to be discussed, but did not participate in the development of the statements or in any of the meetings or discussions involved in developing the Delphi consensus. The statements were, therefore, developed independently of the industry sponsor. The authors from Merck KGaA, Darmstadt, Germany, were only involved in the development of the manuscript, critically revising it for important intellectual content, especially in the Introduction, Results and Discussion sections, but could not alter the consensus statements in any way.
Consensus Participants
The Delphi consensus involved a Scientific Board, comprising two Scientific Coordinators (AC and FT) and nine additional experts ( Table 2 ). Scientific Board members were selected based on their recognized expertise in Reproductive Genetics proven by literature contributions in this field ( Supplementary Table 1 ). Our goal was to have diverse coverage involving experienced panel members from across the world. Each member of the Scientific Board suggested an additional two or three experts, resulting in a panel of 36 experts (the Extended Panel), which comprised nine members of the Scientific Board (excluding the two Scientific Coordinators) plus 27 additional experts. Written informed consent was obtained from all Consensus participants for the publication of their name in Table 2 .
Table 2.
Participants involved in rounds 1–3 of the consensus.
| Name | Country | Round 1 (Web conference*) | Round 2 (Online survey) | Round 3 (Web conference*) | ||
|---|---|---|---|---|---|---|
| 06 October 2020 (09:00 CET) | 08 October 2020 (16:30 CET) | 10 November to 01 December 2020 |
02 December 2020 (09:00, CET) | 14 December 2020 (16:30, CET) | ||
| Scientific Coordinators | ||||||
| Alessandro Conforti | Italy | X | X | X | X | |
| Frank Tüttelmann | Germany | X | X | X | X | |
| Scientific Board | ||||||
| Carlo Alviggi | Italy | X | X | X | ||
| Hermann M. Behre | Germany | X | X | X | ||
| Robert Fischer | Germany | X | X | X | ||
| José Gonçalves Franco Junior | Brazil | X | X | X | ||
| Liang Hu | China | X | X | X | ||
| Nikolaos P. Polyzos | Spain | X | X | X | ||
| Gottumukkala Achyuta Rama Raju | India | X | X | X | ||
| Manuela Simoni | Italy | X | X | X | ||
| Sesh K. Sunkara | UK | X | X | X | ||
| Extended Panel | ||||||
| Claus Yding Andersen | Denmark | X | ||||
| Rafael Bernabeu | Spain | X | ||||
| Bianca Bianco | Brazil | X | ||||
| Peter Humaidan | Denmark | X | ||||
| Kim Jonas | UK | X | ||||
| João Sabino Lahorgue da Cunha Filho | Brazil | X | ||||
| Dimitris Loutradis | Greece | X | ||||
| Joop Se Laven | Netherlands | X | ||||
| Dolors Manau | Spain | X | ||||
| Ana Neves | Portugal | X | ||||
| Caio Parente Barbosa | Brazil | X | ||||
| Matheus Roque | Brazil | X | ||||
| Ippokratis Sarris | UK | X | ||||
| Laura Vagnini | Brazil | X | ||||
| Daniele Santi | Italy | X | ||||
| Antonio La Marca | Italy | X | ||||
*Due to different time zones one web conference was held in the morning and a second in the afternoon.
Written informed consent was obtained from all Consensus participants for the publication of their name in Table 2 .
The Consensus Process
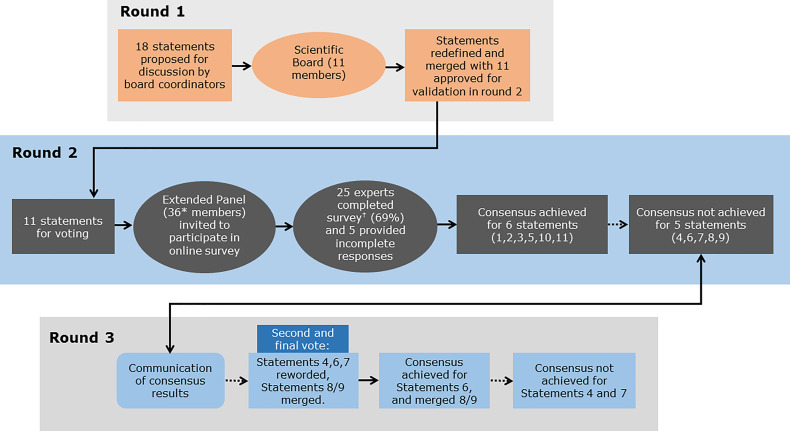
The Delphi consensus comprised three rounds ( Figure 1 ). During Round 1, statements and supporting references initially developed by the two Scientific Coordinators were discussed and amended by the 11 members of the Scientific Board during two web conferences ( Table 2 ). The statements and references to be used in Round 2 were approved by the Scientific Board. During Round 2, an online survey was conducted, in which the Extended Panel of 36 experts were invited to vote anonymously on their level of agreement or disagreement with the statements approved by the Scientific Board in Round 1. Voting was conducted using a five-point Likert-type scale (1=Absolutely agree; 2=Agree; 3=Neither agree nor disagree; 4=Disagree; 5=Absolutely disagree). Participants were also asked to provide the main reason(s) for their response in an open-ended response field. Of the 36 invited experts, 25 completed the survey (nine members of the Scientific Board [excluding the two Scientific Coordinators] plus 16 additional experts) and five provided incomplete survey responses. Consensus was considered to be achieved if the proportion of participants either agreeing with a statement (responding “agree” or “absolutely agree”) or disagreeing with a statement (responding “disagree” or “absolutely disagree”) exceeded 66% (48, 49). During Round 3, the consensus results were communicated to the participating experts via two web conferences ( Table 2 ). Statements that did not achieve consensus in Round 2 were discussed, revised and/or merged by the scientific board. The newly reworded statements were shared with the Extended Panel for voting on their level of agreement or disagreement through an online survey during Round 3.
Figure 1.
Overview of the Delphi consensus process and outcomes. Round 1: Statements and supporting references initially developed by the two Scientific Coordinators were discussed and amended by the 11 members of the Scientific Board. Round 2: An Extended Panel of 36 experts were invited to vote on their level of agreement or disagreement with each statement in an online survey, of which 25 experts completed the survey and five provided incomplete survey responses. Round 3: The consensus results were communicated to the participating experts. Statements that did not achieve consensus in Round 2 were discussed and revised or merged. The Extended Panel then voted on their level of agreement or disagreement with the revised statements. *9 members of the Scientific Board and 27 additional experts suggested by the Scientific Board; †9 members of the Scientific Board and 16 additional experts suggested by the Scientific Board.
Results of the Consensus and Actionable Recommendations (Including Supportive Evidence)
Overall Results
A total of 18 statements with supporting references were proposed by the Scientific Coordinators. Following discussion and refinement of these statements by the Scientific Board during Round 1, a total of 11 statements with supporting references were approved by the Scientific Board and included in the online survey in Round 2 ( Table 3 ). The Extended Panel who participated in the online survey comprised fertility experts from a number of different regions, including Europe, Asia, and South America.
Table 3.
Statements for online voting in rounds 2 and 3.
| Statement | |
|---|---|
| Statements related to SNP of FSHR: rs6166, c.2039 A>G, p.Asn680Ser | |
| 1. | Ser/Ser carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) require higher amounts of gonadotropin during ovarian stimulation than Asn/Asn carriers |
| 2. | Ser/Ser carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) showed higher basal levels of FSH compared with Asn/Asn carriers |
| 3. | Ser/Ser carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) produce fewer oocytes in response to ovarian stimulation than Asn/Asn and Asn/Ser carriers |
| 4. | Ser/Ser carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) produce fewer metaphase II oocytes after ovarian stimulation than Asn/Asn and Asn/Ser carriers |
| 4. (revote*) | Available data in the literature suggest that Ser/Ser carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) tend to produce fewer metaphase II oocytes after ovarian stimulation than Asn/Asn and Asn/Ser carriers |
| 5. | There is mixed evidence supporting an association between the FSHR Asn680Ser variant (FSHR rs6166, c.2039A>G, p.Asn680Ser) and ovarian hyperstimulation syndrome |
| Statements related to SNP of FSHR: rs6165, c.919G>A, p.Thr307Ala | |
| 6. | Thr/Thr carriers of FSHR (FSHR rs6165, c.919G>A, p.Thr307Ala) require a shorter duration of gonadotropin stimulation than Thr/Ala carriers† |
| 6. (revote*) | Few studies suggest that Thr/Thr carriers of FSHR (FSHR rs6165, c.919G>A, p.Thr307Ala) require a shorter duration of gonadotropin stimulation than Thr/Ala and Ala/Ala carriers |
| 7. | Thr/Thr carriers of FSHR (FSHR rs6165, c.919G>A, p.Thr307Ala) produce a higher number of oocytes in response to ovarian stimulation than Thr/Ala and Ala/Ala carriers† |
| 7. (revote*) | Ala/Ala carriers of FSHR (FSHR rs6165, c.919G>A, p.Thr307Ala), which is in strong linkage disequilibrium with the Ser/Ser FSHR variant (FSHR rs6166, c.2039A>G, p.Asn680Ser), produce fewer oocytes in response to ovarian stimulation than Thr/Ala and Thr/Thr carriers† |
| Statements related to SNP of FSHR: rs1394205, −29G>A | |
| 8. | Limited data suggest that A/A allele carriers of the FSHR −29 variant (rs1394205, −29G>A) require higher amounts of gonadotropin during ovarian stimulation than G/G carriers |
| 9. | Limited data suggest that A/A allele carriers of the FSHR −29 variant (rs1394205, –29G>A) produce fewer oocytes in response to ovarian stimulation than GG carriers |
| 8/9 merged (revote*) | Limited data in specific ethnic groups suggest that A/A allele carriers of the FSHR −29 variant (rs1394205, −29G>A) require higher amounts of gonadotropin and produce fewer oocytes in response to ovarian stimulation than G/G carriers |
| Statements related to SNP of FSHB: rs10835638, −211G>T | |
| 10. | The data on an association of FSHB (FSHB rs10835638, −211G>T) with basal levels of FSH and production of oocytes in response to ovarian stimulation are contradictory |
| Statements related to SNPs of the LHB/LHCGR genes | |
| 11. | Limited data suggest that polymorphisms of the LHB/LHCGR genes (V-betaLH rs1800447, c.82T>C, p.Trp8Arg; V-betaLH rs34349826, c.104 A>G, p.Ile35Thr; LHCGR rs2293275, c.935A>G, p.Asn312Ser) can influence ovarian stimulation outcomes and may represent targets for pharmacogenomic research in ART |
*Any statement that did not achieve consensus in Round 2 was discussed and reworded in Round 3, in order to revote with the Extended Panel. †FSHR rs6166 (c.2039A>G, p.Asn680Ser) and FSHR rs6165 (c.919G>A, p.Thr307Ala) are in linkage disequilibrium, except in some African populations.
A, adenine; Ala, alanine; Asn, asparagine; Arg, arginine; ART, assisted reproductive technology; c., coding DNA sequence; C, cytosine; FSH, follicle-stimulating hormone; FSHB, follicle-stimulating hormone β-chain; FSHR, follicle-stimulating hormone receptor; G, guanine; Ile, isoleucine; LH, luteinizing hormone; LHB, luteinizing hormone β-chain; LHCGR, luteinizing hormone/choriogonadotropin receptor; SNP, single nucleotide polymorphism; T, thymine; Trp, tryptophan; Thr, threonine.
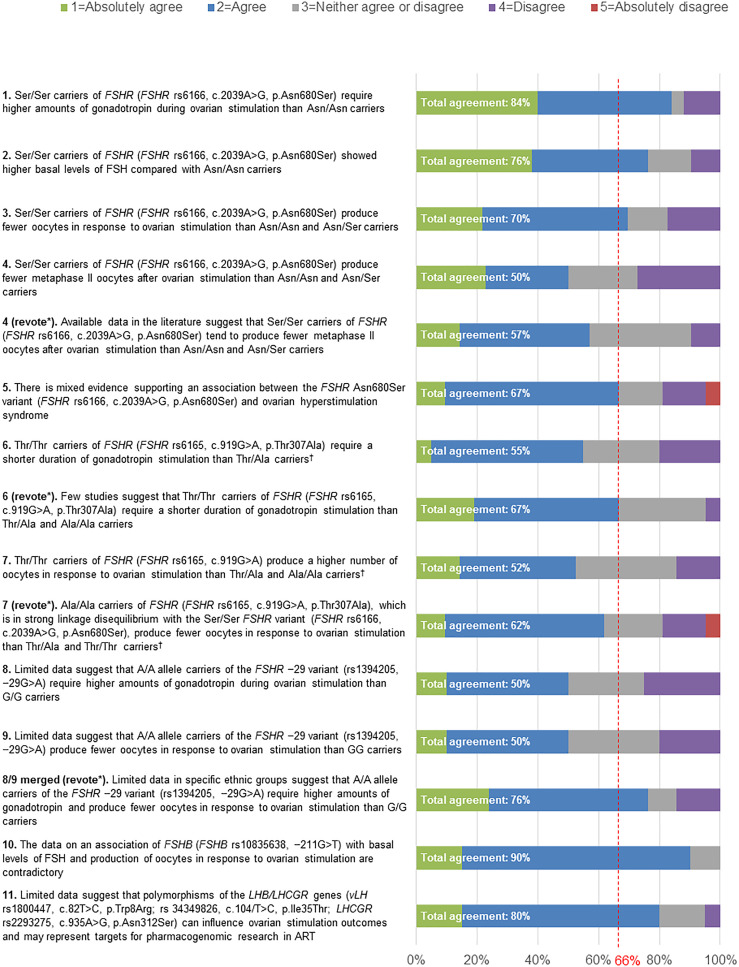
No statements achieved 100% agreement. Consensus was achieved for six statements (Statements 1, 2, 3, 5, 10 and 11). A high level of agreement (≥80% of votes were ‘agree’ or ‘absolutely agree’) was achieved for three statements (Statements 1, 10 and 11). Five statements failed to reach consensus (Statements 4, 6, 7, 8, and 9) and were discussed and amended during Round 3. Statements 4 and 7, which had a total agreement level of 50% (i.e. only 50% of votes were ‘agree’ or ‘absolutely agree’) and 52%, respectively, after the first vote, were reworded but failed to reach consensus after a second round of voting (57% and 62% total agreement, respectively). Statement 6, which had a total agreement level of 55% after the first vote, was reworded and reached consensus after a second vote (67% total agreement). Statements 8 and 9, which both had a total agreement level of 50% after the first vote, were merged and reworded, and achieved consensus after a second vote (76% total agreement). For two statements (Statements 5 and 7 [revote]) one expert voted ‘absolutely disagree’. The reasons that experts provided when voting to disagree with a given statement are shown in Supplementary Table 2 .
Statements Related to SNP of FSHR: rs6166, c.2039A>G, p.Asn680Ser
Statement 1: Ser/Ser Carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) Require Higher Amounts of Gonadotropin During Ovarian Stimulation than Asn/Asn Carriers
This statement received 84% agreement from the Extended Panel ( Figure 2 ). The reasons provided by participants for disagreeing with this statement included the absence of data from RCTs of an adequate size and the fact that a meta-analysis failed to show significant differences in gonadotropin consumption. Furthermore, one expert suggested that the extent to which FSHR rs6166 affected gonadotropin consumption depended on the study population ( Supplementary Table 2 ).
Figure 2.
Level of agreement/disagreement with each statement (Rounds 2 and 3). The Extended Panel voted on their level of agreement or disagreement with each of the 11 statements using a 5-point Likert scale (1=Absolutely agree; 2=Agree; 3=Neither agree nor disagree; 4=Disagree; 5=Absolutely disagree). Consensus was considered to have been achieved if the proportion of participants either agreeing with the statement (responding 1 or 2) or disagreeing with the statement (responding 4 or 5) exceeded 66%. *Any statement that did not achieve consensus in Round 2 was discussed and reworded in Round 3, in order to revote with the Extended Panel. † FSHR rs6166 (c.2039A>G, p.Asn680Ser) and FSHR rs6165 (c.919G>A, p.Thr307Ala) are in linkage disequilibrium, except in some African populations.
A number of studies support a role for the FSHR rs6166 (c.2039A>G, p.Asn680Ser) variant as a prognostic indicator of ovarian response to FSH stimulation (33–37). The Ser/Ser variant was associated with higher basal levels of FSH (33, 34, 36), a higher total dose of gonadotropins required during ovarian stimulation (33, 34, 36), lower peak estradiol levels (34–36) and fewer retrieved oocytes (36). Collectively these studies suggest that the Ser/Ser variant is associated with a reduced sensitivity of the FSHR to exogenous FSH. A randomized controlled trial (RCT) demonstrated that this reduced sensitivity of the FSHR may be overcome by increasing the FSH dose (35).
In a study by Perez Mayorga et al. in 161 women aged <40 years undergoing ovarian stimulation in Germany, significantly more exogenous FSH was required to achieve ovulation stimulation and oocyte retrieval with the Ser/Ser variant compared with the Asn/Asn variant or the Asn/Ser variant (mean number of FSH ampoules [SEM]: 46.8 [5.0] vs 31.8 [2.4] vs 40.7 [2.3], respectively; p<0.01). Multiple linear regression analysis revealed that the number of ampoules of exogenous FSH could be predicted from both the type of polymorphism and basal FSH level (p<0.001) (33). Furthermore, a study in 522 Japanese women (mean age 31.8 years) reported that the distribution of FSHR rs6166 polymorphisms was 12.1% for the Ser/Ser variant compared with 41.0% for the Asn/Asn variant and 46.9% for the Asn/Ser variant (34). A higher dose of exogenous FSH was required to achieve ovulation stimulation in women with the Ser/Ser variant than women with the Asn/Ser variant (25%; p<0.05) or Asn/Asn variant (16%), although the latter comparison did not reach statistical significance.
In a study in 263 Korean women aged <40 years, Jun et al. reported significantly higher basal FSH levels in women with the Ser/Ser variant compared with those with the Asn/Asn or Asn/Ser variants (mean [SEM] 8.2 [0.9] IU/L vs 5.7 [0.3] IU/L and 6.0 [0.3] IU/L, respectively) (36). There was a trend towards lower peak estradiol levels and a higher exogenous FSH dose required for ovarian stimulation, but these did not reach statistical significance. The number of oocytes retrieved was lower in women with the Ser/Ser variant compared with those with the Asn/Asn or Asn/Ser variants (mean [SEM] 7.9 [0.8] vs 9.6 [0.6] and 10.2 [0.6], respectively). Furthermore, the clinical pregnancy rate was significantly lower in women with the Ser/Ser or Asn/Ser variants compared with those with the Asn/Asn variant (28.1% vs 31.1% vs 45.7%, respectively; p=0.013) (36). Similarly, in a study of 1250 Chinese women aged ≤38 years undergoing IVF/ICSI treatment (50), women with the Ser/Ser variant had higher basal FSH levels, required a higher dose of exogenous recombinant human FSH (r-hFSH) for ovarian stimulation and had fewer oocytes retrieved compared with women with the Asn/Asn or Asn/Ser variants. A logistic regression analysis demonstrated that the odds ratio (OR) of a poor ovarian response was 2.25 (95% CI 1.40, 3.58; p<0·001) for the Ser/Ser variant, compared with 1.79 (95% CI 1.28, 2.61; p<0.001) for the Asn/Ser variant (50).
In a prospective RCT of women aged <40 years undergoing ovarian stimulation for IVF/ICSI, carriers of the Ser/Ser variant were randomly assigned to receive hFSH (recombinant or urinary) 150 IU/day (n=24) or hFSH 225 IU/day (n=25), whereas carriers of the Asn/Asn variant (n=44) received hFSH 150 IU/day (35). Peak estradiol levels were significantly lower in the Ser/Ser variant carriers receiving hFSH 150 IU/day compared with the Asn/Asn variant carriers (mean [SEM] 5680 [675] pmol/L and 8679 [804] pmol/L, respectively; p=0.028); however, increasing the hFSH dose to 225 IU/day overcame the lower estradiol response in women with the Ser/Ser variant (7804 [983] pmol/L). This study suggests that the lower FSHR sensitivity associated with the Ser/Ser variant could be overcome by using higher FSH doses (35). In addition, a retrospective evaluation in 42 women aged <37 years compared genetic and clinical characteristics in those who required a higher cumulative dose of r-hFSH (>2500 IU; n=17) with those who required a lower cumulative dose of r-hFSH (<2500 IU; n=24) (37). The number of oocytes retrieved (p=0.0005) and embryos transferred (p=0.001) were significantly greater in women with a higher r-hFSH consumption compared with those with a lower r-hFSH consumption. The incidence of the Ser/Ser variant was higher in patients requiring a higher r-hFSH dose (p=0.02), suggesting that this genotype may be associated with a poor response to FSH, that may be overcome by higher FSH doses (37).
Statement 2: Ser/Ser Carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) Showed Higher Basal FSH Levels Compared With Asn/Asn Carriers
This statement received 76% agreement from the Extended Panel ( Figure 2 ). The reasons given by participants for disagreeing with this statement included poor replication of data between studies, the fact that most studies were conducted in homogeneous populations and that the extent to which FSHR rs6166 affected basal FSH levels depended on the study population ( Supplementary Table 2 ).
A number of studies have reported elevated basal FSH levels in Ser/Ser carriers of FSHR rs6166 (31, 33, 36, 46, 50–53). In a meta-analysis by Alviggi et al, Ser/Ser carriers had significantly higher basal FSH levels than Asn/Asn carriers (fixed random weighted mean difference [WMD] for Asn/Asn versus Ser/Ser −0.54 [95% CI −0.72, −0.36], p<0.00001, Bonferroni adjusted p<0.0001, I2 = 21%); based on data from one study (31). Furthermore, a study assessing serum FSH, LH and AMH levels in the follicular stage of the menstrual cycle in eumenorrheic healthy women without known fertility problems (n=169) and female partners of infertile couples (n=186) reported that the Ser/Ser variant was associated with significantly higher basal FSH levels compared with the Asn/Ser or Asn/Asn variants in the healthy group (G-allele affect [SE] 0.56 [0.19]; p=0.0046) (46).
A retrospective study in 1250 Chinese women with poor (≤5 oocytes) or good (>5 oocytes) ovarian response who were undergoing IVF/ICSI reported higher basal FSH levels in women with the Ser/Ser variant compared with the Asn/Asn or Asn/Ser variants (50). In another retrospective study in 136 women with either poor response (n=22), normal response (n=57) or high response (n=57), basal FSH levels (Day 2) were significantly higher in women with the Ser/Ser variant compared with the Asn/Ser or Asn/Asn variants (p<0.05) (53). Another retrospective study in 263 Korean women aged <40 years also reported significantly higher basal FSH levels in women with the Ser/Ser variant compared with the Asn/Asn or Asn/Ser variants (mean [SEM] 8.2 [0.9] IU/L vs 5.7 [0.3] IU/L and 6.0 [0.3] IU/L, respectively; p=0.001) (36).
Falconer et al. reported a higher distribution of the Ser/Ser variant compared with the Asn/Ser or Asn/Asn variants (41%, 24% and 35%, respectively) in 68 infertile women in Sweden (median age 33 [range 23–38] years). There was no significant difference in basal (Day 3) FSH levels between the Ser/Ser, Asn/Ser or Asn/Asn variants in a subpopulation of women with normal ovulatory reserve (mean [SE] 5.7 [1.7] vs 6.7 [1.3] vs 5.6 [1.9], respectively), although significantly higher FSH levels were detected at Day 10 in the women with the Ser/Ser variant compared with women with the Asn/Ser or Asn/Asn variants (mean [SE] 8.3 [2.8] vs. 6.3 [1.7] vs 6.9 [1.9], respectively; p<0.01). Additionally, FSH levels in women with the Ser/Ser variant at Day 3 of the cycle were significantly higher than at Day 10 (p<0.05) (52). FSHR genotypes were also evaluated in a cross-sectional study among 178 women (148 normogonadotropic anovulatory women, of whom 61 had polycystic ovary syndrome [PCOS], and 30 normo-ovulatory controls) (51). The Ser/Ser variant was significantly more prevalent in the anovulatory group compared with the normo-ovulatory control group (40% versus 16%). Furthermore, anovulatory women with the Ser/Ser variant had higher basal FSH serum levels (5.2 IU/L [range, 2.4–9.7 IU/L) than those with the Asn/Asn (4.6 IU/L [range, 1.4–5.8 IU/L) or Asn/Ser (4.5 IU/L [range, 1.8–9.7 IU/L) variants (p<0.01) (51). Finally, Perez Mayorga et al. assessed FSHR polymorphisms in 161 ovulatory women aged <40 years with couple-infertility attributed to male causes, tubal factor, or both (33). Distributions for the Ser/Ser, Asn/Asn and Asn/Ser variants were 26%, 29% and 45%, respectively. Basal FSH levels were significantly higher for women with the Ser/Ser variant compared with women with the Asn/Asn and Asn/Ser variants (p<0.05).
Statement 3: Ser/Ser Carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) Produce Fewer Oocytes in Response to Ovarian Stimulation than Asn/Asn and Asn/Ser Carriers
This statement received 70% agreement from the Extended Panel ( Figure 2 ). The main reasons stated by participants for disagreeing with this statement included the fact that most data were from observational studies with a limited number of patients, and the absence of data from RCTs. Furthermore, one participant suggested that the extent to which FSHR rs6166 affected oocyte number was dependent on the study population ( Supplementary Table 2 ).
A number of studies suggest that women with the FSHR rs6166 (c.2039A>G, p.Asn680Ser) Ser/Ser variant produce fewer oocytes in response to ovarian stimulation than women with the Asn/Asn or Asn/Ser variants, despite there being no statistically significant difference in the gonadotropin dose among women with the different polymorphisms (31, 36, 50, 54–59).
In a retrospective study in Mexican Mestizo women (n=224) there was a lower distribution for the Ser/Ser variant (9.8%) compared with the Asn/Asn variant (41.9%) or Asn/Ser variant (48.2%) (59). The Ser/Ser variant was associated with a significantly reduced number of retrieved oocytes following ovarian stimulation compared with the Asn/Asn or Asn/Ser variants (p<0.01) in normal oocyte donors. There was also a trend towards lower pregnancy rates in women with the Ser/Ser variant, which was stronger in a separate analysis of women with more Native American ancestry (OR 2.0 [95% CI 1.03, 3.90], p=0.04) (59). Furthermore, Alviggi et al. conducted a systematic review and meta-analysis of 33 studies, 21 of which (including 4425 women) reported the number of oocytes retrieved in relation to FSHR rs6166 (31). The number of oocytes retrieved was significantly lower in women with the Ser/Ser variant compared with women with the Asn/Asn variant (random WMD 0.84 [95% CI 0.19,1.49], p=0.01, Bonferroni adjusted p=0.03, I2 = 76%), and was significantly lower in women with the Ser/Ser variant compared with the Asn/Ser variant (random WMD 0.88 [95% CI 0.12, 1.63], p=0.02, Bonferroni adjusted p=0.04, I2 = 76%) (31). In addition, a cohort study in 1250 Chinese women aged ≤38 years undergoing IVF/ICSI treatment reported that women with the Ser/Ser variant had higher basal FSH levels, required a higher dose of exogenous gonadotropin for ovarian stimulation and had fewer oocytes retrieved compared with women carrying the Asn/Asn or Asn/Ser variants (50). A logistic regression analysis demonstrated that the OR of a poor ovarian response for women with the Ser/Ser variant was 2.25 (95% CI 1.40, 3.58; p<0.001) compared with the Asn/Asn variant (50).
A meta-analysis conducted by Tang et al. of 16 cohort studies (4278 women) determined, using a random effects model, that the number of oocytes retrieved in women with the Ser/Ser variant was significantly lower than in those with the Asn/Asn or Asn/Ser variants (WMD −1.36 [95% CI −1.85, −0.87] (58). Another meta-analysis of 11 studies (4020 women) demonstrated that women with the Ser/Ser variant were more likely to be poor responders (previously defined in other studies on this polymorphism as no more than 4–5 oocytes retrieved following ovarian stimulation) (50, 60) compared with the Asn/Asn or Asn/Ser variants (OR 1.61, p=0.08) (57). Furthermore, a prospective study in 450 Chinese women receiving ovarian stimulation for ART (56) reported higher basal levels of FSH in women with the Ser/Ser variant compared with the Asn/Asn or Asn/Ser variants (p<0.05), with numerically fewer oocytes retrieved in the women with the Ser/Ser variant compared with women with the Asn/Asn variant or the Asn/Ser variant (mean number of oocytes [SEM]: 11.12 [7.29] vs 13.07 [6.76] vs 13.20 [6.17], respectively). Moreover, the Ser/Ser variant was associated with an increased risk of poor response compared with the other two variants (p<0.05) (56).
A genotyping study that assessed the effect of genotype on ovarian responses in 300 women undergoing IVF/ICSI treatment, compared with a control group of 300 women with successful child birth, reported a reduction in the number of oocytes retrieved in women with the Ser/Ser variant compared with women with the Asn/Asn variant (p<0.02) (55). In addition, a study in 263 Korean women aged <40 years reported the retrieval of fewer oocytes in women with the Ser/Ser variant compared with the Asn/Asn or Asn/Ser variants (mean [SEM] 7.9 [0.8] vs 9.6 [0.6] vs 10.2 [0.6], respectively) (36). In another study, Loutradis et al. analysed polymorphisms in 125 women classified as ‘subfertile’ (n=79; defined as women who had previously undergone ovarian stimulation and who had a Day 3 FSH level of ≥9 IU/L [normal range 2–9 IU/L]) or ‘normo-ovulatory’ (n=46) (54). The distribution of the Ser/Ser, Asn/Ser and Asn/Asn variants were 45.5%, 22.7% and 31.8%, respectively, in the subfertile women. The number of oocytes retrieved for women with the Ser/Ser variant was significantly lower than those with the Asn/Ser variant (p<0.01) (54).
In a retrospective study in 170 women in Spain with conserved ovarian function undergoing ovarian stimulation (mean [SD] age 33 [2.55] years), the frequency of the Ser/Ser variant was higher in women with poor response (≤3 ovarian follicles) compared with the Ser/Asn or Asn/Asn variants (30% vs 13.9% vs 14.5% respectively; p=0.005). The number of oocytes retrieved for each variant was not reported in this study (61). Finally, in another retrospective study in Spain by the same author in 102 women undergoing ovarian stimulation (mean [SD] age 33 [2.55] years), 37% of women with poor ovarian response (≤3 ovarian follicles) had the Ser/Ser variant, compared with 21% of women with the Asn/Asn variant. However, in contrast to the other studies reported here, there was no difference in the number of oocytes retrieved between the Ser/Ser variant and the Asn/Asn or Asn/Ser variants (mean [range] number of oocytes: Ser/Ser: 5 [0–14] vs Asn/Asn + Asn/Ser: 5.3 [0–21]; p=0.85) (62). This may be due to the small sample size (102 women, of whom only 19 patients carried the Ser/Ser variant). Furthermore, the overall number of oocytes retrieved was low, regardless of variant (5.2 [range 0–21]), despite the fact that only 19% of patients were categorized as poor responders (62).
Statement 4 (Revote): Available Data in the Literature Suggest that Ser/Ser Carriers of FSHR (FSHR rs6166, c.2039A>G, p.Asn680Ser) Tend to Produce Fewer Metaphase II Oocytes After Ovarian Stimulation Than Asn/Asn and Asn/Ser Carriers
This statement reached 50% agreement in the first round of voting ( Figure 2 ). The wording of the statement was revised to reflect the fact that there are few large, prospective studies and no RCTs available regarding the influence of genotype on gonadotropin stimulation protocols. Furthermore, there were insufficient data on the comparison of homozygotic carriers of FSHR rs6166 (c.2039A>G, p.Asn680Ser) with grouped heterozygotic carriers. The reworded statement reached 57% agreement during re-voting ( Figure 2 ); therefore, this statement did not achieve consensus. This statement received 10% disagreement from the Extended Panel, with 33% of experts neither agreeing nor disagreeing with the statement. The main motivation for disagreement was insufficient evidence to support this statement ( Supplementary Table 2 ).
In a study including 455 consecutively enrolled women and 210 unselected women aged <40 years, the number of MII oocytes in a subgroup of women who underwent ICSI (n=317) was significantly lower in Ser/Ser carriers compared with Asn/Ser carriers or Asn/Asn carriers (mean [SD]: 6.1 [3.0] vs 7.1 [4.1] vs 8.2 [4.5]; unadjusted p=0.012; adjusted p=0.009 [adjusted for age]). (40). Furthermore, in a study of 104 prospectively enrolled women of Albanian ethnic population from the Kosovo Dukagjin region undergoing ICSI for male factor infertility, women with the Ser/Ser variant had a lower rate of MII oocytes (78.6%) compared with women with Asn/Asn (84.7%) or Asn/Ser (89.7%) variants (p=0.0258). In addition, there was a lower rate of immature MI oocytes progressing to MII state after 2-6 hours of in vitro incubation (5.6%) in women with the Ser/Ser variant compared with women with the Asn/Ser (11.6%) or Asn/Asn (8.4%) variants (p=0.0031) (65).
However, in contrast to these studies, a cross-sectional study of 384 women aged <40 years (mean age [SD]: 32.0 [3.82] years) undergoing IVF reported no significant difference in the number of mature oocytes retrieved in Ser/Ser variant carriers compared with Asn/Ser or Asn/Asn variant carriers (mean [SD]: 9.03 [5.6] vs 8.85 [5.3] vs 9.25 [6.0]). There was a trend towards a difference in the a posteriori enrolled validation cohort (n=233; mean [SD]: Ser/Ser 9.18 [5.2], Asn/Ser 10.5 [7.1], Asn/Asn 11.3 [7.1]) and the merged cohort (mean [SD]: Ser/Ser 9.05 [5.4], Asn/Ser 9.43 [6.1], Asn/Asn 10.1 [6.5]) (41). However, the differences between the variants were not significant in the study, validation or merged cohorts, suggesting no influence of the polymorphisms on receptor sensitivity for in vitro stimulation response (41).
A meta-analysis of five studies [n=1185 women (also including 41)] reported a numerically lower number of oocytes in women with the Ser/Ser variant compared with women with the Asn/Asn variant, although the difference was not significant after Bonferroni correction (fixed weighted mean difference 1.03, 95% CI 0.01 to 2.05; p=0.05. Bonferroni adjusted p=0.14; I2 = 0%). No significant differences were observed between women with the Asn/Asn variant and those with the Asn/Ser variant (fixed weighted mean difference 0.79, 95% CI –0.05 to 1.62; I2 = 0%) or between those with the Ser/Ser variant and those with the Asn/Ser variant (fixed weighted mean difference 0.34, 95% CI –0.57 to 1.26; I2 = 49%) (31).
Statement 5: There Is Mixed Evidence Supporting an Association Between the FSHR Asn680Ser Variant (FSHR rs6166, c.2039A>G, p.Asn680Ser) and Ovarian Hyperstimulation Syndrome
This statement reached 67% agreement during the first round of voting ( Figure 2 ). The main reasons stated by participants for disagreeing with this statement included insufficient evidence and the fact that most data were from observational studies, with an absence of data from RCTs, or from studies in specific populations ( Supplementary Table 2 ).
This statement was supported by evidence from three studies (38, 58, 66). A retrospective study of 586 women undergoing their first IVF treatment determined whether the FSHR rs6166 (c.2039A>G, p.Asn680Ser) predicted the likelihood of developing OHSS (38). In this study, 36 women (6%) developed OHSS, of whom none carried the Ser/Ser variant. FSHR rs6166 was associated with OHSS (P trend = 0.004 and P allele = 0.038), with carriers of Asn having an OR of 1.7 (95% CI 1.0, 2.8; p<0.04), compared with carriers of Ser. Women who developed OHSS were exposed to a lower total hormonal dose, yet produced more oocytes than those without OHSS (16 ± 8 vs 11 ± 6; p=0.001) (38). Conversely, a meta-analysis conducted by Tang et al., comprising 16 cohort studies and a total of 4287 women, reported no evidence for an association between FSHR rs6166 variants and OHSS (OR: 1.58, 95% CI: 0.41, 6.07) (58). Finally, in their retrospective study of 150 Indian women (n=50 in an assisted reproductive technology [ART] program and n=100 with proven fertility [control group]), Achrekar et al. explored the association between FSHR rs6166 variants and variable ovarian response, including the occurrence of OHSS (66). The distributions were 31%, 56%, and 13% in controls and 42%, 46%, and 12% in ART patients, for the Asn/Asn, Asn/Ser and Ser/Ser variants, respectively. (66). Patient age, basal FSH and LH levels, progesterone levels before and on the day of human choriogonadotropin (hCG) administration, number of pre-ovulatory follicles, number of oocytes retrieved, and pregnancy rates showed no statistically significant differences among groups, suggesting that treatment outcome was independent of the FSHR rs6166 variants. There were no statistically significant differences in any of the clinical parameters among women with the different variants, although women with the Ser/Ser variant showed higher serum estradiol levels before or on the day of hCG administration. OHSS developed in 50%, 26%, and 29% of women with the Ser/Ser, Asn/Ser, and Asn/Asn variants, respectively, although these values were not statistically significant (OR: 2.67) (66).
Statements Related to SNP of FSHR: rs6165, c.919G>A, p.Thr307Ala
Statement 6 (Revote): Few Studies Suggest That Thr/Thr Carriers of FSHR (FSHR rs6165, c.919G>A, p.Thr307Ala) Require a Shorter Duration of Gonadotropin Stimulation Than Thr/Ala and Ala/Ala Carriers
The original wording of this statement received 55% agreement during the first round of voting ( Figure 2 ). Following discussion regarding the lack of prospective studies reporting on this outcome, the wording was revised and received 67% agreement after re-voting ( Figure 2 ). The reasons given by participants for disagreeing with this statement included the fact that there was a limited number of studies and that the available studies had been poorly designed ( Supplementary Table 2 ).
In one study including 450 Chinese women undergoing IVF due to male factor, tubal factor, or both, the length of stimulation was significantly different among women with different variants (although only a small absolute clinical difference was observed), with the longest duration of stimulation reported in women with the Ala/Ala variant: Thr/Thr 11.32 (2.15) days, Thr/Ala 12.02 (2.44) days and Ala/Ala 12.62 (2.92) days; p<0.05 (56). A prospective, cross-sectional study of 149 women in Brazil undergoing ART treatment due to male factor (n=93) or tubal factor (n=56) did not report on the stimulation length in women with different variants, but there was no significant difference in mean (SD) basal FSH levels (Thr/Thr 6.0 [2.0] versus Ala/Ala 6.41 [1.96] and Thr/Ala 6.49 [1.73], p=0.402), suggesting a comparable ovarian response for the alleles related to this variant (32).
In a meta-analysis of three studies (679 patients, including Yan 2013), a shorter duration of stimulation that approached statistical significance was reported between women with the Thr/Thr and Ala/Ala variants (random weighted mean difference −0.59 [95% CI −1.24, 0.05], I2 = 60%, p=0.07). However, the duration of stimulation was significantly shorter in women with the Thr/Thr variants than in those with the Thr/Ala variant (fixed weighted mean difference −0.48 [95% CI −0.87, −0.10], p-0.01; Bonferroni adjusted p=0.04; I2 = 44%), although there was no difference between women with the Ala/Ala variant and those with the Thr/Ala variant (fixed weighted mean difference −0.29 [95% CI −0.95, 0.37]; I2 = 0%) (31).
Statement 7 (Revote): Ala/Ala Carriers of FSHR (FSHR rs6165, c.919G>A, p.Thr307Ala), Which Is in Strong Linkage Disequilibrium With the Ser/Ser FSHR Variant (FSHR rs6166, c.2039A>G, p.Asn680Ser), Produce Fewer Oocytes in Response to Ovarian Stimulation Than Thr/Ala and Thr/Thr Carriers
This statement reached 52% total agreement during the first round of voting ( Figure 2 ). The statement was revised to highlight that the FSHR variant (FSHR rs6165, c.919G>A) is in linkage disequilibrium ( Box 2 ) with the FSHR rs6166 (c.2039 A>G, p.Asn680Ser), and following re-voting the revised statement reached 62% agreement ( Figure 2 ); therefore, this statement did not achieve consensus. This statement received 19% disagreement from the Extended Panel, with an additional 19% of experts neither agreeing nor disagreeing with the statement. The motivations supporting these disagreements are outlined in Supplementary Table 2 , with some experts believing there was insufficient data to support this statement and others suggesting that it depended on how the gonadotropin dose was adjusted.
Box 2. Linkage Disequilibrium.
Linkage disequilibrium is defined as the non-random association of alleles of different loci that are inherited co-ordinately.
Linkage disequilibrium differs between ethnic groups, resulting in various combinations of the different SNPs (30).
Such genetic distinctions could potentially explain the significant disparities reported in assisted reproductive technology (ART) outcomes according to ethnicity (63) and should be taken into account when assessing studies in different populations (64).
For example, FSHR rs6166 (c.2039A>G, p.Asn680Ser) and FSHR rs6165 (c.919G>A, p.Thr307Ala) are in linkage disequilibrium, except in some African populations (30, 31).
In a meta-analysis of five studies [including (66) and (56)] comprising 1020 women, the number of oocytes retrieved was lower in women with the Ala/Ala variant than in those with the Thr/Thr variant (fixed weighted mean difference 1.85 [95% CI 0.85, 2.85], p<0.01, Bonferroni adjusted p=0.008, I2 = 0%) (31). No difference was reported between women with the Thr/Ala variant and those with the Ala/Ala variant (fixed weighted mean difference −0.37 [95% CI −1.51, 0.78]; I2 = 18%) or between those with the Thr/Ala variant and those with the Thr/Thr variant (random weighted mean difference 1.62 [95% CI 0.28, 2.95], p=0.02, Bonferroni adjusted p=0.052, I2 = 56%) (31). However, in contrast to this study, another study of 50 normogonadotropic women with infertility due to male factor or tubal factor in India, who were independently segregated according to genotype, reported no significant difference for the mean (SD) number of oocytes retrieved among women with different variants: Thr/Thr 16.27 (2.4), Thr/Ala 14.24 (1.2), Ala/Ala 13.86 (3.3) (66).
Two studies further categorized patients according to ovarian response based on the number of oocytes retrieved. In a prospective study of 216 Egyptian women undergoing IVF treatment for unexplained infertility, patients were classified according to ovarian response (good responders [n=111]: ≥5 oocytes retrieved; poor responders [n=105]: ≤4 oocytes retrieved). In the good ovarian responders, no statistically significant difference was observed in mean (SD) oocyte number retrieved between women with the Thr/Thr variant (13.00 [2.65]), Thr/Ala variant (11.07 [2.73]) or the Ala/Ala variant (10.20 [0.84]) (p=0.078). However, a statistically significant difference in oocyte number was reported among women with different variants in the poor ovarian responders (Ala/Ala 2.42 [0.51]; Thr/Ala 1.29 [1.14]; Thr/Thr 2.50 [0.58]; p=0.005). The Ala/Ala variant was threefold higher in poor ovarian responders compared with good ovarian responders, and the presence of a G allele significantly increased the probability of a poor ovarian response (67). Finally, in a study of 450 Chinese women who were categorized according to ovarian response (poor <5 oocytes retrieved; normal 5–14 oocytes retrieved; high >14 oocytes retrieved), the proportion with the Ala/Ala variant was significantly higher in poor ovarian responders than the proportions with the Thr/Thr variant or with the Thr/Ala variant (p<0.001) (56).
Statements Related to SNP of FSHR: rs1394205, –29G>A
Statement 8/9 (Revote): Limited Data in Specific Ethnic Groups Suggest That A/A Allele Carriers of the FSHR −29 Variant (rs1394205, −29G>A) Require Higher Amounts of Gonadotropin and Produce Fewer Oocytes in Response to Ovarian Stimulation than G/G Carriers
Statements 8 and 9 each received 50% total agreement in the first round of voting ( Figure 2 ). The same concerns were identified for each statement and so they were softened and merged with the specification that the effect can be seen in ‘at least some ethnic groups’ for the second round of voting, during which 76% total agreement was reached. The reasons given by participants for disagreeing with this statement are shown in Supplementary Table 2 . One participant explained that ethnicity had been shown to play a fundamental role in SNPs and that 1% of the population would need to carry the FSHR −29 variant in order for it to have a clinical application, and another participant stated that the studies supporting this statement had not been correctly planned.
The FSHR −29 variant (rs1394205, −29G>A) has been associated with reduced transcriptional activity, primary or secondary amenorrhea, and poor response to exogenous FSH (31, 59, 68). The latter has not been found consistently in other studies (59). There is controversial evidence for the influence of this variant on ovarian stimulation. Studies suggest that carriers of the A allele, which has a frequency of 49–55% in a Hispano-American population (59), have reduced FSHR expression, are more likely to have a poor ovarian response (31, 68) and may require a higher amount of FSH during OS; exogenous FSH consumption was significantly higher (p<0.001) in A/A carriers than in G/G or G/A carriers in a retrospective study of 50 women undergoing ART (69). A meta-analysis of three studies (n=709 women) also found that A/A carriers needed significantly higher doses of FSH during OS (31). These studies were conducted in only a few homogenous populations where the polymorphism has been found to be highly prevalent (31) and the results may not be generalizable to different populations.
This variant may be associated with fewer oocytes being retrieved during ART. A review of three studies reporting on this polymorphism showed that a lower number of oocytes were retrieved in A/A carriers than A/G or G/G carriers, although the association was not found to be significant (31). One of these studies reported that the mean (SD) number of oocytes retrieved was significantly lower in A/A carriers (6.00 [1.09]) than in G/G carriers (17.88 [1.75]; P = 0.003) (66). A study by Desai et al. (70) found that the mean (SD) number of oocytes retrieved in A/A carriers (10.50 [1.19]) was significantly lower when compared to G/G carriers (16.43 [1.50]; p=0.046) (70). This association has also been found in another study by the same group (68), who reported that increasing the dose of exogenous FSH did not improve oocyte development, probably due to insufficiency of FSHR expression in granulosa cells (68). These studies from one group were restricted to a small, homogenous population, and further studies in mixed populations are needed to generalize the results.
Statements Related to SNP of FSHB: rs10835638, −211G>T
Statement 10: The Data on an Association of FSHB (FSHB rs10835638, −211G>T) With Basal Levels of FSH and Production of Oocytes in Response to Ovarian Stimulation Are Contradictory
This statement reached 90% agreement during the first round of voting ( Figure 2 ). The consensus participants did not give any reasons for disagreeing with this statement ( Supplementary Table 2 ).
This statement was supported by evidence from three studies (45–47). A cross-sectional study evaluated the potential effects of the FSHB rs10835638 (−211G>T) variant on hormonal profiles and IVF/ICSI outcomes in 140 normo-ovulatory women in Brazil (47). The distributions of the GG (wild-type) variant (n=102) and GT variant (n=38) were 86.4% and 13.6%, respectively; the TT variant was not detected in any women. A poor response to ovarian stimulation was more common in women with the GT variant compared with those with the GG variant (47.4% vs. 26.5%; p=0.010), and fewer oocytes were retrieved from those with the GT variant than from those with the GG variant (3.0 vs. 5.0; p=0.03). However, no difference in pregnancy rates were reported between women with different variants.
Two studies assessing the effects of FSHB rs10835638 on basal FSH levels, in women with known infertility compared with a control group of healthy women, reported that women expressing the T allele showed significantly higher FSH basal levels. One study reported that the T allele was associated with significantly higher basal FSH levels in both non-pregnant healthy women (n=169) and female partners in infertile couples (n=186) (T-allele effect: 0.80 IU/L, p=0.001 after Bonferroni testing) (46). FSHB rs10835638 was estimated to explain 3.5% of the total variance of the measured serum FSH levels in healthy women and 1.6% in the female partners of infertile couples, and could have a diagnostic value in fertility clinics to detect female patients with genetically inherited elevated basal FSH and LH levels (46). In the other study, eumenorrheic women attending an IVF unit for predominantly male-factor infertility (n=365) were compared with a control group of women with proven fertility (n=438) (45). The distribution of the variants was 2.5% for the TT variant (n=9), 23.8% for the GT variant (n=87) and 73.7% (n=269) for the GG variant. The TT variant was strongly associated with an elevated mean (SD) basal FSH (TT 9.6 [2.4] U/L vs. GT 7.4 [1.8] U/L vs. GG 7.7 [2.2] U/L; TT-homozygosity effect 2.05 U/L, p=0.003) (45).
Statements Related to SNPs of the LHB/LHCGR Genes
Statement 11: Limited Data Suggest That Polymorphisms of the LHB/LHCGR Genes (V-betaLH rs1800447, c.82T>C, p.Trp8Arg; V-betaLH rs34349826, c.104 A>G, p.Ile35Thr; LHCGR rs2293275, c.935A>G, p.Asn312Ser) Can Influence Ovarian Stimulation Outcomes and May Represent Targets for Pharmacogenomic Research in ART
This statement reached 80% agreement during the first round of voting ( Figure 2 ). The reason given by participants for disagreeing with this statement was that it had not been proven ( Supplementary Table 2 ).
Four studies supported this statement, the collective results of which suggest that clinicians should be aware of patients with LHB polymorphisms, who may, consequently, fail to respond to ovarian stimulation (43, 71–73).
Hypo-sensitivity to exogenous FSH was observed in a retrospective study of 220 normogonadotropic Danish women undergoing controlled ovarian stimulation who were carriers of V-betaLH (rs1800447 [c.82 T>C, p.Trp8Arg] and rs34349826 [c.104 A>G, p.Ile135Thr] polymorphisms), which are common genetic variants of LHB (43). Daily doses of r-hFSH were administered on an individualized basis, tailored to age, body mass index, baseline FSH, and antral follicle count. The LHB genotype was assessed by immunofluorometric assay. A total of 24 women carried the V-betaLH variant, of whom 21 were heterozygous and three were homozygous; 196 of the women were wild type. The differences in the mean number of oocytes retrieved and the fertilization and pregnancy rates for each cycle were not statistically significant between the V-betaLH genotypes, but carriers of V-betaLH variant received a significantly higher cumulative dose of r-hFSH compared with women with wild type LH (2435.86 ± 932.8 IU vs. 1959.8 ± 736.45 IU; p=0.048). A within-design one-way ANOVA analysis showed that the V-betaLH variants had a statistically significant effect (p<0.01) on the cumulative dose of r-hFSH, with a mean (SD) increase from 1959.8 (736.45) IU for wild type carriers, to 2267.5 (824.3) IU and 3558.3 (970.9) IU, for heterozygotic and homozygotic carriers, respectively. These results confirm that carriers of V-betaLH variants have hypo-sensitivity to exogenous FSH during controlled ovarian stimulation (43).
In their systematic review of the current status of pharmacogenetic analyses of controlled ovarian hyperstimulation, Altmäe et al. concluded that there is accumulating data to suggest that the ovarian response to COH is mediated by various polymorphisms, including variants of the V-betaLH and LHCGR genes (72). However, further studies investigating the predictive value of such genetic polymorphisms as markers of COH in subgroups of women who may require supplementation with exogenous LH during ovarian stimulation are needed (72). In addition, the results from an observational preliminary trial of 60 normogonadotropic patients undergoing IVF/ICSI suggested that women with the most common polymorphism of LHB (V-betaLH) were hyporesponsive to r-hFSH (72). A greater proportion (31.8%) of carriers of V-betaLH were identified among 22 women who required a cumulative dose of r-hFSH of >3500 IU, relative to those who required between 2000 and 3500 IU r-hFSH (one woman [6.7%] V-betaLH of 15 women). V-betaLH variants were detected in 23 women who required <2000 IU r-hFSH (73). Lastly, sequence analysis indicated that heterozygous point mutations in the LHB gene (Trp8Arg and Ile15Thr) were present in a 35-year-old woman who had failed to conceive after six cycles of human menopausal gonadotrophin (hMG) therapy for ovarian induction (71). The observed LH hypersecretion was likened to that seen in PCOS. A further cycle of ovarian stimulation with hMG, during which estrogen–progesterone replacement therapy effectively controlled basal LH and FSH, led to successful conception and delivery outcomes (71).
Discussion
This Delphi consensus provides clinical perspectives from a diverse international group of experts. It generates a series of literature-supported consensus statements regarding the influence of specific gonadotropin and gonadotropin receptor variants on clinical ovarian stimulation outcomes that will be useful to optimize current stimulation protocols. The consensus results suggest that there is evidence to support a link between SNP variants in gonadotropin and gonadotropin receptors and ovarian stimulation outcomes, although data for some variants are still lacking.
Our consensus demonstrates that polymorphisms of gonadotropins (FSH and LH) and their receptors may impact ovarian stimulation in a number of ways. SNP of FSHR have been shown to influence basal FSH levels (FSHR rs6166, c.2039A>G, p.Asn680Ser), gonadotropin consumption (FSHR rs6166, c.2039A>G, p.Asn680Ser; FSHR rs1394205, −29G>A), oocyte number (FSHR rs6166, c.2039A>G, p.Asn680Ser; FSHR rs1394205, −29G>A), and may affect duration of gonadotropin stimulation (FSHR rs6165, c.919G>A, p.Thr307Ala) and risk of OHSS (FSHR rs6166, c.2039A>G, p.Asn680Ser). Evidence supporting an association between SNP of FSHB (rs10835638, −211G>T) and basal FSH levels or oocyte number are currently limited. Furthermore, our consensus highlights that there are limited data that polymorphisms of the LHB/LHCGR genes can influence ovarian stimulation outcomes and could potentially represent future targets for pharmacogenomic research in ART.
Although there is strong supporting evidence for the impact of polymorphisms of FSHR on several outcomes, this Delphi Consensus shows there are only modest data on the clinical relevance that would support these polymorphisms as the basis for pharmacogenetic approaches to treatment, although we acknowledge that this is an area where new data are being published. A recent multicentre, multinational, prospective study in 368 predicted normal responder women from Vietnam, Belgium, and Spain, published since the selection of the literature to be considered in this Delphi consensus, reported only minimal clinical impact of genotyping for FSHR SNPs rs6165 (c.919G>A, p.Thr307Ala), rs6166 (c.2039A>G, p.Asn680Ser), rs1394205 (−29G>A) and FSHB SNP rs10835638 (−211G>T) prior to initiating ovarian stimulation with r-hFSH (74). Although the study reported a significantly lower number of oocytes retrieved in heterozygous patients for the FSHR variants rs6166 and rs1394205, as well as a significantly higher rate of hypo-response in heterozygous patients for the FSHR variant rs6166, this resulted in a change of just one or two oocytes in a population of normal responders (74).
Currently, there is contradictory evidence for the clinical impact of polymorphisms in FSHB on ovarian stimulation. Three studies supporting this consensus reported that women expressing the T-allele of the FSHB rs10835638 (−211G>T) polymorphism had fewer retrieved oocytes (47) and significantly higher basal FSH levels compared with women carrying the wild type (GG) variant (45, 46). However, in contrast to the aforementioned studies, La Marca et al. observed that FSHB rs10835638 does not affect FSH basal levels per se. The authors observed a significant reduction of FSH basal levels in women expressing the T-allele compared with the wild-type genotype (75).
The inconsistency between some studies, which is also highlighted in the Alviggi 2018 systematic review and meta-analysis (31), is likely due to differences in inclusion criteria between studies, as well as the use of different gonadotropin products and doses, and allowance for dose adjustments during treatment, highlighting the need for new prospective studies in this field.
As already stated in this Delphi consensus, there are limited data on the influence of polymorphisms of the LHB/LHCGR genes on ovarian stimulation outcomes and their usefulness in pharmacogenomic research in ART. While the usefulness of polymorphisms in these genes per se remains to be determined, a recent study (also published since the selection of the literature for consideration in this Delphi Consensus) may indicate some clinical value in determining the need for LH supplementation compared with current supplementation protocols, reporting higher clinical pregnancy rates (p=0.049) and a trend towards improved live birth rates (p=0.082) in 193 women when supplementation was based on a woman’s SNP profile compared with conventional methods (76). Furthermore, in a cross-sectional study of LHCGR rs2293275 (c.935 A>G, p.Asn312Ser), Lindgren et al. reported no significant difference in the number of oocytes retrieved or obvious differences in embryo quality between Ser/Ser, Asn/Ser or Asn/Asn carriers. However, significantly higher clinical pregnancy rates were reported in Ser/Ser carriers compared with Asn/Asn carriers (OR 1.61 [95% CI 1.13, 2.29], p=0.008) (41). These studies suggest that individualising protocols based on specific genotypes, rather than the number and morphological characteristics of the embryos retrieved, may be beneficial in terms of improved pregnancy rates.
Strengths
This consensus has a number of strengths, including the fact that each of the statements in the consensus were supported by a number of peer-reviewed studies. Furthermore, the majority of women included in these studies were aged <35 years (mean/median age ~32 years). As advanced maternal age (>35 years) is associated with a reduction in ovarian reserve, oocyte/embryo competence and cumulative live birth rate (77–79), the fact that most women in these studies were aged <35 years suggests that age was less likely to impact ovarian response, making it easier to discern the influence of SNP variants. Another strength was that the participants of the consensus were fertility experts from across the globe, representing different regions, including Europe, Asia, and South America, reflecting the quality of healthcare and different approaches to infertility treatment in different parts of the world.
Limitations
The consensus does have some limitations that should be acknowledged. Firstly, the consensus does not represent an exhaustive list of statements referring to all polymorphisms potentially affecting ovarian stimulation. Furthermore, the statements and supporting literature were chosen according to the expert opinion of the Scientific Coordinators and the Scientific Board, and the inclusion of the statements and supporting literature was based on the expert opinion of the Extended Panel. Another limitation was that none of the statements reached 100% agreement, with most statements reaching consensus even though some participants disagreed with them, and only three statements (Statements 1, 10 and 11) achieved a high level of agreement (defined as ≥80% of participants voting ‘agree’ or ‘absolutely agree’). Furthermore, not all statements reached consensus, with Statements 4 and 7 failing to reach consensus even after the statements were revised and revoted. The main reasons stated by participants for disagreeing with a given statement included a limited number of studies, small sample sizes, poor replication of data between studies, and the fact that most data were from observational studies based on homogeneous populations and with homogeneous protocols of ovarian stimulation. Furthermore, a number of participants cited the absence of data from RCTs as a reason for disagreeing with a given statement.
Future Research
Our consensus represents an opportunity to encourage researchers to initiate studies to verify whether a pharmacogenomic approach (i.e. the individualization of treatment based on a patient’s genetic profile) could lead to an improved patient-tailored approach to ovarian stimulation. This could be beneficial, as a more unified approach among studies will enable us to reach future clinical decisions. Given the lack of studies on pharmacogenomic approaches, the consensus was unable to include any statement(s) concerning a pharmacogenomic approach to ovarian stimulation. Conversely, we believe that there is sufficient evidence from the results of our consensus to support the fact that specific genetic variants could represent interesting causative factors of impaired ovarian response that cannot be explained by other parameters such as ovarian reserve markers. Gene association studies could focus on all SNPs known to influence ovarian response (including those highlighted in this Delphi consensus), in order to examine whether an individualized treatment approach may significantly improve ovarian stimulation outcomes, compared with a standard approach. Ideally, the ovarian response would be correlated with the “global genetic background” assessed by SNP genotyping, exome sequencing or even whole genome sequencing combined with artificial intelligence in large, multicenter studies. Greater efforts should focus on increasing the number of observations, for example, by utilizing large-scale datasets such as the UKBioBank (80). Hypothetically, the development of a large international registry concerning genome-wide association studies in IVF could also be beneficial. This strategy would lead to “real-world” data that could provide interesting findings concerning the importance of genetics in Reproductive Genetics and ART (81, 82). Nonetheless, the quality of association studies in Reproductive Genetics should take into account selection bias, different treatments and differential follow-ups among IVF centers. In addition, the use of check-list proposed STROBE or GRACE guidelines should be encouraged to increase the overall quality of published articles (21, 83, 84).
Specifically, further research could include investigating an association between FSHR rs6166 (c.2039A>G, p.Asn680Ser) and ovarian morphology, AFC following ovarian stimulation and basal AFC in different age groups. A multivariate analysis assessing the impact of r-hLH supplementation according to SNP status (specifically, FSHR rs6166 [c.2039A>G, p.Asn680Ser] and LHCGR rs2293275 [c.935A>G, p.Asn312Ser]) would also be relevant, as would investigating the effect of follicular fluid steroid hormone levels in different FSHR and LHCGR SNPs and their implication as a biomarker for oocyte preservation in women postponing pregnancy. Furthermore, it would be of interest to test the hypothesis that different r-hFSH doses with or without r-hLH supplementation may improve ongoing pregnancy and live birth rates per started cycle compared with a standard dose, taking into account age and ovarian reserve, and according to SNP status.
Conclusions
This Delphi consensus supports a link between some genetic variants in gonadotropin and gonadotropin receptors and ovarian stimulation outcomes. The consensus results reinforce the idea that pharmacogenomics may provide a promising new field examining genotype-specific responses to ovarian stimulation medication that may help tailor ovarian stimulation therapies to individual patients, optimizing ART success outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from all Consensus participants for the publication of their name in Table 2 .
Author Contributions
AC and FT were Scientific Coordinators for the Delphi consensus; AC, FT CA, HMB, RF, LH, NPP, GARR, MS, and SKS were members of the Scientific Board. One Scientific Board member declined authorship of the article owing to personal reasons. All authors made substantial contributions to the concept and design, or the acquisition, analysis or interpretation of data, and to the drafting of the manuscript or revising it critically for important intellectual content. In addition, all authors provided final approval of the manuscript.
Funding
The work was funded by Merck KGaA, Darmstadt, Germany.
Conflict of Interest
The Delphi consensus was coordinated by a healthcare consulting and training company (Sanitanova Srl, Milan, Italy). The consensus concept was initiated and funded by Merck KGaA, Darmstadt, Germany. The sponsor was involved early in the process, defining the overarching topic to be discussed, but did not participate in the development of the statements or in any of the meetings or discussions involved in developing the Delphi consensus. The statements were, therefore, developed independently of the industry sponsor. The authors from Merck KGaA, Darmstadt, Germany, were only involved in the development of the manuscript, critically revising it for important intellectual content, especially in the Introduction, Results and Discussion sections, but could not alter the consensus statements in any way.
DC, TD’H, and SL are employees of Merck KGaA, Darmstadt, Germany. AC has received of honoraria and consultation from Merck KGaA, Darmstadt, Germany and Event Planet SpA. CA has received of honoraria for lectures from Merck KGaA, Darmstadt, Germany and Event Planet SpA. HB has been scientific advisor for Merck KGaA, Darmstadt, Germany and MSD. RF has received honoraria from Merck KGaA, Darmstadt, Germany and affiliates for lectures. NP received research grants or honoraria for lectures from: Merck KGaA, Darmstadt, Germany, MSD, Ferring Pharmaceuticals, Besins International, Roche Diagnostics, IBSA, Theramex, Gedeon Richter. MS received honoraria and research grants from Merck KGaA, Darmstadt, Germany, Ferring and IBSA. SS was a speaker at non-promotional educational symposia by Merck KGaA, Darmstadt, Germany and Ferring, and received independent research grants from Merck KGaA, Darmstadt, Germany and Ferring.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Medical writing support was provided by Helen Brereton and Nichola Cruickshanks, inScience Communication, and funded by Merck KGaA, Darmstadt, Germany. The Delphi consensus process was coordinated by Sanitanova Srl. The authors would like to thank the non-author members of the Extended Panel listed in Table 2 for their participation in the consensus.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.797365/full#supplementary-material
References
- 1. Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, Regional, and National Prevalence and Disability-Adjusted Life-Years for Infertility in 195 Countries and Territories 1990-2017: Results From a Global Burden of Disease Study 2017. Aging (Albany NY) (2019) 11(23):10952–91. doi: 10.18632/aging.102497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain T, Grainger DA, Ball GD, Gibbons WE, Rebar RW, Robins JC, et al. 30 Years Of Data: Impact Of The United States In Vitro Fertilization Data Registry On Advancing Fertility Care. Fertil Steril (2019) 111(3):477–88. doi: 10.1016/j.fertnstert.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 3. Smeltzer S, Acharya K, Truong T, Pieper C, Muasher S. Clinical Pregnancy (CP) and Live Birth (LB) Increase Significantly With Each Additional Fertilized Oocyte Up to Nine, and CP and LB Decline After That: An Analysis of 15,803 First Fresh In Vitro Fertilization Cycles From the Society for Assisted Reproductive Technology Registry. Fertil Steril (2019) 112(3):520–6.e521. doi: 10.1016/j.fertnstert.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 4. De Geyter C, Wyns C, Calhaz-Jorge C, de Mouzon J, Ferraretti AP, Kupka M, et al. 20 Years of the European IVF-Monitoring Consortium Registry: What Have We Learned? A Comparison With Registries From Two Other Regions. Hum Reprod (2020) 35(12):2832–49. doi: 10.1093/humrep/deaa250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European IVF Monitoring Consortium. Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, et al. ART in Europ : Results Generated From European Registries by ESHRE. Hum Reprod Open (2020) 2020(3):hoaa032. doi: 10.1093/hropen/hoaa032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banker M, Dyer S, Chambers GM, Ishihara O, Kupka M, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART): World Report on Assisted Reproductive Technologies 2013. Fertil Steril (2021) 116(3):741–56. doi: 10.1016/j.fertnstert.2021.03.039 [DOI] [PubMed] [Google Scholar]
- 7. Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association Between the Number of Eggs and Live Birth in IVF Treatment: An Analysis of 400 135 Treatment Cycles. Hum Reprod (2011) 26(7):1768–74. doi: 10.1093/humrep/der106 [DOI] [PubMed] [Google Scholar]
- 8. Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative Live Birth Rates According to the Number of Oocytes Retrieved After the First Ovarian Stimulation for In Vitro Fertilization/Intracytoplasmic Sperm Injection: A Multicenter Multinational Analysis Including Approximately 15,000 Women. Fertil Steril (2018) 110(4):661–670 e661. doi: 10.1016/j.fertnstert.2018.04.039 [DOI] [PubMed] [Google Scholar]
- 9. Griesinger G, Verweij PJ, Gates D, Devroey P, Gordon K, Stegmann BJ, et al. Prediction of Ovarian Hyperstimulation Syndrome in Patients Treated With Corifollitropin Alfa or rFSH in a GnRH Antagonist Protocol. PloS One (2016) 11(3):e0149615. doi: 10.1371/journal.pone.0149615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kligman I, Rosenwaks Z. Differentiating Clinical Profiles: Predicting Good Responders, Poor Responders, and Hyperresponders. Fertil Steril (2001) 76(6):1185–90. doi: 10.1016/S0015-0282(01)02893-X [DOI] [PubMed] [Google Scholar]
- 11. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A Systematic Review of Tests Predicting Ovarian Reserve and IVF Outcome. Hum Reprod Update (2006) 12(6):685–718. doi: 10.1093/humupd/dml034 [DOI] [PubMed] [Google Scholar]
- 12. Olivennes F, Howies CM, Borini A, Germond M, Trew G, Wikland M, et al. Individualizing FSH Dose for Assisted Reproduction Using a Novel Algorithm: The CONSORT Study. Reprod BioMed Online (2011) 22 Suppl 1:S73–82. doi: 10.1016/S1472-6483(11)60012-6 [DOI] [PubMed] [Google Scholar]
- 13. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Mullerian Hormone: Ovarian Reserve Testing and Its Potential Clinical Implications. Hum Reprod Update (2014) 20(5):688–701. doi: 10.1093/humupd/dmu020 [DOI] [PubMed] [Google Scholar]
- 14. Iliodromiti S, Kelsey TW, Wu O, Anderson RA, Nelson SM. The Predictive Accuracy of Anti-Mullerian Hormone for Live Birth After Assisted Conception: A Systematic Review and Meta-Analysis of the Literature. Hum Reprod Update (2014) 20(4):560–70. doi: 10.1093/humupd/dmu003 [DOI] [PubMed] [Google Scholar]
- 15. Tal R, Seifer DB. Ovarian Reserve Testing: A User's Guide. Am J Obstet Gynecol (2017) 217(2):129–40. doi: 10.1016/j.ajog.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 16. Alviggi C, Esteves SC, Conforti A. Ovarian Reserve Tests: Are They Only a Quantitative Measure? Fertil Steril (2020) 113(4):761–2. doi: 10.1016/j.fertnstert.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 17. Conforti A, Esteves SC, Cimadomo D, Vaiarelli A, Di Rella F, Ubaldi FM, et al. Management of Women With an Unexpected Low Ovarian Response to Gonadotropin. Front Endocrinol (Lausanne) (2019) 10:387. doi: 10.3389/fendo.2019.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosch E, Alviggi C, Lispi M, Conforti A, Hanyaloglu AC, Chuderland D, et al. Reduced FSH and LH Action: Implications for Medically Assisted Reproduction. Hum Reprod (2021) 36(6):1469–80. doi: 10.1093/humrep/deab065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conforti A, Esteves SC, Picarelli S, Iorio G, Rania E, Zullo F, et al. Novel Approaches for Diagnosis and Management of Low Prognosis Patients in Assisted Reproductive Technology: The POSEIDON Concept. Panminerva Med (2019) 61(1):24–9. doi: 10.23736/S0031-0808.18.03511-5 [DOI] [PubMed] [Google Scholar]
- 20. Poseidon G, Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, et al. A New More Detailed Stratification of Low Responders to Ovarian Stimulation: From a Poor Ovarian Response to a Low Prognosis Concept. Fertil Steril (2016) 105(6):1452–3. doi: 10.1016/j.fertnstert.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 21. Esteves SC, Conforti A, Sunkara SK, Carbone L, Picarelli S, Vaiarelli A, et al. Improving Reporting of Clinical Studies Using the POSEIDON Criteria: POSORT Guidelines. Front Endocrinol (Lausanne) (2021) 12:587051. doi: 10.3389/fendo.2021.587051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S, et al. Understanding Ovarian Hypo-Response to Exogenous Gonadotropin in Ovarian Stimulation and Its New Proposed Marker-The Follicle-To-Oocyte (FOI) Index. Front Endocrinol (Lausanne) (2018) 9:589. doi: 10.3389/fendo.2018.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hrdlickova B, de Almeida RC, Borek Z, Withoff S. Genetic Variation in the Non-Coding Genome: Involvement of Micro-RNAs and Long Non-Coding RNAs in Disease. Biochim Biophys Acta (2014) 1842(10):1910–22. doi: 10.1016/j.bbadis.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 24. Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A "Silent" Polymorphism in the MDR1 Gene Changes Substrate Specificity. Science (2007) 315:(5811)525–8. doi: 10.1126/science.1135308 [DOI] [PubMed] [Google Scholar]
- 25. Chu D, Wei L. Nonsynonymous, Synonymous and Nonsense Mutations in Human Cancer-Related Genes Undergo Stronger Purifying Selections Than Expectation. BMC Cancer (2019) 19(1):359. doi: 10.1186/s12885-019-5572-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conforti A, Vaiarelli A, Cimadomo D, Bagnulo F, Peluso S, Carbone L, et al. Pharmacogenetics of FSH Action in the Female. Front Endocrinol (Lausanne) (2019) 10:398. doi: 10.3389/fendo.2019.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boudjenah R, Molina-Gomes D, Torre A, Bergere M, Bailly M, Boitrelle F, et al. Genetic Polymorphisms Influence the Ovarian Response to rFSH Stimulation in Patients Undergoing In Vitro Fertilization Programs With ICSI. PloS One (2012) 7(6):e38700. doi: 10.1371/journal.pone.0038700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lledo B, Ortiz JA, Llacer J, Bernabeu R. Pharmacogenetics of Ovarian Response. Pharmacogenomics (2014) 15(6):885–93. doi: 10.2217/pgs.14.49 [DOI] [PubMed] [Google Scholar]
- 29. Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the Follicle-Stimulating Hormone Receptor Gene Causes Hereditary Hypergonadotropic Ovarian Failure. Cell (1995) 82(6):959–68. doi: 10.1016/0092-8674(95)90275-9 [DOI] [PubMed] [Google Scholar]
- 30. Simoni M, Casarini L. Mechanisms in Endocrinology: Genetics of FSH Action: A 2014-and-Beyond View. Eur J Endocrinol (2014) 170(3):R91–107. doi: 10.1530/EJE-13-0624 [DOI] [PubMed] [Google Scholar]
- 31. Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, et al. Clinical Relevance of Genetic Variants of Gonadotrophins and Their Receptors in Controlled Ovarian Stimulation: A Systematic Review and Meta-Analysis. Hum Reprod Update (2018) 24(5):599–614. doi: 10.1093/humupd/dmy019 [DOI] [PubMed] [Google Scholar]
- 32. Trevisan CM, Peluso C, Cordts EB, de Oliveira R, Christofolini DM, Barbosa CP, et al. Ala307Thr and Asn680Ser Polymorphisms of FSHR Gene in Human Reproduction Outcomes. Cell Physiol Biochem (2014) 34(5):1527–35. doi: 10.1159/000366356 [DOI] [PubMed] [Google Scholar]
- 33. Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian Response to Follicle-Stimulating Hormone (FSH) Stimulation Depends on the FSH Receptor Genotype. J Clin Endocrinol Metab (2000) 85(9):3365–9. doi: 10.1210/jcem.85.9.6789 [DOI] [PubMed] [Google Scholar]
- 34. Sudo S, Kudo M, Wada S, Sato O, Hsueh AJ, Fujimoto S. Genetic and Functional Analyses of Polymorphisms in the Human FSH Receptor Gene. Mol Hum Reprod (2002) 8(10):893–9. doi: 10.1093/molehr/8.10.893 [DOI] [PubMed] [Google Scholar]
- 35. Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a Common Single Nucleotide Polymorphism in Exon 10 of the Follicle-Stimulating Hormone (FSH) Receptor Gene for the Ovarian Response to FSH: A Pharmacogenetic Approach to Controlled Ovarian Hyperstimulation. Pharmacogenet Genomics (2005) 15(7):451–6. doi: 10.1097/01.fpc.0000167330.92786.5e [DOI] [PubMed] [Google Scholar]
- 36. Jun JK, Yoon JS, Ku SY, Choi YM, Hwang KR, Park SY, et al. Follicle-Stimulating Hormone Receptor Gene Polymorphism and Ovarian Responses to Controlled Ovarian Hyperstimulation for IVF-ET. J Hum Genet (2006) 51(8):665–70. doi: 10.1007/s10038-006-0005-5 [DOI] [PubMed] [Google Scholar]
- 37. Alviggi C, Conforti A, Caprio F, Gizzo S, Noventa M, Strina I, et al. In Estimated Good Prognosis Patients Could Unexpected "Hyporesponse" to Controlled Ovarian Stimulation be Related to Genetic Polymorphisms of FSH Receptor? Reprod Sci (2016) 23(8):1103–8. doi: 10.1177/1933719116630419 [DOI] [PubMed] [Google Scholar]
- 38. Nenonen HA, Lindgren IA, Prahl AS, Trzybulska D, Kharraziha I, Hulten M, et al. The N680S Variant in the Follicle-Stimulating Hormone Receptor Gene Identifies Hyperresponders to Controlled Ovarian Stimulation. Pharmacogenet Genomics (2019) 29(5):114–20. doi: 10.1097/FPC.0000000000000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wunsch A, Ahda Y, Banaz-Yasar F, Sonntag B, Nieschlag E, Simoni M, et al. Single-Nucleotide Polymorphisms in the Promoter Region Influence the Expression of the Human Follicle-Stimulating Hormone Receptor. Fertil Steril (2005) 84(2):446–53. doi: 10.1016/j.fertnstert.2005.02.031 [DOI] [PubMed] [Google Scholar]
- 40. Lindgren I, Nenonen H, Henic E, Bungum L, Prahl A, Bungum M, et al. Gonadotropin Receptor Variants Are Linked to Cumulative Live Birth Rate After In Vitro Fertilization. J Assist Reprod Genet (2019) 36(1):29–38. doi: 10.1007/s10815-018-1318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lindgren I, Baath M, Uvebrant K, Dejmek A, Kjaer L, Henic E, et al. Combined Assessment of Polymorphisms in the LHCGR and FSHR Genes Predict Chance of Pregnancy After In Vitro Fertilization. Hum Reprod (2016) 31(3):672–83. doi: 10.1093/humrep/dev342 [DOI] [PubMed] [Google Scholar]
- 42. Ramaraju GA, Cheemakurthi R, Prathigudupu K, Balabomma KL, Kalagara M, Thota S, et al. Role of Lh Polymorphisms and r-hLh Supplementation in GnRh Agonist Treated ART Cycles: A Cross Sectional Study. Eur J Obstet. Gynecol. Reprod Biol (2018) 222:119–25. doi: 10.1016/j.ejogrb.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 43. Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P, et al. A Common Polymorphic Allele of the LH Beta-Subunit Gene Is Associated With Higher Exogenous FSH Consumption During Controlled Ovarian Stimulation for Assisted Reproductive Technology. Reprod Biol Endocrinol (2013) 11:51. doi: 10.1186/1477-7827-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Batista MC, Duarte Ede F, Borba MD, Zingler E, Mangussi-Gomes J, dos Santos BT, et al. Trp28Arg/Ile35Thr LHB Gene Variants Are Associated With Elevated Testosterone Levels in Women With Polycystic Ovary Syndrome. Gene (2014) 550(1):68–73. doi: 10.1016/j.gene.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 45. Schuring AN, Busch AS, Bogdanova N, Gromoll J, Tuttelmann F. Effects of the FSH-Beta-Subunit Promoter Polymorphism -211G->T on the Hypothalamic-Pituitary-Ovarian Axis in Normally Cycling Women Indicate a Gender-Specific Regulation of Gonadotropin Secretion. J Clin Endocrinol Metab (2013) 98(1):E82–86. doi: 10.1210/jc.2012-2780 [DOI] [PubMed] [Google Scholar]
- 46. Rull K, Grigorova M, Ehrenberg A, Vaas P, Sekavin A, Nommemees D, et al. FSHB -211 G>T Is a Major Genetic Modulator of Reproductive Physiology and Health in Childbearing Age Women. Hum Reprod (2018) 33(5):954–66. doi: 10.1093/humrep/dey057 [DOI] [PubMed] [Google Scholar]
- 47. Trevisan CM, de Oliveira R, Christofolini DM, Barbosa CP, Bianco B. Effects of a Polymorphism in the Promoter Region of the Follicle-Stimulating Hormone Subunit Beta (FSHB) Gene on Female Reproductive Outcomes. Genet Test Mol Biomarkers (2019) 23(1):39–44. doi: 10.1089/gtmb.2018.0182 [DOI] [PubMed] [Google Scholar]
- 48. Concolino D, Degennaro E, Parini R, g. Fabry Delphi working. g. Fabry Delphi working . Delphi Consensus on the Current Clinical and Therapeutic Knowledge on Anderson-Fabry Disease. Eur J Intern Med (2014) 25(8):751–6. doi: 10.1016/j.ejim.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 49. Girolomoni G, Altomare G, Ayala F, Berardesca E, Calzavara Pinton P, Chimenti S, et al. Differential Management of Mild-to-Severe Psoriasis With Biologic Drugs: An Italian Delphi Consensus Expert Panel. J Dermatolog. Treat (2015) 26(2):128–33. doi: 10.3109/09546634.2014.907466 [DOI] [PubMed] [Google Scholar]
- 50. Huang X, Li L, Hong L, Zhou W, Shi H, Zhang H, et al. The Ser680Asn Polymorphism in the Follicle-Stimulating Hormone Receptor Gene Is Associated With the Ovarian Response in Controlled Ovarian Hyperstimulation. Clin Endocrinol (Oxf) (2015) 82(4):577–83. doi: 10.1111/cen.12573 [DOI] [PubMed] [Google Scholar]
- 51. Laven JS, Mulders AG, Suryandari DA, Gromoll J, Nieschlag E, Fauser BC, et al. Follicle-Stimulating Hormone Receptor Polymorphisms in Women With Normogonadotropic Anovulatory Infertility. Fertil Steril (2003) 80(4):986–92. doi: 10.1016/S0015-0282(03)01115-4 [DOI] [PubMed] [Google Scholar]
- 52. Falconer H, Andersson E, Aanesen A, Fried G. Follicle-Stimulating Hormone Receptor Polymorphisms in a Population of Infertile Women. Acta Obstet. Gynecol. Scand (2005) 84(8):806–11. doi: 10.1111/j.0001-6349.2005.00736.x [DOI] [PubMed] [Google Scholar]
- 53. Huang S, Yang J, Yin T, Xing LI, Jie LI, Xu W. Association of Gene Polymorphism of Follicle Stimulating Hormone Receptor With Ovarian Response in IVF Cycles. Med J Wuhan Univ (2010) 31(3):334–8. doi: 10.14188/j.1671-8852.2010.03.020 [DOI] [Google Scholar]
- 54. Loutradis D, Patsoula E, Minas V, Koussidis GA, Antsaklis A, Michalas S, et al. FSH Receptor Gene Polymorphisms Have a Role for Different Ovarian Response to Stimulation in Patients Entering IVF/ICSI-ET Programs. J Assist Reprod Genet (2006) 23(4):177–84. doi: 10.1007/s10815-005-9015-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lazaros LA, Hatzi EG, Pamporaki CE, Sakaloglou PI, Xita NV, Markoula SI, et al. The Ovarian Response to Standard Gonadotrophin Stimulation Depends on FSHR, SHBG and CYP19 Gene Synergism. J Assist Reprod Genet (2012) 29(11):1185–91. doi: 10.1007/s10815-012-9849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yan Y, Gong Z, Zhang L, Li Y, Li X, Zhu L, et al. Association of Follicle-Stimulating Hormone Receptor Polymorphisms With Ovarian Response in Chinese Women: A Prospective Clinical Study. PloS One (2013) 8(10):e78138. doi: 10.1371/journal.pone.0078138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pabalan N, Trevisan CM, Peluso C, Jarjanazi H, Christofolini DM, Barbosa CP, et al. Evaluating Influence of the Genotypes in the Follicle-Stimulating Hormone Receptor (FSHR) Ser680Asn (Rs6166) Polymorphism on Poor and Hyper-Responders to Ovarian Stimulation: A Meta-Analysis. J Ovarian Res (2014) 7:285. doi: 10.1186/s13048-014-0122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang H, Yan Y, Wang T, Zhang T, Shi W, Fan R, et al. Effect of Follicle-Stimulating Hormone Receptor Asn680Ser Polymorphism on the Outcomes of Controlled Ovarian Hyperstimulation: An Updated Meta-Analysis of 16 Cohort Studies. J Assist Reprod Genet (2015) 32(12):1801–10. doi: 10.1007/s10815-015-0600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garcia-Jimenez G, Zarinan T, Rodriguez-Valentin R, Mejia-Dominguez NR, Gutierrez-Sagal R, Hernandez-Montes G, et al. Frequency of the T307A, N680S, and -29G>A Single-Nucleotide Polymorphisms in the Follicle-Stimulating Hormone Receptor in Mexican Subjects of Hispanic Ancestry. Reprod Biol Endocrinol (2018) 16(1):100. doi: 10.1186/s12958-018-0420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sheikhha MH, Eftekhar M, Kalantar SM. Investigating the Association Between Polymorphism of Follicle-Stimulating Hormone Receptor Gene and Ovarian Response in Controlled Ovarian Hyperstimulation. J Hum Reprod Sci (2011) 4(2):86–90. doi: 10.4103/0974-1208.86089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Castro F, Moron FJ, Montoro L, Galan JJ, Hernandez DP, Padilla ES, et al. Human Controlled Ovarian Hyperstimulation Outcome Is a Polygenic Trait. Pharmacogenetics (2004) 14(5):285–93. doi: 10.1097/00008571-200405000-00003 [DOI] [PubMed] [Google Scholar]
- 62. de Castro F, Ruiz R, Montoro L, Perez-Hernandez D, Sanchez-Casas Padilla E, Real LM, et al. Role of Follicle-Stimulating Hormone Receptor Ser680Asn Polymorphism in the Efficacy of Follicle-Stimulating Hormone. Fertil Steril (2003) 80(3):571–6. doi: 10.1016/S0015-0282(03)00795-7 [DOI] [PubMed] [Google Scholar]
- 63. Fujimoto VY, Luke B, Brown MB, Jain T, Armstrong A, Grainger DA, et al. Racial and Ethnic Disparities in Assisted Reproductive Technology Outcomes in the United States. Fertil Steril (2010) 93(2):382–90. doi: 10.1016/j.fertnstert.2008.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim HH. Gonadotropin Dosing for Assisted Reproductive Technology: Does Race Matter? Fertil Steril (2021) 115(6):1430–1. doi: 10.1016/j.fertnstert.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 65. Gashi Z, Elezaj S, Zeqiraj A, Grabanica D, Shabani I, Gruda B, et al. Relationship Between Genotype Variants Follicle-Stimulating Hormone Receptor Gene Polymorphisms (FSHR) and Morphology of Oocytes Prior to ICSI Procedures. Med Arch (2016) 70(5):364–8. doi: 10.5455/medarh.2016.70.364-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Follicle-Stimulating Hormone Receptor Polymorphism (Thr307Ala) Is Associated With Variable Ovarian Response and Ovarian Hyperstimulation Syndrome in Indian Women. Fertil Steril (2009) 91(2):432–9. doi: 10.1016/j.fertnstert.2007.11.093 [DOI] [PubMed] [Google Scholar]
- 67. Motawi TMK, Rizk SM, Maurice NW, Maged AM, Raslan AN, Sawaf AH. The Role of Gene Polymorphisms and AMH Level in Prediction of Poor Ovarian Response in Egyptian Women Undergoing IVF Procedure. J Assist Reprod Genet (2017) 34(12):1659–66. doi: 10.1007/s10815-017-1013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Desai SS, Achrekar SK, Pathak BR, Desai SK, Mangoli VS, Mangoli RV, et al. Follicle-Stimulating Hormone Receptor Polymorphism (G-29A) Is Associated With Altered Level of Receptor Expression in Granulosa Cells. J Clin Endocrinol Metab (2011) 96(9):2805–12. doi: 10.1210/jc.2011-1064 [DOI] [PubMed] [Google Scholar]
- 69. Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor Ovarian Response to Gonadotrophin Stimulation Is Associated With FSH Receptor Polymorphism. Reprod BioMed Online (2009) 18(4):509–15. doi: 10.1016/S1472-6483(10)60127-7 [DOI] [PubMed] [Google Scholar]
- 70. Desai SS, Roy BS, Mahale SD. Mutations and Polymorphisms in FSH Receptor: Functional Implications in Human Reproduction. Reproduction (2013) 146(6):R235–48. doi: 10.1530/REP-13-0351 [DOI] [PubMed] [Google Scholar]
- 71. Takahashi K, Ozaki T, Kanasaki H, Miyazaki K. Successful Pregnancy in a Woman With Ovarian Failure Associated With Mutation in the Beta-Subunit of Luteinizing Hormone. Horm Res (2001) 55(5):258–63. doi: 10.1159/000050007 [DOI] [PubMed] [Google Scholar]
- 72. Altmae S, Hovatta O, Stavreus-Evers A, Salumets A. Genetic Predictors of Controlled Ovarian Hyperstimulation: Where Do We Stand Today? Hum Reprod Update (2011) 17(6):813–28. doi: 10.1093/humupd/dmr034 [DOI] [PubMed] [Google Scholar]
- 73. Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I, et al. Suboptimal Response to GnRHa Long Protocol Is Associated With a Common LH Polymorphism. Reprod BioMed Online (2011) 22 Suppl 1:S67–72. doi: 10.1016/S1472-6483(11)60011-4 [DOI] [PubMed] [Google Scholar]
- 74. Polyzos NP, Neves AR, Drakopoulos P, Spits C, Alvaro Mercadal B, Garcia S, et al. The Effect of Polymorphisms in FSHR and FSHB Genes on Ovarian Response: A Prospective Multicenter Multinational Study in Europe and Asia. Hum Reprod (2021) 36(6):1711–21. doi: 10.1093/humrep/deab068 [DOI] [PubMed] [Google Scholar]
- 75. La Marca A, Papaleo E, Alviggi C, Ruvolo G, De Placido G, Candiani M, et al. The Combination of Genetic Variants of the FSHB and FSHR Genes Affects Serum FSH in Women of Reproductive Age. Hum Reprod (2013) 28(5):1369–74. doi: 10.1093/humrep/det061 [DOI] [PubMed] [Google Scholar]
- 76. Ga R, Cheemakurthi R, Kalagara M, Prathigudupu K, Balabomma KL, Mahapatro P, et al. Effect of LHCGR Gene Polymorphism (Rs2293275) on LH Supplementation Protocol Outcomes in Second IVF Cycles: A Retrospective Study. Front Endocrinol (Lausanne) (2021) 12:628169. doi: 10.3389/fendo.2021.628169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moragianni VA, Penzias AS. Cumulative Live-Birth Rates After Assisted Reproductive Technology. Curr Opin Obstet Gynecol (2010) 22(3):189–92. doi: 10.1097/GCO.0b013e328338493f [DOI] [PubMed] [Google Scholar]
- 78. Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Buhler K, et al. Recombinant Luteinizing Hormone Supplementation in Assisted Reproductive Technology: A Systematic Review. Fertil Steril (2018) 109(4):644–64. doi: 10.1016/j.fertnstert.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 79. Ubaldi FM, Cimadomo D, Vaiarelli A, Fabozzi G, Venturella R, Maggiulli R, et al. Advanced Maternal Age in IVF: Still a Challenge? The Present and the Future of Its Treatment. Front Endocrinol (Lausanne) (2019) 10:94. doi: 10.3389/fendo.2019.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. UK BioBank . "UK Biobank " (2021). Available at: https://www.ukbiobank.ac.uk/.
- 81. Esteves SC, Yarali H, Vuong LN, Carvalho JF, Ozbek IY, Polat M, et al. Cumulative Delivery Rate Per Aspiration IVF/ICSI Cycle in POSEIDON Patients: A Real-World Evidence Study of 9073 Patients. Hum Reprod (2021) 36(8):2157–69. doi: 10.1093/humrep/deab152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jungheim ES, Carson KR. Leveraging Real-World Data to Move Toward More Personalized Fertility Treatment. Fertil Steril (2018) 109(4):608–9. doi: 10.1016/j.fertnstert.2018.01.036 [DOI] [PubMed] [Google Scholar]
- 83. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int J Surg (2014) 12(12):1495–9. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 84. Dreyer NA, Bryant A, Velentgas P. The GRACE Checklist: A Validated Assessment Tool for High Quality Observational Studies of Comparative Effectiveness. J Manag Care Spec Pharm (2016) 22(10):1107–13. doi: 10.18553/jmcp.2016.22.10.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.