Abstract
Background
Previous studies comparing immigrant ethnic groups and native patients with IBD have yielded clinical and phenotypic differences. To date, no study has focused on the immigrant IBD population in Spain.
Methods
Prospective, observational, multicenter study comparing cohorts of IBD patients from ENEIDA-registry who were born outside Spain with a cohort of native patients.
Results
We included 13,524 patients (1,864 immigrant and 11,660 native). The immigrants were younger (45 ± 12 vs. 54 ± 16 years, p < 0.001), had been diagnosed younger (31 ± 12 vs. 36 ± 15 years, p < 0.001), and had a shorter disease duration (14 ± 7 vs. 18 ± 8 years, p < 0.001) than native patients. Family history of IBD (9 vs. 14%, p < 0.001) and smoking (30 vs. 40%, p < 0.001) were more frequent among native patients. The most prevalent ethnic groups among immigrants were Caucasian (41.5%), followed by Latin American (30.8%), Arab (18.3%), and Asian (6.7%). Extraintestinal manifestations, mainly musculoskeletal affections, were more frequent in immigrants (19 vs. 11%, p < 0.001). Use of biologics, mainly anti-TNF, was greater in immigrants (36 vs. 29%, p < 0.001). The risk of having extraintestinal manifestations [OR: 2.23 (1.92–2.58, p < 0.001)] and using biologics [OR: 1.13 (1.0–1.26, p = 0.042)] was independently associated with immigrant status in the multivariate analyses.
Conclusions
Compared with native-born patients, first-generation-immigrant IBD patients in Spain were younger at disease onset and showed an increased risk of having extraintestinal manifestations and using biologics. Our study suggests a featured phenotype of immigrant IBD patients in Spain, and constitutes a new landmark in the epidemiological characterization of immigrant IBD populations in Southern Europe.
Keywords: immigrant, phenotype, biologics, inflammatory bowel disease, Crohn's disease, ulcerative colitis
HIGHLIGHTS
What is known?
- Studies evaluating phenotypic characteristics of immigrant IBD populations show specific features that are not always reproduced in native patients.
- Few studies have evaluated the characteristics of immigrant IBD patients in Southern Europe and, to date, none has focused on the immigrant IBD population in Spain.
What is new here?
- The main ethnic groups of immigrant IBD patients in Spain are Caucasian, Latin American and Arab.
- Immigrant IBD patients in Spain are younger and have more extraintestinal manifestations than native-born patients. Accordingly, the use of biologics is more frequent among immigrants.
- The study represents a new landmark in the epidemiologic characterization of immigrant patients with IBD in Southern Europe.
Introduction
Inflammatory bowel disease (IBD) is a term that covers Crohn's disease (CD) and ulcerative colitis (UC), which are chronic, progressive and disabling diseases characterized by cycles of remission and relapse. Though the cause of IBD is not yet fully understood, its pathogenesis has been linked to complex interactions between genetic, epithelial, immune, microbial and environmental factors (1, 2). Although more than 240 IBD-associated risk variants have been identified to date by large genome-wide association studies (3), genetic background seems only to partially explain susceptibility to the disease and in most cases, environmental risk factors are also involved in disease onset and progression (4, 5). However, studies focusing on causative environmental factors are challenging and have produced conflicting results (6).
The epidemiology of IBD has changed over time and is influenced by geographical variations, suggesting that environmental factors play a role in modifying its expression (7). In fact, newer epidemiologic studies define IBD as a global disease, suggesting that its incidence either continues to grow steadily or remains stable in western countries while rapidly increasing in developing countries (8), possibly owing to their increasingly westernized lifestyle (9). Moreover, studies carried out among migrants show that they tend to acquire the disease incidence of their adopted country rather than the country of origin, reflecting the importance of the environmental risk factors such as lifestyle, pollution and diet (10–12).
Migration has increased in the past decades together with globalization. Several studies have been conducted in large, well-defined migrant groups such as Mexicans living in the USA and people from India living in Europe, the USA or the Middle East (13). A recent review on the epidemiology of IBD in migrant racial and ethnic groups revealed differences in disease incidence, prevalence and phenotypes between immigrants and native patients in individual studies (14). However, few studies of this kind have analyzed European populations. Although there is evidence showing an increased risk of IBD in Spanish people who have migrated to Western Europe (15), to date no study has explored the characteristics of immigrant IBD patients in Spain, which has a population of 47 million people and more than 5 million foreign residents (www.ine.es/en).
This study aimed to explore the characteristics of immigrant IBD patients in Spain compared with native IBD patients, focusing on potential differences in age of disease onset, IBD phenotype, and therapeutic requirements.
Methods
Study Design
This was an observational, prospective, multicenter, nationwide study carried out with data from ENEIDA project (16). ENEIDA is a large, prospectively maintained nationwide registry of IBD patients in Spain. It was set up in 2006 by the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) and undergoes continuous external monitoring to ensure completeness and consistency of the entered data. At the time of the study, the database contained data from over 69,000 patients from ~100 actively participating centers.
Study Population and Data Collection
The immigrant cohort of our study comprised all adult IBD patients born outside of Spain (first-generation immigrants) and included in the ENEIDA registry. Any conflicting data regarding date of migration to Spain, country of birth, ethnicity, or date of IBD diagnosis were checked against patient's clinical records by ENEIDA project investigators. The second cohort was a large, randomly selected sample of adult native IBD patients included in the ENEIDA database. Native IBD patients were born in Spain and were all Caucasians. Inclusion criteria were diagnosis with UC, CD or IBD unclassified (IBDU) based on the accepted criteria of the European Crohn's and Colitis Organization (ECCO) (17); diagnosis before 2015, to ensure follow-up of at least 5 years; and age 16 years or older. The ENEIDA project was approved by research ethics committees in all participating centers and the GETECCU scientific committee. All patients gave written informed consent before being enrolled in the ENEIDA registry.
Patient Demographics, Disease Phenotype and Disease Course
We collected the following demographic characteristics of the included patients: age, sex, smoking status, ethnicity, country of birth, date of migration to Spain, date of IBD diagnosis, duration of the disease, and family history of IBD. The ethnic groups were classified as Caucasian, Latin American, Arab, Asian, African, Roma, and Jewish. Using the United Nations Standard Country or Area Codes for Statistical Use, M49 standard (https://unstats.un.org/unsd/methodology/m49/) we categorized the birthplace of immigrants into the following geographical regions: Northern Africa, Central Africa, South America, Northern America, Central America, Caribbean, Eastern Europe, Western Europe, Southern Europe, Northern Europe, Southern Asia, Western Asia, Eastern Asia, Southeast Asia, and Oceania.
We used the Montreal classification of IBD to classify disease phenotypes according to extent of UC and location and behavior of CD (18). Surgical procedures were defined as interventions related directly to IBD or its complications, including both abdominal and perianal surgeries. Pharmacological treatment collected included type and time to initiation of first biologic agent and use of immunomodulator agents (methotrexate, azathioprine, or 6-mercaptopurine).
Statistical Analysis
Continuous variables are reported as means with standard deviations and categorical variables as absolute and relative frequencies. Differences between immigrant and native populations were analyzed using the two-tailed t test and Mann-Whitney U test for normally and non-normally distributed quantitative variables, respectively. We analyzed categorical variables using the Chi-square test. Univariate and multivariate logistic regression analyses were performed to assess potential risk factors for the development of EIMs, use of biologics, surgery, and death. Only significant variables from the univariable analysis were entered into the regression model. Estimations of crude odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated using logistic regression analysis. Timeframe between study entry and surgery or initiation of treatment with biologics was analyzed using Kaplan-Meier life-table analysis, as precise dates were known. Log-rank test was used to compare survival curves. All reported P-values are 2-sided, and P-values lower than 0.05 are considered to indicate statistical significance. All analyses were carried out in the R software environment (Core Team 2014, Vienna, Austria).
Results
Demographic Characteristics
A total of 13,524 patients were included in this study, 1,864 of whom were immigrants born outside Spain. The comparative cohort included 11,660 Caucasian IBD patients born in Spain. Table 1 summarizes the main demographic characteristics of immigrants and native patients. Median age of the immigrant group at the time of arrival in Spain was 26 years (range 0–78 years). No differences in IBD diagnosis and sex were observed between the two groups comprising all IBD patients, but more native than immigrant patients diagnosed with UC were men. Immigrant IBD patients were younger at inclusion, were diagnosed earlier in life, and had a shorter disease duration than native patients. These differences persisted when CD and UC patients were analyzed separately (Supplementary Tables 1, 2). Immigrants aged younger than 15 years on arrival in Spain were significantly younger at disease onset than those who migrated after age 15 (23.24 ± 11.34 vs. 32.12 ± 11.28, p < 0.001, Supplementary Table 3). Family history of IBD and smoking (present or past) were more frequent in native patients than in immigrants (14.3 vs. 9.4%, p = 0.001; and 40.6 vs. 28%, p = 0.001, respectively). No differences were found regarding the frequency of appendectomies between the two groups. Seventy-one percent of immigrant patients were diagnosed with IBD in Spain. Supplementary Table 4 compares the main demographic characteristics of immigrants diagnosed in Spain vs. outside Spain.
Table 1.
Demographic and clinical characteristics of the immigrant and native-born IBD population in Spain.
| Immigrants | Natives | P -value | |
|---|---|---|---|
| (n = 1,864) | (n = 11,660) | ||
| Gender, female (n,%) | 936 (50.2) | 5444 (46.7) | 0.005 |
| Mean current age (SD) | 45.3 (12.6) | 54.5 (16.0) | <0.001 |
| Mean age at IBD diagnosis | 31.2 (12.0) | 36.5 (15.6) | <0.001 |
| Mean duration of disease (SD) | 14.0 (7.2) | 18.0 (8.7) | <0.001 |
| Smoking habit | |||
| no (n,%) | 1181 (69.3) | 6952 (59.5) | <0.001 |
| yes (n,%) | 296 (17.4) | 2227 (19.1) | |
| Ex (n,%) | 227 (13.3) | 2508 (21.5) | |
| Crohn's disease (n,%) | 777 (41.8) | 4755 (40.8) | 0.068 |
| Ulcerative colitis (n,%) | 1029 (55.4) | 6665 (57.2) | |
| Unclassified IBD (n,%) | 52 (2.8) | 240 (2.1) | |
| Crohn's age at diagnosis | |||
| A1 (<16y) (n,%) | 67 (8.6) | 275 (5.8) | <0.001 |
| A2 (16-40y) (n,%) | 586 (75.3) | 3223 (67.8) | |
| A3 (>40y) (n,%) | 125 (16.1) | 1257 (26.4) | |
| Crohn's location | |||
| L1 (ileal) (n,%) | 154 (28.5) | 1025 (28.4) | 0.368 |
| L2 (colonic) (n,%) | 112 (20.7) | 640 (17.7) | |
| L3 (ileocolonic) (n,%) | 233 (43.1) | 1636 (45.3) | |
| L4 (upper GI) (n,%) | 42 (7.8) | 308 (8.5) | |
| Crohn's behavior | |||
| B1 (inflammatory) | 482 (63.9) | 2744 (57.7) | 0.002 |
| B2 (stricturing) (n,%) | 144 (19.1) | 1158 (24.4) | |
| B3 (perforating) (n,%) | 128 (17.0) | 853 (17.9) | |
| Perianal perforating (n;%) | 242 (13.7) | 1776 (15.4) | 0.069 |
| Ulcerative colitis extent | |||
| Proctitis (n,%) | 186 (29.7) | 1478 (31.9) | 0.031 |
| Left sided colitis (n,%) | 357 (57.0) | 2689 (58.1) | |
| Extensive colitis (n,%) | 83 (13.3) | 459 (9.9) | |
| Extraintestinal manifestations (n;%) | 318 (18.9) | 1275 (10.9) | <0.001 |
| Family history of IBD (n,%) | 155 (9.4) | 1676 (14.3) | <0.001 |
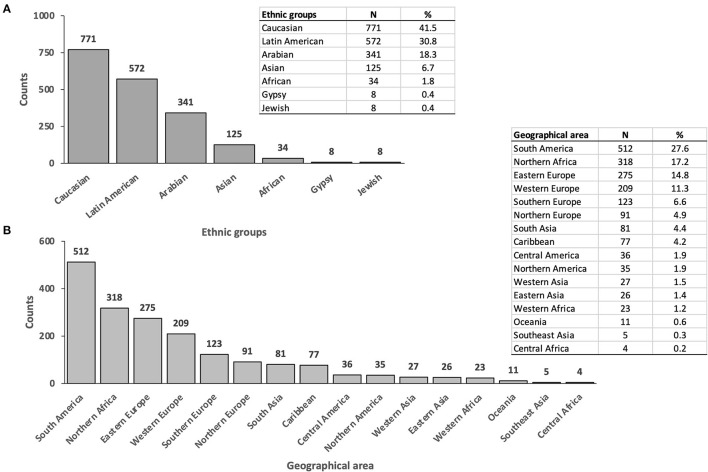
Figure 1A shows the distribution by ethnicity of the immigrant cohort. The most prevalent ethnic group was Caucasian (41.5%, 771), followed by Latin American (30.8%, 572) and Arab (18.3%, 341), whereas Asians represented a minority (6.7%, 125). Supplementary Table 5 shows the characteristics of immigrant patients by ethnicity. UC diagnosis was more common among Latin American and Asian IBD patients compared with Caucasians and Arabs. The distribution of the immigrant population by geographical area of birth is represented in Figure 1B. South America was the most frequent birthplace, followed by North Africa and Eastern Europe.
Figure 1.
(A) Distribution of immigrant population by ethnicity. (B) Distribution of immigrant population by geographical area of birth according to OMS Standard Country or Area Codes for Statistical Use, M49 standard.
Disease Phenotype
Phenotypic IBD characteristics of both cohorts are displayed in Table 1. Disease location in CD patients did not differ between the two groups. Regarding the age of disease onset, the number of patients diagnosed under 16 years (A1) was significantly higher among immigrant compared with native CD patients. In contrast, the number of patients diagnosed over 40 years (A3) was significantly higher in the native group. Stricturing behavior was more frequent among native CD patients, although the multivariate analysis found no independent association between stricturing behavior and being native. Similarly, stricturing behavior was not independently associated with smoking but was associated with longer disease duration (Supplementary Table 6). We found no significant difference in the prevalence of perianal disease between the two groups. In UC patients, the distribution of ulcerative proctitis, left sided UC and extensive UC was similar in immigrant and native patients.
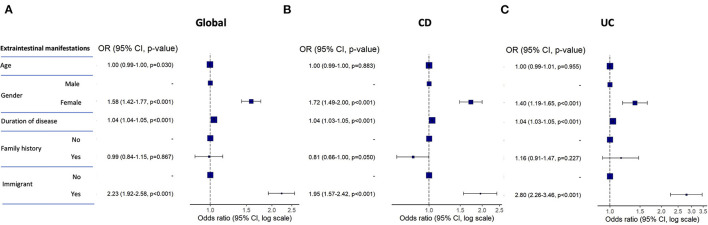
EIMs were significantly more frequent among immigrant compared with native IBD patients (Table 1). The list of EIMs is detailed in Table 2. Musculoskeletal affections were the most frequent EIM among patients in both groups. Primary sclerosing cholangitis was more frequent in immigrant compared with native patients only in the cohort of UC patients. In the multivariate analysis, the risk of suffering EIMs among the complete series of IBD patients was independently associated with female sex, longer duration of disease and being an immigrant, while family history of IBD was unrelated (Figure 2A). These factors remained independently associated with an increased risk of EIMs when the patients were divided into CD and UC patients (Figures 2B,C). Supplementary Table 7 describes the complete univariate and multivariate analyses for EIMs in IBD patients.
Table 2.
Extraintestinal manifestations in the immigrant and native-born IBD population in Spain.
| Immigrants | Natives | p -value | |
|---|---|---|---|
| N (%) | (n = 395) | (n = 1,608) | |
| Peripheral arthritis | 164 (42.3) | 582 (25.3) | <0.001 |
| Ankylosing spondylitis | 52 (14.2) | 158 (6.1) | <0.001 |
| Isolated sacroiliitis | 29 (13.9) | 166 (18.2) | 0.176 |
| Erythema nodosum | 40 (17.6) | 221 (10.5) | 0.002 |
| Pyoderma gangrenosum | 11 (6.0) | 67 (3.2) | 0.078 |
| Aphtous stomatitis | 31 (8.8) | 156 (6.0) | 0.005 |
| Uveitis | 25 (12.2) | 76 (3.8) | <0.001 |
| Episcleritis | 6 (3.5) | 64 (3.2) | 1.000 |
| Primary sclerosing cholangitis | 21 (5.9) | 41 (1.6) | <0.001 |
| Thrombosis | 16 (4.5) | 77 (3.0) | 0.167 |
Figure 2.
Forest plots representing the odds ratio for presenting with EIMs in the multivariate analysis for the global (A), CD (B), and UC (C) cohorts of patients. CD, Crohn's disease; UC, ulcerative colitis.
Therapeutic Requirements and Disease Course
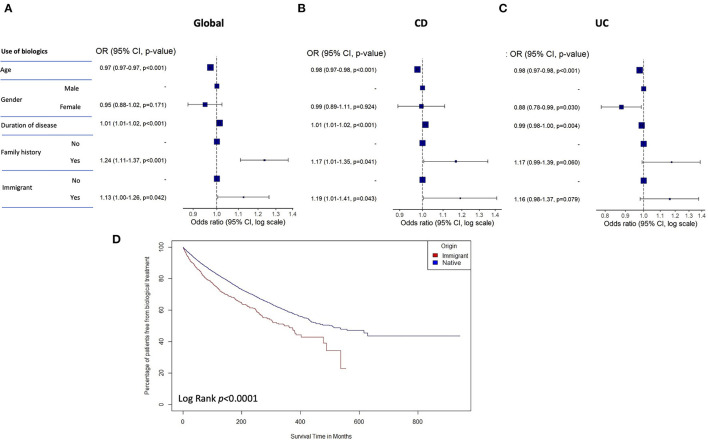
Table 3 summarizes the different medical treatments and surgical procedures in both groups of patients. Endoscopic treatment was more frequent among native patients, while no significant differences between groups were present regarding the use of immunosuppressants (mainly thiopurines). In contrast, the use of biologics was significantly more frequent in the subgroup of immigrants compared with native patients. Of the different biologics, anti-TNF was the most frequently prescribed in both subgroups. Younger age, longer duration of disease, family history of IBD and being a immigrant were also independently associated with the use of biological therapy in the complete series of IBD patients in the multivariate analysis (Figure 3A). While all these variables remained independently associated with the use of biologics in the cohort of CD patients, family history and being a immigrant were not independently associated with an increased risk of using biologics in the multivariate analysis among UC patients (Figures 3B,C). Supplementary Table 8 describes the univariate and multivariate analyses for the use of biologics in IBD patients. Time to first biologic drug during follow-up was significantly earlier in immigrant vs. native patients according to the performed survival analysis of the time to first biologic drug (Figure 3D).
Table 3.
Medical treatments and surgical procedures in the immigrant and native-born IBD population in Spain.
| Immigrants | Natives | p -value | |
|---|---|---|---|
| N (%) | |||
| Endoscopic treatment | 24/1517 (1.6) | 425/10663 (3.9) | <0.001 |
| Immunosuppresants | 1006/1835 (54.8) | 6126/11651 (52.6) | 0.082 |
| Biologics | 643/1766 (36.4) | 3469/11651 (29.8) | <0.001 |
| AntiTNF | 637 (34.2) | 3405 (29.2) | 0.038 |
| Vedolizumab | 2 (0.1) | 41 (2.5) | |
| Ustekinumab | - | 19 (0.16) | |
| Others | 2 (0.1) | 4 (0.03) | |
| IBD-related surgery | 389/1763 (22.1) | 2742/11651 (23.5) | 0.184 |
| Abdominal surgery for CD* | 276 (29.4) | 2253 (34.5) | 0.002 |
| Total proctocolectomy for UC** | 37 (3.5) | 240 (3.4) | 1 |
| Perianal surgery | 154 (8.3) | 1372 (11.8) | 0.184 |
Percentages are calculated based on patients with available data.
Percentage calculated on CD patients.
Percentage calculated on UC patients.
Figure 3.
Forest plots representing the odds ratio for using biologics in the multivariate analysis for the global (A), CD (B), and UC (C) cohorts of patients. (D) Survival analysis of the time to first biologic drug in immigrant vs. native patients. CD, Crohn's disease; UC, ulcerative colitis.
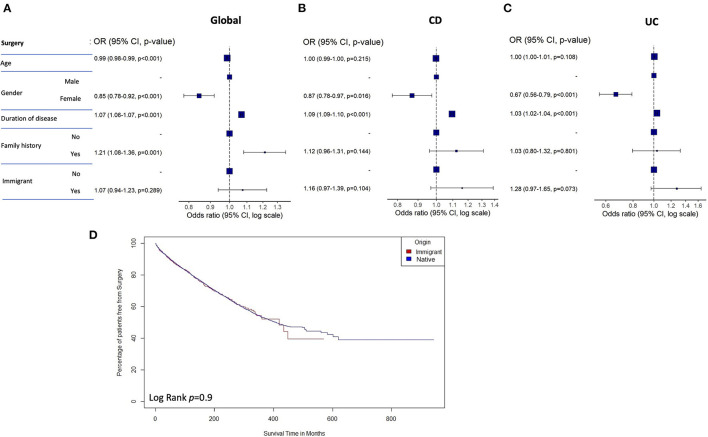
No significant differences were found in the frequency of IBD-related abdominal and perianal surgeries between all immigrant and native IBD patients, or in the frequency of proctocolectomy between native and immigrant UC patients. However, abdominal surgery was significantly more frequent among native vs. immigrant CD patients. The risk of surgical treatment was not associated with immigrant status. Female sex, longer duration of disease, and family history of IBD were associated with this parameter in the global cohort of all IBD patients (Figure 4A), although only female sex and longer duration of disease remained independently associated, with a significantly decreased risk of surgical treatment, when CD and UC cohorts of patients were analyzed separately (Figures 4B,C). Supplementary Table 9 shows the univariate and multivariate analyses for the risk for surgical treatment in the series of IBD patients. Time to surgery during follow-up was not different between native vs. immigrant patients according to the performed survival analysis of the time to intestinal surgery (Figure 4D).
Figure 4.
Forest plots representing the odds ratio of surgery in the multivariate analysis for the global (A), CD (B), and UC (C) cohorts of patients. (D) Survival analysis of the time to surgery in immigrant vs. native patients. CD, Crohn's disease; UC, ulcerative colitis.
Loss to follow-up was more frequent among immigrant IBD patients (n = 265, 14.6 vs. n = 1,216, 10.4%; p = 0.001). Mean follow-up was 5.6 ± 6.2 years in the immigrant subgroup and 6.1 ± 4.5 years in the subgroup of native patients (p = 0.016). Significantly more native than immigrant patients died during the study period (2.6%, n = 300 vs. 0.9%, n = 17; p < 0.01). The cause of death was available for 179 natives (59.6%) and 3 immigrants (17.6%). The most frequent causes of death in native patients were neoplasm (n = 97, 54.2%) and infection (n = 30, 16.7%). The three recorded causes of death among immigrants were neoplasm (n = 1), infection (n = 1), and others (n = 1). Supplementary Table 10 shows the recorded causes of death in both subgroups.
Discussion
We present the first study exploring the characteristics of first-generation immigrant IBD population in Spain, extracted from the vast ENEIDA nationwide registry of GETECCU. The immigrant cohort (>1,800 patients) was younger at disease onset and showed an increased risk of having EIMs and using biologics compared with the native cohort (>11,000 patients). Our results add to the scarce epidemiologic data characterizing immigrant IBD patients in Southern Europe, and suggest that immigrant IBD patients in Spain may have a distinct IBD phenotype.
The population of first-generation immigrants diagnosed with IBD and living in Spain is mainly Caucasian and Latin American, though a large proportion of patients are of Arab ethnicity. Asian patients make up a far smaller section of the population, unlike in series evaluated in other studies—mainly from the USA and the UK—in which South Asians constitute the most prevalent ethnic group (19–21). While those same studies found no differences in age at disease onset, we present data showing that the first-generation-immigrant IBD population in Spain is younger than native patients at disease onset and has a shorter disease duration. To explain the observed difference in age at onset, we may speculate on the relevance of the so called exposome (22), or the accumulation of environmental exposures, including several disease triggering factors such as microbiota dysbiosis, smoking and use of antibiotics. A study by Damas et al. (23) found earlier IBD onset among young Cuban migrants to America compared with previous migratory cohorts, suggesting a more intensive exposure to environmental triggering factors in the more recent arrivals. We also found that patients who were younger than 15 years on arrival in Spain were significantly younger at disease onset than those who migrated after 15. This fact highlights the relevance of early-life exposure to environmental risk factors for disease. Age of migration is an important factor in IBD development and phenotype, with younger ages being more affected in terms of incidence. In line with observations from our study, South Asians younger than 5 years-old at migration to US, or descendants who were already born in US, were younger at IBD onset than those who were older than 5 years-old at migration (24).
UC predominance over CD in IBD diagnosis has been described in certain ethnic groups such as South Asian and Hispanic (25–27). Our study also found a higher proportion of UC among Latin American and Asian patients compared with other ethnicities. This finding is consistent with findings reported of South Asians cohorts in the UK and North America, where the burden of UC is believed to be twice that of CD (19, 21, 24). Indeed, one meta-analysis showed that UC was more frequent among Hispanic vs. non-Hispanic Americans (28), and studies conducted in Mexico and South America have reported UC to be the predominant IBD subtype (29–32). Interestingly, other series have found that Hispanic patients are not only more likely to have UC but are also less likely than the non-Hispanic white population to have a family history of IBD (33, 34). We also found a lower frequency of family history of the disease among our immigrant cohort, which suggests that the cause is more likely to be de novo or sporadic. This observation is in line with other reports of sporadic disease among non-Caucasian ethnic groups (35, 36). On the other hand, several studies have reported the inverse association between smoking and presenting UC (37), which may also help to explain the predominance of UC in Latin American and Asian patients, who are less likely to smoke than Caucasian immigrants and native patients.
Several studies have reported a more aggressive phenotype, defined as more frequently colonic and perianal, and with more penetrating and stricturing behavior, in South Asian migrants (19, 20, 24). We did not observe differences in either CD location or UC extent in our study, possibly because Asian patients represented only a small part of the immigrant series. We did find more frequent stricturing behavior among native vs. immigrant CD patients, although stricturing was only associated with longer disease duration in the multivariate analysis. The longer disease history probably reflects the accumulation of disease progression milestones. Similar results were obtained in a multicenter study on African-American compared with Caucasian patients (38). Although other studies showed no differences in behavior between immigrant and host populations (20), one potential explanation for increased stricturing in our native cohort may derive from the genetic perspective. The allele frequency of various mutations within NOD2/ CARD15 may help to explain differences in ethnic risks (39). Indeed, NOD2 variations are less frequent among African Americans (40), and South Asian patients compared with Caucasian patients (41, 42). A second explanation may relate to the higher prevalence of smoking habit among native vs. immigrant patients. Smoking has been widely described as a risk factor for developing CD, determining disease location and the development of strictures or fistulae, and increasing the risk of surgery (43–45).
The two main differences between first-generation immigrant and native IBD populations in Spain were the increased risk of EIMs and use of biologics among immigrants. Previous studies on immigrant populations have not reported these findings (19, 21, 24). In fact, a lower rate of EIMs has previously been described in Asian patients (46, 47). It is possible that we found the opposite association because our cohort contained relatively few Asian patients, and indeed, our sub-analysis of the different ethnic groups showed increased rates of EIMs in Caucasian, Latin American and Arab patients, but not in Asian patients. Although the physiological mechanisms of EIM development are poorly understood, several risks factors have been described, including longer disease duration, colonic CD, and female sex (48, 49). In our study, longer disease duration, female sex and immigrant status were risk factors independently associated with EIM development. Increased risk of EIMs can therefore be considered a component of the IBD phenotype in our immigrant population.
The use of biologics, meanwhile, was independently associated with longer disease duration, younger age, and immigrant status. The increased frequency of EIMs among immigrants may explain this result, as anti-TNF is one of the most effective treatments for managing EIMs in IBD (49, 50). In addition, younger disease onset, as found in our immigrant cohort, is a poor prognostic factor (51), which may also help to explain the increased use of biologics among our immigrant population. Interestingly, time to first biologic treatment during follow-up was shorter in the immigrant population. Economic factors are thought to be the primary driver of racial and ethnic disparities in health services utilization, including use of prescription medication (52). However, our results show that both native and immigrant populations have full access to biologic therapies under the universal healthcare coverage provided by Spain's public health service, and low socioeconomic status is therefore unlikely to have contributed to differences in prescription patterns in this study. On the other hand, clinical practice among ENEIDA researchers is fairly uniform due to Spanish program of certification of quality of care at inflammatory bowel disease units (53), which sets the standards of care in IBD all over the country. An alternative explanation is that immigrant patients may have a distinctive disease course. No differences in the risk of IBD-related surgery were found between first-generation-immigrant and native IBD populations in Spain. However, intestinal resection was more frequent among native CD patients. This may be related to the increased rate of stricturing behavior and smoking habit, both described as risk factors for surgery in CD (44).
We must acknowledge that the retrospective study design and the lack of information regarding patient's genetic background, dietary habits and socioeconomical status constitute limitations of the present study. On the other hand, the main strengths of our study were the large cohorts; the representation of several ethnicities, including poorly studied groups such as Latin American or Arab immigrants; and the inclusion of patients diagnosed before 2015 to guarantee a follow-up period of at least 5 years.
In summary, we present the first study exploring the characteristics of first-generation immigrants with IBD in Spain.Immigrant patients were younger at disease onset and showed an increased risk of having EIMs and using biologics compared with native patients. These factors could constitute features of an IBD phenotype for the immigrant population in Spain. Our study represents a new landmark in the epidemiologic characterization of IBD immigrant patients in Southern Europe.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee from Hospital General Universitario de Alicante. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AG and RF: planning and/or conducting the study. AG, ER, MG-V, JG, DO, IV, MM, JPG, MA, ES-R, MB-W, VL, BC, IM-J, YZ, MM-A, RM, MN, ESi, LM, MVe, JP-C, ESa, XC, LA, VM, FB, LF-S, MVa, LD, CR, CM-V, RL, MRi, EI, BH, DB, JR, MM-M, MRo, OR, EH, MS, JB, RD, JH, OM, DC, DG, FM, MP, PA, FA-A, GA, LB, NM, AL, PV, IR-L, LR, LS, ESe, MB-d, and ED: collecting data. AG, RF, and PZ: interpreting data and drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The ENEIDA registry of GETECCU is supported by AbbVie, Galápagos, Janssen, Biogen, Takeda, Pfizer. AG is recipient of a grant by Instituto de Salud Carlos III, Madrid, Spain (PI21/1702).
Conflict of Interest
AG has served as speaker, consultant and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Tillotts Pharma, Chiesi and Otsuka Pharmaceutical. ER has provided scientific advice/participated in medical meetings/received research funding from/received payment for presentations and advice from: MSD, Schering-Plow, Ferring, Abbvie, Takeda, Janssen, Fresenius Kabi, Pfizer. IV has served as a speaker, or has received research or education funding from Abbvie, MSD, Pfizer, Takeda, Janssen, Tillotts Pharma, Ferring and Shire Pharmaceuticals. MM has served as speaker or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Biogen, Tillotts Pharma, Chiesi and Adacyte. JPG has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene, Gilead/Galapagos, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, and Vifor Pharma. YZ has received support for conference attendance, speaker fees, research support and consulting fees from Abbvie, Adacyte, Almirall, Amgen, Dr. Falk, FAES Pharma, Ferring, Janssen, MSD, Otsuka, Pfizer, Shire, Takeda and Tillots. XC has received grants for research from Abbvie, MSD, Vifor fees for advisory boards form Abbvie, MSD, Takeda, Pfizer, Janssen and VIFOR and has given lectures for Abbvie, MSD, Janssen, Pfizer, Takeda, Shire and Allergan. FB has served as a speaker, a consultant and advisory member for or has received research funding from MSD, Abbvie, Takeda, Janssen, Pfizer, Biogen, Amgen, Ferring, Faes Farma, Tillotts Pharma, Falk Pharma, Chiesi, Gebro Pharma, Vifor Pharma. MM-M has served as speaker, consultant and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Shire Pharmaceuticals and Otsuka Pharmaceutical. MR has served as speaker, consultant or advisory member for MSD, Abbvie, Pfizer, Takeda, Janssen, Ferring and Chiesi. MP has served as speaker or has received research funding from Abbvie, Takeda and Janssen. IR-L has received financial support for traveling and educational activities from or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Shire Pharmaceuticals, Roche, Celltrion, Faes Farma, Ferring, Dr. Falk Pharma, Otsuka Pharmaceutical and Adacyte. Financial support for research from Tillotts Pharma. RL has served as a speaker, or has received research or education funding from MSD, Abbvie, Pfizer, Takeda, Janssen and Dr. Falk. LA has served as speaker, or has received research or education funding from MSD, Abbvie, Kern Pharma, Ferring, FaesFarma, Shire Pharmaceuticals, Pfizer, Takeda, Janssen, Tillotts Pharma, and Otsuka Pharmaceutical. FA-A has served as speaker, consultant and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Tillotts Pharma, Chiesi and Dr. Falk. LR has served as a speaker, or has received education funding from MSD, Abbvie, Adacyte, Takeda, Pfizer, Janssen and Ferring. MB-d has served as a speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Sandoz, Biogen, Fresenius, Ferring, Faes Farma, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma. ED has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Gilead, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Tillots, Thermofisher. RF has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Janssen, Takeda, Adacyte Therapeutics, Sanofi, GlaxoSmithKline, Almirall. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.823900/full#supplementary-material
References
- 1.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. (2017) 389:1756–70. 10.1016/S0140-6736(16)32126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. (2017) 389:1741–55. 10.1016/S0140-6736(16)31711-1 [DOI] [PubMed] [Google Scholar]
- 3.Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. (2020) 578:527–39. 10.1038/s41586-020-2025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Sloot KWJ, Amini M, Peters V, Dijkstra G, Alizadeh BZ. Inflammatory bowel diseases: review of known environmental protective and risk factors involved. Inflamm Bowel Dis. (2017) 23:1499–509. 10.1097/MIB.0000000000001217 [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. (2018) 15:39–49. 10.1038/nrgastro.2017.136 [DOI] [PubMed] [Google Scholar]
- 6.Ho SM, Lewis JD, Mayer EA, Plevy SE, Chuang E, Rappaport SM, et al. Challenges in IBD research: environmental triggers. Inflamm Bowel Dis. (2019) 25:S13–23. 10.1093/ibd/izz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Windsor JW, Kaplan GG. Evolving epidemiology of IBD. Curr Gastroenterol Rep. (2019) 21:40. 10.1007/s11894-019-0705-6 [DOI] [PubMed] [Google Scholar]
- 8.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 9.Kedia S, Ahuja V. Epidemiology of inflammatory bowel disease in india: the great shift east. Inflamm Intest Dis. (2017) 2:102–15. 10.1159/000465522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benchimol EI, Mack DR, Guttmann A, Nguyen GC, To T, Mojaverian N, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. (2015) 110:553–63. 10.1038/ajg.2015.52 [DOI] [PubMed] [Google Scholar]
- 11.Li X, Sundquist J, Hemminki K, Sundquist K. Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: a nationwide follow-up study. Inflamm Bowel Dis. (2011) 17:1784–91. 10.1002/ibd.21535 [DOI] [PubMed] [Google Scholar]
- 12.Hammer T, Lophaven SN, Nielsen KR, von Euler-Chelpin M, Weihe P, Munkholm P, et al. Inflammatory bowel diseases in faroese-born danish residents and their offspring: further evidence of the dominant role of environmental factors in IBD development. Aliment Pharmacol Ther. (2017) 45:1107–14. 10.1111/apt.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis KF, D'Odorico P, Laio F, Ridolfi L. Global spatio-temporal patterns in human migration: a complex network perspective. PLoS ONE. (2013) 8:e53723. 10.1371/journal.pone.0053723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra R, Faiz O, Munkholm P, Burisch J, Arebi N. Epidemiology of inflammatory bowel disease in racial and ethnic migrant groups. World J Gastroenterol. (2018) 24:424–37. 10.3748/wjg.v24.i3.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreiro-de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez-Munoz JE. Emigration to western industrialized countries: a risk factor for developing inflammatory bowel disease. J Crohns colitis. (2011) 5:566–9. 10.1016/j.crohns.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 16.Zabana Y, Panes J, Nos P, Gomollon F, Esteve M, Garcia-Sanchez V, et al. The ENEIDA registry (Nationwide study on genetic and environmental determinants of inflammatory bowel disease) by GETECCU: design, monitoring and functions. Gastroenterol Hepatol. (2020) 43:551–8. [DOI] [PubMed] [Google Scholar]
- 17.Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. (2019) 13:144–64. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 montreal world congress of gastroenterology. Can J Gastroenterol. (2005) 19:5–36. 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 19.Walker DG, Williams HR, Kane SP, Mawdsley JE, Arnold J, McNeil I, et al. Differences in inflammatory bowel disease phenotype between South Asians and Northern Europeans living in North West London, UK. Am J Gastroenterol. (2011) 106:1281–9. 10.1038/ajg.2011.85 [DOI] [PubMed] [Google Scholar]
- 20.Goodhand JR, Kamperidis N, Joshi NM, Wahed M, Koodun Y, Cantor EJ, et al. The phenotype and course of inflammatory bowel disease in UK patients of Bangladeshi descent. Aliment Pharmacol Ther. (2012) 35:929–40. 10.1111/j.1365-2036.2012.05043.x [DOI] [PubMed] [Google Scholar]
- 21.Bodiwala V, Marshall T, Das KM, Brant SR, Seril DN. Comparison of disease phenotypes and clinical characteristics among South Asian and white patients with inflammatory bowel disease at a tertiary referral center. Inflamm Bowel Dis. (2020) 26:1869–77. 10.1093/ibd/izaa019 [DOI] [PubMed] [Google Scholar]
- 22.Rogler G, Vavricka S. Exposome in IBD: recent insights in environmental factors that influence the onset and course of IBD. Inflamm Bowel Dis. (2015) 21:400–8. 10.1097/MIB.0000000000000229 [DOI] [PubMed] [Google Scholar]
- 23.Damas OM, Avalos DJ, Palacio AM, Gomez L, Quintero MA, Deshpande AR, et al. Inflammatory bowel disease is presenting sooner after immigration in more recent US immigrants from Cuba. Aliment Pharmacol Ther. (2017) 46:303–9. 10.1111/apt.14145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jangi S, Ruan A, Korzenik J, de Silva P. South Asian patients with inflammatory bowel disease in the United States demonstrate more fistulizing and perianal crohn phenotype. Inflamm Bowel Dis. (2020) 26:1933–42. 10.1093/ibd/izaa029 [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Hann HJ, Hong SN, Kim KH, Ahn IM, Song JY, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. (2015) 21:623–30. 10.1097/MIB.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 26.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific crohn's and colitis epidemiology study. Gastroenterology. (2013) 145:158–65. 10.1053/j.gastro.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 27.Wei SC, Lin MH, Tung CC, Weng MT, Kuo JS, Shieh MJ, et al. A nationwide population-based study of the inflammatory bowel diseases between 1998 and 2008 in Taiwan. BMC Gastroenterol. (2013) 13:166. 10.1186/1471-230X-13-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avalos DJ, Mendoza-Ladd A, Zuckerman MJ, Bashashati M, Alvarado A, Dwivedi A, et al. Hispanic Americans and non-hispanic white Americans have a similar inflammatory bowel disease phenotype: a systematic review with meta-analysis. Dig Dis Sci. (2018) 63:1558–71. 10.1007/s10620-018-5022-7 [DOI] [PubMed] [Google Scholar]
- 29.Juliao F, Marquez J, Aristizabal N, Yepes C, Zuleta J, Gisbert JP. Clinical efficacy of infliximab in moderate to severe ulcerative colitis in a latin american referral population. Digestion. (2013) 88:222–8. 10.1159/000355529 [DOI] [PubMed] [Google Scholar]
- 30.Paredes Mendez J, Otoya Moreno G, Mestanza Rivas Plata AL, Lazo Molina L, Acuna Ordonez K, Arenas Gamio JL, et al. [Epidemiological and clinical characteristics of inflammatory bowel disease in a tertiary referral hospital in Lima-Peru]. Rev Gastroenterol Peru. (2016) 36:209–18. [PubMed] [Google Scholar]
- 31.Simian D, Fluxa D, Flores L, Lubascher J, Ibanez P, Figueroa C, et al. Inflammatory bowel disease: a descriptive study of 716 local chilean patients. World J Gastroenterol. (2016) 22:5267–75. 10.3748/wjg.v22.i22.5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto-Furusho JK. Clinical epidemiology of ulcerative colitis in Mexico: a single hospital-based study in a 20-year period (1987-2006). J Clin Gastroenterol. (2009) 43:221–4. 10.1097/MCG.0b013e31817a76b4 [DOI] [PubMed] [Google Scholar]
- 33.Betteridge JD, Armbruster SP, Maydonovitch C, Veerappan GR. Inflammatory bowel disease prevalence by age, gender, race, and geographic location in the U. S military health care population. Inflamm Bowel Dis. (2013) 19:1421–7. 10.1097/MIB.0b013e318281334d [DOI] [PubMed] [Google Scholar]
- 34.Nguyen GC, Chong CA, Chong RY. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns colitis. (2014) 8:288–95. 10.1016/j.crohns.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 35.Kuwahara E, Asakura K, Nishiwaki Y, Inoue N, Watanabe M, Hibi T, et al. Effects of family history on inflammatory bowel disease characteristics in Japanese patients. J Gastroenterol. (2012) 47:961–8. 10.1007/s00535-012-0558-3 [DOI] [PubMed] [Google Scholar]
- 36.Chung SH, Park SJ, Lee HS, Hong SP, Cheon JH, Kim TI, et al. Similar clinical characteristics of familial and sporadic inflammatory bowel disease in South Korea. World J Gastroenterol. (2014) 20:17120–6. 10.3748/wjg.v20.i45.17120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. (2015) 12:205–17. 10.1038/nrgastro.2015.34 [DOI] [PubMed] [Google Scholar]
- 38.Nguyen GC, Torres EA, Regueiro M, Bromfield G, Bitton A, Stempak J, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. (2006) 101:1012–23. 10.1111/j.1572-0241.2006.00504.x [DOI] [PubMed] [Google Scholar]
- 39.Bonen DK, Ogura Y, Nicolae DL, Inohara N, Saab L, Tanabe T, et al. Crohn's disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. (2003) 124:140–6. 10.1053/gast.2003.50019 [DOI] [PubMed] [Google Scholar]
- 40.Dassopoulos T, Nguyen GC, Talor MV, Datta LW, Isaacs KL, Lewis JD, et al. NOD2 mutations and anti-saccharomyces cerevisiae antibodies are risk factors for Crohn's disease in African Americans. Am J Gastroenterol. (2010) 105:378–86. 10.1038/ajg.2009.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juyal G, Amre D, Midha V, Sood A, Seidman E, Thelma BK. Evidence of allelic heterogeneity for associations between the NOD2/CARD15 gene and ulcerative colitis among North Indians. Aliment Pharmacol Ther. (2007) 26:1325–32. 10.1111/j.1365-2036.2007.03524.x [DOI] [PubMed] [Google Scholar]
- 42.Pugazhendhi S, Amte A, Balamurugan R, Subramanian V, Ramakrishna BS. Common NOD2 mutations are absent in patients with Crohn's disease in India. Indian J Gastroenterol. (2008) 27:201–3. [PubMed] [Google Scholar]
- 43.Picco MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn's disease. Am J Gastroenterol. (2003) 98:363–8. 10.1111/j.1572-0241.2003.07240.x [DOI] [PubMed] [Google Scholar]
- 44.Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn's disease: a meta-analysis of observational studies. Int J Colorectal Dis. (2008) 23:1213–21. 10.1007/s00384-008-0542-9 [DOI] [PubMed] [Google Scholar]
- 45.Quezada SM, Langenberg P, Cross RK. Cigarette smoking adversely affects disease activity and disease-specific quality of life in patients with Crohn's disease at a tertiary referral center. Clin Exp Gastroenterol. (2016) 9:307–10. 10.2147/CEG.S104652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. (2016) 14:111–9. 10.5217/ir.2016.14.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. AmJ Gastroenterol. (2009) 104:2100–9. 10.1038/ajg.2009.190 [DOI] [PubMed] [Google Scholar]
- 48.Hedin CRH, Vavricka SR, Stagg AJ, Schoepfer A, Raine T, Puig L, et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohns Colitis. (2019) 13:541–54. 10.1093/ecco-jcc/jjy191 [DOI] [PubMed] [Google Scholar]
- 49.Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, et al. The first european evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. (2016) 10:239–54. 10.1093/ecco-jcc/jjv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peyrin-Biroulet L, Van Assche G, Gomez-Ulloa D, Garcia-Alvarez L, Lara N, Black CM, et al. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol. (2017) 15:25–36. 10.1016/j.cgh.2016.06.025 [DOI] [PubMed] [Google Scholar]
- 51.Torres J, Caprioli F, Katsanos KH, Lobaton T, Micic D, Zeroncio M, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis. (2016) 10:1385–94. 10.1093/ecco-jcc/jjw116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Zuckerman IH, Miller NA, Shaya FT, Noel JM, Mullins CD. Utilizing new prescription drugs: disparities among non-Hispanic whites, non-Hispanic blacks, and Hispanic whites. Health Serv Res. (2007) 42:1499–519. 10.1111/j.1475-6773.2006.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barreiro-de Acosta M, Gutierrez A, Zabana Y, Beltran B, Calvet X, Chaparro M, et al. Inflammatory bowel disease integral care units: Evaluation of a nationwide quality certification programme. The GETECCU experience. United European Gastroenterol J. (2021) 9:766–72. 10.1002/ueg2.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.