Abstract
Background
It is unclear whether diabetes or prediabetes affects unfavorable treatment outcomes and death in people with tuberculosis (PWTB).
Methods
Culture-confirmed, drug-susceptible PWTB, enrolled in the Regional Prospective Observational Research in Tuberculosis (RePORT)–Brazil cohort between 2015 and 2019 (N = 643) were stratified based on glycemic status according to baseline glycated hemoglobin. Unfavorable tuberculosis (TB) outcome was defined as treatment failure or modification, recurrence, or death; favorable outcome was cure or treatment completion. We corroborated the findings using data from PWTB reported to the Brazilian National System of Diseases Notification (SINAN) during 2015–2019 (N = 20 989). Logistic regression models evaluated associations between glycemic status and outcomes.
Results
In both cohorts, in univariate analysis, unfavorable outcomes were more frequently associated with smoking, illicit drug use, and human immunodeficiency virus infection. Diabetes, but not prediabetes, was associated with unfavorable outcomes in the RePORT-Brazil (adjusted relative risk [aRR], 2.45; P < .001) and SINAN (aRR, 1.76; P < .001) cohorts. Furthermore, diabetes was associated with high risk of death (during TB treatment) in both RePORT-Brazil (aRR, 2.16; P = .040) and SINAN (aRR, 1.93; P = .001).
Conclusions
Diabetes was associated with an increased risk of unfavorable outcomes and mortality in Brazilian PWTB. Interventions to improve TB treatment outcomes in persons with diabetes are needed.
Keywords: diabetes, prediabetes, treatment outcome, SINAN, Mycobacterium tuberculosis
In a multicenter prospective cohort study from Brazil, diabetes was associated with an increased risk of unfavorable treatment outcomes, including mortality, in people with pulmonary tuberculosis. These observations were corroborated in the Brazilian National Disease Notification System during the same period.
Diabetes mellitus (DM) is recognized by the World Health Organization (WHO) as a global epidemic [1]. This metabolic disease triples the risk of active tuberculosis (TB) in patients with latent Mycobacterium tuberculosis (Mtb) infection [2]. In 2019, approximately 400 000 people with TB (PWTB) worldwide were also diagnosed with DM [3]. Importantly, DM prevalence is increasing globally, including settings with a high TB burden, such as China and India [4].
The high prevalence of DM among TB patients (10%–30%) in high-TB-burden countries and the negative impact of TB-DM comorbidity has been previously described by many groups [5–8], including higher mycobacterial loads and prevalence of sputum acid-fast bacilli positive, treatment failure, and death outcomes during TB treatment, among others.
We have reported an association between DM and more severe TB clinical presentation (higher frequency of cough, night sweats, hemoptysis, and malaise) [9], increased lung pathology reflected by severe radiographic manifestations (higher number of pulmonary lesions, including cavitation) [10], increased bacterial load in sputum [11], and delayed sputum conversion after anti-TB treatment initiation [12]. Furthermore, activation of tissue remodeling responses [12] and increased and persistent systemic inflammation [13] have been reported during treatment in TB-DM patients. Thus, the increase in the number of people with DM may further complicate care and control of TB, especially in areas with a high burden of both diseases [14].
There is also evidence that patients with concomitant TB and DM have an increased risk of unfavorable anti-TB treatment outcome such as failure, recurrence, and death compared to normoglycemic patients [15–17]. However, the findings have not been consistent [18, 19]. In the present study we evaluated the effect of dysglycemia (DM or prediabetes [pre-DM]) on anti-TB treatment outcomes in a prospective Brazilian cohort of patients with pulmonary TB (Regional Prospective Observational Research in Tuberculosis [RePORT]–Brazil), and also among PWTB reported to the Brazilian National TB Registry through the National System of Diseases Notification (SINAN).
METHODS
Ethics Statement
The study was conducted according to the principles in the Declaration of Helsinki. The RePORT-Brazil protocol was approved by the institutional review boards at each study site and at Vanderbilt University Medical Center. Participation in RePORT-Brazil was voluntary, and written informed consent was obtained from all participants. All data extracted from SINAN were public and freely accessible. All data were de-identified to preserve the anonymity of study participants.
Overall Study Design
RePORT-Brazil includes 5 study sites, located in 3 cities with high TB burden: 1 site each in Salvador and Manaus, and 3 sites in Rio de Janeiro [20]. One main objective of the consortium is to describe the clinical outcomes of TB treatment and latent Mtb infection in Brazil. The details of the sites and representativeness of the RePORT-Brazil cohort to all TB patients in Brazil have been described previously [20].
For the current analysis, we selected RePORT-Brazil participants meeting the following inclusion criteria: pulmonary TB, age ≥18 years, and enrolled between June 2015 and June 2019 (Figure 1A), with new or previously diagnosed culture-positive sputum (Lowenstein–Jensen medium or BD BACTEC MGIT), who received anti-TB treatment and had a treatment outcome recorded in the study database. For this study we only used information from patients with confirmed anti-TB drug susceptibility to the first-line scheme drugs, in RePORT-Brazil and from the SINAN database. Clinical and epidemiological information was collected at 3 in-person visits: (i) at TB diagnosis and anti-TB treatment initiation (baseline); (ii) 2 months after initiating treatment; and (iii) at the completion of anti-TB treatment. In addition, telephone follow-up was performed for all participants every 6 months until up to month 24. All data collected were stored in REDCap [21].
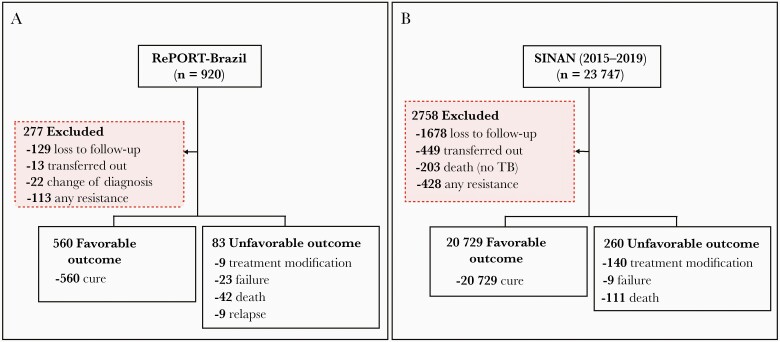
Figure 1.
Study flowchart presenting the people with tuberculosis (TB) included and excluded from Regional Prospective Observational Research in Tuberculosis (RePORT)–Brazil (A) and reported to the Brazilian National System of Diseases Notification (SINAN, B) between 2015 and 2019.
To establish the glycemic status of PWTB, baseline glycated hemoglobin (HbA1c) (prior to TB treatment) in blood was measured. DM was defined according to American Diabetes Association guidelines [22]. Patients were classified as having DM (HbA1c ≥6.5%), pre-DM (HbA1c = 5.7%–6.4%), or normoglycemia (HbA1c <5.7%). HbA1c ≥5.7% was classified as dysglycemia.
SINAN [23] includes diseases that require notification in all states and municipalities of Brazil; TB is a notifiable disease. The details of SINAN have been previously described [20]. PWTB reported to SINAN are diagnosed following the criteria in the Brazilian Manual of Recommendations for Tuberculosis Control [24]. Diagnostic criteria include (i) clinical and epidemiologic factors (presumptive diagnosis); (ii) bacteriology (sputum smear positive) or positive culture for Mtb (solid or liquid media); (iii) positive Xpert MTB/RIF; (iv) chest radiography; or (v) in the case of extrapulmonary TB, histopathology [24]. For each TB case reported, characteristics such as sex, age, race, education, alcohol consumption, illicit drug use, smoking habits, comorbidities, presence of human immunodeficiency virus (HIV) infection, and test results, among others, were also reported. Glycemic status was reported to SINAN as a diagnosis of DM (yes/ no), not exclusively based on HbA1c level.
We used the SINAN information from the years 2015–2019 to match the enrollment period of RePORT-Brazil [20] (Figure 1B). Of note, in 2014 the “strategies for care of people with chronic diseases,” addressing comprehensive basic care for patients with TB and DM among others [25], was implemented in Brazil. In addition, we used data reported from Salvador, Manaus, and Rio de Janeiro, the cities with RePORT-Brazil study sites.
Outcome Definition
The primary outcome in this study was an unfavorable treatment outcome, defined as treatment modification, treatment failure, recurrence, or death (during TB treatment). The secondary outcome was mortality (TB patient who dies for any reason before starting or during the course of treatment) [26]. All definitions of outcome treatments were established in accordance with the Manual of Recommendations for the Control of TB of Brazil [24]. The treatment outcome definitions used in RePORT-Brazil and SINAN are depicted in Supplementary Table 1. A favorable outcome was defined as cure or treatment completion (defined as at least 90% of the total number of doses over 1 year for drug-susceptible TB). Patients who were lost to follow-up or transferred out or had a change in diagnosis (not a TB case) as the treatment outcome were excluded. The numbers of participants per outcome in this study are shown in more detail in Figure 1.
Data Analysis
All analyses were prespecified. Medians and interquartile ranges (IQRs) were used as measures of central tendency. Categorical variables were represented as percentages and compared using a 2-sided Pearson χ 2 test with Yates correction or the Fisher 2-tailed test in 2 × 3 or 2 × 2 tables, respectively. Quantitative variables were compared using the Mann–Whitney U test. We performed a logistic regression in both the RePORT-Brazil and SINAN cohorts, using variables with univariate P value ≤ .2 to assess the risk factors among characteristics of TB patients (1 model for each stratification strategy of the glycemic status: dysglycemia, DM, pre-DM, and HbA1c value) with the composite unfavorable treatment outcome or with mortality alone. Results from both regression approaches were presented in terms of relative risk (RR) and 95% confidence intervals (CIs). P values < .05 were considered statistically significant. Statistical analyses were performed using SPSS 25.0 (IBM Corporation, Armonk, New York), Graph Pad Prism 9.0 (GraphPad Software, San Diego, California), and JMP 13.0 (SAS Institute, Cary, North Carolina) software.
RESULTS
Factors Associated With TB Treatment Outcomes in RePORT-Brazil
The RePORT cohort included 643 participants with culture-confirmed pulmonary TB who were treated with anti-TB drugs for at least 6 months. Patients were grouped according to treatment outcomes: favorable (n = 560 [87.1%]) and unfavorable (n = 83 [12.9%]) (Figure 1A). The median age in the RePORT cohort was 38 years (IQR, 27–52 years), and most study participants were male. Patients with unfavorable outcomes more frequently reported current smoking (P = .025) and illicit drug use (P = .006) (Table 1). These patients also had a higher frequency of DM (P = .003) and HIV infection (P < .001) (Table 1). The proportion of individuals with dysglycemia (ie, with DM or pre-DM) did not differ significantly between those with unfavorable vs favorable outcomes. In contrast, persons with unfavorable outcomes had lower hemoglobin levels (10.6 g/dL [IQR, 8.98–12.4 g/dL] vs 12.2 g/dL [IQR, 10.9–13.4 g/dL]) among those with favorable outcomes (P < .001; Table 1).
Table 1.
Characteristics of People With Tuberculosis in the Regional Prospective Observational Research in Tuberculosis (RePORT)–Brazil Cohort, by Outcome
| Characteristics | Unfavorable (n = 83) | Favorable (n = 560) | P Value |
|---|---|---|---|
| Male sex | 57 (68.67) | 359 (64.11) | .462 |
| Age, y, median (IQR) | 38 (27–52) | 35 (25–49) | .399 |
| Race | .039 | ||
| White | 13 (15.66) | 123 (22) | |
| Black | 18 (21.69) | 146 (26.12) | |
| Asian | 0 (0) | 2 (0.36) | |
| Pardoa | 48 (57.83) | 279 (49.91) | |
| Indigenous | 4 (4.82) | 9 (1.61) | |
| Illiterate | 80 (96.39) | 538 (96.07) | 1 |
| Smokingb | 52 (62.65) | 275 (49.11) | .025 |
| Alcohol consumptionc | 76 (91.57) | 470 (83.93) | .072 |
| Illicit drug used | 35 (42.17) | 152 (27.14) | .006 |
| BMI, kg/m2, median (IQR) | 20.4 (17.1–22.9) | 20.3 (18.4–22.5) | .384 |
| Prior TB | 10 (12.05) | 89 (16.01) | .418 |
| Type of TBe | .61 | ||
| PTB | 68 (81.93) | 502 (89.64) | |
| PTB + EPTB | 15 (18.07) | 58 (10.36) | |
| Abnormal radiograph | 77 (92.77) | 547 (97.68) | .026 |
| Positive smear | 64 (77.11) | 456 (81.43) | .37 |
| Glycemic statusf | |||
| Diabetes mellitus | 31 (37.35) | 108 (19.29) | .003 |
| Prediabetes | 19 (22.89) | 186 (33.21) | .554 |
| Normoglycemia | 33 (39.76) | 266 (47.5) | Ref. |
| Dysglycemia | 50 (60.24) | 294 (52.5) | .197 |
| Metformin useg | 0 (0) | 8 (2.73) | .609 |
| Hemoglobin, g/dL, median (IQR) | 10.6 (8.98–12.4) | 12.2 (10.9–13.4) | <.001 |
| HbA1c, %, median (IQR) | 6 (5.4–6.8) | 5.7 (5.3–6.2) | .082 |
| HIV status | 33 (40.24) | 84 (15.16) | <.001 |
| ARTh | 9 (27.27) | 30 (35.29) | .514 |
| Hypertension | 7 (8.43) | 46 (8.21) | 1 |
| Other comorbiditiesi | 7 (8.43) | 32 (5.71) | .325 |
Data are presented as No. (%) unless otherwise indicated. Favorable: cure/complete treatment. Unfavorable: failure, recurrence, treatment modification, or death. In the Regional Prospective Observational Research in Tuberculosis (RePORT)–Brazil cohort, all patients had a positive culture at baseline. Bold values denote statistical significance at the P < .05 level.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; EPTB, extrapulmonary tuberculosis; HbA1c, glycated hemoglobin; HIV, human immunodeficiency virus; IQR, interquartile range; PTB, pulmonary tuberculosis; TB, tuberculosis.
aDefinition of Pardo race: Mixture of European, black, and Amerindian.
bPast or current cigarette smoker.
cPast or current consumption of alcohol (any).
dPast or current illicit drug use (marijuana, cocaine, heroin, or crack).
eAll individuals from the RePORT cohort had a diagnosis of PTB; some also had involvement at extrapulmonary sites.
fOf participants with diabetes mellitus (DM), 12 with an unfavorable outcome and 67 with a favorable outcome had a diagnosis of DM prior to TB diagnosis.
gFrequency of use of metformin was calculated only in patients with DM.
hART frequency was calculated among the persons living with HIV.
iCancer, kidney disease, chronic obstructive pulmonary disease, emphysema, and asthma.
Regarding TB clinical presentation at study enrollment, patients who developed unfavorable outcomes were more likely to be living with HIV (P < .001) and to present with fatigue (P < .001), whereas those who experienced a favorable treatment outcome more frequently presented with cough (P < .001) and hemoptysis (P = .043) (Supplementary Figure 1A and 1B).
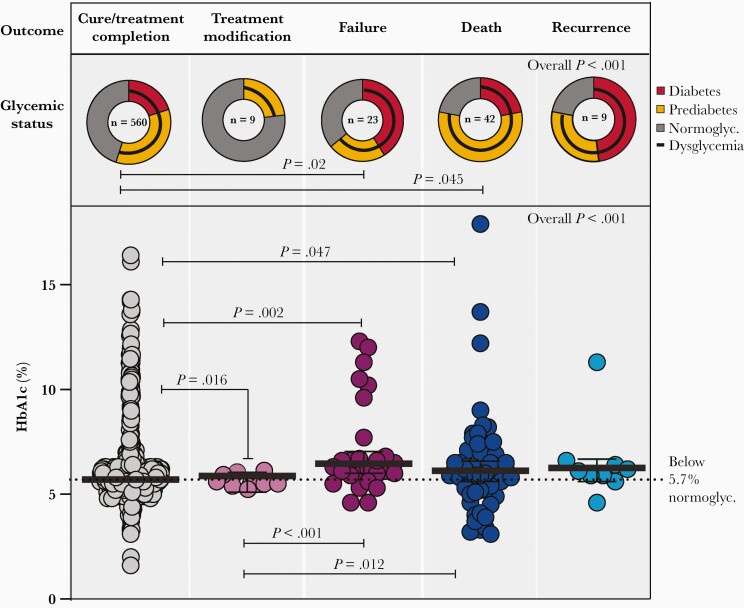
We next evaluated the impact of baseline glycemic status on each type of treatment outcome (cure, treatment modification, failure, death, recurrence). Dysglycemia was more frequent in TB patients with treatment failure (P = .02) or who died (P = .045) during TB treatment (Figure 2). Baseline HbA1c values were distinguishable between the subgroups of participants who further developed favorable and unfavorable outcome when the outcomes were examined individually (treatment modification, failure, death, recurrence, and cure). Individuals with favorable treatment outcomes had lower HbA1c levels than those who experienced treatment modification (P = .016), treatment failure (P = .002), or death (P = .047) during treatment follow-up. Among individuals with unfavorable outcomes, those who experienced treatment modification had lower HbA1c values than patients who had treatment failure (P < .001) or died (P = .012) (Figure 2). Furthermore, TB patients who experienced treatment failure, recurrence, or death exhibited a median HbA1c of 6.6 g/dL (IQR, 5.9–9.6 g/dL).
Figure 2.
Distribution of glycemic status and glycated hemoglobin (HbA1c) levels according to treatment outcomes in patients with tuberculosis (TB) in the Regional Prospective Observational Research in Tuberculosis–Brazil cohort. Frequency of TB patients with diabetes, prediabetes, normoglycemia, and dysglycemia (diabetes + prediabetes) diagnosed using HbA1c levels is shown according to TB treatment outcome (cure, treatment modification, failure, death, and recurrence). Only comparisons (frequency dysglycemia status between TB treatment outcomes) with significant P values are displayed. Scatterplots depict the frequency of HbA1c values in TB patients according to TB treatment outcome. Lines represent median and interquartile range. The differences in median values of HbA1c between groups were compared using the Kruskal–Wallis test with Dunn multiple comparisons posttest. Only comparisons with significant P values are displayed. Abbreviations: HbA1c, glycated hemoglobin, normoglyc., normoglycemia.
Determinants of TB Treatment Outcomes in SINAN
In the SINAN cohort, 23 747 PWTB were notified in the 3 cities where RePORT study sites are located. A total of 2758 were excluded for reasons listed in Figure 1, resulting in a cohort of 20 989 PWTB. Males represented >60% of patients in both groups, and patients with unfavorable outcomes were older (39 years [IQR, 33–47 years]; P < .001) and more likely to report smoking (P = .003), alcohol consumption (P = .019), and illicit drug use (P = .014) than those with favorable outcomes. Among patients with HIV coinfection, those with unfavorable outcome were less likely to report antiretroviral therapy (P < .001) than patients with favorable outcome. Unfavorable outcome was associated with previous TB (P < .001), DM (P = .022), HIV coinfection (P < .001), and other comorbidities (P = .004) (Table 2, Supplementary Figure 1C).
Table 2.
Characteristics of People With Tuberculosis in the Brazilian National System of Diseases Notification (SINAN) Cohort (2015–2019), by Outcome
| Characteristic | Unfavorable (n = 260) | Favorable (n = 20 729) | P Value |
|---|---|---|---|
| Male sex | 137 (69.9) | 12 827 (62.8) | .046 |
| Age, y, median (IQR) | 39 (33–47) | 38 (28–52) | |
| Race | .234 | ||
| White | 49 (27.5) | 5029 (26.4) | |
| Black | 38 (21.3) | 3421 (17.9) | |
| Asian | 4 (2.2) | 166 (0.9) | |
| Pardoa | 87 (48.9) | 10 381 (54.5) | |
| Indigenous | 0 (0.0) | 62 (0.3) | |
| Illiterate | 28 (20.4) | 2382 (15.3) | .092 |
| Smokingb | 46 (26.9) | 3465 (17.9) | .003 |
| Alcohol consumptionc | 33 (19.4) | 2556 (13.1) | .019 |
| Illicit drug used | 26 (15.3) | 1828 (9.5) | .014 |
| Prior TB | 81 (41.3) | 3155 (15.4) | <.001 |
| Type of TBe | <.001 | ||
| PTB | 184 (93.9) | 19 780 (96.8) | |
| EPTB | 3 (1.5) | 117 (0.6) | |
| PTB + EPTB | 9 (4.6) | 536 (2.6) | |
| Abnormal radiograph | 170 (97.1) | 17 662 (97.3) | .811 |
| Positive smear | 108 (75.0) | 10 512 (76.1) | .775 |
| Diabetes mellitus | 28 (15.8) | 2047 (10.5) | .022 |
| HIV status | 21 (70.0) | 1880 (10.7) | <.001 |
| ARTf | 38 (23.8) | 1154 (76.2) | <.001 |
| Hypertension | 5 (2.6) | 559 (2.7) | 1.000 |
| Other comorbiditiesg | 16 (8.5) | 782 (4.0) | .004 |
Data are presented as No. (%) unless otherwise indicated. Favorable: cure/complete treatment. Unfavorable: failure, recurrence, treatment modification, or death. Bold values denote statistical significance at the P < .05 level.
Abbreviations: ART, antiretroviral therapy; EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis; TB, tuberculosis.
aDefinition of Pardo race: mixture of European, black, and Amerindian.
bPast or current cigarette smoker.
cPast or current consumption of alcohol (any).
dPast or current illicit drug use (marijuana, cocaine, heroin, or crack).
eOnly individuals from the SINAN 2015–2019 cohort who had a diagnosis of PTB, in some cases with presence in other anatomical sites, were included.
fART frequency was calculated among the persons living with HIV.
gCancer, kidney disease, chronic obstructive pulmonary disease, emphysema, and asthma.
We likewise evaluated the impact of glycemic status on the TB clinical presentation by the study participants in both cohorts (Supplementary Figure 2). In RePORT patients, weight loss was more commonly observed in people with DM than in those with pre-DM or normoglycemia (P < .001). In patients from SINAN, there was a higher proportion of PWTB with dysglycemia presenting with a positive smear at baseline than in those with normoglycemia (P < .001). In addition, frequency of HIV infection was higher in the normoglycemic patients from the SINAN dataset (P < .001). Additional details of other clinical factors are displayed in Supplementary Figure 2.
Multivariable Logistic Regression to Assess Association Between Glycemic Status and TB Treatment Outcomes
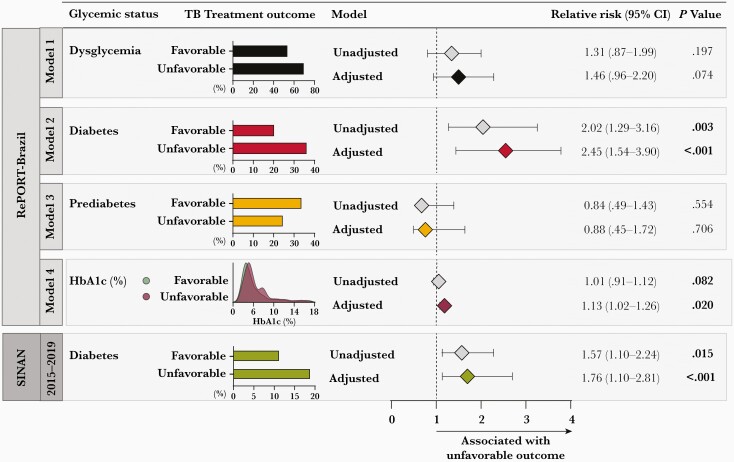
A logistic regression analysis was performed to determine the RR according to glycemic status (dysglycemia, DM, pre-DM, or HbA1c values) and treatment outcome in the RePORT cohort. DM was associated with unfavorable treatment outcomes independent of the other factors (adjusted RR [aRR], 2.45 [95% CI, 1.54–3.90]; P < .001) (Figure 3, model 2). HbA1c values were also independently associated with unfavorable treatment outcomes (aRR, 1.13 [95% CI, 1.02–126]; P = .02) (Figure 3, model 4). Pre-DM and the overall category of dysglycemia were not significantly associated with unfavorable treatment outcome (Figure 3). This analysis also was performed in the SINAN cohort, and DM was independently associated with unfavorable TB treatment outcomes (aRR, 1.76 [95% CI, 1.104–2.81]; P < .001) (Figure 3). Details of the logistic regression models are shown in Supplementary Table 2.
Figure 3.
Association between glycemic status and tuberculosis (TB) treatment outcomes among TB patients from the Regional Prospective Observational Research in Tuberculosis (RePORT)–Brazil and the Brazilian National System of Diseases Notification (SINAN) cohorts. In the RePORT cohort (upper panel), logistic regression was performed to evaluate the independent associations between glycemic status of TB patients (model 1: dysglycemia; model 2: diabetes; model 3: prediabetes; model 4: increases of 1 unit in glycated hemoglobin level) and variables with P value < .2 in the univariate analyses (Table 1) and unfavorable treatment outcome (treatment modification, failure, recurrence, and death). Comparisons of diabetes, prediabetes, and dysglycemia were performed using normoglycemia as the reference. In the SINAN cohort (lower panel), logistic regression was performed to evaluate the independent associations between diabetes and unfavorable TB treatment outcome (treatment modification, treatment failure, and death). Variables with P < .2 in the univariate analyses (Table 2) were included. Details of the bivariate binomial logistic regression models are shown in Supplementary Table 2. Abbreviations: CI, confidence interval; HbA1c, glycated hemoglobin; RePORT, Regional Prospective Observational Research for Tuberculosis; SINAN, National System of Diseases Notification; TB, tuberculosis.
Mortality During TB Treatment
We next compared patients with cure outcome vs those who died during TB treatment (n = 42) in the RePORT cohort. Mortality was associated with male sex (P = .029), increasing age (P = .035), illicit drug use (P = .034), HIV infection (P = .001), DM (P = .028), and lower concentrations of hemoglobin (death: 9.45 g/dL [IQR, 8.00–10.7 g/dL]; cure: 12.2 g/dL [IQR, 10.9–13.4 g/dL]; P < .001) and higher values of HbA1c (death: 6% [IQR, 5.2%–6.9%]; cure: 5.7% [IQR, 5.3%–6.2%]; P < .001) (Supplementary Table 3).
In the SINAN cohort, death was associated with the same statistically significant variables as those that predicted the composite unfavorable outcome vs cure (Supplementary Table 4). Mortality was associated with smoking (P = .003), alcohol consumption (P = .003), illicit drug use (P = .009), HIV infection (P < .001), and other comorbidities (P = .006). In SINAN, patients who died had a higher frequency of DM (P = .024) and a lower frequency of antiretroviral therapy use in individuals with HIV infection (P = .004).
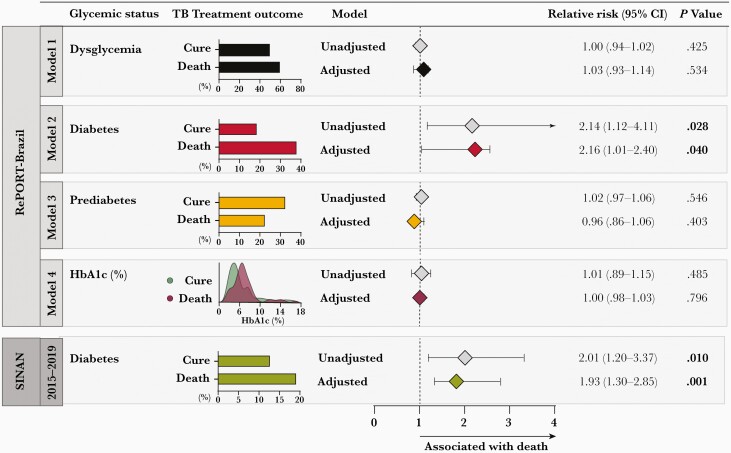
A logistic regression analysis was performed comparing cure and death and presented similar results to the first model exploring the composite unfavorable outcome. In the RePORT cohort, DM was once again strongly associated with mortality (aRR, 2.16 [95% CI, 1.01–2.40]; P = .040) (Figure 4, model 2). Pre-DM and dysglycemia were not significantly associated with death (Figure 4). In the SINAN cohort, DM was also significantly associated with death (aRR, 1.93 [95% CI, 1.30–2.85]; P = .001) (Figure 4). Details of the logistic regression models are shown in Supplementary Table 2.
Figure 4.
Association between glycemic status and death during antituberculosis treatment among tuberculosis (TB) patients from the Regional Prospective Observational Research for Tuberculosis (RePORT)–Brazil and the Brazilian National System of Diseases Notification (SINAN) cohorts. In the RePORT cohort (upper panel), logistic regression was performed to evaluate the independent associations between glycemic status of TB patients (model 1: dysglycemia; model 2: diabetes; model 3: prediabetes; model 4: increases of 1 unit in glycated hemoglobin level) and variables with P < .2 in the univariate analyses (Supplementary Table 3) and death. Comparisons of diabetes, prediabetes, and dysglycemia were performed using normoglycemia as reference. In the SINAN cohort (lower panel), logistic regression was performed to evaluate the independent associations between diabetes in TB patients in the period 2015–2019 and variables with P < .2 in the univariate analyses (Supplementary Table 4) and death. Details of the bivariate binomial logistic regression models are shown in Supplementary Table 5. Abbreviations: CI, confidence interval; HbA1c, glycated hemoglobin; RePORT, Regional Prospective Observational Research for Tuberculosis; SINAN, National System of Diseases Notification; TB, tuberculosis.
DISCUSSION
There has recently been increased recognition of DM as an important risk factor for developing active TB and experiencing unfavorable TB treatment outcomes [14–16, 27]. As the prevalence of DM increases [28], particularly in developing countries, it is necessary to determine the public health impact of this syndemic in large populations. Our study analyzed data from PWTB from 2 data sources (a longitudinal cohort and the nationwide disease notification system) in the same period and the same 3 cities to determine the impact of DM on TB treatment outcomes in a Brazilian population.
Our results show that the frequency of unfavorable outcomes of anti-TB treatment in the RePORT-Brazil cohort was similar to those reported by other studies [19] We have also demonstrated that TB patients in RePORT-Brazil are comparable to/representative of all PWTB reported in Brazil. [20].
It is interesting to note that the factors consistently associated with poor outcomes in the 2 datasets analyzed—namely, substance use (alcohol, tobacco, and illicit drug) and HIV infection—were all in accordance with previous literature [19, 29] but different from other studies [30]. The analyses from RePORT also identified other drug resistance, anemia, and normal chest radiograph as factors associated with worse outcomes. This latter observation may be because RePORT performs systematic collection of these variables, which coincides with findings from other studies [19, 31].
In addition to the above factors, we found an association between higher HbA1c levels and treatment modification, treatment failure, and death, when compared with the HbA1c levels among TB patients who were successfully treated, and this was reflected in the significantly higher proportion of people with DM among those with unfavorable anti-TB treatment outcomes. In the RePORT cohort, the risk of having an unfavorable outcome was 2.45 times higher in DM patients compared with normoglycemic individuals. A risk of 1.76 was found in the SINAN dataset. Additionally, patients with higher HbA1c values were also at a higher risk of experiencing unfavorable outcomes. The relatively lower detection of DM cases in the SINAN dataset probably led to an underestimation of the effect of DM in TB outcomes. Our results are similar to those from previous studies [13, 16]. The risk of death in the DM groups was 2.16 times higher than in normoglycemic patients in the RePORT dataset and 1.93 in the SINAN dataset. The risk of death in the RePORT cohort was more compatible with what has been described in literature [16]. Previous studies showed how TB-DM comorbidity is associated with a higher burden of immune pathology and systemic inflammation compared to normoglycemic PWTB [13, 32]. Furthermore, a defective regulation of the innate immune response in TB-DM patients could maintain inflammatory foci despite anti-TB treatment, resulting in worse treatment results [13].
Moreover, all participants from RePORT had HbA1c measured at the baseline visit, whereas in SINAN the diagnosis of DM was self-reported in most cases, possibly missing many diagnoses. Of note, a longitudinal cohort study done in Brazil found that 50% of individuals diagnosed with DM did not know of their diagnosis [33]. Thus, in the SINAN cohort, with increasing clinical evidence that DM is a risk factor for developing active TB [2] and a more severe clinical TB presentation [9], screening for DM has increased in recent years. But the problem of underdiagnosis and subnotification still exists.
The findings presented here suggest that the impact of DM on TB in Brazil is underestimated. While the results reiterate the value of cohorts like RePORT in better delineating the local epidemiology, they highlight the importance of guidelines recommending laboratory investigation of DM in all TB incident cases and to establish specific treatment recommendations for this population. This implies that long-term glycemic control may improve the outcome of TB treatment in patients with TB-DM comorbidity [34]. The integrated care of individuals with both diseases has specific challenges, such as the interaction between oral antidiabetic and anti-TB drugs [35] and the greater risk for adverse events [36].
We did not find an association between unfavorable outcome and death with either pre-DM or dysglycemia in the RePORT cohort; these associations were observed only with DM. Previous studies reported the normalization of glycemic levels during TB treatment among persons who were dysglycemic prior to anti-TB treatment [37, 38]. Therefore, the deleterious effects on the immune response and treatment outcome caused by chronic DM and associated hyperglycemia may be less likely with pre-DM, which could explain the lack of association with unfavorable and death outcomes in the latter group [37, 38]. The dysglycemia group included pre-DM, and this likely influenced the results.
This study had several limitations. First, RePORT and SINAN are both observational cohorts; unmeasured or residual confounding could have explained the findings. However, the consistent results across both cohorts support their reproducibility. Second, SINAN is a disease notification system that is not part of a study protocol. While SINAN represents TB cases from all of Brazil, there could be incomplete data collection or endpoint ascertainment, as well as different sources of information (ie, self-reported vs information from medical records) for different patients. Third, we did not have information on serum drug levels; low drug levels have been associated with unfavorable TB treatment outcomes, including in PWTB with advanced HIV and DM [39]. Fourth, HbA1c levels could be affected by hemoglobin levels and, in RePORT, HbA1c was the only method used to define DM; the rationale for this was that obtaining fasting glucose levels on all study participants was not feasible. Fifth, the number of patients with a follow-up outcome who were excluded from the study may affect the interpretation of outcomes. Finally, we did not have glycemic status in the following months or at the end of treatment, so we did not have data to identify transient hyperglycemia. Nevertheless, we believe our study provides valuable information on the impact of TB-DM on a population level.
Diabetes is a disease with increasing prevalence and a major risk factor for unfavorable outcomes, including death during treatment, in individuals with TB, along with HIV infection and substance use. Actions prioritizing these groups are essential for the control of TB in Brazil.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
RePORT-Brazil Consortium. Corporate authorship: Alice M S Andrade (Laboratório de Inflamação e Biomarcadores, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil; Multinational Organization Network Sponsoring Translational and Epidemiological Research [MONSTER] Initiative, Salvador, Brazil); Vanessa Nascimento (MONSTER Initiative, Salvador, Brazil; Instituto Brasileiro para Investigação da Tuberculose, Fundação José Silveira, Salvador, Brazil); Hayna Malta-Santos and Jéssica Rebouças-Silva (Laboratório de Inflamação e Biomarcadores, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil; Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Brazil); Alysson G Costa (Fundação Medicina Tropical Dr Heitor Vieira Dourado, Manaus, Brazil; Programa de Pós-Graduação em Medicina Tropical, Universidade do Estado do Amazonas, Manaus, Brazil); Jaquelane Silva (Fundação Medicina Tropical Dr Heitor Vieira Dourado, Manaus, Brazil); Jamile G de Oliveira (Secretaria Municipal de Saúde do Rio de Janeiro, Rio de Janeiro, Brazil); Aline Benjamin, Adriano Gomes-Silva, Flavia M Sant’Anna, and Francine P Ignácio (Laboratório de Pesquisa Clínica em Micobacteriose, Instituto Nacional de Infectologia Evandro Chagas, Fiocruz, Rio de Janeiro, Brazil), Maria Cristina Lourenço (Bacteriology and Bioassay Laboratory, National Institute of Infectious Diseases Evandro Chagas, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil); Elisangela C Silva and Adriana S R Moreira (Programa Acadêmico de Tuberculose da Faculdade de Medicina, Universidade Federal do Rio de Janeiro, Brazil); and Mayla Mello (Laboratório de Inflamação e Biomarcadores, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil).
Notes
Author contributions. Conceptualization: T. R. S., M. C. F., M. C. S., V. C. R., and B. B. A. Data curation: M. B. A., M. A.-P., A. T. L. Q., M. M. S. R., and B. B. A. Investigation: M. B. A., B. B.-D., M. A.-P., B. N., A. B. S., M. S. R., A. B., R. S.-G., M. C. F., B. D., J. R. L. S., A. L. K., S. C., V. C. R., T. R. S., M. C. S., and B. B. A. Formal analysis: M. B. A., M. A.-P., A. T. L. Q., M. M. S. R., and B. B. A. Funding acquisition: B. D., J. R. L. S., A. L. K., S. C., V. C. R., T. R. S., M. C. S., M. C. F., and B. B. A. Methodology: M. B. A., B. B.-D., M. A.-P., and B. B. A. Project administration: M. C. F., T. R. S., and B. B. A. Resources: M. B. A., G. A., T. R. S., and B. B. A. Software: M. B. A., M. A.-P., A. T. L. Q., M. M. S. R., M. C. F., T. R. S., and B. B. A. Supervision: T. R. S. and B. B. A. Writing of the original draft: M. B. A., B. B.-D., M. A.-P., B. N., M. V. C. N. S. F., and B. B. A. Review and editing of the manuscript: All authors. All authors have read and agreed to the submitted version of the manuscript.
Acknowledgments. The authors thank the study participants and the teams of clinical and laboratory platforms of RePORT-Brazil. Special thanks go to Elze Leite (Fiocruz, Salvador, Brazil), Eduardo Gama (Fiocruz, Rio de Janeiro, Brazil), Elcimar Junior (Fundação de Medicina Tropical Heitor Vieira Dourado [FMT-HVD] Manaus, Brazil), Hilary Vansell (Vanderbilt University Medical Center [VUMC], Nashville, Tennessee), and Letícia C. M. Linhares (VUMC) for administrative and logistical support.
Disclaimer. The funder of the study had no role in the study design, data collection, or data analysis; however, a representative of the Brazilian Ministry of Health (A. L. K.) was involved in data interpretation and writing of the report.
Financial support. This work was supported by the Intramural Research Program of the Fundação Oswaldo Cruz, Intramural Research Program of the Fundação José Silveira, Departamento de Ciência e Tecnologia, Secretaria de Ciência e Tecnologia, Ministério da Saúde, Brazil (25029.000507/2013-07); the Civilian Research and Development Foundation (DAA3-17-63144); and the National Institute of Allergy and Infectious Diseases (U01-AI069923). M. B. A. received a fellowship from the Fundação de Amparo à Pesquisa da Bahia. M. A.-P. and B. B.-D. received a fellowship from Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (finance code: 001). B. B. A., J. R. L.S., and A. L. K. are senior investigators of the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
María B Arriaga, Laboratório de Inflamação e Biomarcadores, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil; Multinational Organization Network Sponsoring Translational and Epidemiological Research Initiative, Salvador, Brazil; Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Brazil.
Mariana Araújo-Pereira, Laboratório de Inflamação e Biomarcadores, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil; Multinational Organization Network Sponsoring Translational and Epidemiological Research Initiative, Salvador, Brazil; Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Brazil.
Beatriz Barreto-Duarte, Laboratório de Inflamação e Biomarcadores, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil; Multinational Organization Network Sponsoring Translational and Epidemiological Research Initiative, Salvador, Brazil; Curso de Medicina, Universidade Salvador, Salvador, Brazil.
Betânia Nogueira, Multinational Organization Network Sponsoring Translational and Epidemiological Research Initiative, Salvador, Brazil; Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Brazil; Instituto Brasileiro para Investigação da Tuberculose, Fundação José Silveira, Salvador, Brazil.
Maria Vitória C N S Freire, Curso de Medicina, Escola Bahiana de Medicina e Saúde Pública, Salvador, Brazil.
Artur T L Queiroz, Multinational Organization Network Sponsoring Translational and Epidemiological Research Initiative, Salvador, Brazil; Center of Data and Knowledge Integration for Health, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil.
Moreno M S Rodrigues, Laboratório de Análise e Visualização de Dados, Fundação Oswaldo Cruz, Porto Velho, Brazil.
Michael S Rocha, Multinational Organization Network Sponsoring Translational and Epidemiological Research Initiative, Salvador, Brazil; Instituto Brasileiro para Investigação da Tuberculose, Fundação José Silveira, Salvador, Brazil.
Alexandra B Souza, Fundação Medicina Tropical Dr Heitor Vieira Dourado, Manaus, Brazil; Programa de Pós-Graduação em Medicina Tropical, Universidade do Estado do Amazonas, Manaus, Brazil.
Renata Spener-Gomes, Fundação Medicina Tropical Dr Heitor Vieira Dourado, Manaus, Brazil; Programa de Pós-Graduação em Medicina Tropical, Universidade do Estado do Amazonas, Manaus, Brazil; Universidade Federal do Amazonas, Manaus, Brazil.
Anna Cristina C Carvalho, Programa Acadêmico de Tuberculose da Faculdade de Medicina, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; Laboratório de Inovações em Terapias, Ensino e Bioprodutos, Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, Brazil.
Marina C Figueiredo, Division of Infectious Diseases, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
Megan M Turner, Division of Infectious Diseases, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
Betina Durovni, Secretaria Municipal de Saúde do Rio de Janeiro, Rio de Janeiro, Brazil.
José R Lapa-e-Silva, Programa Acadêmico de Tuberculose da Faculdade de Medicina, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil.
Afrânio L Kritski, Programa Acadêmico de Tuberculose da Faculdade de Medicina, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil.
Solange Cavalcante, Secretaria Municipal de Saúde do Rio de Janeiro, Rio de Janeiro, Brazil.
Valeria C Rolla, Laboratório de Pesquisa Clínica em Micobacteriose, Instituto Nacional de Infectologia Evandro Chagas, Fiocruz, Rio de Janeiro, Brazil.
Marcelo Cordeiro-Santos, Fundação Medicina Tropical Dr Heitor Vieira Dourado, Manaus, Brazil; Programa de Pós-Graduação em Medicina Tropical, Universidade do Estado do Amazonas, Manaus, Brazil; Universidade Nilton Lins, Manaus, Brazil.
Timothy R Sterling, Division of Infectious Diseases, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
Bruno B Andrade, Laboratório de Inflamação e Biomarcadores, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brazil; Multinational Organization Network Sponsoring Translational and Epidemiological Research Initiative, Salvador, Brazil; Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Brazil; Curso de Medicina, Universidade Salvador, Salvador, Brazil; Curso de Medicina, Escola Bahiana de Medicina e Saúde Pública, Salvador, Brazil; Division of Infectious Diseases, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
Regional Prospective Observational Research in Tuberculosis (RePORT)–Brazil Consortium:
Alice M S Andrade, Vanessa Nascimento, Hayna Malta-Santos, Jéssica Rebouças-Silva, Alysson G Costa, Jaquelane Silva, Jamile G de Oliveira, Aline Benjamin, Adriano Gomes-Silva, Flavia M Sant’Anna, Francine P Ignácio, Maria Cristina Lourenço, Elisangela C Silva, Adriana S R Moreira, and Mayla Mello
References
- 1. World Health Organization. Global report on diabetes. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Global tuberculosis report 2020. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 4. World Health Organization. Collaborative framework for care and control of tuberculosis and diabetes. Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 5. Cheng J, Zhang H, Zhao YL, Wang LX, Chen MT. Mutual impact of diabetes mellitus and tuberculosis in China. Biomed Environ Sci 2017; 30:384–9. [DOI] [PubMed] [Google Scholar]
- 6. Kreisel CF, Passannante MR, Lardizabal AA. The negative clinical impact of diabetes on tuberculosis: a cross-sectional study in New Jersey. J Endocr Soc 2019; 3:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017; 152:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sembiah S, Nagar V, Gour D, Pal DK, Mitra A, Burman J. Diabetes in tuberculosis patients: an emerging public health concern and the determinants and impact on treatment outcome. J Family Community Med 2020; 27:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gil-Santana L, Almeida-Junior JL, Oliveira CA, et al. Diabetes is associated with worse clinical presentation in tuberculosis patients from Brazil: a retrospective cohort study. PLoS One 2016; 11:e0146876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barreda NN, Arriaga MB, Aliaga JG, et al. Severe pulmonary radiological manifestations are associated with a distinct biochemical profile in blood of tuberculosis patients with dysglycemia. BMC Infect Dis 2020; 20:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Almeida-Junior JL, Gil-Santana L, Oliveira CA, et al. Glucose metabolism disorder is associated with pulmonary tuberculosis in individuals with respiratory symptoms from Brazil. PLoS One 2016; 11:e0153590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mesquita ED, Gil-Santana L, Ramalho D, et al. ; Rede-TB Study Group. Associations between systemic inflammation, mycobacterial loads in sputum and radiological improvement after treatment initiation in pulmonary TB patients from Brazil: a prospective cohort study. BMC Infect Dis 2016; 16:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar NP, Fukutani KF, Shruthi BS, et al. Persistent inflammation during anti-tuberculosis treatment with diabetes comorbidity. Elife 2019; 8:e46477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Restrepo BI. Diabetes and tuberculosis. Microbiol Spectr 2016; 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dousa KM, Hamad A, Albirair M, et al. Impact of diabetes mellitus on the presentation and response to treatment of adults with pulmonary tuberculosis in Qatar. Open Forum Infect Dis 2019; 6:ofy335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huangfu P, Ugarte-Gil C, Golub J, Pearson F, Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis 2019; 23:783–96. [DOI] [PubMed] [Google Scholar]
- 17. Yoon YS, Jung JW, Jeon EJ, et al. The effect of diabetes control status on treatment response in pulmonary tuberculosis: a prospective study. Thorax 2017; 72:263–70. [DOI] [PubMed] [Google Scholar]
- 18. Kornfeld H, Sahukar SB, Procter-Gray E, et al. Impact of diabetes and low body mass index on tuberculosis treatment outcomes. Clin Infect Dis 2020; 71:e392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaves Torres NM, Quijano Rodríguez JJ, Porras Andrade PS, Arriaga MB, Netto EM. Factors predictive of the success of tuberculosis treatment: a systematic review with meta-analysis. PLoS One 2019; 14:e0226507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arriaga MB, Amorim G, Queiroz ATL, et al. Novel stepwise approach to assess representativeness of a large multicenter observational cohort of tuberculosis patients: the example of RePORT Brazil. Int J Infect Dis 2021; 103:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Diabetes Association. Glycemic targets: standards of medical care in diabetes. Diabetes Care 2020; 42:S187–93. [DOI] [PubMed] [Google Scholar]
- 23. Ministério da Saúde do Brasil, Secretaria de Vigilância em Saúde. Sistema de Informação de Agravos de Notificação [in Portuguese]. http://portalsinan.saude.gov.br/. Accessed 14 August 2020.
- 24. Ministério da Saúde. Brasília: Ministério da Saúde. Brasilia, Brasil: Manual de recomendações para o controle da tuberculose no Brasil. 2013:288.
- 25. Ministerio da Saude do Brasil, Secretaria de Vigilancia em Saude. Estrategias para o cuidado da pessoa com doenca cronica [in Portuguese]. Brasilia, Brazil: Cadernos de Atencao Basica [in Portuguese]. 2014:146. [Google Scholar]
- 26. World Health Organization. Definitions and reporting framework for tuberculosis - 2013 revision: updated December 2014 and January 2020. World Health Organization, 2013. https://apps.who.int/iris/handle/10665/79199
- 27. Shewade HD, Jeyashree K, Mahajan P, et al. Effect of glycemic control and type of diabetes treatment on unsuccessful TB treatment outcomes among people with TB-diabetes: a systematic review. PLoS One 2017; 12:e0186697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019. [Google Scholar]
- 29. Myers B, Bouton TC, Ragan EJ, et al. Impact of alcohol consumption on tuberculosis treatment outcomes: a prospective longitudinal cohort study protocol. BMC Infect Dis 2018; 18:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Q, Ma A, Han X, et al. Prevalence of type 2 diabetes among newly detected pulmonary tuberculosis patients in China: a community based cohort study. PLoS One 2013; 8:e82660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calderon RI, Arriaga MB, Lopez K, et al. High prevalence and heterogeneity of dysglycemia in patients with tuberculosis from Peru: a prospective cohort study. BMC Infect Dis 2019; 19:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prada-Medina CA, Fukutani KF, Pavan Kumar N, et al. Systems immunology of diabetes-tuberculosis comorbidity reveals signatures of disease complications. Sci Rep 2017; 7:1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmidt MI, Hoffmann JF, de Fátima Sander Diniz M, et al. High prevalence of diabetes and intermediate hyperglycemia—the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Diabetol Metab Syndr 2014; 6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Critchley JA, Restrepo BI, Ronacher K, et al. Defining a research agenda to address the converging epidemics of tuberculosis and diabetes: part 1: epidemiology and clinical management. Chest 2017; 152:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arbex MA, Varella Mde C, Siqueira HR, Mello FA. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 1: first-line drugs. J Bras Pneumol 2010; 36:626–40. [DOI] [PubMed] [Google Scholar]
- 36. Siddiqui AN, Khayyam KU, Sharma M. Effect of diabetes mellitus on tuberculosis treatment outcome and adverse reactions in patients receiving directly observed treatment strategy in India: a prospective study. Biomed Res Int 2016; 2016:7273935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boillat-Blanco N, Ramaiya KL, Mganga M, et al. Transient hyperglycemia in patients with tuberculosis in Tanzania: implications for diabetes screening algorithms. J Infect Dis 2016; 213:1163–72. [DOI] [PubMed] [Google Scholar]
- 38. Magee MJ, Salindri AD, Kyaw NTT, Auld SC, Haw JS, Umpierrez GE. Stress hyperglycemia in patients with tuberculosis disease: epidemiology and clinical implications. Curr Diab Rep 2018; 18:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park JS, Lee JY, Lee YJ, et al. Serum levels of antituberculosis drugs and their effect on tuberculosis treatment outcome. Antimicrob Agents Chemother 2016; 60:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.