Tumour-associated macrophages (TAMs) are the most abundant innate immune cells and constitute up to 50% of the cell mass within the tumour microenvironment (TME) of most solid tumours. TAMs affect all aspects of tumour progression, namely, tumour cell proliferation, angiogenesis, metastasis, immune suppression, immune escape, and drug resistance. Unsurprisingly, TAMs play an indispensable role in anticancer therapy. Therapeutic strategies usually target TAMs directly, which rely on the natural characteristics of TAMs, tumour cells, the TME, and the interactions between these components by depleting TAMs, inhibiting TAM recruitment, and reprogramming TAMs. Although TAM-targeted therapeutic approaches have attained promising antitumour efficacy in some settings, several specific limitations remain. First, the complexity of the mechanisms behind these approaches is much higher than expected, including the ontogeny, plasticity of TAMs, and high intertumoural and intratumoural heterogeneity of TAMs. These results lead to limited efficacy and high off-target toxicity. In addition, current drugs do not have the necessary specificity. Targeting TAMs requires preferential drug delivery to the M2 phenotype over tissue-resident macrophages or the M1 phenotype; this is not possible using today's therapies. Due to limitations of the commonly used therapies, biotechnology and gene engineering approaches have been applied to optimise macrophages to potentially revolutionise TAM-targeted anticancer therapies.
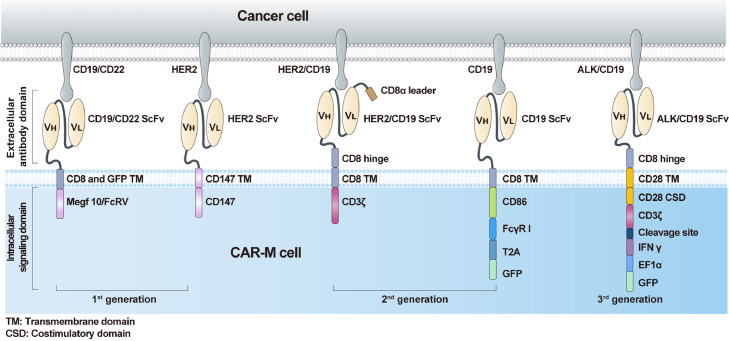
The chimeric antigen receptor (CAR) has become a promising approach to increase the cancer recognition capacity of immune cells. Based on their ability to penetrate solid tumours and traffic through the inhibitory TME, TAMs engineered with CAR constructs demonstrate sufficient potency. Similar to CAR-T, the core components of CAR-M contain an extracellular domain that provides specific recognition by a single-chain variable fragment (scFv) (eg, CD19 and HER2), a hinge domain, a transmembrane domain (mostly CD8), and an intracellular domain that presents dedicated downstream signalling (eg, CD3ζ, FcγR) (Figure 1). First-generation CAR-M cells are modified with edited CARs to target specific antigens to recognise tumour cells and improve their phagocytic ability. However, the CAR-M structure merely uses unique macrophage properties, mainly phagocytosis. As a representative structure, CAR-phagocytes (CAR-Ps) from Morrissey et al.1 were constructed to enhance phagocytosis. The penetration capacity of macrophages was fully used in the original generation. Zhang et al.2 constructed CAR-HER2-CD147 to activate the expression of matrix metalloproteinases, which are stimulated by CD147 and can degrade the tumour extracellular matrix to overcome physical barriers.
Figure 1.
The structures of various generations of CAR-Ms which differ in their intracellular domain.
Second-generation CAR-M cells are in development. In addition to maintaining the characteristics of first-generation CAR-M technology, the goals of second-generation therapies comprise improving tumour-associated antigen presentation and T cell activation. The ideal method is to add an intracellular cytoplasmic domain to the CAR structure. Second, the induction and maintenance of anticancer phenotypes to overcome the plasticity of TAMs must be considered. Third, although macrophages cannot be expanded in vitro, we expected that the CAR-engineered macrophages would expand considerably and last a relatively long time to achieve satisfactory therapeutic effects in a small infusion dose. Based on these concepts, researchers have attempted to engineer murine-origin or human-origin bone marrow-derived macrophages to express CAR through chimeric vector transduction and then obtain the agents after expansion, concentration, and purification in vitro. The anti-HER2 CAR-M (CT-0508) from Klichinsky et al.3 successfully demonstrated these improvements and has been evaluated in a first-in-human phase 1 clinical trial that focused on patients with recurrent or metastatic HER-2 overexpressing solid tumours (NCT04660929). Encouragingly, the United States Food and Drug Administration granted fast track designation to CT-0508 in September 2021, which demonstrates the critical need for CAR-based TAM therapy development. However, the product cannot overcome the expansion obstacle, and the minimal effective dose requires further clinical investigation. To solve the expansion problem, Zhang et al.4 derived induced pluripotent stem cells (iPSCs) from peripheral blood monocyte cells of a healthy donor with nonintegrating episomal vectors encoding reprogramming factors, introduced CAR into iPSCs by lentiviral transduction, and established a protocol for myeloid/macrophage differentiation to induce CAR-iPSCs towards myeloid cell lineages, to generate CAR-iMac in sufficient quantities. They found that CAR-iMac cells can expand above 50 folds and persist for more than 30 days. Additionally, CAR transgene expression is up to 85% in CAR-iMac cells. Thus, the features of iPSC-derived engineered immune cells, such as CAR-M, pave the way towards manufacturing clonal, homogeneous, and abundant products that fulfil clinical needs. These approaches employ a sophisticated process of ex vivo CAR-M production, which requires accurate cell product manufacturing and quality control to support broader clinical applications.
We expect the third-generation CAR-M technology to extensively enhance anticancer efficacy. A new concept of reprogramming CAR-M in vivo via a nonviral vector has been utilised in the designation of the third generation to overcome these challenges, owing to its cost-effective, simple manufacturing process. Moreover, in the new generation, the adaptation of cytokine receptor domains is also necessary to further improve the immune modulation and tumoricidal capability of CAR-M products. Kang et al.5 attempted to deliver the genes encoding CAR and interferon-γ (IFN-γ) into macrophages in vivo using macrophage-targeting polymer nanocarriers. The IFN-γ gene is designed to further enhance antitumour potency by re-polarising CAR-M from an M2 phenotype to an M1 phenotype. Nanobiotechnology, another promising cancer immunotherapy platform, was first combined with CAR-M therapy. The effective antitumour results support our perspective on the development of CAR-M cells. However, concerns regarding expansion ability and transduction efficiency from a nonviral vector must be solved through a direct comparison of the therapeutic efficacy of ex vivo-generated and in vivo-programmed CAR-M.
Although CAR-M therapy may become a promising anticancer therapy, its realisation has not been achieved (Suppl. Table 1). First, TAMs are extremely plastic and can adapt to their phenotype and function in response to environmental stimuli. Second, the limited expansion of CAR-Ms in vivo with current technology may obstruct therapeutic efficacy. Furthermore, safety should also be considered when developing CAR-M cells. The majority of targeted tumour antigens tend to be expressed in subsets of healthy cells, leading to potential off-target toxicity. In addition, macrophages are distributed throughout the body, with the highest enrichment in the liver, leading to unexpected toxicity and limited efficacy. Another concern is the potential immunogenicity of the therapy due to specific anti-CAR immune responses to nonself-components of the CAR construct or originating from gene-transfer vectors once entering the clinical phase. We expect an optimised CAR-M product engineered with a mature technology platform to overcome the aforementioned difficulties. Strategies for new-generation CAR-M will equip CAR-M with the characteristics of specific tumour antigen selection, improved expansion efficiency, high calibre or homogeneity, feasible genetic modification, and strict control of safety, which would enhance the immune efficacy extensively through specific phagocytosis, antigen presentation, and cytotoxic T cell activation.
Contributors
SHW, YQY conceptualization, writing original draft, review & editing. JZ, NL conceptualization, revising the draft. YZ, AHL and PWM review & editing. SHW and YQY contributed equally to this manuscript. All authors have approved the final manuscript.
Declaration of interests
All authors declare no conflicts of interest.
Acknowledgements
The commentary was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Platform Improvement of Clinical Trial Capability 2020‐I2M‐2‐007), and the Beijing Municipal Health Commission, Beijing Demonstration Research Ward (BCRW20200303).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2022.103873.
Appendix. Supplementary materials
References
- 1.Morrissey M.A., Williamson A.P., Steinbach A.M., et al. Chimeric antigen receptors that trigger phagocytosis. eLife. 2018;7:e36688. doi: 10.7554/eLife.36688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W., Liu L., Su H., et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121(10):837–845. doi: 10.1038/s41416-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klichinsky M., Ruella M., Shestova O., et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Tian L., Dai X., et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13(1):153. doi: 10.1186/s13045-020-00983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang M., Lee S.H., Kwon M., et al. Nanocomplex-mediated in vivo programming to chimeric antigen receptor-M1 macrophages for cancer therapy. Adv Mater. 2021;33(43) doi: 10.1002/adma.202103258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.