Abstract
目的
研究BMSCs成骨分化过程中环指蛋白11(ring finger protein 11,RNF11)对Akt信号通路的调节作用,为进一步阐明BMSCs成骨分化机制和用于临床治疗提供思路。
方法
从健康人体新鲜骨髓中分离培养BMSCs并传代,取第4代细胞经流式细胞术,成骨、成软骨和成脂诱导培养鉴定后用于实验。BMSCs成骨诱导分化培养0~14 d,通过茜素红染色和ALP染色检测其成骨分化程度,并用Western blot法检测RNF11蛋白表达。取第4代BMSCs,分为空白对照组(A组)、空载慢病毒(Lv-NC)组(B组)和敲低RNF11(Lv-ShRNF11)组(C组),成骨诱导分化培养0~14 d,采用Western blot检测RNF11蛋白表达,茜素红染色和ALP染色检测其成骨分化程度,14 d实时荧光定量PCR(real-time fluorescence quantitative PCR,qRT-PCR)检测BMSCs成骨标志物Runx2、骨钙素(osteocalcin,OCN)及骨桥蛋白(osteopontin,OPN)的mRNA相对表达量;采用Western blot检测Akt、Smad1/5/8及β-catenin信号通路蛋白相对表达量,以磷酸化前后比值表示。为研究RNF11对Akt信号通路的影响机制,取第4代BMSCs分为Lv-NC转染组(A1组)、Lv-ShRNF11转染组(B1组)和添加Akt信号通路激活剂SC79的Lv-ShRNF11转染组(C1组),14 d时采用Western blot检测RNF11和Akt信号通路蛋白相对表达量,茜素红染色、ALP染色及qRT-PCR检测成骨相关指标。
结果
流式细胞术及成骨、成软骨和成脂诱导培养鉴定显示分离培养细胞为BMSCs。RNF11蛋白相对表达量随成骨分化时间延长而逐渐增加(P<0.05);下调RNF11后,茜素红和ALP染色示C组相较于A、B组BMSCs成骨分化程度降低,qRT-PCR检测示Runx2、OCN、OPN mRNA相对表达量减少(P<0.05)。随成骨分化时间延长,RNF11与Akt信号通路蛋白相对表达量均上升(P<0.05)。下调RNF11后,C组相较于A、B组,其Akt信号通路蛋白相对表达量显著降低(P<0.05),而对Smad1/5/8以及β-catenin信号通路蛋白相对表达量无明显影响(P>0.05)。B1、C1组相较于A1组,其RNF11蛋白相对表达量减少(P<0.05),B1组相较于A1、C1组,其Akt信号通路蛋白相对表达量降低(P<0.05);茜素红与ALP染色示C1组BMSCs成骨分化程度稍低于A1组(P>0.05),而明显高于B1组(P<0.05);qRT-PCR检测示C1组Runx2、OCN、OPN mRNA相对表达量稍低于A1组(P>0.05),而明显高于B1组(P<0.05)。
结论
RNF11通过正向调控Akt信号通路激活水平促进BMSCs向成骨细胞分化。RNF11可作为提高BMSCs修复骨缺损疗效以及治疗骨代谢病的潜在靶点。
Keywords: BMSCs, 成骨分化, 环指蛋白11, Akt信号通路, 组织工程骨, 骨缺损修复
Abstract
Objective
To investigate the role and regulatory mechanism of ring finger protein 11 (RNF11) on Akt signaling pathway in the process of osteogenesis of bone marrow mesenchymal stem cells (BMSCs) to provide ideas for further clarifying its osteogenesis mechanism and its use in clinical treatment in the future.
Methods
BMSCs were isolated and cultured from fresh bone marrow of healthy donors and subcultured. The 4th generation cells were used in experiments after identification by flow cytometry, and osteogenic, chondrogenic, and adipogenic induction. BMSCs were cultured in osteogenic differentiation medium for 0-14 days. The degree of osteogenic differentiation was detected by Alizarin red staining and alkaline phosphatase (ALP) staining, and the protein expression of RNF11 was detected by Western blot. The 4th generation BMSCs were divided into blank control group (group A), empty lentivirus (Lv-NC) group (group B), and knockdown RNF11 (Lv-ShRNF11) group (group C). Osteogenesis was induced and cultured for 0-14 days. The expression of RNF11 protein was detected by Western blot, the degree of osteogenic differentiation was detected by Alizarin red staining and ALP staining, and the relative mRNA expressions of Runx2, osteocalcin (OCN), and osteopontin (OPN) were detected by real-time fluorescence quantitative PCR (qRT-PCR). The protein relative expressions of Akt, Smad1/5/8, and β-catenin signaling pathway were detected by Western blot, expressed as the ratio before and after phosphorylation. In order to study the effect mechanism of RNF11 on Akt signaling pathway, the 4th generation BMSCs were divided into Lv-NC transfection group (group A1), Lv-ShRNF11 transfection group (group B1), and Lv-ShRNF11 transfection supplemented with Akt signaling pathway activator SC79 group (group C1). The protein relative expressions of RNF11 and Akt signaling pathway were detected by Western blot, the related osteogenesis indexes were detected by Alizarin red staining, ALP staining, and qRT-PCR.
Results
The flow cytometry, and osteogenic, chondrogenic, adipogenic induction culture identification showed that the isolated and cultured cells were BMSCs. The protein relative expression of RNF11 increased gradually with the extension of osteogenic differentiation time (P<0.05); after knockdown RNF11, Alizarin red and ALP stainings showed that the degree of osteogenic differentiation of BMSCs in group C were significantly lower than those in groups A and B, and qRT-PCR detection showed that the relative expression of Runx2, OCN, and OPN mRNA significantly decreased (P<0.05). The protein relative expressions of RNF11 and Akt signaling pathway significantly increased with the extensions of osteogenic differentiation time (P<0.05). After knockdown RNF11, the protein relative expression of Akt signaling pathway in group C was significantly lower than that in groups A and B (P<0.05), while Smad1/5/8 and β-catenin signaling pathway had no significant effect (P>0.05). Compared with group A1, the protein relative expression of RNF11 in groups B1 and C1 significantly decreased (P<0.05). Compared with groups A1 and C1, the protein relative expression of Akt signaling pathway in group B1 was significantly lower (P<0.05); Alizarin red and ALP stainings showed that the degree of osteogenic differentiation of BMSCs in group C1 were slightly lower than that of group A1 (P>0.05), but significantly higher than that of group B1 (P<0.05); qRT-PCR detection showed that the relative expressions of Runx2, OCN, and OPN mRNA in group C1 were slightly lower than those of group A1 (P>0.05), but were significantly higher than those of group B1 (P<0.05).
Conclusion
RNF11 promotes the differentiation of BMSCs into osteoblasts by positively regulating the activation level of Akt signaling pathway. RNF11 can be used as a potential target to improve the bone repair efficacy of BMSCs and treat bone metabolic diseases.
Keywords: Bone marrow mesenchymal stem cells, osteogenesis, ring finger protein 11, Akt signaling pathway, tissue engineered bone, bone defect repair
骨缺损是骨科常见疾病之一,其治疗手段主要有自体骨、同种异体骨或人工骨移植修复,但自体骨移植取材较复杂且增加了患者痛苦,同种异体骨移植存在免疫排斥、疾病传播等问题,人工骨移植优点较多,但也存在诸多局限[1-2]。组织工程骨技术是骨缺损治疗的主要发展方向。MSCs是一种在体内分布广泛、来源丰富的多能祖细胞,免疫原性低,具有成脂、成软骨和成骨多向分化潜能[1-3]。在附着于生物材料的情况下,MSCs可快速分化为成骨细胞,形成膜内骨[4-5],不仅极大程度解决了免疫排斥问题,还可以结合3D打印技术实现精准、可控的骨缺损修复[6-7]。Maruyama等[8-9]研究还发现,MSCs与免疫细胞共同参与骨愈合的炎症阶段,相互拮抗以调节骨愈合过程。然而,调控MSCs成骨分化的分子机制尚未完全阐明,阻碍了临床上基于MSCs的骨缺损细胞疗法进一步发展[1]。因此,有必要进一步阐明MSCs成骨分化的分子调控机制。
Akt是PI3K的下游效应分子,PI3K/Akt信号通路在细胞增殖、分化、凋亡过程中起着重要作用,尤其是软骨细胞、成骨细胞、成肌细胞和脂肪细胞[10]。PI3K/Akt信号通路的活化有助于抑制骨质疏松[11]。Peng等[12]发现当敲除Akt1和Akt2基因时,小鼠会表现出严重的生长发育障碍,包括骨骼发育迟缓、皮肤发育不全、骨骼肌萎缩和成脂障碍。环指蛋白11(ring finger protein 11,RNF11)是环指蛋白家族中的重要成员之一,由154个氨基酸构成,在生物进化过程中有高度保守性[13-15]。RNF11在生物体内发挥重要作用,包括细胞中的物质运输、信号传导以及肿瘤发生等[16],其通过E3泛素连接酶属性参与多种生理及病理过程[14,17-19]。既往研究已发现RNF11在骨骼中表达丰度很高[20],但其在骨骼发育中的作用却鲜有研究。本研究通过敲低RNF11在BMSCs中的表达后,检测Akt信号通路蛋白的表达,探讨RNF11如何参与调控BMSCs成骨分化过程。
1. 材料与方法
1.1. 主要试剂与仪器
健康人体新鲜骨髓样品由志愿者捐赠。293T细胞(武汉普诺赛生命科技有限公司)。DMEM培养基(HyClone公司,美国);聚偏氟乙烯(polyvinylidene fluoride,PVDF)膜(Millipore公司,德国);Trizol(Invitrogen公司,美国);重组慢病毒载体(上海吉凯基因医学科技股份有限公司);PrimeScript Ⅱ cDNA合成试剂盒(D6210A)(Takara公司,日本);茜素红(上海中乔新舟生物科技有限公司);阿利新蓝、ALP、油红O染色试剂盒(上海碧云天生物技术有限公司)。Merinton MA1000显微分光光度计(北京美林恒通仪器有限公司);LightCycler480 PCR system(Roche公司,瑞士);Immobilon Western Chemiluminescent辣根过氧化物酶(horseradish peroxidase,HRP)底物曝光(Millipore公司,德国);Coulter流式细胞仪系统(Beckman Coulter公司,美国);石蜡切片机、石蜡包埋机(Leica公司,德国)。
1.2. BMSCs分离、培养及鉴定
1.2.1. BMSCs分离培养
取健康人体新鲜骨髓样品,分离、纯化后接种至12孔板,使用含10%FBS的DMEM培养基进行重悬和培养(培养条件为37℃、5%CO2),每3天更换1次培养液。当细胞融合度达90%左右时进行传代,取第4代细胞进行以下实验。
1.2.2. BMSCs鉴定
① 流式细胞术:取获得的第4代细胞与抗CD14、CD45、HLA-DR、CD29、CD44、CD105抗体于4℃下孵育30 min,然后离心洗涤细胞,通过流式细胞仪系统进行分析。② BMSCs分化潜能检测:取获得的第4代细胞,行成骨、成软骨及成脂诱导分化14 d后,分别行茜素红染色、阿利新蓝染色和油红O染色观察。
1.3. RNF11在BMSCs成骨分化过程中的表达
1.3.1. BMSCs成骨分化规律
于BMSCs成骨诱导分化0、3、7、10、14 d,分别行茜素红染色[以562 nm处吸光度(A)值作为阳性染色钙结节数量]和ALP染色(检测ALP活性)定量检测,观察BMSCs的成骨分化规律。
1.3.2. Western blot检测RNF11在BMSCs成骨分化过程中的表达
于BMSCs成骨诱导分化第0、3、7、10、14天,使用RIPA裂解BMSCs,提取细胞裂解液,于4℃以离心半径20 cm、12 000 r/min离心30 min,然后加入Loading buffer混匀煮沸。用等量蛋白质上样至10%SDS-PAGE中,电泳分离,再转印至PVDF膜,加入5%脱脂奶粉封闭1 h;加入GAPDH、RNF11一抗(1∶1 000)孵育过夜,TBST洗涤3次;用HRP偶联的二抗(1∶3 000)室温孵育1 h,TBST洗涤3次;最后用Immobilon Western Chemiluminescent HRP底物曝光检测RNF11蛋白相对表达量。
1.4. 下调RNF11对BMSCs体外成骨分化能力的影响
1.4.1. 实验分组
取第4代BMSCs,分为空白对照组(A组)、空载慢病毒(Lv-NC)组(B组)和敲低RNF11(Lv-ShRNF11)组(C组)。A组不作任何处理,B、C组细胞分别转染慢病毒Lv-NC和Lv-ShRNF11。慢病毒转染方法:选一段最佳敲除序列构建慢病毒载体,然后通过转染将质粒包装至293T细胞中;转染72 h后以离心半径20 cm、12 000 r/min离心30 min,将含有慢病毒的上清液转移至另一管中并浓缩;将浓缩液与聚丙烯添加至BMSCs中孵育24 h,然后将培养基更换为成骨诱导分化培养基。
1.4.2. 观测指标
① 各组成骨诱导分化3、7、10、14 d,同上法采用Western blot检测RNF11蛋白表达,茜素红染色定量检测阳性钙结节数量,ALP染色检测ALP活性。② 实时荧光定量PCR(real-time fluorescence quantitative PCR,qRT-PCR)检测:各组成骨诱导分化14 d去除培养基,加入1 mL Trizol裂解细胞,室温孵育5 min,Trizol提取细胞RNA,用Merinton MA1000显微分光光度计在260 nm和280 nm处测量A值,用PrimeScript Ⅱ cDNA合成试剂盒(D6210A)生成cDNA模板,然后用LightCycler480 PCR system进行qRT-PCR检测,以GAPDH为内参,采用2−ΔΔCt法计算BMSCs成骨标志物Runx2、骨钙素(osteocalcin,OCN)及骨桥蛋白(osteopontin,OPN)的mRNA相对表达量。引物序列见表1。
表 1.
Primer sequences of qRT-PCR genes
qRT-PCR各基因引物序列
| 基因
Gene |
引物序列(5'→3')
Primer sequence (5'→3') |
|
| 上游
Forward |
下游
Reverse |
|
| GAPDH | GGAGCGAGATCCCTCCAAAAT | CTCCTTAATGTCACGCACGAT |
| Runx2 | TCAACGATCTGAGATTTGTGGG | GGGGAGGATTTGTGAAGACGG |
| OCN | CACTCCTCGCCCTATTGGC | CCCTCCTGCTTGGACACAAAG |
| OPN | GAAGTTTCGCAGACCTGACAT | GTATGCACCATTCAACTCCTCG |
1.5. RNF11对Akt信号通路的影响
1.5.1. RNF11与Akt信号通路蛋白相对表达量在BMSCs成骨分化过程中的表达
取第4代BMSCs行成骨诱导分化培养,培养0、7、14 d时,同上法采用Western blot检测RNF11及Akt信号通路蛋白相对表达量,后者以磷酸化Akt(phosphorylated Akt,p-Akt)/Akt比值表示。
1.5.2. 下调RNF11对Akt信号通路的影响
同1.4.1方法分组,于各组成骨诱导分化培养14 d,采用Western blot检测β-catenin、Akt及Smad1/5/8信号通路蛋白相对表达量,分别以p-β-catenin/β-catenin、p-Akt/Akt、p-Smad1/Smad1比值表示。
1.6. RNF11对Akt信号通路的影响机制
1.6.1. 实验分组
取第4代BMSCs,分为Lv-NC转染组(A1组)、Lv-ShRNF11转染组(B1组)和添加Akt信号通路激活剂SC79(3 μg/mL)的Lv-ShRNF11转染组(C1组)。慢病毒转染方法同1.4.1。转染后各组行成骨诱导分化。
1.6.2. 观测指标
各组成骨诱导分化14 d,同上法采用Western blot检测RNF11和Akt信号通路蛋白相对表达量,茜素红染色定量检测阳性钙结节数量,ALP染色检测ALP活性,qRT-PCR检测Runx2、OCN、OPN mRNA相对表达量。
1.7. 统计学方法
采用SPSS22.0统计软件进行分析。计量资料均符合正态分布,以均数±标准差表示,组间比较采用单因素方差分析,两两比较采用LSD检验;检验水准α=0.05。
2. 结果
2.1. BMSCs鉴定
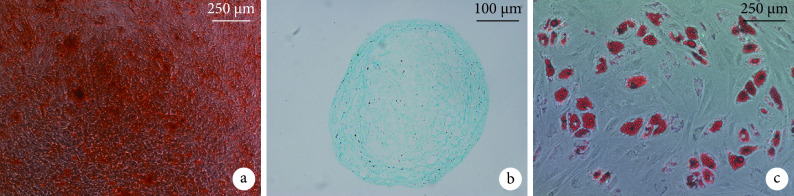
流式细胞术检测示,所培养细胞表面标志物CD14、CD45、HLA-DR表达呈阴性,而CD29、CD44及CD105表达呈阳性,与MSCs表面标志物表达模式一致。见图1。成骨诱导分化14 d后茜素红染色可见红色钙结节,成软骨诱导分化14 d阿利新蓝染色可见深蓝色结节,成脂诱导分化14 d油红O染色可见红色脂滴。见图2。提示分离获得的细胞为BMSCs。
图 1.
Identification of BMSCs by flow cytometry
BMSCs流式细胞术鉴定
a. CD14;b. CD45;c. HLA-DR;d. CD29;e. CD44;f. CD105
a. CD14; b. CD45; c. HLA-DR; d. CD29; e. CD44; f. CD105
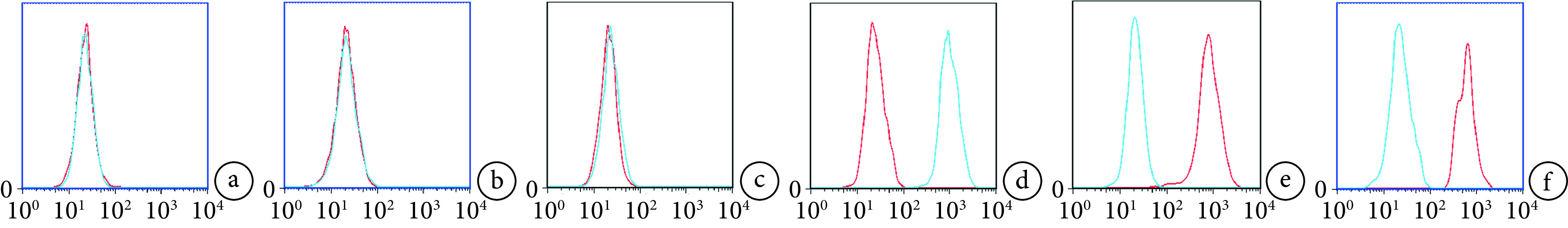
图 2.
Identification of multipotential differentiation of BMSCs
BMSCs各向分化潜能鉴定
a. 茜素红染色(×40);b. 阿尔新蓝染色(×100);c. 油红O染色(×40)
a. Alizarin red staining (×40); b. Alcian blue staining (×100); c. Oil red O staining (×40)
2.2. RNF11在BMSCs成骨分化过程中的表达
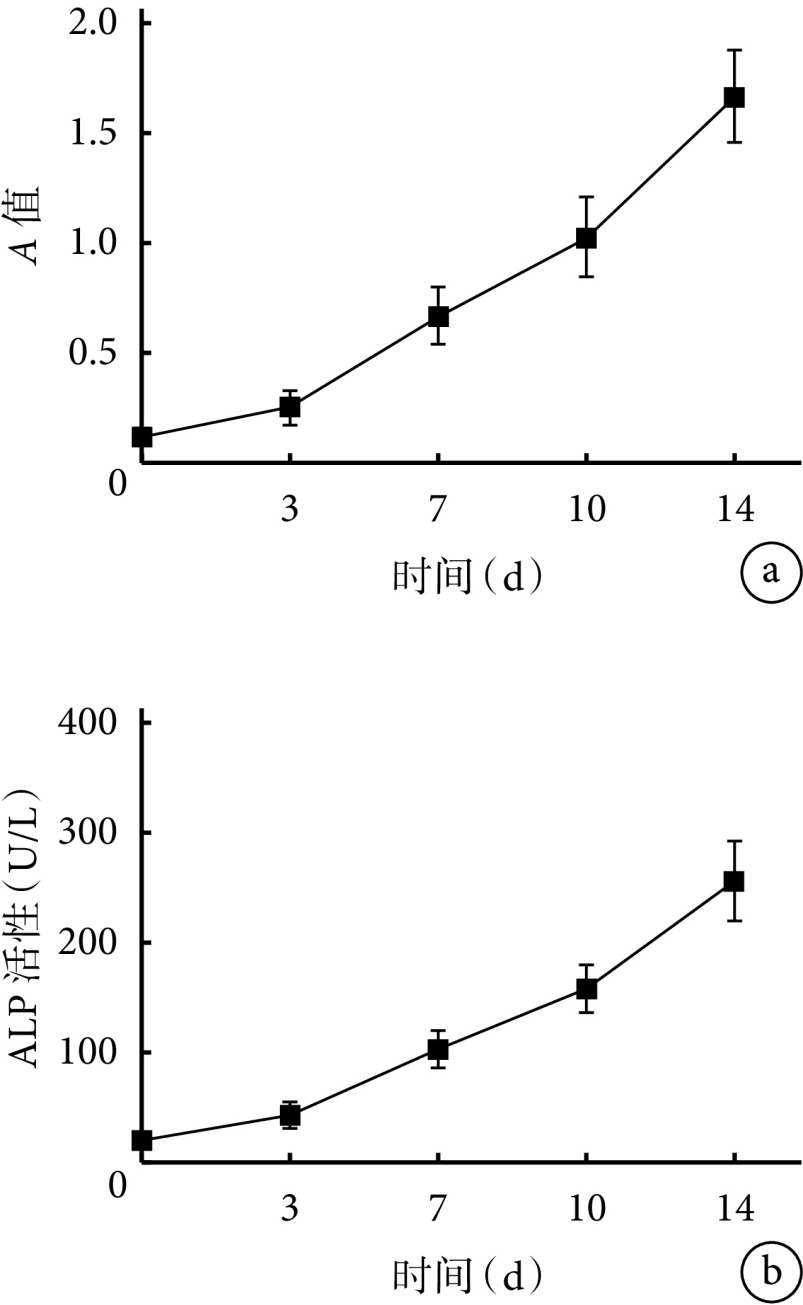
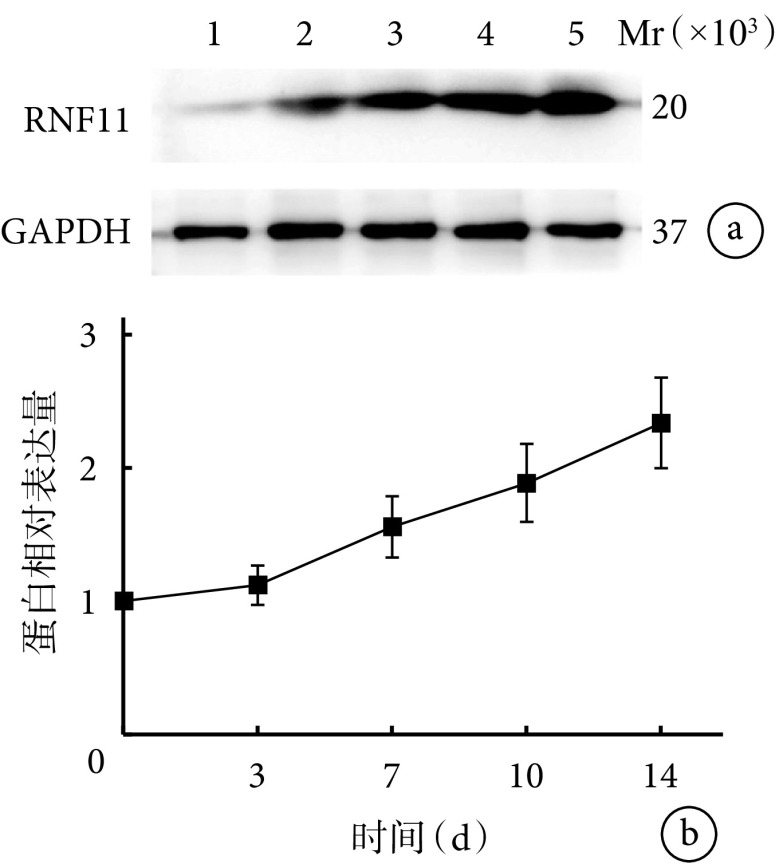
随BMSCs成骨诱导分化时间延长,茜素红染色阳性钙结节数量和ALP活性均逐渐增加,成熟程度逐渐上升,各时间点间差异均有统计学意义(P<0.05),符合MSCs成骨的一般规律。见图3、4。Western blot检测示,随BMSCs成骨诱导分化时间延长,RNF11蛋白相对表达量亦呈逐渐增加趋势,各时间点间差异均有统计学意义(P<0.05),提示RNF11可能参与了BMSCs成骨分化过程。见图5。
图 3.
Observation on the osteogenic differentiation regularity of BMSCs (×40)
BMSCs成骨分化规律观察(×40)
从左至右分别为培养0、3、7、10、14 d a. 茜素红染色;b. ALP染色
From left to right for cultured 0, 3, 7, 10, and 14 days a. Alizarin red staining; b. ALP staining
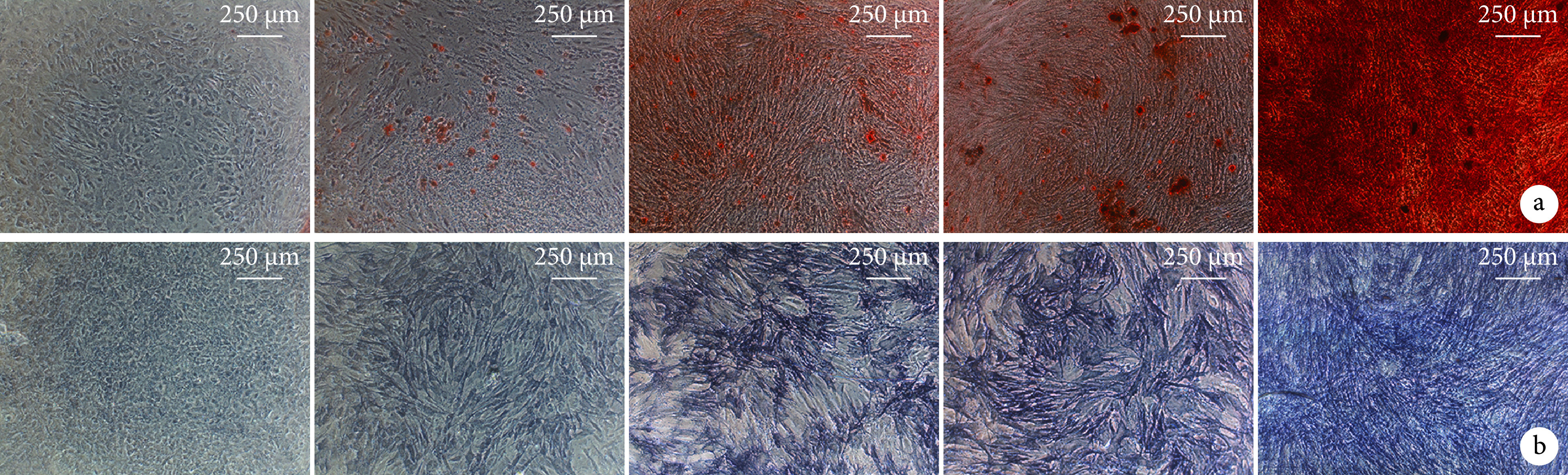
图 4.
Quantitative detection of BMSCs osteogenesis
BMSCs成骨分化定量检测
a. 茜素红染色;b. ALP活性
a. Alizarin red staining; b. ALP activity
图 5.
Expression of RNF11 during BMSCs osteogenesis detected by Western blot
Western blot检测RNF11在BMSCs成骨分化过程中的表达
a. 电泳图 Mr:相对分子质量 1:0 d 2:3 d 3:7 d 4:10 d 5:14 d;b. RNF11蛋白相对表达量
a. Electrophoresis Mr: Relative molecular weight 1: 0 day 2: 3 days 3: 7 days 4: 10 days 5: 14 days; b. RNF11 protein relative expression
2.3. 下调RNF11对BMSCs体外成骨分化能力的影响
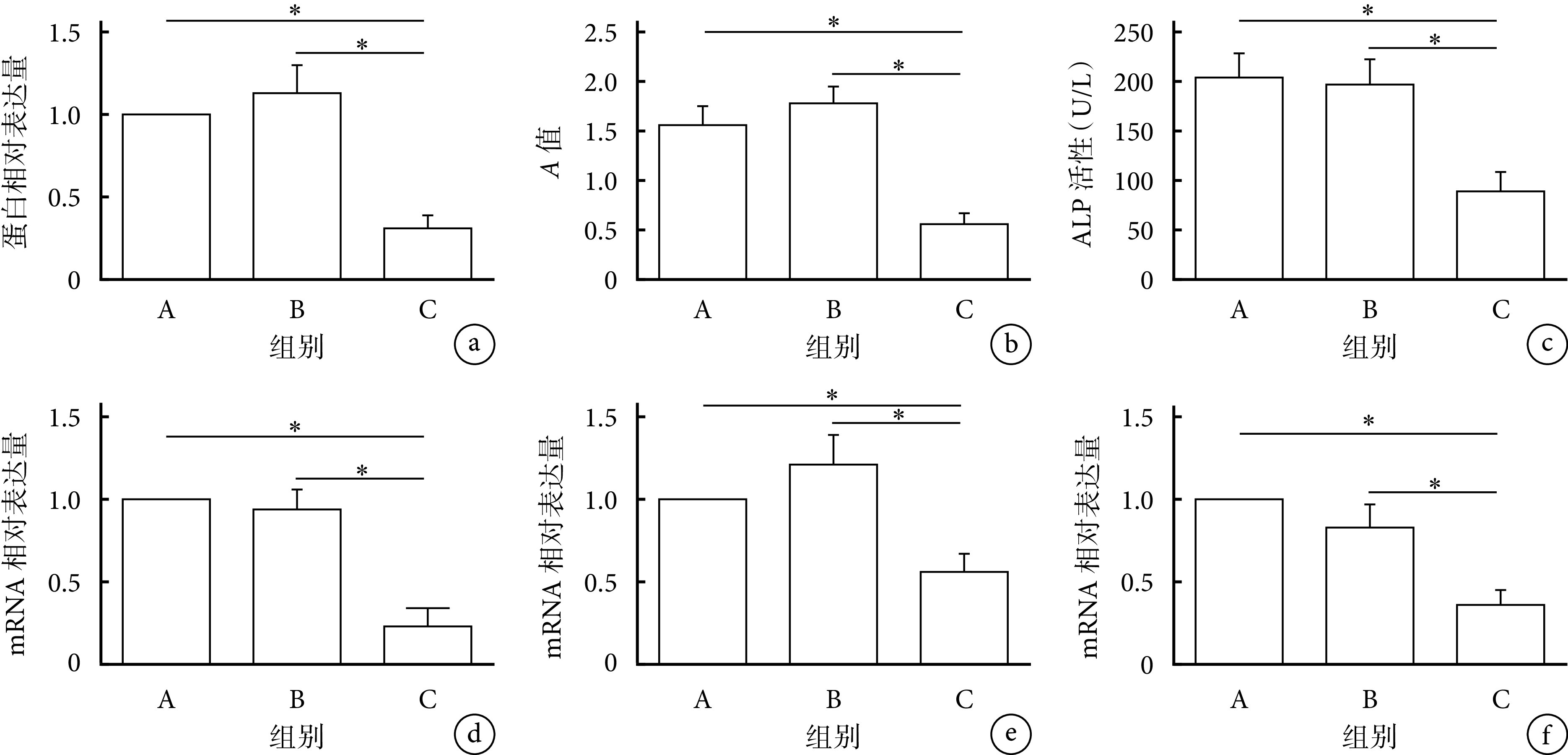
各组成骨诱导分化14 d检测示,C组RNF11蛋白相对表达量、阳性钙结节数量、ALP活性及Runx2、OCN、OPN mRNA相对表达量均显著低于A、B组,差异均有统计学意义(P<0.05)。提示下调BMSCs中RNF11表达将抑制其成骨分化能力。见图6、7。
图 6.
The influence of knockdown RNF11 on osteogenic differentiation of BMSCsin vitro
下调RNF11对BMSCs体外成骨分化能力的影响
a. Western blot检测电泳图 Mr:相对分子质量 1:A组 2:B组 3:C组;b. 茜素红染色(×40) 从左至右依次为A、B、C组;c. ALP染色(×40) 从左至右依次为A、B、C组
a. Electrophoresis of Western blot Mr: Relative molecular weight 1: Group A 2: Group B 3: Group C; b. Alizarin red staining (×40) From left to right for groups A, B, and C, respectively; c. ALP staining (×40) From left to right for groups A, B, and C, respectively
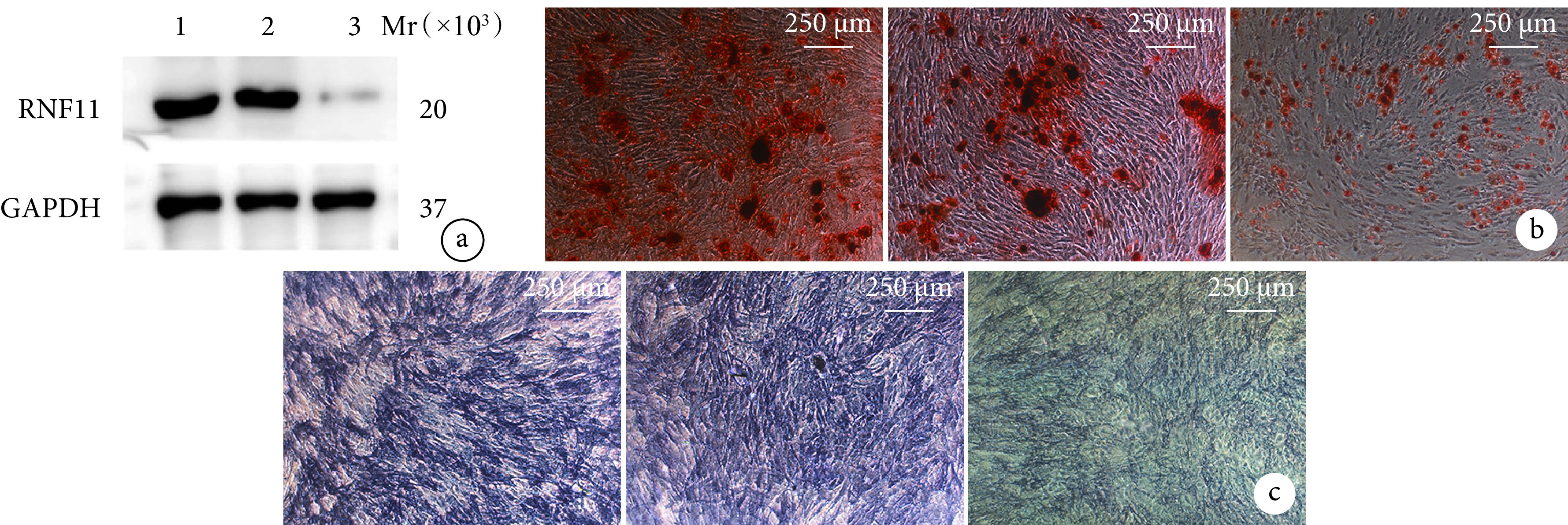
图 7.
Quantitative analysis of the influence of knockdown RNF11 on osteogenic differentiation of BMSCsin vitro
下调RNF11对BMSCs体外成骨分化能力影响定量分析
*P<0.05 a. RNF11蛋白相对表达量;b. 茜素红染色;c. ALP活性;d~f. qRT-PCR检测Runx2、OCN、OPN mRNA相对表达量
*P<0.05 a. RNF11 protein relative expression; b. Alizarin red staining; c. ALP activity; d-f. Relative expressions of Runx2, OCN, and OPN mRNA detected by qRT-PCR
2.4. RNF11对Akt信号通路的影响
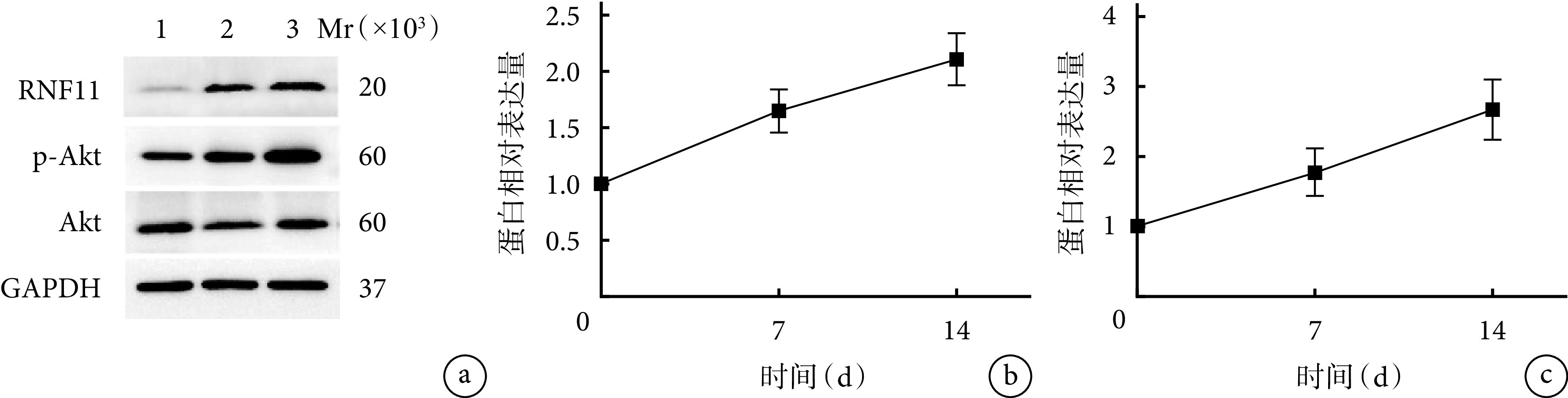
随BMSCs成骨诱导分化时间延长,RNF11与Akt信号通路蛋白相对表达量均逐渐增加,各时间点间差异有统计学意义(P<0.05)。见图8。
图 8.
Western blot detection of RNF11 and Akt signaling pathway protein expressions before and after osteogenic differentiation
Western blot检测成骨分化前后RNF11与Akt信号通路蛋白表达
a. 电泳图 Mr:相对分子质量 1:0 d 2:7 d 3:14 d;b. RNF11蛋白相对表达量;c. Akt信号通路蛋白相对表达量
a. Electrophoresis Mr: Relative molecular weight 1: 0 day 2: 7 days 3: 14 days; b. RNF11 protein relative expression; c. Relative protein expression of Akt signaling pathway
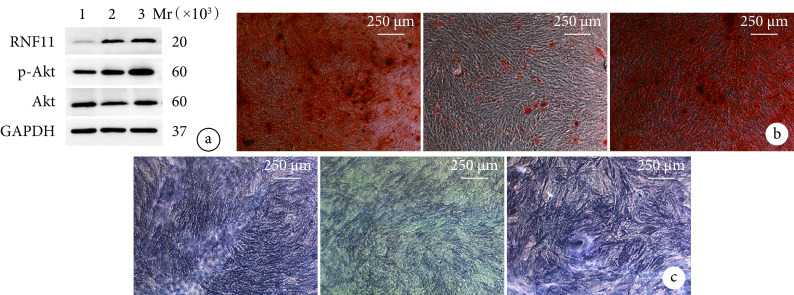
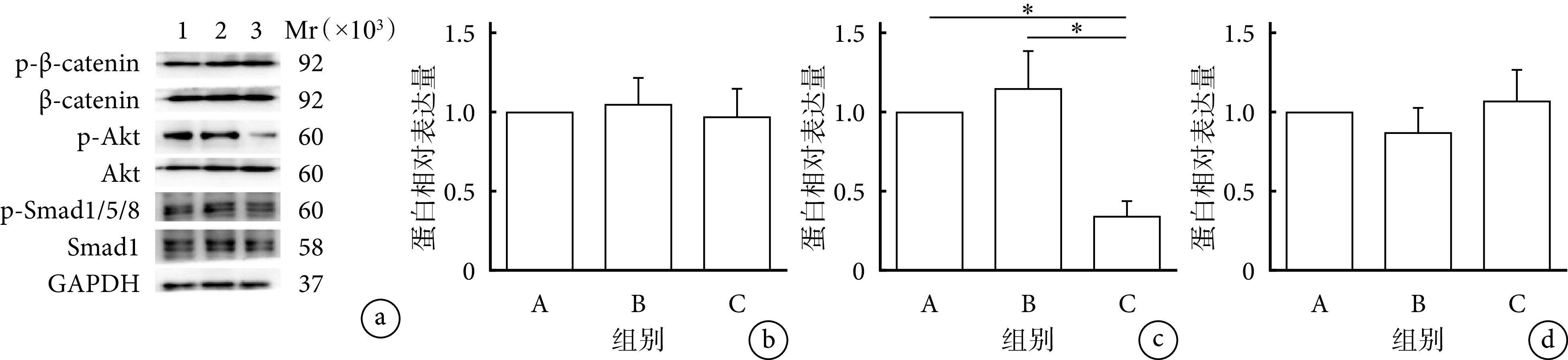
下调RNF11实验中,各组β-catenin及Smad1/5/8信号通路蛋白相对表达量差异均无统计学意义(P>0.05);C组Akt信号通路蛋白相对表达量显著低于A、B组,差异有统计学意义(P<0.05)。见图9。提示RNF11可能是通过影响Akt信号通路激活来参与BMSCs的体外成骨分化过程。
图 9.
The influence of knockdown RNF11 on Akt signaling pathway
下调RNF11对Akt信号通路的影响
a. Western blot检测电泳图 Mr:相对分子质量 1:A组 2:B组 3:C组;b~d. 分别为β-catenin、Akt、Smad1/5/8信号通路蛋白相对表达量 *P<0.05
a. Electrophoresis of Western blot Mr: Relative molecular weight 1: Group A 2: Group B 3: Group C; b-d. Protein relative expressions of β-catenin, Akt, and Smad1/5/8 signaling pathway, respectively*P<0.05
2.5. RNF11对Akt信号通路的影响机制
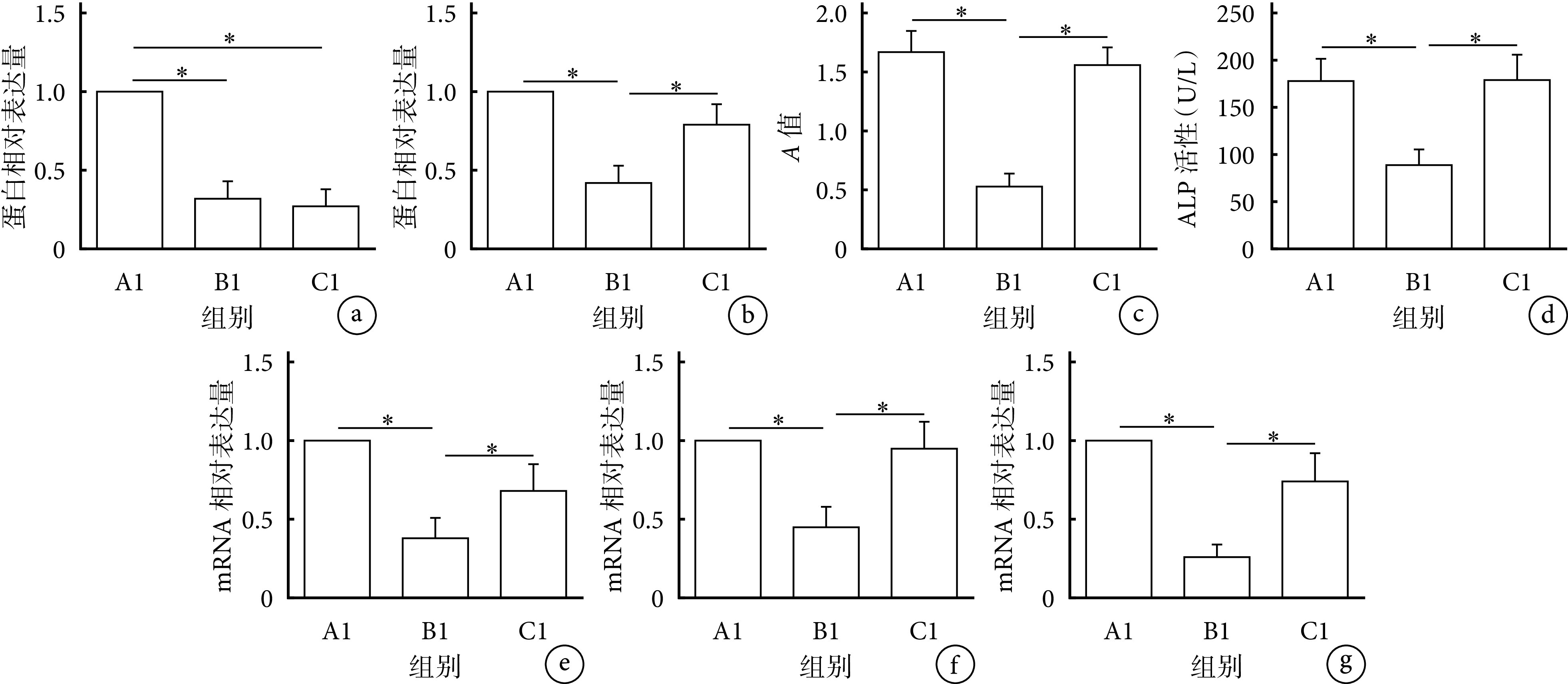
B1、C1组RNF11蛋白相对表达量显著低于A1组,差异有统计学意义(P<0.05);B1、C1组间差异无统计学意义(P>0.05)。与A1、C1组比较,B1组Akt信号通路蛋白相对表达量、阳性钙结节数量、ALP活性及成骨标志物Runx2、OCN、OPN mRNA相对表达量均显著下调,差异有统计学意义(P<0.05);添加了SC79的C1组可逆转上述效果,与A1组比较上述指标差异均无统计学意义(P>0.05)。见图10、11。提示RNF11可能通过激活Akt信号通路促进BMSCs成骨分化。
图 10.
The influence of activating Akt signaling pathway on osteogen
激活Akt信号通路对下调RNF11后BMSCs成骨分化的影响
a. Western blot检测电泳图 Mr:相对分子质量 1:A1组 2:B1组 3:C1组;b. 茜素红染色(×40) 从左至右依次为A1、B1、C1组;c. ALP染色(×40) 从左至右依次为A1、B1、C1组
a. Electrophoresis of Western blot Mr: Relative molecular weight 1: Group A1 2: Group B1 3: Group C1; b. Alizarin red staining (×40) From left to right for groups A1, B1, and C1, respectively; c. ALP staining (×40) From left to right for groups A1, B1, and C1, respectively
图 11.
Quantitative analysis of the influence of activating Akt signaling pathway on osteogenic differentiation of BMSCs after knockdown RNF11
激活Akt信号通路对下调RNF11后BMSCs成骨分化影响的定量分析
*P<0.05 a. RNF11蛋白相对表达量;b. Akt信号通路蛋白相对表达量;c. 茜素红染色;d. ALP活性;e~g. qRT-PCR检测Runx2、OCN、OPN mRNA相对表达量
*P<0.05 a. Relative protein expression of RNF11; b. Relative protein expression of Akt signaling pathway; c. Alizarin red staining; d. ALP activity; e-g. Relative expressions of Runx2, OCN, and OPN mRNA detected by qRT-PCR
3. 讨论
组织工程骨技术修复骨缺损虽然目前仍在研究阶段,但有极大临床应用前景。BMSCs作为组织工程骨的三大要素之一,研究其成骨分化过程的调控因子和机制一直是此领域的热点。
RNF11是环指蛋白家族中的重要成员之一,通过PY基序结合HECT型E3泛素连接酶Smurf2和AIP4,介导多种细胞蛋白的降解[21]。尽管已有研究表明RNF11减少或功能受损可能与神经退行性疾病有关[22-23],但与BMSCs成骨分化相关研究较少。Gao等[20]在研究成骨机制过程中发现,在小鼠成骨发育的体外模型中,胚胎成骨过程中最早阶段表达的转录因子Est1能够结合RNF11的启动子,且RNF11水平与成骨分化程度成正相关,但并未深入研究。本研究中,我们发现RNF11在BMSCs成骨分化过程中表达明显上调,且与之成正相关;以慢病毒构建敲低RNF11的BMSCs模型,茜素红、ALP染色示C组成骨分化程度低于A、B组,提示RNF11参与BMSCs成骨分化过程,与Gao等的研究结果一致。
为进一步探究RNF11如何影响BMSCs成骨分化,分别检测A、B、C组与BMSCs成骨分化密切相关的Akt、Smad1/5/8、β-catenin信号通路的激活水平[24]。Western blot结果显示,相较于A、B组,C组的Akt信号通路显著受抑制,而对Smad1/5/8和β-catenin信号通路激活水平无明显影响,且RNF11蛋白表达和Akt信号通路激活水平均与BMSCs成骨分化程度成正相关。提示在此过程中两者可能共同发挥作用。为探究两者的关系,我们在实验中添加Akt信号通路激活剂SC79[25],茜素红、ALP染色以及对成骨标志物Runx2、OCN及OPN mRNA的定量分析结果显示,C1组成骨分化水平略低于A1组,但明显高于B1组。分析原因可能是RNF11在BMSCs分化过程中通过激活Akt信号通路表达来促进成骨分化,成骨细胞合成大量Runx2、OCN及OPN,因此下调RNF11时,Akt信号通路激活受抑制,成骨分化水平降低;添加SC79使Akt信号通路激活水平升高后,成骨分化水平重新升高,但激活不完全,因而略低于A1组。
RNF11在机体还具有抑制炎症反应的作用。据报道,由A20-TAX1BP1-Itch-RNF11共同构成的A20泛素编辑酶复合物能够下调NF-κB信号通路,确保炎症反应的短暂性,其中RNF11的PPXY基序负责招募Itch到TAX1BP1形成A20泛素编辑酶复合物[26]。由此推断,若将RNF11运用到BMSCs对骨缺损的临床治疗中,可能有一定抗炎作用,能减轻机体损伤,减少其他抗炎药物的使用,但有待进一步实验研究明确。
近年有很多关于Akt信号通路调控成骨分化的分子机制研究。Zhao等[27]研究显示巨噬细胞MSR1通过激活Akt信号通路促进BMSCs成骨分化,Yang等[28]研究表明miRNA-21通过激活Akt信号通路促进BMSCs成骨分化来促进犬颌面骨再生。提示Akt信号通路调控成骨分化受到多种因素的共同作用,分子间相互协同,共同完成骨修复过程。
综上述,本研究结果表明RNF11通过正向调控Akt信号通路激活水平促进BMSCs向成骨细胞分化。但本研究也存在不足之处:首先,实验采用离体培养的BMSCs,未在动物体内验证,可能会造成实验结果偏倚,也无法验证将其运用于机体时是否会对其他系统产生影响。第二,未探究在病理条件下RNF11对BMSCs的成骨分化作用是否会受影响。第三,未探究RNF11在BMSCs成骨分化其他重要过程中的作用,如破骨细胞形成、炎症等。以上不足有待进一步研究完善。
作者贡献:邓雯负责研究设计、数据收集整理、统计分析;龙婷负责论文撰写;杜英负责对文章的知识性内容作批评性审阅。
利益冲突:所有作者声明,在课题研究和文章撰写过程中不存在利益冲突。
机构伦理问题:研究方案经郑州大学生命科学伦理审查委员会批准。
References
- 1.Oryan A, Kamali A, Moshiri A, et al. Role of mesenchymal stem cells in bone regenerative medicine: What is the evidence? Cells Tissues Organs, 2017, 204(2): 59-83.
- 2.Aicher WK, Bühring HJ, Hart M, et al Regeneration of cartilage and bone by defined subsets of mesenchymal stromal cells-potential and pitfalls. Adv Drug Deliv Rev. 2011;63(4-5):342–351. doi: 10.1016/j.addr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Marie PJ, Fromigué O Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med. 2006;1(4):539–548. doi: 10.2217/17460751.1.4.539. [DOI] [PubMed] [Google Scholar]
- 4.Shih YR, Chen CN, Tsai SW, et al Growth of mesenchymal stem cells on electrospun type Ⅰ collagen nanofibers. Stem Cells. 2006;24(11):2391–2397. doi: 10.1634/stemcells.2006-0253. [DOI] [PubMed] [Google Scholar]
- 5.Bruder SP, Kraus KH, Goldberg VM, et al The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg (Am) 1998;80(7):985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 6.袁宇, 徐林 骨髓间充质干细胞联合3D生物打印技术治疗骨缺损的研究进展. 中国医学物理学杂志. 2021;38(1):110–126. doi: 10.3969/j.issn.1005-202X.2021.01.019. [DOI] [Google Scholar]
- 7.Tse WT, Pendleton JD, Beyer WM, et al Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama M, Moeinzadeh S, Guzman RA, et al. The efficacy of lapine preconditioned or genetically modified IL4 over-expressing bone marrow-derived mesenchymal stromal cells in corticosteroid-associated osteonecrosis of the femoral head in rabbits. Biomaterials, 2021, 275: 120972. doi: 10.1016/j.biomaterials.2021.120972.
- 9.Maruyama M, Rhee C, Utsunomiya T, et al. Modulation of the inflammatory response and bone healing. Front Endocrinol (Lausanne), 2020, 11: 386. doi: 10.3389/fendo.2020.00386.
- 10.Fujita T, Azuma Y, Fukuyama R, et al Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166(1):85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.史东梅, 董明, 陆颖, 等. PI3K/Akt信号通路与骨破坏: 问题与机制. 中国组织工程研究, 2020, 24(23): 3716-3722.
- 12.Peng XD, Xu PZ, Chen ML, et al Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitching R, Wong MJ, Koehler D, et al The RING-H2 protein RNF11 is differentially expressed in breast tumours and interacts with HECT-type E3 ligases. Biochim Biophys Acta. 2003;1639(2):104–112. doi: 10.1016/j.bbadis.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Azmi P, Seth A RNF11 is a multifunctional modulator of growth factor receptor signalling and transcriptional regulation. Eur J Cancer. 2005;41(16):2549–2560. doi: 10.1016/j.ejca.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Connor MK, Azmi PB, Subramaniam V, et al Molecular characterization of ring finger protein 11. Mol Cancer Res. 2005;3(8):453–461. doi: 10.1158/1541-7786.MCR-04-0166. [DOI] [PubMed] [Google Scholar]
- 16.Mattioni A, Castagnoli L, Santonico E. RNF11 at the crossroads of protein ubiquitination. Biomolecules, 2020, 10(11): 1538. doi: 10.3390/biom10111538.
- 17.Santonico E, Belleudi F, Panni S, et al Multiple modification and protein interaction signals drive the Ring finger protein 11 (RNF11) E3 ligase to the endosomal compartment. Oncogene. 2010;29(41):5604–5618. doi: 10.1038/onc.2010.294. [DOI] [PubMed] [Google Scholar]
- 18.Malonis RJ, Fu W, Jelcic MJ, et al RNF11 sequestration of the E3 ligase SMURF2 on membranes antagonizes SMAD7 down-regulation of transforming growth factor β signaling. J Biol Chem. 2017;292(18):7435–7451. doi: 10.1074/jbc.M117.783662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budhidarmo R, Zhu J, Middleton AJ, et al The RING domain of RING Finger 11 (RNF11) protein binds Ubc13 and inhibits formation of polyubiquitin chains. FEBS Lett. 2018;592(8):1434–1444. doi: 10.1002/1873-3468.13029. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Ganss BW, Wang H, et al The RING finger protein RNF11 is expressed in bone cells during osteogenesis and is regulated by Ets1. Exp Cell Res. 2005;304(1):127–135. doi: 10.1016/j.yexcr.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Subramaniam V, Li H, Wong M, et al The RING-H2 protein RNF11 is overexpressed in breast cancer and is a target of Smurf2 E3 ligase. Br J Cancer. 2003;89(8):1538–1544. doi: 10.1038/sj.bjc.6601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pranski EL, Dalal NV, Herskowitz JH, et al. Neuronal RING finger protein 11 (RNF11) regulates canonical NF-κB signaling. J Neuroinflammation, 2012, 9: 67. doi: 10.1186/1742-2094-9-67.
- 23.Pranski EL, Dalal NV, Sanford CV, et al RING finger protein 11 (RNF11) modulates susceptibility to 6-OHDA-induced nigral degeneration and behavioral deficits through NF-κB signaling in dopaminergic cells. Neurobiol Dis. 2013;54:264–279. doi: 10.1016/j.nbd.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.池玉磊, 卜宪敏, 查玉梅, 等 骨髓间充质干细胞复合支架材料治疗骨缺损: 研究现状及前景展望. 中国组织工程研究. 2019;23(29):4749–4756. doi: 10.3969/j.issn.2095-4344.1819. [DOI] [Google Scholar]
- 25.Jo H, Mondal S, Tan D, et al Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc Natl Acad Sci U S A. 2012;109(26):10581–10586. doi: 10.1073/pnas.1202810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shembade N, Parvatiyar K, Harhaj NS, et al The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling. EMBO J. 2009;28(5):513–522. doi: 10.1038/emboj.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao SJ, Kong FQ, Jie J, et al Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics. 2020;10(1):17–35. doi: 10.7150/thno.36930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C, Liu X, Zhao K, et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res Ther, 2019, 10(1): 65. doi: 10.1186/s13287-019-1168-2.