Abstract
Introduction
Trifluridine/tipiracil (FTD/TPI) improved both overall and progression-free survival (OS, PFS) of patients with pre-treated metastatic colorectal cancer (mCRC) in the pivotal phase III RECOURSE trial. However, health-related quality of life (HRQoL) was not assessed directly. To this end and to generate post-authorisation data, the TALLISUR trial was conducted.
Methods
In this prospective, multi-centre, Germany-wide, phase IV study, patients with pre-treated mCRC were given the choice to receive either FTD/TPI or best supportive care (BSC). A validated questionnaire, EORTC QLQ-C30, was employed to assess HRQoL. Secondary endpoints included OS, PFS and safety.
Results
Of 194 eligible patients, 185 decided to receive FTD/TPI and 9 to receive BSC. The low number of patients in the BSC-arm did not allow statistically meaningful analyses. On the other hand, treatment with FTD/TPI was associated with maintained HRQoL. Median OS was 6.9 months [95% confidence interval (CI) 6.1-8.2 months] and median PFS was 2.5 months (95% CI 2.1-2.9 months). The most frequent treatment-emergent adverse events were neutropenia (27.6%) and anaemia (22.7%). Febrile neutropenia occurred in 1.1%.
Conclusions
Treatment of patients suffering from pre-treated mCRC with FTD/TPI was associated not only with prolonged survival and delayed progression but also with maintained HRQoL.
Key words: metastatic colorectal cancer, phase IV, trifluridine/tipiracil
Highlights
-
•
First patient-reported HRQoL and post-authorisation efficacy and safety data from German mCRC patients receiving FTD/TPI.
-
•
Results were consistent with results of pivotal phase III and other studies.
-
•
FTD/TPI prolonged survival and delayed progression with manageable toxicity profile.
-
•
Treatment with FTD/TPI was associated with maintained HRQoL.
Introduction
With an estimated over 1.8 million new cases and 881 000 deaths annually, colorectal cancer (CRC) is the third most common and the second deadliest malignancy worldwide.1 In Germany, nearly 60 000 new cases of CRC are diagnosed every year.2
Over the past two decades, combination of chemotherapies with monoclonal antibodies has increased the survival of patients with metastatic CRC (mCRC).3, 4, 5 However, patients with mCRC who are refractory or intolerant to all approved drugs have an unmet medical need.6
Trifluridine/tipiracil (FTD/TPI) is an orally administered anti-neoplastic agent consisting of FTD, a thymidine-based nucleoside analogue, and TPI, a thymidine phosphorylase inhibitor, which improves the bioavailability of FTD.7
In April 2016, FTD/TPI was approved by the European Medicines Agency as monotherapy for the treatment of adult patients with mCRC who have been previously treated with or are not considered candidates for available therapies including fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-vascular endothelial growth factor (VEGF) and -epidermal growth factor receptor (EGFR) agents.8
Approval was granted based on results of the pivotal phase III RECOURSE trial.9,10 Here, 800 patients with pre-treated mCRC were randomised (2 : 1) to receive either FTD/TPI or placebo plus best supportive care (BSC). Compared to placebo treatment, FTD/TPI was shown to significantly improve both overall survival [OS; 7.2 versus 5.2 months; hazard ratio (HR) 0.69, P < 0.0001] and progression-free survival (PFS; 2.0 versus 1.7 months; HR 0.48, P < 0.001) while maintaining a manageable toxicity profile, with neutropenia (38%) and leukopenia (21%) being the most frequently observed clinically significant adverse events. Notably, merely 4% of patients receiving FTD/TPI suffered from febrile neutropenia. Although time to deterioration of the Eastern Cooperative Oncology Group performance status (ECOG PS) from 0/1 to ≥2 was significantly longer in patients treated with FTD/TPI (5.7 versus 4.0 months; HR 0.66, P < 0.001), health-related quality of life (HRQoL) was not formally assessed by direct means.
Ideally, however, novel therapies for late-stage cancer patients bring in-line efficacy, safety and HRQoL. Therefore, assessment of their potential on all three levels is paramount and preferably confirmed by post-authorisation studies.
In order to obtain post-authorisation efficacy and safety data and to assess HRQoL following FTD/TPI treatment, the prospective, interventional, multi-centre, Germany-wide, open-label, non-randomised, phase IV TALLISUR (Trifluridine/tipirAcil quaLity of LIfe StUdy in mCRC patients) study was conducted.
Upon informed consent, patients with pre-treated mCRC were given the freedom of choice to receive either FTD/TPI according to the summary of product characteristics (SmPC) or BSC without any anti-neoplastic treatment. The primary objective of this study was to evaluate the effect of treatment with FTD/TPI on HRQoL. Here, we present patient-reported HRQoL as well as post-authorisation efficacy and safety data.
Methods
Study design, patients and treatment
The TALLISUR study (EudraCT-Number 2017-000292-83) was designed according to the explicit request by the German Federal Joint Committee (Gemeinsamer Bundesausschuss) to generate HRQoL data required for the health technology assessment.
In total, 202 patients (≥18 years old) suffering from mCRC [histologically or cytologically confirmed union internationale contre le cancer (UICC) stage IV carcinoma of colon or rectum] were enrolled of which 194 entered the trial. Assessment of the RAS and BRAF status was not obligatory. For 76 patients (39.2%) a RAS wild-type status and for 104 patients (53.6%) a RAS mutant status have been determined. The BRAF status remains unknown for most patients (127, 65.5%). Tumours of 66 patients (34.0%) carried BRAF wild-type alleles and the tumour of one patient carried a BRAF V600E mutant allele (0.5%). Patients were eligible who had previously been treated with or were not considered candidates for available therapies including fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-VEGF and -EGFR agents. The ECOG PS was not an exclusion criteria.
After prior informed consent, each patient and the respective treating physician mutually decided to opt either for FTD/TPI according to SmPC or for BSC without any anti-neoplastic treatment. FTD/TPI was administered orally twice daily on days 1 to 5 and days 8 to 12 of each 28-day cycle as long as a benefit was observed or until unacceptable toxicity occurred. Calculated based on body surface area, the dose was 35 mg/m2 given twice daily and was not allowed to exceed 80 mg per dose. Depending on individual safety and tolerability, a maximum of three dose reduction levels (30, 25 and 20 mg/m2) were permitted. In the event of toxicities, the dose interruption, resumption and reduction criteria as stated in the SmPC were followed.
In the BSC group, each observation cycle was 28 days in order to be comparable to the FTD/TPI group. Close observation was carried out until radiological or clinical progression or until a patient received any anti-tumour therapy.
Patient-reported HRQoL
A validated and widely accepted questionnaire was employed to assess HRQoL: European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 Version 3.0.11, 12, 13 Questionnaires were scheduled to be filled in within 2 days before or on the first day of any treatment/observation cycle and afterwards, during follow-up, monthly from month 1 until month 6, after month 9 and after month 12 for a maximum of 1 year after the start of treatment/observation. In order to obtain additional information, an extended time period was analysed including questionnaires being filled in between the day after the last administration (FTD/TPI group)/day 12 (BSC group) of the previous cycle and the first day of the respective cycle.
The primary endpoint was pre-defined as the rate of responders with stabilised (≥10 and <10 scores) or improved (≥10 scores) HRQoL response (HRQoL-RR). Response was calculated as the mean score of the EORTC QLQ-C30 global health status/QoL scale from the second cycle until the end of treatment/observation compared to the baseline score. A HRQoL-RR of 45% ± 10% for the FTD/TPI group and 45% ± 20% for the BSC group was considered statistically acceptable. The study was considered positive, if the lower boundary of the two-sided 95% confidence interval (CI) was ≥35% and ≥25%, respectively.
To be considered assessable for the primary endpoint, a patient must have filled in at least the baseline and one additional questionnaire. Additionally, patients in the FTD/TPI group must have received at least two cycles of treatment administered on at least 5 days of the second cycle.
Efficacy
Tumour response was assessed by imaging procedures (magnetic resonance imaging or computed tomography scan) and evaluated according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.14 It was anticipated that imaging procedures to evaluate tumour response would not be carried out in many patients receiving BSC. Therefore, the date of clinical disease progression was permitted for computing PFS.
OS was defined as the duration from first administration of FTD/TPI or day 1 of the first observation cycle to the day of death by any cause.
PFS was defined as the duration from the first administration of FTD/TPI or day 1 of the first observation cycle to the day of radiological or clinical tumour progression or death by any cause, whichever came first.
Safety
Treatment-emergent adverse events (TEAEs) were classified into defined categories of severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Adverse events had to be documented from the first administration/from day 1 of the first observation cycle until 28 days after the last administration/until the end of close observation. Grade 5 TEAEs are summarised in Table 2.
Table 2.
Treatment-emergent adverse events
| FTD/TPI |
BSC |
|||||||
|---|---|---|---|---|---|---|---|---|
|
N = 185 |
N = 9 |
|||||||
| Any grade |
Grade ≥3 |
Any grade |
Grade ≥3 |
|||||
| AE | n (%) | AE | n (%) | AE | n (%) | AE | n (%) | |
| Any | 1 069 | 177 (95.7) | 297 | 122 (65.9) | 29 | 7 (77.8) | 12 | 6 (66.7) |
| Blood and lymphatic system disorders | ||||||||
| Neutropenia | 105 | 51 (27.6) | 61 | 34 (18.4) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Leukopenia | 77 | 35 (18.9) | 32 | 15 (8.1) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Anaemia | 57 | 42 (22.7) | 16 | 13 (7.0) | 1 | 1 (11.1) | 1 | 1 (11.1) |
| Gastrointestinal disorders | ||||||||
| Diarrhoea | 45 | 38 (20.5) | 9 | 8 (4.3) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Abdominal pain | 12 | 12 (6.5) | 3 | 3 (1.6) | 2 | 1 (11.1) | 1 | 1 (11.1) |
| Ascites | 16 | 10 (5.4) | 6 | 3 (1.6) | 1 | 1 (11.1) | 1 | 1 (11.1) |
| Vomiting | 32 | 25 (13.5) | 2 | 2 (1.1) | 1 | 1 (11.1) | 0 | 0 (0.0) |
| Nausea | 46 | 40 (21.6) | 1 | 1 (0.5) | 1 | 1 (11.1) | 0 | 0 (0.0) |
| Constipation | 21 | 20 (10.8) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| General disorders and administration site conditions | ||||||||
| Pain | 10 | 9 (4.9) | 5 | 5 (2.7) | 2 | 2 (22.2) | 0 | 0 (0.0) |
| Fatigue | 52 | 40 (21.6) | 4 | 4 (2.2) | 3 | 2 (22.2) | 1 | 1 (11.1) |
| General physical health deterioration | 10 | 9 (4.9) | 4 | 4 (2.2) | 1 | 1 (11.1) | 0 | 0 (0.0) |
| Oedema | 21 | 18 (9.7) | 2 | 2 (1.1) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Pyrexia | 19 | 16 (8.6) | 1 | 1 (0.5) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Infections and infestations | ||||||||
| Infection | 11 | 9 (4.9) | 5 | 5 (2.7) | 1 | 1 (11.1) | 1 | 1 (11.1) |
| Metabolism and nutrition disorders | ||||||||
| Decreased appetite | 29 | 26 (14.1) | 4 | 3 (1.6) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | ||||||||
| Neoplasm progression | 25 | 25 (13.5) | 21 | 21 (11.4) | 1 | 1 (11.1) | 1 | 1 (11.1) |
| Nervous system disorders | ||||||||
| Polyneuropathy | 10 | 10 (5.4) | 1 | 1 (0.5) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Respiratory, thoracic and mediastinal disorders | ||||||||
| Dyspnoea | 22 | 19 (10.3) | 1 | 1 (0.5) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Skin and subcutaneous tissue disorders | ||||||||
| Alopecia | 12 | 12 (6.5) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) |
AE, adverse event; BSC, best supportive care; FTD/TPI, trifluridine/tipiracil.
Statistical analyses
Time-to-event data were analysed according to the Kaplan–Meier analysis (product-limit method). Patients who had not reached the endpoint by the time of the analyses were censored at the last date at which it was known that they had been event free.
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and with the International Council on Harmonisation guideline for good clinical practice. The protocol was approved by the ethics committee of the Medical Faculty of the Ludwig-Maximilians-University, Munich, (reference number: 17-429). All patients provided written informed consent prior to entering the trial.
Results
Patient enrolment and demographics
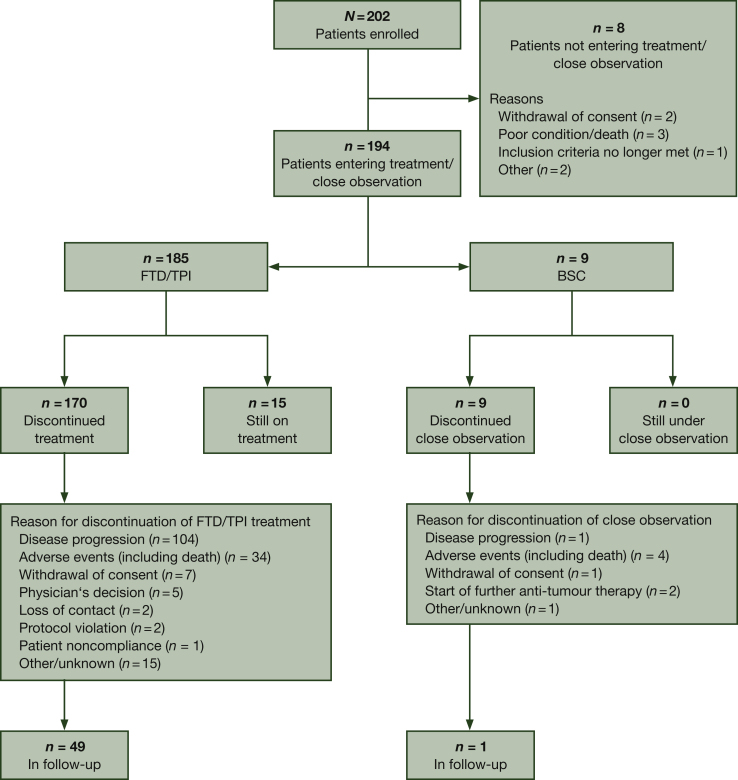
Between 22 September 2017 (first patient included) and 08 January 2019 (last patient included), 194 patients entered the trial across 44 study centres in Germany (Figure 1). Due to the inherent nature of the trial design, both study arms were utterly imbalanced with respect to the number of patients (185 in the FTD/TPI group versus 9 in the BSC group). Therefore, it was not possible to conduct any statistically meaningful analyses using data obtained from patients receiving BSC.
Figure 1.
Trial profile.
BSC, best supportive care; FTD, trifluridine; TPI, tipiracil.
By the data cut-off date of this analysis (26 August 2019), 92.3% of the included patients had discontinued treatment (170 patients) or close observation (9 patients). Of them, 50 patients (25.8%) were in the follow-up after treatment (49 patients) or after close observation (1 patient). In contrast, 15 patients (7.7%) were still under FTD/TPI treatment. The most common reasons for discontinuation were disease progression (105 patients, 54.1%), adverse events including death (38 patients, 19.6%) and withdrawal of consent (8 patients, 4.1%).
The median duration of FTD/TPI treatment was 2.24 months (range 1-537 days) and the median number of cycles was 3 (range 1-18 cycles). The median cumulative FTD/TPI dose administered was 657.6 mg/m2 (range 32.5-743.9 mg/m2) and the median relative dose intensity was 97.6% (range: 4.7%-110.0%). Throughout the study, 68 patients (36.8%) had at least one cycle with a dose reduction to <90% of the target dose. During the treatment period, 12 patients (6.5%) were given at least one dose of granulocyte colony-stimulating factor.
Baseline characteristics and demographics are shown in Table 1. Enrolled patients were median 67 or 78 years old and 63.2% or 55.6% male in the FTD/TPI and BSC groups, respectively. 89.2% of patients in the FTD/TPI group and 44.4% of patients in the BSC group presented an ECOG PS of 0-1. In particular, no patient in the BSC group had an ECOG PS of 0. All patients presented a UICC stage IV metastatic carcinoma of the colon or the rectum. Primary tumours were located more often on the left than on the right side of the colon (73.0% versus 22.7% in the FTD/TPI and 66.7% versus 22.2% in the BSC group). Most patients received FTD/TPI as a third-(38.4%) or fourth- (28.6%) line therapy option.
Table 1.
Patient baseline characteristics and demographics
| Demographic variable | FTD/TPI |
BSC |
|---|---|---|
| N = 185 | N = 9 | |
| Age, years | ||
| Arithmetic mean | 66.2 | 71.4 |
| SD | 9.8 | 11.0 |
| Median | 67.0 | 78.0 |
| Range | 40.0-88.0 | 54.0-82.0 |
| Sex, n (%) | ||
| Men | 117 (63.2) | 5 (55.6) |
| Women | 68 (36.8) | 4 (44.4) |
| Ethnicity, n (%) | ||
| Caucasian | 184 (99.5) | 8 (88.9) |
| Hispanic | 1 (0.5) | 0 (0.0) |
| Other | 0 (0.0) | 1 (11.1) |
| ECOG performance status, n (%) | ||
| 0 | 73 (39.5) | 0 (0.0) |
| 1 | 92 (49.7) | 4 (44.4) |
| 2 | 16 (8.6) | 3 (33.3) |
| 3 | 0 (0.0) | 1 (11.1) |
| Unknown | 4 (2.2) | 1 (11.1) |
| Primary tumour site, n (%) | ||
| Colon | 100 (54.1) | 4 (44.4) |
| Rectum | 73 (39.5) | 3 (33.3) |
| Colon, rectum | 10 (5.4) | 2 (22.2) |
| Unknown | 2 (1.1) | 0 (0.0) |
| Sidedness of primary tumour, n (%) | ||
| Left side | 135 (73.0) | 6 (66.7) |
| Right side | 42 (22.7) | 2 (22.2) |
| Both sides | 6 (3.2) | 1 (11.1) |
| Unknown | 2 (1.1) | 0 (0.0) |
| Metastatic sites, n (%) | ||
| Liver | 148 (80.0) | 8 (88.9) |
| Lung | 132 (71.4) | 5 (55.6) |
| Lymph node | 91 (49.2) | 5 (55.6) |
| Peritoneum | 39 (21.1) | 2 (22.2) |
| Other | 48 (25.9) | 1 (11.1) |
| Number of metastatic sites, n (%) | ||
| 1 | 24 (13.0) | 1 (11.1) |
| 2 | 74 (40.0) | 3 (33.3) |
| 3 | 61 (33.0) | 4 (44.4) |
| 4 | 22 (11.9) | 0 (0.0) |
| 5 | 3 (1.6) | 0 (0.0) |
| Unknown | 1 (0.5) | 1 (11.1) |
| Metastatic manifestation, n (%) | ||
| Synchronous | 96 (51.9) | 3 (33.3) |
| Metachronous | 58 (31.4) | 3 (33.3) |
| Unknown | 31 (16.8) | 3 (33.3) |
| Grading (World Health Organization), n (%) | ||
| G1 | 4 (2.2) | 0 (0.0) |
| G2 | 127 (68.6) | 7 (77.8) |
| G2-3 | 3 (1.6) | 0 (0.0) |
| G3 | 27 (14.6) | 1 (11.1) |
| G3-4 | 2 (1.1) | 0 (0.0) |
| G4 | 3 (1.6) | 0 (0.0) |
| GX | 6 (3.2) | 0 (0.0) |
| Unknown | 13 (7.0) | 1 (11.1) |
| RAS status, n (%) | ||
| Wild-type | 72 (38.9) | 4 (44.4) |
| Mutant | 101 (54.6) | 3 (33.3) |
| Unknown | 12 (6.5) | 2 (22.2) |
| BRAF V600E status, n (%) | ||
| Wild-type | 65 (35.1) | 1 (11.1) |
| Mutant | 1 (0.5) | 0 (0.0) |
| Unknown | 119 (64.3) | 8 (88.9) |
| Surgery of primary tumour, n (%) | ||
| No | 29 (15.7) | 2 (22.2) |
| Yes | 156 (84.3) | 7 (77.8) |
| Surgery of metastases, n (%) | ||
| No | 102 (55.1) | 4 (44.4) |
| Yes | 83 (44.9) | 5 (55.6) |
| Radiation, n (%) | ||
| No | 135 (73.0) | 6 (66.7) |
| Yes | 50 (27.0) | 3 (33.3) |
| Number of previous therapy lines for the treatment of mCRC, n (%) | ||
| 0 | 4 (2.2) | 3 (33.3) |
| 1 | 25 (13.5) | 1 (11.1) |
| 2 | 71 (38.4) | 3 (33.3) |
| 3 | 53 (28.6) | 0 (0.0) |
| ≥4 | 32 (17.3) | 2 (22.2) |
| Substances of previous systemic anti-CRC therapies, n (%) | ||
| Fluoropyrimidine | 184 (99.5) | 8 (88.9) |
| Irinotecan | 169 (91.4) | 5 (55.6) |
| Oxaliplatin | 173 (93.5) | 6 (66.7) |
| Bevacizumab | 149 (80.5) | 3 (33.3) |
| Anti-EGFR antibodies | 71 (38.4) | 3 (33.3) |
| Regorafenib | 0 (0.0) | 0 (0.0) |
| Other than the above | 47 (25.4) | 0 (0.0) |
| No previous therapy documented | 1 (0.5) | 1 (11.1) |
BSC, best supportive care; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group; FTD/TPI, trifluridine/tipiracil; mCRC, metastatic CRC; SD, standard deviation.
Primary endpoint—rate of HRQoL-responders
Questionnaires from 109 patients receiving FTD/TPI were assessable for HRQoL-RR. The primary endpoint was reached for FTD/TPI-treated patients with 59.6% HRQoL-RR (95% CI 49.8% to 68.9%). When allowing for the above-mentioned extended period to fill in the questionnaires, HRQoL-RR was reported to be even higher with 67.0% (95% CI 57.3% to 75.7%).
Efficacy
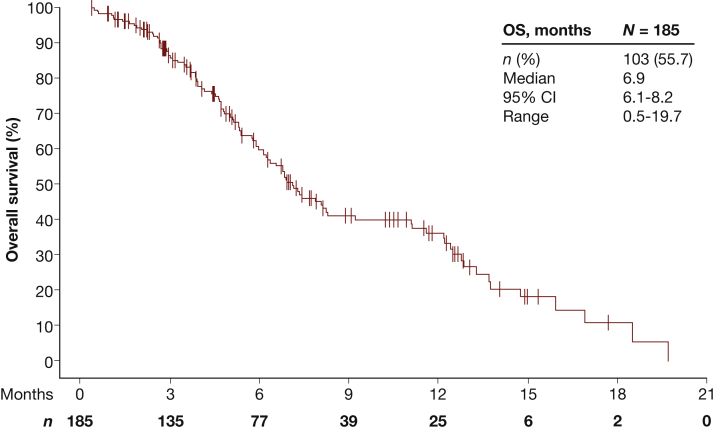
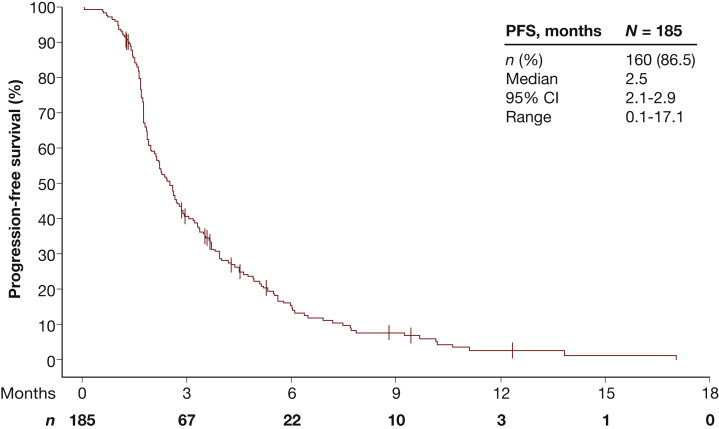
Treatment with FTD/TPI was associated with a median OS of 6.9 months (95% CI 6.1-8.2 months) (Figure 2) and a median PFS of 2.5 months (95% CI 2.1-2.9 months) (Figure 3; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100391). By the time of the cut-off date, events of progression were reported in 150 FTD/TPI-treated patients. Median time to progression was 2.6 months (95% CI 2.2-2.9 months).
Figure 2.
Overall survival.
CI, confidence interval; OS, overall survival.
Figure 3.
Progression-free survival.
CI, confidence interval; PFS, progression-free survival.
Efficacy data obtained from patients receiving BSC is summarised in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100391.
Safety
By the data cut-off date, TEAEs occurred in 177 FTD/TPI-treated patients (95.7%) and in 7 patients receiving BSC (77.8%) (Table 2). At least one TEAE of grade ≥3 was experienced by 122 FTD/TPI-treated patients (65.9%) and by 6 patients receiving BSC (66.7%). Serious TEAEs were reported for 82 FTD/TPI-treated patients (44.3%) and for 5 patients receiving BSC (55.6%). Serious TEAEs of grade 5 that resulted in death occurred in 24 FTD/TPI-treated patients (13.0%) and in 1 patient receiving BSC (11.1%). Of the 185 FTD/TPI-treated patients, 57 (30.8%) discontinued study treatment, 23 (12.4%) experienced a dose reduction and 55 (29.7%) a dose delay due to a TEAE. The most frequently reported TEAEs for FTD/TPI-treated patients were neutropenia (27.6%), anaemia (22.7%), fatigue (21.6%), nausea (21.6%), diarrhoea (20.5%), leukopenia (18.9%), decreased appetite (14.1%), neoplasm progression (13.5%), vomiting (13.5%), constipation (10.8%) and dyspnoea (10.3%). Febrile neutropenia of grade 3 did not occur in any patient receiving BSC but in two patients treated with FTD/TPI (1.1%).
Discussion
Approval of FTD/TPI was granted based on the results of the pivotal phase III RECOURSE trial.9,10 In order to complement this trial with post-authorisation efficacy and safety data and patient-reported HRQoL data from German patients, the TALLISUR study was conducted.
Here, the median OS of FTD/TPI-treated patients was 6.9 months and therefore slightly shorter than 7.2 months reported in the RECOURSE trial. In contrast, the median PFS was longer in patients of the TALLISUR study, with 2.5 months compared to 2.0 months. The safety profiles of both studies were comparable. Neutropenia (18.4% versus 38%) and leukopenia (8.1% versus 21%) were the most common TEAEs of grade ≥3 in the TALLISUR versus RECOURSE trial. Febrile neutropenia was reported in 1.1% receiving FTD/TPI compared to 4% in the RECOURSE study. Although cross-study comparisons must be taken with caution, results from the TALLISUR trial confirmed efficacy and safety of FTD/TPI reported in the RECOURSE trial in a post-authorisation setting.
In addition, the TALLISUR study provides patient-reported outcome on HRQoL which was not assessed in the pivotal phase III RECOURSE study. Based on the EORTC QLQ-C30 questionnaire, 67% of patients treated with FTD/TPI reported a stabilised or improved HRQoL score when considering the extended time period to fill in questionnaires.
With a similar objective as the TALLISUR study, two studies were recently conducted in order to obtain patient-reported HRQoL data from mCRC patients receiving FTD/TPI. In the international, single-arm, phase IIIb PRECONNECT study, 793 patients with pre-treated mCRC were recruited in 13 countries (Australia, Belgium, Bulgaria, Croatia, France, Ireland, Italy, Panama, Poland, Portugal, Slovakia, Slovenia and Turkey) and treated with FTD/TPI.15 Here, the median duration of treatment was 2.84 months (3 cycles) compared to median 2.24 months (3 cycles) in the TALLISUR trial. The EORTC QLQ-C30 questionnaire was filled in by patients before the start of each cycle and at the end of treatment. In the end, the EORTC QLQ-C30 global health status was evaluated up to the seventh cycle and shown to remain unchanged throughout, which is comparable to our results. The most common TEAE of grade ≥3 was neutropenia (39.1%). Febrile neutropenia occurred in 1.4%. Median PFS was slightly longer in the PRECONNECT trial (2.8 months) compared to the TALLISUR trial (2.5 months).
A Canadian non-interventional study assessed HRQoL in 50 FTD/TPI-treated patients and 55 patients receiving BSC.16 Unlike the TALLISUR and PRECONNECT trial, QoL was assessed using the Rotterdam Symptom Checklist and the CRC-specific FACT Colorectal Cancer Symptom Index (FCSI). According to the former assessment, physical and psychological distress, activity level and overall global QoL were statistically significantly better in patients receiving FTD/TPI compared to patients receiving BSC. Similarly, according to the FCSI, patients treated with FTD/TPI reported a statistically significantly higher mean score indicating less symptomology compared to patients receiving BSC.
Therefore, comparable to results from the TALLISUR trial, these two studies reported a maintained HRQoL in mCRC patients receiving FTD/TPI.
Results of the phase IV TALLISUR trial were based on post-authorisation data. In concordance, 8.6% of FTD/TPI-treated patients had a baseline ECOG PS of 2, which contrasts with the exclusion criteria ECOG PS > 1 often applied in pivotal trials such as the RECOURSE trial. This might also have had an impact on the OS results described above. Furthermore, when given the freedom of choice, the vast majority of patients chose FTD/TPI over BSC (185 versus 9) illustrating the wish and capability of these patients to receive palliative chemotherapy beyond the second line.
In summary, these results of the TALLISUR trial demonstrated for the first time patient-reported HRQoL and post-authorisation efficacy and safety data from German patients with pre-treated mCRC receiving FTD/TPI. Consistent with results of studies conducted in other countries, FTD/TPI prolonged survival and delayed progression with a manageable toxicity profile while concomitantly being associated with maintained HRQoL.
Acknowledgements
We thank the patients who participated in our study as well as their families and caretakers. We also thank all the TALLISUR investigators who included the patients in the analyses described in this paper. Furthermore, we acknowledge Swantje Held and Filippina Termini from the clinical research organisation ClinAssess GmbH (51379 Leverkusen, Germany) for their contribution to this study. Finally, we recognise Marion Röhlich for her support in illustrating Figures 2 and 3. This design assistance was funded by the Institut de Recherches Internationales Servier, France.
Funding
The TALLISUR study was sponsored by the Institut de Recherches Internationales Servier, France (no grant number).
Disclosure
LW reports receiving honoraria for talks from Roche; MK reports receiving fees for serving on advisory boards and travel support from Servier; MW reports receiving fees for serving on advisory boards from Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Celgene, Gilead, Hexal, Janssen, Lilly, Medac, Novartis, Roche and SANOFI; HP reports being an employee of PiTri Studien GmbH and receiving fees for serving on advisory boards and travel support from Roche Pharma AG, Bristol-Myers Squibb, Pfizer, Bayer, Novartis, MSD, Takeda, Janssen-Cilag, Rönsberg, Ingress-Health, Gilead, Amgen, Alexion, Abbvie and Uniklinik Freiburg; JH, TR and AK report being employees of Servier Deutschland GmbH. VH reported receiving honoraria from Merck, Roche, Celgene, AMGEN, Sanofi, Lilly, SIRTEX, Boehringer-Ingelheim, Taiho, Servier; consulting of Merck, Roche, AMGEN, Sanofi, SIRTEX, Servier, Celgene, Boehringer-Ingelheim, Halozyme, MSD, BMS; research funding for the institution of Merck, Roche, AMGEN, SIRTEX, Servier, Celgene, Boehringer-Ingelheim, Shire and Travel accommodation expenses from Merck, Roche, AMGEN, SIRTEX, Servier, Shire, MSD, BMS. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Robert Koch Institute and the Association of Population-Based Cancer Registries in Germany . 12th ed. German Ministry; 2019. Cancer in Germany 2015/2016. [Google Scholar]
- 3.Cremolini C., Loupakis F., Antoniotti C., et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann V., von Weikersthal L.F., Decker T., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 5.Venook A.P., Niedzwiecki D., Lenz H.-J., et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) J Clin Oncol. 2014;32(suppl 9):LBA3. [Google Scholar]
- 6.Van Cutsem E., Cervantes A., Adam R., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima M., Suzuki N., Emura T., et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2'-deoxyribonucleosides. Biochem Pharmacol. 2000;59:1227–1236. doi: 10.1016/s0006-2952(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 8.Peeters M., Cervantes A., Moreno Vera S., Taieb J. Trifluridine/tipiracil: an emerging strategy for the management of gastrointestinal cancers. Future Oncol. 2018;14:1629–1645. doi: 10.2217/fon-2018-0147. [DOI] [PubMed] [Google Scholar]
- 9.Mayer R.J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E., Mayer R.J., Laurent S., et al. The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. Eur J Cancer. 2018;90:63–72. doi: 10.1016/j.ejca.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 12.King M.T. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 13.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Bachet J.B., Wyrwicz L., Price T., et al. Safety, efficacy and patient-reported outcomes with trifluridine/tipiracil in pretreated metastatic colorectal cancer: results of the PRECONNECT study. ESMO Open. 2020;5:e000698. doi: 10.1136/esmoopen-2020-000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung W.Y., Kavan P., Dolley A. Quality of life in a real-world study of patients with metastatic colorectal cancer treated with trifluridine/tipiracil. Curr Oncol. 2020;27:e451–e458. doi: 10.3747/co.27.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.