Abstract
Objectives
The effect of early enteral nutrition (EN) in patients with acute pancreatitis (AP) has been confirmed. In recent years, some researchers provided new strategy that immediate EN was offered after admission. The effect and safety of immediate EN was unclear because of the different results among studies. The study aimed to implement the meta analysis of randomized controlled trials (RCT) to confirm the effect and safety between the immediate EN group and the early refeeding group.
Methods
Four electronic databases including PubMed, EMBASE, the Cochrane Library and China National Knowledge Internet (CNKI) were searched from inception to July 2021. Endnote X7.0 software was used to manage all the relevant citations. Then data extraction and evaluation of risk of bias for included studies were performed after initial selection and full-text selection. All statistical analyses were performed by Review Manager 5.3 version software.
Results
5 randomized controlled trials (RCT) involving 372 patients were included in the present study. The meta analysis revealed that immediate EN after admission in patients with AP could significantly decrease the length of hospital stay (LOHS) (Mean difference [MD] = 2.57, 95% confidence interval [CI] = 0.41–4.72) and the intolerance of feeding (risk ratio [RR] = 0.78, 95%CI = 0.63–0.95), compared with early refeeding. But immediate EN couldn't significantly decrease the incidence of readmission after discharging (RR = 0.51, 95%CI = 0.12–2.27), the incidence of progression to severe pancreatitis (RR = 0.76, 95%CI = 0.15–3.76), the incidence of complications (RR = 1.12, 95%CI = 0.50–2.49) and the values of C-reactive protein (CRP) and leukocyte counts (MD = 1.05, 95%CI = 0.15–2.26 and MD = 0.11, 95%CI = 0.59–0.80), compared with early refeeding.
Conclusions
Compared with early refeeding, immediate EN after admission could safely reduce LOHS and intolerance of feeding in patients with AP.
Keywords: Immediate, Enteral nutrition, Mild acute pancreatitis, Meta analysis
Immediate; Enteral nutrition; Mild acute pancreatitis; Meta analysis.
1. Introduction
Acute pancreatitis (AP) is a common gastrointestinal condition involving hospitalization worldwide [1]. Every year more than 275,000 patients are hospitalized for AP in the United States, and it is estimated that approximately $2.6 billion is consumed per year [2]. When patients suffer from AP, premature activation of digestive enzymes leads to auto-digestion of the pancreatic gland, followed by an increased risk of developing systematic inflammatory response and multi-organ dysfunction [3, 4, 5]. Based on the 2012 revised Atlanta Classification, the severity of AP is categorized as mild, moderate or severe, with vast majority (80%) of the mild type [6, 7]. Patients in this group often develop the symptoms of pancreatic edema, without local or systemic complications or transient organ failure. And they can usually recover within 1–2 weeks without incident [6]. With the aim of minimizing stimulation of pancreatic secretion and thus putting inflamed pancreas at rest, the initial treatment of mild AP traditionally was consist of three basic elements: initial fasting for 3–7 days, administration of parenteral fluids and analgesia [8, 9, 10]. While the rational for pancreatic rest is questioned by emerging evidence which suggests that the secretion of pancreatic juice and trypsin may not increase over the first days of AP [11, 12]. Besides, it has been reported that fasting may induce intestinal mucosal atrophy and bacterial translocation which are the risk factors of AP complications [13, 14].For initial management of AP, the American Gastroenterological Association (AGA) released a guideline in 2018 and recommended that oral feeding (within 24 h) should be used as soon as possible if the patients are tolerant of oral feeding [7]. And if not, enteral nutrition (EN) is to be given preference. Early EN is a more natural approach to provide nutrients to the intestinal tract. What's more, it has a beneficial effect on maintenance of intestinal mucosal integrity and promotion of normal bowel function [15, 16, 17, 18]. A recent meta-analysis evaluating 6 studies demonstrated that EN within 48 h, compared with delayed enteral feeding, lowered the risks of multiple organ failure. This review also suggested a tendency for decreased systematic inflammatory syndrome (SIRS) with early initiation of EN [19]. To date, the optimal timing for recommencing EN was unclear. The traditional opinion considers that whether EN (oral or enteral tube) can be restarted according to resolution of abdominal pain, recovery of gastrointestinal function and normalization of pancreatic enzymes [10, 20]. Contrary to this, some recent studies stated that immediate EN seems to be a safe approach. Two RCTs demonstrated this way may accelerate recovery without increasing adverse gastrointestinal events [15, 21]. However, this result was not observed in others [22, 23]. We therefore conducted a systematic review and meta-analysis to assess the efficacy and feasibility of immediate EN in patients with mild AP. We also tried to define the optimum time to start EN after disease onset.
2. Methods
2.1. Searching strategy
This meta analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) although the protocol of the present meta analysis was not registered in a public platform [24]. Two investigators (QHG and XYT) independently searched the four electronic databases including PubMed, EMBASE, the Cochrane Library and China National Knowledge Internet (CNKI) from inception to July 2021. In order to obtain more eligible studies, the manual searching was conducted by reviewing the references of all included studies and related reviews. The Medical Subject Headings (MeSH) and the key words were combined in the search algorithms. The key words were consisted of ‘acute pancreatitis’, ‘random∗’, ‘nutrition’ and ‘immediately’. The language of published studies was not restricted. The complete search strategy for EMBASE was documented in Supplemental Table 1. Finally, all the relevant literatures were imported to Endnote X7.0 software.
2.2. Selecting criteria
Before completing the meta analysis, we designed the selecting criteria according to the five aspects including patients, intervention, comparison, outcome measures and study designs (PICOS) [25]. The studies which met the following the inclusion criteria would be included.
(P): All the patients were aged 16 and above, and the gender was not limited. All the patients were clearly diagnosed with mild acute pancreatitis, which pancreas amylase was 3 times and above than normal, onset of abdominal pain within 48 h, acute physiological and chronic health evaluation score (APACHE) II < 8 based on the Atlanta classification system [15].
(I): Enteral nutrition initiated immediately after admission.
(C): Enteral nutrition after pain relief or bowel sound after admission or parenteral nutrition.
(O): Length of hospital stay, pain relief time, adverse effects, local complications, mortality, and gastrointestinal symptoms including nausea, vomiting and diarrhea.
(S): Randomized controlled trials (RCT).
Studies were excluded if they met the exclusion criteria.
(S): Not RCT, animal experiment.
If the studies couldn't offer sufficient data, they would be excluded.
2.3. Data extraction
Two investigators (YLQ and XTH) were independently asked to finish reviewing the title and abstract of all the relevant literatures to judge whether literatures met inclusion criteria. If a study met the selection criteria based on the title and abstract, full-text was obtained to further judge its eligibility. After ensuring the eligibility of included studies, two investigators (QHG and XYT) independently extracted the basic information including the first author, publication year, country, age and sex of patients, random method, the number of dropout, intervention regimes and outcome measures. If there was any disagreements between the two investigators, the third investigator (WHW) would make the ultimate decision.
2.4. Evaluation of risk of bias of included studies
The Cochrane risk of bias assessment tool including seven domains was used to evaluate the risk of bias of all the included studies by two investigators (YLQ and XTH). The seven domains were randomization sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases [26]. After judging the risk of bias of each eligible study, we finally graded the overall quality moderate if most of the eligible study was evaluated as unclear or low risk of bias. The third investigator (WHW) would deal with the discrepancy between the two investigators.
2.5. Statistical analysis
We used mean difference (MD) or standardized mean difference (SMD) with 95% confidence interval (CI) for the continuous data and relative risk (RR) with 95% CI for categorical data. Random-effect model was chosen to perform statistical analysis of each outcome measure in the present study, which simultaneously considering the heterogeneity within and across trials [27]. The qualitative description by chi-square test and the quantitative description by I2 statistic were used to express the heterogeneity [28]. While the number of included studies for each outcome measure was less than 10, we didn't draw the funnel plot to analyze the publication bias [29].
3. Results
3.1. Results of searching and selecting
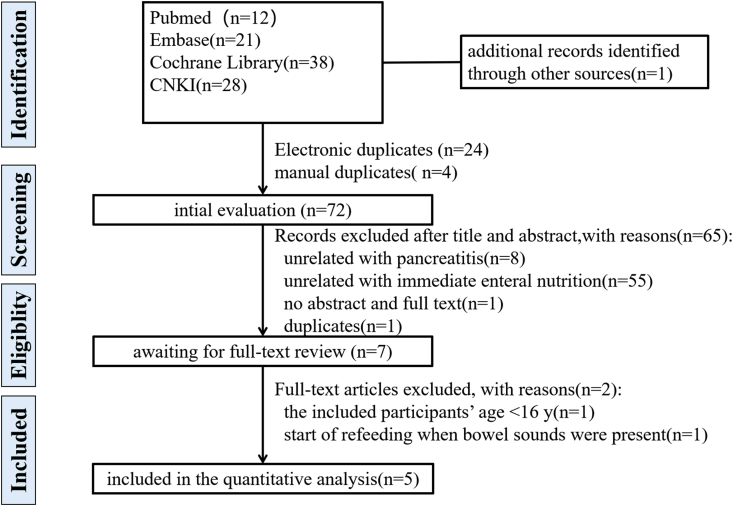
A total of 99 articles were captured from four electronic databases and one article was found by manual searching of references of all the included studies and related reviews. After initial selection and full-text selection, 5 studies involving 372 patients were included in the present study [15, 21, 22, 23, 30]. The flow diagram of searching and selecting of articles was displayed in Figure 1.
Figure 1.
The flow diagram of searching and selecting of articles.
3.2. The basic characteristics of 5 included studies
We concluded the basic characteristics of 5 included studies, which presented in Table 1. For the 5 included studies in the meta analysis, the sample size of each study ranged from 26 to 143 and all patients were diagnosed with mild acute pancreatitis. The country of publication of each study was different. The publication language included English, Chinese and Spanish. The article published in Spanish was translated into English by two native speakers who had a high level of competence in English [30]. The experiment group of each study performed the immediate enteral nutrition after admission, but the time and method of enteral nutrition in the control group of each study was slightly different.
Table 1.
The basic characteristics of the five trials included into the study.
| Study ID | Country | AP | NO. of Patients |
Age of Patients T/C (years) |
Drop out T/C | Interventions |
Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| T (M/F) | C (M/F) | T | C | ||||||
| Eckerwall 2007 [15] | Sweden | mild | 13/17 | 14/16 | 56(48–72)/ 52(38–60) |
1/0 | immediately allowed to drink and eat freely as tolerated | firstly fasting and oral fluids and diet reintroduced in a traditional stepwise manner | pancreas-specific amylase, CRP and leukocytes, Intolerance, LOHS |
| Teich 2010 [21] | Germany | mild | 42/27 | 51/23 | (45.4 ± 1.6)/ (46.7 ± 1.8) |
11/27 | self-selected eating | lipase level < 2-fold of ULM | LOHS, Pain relief, Complications |
| Yang 2018 [22] | China | mild | 23/19 | 20/22 | (44.38 ± 9.38)/ (44.69 ± 2.12) |
0 | immediately allowed to drink and eat freely | drink and eat when bowel sounds are present and abdominal pain is relieved | LOHS, Mortality rate, Intolerance, Complications |
| Horibe 2020 [23] | Japan | mild | 10/3 | 8/5 | (50.1 ± 5.3)/ (60.8 ± 5.3) |
0/1 | immediate oral intake of low-fat (15 g/d) solid food | a gradually increasing amount of dietary fat after a fast | LOHS, Rehospitalization rate, Progression from mild to severe AP |
| Esmer 2021 [31] | Mexico | mild | 3/26 | 5/25 | 37(20–49)/ 38(18–89) |
0 | immediate oral feeding within 8 h | early oral feeding within 48 h | Intolerance, LOHS |
Abbreviations: AP: acute pancreatitis, T-study group, C-control group, M-male, F-female, LOHS-length of hospital stay.
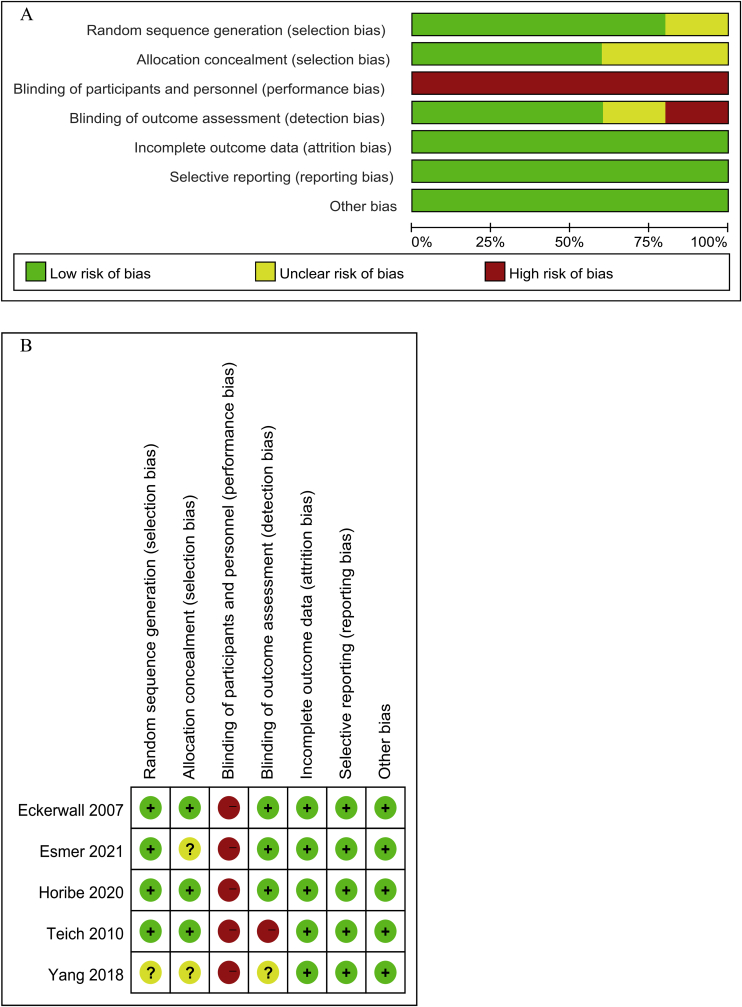
3.3. Results of assessment of risk of bias of 5 included studies
The 5 included studies all reported randomization and only one study didn't mention the method of randomization sequence generation adequately [23]. Three studies reported the use of allocation concealment [15, 21, 22]. Because the time and method of enteral nutrition were different between the experiment group and the control group, it was impossible to blind the participants and study personnel. Therefore, all the included studies were evaluated as a high risk of bias in the domain of the blinding of participants and study personnel. There were three studies performed the blinding of outcome assessment [15, 21, 30]. In all, the overall methodological quality of five included studies could be rated as moderate level. More details of results of risk of bias were presented in Figure 2.
Figure 2.
Risk of bias. (A) risk of bias graph and (B) risk of bias summary.
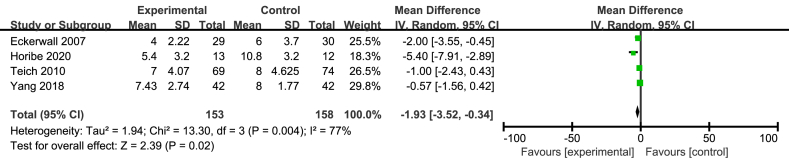
3.4. Length of hospital stay
The five included studies reported LOHS but only four studies involving 227 patients offered the specific data [15, 21, 22, 23]. The meta analysis suggested that immediate EN could significantly decrease the LOHS, compared with early refeeding (MD = 2.57; 95%CI, 0.41–4.72; P = 0.02; I2 = 78%; presented as Figure 3).
Figure 3.
Meta analysis of LOHS.
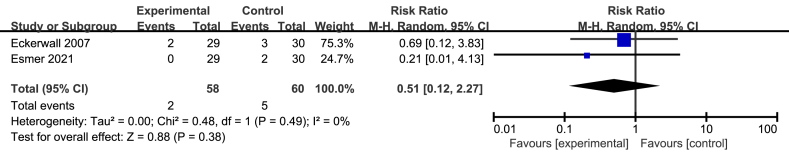
3.5. Readmission after discharging
Only two studies involving 118 patients offered the specific information concerning readmission [15, 30]. The pooled result of the two studies showed immediate EN had no significant decrease compared with early refeeding (RR = 0.51; 95%CI, 0.12–2.27; P = 0.38; I2 = 0%; presented as Figure 4).
Figure 4.
Meta analysis of incidence of readmission after discharging.
3.6. Abdominal pain
The included three studies reported the assessment of pain before and after refeeding [15, 22, 30]. But only one studies offered the specific data, and thus qualitative description was performed [30]. The included three studies all showed no significant differences between two groups concerning abdominal pain.
3.7. Progression to severe pancreatitis
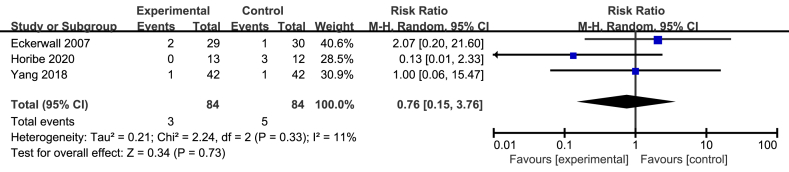
There were three studies providing the specific information about progression to severe pancreatitis [15, 21, 23]. The meta analysis of three studies involving 168 patients revealed that immediate EN didn't significantly increase the number of progression to severe pancreatitis compared with early refeeding (RR = 0.76; 95%CI, 0.15–3.76; P = 0.73; I2 = 11%; presented as Figure 5).
Figure 5.
Meta analysis of incidence of progression to severe pancreatitis.
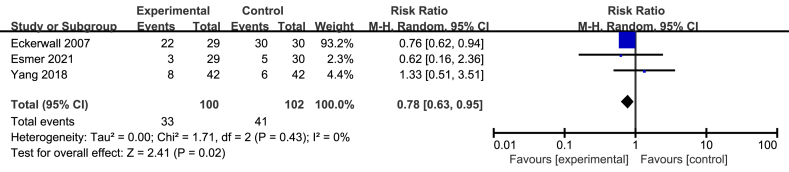
3.8. Intolerance of feeding
Only three studies involving 202 patients reported the patients’ intolerance of feeding [15, 23, 30]. The intolerance of feeding included vomiting, nausea and other gastrointestinal symptoms. The meta analysis of three studies revealed that immediate EN could significantly decrease the intolerance of feeding, compared with early refeeding (RR = 0.78; 95%CI, 0.63–0.95; P = 0.02; I2 = 0%; presented as Figure 6).
Figure 6.
Meta analysis of incidence of Intolerance of feeding.
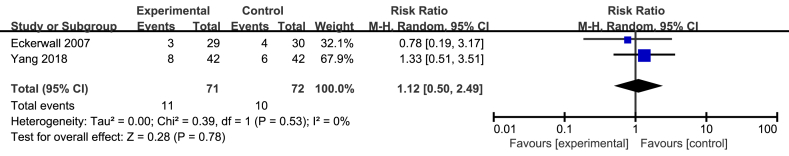
3.9. Complications
Only two studies involving 143 patients displayed the number of complications [15, 23]. The pooled result showed that there was no significant difference between the two groups (RR = 1.12; 95%CI, 0.50–2.49; P = 0.78; I2 = 0%; presented as Figure 7).
Figure 7.
Meta analysis of incidence of complications.
3.10. Systemic inflammatory response
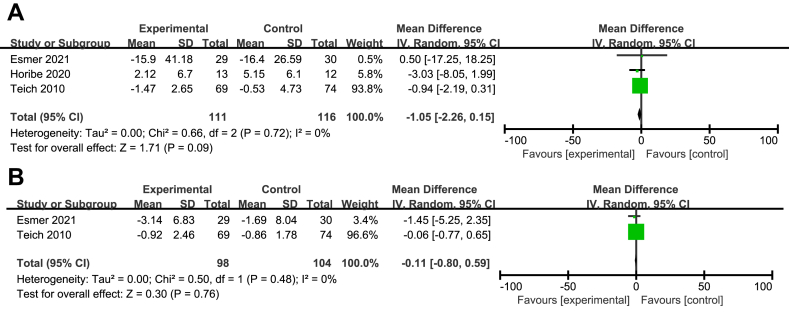
3.10.1. C-reactive protein
Only three studies involving 227 patients offered the specific data concerning difference of the values of C-reactive protein (CRP) before and after EN [21, 22, 30]. The meta analysis of three studies showed that no significant difference between the two groups was found (MD = 1.05; 95%CI, 0.15–2.26; P = 0.09; I2 = 0%; presented as Figure 8A). Because Eckerwall et al. only offered the values of CRP after EN, a qualitative description was displayed, which a similar result was found with the meta analysis [15].
Figure 8.
Meta analysis of CRP(A) and Leukocyte counts(B).
3.10.2. Leukocyte counts
Only two studies involving 202 patients reported the specific data concerning difference of the leukocyte counts before and after EN [22, 30]. The meta analysis of two studies showed that no significant difference between the two groups was found (MD = 0.11; 95%CI, 0.59–0.80; P = 0.76; I2 = 0%; presented as Figure 8B). Eckerwall et al. found the similar result with the meta analysis [15].
4. Discussion
Most of people (80%) presenting with AP undergo only slight symptoms [6]. Nutritional support seems to play a pivotal role in the course of recovery in patients with mild type. The AGA for clinical practice guidelines for the nutritional support of mild AP recommends initiation of early EN (oral or enteral tube) instead of parenteral nutrition [7]. However, it is unclear how early the EN could be offered. Hence, in recent years, some researchers explored the effect and safety of immediate EN after admission compared with the early EN after bowel sounds existing or pain stopping [15, 21, 22, 23, 30]. But the conclusion couldn't be confirmed owing to inconsistent results among studies.
The present study is the first meta analysis to clarify the effect and safety of immediate EN after admission in patients with mild AP. In the meta analysis, we found the results that immediate EN after admission could not only make the LOHS shorter (MD = 2.57, 95% CI = 0.41–4.72) but also relieve the intolerance of feeding (RR = 0.78, 95%CI = 0.63–0.95). Meanwhile, compared with early refeeding, immediate EN after admission didn't result in the significant increase of the incidence of readmission after discharging (RR = 0.51, 95%CI = 0.12–2.27), the incidence of progression to severe pancreatitis (RR = 0.76, 95%CI = 0.15–3.76), the incidence of complications (RR = 1.12, 95%CI = 0.50–2.49) and the values of CRP and leukocyte counts (MD = 1.05, 95%CI = 0.15–2.26 and MD = 0.11, 95%CI = 0.59–0.80).
According to the traditional views, fasting and PN were performed in patients with AP because fasting and PN were believed to decrease auto-digestion of the pancreas and tissue damage by reducing pancreatic secretion of enzymes and minimizing the impact on the pancreatic gland [31, 32]. But several clinical studies and animal experiments found that EN could prevent the damage of integrity and function of the intestinal barrier by affecting the intestinal permeability, immunocompetent cells and bacteria translocation, which promoting recovery and decreasing the mortality of AP [13, 20, 22]. During the period of AP, the gut function damage could occur as early as 28–72 h after the development of AP [5]. Earlier EN and shorter fasting time were associated with accelerated recovery, shorter LOHS and decreased intolerance of feeding. Hence, immediate EN could significantly make LOHS shorter and relieve intolerance of feeding in the present study. Immediate oral feeding may suppress the progression of severe AP but the present meta analysis did not show meaningful result due to a low number of events and participants which was consistent with the three original studies [15, 21, 23].
At present, some published meta-analyses explored the optimal time of EN in AP [19, 33, 34, 35, 36]. However, only one of five published meta-analysis performed subgroup analysis to explore the effect of immediate EN on LOHS [36]. But the subgroup analysis only included two studies. The other meta analyses included all the studies which EN was offered within 24 h or 48 h, and so they couldn't analyze the effect of immediate EN separately. Meanwhile, the patients included in the other meta analyses were mild to moderate AP or severe AP. Therefore, these limitations destroyed the robustness and reliability of the results of the effect of immediate EN in patients with mild AP. However, the present study only included RCT and the patients with mild AP were offered immediate EN. More importantly, more recent published studies were searched and included in this meta analysis. Thus, more reliable and rigorous findings could be obtained in the present study compared with published meta-analysis. For other secondary outcome measures including readmission after discharging, progression to severe pancreatitis, complications and et al, the results of included studies in meta analysis and published meta-analysis were consistent with the results of the present study, which revealing immediate EN were safe.
We have to acknowledge that there are still some limitations in the meta analysis including 5 studies involving 372 patients. The heterogeneity within and across 5 trials was a little high and we couldn't perform subgroup analysis to explore the reason according to the intervention methods in experiment group or control group because of the inadequate number of included studies. Moreover, the sample size is not adequately large, which limited its wider clinical application. A large multi-center clinical trial is still required.
5. Conclusions
With the present findings, immediate EN after admission could safely reduce LOHS and intolerance of feeding in patients with mild AP, compared with early refeeding. However, large multi-center studies with more rigorous methodology are required to improve the quality of evidence before determining clinical decisions owing to the presence of limitations.
Declarations
Author contribution statement
Qing Hua Guo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Xin Yi Tian: Performed the experiments; Wrote the paper.
Yue Lan Qin and Xiao Tong Han: Performed the experiments.
Wei Hong Wang: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Peery A.F., Crockett S.D., Murphy C.C., Lund J.L., Dellon E.S., Williams J.L., et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254–272. doi: 10.1053/j.gastro.2018.08.063. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery A.F., Crockett S.D., Barritt A.S., Dellon E.S., Eluri S., Gangarosa L.M., et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149(7):1731–1741. doi: 10.1053/j.gastro.2015.08.045. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandol S.J., Saluja A.K., Imrie C.W., Banks P.A. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Pan L.L., Li J., Shamoon M., Bhatia M., Sun J. Recent advances on nutrition in treatment of acute pancreatitis. Front. Immunol. 2017;8:762. doi: 10.3389/fimmu.2017.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson E., Andersson R. Exocrine insufficiency in acute pancreatitis. Scand. J. Gastroenterol. 2004;39(11):1035–1039. doi: 10.1080/00365520410003164. [DOI] [PubMed] [Google Scholar]
- 6.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 7.Crockett S.D., Wani S., Gardner T.B., Falck-Ytter Y., Barkun A.N. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154(4):1096–1101. doi: 10.1053/j.gastro.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Banks P.A., Freeman M.L. Practice guidelines in acute pancreatitis. Am. J. Gastroenterol. 2006;101(10):2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 9.Whitcomb D.C. Clinical practice. Acute pancreatitis. N. Engl. J. Med. 2006;354(20):2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 10.Meier R., Ockenga J., Pertkiewicz M., Pap A., Milinic N., Macfie J., et al. ESPEN guidelines on enteral nutrition: pancreas. Clin. Nutr. (Edinb. Scotl.) 2006;25(2):275–284. doi: 10.1016/j.clnu.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Domínguez-Muñoz J.E., Pieramico O., Büchler M., Malfertheiner P. Exocrine pancreatic function in the early phase of human acute pancreatitis. Scand. J. Gastroenterol. 1995;30(2):186–191. doi: 10.3109/00365529509093260. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe S.J., Lee R.B., Li J., Stevens S., Abou-Assi S., Zhou W. Trypsin secretion and turnover in patients with acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289(2):G181–G187. doi: 10.1152/ajpgi.00297.2004. [DOI] [PubMed] [Google Scholar]
- 13.Mirtallo J.M., Forbes A., McClave S.A., Jensen G.L., Waitzberg D.L., Davies A.R. International consensus guidelines for nutrition therapy in pancreatitis. JPEN - J. Parenter. Enter. Nutr. 2012;36(3):284–291. doi: 10.1177/0148607112440823. [DOI] [PubMed] [Google Scholar]
- 14.Buchman A.L., Moukarzel A.A., Bhuta S., Belle M., Ament M.E., Eckhert C.D., et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN - J. Parenter. Enter. Nutr. 1995;19(6):453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 15.Eckerwall G.E., Tingstedt B.B., Bergenzaun P.E., Andersson R.G. Immediate oral feeding in patients with mild acute pancreatitis is safe and may accelerate recovery--a randomized clinical study. Clin. Nutr. (Edinb. Scotl.) 2007;26(6):758–763. doi: 10.1016/j.clnu.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Faghih M., Fan C., Singh V.K. New advances in the treatment of acute pancreatitis. Curr. Treat. Options Gastroenterol. 2019;17(1):146–160. doi: 10.1007/s11938-019-00223-8. [DOI] [PubMed] [Google Scholar]
- 17.Ammori B.J. Role of the gut in the course of severe acute pancreatitis. Pancreas. 2003;26(2):122–129. doi: 10.1097/00006676-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hua Z., Su Y., Huang X., Zhang K., Yin Z., Wang X., et al. Analysis of risk factors related to gastrointestinal fistula in patients with severe acute pancreatitis: a retrospective study of 344 cases in a single Chinese center. BMC Gastroenterol. 2017;17(1):29. doi: 10.1186/s12876-017-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng P., He C., Liao G., Chen Y. Early enteral nutrition versus delayed enteral nutrition in acute pancreatitis: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2017;96(46) doi: 10.1097/MD.0000000000008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moraes J.M., Felga G.E., Chebli L.A., Franco M.B., Gomes C.A., Gaburri P.D., et al. A full solid diet as the initial meal in mild acute pancreatitis is safe and result in a shorter length of hospitalization: results from a prospective, randomized, controlled, double-blind clinical trial. J. Clin. Gastroenterol. 2010;44(7):517–522. doi: 10.1097/MCG.0b013e3181c986b3. [DOI] [PubMed] [Google Scholar]
- 21.Horibe M., Iwasaki E., Nakagawa A., Matsuzaki J., Minami K., Machida Y., et al. Efficacy and safety of immediate oral intake in patients with mild acute pancreatitis: a randomized controlled trial. Nutrition (Burbank, Los Angeles County, Calif) 2020;74 doi: 10.1016/j.nut.2020.110724. [DOI] [PubMed] [Google Scholar]
- 22.Teich N., Aghdassi A., Fischer J., Walz B., Caca K., Wallochny T., et al. Optimal timing of oral refeeding in mild acute pancreatitis: results of an open randomized multicenter trial. Pancreas. 2010;39(7):1088–1092. doi: 10.1097/MPA.0b013e3181d3ce05. [DOI] [PubMed] [Google Scholar]
- 23.Yang J., Qin Q., Wang T., Zhuang Y-z, Xiang H., Zhao Y., et al. Effect of very early feeding on clinical outcomes in patients with mild acute pancreatitis. Parent. Enter. Nutr. 2018;25(5):301–3+7. [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng X., Zhang Y., Kwong J.S., Zhang C., Li S., Sun F., et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evidence-based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palma Pérez S., Delgado Rodríguez M. Practical considerations on detection of publication bias. Gac. Sanit. 2006;20(Suppl 3):10–16. doi: 10.1157/13101085. [DOI] [PubMed] [Google Scholar]
- 30.Esmer D., Rivera-Villalobos O., Hernández-Sierra J.F., Valencia-Sánchez L.D., Sánchez M. Immediate feeding tolerance in patients with mild acute biliary pancreatitis. Cirugía Cir. (Engl. Ed.) 2021;89(2):243–247. doi: 10.24875/CIRU.19001724. [DOI] [PubMed] [Google Scholar]
- 31.Koh Y.Y., Jeon W.K., Cho Y.K., Kim H.J., Chung W.G., Chon C.U., et al. The effect of intestinal permeability and endotoxemia on the prognosis of acute pancreatitis. Gut Liver. 2012;6(4):505–511. doi: 10.5009/gnl.2012.6.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leach S.D., Modlin I.M., Scheele G.A., Gorelick F.S. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J. Clin. Invest. 1991;87(1):362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Ma F., Jia K. Early enteral nutrition within 24 hours or between 24 and 72 hours for acute pancreatitis: evidence based on 12 RCTs. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2014;20:2327–2335. doi: 10.12659/MSM.892770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Zhu S., Tan D., Ma A., Yang Y., Xu J. A meta-analysis of early oral refeeding and quickly increased diet for patients with mild acute pancreatitis. Saudi J. Gastroenterol.: Off. J. Saudi Gastroenterol. Asso. 2019;25(1):14–19. doi: 10.4103/sjg.SJG_240_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu P., Li L., Sun W. Efficacy comparisons of enteral nutrition and parenteral nutrition in patients with severe acute pancreatitis: a meta-analysis from randomized controlled trials. Biosci. Rep. 2018;38(6) doi: 10.1042/BSR20181515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horibe M., Nishizawa T., Suzuki H., Minami K., Yahagi N., Iwasaki E., et al. Timing of oral refeeding in acute pancreatitis: a systematic review and meta-analysis. Unit. Euro. Gastroenterol. J. 2016;4(6):725–732. doi: 10.1177/2050640615612368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.